Abstract
A specific protein profile that accompanies neoplastic transformation in the premalignant airway epithelium could provide an opportunity for early diagnosis of lung cancer. The aim of this study was to screen and identify early candidate biomarkers of non–small cell lung cancer. Thirteen non–small cell lung cancer samples were obtained within 30 minutes after a surgical resection. Laser capture microdissection was performed to enrich the normal lung cell and squamous metaplasia or atypical adenomatous hyperplasia cell populations. The resulting tandem mass spectrum was automatically searched for proteins against International Protein Index (IPI) human protein database using the TurboSEQUEST searching engine. The molecular function and biological processes of identified proteins were determined based on universal bioinformatics tools. The 2 proteins of interest, focal adhesion kinase and C-terminal Src kinase, were validated using Western blot method. A total of 863 proteins were identified by automatically searching the tandem mass spectrum, among which 427 were dysregulated expression in premalignant airway epithelium compared with those of normal lung cells. The 427 proteins were mainly distributed in 24 sorts of cellular components, 22 molecular function, 15 biological processes, and 10 significant perturbations of pathways. The most significant network included 48 genes and was related to energy production, cell cytoskeleton, cell adhesion, metabolism, oxidative stress, and small molecule biochemistry. Focal adhesion kinase and C-terminal Src kinase were significantly overexpressed in premalignant lung lesion cells compared with the normal lung cells in 13 cases. We identified that there were 427 proteins involved in non–small cell lung cancer carcinogenic process and confirmed the key biological pathways in premalignant lung tissue. The significantly upregulated focal adhesion kinase and C-terminal Src kinase could be considered as molecular biomarkers for early diagnosis and prognosis of non–small cell lung cancer.
Keywords: NSCLC, early diagnosis, biomarkers, proteomics, FAK, C-Src
Introduction
Nowadays, lung cancer has the highest incidence in all kinds of malignant tumors and is the most cause of cancer-related death worldwide as well. In 2014, in the United States, 224210 new cases of lung cancer were diagnosed, including 159260 cancer-related deaths.1 With increase in the count of smokers and in pollution in the environment, it is estimated that more than 1 000 000 patients are diagnosed with lung cancer every year in China.2 Therefore, the timely diagnosis and effective treatment of lung cancer become more and more important. In the last decade, a significant progress has been made in lung cancer screening using minimally invasive techniques for diagnosis, and the treatment included targeted molecular therapies.3 However, only 16.6% of patients with lung cancer survived 5 years or more after the diagnosis.4 Currently, several molecular biomarkers are commonly used in clinical practice to identify a variety of solid tumors, such as carcinoembryonic antigen, cytokeratin 19 fragment (CYFRA21-1), neuron-specific enolase, and so on, but none of them is able to identify lung cancer in early stage.
The molecular pathogenesis of lung cancer shows that the development of lung cancer involves several stages, which transformed from a normal airway epithelium to a premalignant lesion, squamous metaplasia or atypical adenomatous hyperplasia (AAH), and to a malignant lesion, carcinoma in situ, and invasive carcinoma. When lung cancer is diagnosed in precancerous stage, the survival rate can be significantly improved by some effective intervention measures. Therefore, it is very crucial to differentiate the critical molecule of normal lung and cancerous lung in the intermediate stage, which can show lung neoplastic transformation.
Some experts have found some valuable biomarkers in the diagnosis of premalignant lung lesion, for instance, Lowe et al5 used a novel autoantibody test to detect the preneoplastic lung lesions, and Rice et al6 identified plasma apolipoprotein E as being associated with smoking and a marker for squamous metaplasia of the lung. Unfortunately, there are seldom studies that are relevant to proteomics of lung squamous metaplasia and AAH cell, considering the difficulties in collecting even modest number of cell samples from patients with premalignant lung lesions.
In this study, we first successfully collected squamous metaplasia and AAH cell samples from patients with premalignant lung lesions using laser capture microdissection (LCM). We further applied advanced mass spectrometry–based proteomics to identify the different protein profiles between atypical premalignant lung lesions that accompany neoplastic transformation and normal lung cell. The 10 interesting proteins that can be taken as promising candidate biomarkers for early lung cancer were screened via bioinformatics analysis and biochemical validations. Our results indicated that screening and identification of protein profiles of premalignant lung lesion could be a feasible and effective strategy used to search for the potential early detection biomarkers for lung cancer.
Materials and Methods
Patient Populations
Thirteen non–small cell lung cancer (NSCLC) tumor samples (7 squamous cell cancers and 6 adenocarcinomas) were obtained within 30 minutes after a surgical resection and stored in liquid nitrogen until use. The study was approved by the institutional review board, and informed consent was obtained from all individuals. Tumor specimens were diagnosed by histopathology as 4 cases well differentiated, 7 moderate, and 2 poor and no metastasis of distant organs. Cases were reviewed prior to inclusion according to pathomorphism features. Paraffin sections from the surgical resection tumor specimens stained with hematoxylin–eosin were reviewed to assure presence of the normal lung and premalignant lung lesions. Squamous metaplasia or AAH was histologically identified by 2 veteran pathologists with complete agreement according to cell density, cell size, nuclear size, and nucleolar morphology. Snap-frozen tissue blocks of normal lung and premalignant lung lesions, embedded in optimum cutting temperature medium, were cut with a cryostat into 10 μm sections and mounted on uncoated glass slide. Cryostat sections were stored at −80°C until the time of the LCM.
Laser Capture Microdissection for Cell Collection
Laser capture microdissection was performed to enrich the normal lung cells and squamous metaplasia or AAH cell populations. The Arcturus PixCell II system (Arcturus, Mountain View, California) incorporates an Olympus IX-50 microscope containing a microscope slide stage that is moved by a joystick. The system uses a pulsed infrared laser to activate a thermoplastic film placed over the cells of interest, causing the film to fuse the cells. Laser shots were repeated until all cells of interest were collected into a film-coated plastic cap. The plastic-fused cells were removed from the tissue specimen and frozen at −80°C immediately. Then, all LCM cap samples were thawed on ice for 30 minutes to 1 hour. Cells were then transferred into an Eppendorf tube and further crushed with ultrasound for 50 seconds. The supernatant was taken and added 5-folds of acetone to precipitate protein and remove staining reagents. The precipitation was dissolved in lysis buffer and quantitated by Bradford protein assay using bovine serum albumin as a protein standard. The precipitation was dissolved in 100 μL reduction buffer. The sample was exchanged into 100 mmol/L ammonium bicarbonate buffer (pH 8.5). The buffer-exchanged sample was incubated with trypsin (50:1) and incubated at 37°C overnight. The solution was stored at −80°C until ready to be subsequently processed for analysis.
Proteomics Analysis
One run of high-performance liquid chromatography (HPLC) and ion trap (IT) tandem mass spectrum (MS/MS) was performed from the total pooled solution of LCM premalignant and normal lung cells samples. Chromatography was performed using a surveyor HPLC system (Thermo Finnigan, San Jose, California) on C18 reverse phase column (180 μm × 150 mm, BioBasic C18, 5 μm; Thermo Hypersil-Keystone, America). The column effluent from the reverse phase column was analyzed by linear trap quadrupole (LTQ)-IT mass spectrometer. The microelectrospray interface used a 30-μm metal needle that was orthogonal to the inlet of the LTQ. The resulting MS/MS spectra were automatically searched for proteins against International Protein Index (IPI) human protein database using the TurboSEQUEST searching engine of the BioWorks 3.0 software.
Bioinformatics Analysis
Protein function family cluster, including cellular component, molecular function, and biological process, was determined based on universal gene ontology annotation terms (http://www.ebi.ac.uk/GOA/) by extracting the number of identified proteins in IPI using Blast2GO program.7 The identified proteins showing obvious correlation with tumor genesis were analyzed using GeneGo MetaCore software (version 5.3; http://www.genego.com). The gene content of the uploaded files was used as the input list for the generation of biological networks using analyze network algorithm with default settings. This is a variant of the shortest path algorithm with main parameters of relative enrichment with the uploaded data and relative saturation of networks with canonical pathways. The Fisher exact test was used to calculate a P value determining the probability that the association between the genes in the data set and the canonical pathway was explained by chance alone.
Western Blot Analysis
We selected the identified protein focal adhesion kinase (FAK) and C-terminal Src kinase (C-Src) with the highest expression rate to be validated using Western blot methods. The protein samples (80 μg) of premalignant lung lesions and normal lung were run on 12% sodium dodecyl sulfate–polyacrylamide gel electrophoresis, transferred onto polyvinylidene fluoride membranes, and then were blocked with 5% nonfat milk and incubated with the respective primary antibodies (FAK and C-Src antibody; Biosource International, Camarillo, California) for 1 hour at room temperature. The protein abundance of glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as a control for protein loading. Afterward, the membranes were incubated with the respective horseradish peroxidase (HRP)-conjugated secondary antibody (The Jackson Laboratory, Bar Harbor, Maine) and an HRP-conjugated anti-GAPDH to confirm equal protein loading in each lane for 1 to 2 hours at 37°C or room temperature. The samples were washed and detected with enhanced chemiluminescence for 30 to 60 seconds (Minipore, America).
Results
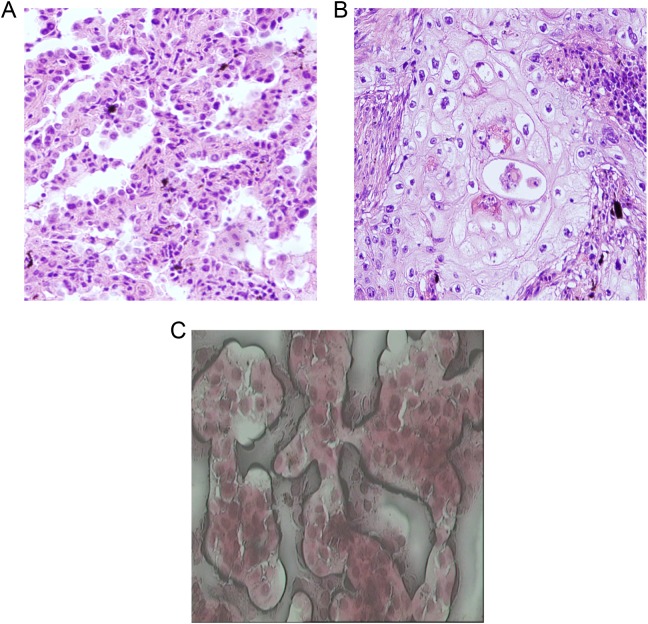
The premalignant lung lesions including squamous metaplasia or AAH cells are characterized by relatively more cell counts and high cell density. The cells are slightly larger than the normal lung alveolar epithelial cell, and the nuclear size is approximately 2-fold than that of small lymphocytes (Figure 1A and B). Laser capture microdissection was performed with capture of about 70 000 cells from the premalignant lung lesions and normal lung tissue, respectively (Figure 1C). Cells samples were prepared for subsequent proteomics analysis.
Figure 1.
Examples of laser capture microdissected representative sections from the premalignant lung lesion and normal lung submitted for proteomics analysis. Histology was confirmed with hematoxylin–eosin (H&E): atypical adenomatous hyperplasia cells (A), squamous metaplasia cells (B), and laser capture microdissection (LCM) acquisition of atypical premalignant lung cells (C).
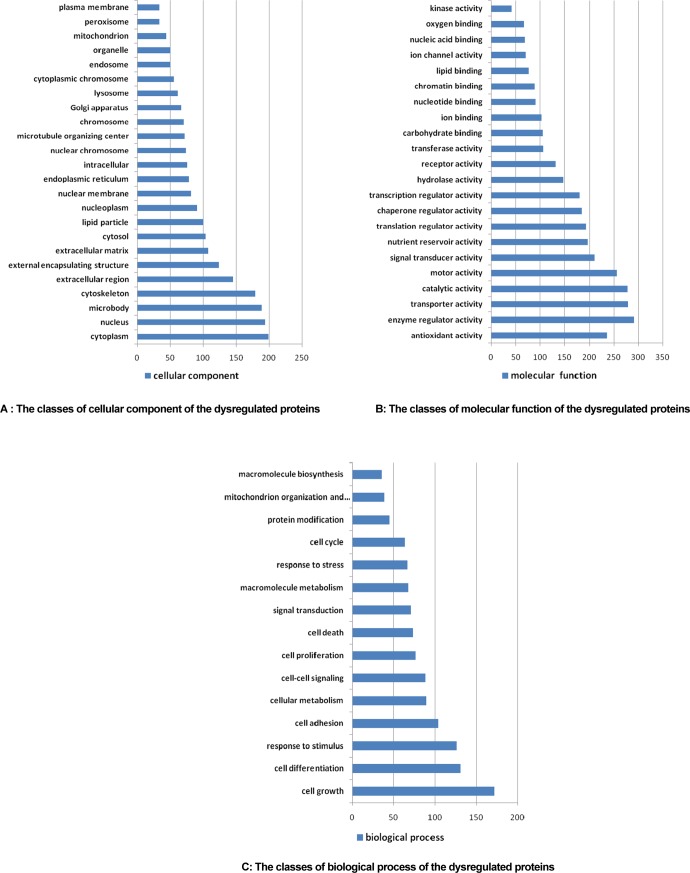
A total of 863 proteins were identified by automatically searching the MS/MS spectrum, among which 427 were dysregulated. The proteins in premalignant lung lesion could be classified by their cell component, molecular function, and biological process with the tools on www.geneontology.org (Figure 2A–C). The expression proteins were mainly distributed in 24 cellular components, among which 9 kinds of components with more than 100 proteins were cell cytoplasm (199, 23.1%), nucleus (194, 22.4%), microbody (189, 21.9%), cytoskeleton (179, 20.7%), extracellular region (145, 16.8%), and external encapsulating structure (124, 14.4%), respectively. Molecular function was classified into 22 sorts, including enzyme regulator activity (291, 33.7%), transporter activity (279, 32.3%), catalytic activity (278, 32.2%), motor activity (256, 29.7%), antioxidant activity (236, 27.3%), signal transducer activity (211, 24.4%), and so on. Biological process included cell growth (172, 19.9%), cell differentiation (131, 15.2%), response to stimulus (126, 14.6%), cell adhesion (104, 12.1%), and so on.
Figure 2.
The 427 dysregulated proteins could be classified by their cell component (A), molecular function (B), and biological process (C) with the tools on www.geneontology.org.
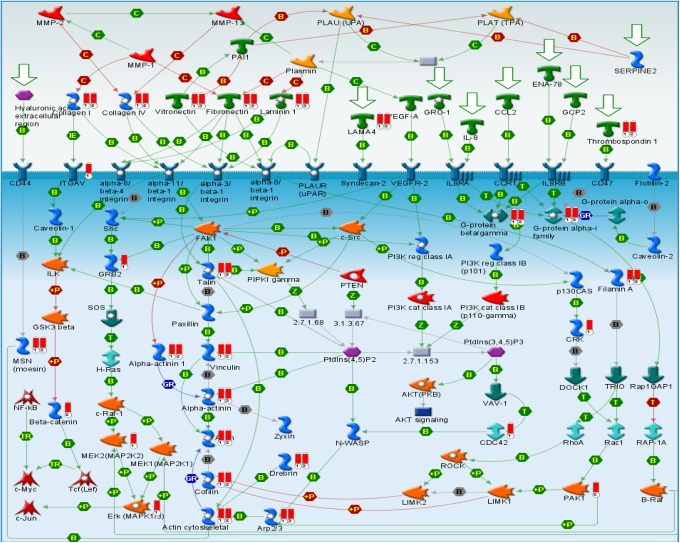
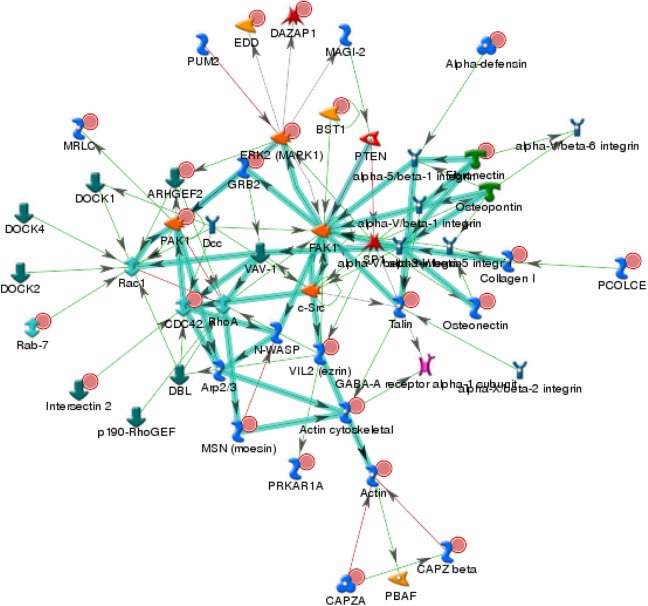
The canonical pathway of expression proteins was analyzed by the GeneGo MetaCore software. Canonical pathway maps represent a set of signaling and metabolic maps covering human biology in a comprehensive way. The analysis showed significant perturbation of GeneGo pathways, including chemokines and adhesion, regulation of actin, cytoskeleton by Rho GTPases, integrin-mediated cell adhesion, cytoskeleton remodeling, integrin-mediated cell adhesion and migration, antigen presentation by major histocompatibility complex class I, regulation of cystic fibrosis transmembrane conductance regulator (CFTR) activity, integrin outside-in signaling, and endothelial cell contacts by nonjunctional and neurofilaments (Table 1). The top scored map of chemokines and adhesion with the lowest P value based on the enrichment distribution sorted by difference set is illustrated in Figure 3. To overview the relations between the upregulated genes and the downregulated ones, we also looked into the integrated total networks that include these genes. The most significant network included 48 genes and was related to energy production, cell cytoskeleton, cell adhesion, metabolism, oxidative stress, and small molecule biochemistry (Figure 4). A list of the 10 most significantly different proteins in terms of the number of identified unique peptide and cover percentage were selected as candidate biomarkers for lung cancer (Table 2).
Table 1.
The Top Pathways Including Upregulated and Downregulated Proteins.a
| GeneGo Pathway Name | P |
|---|---|
| Chemokines and adhesion | 1.936×10−12 |
| Regulation of actin cytoskeleton by Rho GTPases | 5.217×10−12 |
| Integrin-mediated cell adhesion | 1.361×10−10 |
| Cytoskeleton remodeling | 2.305×10−10 |
| Integrin-mediated cell adhesion and migration | 2.890×10−10 |
| Antigen presentation by MHC class I | 2.268×10−8 |
| Regulation of CFTR activity | 3.936×10−8 |
| Integrin outside-in signaling | 5.990×10−8 |
| Endothelial cell contacts by nonjunctional mechanism | 1.415×10−7 |
| Neurofilaments | 2.032×10−7 |
Abbreviations: MHC, major histocompatibility complex; CFTR: cystic fibrosis transmembrane conductance regulator.
a Data sets were analyzed by GeneGo MetaCore software (version 5.3; http://www.genego.com). The significance is expressed as a P value that is calculated using the Fisher exact test.
Figure 3.
The top scored map of chemokines and adhesion with the lowest P value based on the enrichment distribution sorted by difference set. Experimental data from all files are linked to and visualized on the maps as thermometer-like figures. Upward thermometers (red) indicate upregulated signals, and downward (blue) ones indicate downregulated expression levels of the genes. Data set was analyzed by the GeneGo MetaCore software (version 5.3; http://www.genego.com).
Figure 4.
The first scored integrated network with both upregulated and downregulated proteins in premalignant lung compared with the normal lung. The network included 48 proteins and was related with energy production, cell cytoskeleton, cell adhesion, metabolism, oxidative stress, and small molecule biochemistry. Upregulated genes are marked with red circles and downregulated with blue circles. Data set was analyzed by the GeneGo MetaCore software (version 5.3; http://www.genego.com).
Table 2.
The Top 10 Proteins Were Chosen as Candidate Biomarkers for Lung Cancer.
| Protein Name | Unique Peptide Number | Cover Percentage | Function Description |
|---|---|---|---|
| FAK1 | 52 | 61.25 | FAK plays a prominent role in tumor progression and metastasis through its regulation of both cancer cells and their microenvironments |
| C-Src | 49 | 51.66 | Src is involved in the proliferation, survival, adhesion, migration, invasion, and metastasis in multiple tumor types |
| Integrin | 42 | 46.34 | Integrins are transmembrane receptors that are the bridges for cell–cell and cell–extracellular matrix interactions |
| GRB2 | 39 | 41.07 | Grb2 could link the epidermal growth factor receptor tyrosine kinase to the activation of Ras and its downstream kinases, ERK1/2 |
| PAK1 | 37 | 38.41 | PAK proteins are critical effectors that link the Rho family of GTPases to cytoskeleton reorganization and nuclear signaling |
| CDC42 | 34 | 32.09 | CDC42 is a small GTPase of the Rho family, which regulates signaling pathways that control diverse cellular functions including cell morphology, migration, endocytosis, and cell cycle progression |
| Rac1 | 28 | 27.45 | Rac1 is a small signaling G protein, regulates a diverse array of cellular processes, including the cell cycle, cell–cell adhesion, motility, and epithelial differentiation |
| RhoA | 26 | 25.71 | RhoA is primarily involved in actin organization, cell cycle maintenance, cellular development, and transcriptional control |
| VAV1 | 17 | 20.98 | VAV1 initiates profound morphological changes, cytoskeletal rearrangements, and the JNK/SAPK signaling cascade. |
| Talin | 15 | 17.06 | Talin is capable of linking integrins to the actin cytoskeleton either directly or indirectly by interacting with vinculin and α-actinin |
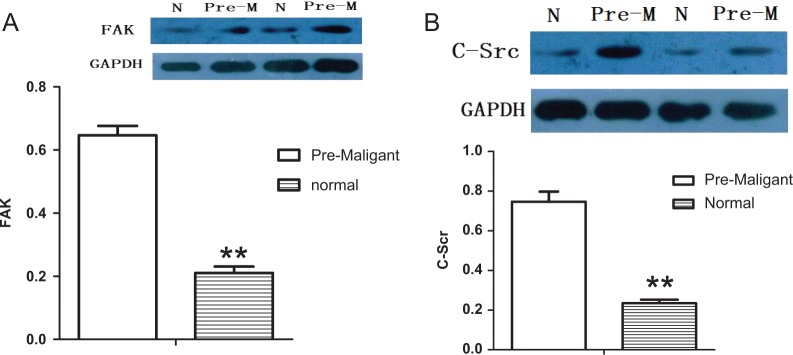
We went ahead to assess FAK and C-Src using Western blot assays because it was the excreted protein with the high rate in the tumor genesis and had already been identified as an upregulated protein in premalignant lung lesion. The results confirmed that the FAK and C-Src significantly overexpressed in premalignant lung lesion cells compared with the normal lung cells for all 13 cases of patients (Figure 5).
Figure 5.
Western blot assay validated the expression of focal adhesion kinase (FAK; A) and C-terminal Src kinase (C-Src; B) according to proteomics analysis. The FAK and C-Src were significantly overexpressed in premalignant lung lesions cells compared with the normal lung cells (P < .05).
Discussion
Early diagnosis and treatment are critical for improving the prognosis of lung cancer. Several lung cancer detection trials showed no reduction in lung cancer mortality among high-risk cohorts of smokers screened with sputum cytology plus radiographic techniques compared with radiographic screening alone.8 We hypothesized that identifying specific protein profile that accompanies neoplastic transformation in the premalignant airway epithelium could provide an opportunity to screen early lung cancer biomarkers. In this study, morphologically recognizable atypical premalignant cell populations from each lung tumor case were successfully harvested with a PixCell II LCM system from Arcturus Engineering (Mountain View, California) based on LCM procedures.9 Each cell populations were determined to be 95% homogeneous by microscopic visualization of the captured cells. A comparative proteomic profile of atypical premalignant lung cells and normal bronchial epithelial cells has been separately acquired using a previously developed high-throughput screening method. This allowed the identification of dysregulated proteins in accordance with the difference expression.
Canonical pathway and integrated network analyses indicated the presence of a dysregulation of the chemokines and adhesion GeneGO pathway in premalignant lung tissue. Chemokines and adhesion molecules are a group of small proteins that act together with their cell surface receptors in growth factor signaling, cell proliferation, cell survival, and cell migration.10,11 Recently, the importance of chemokines and chemokine receptors in inflammation associated with carcinogenesis has been highlighted. Chemokines are produced by epithelial cancer cells, leading to the recruitment of tumor-associated macrophages, tumor-associated neutrophils, lymphocytes, cancer-associated fibroblasts, mesenchymal stem cells, and endothelial cells into the tumor microenvironment.12 Chemokine ligands and receptors are downstream of genetic events that cause neoplastic transformation and are abundantly expressed in chronic inflammatory conditions that predispose to cancer.13 Several studies have suggested that chemokines and their receptors play a key role in the initiation or progression of cancers of the lung, colon, liver, breast, cervix, prostate, bladder, ovary, esophagus, skin, and lymphatics.14,15 Our study confirmed the chemokines and adhesion seem to be a key pathway in the neoplastic progression of lung cancer.
A total of 10 proteins were identified as potential biomarkers. Among the different proteins, the FAK and the C-Src appear to be particularly interesting. Focal adhesion kinase is a 125-kDa nonreceptor tyrosine protein kinase that was first identified in Src-transformed fibroblasts.16 Focal adhesion kinase plays a significant role in regulating signals at sites of cell extracellular matrix adhesion through integrins and at activated growth factor receptors.17 It seems that FAK can also influence cell movement, regulate cytoskeleton remodeling and membrane protrusions, and affect cell proliferation by regulating cell cycle progression from G1 to S phases.18,19 C-terminal Src kinase also is a nonreceptor tyrosine kinase protein encoded by the Src gene in humans. C-terminal Src kinase includes an SH2 domain, an SH3 domain, and a tyrosine kinase domain. An elevated level of activity of C-Src tyrosine kinase is suggested to be linked to cancer progression by promoting other signals.20 The activation of the C-Src pathway has been observed in about 50% of tumors from colon, liver, lung, breast, and the pancreas.21
Recent studies found that the dual-kinase FAK-Src could work as a protein complex in the cellular signaling networks.22 The mutually activated FAK/Src complex initiates a cascade of phosphorylation events of new protein–protein interactions to trigger several signaling pathways that eventually lead to different cellular responses.23,24 Some reports have already suggested an additional central role for this complex in cancer through its ability to promote proliferation, angiogenesis, epithelial mesenchymal transition, and anoikis resistance in tumor tissues.23
Conclusion
In our study, we demonstrated that minor changes in morphology from cells recognized as intermediate in premalignant lung lesion may be linked to increased expression or phosphorylation of some key proteins. We also found a significant upexpression of FAK and C-Src in atypical premalignant lung lesions, which probably indicated the more aggressive and invasive phenotype in early NSCLC. This study is the first step of taking FAK and C-Src as early predicted biomarkers for lung cancer. However, our study was limited by its small samples for MS/MS and Western blot analysis. The mechanism of FAK and C-Src from a normal airway epithelium to a precancerous lesion, carcinoma in situ, and invasive carcinoma is still unclear. We need to further confirm the precise mechanism by high-throughput clinical samples and a large-scale immunochemistry in the future study.
Abbreviations
- AAH
adenomatous hyperplasia
- C-Src
C-terminal Src kinase
- FAK
focal adhesion kinase
- GAPDH
glyceraldehyde-3-phosphate dehydrogenase
- HPLC
high-performance liquid chromatography
- HRP
horseradish peroxidase
- IT
ion trap
- LCM
laser capture microdissection
- LTQ
linear trap quadrupole
- MS/MS
tandem mass spectrum
- NSCLC
non–small cell lung cancer
Footnotes
Authors’ Note: Yandong Nan and Jie Du contributed equally to this work.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by National Nature Science Foundation of China (No. 81001040).
References
- 1. Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014;64(1):9–29. [DOI] [PubMed] [Google Scholar]
- 2. Guo P, Huang ZL, Yu P, Li K. Trends in cancer mortality in China: an update. Ann Oncol. 2012;23(10):2755–2762. [DOI] [PubMed] [Google Scholar]
- 3. Ettinger DS. Ten years of progress in non-small cell lung cancer. J Natl Compr Canc Netw. 2012;10(3):292–295. [DOI] [PubMed] [Google Scholar]
- 4. Howlader N, Noone AM, Krapcho M, et al. SEER Cancer Statistics Review, 1975-2008. Bethesda, MD: National Cancer Institute; 2011. [Google Scholar]
- 5. Lowe FJ, Shen W, Zu J, et al. A novel autoantibody test for the detection of pre-neoplastic lung lesions. Mol Cancer. 2014;13:78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Rice SJ, Liu X, Miller B, et al. Proteomic profiling of human plasma identifies apolipoprotein E as being associated with smoking and a marker for squamous metaplasia of the lung. Proteomics. 2015;15(18):3267–3277. [DOI] [PubMed] [Google Scholar]
- 7. Götz S, García-Gómez JM, Terol J, et al. High-throughput functional annotation and data mining with the Blast2GO suite. Nucleic Acids Res. 2008;36(10):3420–3435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Tockman MS. Survival and mortality from lung cancer in a screened population-the Johns Hopkins Study. Chest. 1986;89(suppl 4):324s–325s.3956302 [Google Scholar]
- 9. Emmert-Buck MR, Bonner RF, Smith PD, et al. Laser capture microdissection. Science. 1996;274(5289):998–1001. [DOI] [PubMed] [Google Scholar]
- 10. Raman D, Sobolik-Delmaire T, Richmond A. Chemokines in health and disease. Exp Cell Res. 2011;317(5):575–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bryan RT. Cell adhesion and urothelial bladder cancer: the role of cadherin switching and related phenomena. Philos Trans R Soc Lond B Biol Sci. 2015;370(1661):20140042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lazennec G, Richmond A. Chemokines and chemokine receptors: new insights into cancer-related inflammation. Trends Mol Med. 2010;16(3):133–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Allavena P, Germano G, Marchesi F, Mantovani A. Chemokines in cancer related inflammation. Exp Cell Res. 2011;317(5):664–673. [DOI] [PubMed] [Google Scholar]
- 14. Ali S, Lazennec G. Chemokines: novel targets for breast cancer metastasis. Cancer Metastasis Rev. 2007;26(3-4):401–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Vindrieux D, Escobar P, Lazennec G. Emerging roles of chemokines in prostate cancer. Endocr Relat Cancer. 2009;16(3):663–673. [DOI] [PubMed] [Google Scholar]
- 16. Halder J, Lin YG, Merritt WM, et al. Therapeutic efficacy of a novel focal adhesion kinase inhibitor TAE226 in ovarian carcinoma. Cancer Res. 2007;67(22):10976–10983. [DOI] [PubMed] [Google Scholar]
- 17. Schaller MD. Cellular functions of FAK kinases: insight into molecular mechanisms and novel functions. J Cell Sci. 2010;123(pt 7):1007–1013. [DOI] [PubMed] [Google Scholar]
- 18. Zhao J, Pestell R, Guan JL. Transcriptional activation of cyclin D1 promoter by FAK contributes to cell cycle progression. Mol Biol Cell. 2001;12(12):4066–4077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wang X, Urvalek AM, Liu J, Zhao J. Activation of KLF8 transcription by focal adhesion kinase in human ovarian epithelial and cancer cells. J Biol Chem. 2008;283(20):13934–13942. [DOI] [PubMed] [Google Scholar]
- 20. Wheeler DL, Iida M, Dunn EF. The role of Src in solid tumors. Oncologist. 2009;14(7):667–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Dehm SM, Bonham K. SRC gene expression in human cancer: the role of transcriptional activation. Biochem Cell Biol. 2004;82(2):263–274. [DOI] [PubMed] [Google Scholar]
- 22. Bolós V, Gasent JM, López-Tarruella S, Grande E. The dual kinase complex FAK-Src as a promising therapeutic target in cancer. Onco Targets Ther. 2010;3:83–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Mitra SK, Schlaepfer DD. Integrin-regulated FAK–Src signaling in normal and cancer cells. Curr Opin Cell Biol. 2006;18(5):516–523. [DOI] [PubMed] [Google Scholar]
- 24. Playford MP, Schaller MD. The interplay between Src and integrins in normal and tumor biology. Oncogene. 2004;23(48):7928–7946. [DOI] [PubMed] [Google Scholar]