Abstract
Glioblastoma multiforme, the most common and aggressive form of primary brain tumor, presents a dismal prognosis. MicroRNAs play a critical role in the initiation, progression, and metastasis of cancer; however, the potential biological role of miRNAs in glioblastoma multiforme remains largely unknown. In our study, we found that microRNA-96 is upregulated in glioma tissues than in normal human brains. Transfection of microRNA-96 mimics into glioma cells significantly decreases apoptosis by suppressing PDCD4, a well-known tumor suppressor that is involved in apoptosis. In contrast, knockdown of microRNA-96 enhanced apoptosis. In vivo, microRNA-96 overexpression inhibits the apoptosis and increases tumor growth. These data suggest that microRNA-96 is a potential molecular target for glioma treatment.
Keywords: microRNA-96, PDCD4, apoptosis, glioma
Introduction
Glioma multiforme is the highly prevalent and most common primary brain cancer, accounting for about 80% of all diagnosed cancers in the central nervous system and is associated with high mortality and a poor prognosis.1 Despite intensive efforts to improve current treatment and explore new therapeutic targets, the median survival rate of malignant glioma has not improved, and patients diagnosed with glioblastoma, the most malignant form of glioma, only live about 1 year after diagnosis.2 Thus, an increased identification of novel therapeutic targets is expected to aid the development of new treatment in glioma.
Studies performed in a variety of organisms have revealed that microRNAs (miRNAs) regulate many cellular processes, demonstrated that they could be involved in cancer.3 MicroRNAs are small, noncoding RNAs of approximately 20 to 24 nucleotides, which have been implicated in the regulation of a variety of cellular events.4 MicroRNAs’ target regulation is canonically mediated by nucleotides 2-8 at the 5′ portion of the miRNA, termed the seed region.5 MicroRNAs negatively regulate gene expression by messenger RNA (mRNA) degradation or repressing mRNA translation through binding to complementary sequences in the 3′-untranslated region (3′UTR).6 Dysregulation of global miRNA expression has been implicated in different human cancers. Therefore, exploring the biological role and mechanism of miRNAs that is aberrantly expressed in tumor is critically important for the diagnosis, prevention, and therapy of cancer.
Aberrant expression of miRNA-96 has been showed in different types of cancers and appears to be markedly associated with the clinical prognosis of patients with tumor. For example, miRNA-96 is significantly upregulated in breast cancer and promotes tumor growth and invasion by targeting RECK.7 MicroRNA-96 was also identified to be markedly upregulated in prostate cancer cells and cancer tissues compared with normal prostate cells and normal prostate tissues by microarray and reverse transcription-polymerase chain reaction (RT-PCR) analysis.8 In the acute myeloid leukemia, the expression of miRNA-96 was downregulated and associated with leukemic burden as well as recurrence-free survival and overall survival.9 However, the role of miRNA-96 in glioma cells is not fully clear.
In our study, we reported that miRNA-96 is higher in human glioma tissue than in normal tissue. Ectopic expression of miRNA-96 decreases apoptosis in glioma cells by targeting PDCD4, indicating that miRNA-96 is a potential molecular target for glioma treatment.
Materials and Methods
Cell Culture
U87 and U251 cell lines were purchased from American Type Culture Collection and were cultured in RPMI-1640 medium with 10% fetal calf serum at 5% carbon dioxide. Antibodies against PDCD4 and actin were purchased from Cell Signaling Technology (Boston, Massachusetts).
RNA Extraction and Quantitative Real-Time Polymerase Chain Reaction
Total RNA was extracted using the TRIzol reagent (Life Technologies, NY, USA) according to the manufacturer’s protocols. To measure the miRNA-96 expression level, the reverse transcription was performed using a TaqMan miRNA reverse transcription kit, and real-time quantitative RT-PCR analysis was performed using SYBR (Roche, Switzerland) and LightCycler (Roche, Switzerland). The reaction was performed at 94°C for 5 minutes followed by 40 cycles of 10 seconds at 94°C, 45 seconds at 60°C. The relative expression level of miRNA-96 was normalized by U48 small nuclear RNA48 expression.
Transfections
All transfections were performed using Lipofectamine 2000 (Invitrogen, Carlsbad, California) according to the manufacturer’s instructions. The miRNA-96 inhibitor, mimics, and the scramble were purchased from GenePharma (Shanghai, China). The miRNA-96 inhibitor and mimics were used at a final concentration of 50 nmol/L.
Vector Construction
To construct the pmirGLO-PDCD4-3′UTR-WT vector, a fragment of the wild-type 3′UTR of human PDCD4 mRNA (GenBank accession number: NM_014456.4) containing the putative miRNA-96 binding sequence was amplified and cloned into the Xho l and Sac l sites downstream of the luciferase reporter gene in the pmirGLO-control vector. pmirGLO-PDCD4-3′UTR-mut, which harbored mutations in the complementary sequence in the seed region of miRNA-96, was generated based on the pmirGLO-PDCD4-3′UTR-WT plasmid by site-specific mutagenesis.
Luciferase Reporter Assay
For the luciferase reporter assay, HEK 293T cells were plated in a 96-well plate and were grown to 60% to 70% confluence. HEK 293T cells were cotransfected with 50 nmol/L of either scramble or miRNA-96 mimics and 200 ng of either pmirGLO-PDCD4-3′UTR-WT or pmirGLO-PDCD4-3′UTR-mut using Lipofectamine 2000. The cells were harvested 48 hours posttransfection and measured using the Dual-Luciferase Reporter Assay System (Promega, Madison, Wisconsin).
Western Blotting
Protein was lysed with radioimmunoprecipitation assay buffer containing inhibitor cocktail (Roche, Basel, Switzerland), quantified with Micro BCA protein kit (Pierce, NY, USA),and then resolved on a sodium dodecyl sulfate polyacrylamide gel electrophoresis gel. The protein was then transferred onto polyvinylidene fluoride membrane (Fisher Scientific, Pittsburgh, Pennsylvania), which was blocked in 5% dry milk in Tris-buffered saline and Tween 20 and incubated with an appropriate primary antibody. After being washed, the membranes were incubated with a secondary antibody. The proteins were detected by chemiluminescence.
Terminal Deoxynucleotidyl Transferase dUTP Nick End Labeling Analysis
For in vivo terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) assay, optical coherence tomography-embedded sections from mouse tumors were fixed with freshly prepared 10% buffered neutral formalin (Fisher Scientific ). After permeabilization with 0.1% Triton X-100 in phosphate-buffered saline (PBS) for 10 minutes, slides were rinsed with PBS and incubated with 3% H2O2 in methanol for 10 minutes at room temperature, followed by PBS washing and dUTP nick end labeling at 37°C for 1 hour in a humidified chamber.
Tumorigenicity Assay in Nude Mice
All experiments involving animals were undertaken in accordance with the Guizhou Aerospace Hospital of Health Guide for the Care and Use of Laboratory Animals. In detail, U2OS cells (1 × 106 cells) stably expressing miRNA-96 or the vector were inoculated bilaterally into the armpit region of 4-week-old immunodeficient nude mice. The tumor weight was measured 30 days after inoculation.
Statistical Analysis
Data are shown as mean ± standard deviation (SD). Statistical analysis was performed with SPSS software. Student t tests were used to determine statistical significance. The significance level was set at P < .05 for all analyses.
Results
MicroRNA-96 Is Overexpressed in Glioma
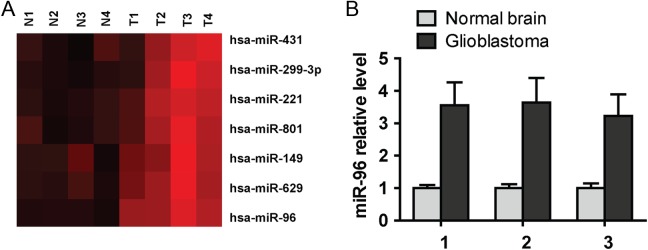
To explore the function of miRNA-96 in human glioma tissues, we analyzed the miRNA expression assay from a previously published datasheet (Gene Expression Omnibus accession GSE25631)10 and showed that miRNA-96 is significantly higher in glioma tissues than in normal tissues (Figure 1A). In addition, we further confirmed the miRNA-96 expression level in glioma. Quantitative RT-PCR data demonstrated that among 3 matched sample pairs, miRNA-96 expression level was markedly higher in clinical glioma tissue than in adjacent normal tissue (Figure 1B).
Figure 1.
MicroRNA (miRNA)-96 is overexpressed in glioma. A, MicroRNA-96 is significantly overexpressed in glioma tissue compared with normal tissue in miRNA expression assays. B, Real-time polymerase chain reaction (PCR) analysis shows microRNA-96 is obviously highly expressed in glioma tissues than in normal tissue.
MicroRNA-96 Inhibits the Apoptosis of Human Glioma Cells In Vitro
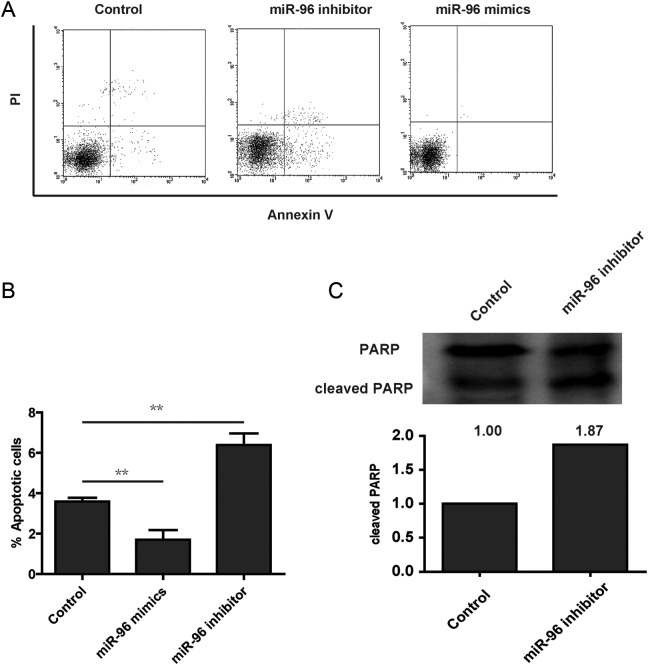
To assess the effects of miRNA-96 expression in glioma cell survival, the glioma cell line U87 was used. Cells were transfected with miRNA-96 mimics, miRNA-96 inhibitor, or scrambled control and were assayed with annexin V, a well-known marker of early apoptosis. Flow cytometry assay showed that a significant decrease in annexin V binding was detected at 48 hours after transfection with miRNA-96 mimics compared to the control cells (Figure 2A and B). As expected, miRNA-96 inhibitor increases the apoptosis cells (Figure 2A and B). To further confirm the effects of miRNA-96 on apoptosis in glioma cells, we performed the Western blot to analyze the cleaved level of PARP, and the result showed that miRNA-96 inhibitor increased the levels of cleaved PARP (Figure 2C). Briefly, these data suggested that overexpression of miRNA-96 protects the tumor cells from apoptosis.
Figure 2.
MicroRNA (miRNA)-96 promotes cell apoptosis. A, U251 cells were transfected with scramble, miRNA-96 mimics, or miRNA-96 inhibitor. Cells were stained with propidium iodide (PI) and annexin V and analyzed using fluorescence-activated cell sorting (FACS). B, The annexin V-positive populations of U2OS were transfected with scramble, microRNA-96 mimics, or microRNA-96 inhibitor. C, Cleaved PARP (poly ADP ribose polymerase) was detected using Western blot in U251 cells transfected with scramble or microRNA-96 mimics.
MicroRNA-96 Reduces PDCD4 Expression, a Tumor Suppressor Involved in Apoptosis
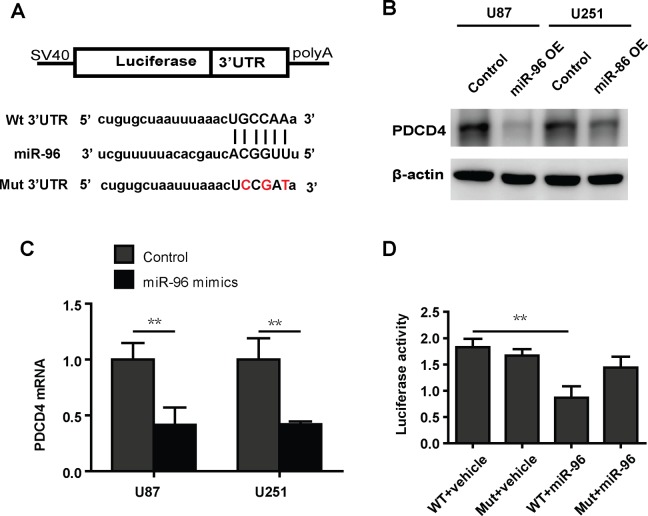
To further detect the underlying mechanism by which miRNA-96 decreases apoptosis in glioma cells, we analyzed the potential targets of miRNA-96 using Targetscan and miRanda to predict potential downstream targets.11,12 We were focused on the potential targets involved in apoptosis. PDCD4 is an apoptosis-related protein that has potential binding regions in the 3′UTR (Figure 3A). We tested that miRNA-96 repressed PDCD4 in glioma cells at both protein and mRNA levels (Figure 3A and B), indicating that miRNA-96 regulates the expression of PDCD4 by degrading the mRNA level. To explore whether PDCD4 is the direct target of miRNA-96, we performed the luciferase reporter assay. The results show that luciferase activity is significantly reduced by miRNA-96 overexpression cells cotransfecting wild-type 3′UTR vector of PDCD4, but not in cells cotransfected with mutant 3′UTR vector of PDCD4 (Figure 3C).
Figure 3.
MicroRNA (miRNA)-96 decreases the expression of PDCD4. A, The sequences in the 3′UTR of PDCD were predicted to potentially bind to miRNA-96. The red nucleotides were mutated to their complementary nucleotides. B, Western blot analysis shows that overexpression of microRNA-96 inhibits the protein level of PDCD in U87 and U251 cells. C, Real-time polymerase chain reaction (PCR) analysis shows that overexpression of microRNA-96 inhibits the mRNA level of PDCD in U87 and U251 cells. D, The luciferase reporter activity assay shows that microRNA-96 suppresses luciferase activity in the wild-type UTR but does not suppress activity in the mutant UTR.
MicroRNA-96 Decreases Tumor Apoptosis In Vivo
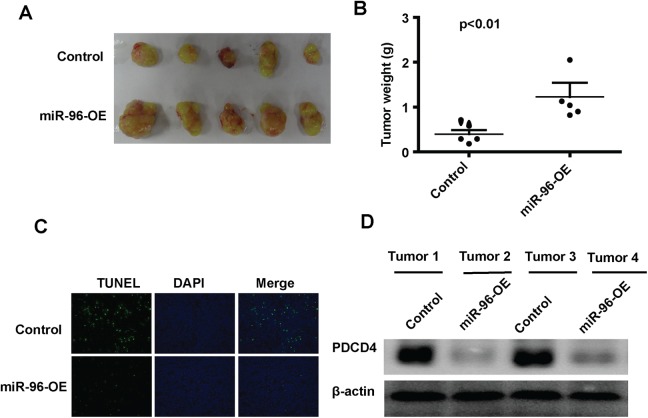
To examine the biological role of miRNA-96 on cancer apoptosis, the control U87 cells or U87 cells overexpressing miRNA-96 were subcutaneously injected into Balb/c nude mice. The tumor weight was measured after killing at the 30th day after injection. As shown in Figure 4A and B, the weight of miRNA-96 overexpression group is significantly increased compared with the control group (Figure 4A and B). In situ TUNEL analysis demonstrated that the tumors overexpressing miRNA-96 had less TUNEL-positive cells than in the control groups (Figure 4C). To further confirm the expression level of PDCD4 in mouse tumors, we lysed 2 tumor samples from the control group and miRNA-96 overexpression group, respectively, and then performed Western blot. The data showed that miRNA-96 decreased the PDCD4 protein level (Figure 4D). These results are consistent with our in vitro data that showed miRNA-96 regulates apoptosis by targeting PDCD4.
Figure 4.
MicroRNA (miRNA)-96 decreases the tumor cell apoptosis in vivo. A, The photo is a representative image of the xenograft tumors. B, The tumor weight was measured in the 2 groups. C, The terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL)-positive cells were analyzed in the tumor sections (original magnification, ×20). D, The expression of PDCD4 in xenograft tumors was analyzed by Western blot.
Discussion
Glioma is the most common primary tumor produced by the brain and spinal cord glial cells.13 The annual incidence rate of glioma is about 3 to 8 per 10 million people.14 Today, treatment of glioma is primarily through tumor resection and subsequent radiotherapy and chemotherapy, typically alkylating agents, for example, temozolomide. Despite intensive efforts to improve current treatment and explore new therapeutic targets, pivotal clinical improvement has remained absent during the last decade.2 Other forms of treatment, such as photodynamic therapy, based on photooxidative reactions and accumulation of photo sensitizers in tumor tissue, can be used to facilitate tumor resection and target cell proliferation.15 Molecular phenotyping of glioma is opening up the potential for molecularly targeted therapies.16 These can take the form of targeting specific components of oncogenic pathways, for example, through delivery of a therapeutic gene or miRNA.16 Several miRNAs are differentially expressed in a variety of malignancies compared to the corresponding healthy tissues.17 Some of these miRNAs have been shown to modulate oncogenes and tumor suppressors.17 However, it is still an ongoing process to elucidate new important deregulated miRNAs and their detailed roles in glioma carcinogenesis and progression.
Recently, a growing number of evidence has demonstrated that various aberrant miRNAs were involved in apoptosis in glioma.18 Some miRNAs may act as antiapoptotic, which are reported upregulated, and others serve as proapoptotic, which are often downregulated, in cell lines and glioma tissues.19 Therefore, increased expression of antiapoptotic miRNAs or decreased expression of proapoptotic miRNAs in cancers could be the potential therapeutic target by inhibiting the expression of antiapoptotic miRNAs or restore the expression of proapoptotic miRNAs, respectively.
Previous reports show that miRNA-96 is aberrantly expressed in various cancer, such as miRNA-96 is decreased in myeloproliferative neoplasms and overexpression of miRNA-96 suppressed hematopoietic cell growth and differentiation by targeting GBP2.20 In this study, we first found that the miRNA-96 was upregulated in clinical glioma tissue compared with the adjacent normal tissue, which is consistent with our analysis of a published microassay data. Previous reports revealed that miRNA-96 was characterized as oncomiR in some other types of cancer, such as lung cancer, colorectal cancer, and endometrial carcinoma.21 In combination with these findings, we further confirmed the role of miRNA-96 as oncomiR in glioma. Overexpression of miRNA-96 reduced apoptosis in glioma cells, in contrast, knockdown of miRNA-96 enhanced cell apoptosis in glioma cells. In addition, we found that miRNA-96 binds the 3′UTR of PDCD4 and thus leads to the decrease in expression both at mRNA and protein levels in vitro. These data indicated that miRNA-96 regulated glioma cell apoptosis via suppressing the expression of PDCD4. In the xenograft tumor model, overexpression of miRNA-96 in U87 cells significantly increased tumor growth. Consistent with our findings in apoptosis in vitro, overexpression of miRNA-96 decreased cell apoptosis. These observations strongly showed that miRNA-96 functioned as oncomiR by regulating apoptosis in glioma in vitro and in vivo.
In conclusion, our study first demonstrated that miRNA-96 is significantly overexpressed in glioma tissues. Moreover, miRNA-96 plays a critical role in apoptosis by inhibiting the expression of PDCD4 in glioma. Therefore, these observations suggest that miRNA-96 might be a molecular target for glioma therapy.
Abbreviations
- miRNAs
microRNAs
- mRNA
messenger RNA
- HEK
Human Embryonic Kidney
- PARP
poly ADP ribose polymerase
- PBS
phosphate-buffered saline
- RECK
reversion-inducing-cysteine-rich protein with kazal motifs
- RT-PCR
reverse transcription-polymerase chain reaction
- SD
standard deviation
- TUNEL
terminal deoxynucleotidyl transferase dUTP nick end labeling
- 3′UTR
3′-untranslated region
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The project received the financial support from Science and Technology Fund of the Bureau of Public Health of Guizhou province (NO. Gzwkj2011-1-098); State Administration of Traditional Chinese Medicine of Guizhou Province (NO. QZYY2012-63); and Science and Technology Department of Guizhou province (Guizhou J [2013] No. 2205).
References
- 1. Omuro A, DeAngelis LM. Glioblastoma and other malignant gliomas: a clinical review. JAMA. 2013;310(17):1842–1850. [DOI] [PubMed] [Google Scholar]
- 2. Paravati AJ, Heron DE, Landsittel D, et al. Radiotherapy and temozolomide for newly diagnosed glioblastoma and anaplastic astrocytoma: validation of Radiation Therapy Oncology Group-Recursive Partitioning Analysis in the IMRT and temozolomide era. J Neurooncol. 2011;104(1)339–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lee YS, Dutta A. MicroRNAs in cancer. Annu Rev Pathol. 2009;4:199–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lages E, Ipas H, Guttin A, Nesr H, Berger F, Issartel JP. MicroRNAs: molecular features and role in cancer. Front Biosci (Landmark Ed). 2012;17:2508–2540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kwak PB, Iwasaki S, Tomari Y. The microRNA pathway and cancer. Cancer Sci. 2010;101(11):2309–2315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Li C, Feng Y, Coukos G, Zhang L. Therapeutic microRNA strategies in human cancer. AAPS J. 2009;11(4):747–757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zhang J, Kong X, Li J, et al. microRNA-96 promotes tumor proliferation and invasion by targeting RECK in breast cancer. Oncol Rep. 2014;31(3):1357–1363. [DOI] [PubMed] [Google Scholar]
- 8. Yu JJ, Wu YX, Zhao FJ, et al. microRNA-96 promotes cell proliferation and clonogenicity by down-regulating of FOXO1 in prostate cancer cells. Med Oncol. 2014;31(3):1357–1363. [DOI] [PubMed] [Google Scholar]
- 9. Zhao J, Lu Q, Zhu J, Fu J, Chen YX. Prognostic value of microRNA-96 in patients with acute myeloid leukemia. Diagn Pathol. 2014;9:76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zhang W, Zhang J, Hoadley K, et al. miR-181d: a predictive glioblastoma biomarker that downregulates MGMT expression. Neuro Oncol. 2012;14(6):712–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Agarwal V, Bell GW, Nam JW, Bartel DP. Predicting effective microRNA target sites in mammalian mRNAs [published online August 12, 2015]. Elife. 2015;4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Betel D, Koppal A, Agius P, Sander C, Leslie C. Comprehensive modeling of microRNA targets predicts functional non-conserved and non-canonical sites. Genome Biol. 2010;11(8):r90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sturm D, Bender S, Jones DT, et al. Paediatric and adult glioblastoma: multiform (epi)genomic culprits emerge. Nat Rev Cancer. 2014;14(2):92–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ng K, Kim R, Kesari S, Carter B, Chen CC. Genomic profiling of glioblastoma: convergence of fundamental biologic tenets and novel insights. J Neurooncol. 2012;107(1):1–12. [DOI] [PubMed] [Google Scholar]
- 15. Mirimanoff RO, Gorlia T, Mason W, et al. Radiotherapy and temozolomide for newly diagnosed glioblastoma: recursive partitioning analysis of the EORTC 26981/22981-NCIC CE3 phase III randomized trial. J Clin Oncol. 2006;24(16):2563–2569. [DOI] [PubMed] [Google Scholar]
- 16. Weller M. Novel diagnostic and therapeutic approaches to malignant glioma. Swiss Med Wkly. 2011;141:w13210. [DOI] [PubMed] [Google Scholar]
- 17. Moller HG, Rasmussen AP, Andersen HH, Johnsen KB, Henriksen M, Duroux M. A systematic review of microRNA in glioblastoma multiforme: micro-modulators in the mesenchymal mode of migration and invasion. Mol Neurobiol. 2013;47(1):131–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zhang S, Wan Y, Pan T, et al. MicroRNA-21 inhibitor sensitizes human glioblastoma U251 stem cells to chemotherapeutic drug temozolomide. J Mol Neurosci. 2012;47(2):346–356. [DOI] [PubMed] [Google Scholar]
- 19. Koshkin PA, Chistiakov DA, Chekhonin VP. Role of microRNAs in mechanisms of glioblastoma resistance to radio- and chemotherapy. Biochemistry (Mosc). 2013;78(4):325–334. [DOI] [PubMed] [Google Scholar]
- 20. Xu D, He X, Chang Y, et al. Inhibition of microRNA-96 expression reduces cell proliferation and clonogenicity of HepG2 hepatoma cells. Oncol Rep. 2013;29(2):653–661. [DOI] [PubMed] [Google Scholar]
- 21. Guo H, Li Q, Li W, Zheng T, Zhao S, Liu Z. MicroRNA-96 downregulates RECK to promote growth and motility of non-small cell lung cancer cells. Mol Cell Biochem. 2014;390(1-2):155–160. [DOI] [PubMed] [Google Scholar]