Abstract
Background
TNFR-associated factor 1 (TRAF1) and TRAF2 have been demonstrated to inhibit apoptosis and promote cell survival in glioblastoma (GBM) cells with experiments in vitro. However, their clinical and prognostic significance have not been elucidated.
Material/Methods
In our study, we for the first time investigated the expression of TRAF1 and TRAF2 in 105 GBM tissues. Furthermore, we evaluated their clinical significance, including their association with clinicopathologic factors and prognostic value. The association with clinicopathologic factors was assessed by chi-square test. The relation of TRAF1/2 expression to survival rate was assessed by Kaplan-Meier method and Cox-regression model.
Results
We demonstrated that TRAF1 expression had no significant prognostic value for GBM. On the contrary, high expression of TRAF2 can predict poorer prognosis of GBM and was identified as an independent biomarker in GBM prognosis.
Conclusions
High expression of TRAF2 was identified as an independent biomarker in GBM prognosis, indicating TRAF2 as a novel drug target in GBM treatment.
MeSH Keywords: Biological Markers, Glioblastoma, Prognosis, TNF Receptor-Associated Factor 1, TNF Receptor-Associated Factor 2
Background
Glioblastoma multiforme (GBM) is the most common and aggressive primary brain tumor in adults; it has strong therapy resistance, high recurrence, and rapid progression [1]. GBM represents 15% to 20% of all primary intracranial tumors [2]. The treatment of GBM has improved significantly in recent years, including new surgical methods, equipment, and multi-drug therapies such as temozolomide [3]. However, the survival rate and survival time of patients with GBM is still very low, with a median survival time about 14.6 months [4]. Standardized treatments of GBM include surgical resection and combined radio-chemotherapy, which have little effect on some aggressive subtypes of GBM [5].
Tumor necrosis factors (TNF) play a pivotal role in cellular apoptosis controlled by binding with their receptors, TNFRs. In GBM, these TNFRs include TNFR1, TRAILDR4, TRAIL-DR5, and Fas, etc. [6,7]. TNFs play an anti-apoptotic role via interacting with some other receptors. Therefore, the effects of TNFs are not certain, mainly depending on the downstream receptors, which determine the balance between pro-apoptotic and anti-apoptotic pathways. In the numerous signaling pathways triggered by TNFs, the TNFR-associated factor (TRAF) family can mediate the TNF-induced NF-κB activation in GBM cells and also in other tumor cells, resulting in both cytokine secretion and resistance to apoptosis [8]. The TRAF family consists of 6 adapter proteins (TRAF1-TRAF6). In the TRAF family, recent evidence suggested the anti-apoptotic activity of TRAF1 and TRAF2 in GBM cells [9]. However, the prognostic value of TRAF1 and TRAF2 is still not known.
In our study, we investigated the expression of TRAF1 and TRAF2 in GBM tissues for the first time. Moreover, we evaluated the influence of TRAF1/2 on clinicopathologic factors. We further performed univariate analysis and multivariate analysis to estimate the prognostic value of TRAF1 and TRAF2 in patients with GBM.
Material and Methods
Patients and follow-ups
A total of 105 patients were diagnosed as having GBM and underwent surgical resection in our study, which were enrolled into our cohort according to the criteria: (1) available follow-up and samples; (2) post-operational survival time more than 2 months; and (3) no adjuvant therapy after operation. All the patients in the cohort underwent macroscopic total or near-total tumor resection and we performed evaluation using the Karnofsky Performance Scale (KPS). The overall survival time was calculated from the operation to the date of death or censored at the date of the last follow-up examination. The average survival time was 7.99 months and the median survival time was 7 months. All the samples were obtained with prior content of patients and the approval of Ethics Committee. The study was approved by the Ethics Committee of Yidu Central Hospital and Linyi People’s Hospital.
Immunohistochemistry
The expression of TRAF1 and TRAF2 was detected with immunohistochemistry (IHC) in a streptavidin-biotin immunoperoxidase method [10,11]. All the slides were first soaked in xylene and degraded ethanol for dewaxing. After that, slides were incubated in 3% hydrogen peroxide to achieve endogenous peroxidase inactivation. Moreover, citrate buffer was used for antigen retrieval and 5% bovine serum albumin was applied for blocking unspecific binding. Primary antibodies of TRAF1 (1: 200, Cell Signaling Technology, Danvers, MA, USA), TRAF2 (1: 100, Abcam, Cambridge, UK), or Ki67(1: 100, DAKO, Denmark) were used to incubate the samples in 4°C overnight, followed by washing in phosphate-buffered saline. Corresponding secondary antibodies and streptavidin peroxidase complex reagent were then applied. Finally, the results were visualized in 3,3′-diaminobenzidine solution.
Score system of IHC results
Slides were blindly scored by 2 independent pathologists unaware of the clinical data. The results of IHC included 2 aspects: the staining intensity and the proportion of positively-stained tumor cells, referring to previous studies [12,13]. The final score of IHC is the product of the score (staining intensity) multiplied by the score (positively-stained tumor cells). The score of positively-stained tumor cells was defined as: score 0 for <5% positive tumor cells; score 1 for 6–30% positive tumor cells; score 2 for 31–50% positive tumor cells; and score 3 for more than 50% positive tumor cells. The staining intensity was defined as: 0 for no staining, 1 for weak staining, 2 for moderate staining, and 3 for strong staining. The cut-off was identified by the ROC curve of IHC score according to a previous study [14], dividing the cohort into TRAF1/2 high-expression or low-expression.
Statistical analysis
All the data in our study were analyzed with SPSS17.0 software (IBM Corporation, Armonk, New York, U.S). The correlation between TRAF1/2 and clinicopathologic features was evaluated by chi-square test. Relevant factors with overall survival rate were investigated by Kaplan-Meier method, and the difference in survival curves was analyzed by log-rank test. The independent prognostic factors were identified with the Cox regression proportional hazards model. P<0.05 was considered statistically significant.
Results
Expression of TRAF1 and TRAF2 in GBM tissue
A total of 105 patients with GBM were enrolled in our study, and their basic information is displayed in Table 1. There were 58 female patients and 47 male patients in our cohort. In formalin-fixed tissues, the expression of TRAF1 and TRAF2 was detected to show their location and abundance. TRAF1 and TRAF2 were mainly expressed in cytoplasm (Figure 1A, 1B). TRAF2 was occasionally observed in the cell nucleus but TRAF1 was not. The cohort was further divided into high-expression and low-expression groups according to the expression of TRAF1 and TRAF2. The percentage of TRAF1 and TRAF2 high expression was 30.48% (32/105) and 40.95% (43/105), respectively.
Table 1.
Characteristics of patients.
| Parameters | Number | Percentage |
|---|---|---|
| Age | ||
| ≤50 | 52 | 49.52% |
| >50 | 53 | 50.48% |
| Gender | ||
| Male | 58 | 55.24% |
| Female | 47 | 44.76% |
| KPS | ||
| <80 | 39 | 37.14% |
| ≥80 | 66 | 62.86% |
| Extent of resection | ||
| Subtotal resection | 37 | 35.24% |
| Gross total resection (95%) | 68 | 64.16% |
| TRAF1 | ||
| Low | 73 | 69.52% |
| High | 32 | 30.48% |
| TRAF2 | ||
| Low | 62 | 59.05% |
| High | 43 | 40.95% |
KPS – Karnofsky Performance Scale; TRAF1 – TNF receptor-associated factor 1; TRAF2 – TNF receptor-associated factor 2.
Figure 1.
Representative images of TRAF1 and TRAF2 for IHC staining. (A) High expression of TRAF1 was displayed. Right: The magnified image of the box in the left. Scale bar: 50 μm. (B) High expression of TRAF2 was displayed. Right: The magnified image of the box in the left.
Association of TRAF1/2 with clinicopathologic factors
The correlation between TRAF1, TRAF2, and clinicopathologic factors, including age, gender, KPS, resection margin, and Ki67 percentage, was analyzed with chi-square test (Table 2). Unfortunately, there was no significant relevant factor of TRAF1 or TRAF2 in our test, indicating that TRAF1/2 expression is not influenced by patient age, gender, or other factors.
Table 2.
Correlation between TRAF1/TRAF2 and clinicopathological factors.
| Parameters | TRAF1 | P* | TRAF2 | P* | ||
|---|---|---|---|---|---|---|
| Low | High | Low | High | |||
| Age | 0.402 | 0.046 | ||||
| ≤50 | 34 | 18 | 29 | 29 | ||
| >50 | 39 | 14 | 33 | 14 | ||
| Gender | 0.291 | 1.000 | ||||
| Male | 43 | 15 | 31 | 21 | ||
| Female | 30 | 17 | 31 | 22 | ||
| KPS | 0.665 | 0.838 | ||||
| <80 | 26 | 13 | 24 | 15 | ||
| ≥80 | 47 | 19 | 38 | 28 | ||
| Extent of resection | 0.270 | 0.218 | ||||
| Subtotal resection | 23 | 14 | 25 | 12 | ||
| Gross total resection (95%) | 50 | 18 | 37 | 31 | ||
| TRAF1 | 0.518 | |||||
| Low | 45 | 28 | ||||
| High | 17 | 15 | ||||
| TRAF2 | 0.518 | |||||
| Low | 45 | 17 | ||||
| High | 28 | 15 | ||||
Means calculated by Chi-square test.
KPS – Karnofsky Performance Scale; TRAF1 – TNF receptor-associated factor 1; TRAF2 – TNF receptor-associated factor 2.
Prognostic significance of TRAF1/2
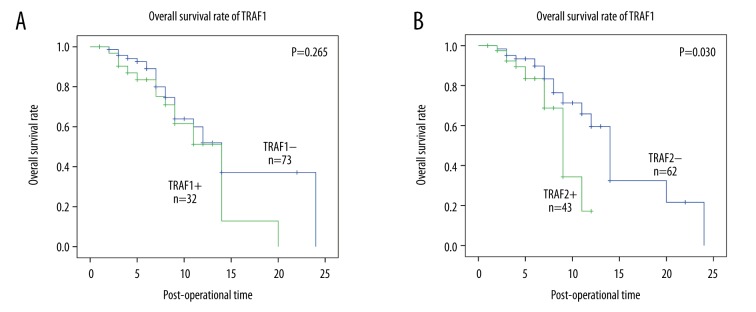
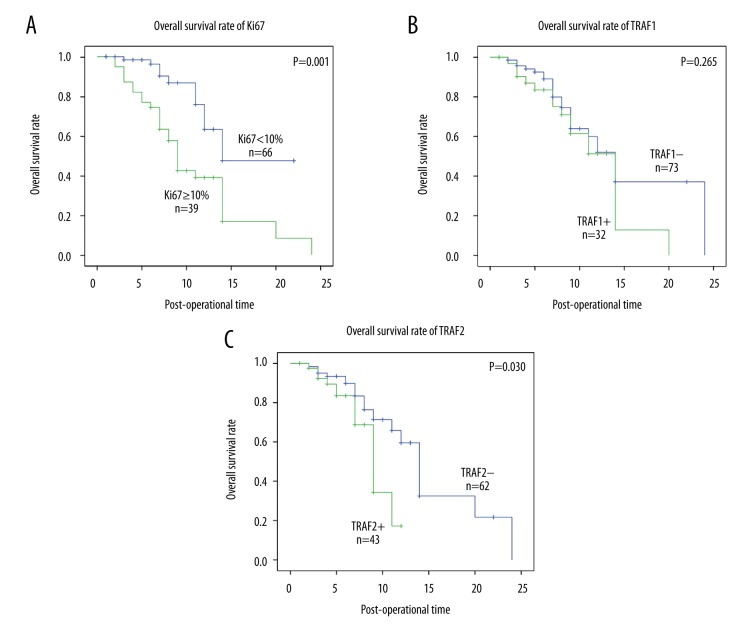
In in vitro experiments, both TRAF1 and TRAF2 have been indicated to promote the progression of GBM cells in previous studies [9,15], but the clinical or prognostic factors of TRAF1/2 in GBM were no investigated. Here we performed univariate analysis and multivariate analysis to evaluate the prognostic value of TRAF1 and TRAF2. Univariate analysis was first carried out with Kaplan-Meier analysis to examine the factors significantly associated with overall survival rate (Table 3). In our study, the extent of resection (P<0.001) and Ki67 (P=0.001) (Figure 2A) were both significantly associated with overall survival rate. Moreover, TRAF2 high expression was proved to predict poorer prognosis, while TRAF1 expression had no similar influence on survival rate (Figure 2B, 2C). Additionally, the KPS score system appeared to influence prognosis of patients with GBM, but the tendency was not statistical significant (P=0.086). Lower KPS score reflects worse overall condition of patients who need more careful nursing and attention. Patients with lower KPS usually had worse survival rates than those with higher KPS.
Table 3.
Prognostic value of TRAF1 and TRAF2.
| Parameters | Univariate analysis | P* | Multivariate analysis | P* | |
|---|---|---|---|---|---|
| 1-year survival rate (%) | HR | 95% CI | |||
| Age | 0.488 | 0.47–1.80 | 0.803 | ||
| ≤50 | 50.3 | 1 | |||
| >50 | 50.5 | 0.92 | |||
| Gender | 0.134 | 0.72–3.0 | 0.284 | ||
| Male | 62.1 | 1 | |||
| Female | 40.1 | 1.47 | |||
| KPS | 0.660 | 0.47–1.88 | 0.859 | ||
| <80 | 50.8 | 1 | |||
| ≥80 | 50.7 | 0.94 | |||
| Extent of resection | <0.001 | 0.13–0.55 | <0.001 | ||
| Subtotal resection | 24.2 | 1 | |||
| Gross total resection (95%) | 63.4 | 0.27 | |||
| TRAF1 | 0.265 | 0.45–1.91 | 0.839 | ||
| Low | 52.0 | 1 | |||
| High | 51.3 | 0.93 | |||
| TRAF2 | 0.030 | 1.08–4.78 | 0.030 | ||
| Low | 59.6 | 1 | |||
| High | 17.2 | 2.27 | |||
Means calculated by log-rank test;
means calculated by Cox-regression model.
KPS – Karnofsky Performance Scale; TRAF1 – TNF receptor-associated factor 1; TRAF2 – TNF receptor-associated factor 2.
Figure 2.
The survival curves of Ki67, TRAF1, and TRAF2. (A) The survival curve of subgroup high Ki67 percentage (≥10%) and low Ki67 (<10%). Patients with high Ki67 had worse prognosis than those with low Ki67 (P=0.001). (B) The survival curve of TRAF1 was drawn by Kaplan-Meier method and stratified by TRAF1 expression. High and low expression of TRAF1 made no significant difference in survival rate. (C) The survival curve of TRAF1 was drawn by Kaplan-Meier method and stratified by TRAF2 expression. Patients with high expression of TRAF2 have worse prognosis than those with low expression of TRAF1 (P=0.030).
To further identify the independent prognostic factors of GBM, multivariate analysis was performed with the Cox regression model. All the clinicopathologic factors were enrolled. The resection extent (P<0.001) was identified as a prognostic risk in our cohort, and it was obvious that positive extent indicated unfavorable prognosis. High percentage of Ki67(P=0.002) alone also predicted worse prognosis of GBM in our study. Additionally, TRAF2 expression was confirmed to be an independent prognostic factor in GBM, meaning TRAF2 high-expression itself can predict worse prognosis of patients with GBM (P=0.046, HR=2.13).
Discussion
TNF receptor superfamily plays pivotal roles in numerous biological processes in eukaryotic cells. TRAF1 and TRAF2 are both components of TNF superfamily signaling complexes, transducing signals following ligation of cytokine receptors, including receptors of TNF. Different TNF family ligands can recruit several different intracellular adaptors, activating multiple signal transduction pathways [16]. Recruitment of TRAF family proteins can result in the activation of transcription factors, such as NF-κB and JNK, which can promote cell survival and differentiation. In contrast, recruitment of adaptors containing death domains, such as Fas-associated death domain or TNFR-associated death domain, can induce apoptosis. Emerging evidence supports the oncologic role of aberrant expression of TRAF1 or TRAF2. For example, TRAF1 activation can promote lymphoid malignancies by its apoptosis-inhibition effect [17]. In GBM cells, activation of NF-κB p65 and its downstream TRAF1 was demonstrated to inhibit cell apoptosis and facilitate cell survival [18]. Angileri et al. demonstrated that NF-κB and its downstream proteins, such as TRAF1 and survivin, are up-regulated in gliomas. For TRAF2, the mRNA level between low-grade astrocytoma and GBM had no significant difference [8]. However, the detection of mRNA level is much different from the protein level detected by IHC, and we focused on comparing the prognostic value of TRAF1 and TRAF2 instead of comparing their mRNA level between normal tissues and glioma tissues.
TRAF2 is considered to play an essential role in TNF signaling, but this hypothesis has not been verified in all tissues. Previous studies suggested that TRAF2 protects cells from TNF-induced death [19]. There are conflicting data on the role of TRAF2 in TNF signaling, and the role of TRAF2 in tumors is also controversial. Schneider recently reported that TRAF2 inhibits the carcinogenesis of hepatocellular carcinoma [20], while Etemadi et al. demonstrated that TRAF2 regulates TNF and NF-κB signaling to suppress apoptosis in in vivo and in vitro experiments [21]. In GBM, TRAF2 silencing blocks the activation of NF-κB signaling and suppresses cell growth, indicating TRAF2 as an attractive drug target for anticancer therapy of GBM [22].
In our cohort, we did not find a significant correlation between age and KPS score with overall survival rate, which has been demonstrated in some previous studies [23–25], possibly because our cohort size was small, but this did not affect the variability of the cohort and our demonstration of TRAF2 as a prognostic factor. The network of TNF receptors and downstream signaling pathways is very complicated. First of all, different TNF receptors and TRAFs have different tissue specificity and a range of affinities for various intracellular adaptors. This could provide tremendous signaling specificities. Additionally, numerous signaling modulators participate in regulation of downstream signal transduction pathways. Their cross-talk provides more complicated signaling abundance and variety [16]. Even in the TNF signaling pathway, TRAF2 exerts multiple receptor-specific functions and mediates cross-talk between TNFR1 and TNFR2. TRAF2 could be a positive or negative regulator of TNF-mediated signaling [26]. In GBM, the exploration of TRAF1/2 and their influence on signaling pathway and oncological effects are not clear. We demonstrated that overexpression of TRAF2 rather than TRAF1 could lead to unfavorable prognosis of GBM. This could provide new insight into the search for effective biomarkers of GBM, and may help stratify high-risk patients more clearly. Unfortunately, we have not explored the mechanisms by which TRAF2 overexpression results in worse prognosis, because of the complicated TNF signaling network described above. However, the study of molecular mechanisms is essential and helpful for finding novel drug targets in TRAF2 downstream signaling. We hope our results trigger interest in TRAF2 in GBM and accelerate associated studies to find more effective therapies.
Conclusions
We, for the first time, investigated the expression of TRAF1 and TRAF2 in GBM tissues and evaluated their clinical significance, including their association with clinicopathologic factors and prognostic value. We demonstrated that high expression of TRAF2, but not TRAF1, can predict worse prognosis of GBM, and it was identified as an independent biomarker in GBM prognosis.
Footnotes
Conflicts of interest
The authors have no conflicts of interest.
Source of support: Departmental sources
References
- 1.Stupp R, Hegi ME, Mason WP, et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009;10:459–66. doi: 10.1016/S1470-2045(09)70025-7. [DOI] [PubMed] [Google Scholar]
- 2.Bhuvanalakshmi G, Arfuso F, Millward M, et al. Secreted frizzled-related protein 4 inhibits glioma stem-like cells by reversing epithelial to mesenchymal transition, inducing apoptosis and decreasing cancer stem cell properties. PloS One. 2015;10:e0127517. doi: 10.1371/journal.pone.0127517. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 3.Li W-Q, Li Y-M, Tao B-B, et al. MicroRNA-328 may contribute to chemoresistance in glioblastoma cancer stem cells by targeting ABCG2. Med Sci Monit. 2010;16(10):HY27–30. [PubMed] [Google Scholar]
- 4.Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. New Engl J Med. 2005;352:987–96. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 5.Koch K, Hartmann R, Schroter F, et al. Reciprocal regulation of the cholinic phenotype and epithelial-mesenchymal transition in glioblastoma cells. Oncotarget. 2016;7(45):73414–31. doi: 10.18632/oncotarget.12337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Choi C, Kutsch O, Park J, et al. Tumor necrosis factor-related apoptosis-inducing ligand induces caspase-dependent interleukin-8 expression and apoptosis in human astroglioma cells. Mol Cell Biol. 2002;22:724–36. doi: 10.1128/MCB.22.3.724-736.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Choi C, Xu X, Oh JW, et al. Fas-induced expression of chemokines in human glioma cells: Involvement of extracellular signal-regulated kinase 1/2 and p38 mitogen-activated protein kinase. Cancer Res. 2001;61:3084–91. [PubMed] [Google Scholar]
- 8.Angileri FF, Aguennouz M, Conti A, et al. Nuclear factor-kappaB activation and differential expression of survivin and Bcl-2 in human grade 2–4 astrocytomas. Cancer. 2008;112:2258–66. doi: 10.1002/cncr.23407. [DOI] [PubMed] [Google Scholar]
- 9.Conti A, Ageunnouz M, La Torre D, et al. Expression of the tumor necrosis factor receptor-associated factors 1 and 2 and regulation of the nuclear factor-kappaB antiapoptotic activity in human gliomas. J Neurosurg. 2005;103:873–81. doi: 10.3171/jns.2005.103.5.0873. [DOI] [PubMed] [Google Scholar]
- 10.Xu YF, Yang XQ, Lu XF, et al. Fibroblast growth factor receptor 4 promotes progression and correlates to poor prognosis in cholangiocarcinoma. Biochem Biophys Res Commun. 2014;446:54–60. doi: 10.1016/j.bbrc.2014.02.050. [DOI] [PubMed] [Google Scholar]
- 11.Liu H, Xu Y, Zhang Q, et al. Prognostic significance of TBL1XR1 in predicting liver metastasis for early stage colorectal cancer. Surg Oncol. 2017;26:13–20. doi: 10.1016/j.suronc.2016.12.003. [DOI] [PubMed] [Google Scholar]
- 12.Hou A, Zhao L, Zhao F, et al. Expression of MECOM is associated with unfavorable prognosis in glioblastoma multiforme. OncoTargets Ther. 2016;9:315–20. doi: 10.2147/OTT.S95831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xu YF, Ge FJ, Han B, et al. High-mobility group box 1 expression and lymph node metastasis in intrahepatic cholangiocarcinoma. World J Gastroenterol. 2015;21:3256–65. doi: 10.3748/wjg.v21.i11.3256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xu Y, Yang X, Li Z, et al. Sprouty2 correlates with favorable prognosis of gastric adenocarcinoma via suppressing FGFR2-induced ERK phosphorylation and cancer progression. Oncotarget. 2017;8(3):4888–900. doi: 10.18632/oncotarget.13982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fortin Ensign SP, Mathews IT, Eschbacher JM, et al. The Src homology 3 domain-containing guanine nucleotide exchange factor is overexpressed in high-grade gliomas and promotes tumor necrosis factor-like weak inducer of apoptosis-fibroblast growth factor-inducible 14-induced cell migration and invasion via tumor necrosis factor receptor-associated factor 2. J Biol Chem. 2013;288:21887–97. doi: 10.1074/jbc.M113.468686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dempsey PW, Doyle SE, He JQ, Cheng G. The signaling adaptors and pathways activated by TNF superfamily. Cytokine Growth Factor Rev. 2003;14(3–4):193–209. doi: 10.1016/s1359-6101(03)00021-2. [DOI] [PubMed] [Google Scholar]
- 17.Zhang B, Wang Z, Li T, et al. NF-kappaB2 mutation targets TRAF1 to induce lymphomagenesis. Blood. 2007;110:743–51. doi: 10.1182/blood-2006-11-058446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee J, Hoxha E, Song HR. A novel NFIA-NFkappaB feed-forward loop contributes to glioblastoma cell survival. Neuro Oncol. 2016 doi: 10.1093/neuonc/now233. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee SY, Reichlin A, Santana A, et al. TRAF2 is essential for JNK but not NF-kappaB activation and regulates lymphocyte proliferation and survival. Immunity. 1997;7:703–13. doi: 10.1016/s1074-7613(00)80390-8. [DOI] [PubMed] [Google Scholar]
- 20.Schneider AT, Gautheron J, Feoktistova M, et al. RIPK1 Suppresses a TRAF2-Dependent Pathway to Liver Cancer. Cancer Cell. 2017;31:94–109. doi: 10.1016/j.ccell.2016.11.009. [DOI] [PubMed] [Google Scholar]
- 21.Etemadi N, Chopin M, Anderton H, et al. TRAF2 regulates TNF and NF-kappaB signalling to suppress apoptosis and skin inflammation independently of Sphingosine kinase 1. Elife. 2015;4 doi: 10.7554/eLife.10592. pii: e10592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zheng M, Morgan-Lappe SE, Yang J, et al. Growth inhibition and radiosensitization of glioblastoma and lung cancer cells by small interfering RNA silencing of tumor necrosis factor receptor-associated factor 2. Cancer Res. 2008;68:7570–78. doi: 10.1158/0008-5472.CAN-08-0632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yang B, Heng L, Du S, et al. Association between RTEL1, PHLDB1, and TREH polymorphisms and glioblastoma risk: A case-control study. Med sci Monit. 2015;21:1983–88. doi: 10.12659/MSM.893723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sun LH, Yang FQ, Zhang CB, et al. Overexpression of paxillin correlates with tumor progression and predicts poor survival in glioblastoma. CNS Neurosci Ther. 2017;23:69–75. doi: 10.1111/cns.12606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang Q, Zhang J, Liu Y, et al. A novel cell cycle-associated lncRNA, HOXA11-AS, is transcribed from the 5-prime end of the HOXA transcript and is a biomarker of progression in glioma. Cancer Lett. 2016;373:251–59. doi: 10.1016/j.canlet.2016.01.039. [DOI] [PubMed] [Google Scholar]
- 26.Borghi A, Verstrepen L, Beyaert R. TRAF2 multitasking in TNF receptor-induced signaling to NF-kappaB, MAP kinases and cell death. Biochem Pharmacol. 2016;116:1–10. doi: 10.1016/j.bcp.2016.03.009. [DOI] [PubMed] [Google Scholar]