ABSTRACT
We recently found that the beneficial fungus Trichoderma harzianum T-78 primes tomato plants for salicylic acid (SA)- and jasmonic acid (JA)-regulated defenses, resulting in enhanced resistance against the root knot nematode Meloidogyne incognita. By using SA- and JA-impaired mutant lines and exogenous hormonal application, here we investigated whether the SA- and JA-pathways also have a role in T-78 root colonization of Arabidopsis thaliana. Endophytic colonization by T-78 was faster in the SA-impaired mutant sid2 than in the wild type. Moreover, elicitation of SA-dependent defenses by SA application reduced T-78 colonization, indicating that the SA-pathway affects T-78 endophytism. In contrast, elicitation of the JA-pathway, which antagonized SA-dependent defenses, resulted in enhanced endophytic colonization by T-78. These findings are in line with our previous observation that SA-dependent defenses are repressed by T-78, which likely aids colonization by the endophytic fungus.
KEYWORDS: Aboveground-belowground interactions, endophyte, jasmonic acid, plant defense, plant-microbe interaction, salicylic acid, Trichoderma
Although the rhizosphere that surrounds the root system is among the most common ecological niches for fungal Trichoderma spp. (hereafter Trichoderma), several species have been found to be facultative endophytes that colonize the interior of the root.1,2 Phylogenetic studies position endophytic Trichoderma isolates in a terminal position within their clades, suggesting that the development of the endophytism in this genus is evolutionary recent.1 In analogy to plant pathogens, endophytic mutualists have to cope with the plant immune system to establish themselves within the plant tissues.2-5 Indeed, a moderate suppression of host immune responses has been reported to be required for the establishment of mutualistic plant-microbe relationships.3 For instance, repression of SA levels and SA-triggered responses occurs during root colonization by several endophytic mutualists like mycorrhizal fungi and Rhizobium bacteria.6-9 Moreover, elicitation of plant immunity can negatively affect root interaction with their rhizobial or mycorrhizal partners.10-12 However, the impact of plant defenses on root colonization by facultative endophytic Trichoderma has remained largely unexplored. Recently, we found that Trichoderma harzianum T-78 (T-78) is able to prime salicylic acid (SA)- and jasmonic acid (JA)-regulated defenses in tomato roots resulting in enhanced resistance against the root knot nematode Meloidogyne incognita.13 Interestingly, in absence of the attacker, we found a moderate repression of the SA marker gene PR1 in T-78-colonized roots. This could hint to a possible role of SA-dependent defenses in the establishment of T-78 in roots. In this addendum, by using the model plant Arabidopsis thaliana (hereafter Arabidopsis) we extend our investigation to better understand the role of SA- and JA-regulated defenses in root colonization by the mutualistic strain T-78.
T-78 colonizes endophytically the roots of Arabidopsis
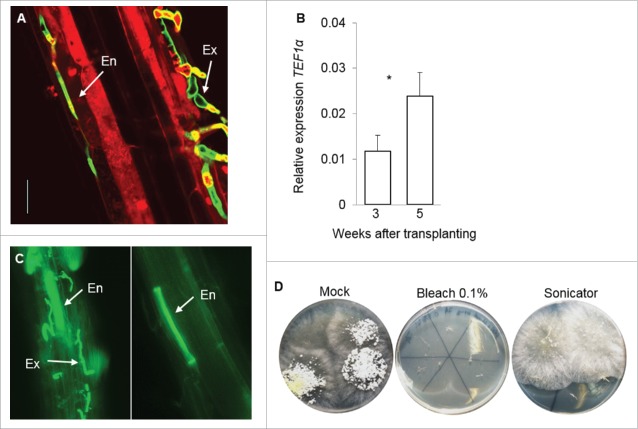
To determine the endophytic colonization ability of T-78, we first imaged the fungus by confocal microscopy on roots of Arabidopsis plants grown for 5 weeks in soil inoculated with the fungus. Plants had been previously grown to the seedling stage for 2 weeks in river sand. Fig. 1A shows that T-78 was able to penetrate Arabidopsis roots and colonize endophytically the outer cell layers, as revealed by the presence of both external (Ex) and endophytic (En) T-78 mycelium. The amount of viable endophytic mycelium was further assessed 3 and 5 weeks after transplanting by analyzing the expression of the Trichoderma constitutively expressed gene Trichoderma translation elongation factor-1α (Tef1α), by using qPCR according to, ref. 13 and the specific primers in Table 1 (Fig. 1B). To selectively determine colonization of the endosphere compartment of the root, we used the sonication method as described in ref. 14. Sonication enabled removing T-78 mycelium from the epiphytic root region, without using chlorine bleach, which might enter roots and destroy RNA (Fig. 1D).15 The absence of mycelium on the root surface in sonicated root fragments was verified by fluorescence microscopy (Fig. 1C). The viability of T-78 mycelium in sonicated roots was verified by plating fragments of sonicated roots onto potato dextrose agar and checking T-78 outgrow from inside the roots (Fig. 1D). Expression of Tef1α was observed in the sonicated roots at 3 weeks and to a higher level at 5 weeks after transplanting (Fig. 1B), further corroborating the ability of T-78 to colonize endophytically Arabidopsis roots.
Figure 1.
T-78 colonizes endophytically the roots of Arabidopsis. (A) T-78 endophytic colonization was assessed in roots of Arabidopsis grown for 5 weeks in soil inoculated with the fungus by using confocal microscopy and 0.05 mg mL-1 wheat germ agglutinin (WGA) Alexa Fluor 488 (green signal). Roots were counterstained with 10 µg mL-1 propidium iodide (PI, red signal). Chromophores were exited using the 488 nm Argon laser and fluorescence was detected at 495–519 (WGA) and 570–620 nm (PI). External (Ex) and endophytic (En) mycelium was observed in roots of inoculated plants. Scale bar = 25 μm. (B) Amount of T-78 endophytic mycelium was estimated by measuring the expression of Tef1α relative to the Arabidopsis At1g13320 gene in sonicated roots at 3 and 5 weeks after transplanting. Values are means ± SE of 4 biological replicates. Each biological replicate consisted of pooled root tissue from 4 independent plants. Asterisk indicates significant difference according to Student's t-test (P < 0.05). These results are representative of 2 independent experiments. (C) Fluorescence microscopy (0.05 mg mL-1 WGA Alexa Fluor 488; green signal) showing (left) roots of Arabidopsis colonized by external (Ex) and endophytic (En) T-78 mycelium and (right) roots after sonication, where only endophytic colonization by T-78 is detected. (D) Multiple root fragments colonized by T-78 were placed on PDA after removing the rhizosphere soil (Mock) and treatments with either bleach (0.1%) or sonication, and 5 d later T-78 outgrow from inside roots was checked.
Table 1.
Primer sequences used for real time qPCR analysis.
| ID | Target gene | Sequence (5′ → 3′) |
|---|---|---|
| AF456892 | Tef1α | GGTACTGGTGAGTTCGAGGCTG GGGCTCGATGGAGTCGATAG |
| At5G24770 | VSP2 | CGGGTCGGTCTTCTCTGTTC CCAAAGGACTTGCCCTA |
| At2g14610 | PR1 | CTCGGAGCTACGCAGAACAACT TTCTCGCTAACCCACATGTTCA |
| At1g13320 | At1g13320 | TAACGTGGCCAAAATGATGC GTTCTCCACAACCGCTTGGT |
T-78 endophytic colonization is faster in the salicylic acid biosynthesis-impaired mutant sid2
To investigate the role of SA- and JA-regulated pathways in T-78 endophytic colonization, we studied the dynamics of T-78 colonization in the SA biosynthesis-impaired mutant sid2 (salicylic acid induction-deficient2; defective in isochorismate synthase16) and in the JA biosynthesis-impaired mutant dde2–2 (delayed dehiscence2; defective in allene oxide synthase17). Tef1α transcript levels were found to be 10 and 20 times higher inside the roots of the sid2 mutant compared with the Col-0 wild type at 3 and 4 weeks after transplanting, respectively (Fig. 2). In line with this, strain T34 of T. harzianum has been recently observed to colonize sid2 to higher levels in comparison to wild-type Arabidopsis plants.18 However, 5 weeks after transplanting, Tef1α mRNA levels had also increased in wild type roots and reached a similar level to that observed in sid2 plants. Hence, impairment of SA-defenses seems to allow an earlier and faster T-78 endophytic colonization. By contrast, no significant differences in Tef1α transcripts were found between the JA biosynthesis-impaired mutant dde2–2 and the wild type in the analyzed time frame (Fig. 2), suggesting that the JA-regulated pathway does not play an important role in T-78 endophytic colonization.
Figure 2.
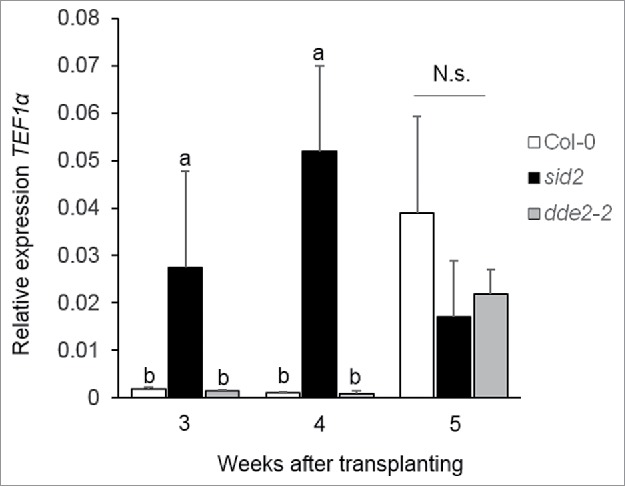
Quantification of T-78 endophytic colonization in roots of Arabidopsis Col-0, sid2 and dde2–2. Amount of T-78 endophytic mycelium was estimated by measuring the relative expression of Tef1α relative to the Arabidopsis At1g13320 gene in sonicated roots at 3, 4 and 5 weeks after transplanting. Values are means ± SE of 3 biological replicates. Each biological replicate consisted of pooled root tissue from 4 independent plants. For each time point, different letters indicate statistically significant differences between treatments (Tukey's HSD test; P < 0.05). N.s.: not significant. These results are representative of 2 independent experiments.
Elicitation of salicylic acid-regulated defenses transiently impairs endophytic colonization by T-78
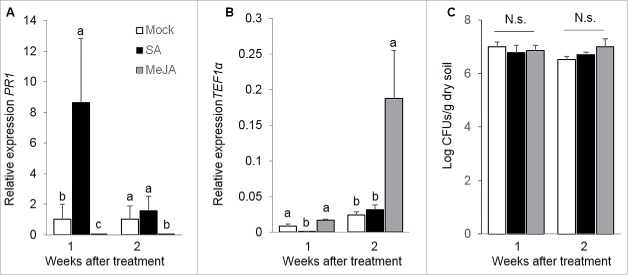
To study the impact of elicitation of plant defenses on T-78 endophytic colonization, the shoot of Arabidopsis plants was treated with 1 mM of SA (Sigma-Aldrich, St. Louis, USA) or 100 µM of MeJA (Serva, Brunschwig Chemie, Amsterdam, the Netherlands), amended with 0.015% (v/v) Silwet L-77, according to ref. 19. Plants were treated twice: 3 and 4 weeks after transplanting to T-78-inoculated soil, according to ref. 11. Subsequently, T-78 endophytic mycelium was assessed in sonicated roots at 1 and 2 weeks after the last hormone application, and compared with mock-treated plants. Shoot application of SA led to the induction of PR1 expression in Arabidopsis roots 1 week after hormone application, however after 2 weeks PR1 levels had returned to basal levels (Fig. 3A). Interestingly, the systemic elicitation of SA-regulated defenses was associated with a strong decrease (about 150-fold) of Tef1α transcripts in sonicated roots 1 week after elicitation (Fig. 3B; endophytic colonization), without significantly affecting T-78 population in the rhizosphere (Fig. 3C; external colonization). However, 2 weeks after treatment, the amount of Tef1α transcripts in SA-elicited plants was similar to that in mock-treated controls (Fig. 3B). These observations indicate a transient negative effect of systemic elicitation of SA-regulated defenses on T-78 internal root colonization. Accordingly, previous studies have reported negative effects of elicitation of SA-regulated defenses on beneficial plant-microbe interactions.10-12 Conversely to SA elicitation, shoot treatment with MeJA did not result in activation of JA-dependent defenses in roots (VSP2 expression data not shown). Interestingly, a strong downregulation of PR1 was observed in MeJA-treated plants (Fig. 3A), highlighting a systemic negative crosstalk between the JA- and the SA-regulated pathways.20 According to the negative impact of activation of SA-regulated defenses on T-78 colonization, PR1 downregulation might explain the increased endophytic colonization observed in MeJA-treated plants (Fig. 3B).
Figure 3.
Impact of elicitation of SA- and JA-regulated defenses on T-78 root endophytic colonization. (A) Expression level of the SA-responsive marker gene PR1 was analyzed in roots of Arabidopsis plants 1 and 2 weeks after application of SA or MeJA to the shoot, and in mock-treated plants. The results were normalized to the Arabidopsis At1g13320 gene expression level and depicted relative to the mock-treated plants. (B) Amount of T-78 endophytic mycelium was estimated by measuring the expression of Tef1α relative to At1g13320 in sonicated roots at 1 and 2 weeks after shoot application of SA or MeJA. (C) Population of T-78 in the rhizosphere of Arabidopsis 1 and 2 weeks after shoot application of SA or MeJA. Values are means ± SE of 4 biological replicates. Each biological replicate consisted of pooled root tissue from 4 independent plants. In (A) and (B) different letters indicate statistically significant differences between treatments per time point (Tukey's HSD test; P < 0.05). N.s.: not significant. These results are representative of 2 independent experiments.
Altogether, our results show that elicitation of SA-regulated defenses has a transient negative effect on the endophytic root colonization by the beneficial fungus T-78. By contrast, our data point to an indirect positive effect of JA-triggered defenses on T-78 endophytic colonization, most likely via a JA/SA crosstalk-mediated downregulation of SA-dependent defenses.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Funding
This research was supported by the Marie Curie Fellowship 301662 (to AMM) and the VIDI grant 11281 of the Netherlands Organization of Scientific Research (to SCMW).
References
- 1.Druzhinina IS, Seidl-Seiboth V, Herrera-Estrella A, Horwitz BA, Kenerley CM, Monte E, Mukherjee PK, Zeilinger S, Grigoriev IV, Kubicek CP. Trichoderma: The genomics of opportunistic success. Nat Rev Microbiol 2011; 9:749-59; PMID:21921934; https://doi.org/ 10.1038/nrmicro2637 [DOI] [PubMed] [Google Scholar]
- 2.Martinez-Medina A, Pozo MJ, Cammue BPA, Vos CMF. Belowground defence strategies in plants: The plant-Trichoderma dialogue In Vos CMF, Kazan K (eds.), Belowground defence strategies in plants (pp. 301-27), 2016. Springer International Publishing; https://doi.org/ 10.1007/978-3-319-42319-7 [DOI] [Google Scholar]
- 3.Zamioudis C, Pieterse CMJ. Modulation of host immunity by beneficial microbes. Mol Plant Microbe Interact 2012; 25:139-50; PMID:21995763; https://doi.org/ 10.1094/MPMI-06-11-0179 [DOI] [PubMed] [Google Scholar]
- 4.Delaye L, García-Guzmán G, Heil M. Endophytes versus biotrophic and necrotrophic pathogens - are fungal lifestyles evolutionarily stable traits? Fungal Divers 2013; 60:125-35; https://doi.org/ 10.1007/s13225-013-0240-y [DOI] [Google Scholar]
- 5.García-Guzmán G, Heil M. Life histories of hosts and pathogens predict patterns in tropical fungal plant diseases. New Phytol 2014; 201:1106-20; PMID:24171899; https://doi.org/ 10.1111/nph.12562 [DOI] [PubMed] [Google Scholar]
- 6.Martinez-Abarca F, Herrera-Cervera JA, Bueno P, Sanjuan J, Bisseling T, Olivares J. Involvement of salicylic acid in the establishment of the Rhizobium meliloti-alfalfa symbiosis. Mol Plant Microbe Interact 1998; 11:153-55; https://doi.org/ 10.1094/MPMI.1998.11.2.153 [DOI] [Google Scholar]
- 7.Herrera Medina MJ, Gagnon H, Piché Y, Ocampo JA, García Garrido JM, Vierheilig H. Root colonization by arbuscular mycorrhizal fungi is affected by the salicylic acid content of the plant. Plant Sci 2003; 164:993-98; https://doi.org/ 10.1016/S0168-9452(03)00083-9 [DOI] [Google Scholar]
- 8.Mitra RM, Long SR. Plant and bacterial symbiotic mutants define three transcriptionally distinct stages in the developmentof the Medicago truncatula/Sinorhizobium meliloti symbiosis. Plant Physiol 2004; 134:595-604; PMID:14739349; https://doi.org/ 10.1104/pp.103.031518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Deguchi Y, Banba M, Shimoda Y, Chechetka SA, Suzuri R, Okusako Y, Ooki Y, Toyokura K, Suzuki A, Uchiumi T, et al.. Transcriptome profiling of Lotus japonicus roots during arbuscular mycorrhiza development and comparison with that of nodulation. DNA Res 2007; 14:117-33; PMID:17634281; https://doi.org/ 10.1093/dnares/dsm014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Faessel L, Nassr N, Lebeau T, Walter B. Chemically-induced resistance on soybean inhibits nodulation and mycorrhization. Plant Soil 2010; 329:259-68; https://doi.org/ 10.1007/s11104-009-0150-7 [DOI] [Google Scholar]
- 11.de Roman M, Fernandez I, Wyatt T, Sahrawy M, Heil M, Pozo MJ. Elicitation of foliar resistance mechanisms transiently impairs root association with arbuscular mycorrhizal fungi. J Ecol 2011; 99:36-45; https://doi.org/ 10.1111/j.1365-2745.2010.01752.x [DOI] [Google Scholar]
- 12.Ballhorn DJ, Younginger BS, Kautz S. An aboveground pathogen inhibits belowground rhizobia and arbuscular mycorrhizal fungi in Phaseolus vulgaris. BMC Plant Biol 2014; 14:321; PMID:25429887; https://doi.org/ 10.1186/s12870-014-0321-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Martinez-Medina A, Fernandez I, Lok GB, Pozo MJ, Pieterse CMJ, Van Wees SCM. Shifting from priming of salicylic acid- to jasmonic acid-regulated defences by Trichoderma protects tomato against the root knot nematode Meloidogyne incognita. New Phytol 2017; 213:1363-77; PMID:27801946; https://doi.org/ 10.1111/nph.14251 [DOI] [PubMed] [Google Scholar]
- 14.Bulgarelli D, Rott M, Schlaeppi K, van Themaat EVL, Ahmadinejad N, Assenza F, Rauf P, Huettel B, Reinhardt R, Schmelzer E, et al.. Revealing structure and assembly cues for Arabidopsis root-inhabiting bacterial microbiota. Nature 2012; 488:91-5; PMID:22859207; https://doi.org/ 10.1038/nature11336 [DOI] [PubMed] [Google Scholar]
- 15.Lundberg DS, Lebeis SL, Paredes SH, Yourstone S, Gehring J, Malfatti S, Tremblay J, Engelbrektson A, Kunin V, del Rio TG, et al.. Defining the core Arabidopsis thaliana root microbiome. Nature 2012; 488:86; PMID:22859206; https://doi.org/ 10.1038/nature11237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wildermuth MC, Dewdney J, Wu G, Ausubel FM. Isochorismate synthase is required to synthesize salicylic acid for plant defence. Nature 2001; 414:562-5; PMID:11734859; https://doi.org/ 10.1038/35107108 [DOI] [PubMed] [Google Scholar]
- 17.von Malek B, van der Graaff E, Schneitz K, Keller B. The Arabidopsis male-sterile mutant dde2-2 is defective in the ALLENE OXIDE SYNTHASE gene encoding one of the key enzymes of the jasmonic acid biosynthesis pathway. Planta 2002; 216:187-92; PMID:12430030; https://doi.org/ 10.1007/s00425-002-0906-2 [DOI] [PubMed] [Google Scholar]
- 18.Alonso-Ramirez A, Poveda J, Martin I, Hermosa R, Monte E, Nicolas C. Salicylic acid prevents Trichoderma harzianum from entering the vascular system of roots. Mol Plant Pathol 2014; 15:823-31; PMID:24684632; https://doi.org/ 10.1111/mpp.12141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Van Wees SCM, Van Pelt JA, Bakker PAHM, Pieterse CMJ. Bioassays for assessing jasmonate-dependent defenses triggered by pathogens, herbivorous insects, or beneficial rhizobacteria. Methods Mol Biol 2013; 1011:35-49; PMID:23615986; https://doi.org/ 10.1007/978-1-62703-414-2_4 [DOI] [PubMed] [Google Scholar]
- 20.Pieterse CMJ, Van der Does D, Zamioudis C, Leon-Reyes A, Van Wees SCM. Hormonal modulation of plant immunity. Ann Rev Cell Dev Bi 2012; 28:489-521; PMID:22559264; https://doi.org/ 10.1146/annurev-cellbio-092910-154055 [DOI] [PubMed] [Google Scholar]