Abstract
Patient: Female, 58
Final Diagnosis: Nephroangiosclerosis
Symptoms: Renal failure
Medication: —
Clinical Procedure: Resuscitation of grafts by hypothermic oxygenated perfusion
Specialty: Transplantology
Objective:
Challenging differential diagnosis
Background:
The recovery of discarded human kidneys has increased in recent years and impels to use of unconventional organ preservation strategies that improve graft function. We report the first case of human kidneys histologically discarded and transplanted after hypothermic oxygenated perfusion (HOPE).
Case Report:
Marginal kidneys from a 78-year-old woman with brain death were declined by Italian transplant centers due to biopsy score (right kidney: 6; left kidney: 7). We recovered and preserved both kidneys through HOPE and we revaluated their use for transplantation by means of perfusion parameters.
The right kidney was perfused for 1 h 20 min and the left kidney for 2 h 30 min. During organ perfusion, the renal flow increased progressively. We observed an increase of 34% for the left kidney (median flow 52 ml/min) and 50% for the right kidney (median flow 24 ml/min). Both kidneys had low perfusate’s lactate levels. We used perfusion parameters as important determinants of the organ discard.
Based on our previous organ perfusion experience, the increase of renal flow and the low level of lactate following 1 h of HOPE lead us to declare both kidneys as appropriate for dual kidney transplantation (DKT). No complications were reported during the transplant and in the post-transplant hospital stay. The recipient had immediate graft function and serum creatinine value of 0.95 mg/dL at 3 months post-transplant.
Conclusions:
HOPE provides added information in the organ selection process and may improve graft quality of marginal kidneys.
MeSH Keywords: Donor Selection, Kidney Transplantation, Pulsatile Flow
Background
In recent years, organ preservation strategies have become a research area of strong interest in the increased trend to recover discarded human kidneys [1]. High-risk donation requires an appropriate evaluation of eligibility and alternative methods of organ preservation to prevent preservation injury, to restore graft viability, and to improve post-transplant function [2].
Based on age, donor history, renal function, perfusion in situ, macroscopic aspects, or histological evaluation, organs are declared transplantable or untransplantable. Donors who are classified as marginal due to age, type of death, hypertension, and renal dysfunction (ECD, expanded criteria donors) [3] need histological analyses to quantify viable renal mass and predict the outcome of kidney transplantation (KT). As reported in animal experiments, an inadequate volume of transplanted nephron mass negatively affects functional recovery of the graft [4]. Similarly, 2 marginal kidneys can be used in the transplantation to prevent graft failure in suboptimal organ donation. Several studies have compared marginal dual kidney transplantation (DKT) and ideal single kidney transplantation (SKT), and the use of 2 marginal kidneys in the same recipient is becoming a widespread and successful procedure. Both transplant options show comparable data on renal function retrieval and graft survival [5].
Various organ perfusion techniques have been developed to optimize the use of high-risk grafts. Several clinical studies demonstrated that hypothermic machine perfusion (HMP) improves graft quality and reduces delayed graft function [6–8]. Oxygenation during HMP provides the additional O2 needed in the hypothermic condition and prevents hypoxia injury [9], increasing adenosine triphosphate (ATP) content [10]. At present, ex vivo normothermic perfusion (EVNP) is used to assess and resuscitate discarded human kidneys [11,12].
Here, we used ex vivo hypothermic oxygenated perfusion (HOPE) to recover 2 marginal human kidneys that were declined due to biopsy score, and used them for DKT, with favorable outcome.
Case Report
Donor kidney selection process
To allocate marginal organs for SKT or DKT, the Italian guidelines recommend a pre-implantation biopsy for histological evaluation.
Donor renal pathology was quantified on the basis of Karpinski histological score [13], which has proven to be a good prognostic factor for graft selection [14].
In accordance with Remuzzi’s histological selection process, organs with renal scores of 3 or less are suitable for SKT, those with renal scores 4 to 6 are appropriate for DKT, and those with renal scores of 7 or more are discarded [5].
Donor data
The ECD donor was a 78-year-old woman who died for an intracranial hemorrhage (ICH) and was a donor after brain death. Donor risk factors were hypertension and arterial vascular disease. Use permission for organ donation was obtained from the relatives. The final donor serum creatinine (sCr) was 0.85 mg/dL and glomerular filtration rate (eGFR) was 55.27 ml/min. Histological analyses were performed by 2 renal pathologists, who both reported a score of 6 for the right kidney and 7 for the left kidney (Table 1A). Due to the histological findings, other transplant centers declined both kidneys. We decided to recover the grafts and preserve them by means of HOPE. Donor aspects are detailed in Table 1A.
Table 1.
Donor, recipient and organ perfusion data.
(A) Demographic, clinical and histological data of the donor.
| Donor | ||
|---|---|---|
| Age | 78 | |
| Sex | Female | |
| BMI | 24 | |
| Blood group | A+ | |
| Cause of death | ICH | |
| sCr (mg/dL) | 0.85 | |
| Cockroft (ml/min) | 55.97 | |
| Karpinski score | Right_K | Left_K |
| Glomerulosclerosis | 1 | 1 |
| Interstitial fibrosis | 1 | 1 |
| Tubular atrophy | 1 | 2 |
| Arterial/arteriolar narrowing | 3 | 3 |
| Total | 6 | 7 |
(B) Demographic and clinical data of therecipient.
| Recipient | |
|---|---|
| Age | 58 |
| Sex | Female |
| BMI | 33.2 |
| Blood group | A+ |
| IRC cause | Nephroangiosclerosis |
| sCr before DKT | 10.91 |
| Cockroft (ml/min) | 3.9 |
(C) Perfusion and metabolic parameters of the discharged kidneys.
| Right_K | Left_K | |
|---|---|---|
| Flow median (ml/min) | 24 | 52 |
| Pressure (mmHg) | 25 | 25 |
| Resistence Ru | 1.04 | 0.48 |
| Temperature | 4°C | 4°C |
| Time | 1 h 20 min | 2 h 30 min |
| pH T0 | <6.80 | 6.82 |
| pCO2 T0(mmHg) | 6 | <6 |
| pO2 T0 (mmHg) | 546 | 423 |
| Lat T0 (mg/dL) | 2.7 | 3.6 |
| pH T1 | 6.93 | <6.80 |
| pCO2 T1 (mmHg) | 8 | 6 |
| pO2 T1 (mmHg) | 648 | 675 |
| Lat T1 (mg/dL) | 1.11 | 1.19 |
Recipient data
A 58-year-old female patient with chronic renal insufficiency due to nephroangiosclerosis and listed for transplantation at our center was selected for DKT. The informed consent to receive an ECD organ preserved by HOPE was signed by the recipient. In the clinical history of the patient, hypertension and hypothyroidism were reported. The peritoneal dialysis time was more than 2 years and sCr was 10.91 mg/dL before transplant. Recipient aspects are detailed in Table 1B.
Ex vivo hypothermic oxygenated perfusion
Following static cold storage with Celsior solution performed during hospital transfer and surgical back-table, organs were connected to the perfusion device through sterile disposable tubes dissectioning and incannulating renal artery with vascular cannula specified for artery size. HOPE was performed using Celsior as fluid perfusion (1 L of total volume) at 4°C, a renal artery pressure of 25 mmHg, and keeping the oxygen partial pressure (pO2) between 600 mmHg and 750 mmHg. Flow, pressure, and temperature values were monitored continuously. Oxygen and carbon dioxide partial pressure (pO2 and pCO2), pH, and lactate production were monitored every 15 min by means of hemo-gas analysis of the effluent perfusate. Data prior to (T0) and after treatment (T1) are reported in Table 1C.
The perfusion time was of 2 h 30 min for the left kidney and 1 h 20 min for the right kidney, without any adverse events. During HOPE, there was a 34% progressive increase of the renal flow for the left kidney (median flow 52 ml/min) and 50% for the right kidney (median flow 24 ml/min). The recommended oxygen levels were adequately provided, and carbon dioxide production was not observed due to extracorporeal CO2 elimination advocated by the oxygenator. Each kidney had perfusate lactate level lower than 1.2 mmol/L at T1.
No bacterial or fungal contamination was detected from microbiological cultures performed on the perfusion fluid before and after HOPE.
Based on our previous organ perfusion experience [15], the increase of renal flow and the low level of lactate following 1 h of HOPE led us to deem both kidneys as transplantable for a dual kidney transplantation.
Kidney transplant and patient outcome
In accordance with the standard procedure, DKT was performed without any complications and both kidneys were implanted into the right iliac fossa after 10 h 50 min of cold ischemic time (CIT) for the left kidney and 11 h 50 min for the right kidney. Each artery and vein anastomosis was constructed to the external iliac vessels and each ureter-bladder anastomosis was over a single stent. Postoperative management and immunosuppression therapy, based on a standard regimen of thymoglobulin infusion, steroids, tacrolimus, and mycophenolate mofetil, were followed according to the standard protocols [16].
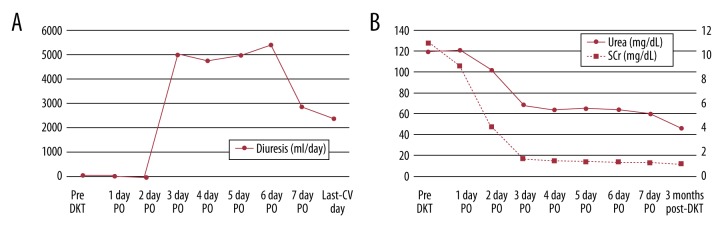
The recipient had immediate graft function (IGF) [17], with 1.18 mg/dL sCr at 5 days after the operation and 1000 cc/die of diuresis (Figure 1A). As result of a good graft function, at discharge, sCr was 1 mg/dL and at 3 months post-DKT, it was 0.95 mg/dL, (Figure 1B).
Figure 1.
(A) Diuresis of the recipient before and after transplantation. (B) Urea and serum creatinine levels of the recipient before and after transplantation.
Discussion
Thanks to the efforts of the Dual Kidney Transplant Group (DKG) [5] during the last 2 decades, the dual transplant of marginal kidneys shows positive results and is helping to increase the number of available allografts. However, the use of marginal kidneys is complex and risky. In determining transplantation eligibility, there are many aspects to assess and compare.
Histology score is not the only aspect to consider for SKT or DKT assignment. Carta et al. reported that an organ with score of 4 or 5 can be allocated for SKT if the eGFR donor is ≥60 ml/min [18]. Some authors had added that, besides the histological score, the parameters of machine perfusion are important determinants in deciding to discard an organ [19]. Our organ allocation system enrolls kidneys with scores of 4 for SKT when donors are under age 75 and have normal sCr. However, it is not reported as a DKT with a total score above 12. We report the first case of dual kidney transplantation with an allocation biopsy score over the limits of transplantation eligibility in according to the Remuzzi’s histological selection process [5].
The kidneys were determined as untransplantable according to histological results. However, the vascular disease resulted in major histological damage, and a normal value of sCr, despite an eGFR of less than 60 ml/min, led us to reevaluate these organs for the machine perfusion. Flow and resistance parameters may help in kidneys selection [19]. Both kidneys were perfused sequentially without prolonged CIT: during the surgical preparation of the second organ (right kidney), HOPE was started for the first organ (left kidney). Despite the worse histology score, the left kidney had a higher flow than the right kidney, but at T1 both organs had an increased flow and decreased resistance, as evidence of the hydrostatic effects of perfusion on intrarenal vasoconstriction [20]. After HOPE, the kidneys were implanted without intra-operative or post-operative complications. The recipient had good IGF and good levels of sCr at 3 months post-DKT.
In this reported case, HOPE appears as a useful tool for reevaluating and improving organ quality of marginal kidneys that were discarded due to the histological score, but with a suitable renal function. In the absence of a standard approach to allocate marginal organs, the information gained during perfusion is useful in the selection process in addition to the histological findings and donor risk factors. There are currently no data about use of HOPE in human KT.
Other authors reported similar cases of discarded kidneys with EVNP [11,12]. This strategy restores organ physiological conditions and permits assay of the graft function during perfusion.
A recent study investigated use of HOPE in rat kidneys obtained from donors after cardiac death, showing HOPE is superior to other preservation techniques, such as EVNP [21]. The efficacy of HOPE in the transplant of marginal human kidneys is unknown, and further study is needed to evaluate the possible effect of this type of perfusion on immunomodulatory molecules such as NGAL or others [22,23]. To the best of our knowledge, this is the first case report of human kidneys histologically discarded and transplanted after HOPE.
Conclusions
HOPE may help clinicians in the organ selection process and improve graft quality of marginal kidneys. A clinical study is required to demonstrate the effects of HOPE on graft outcomes.
Acknowledgments
The authors thank the following for their important scientific contribution: Gabriella Sangiorgi, MD; Giovanni Liviano, MD; Vania Cuna, MD; Matteo Cescon, Professor.
Footnotes
Conflict of interest
None.
Source of support: Funding sources were provided by “Fondazione del Monte” and University of Bologna
References:
- 1.Jochmans I, Akhtar MZ, Nasralla D, et al. Past, present, and future of dynamic kidney and liver preservation and resuscitation. Am J Transplant. 2016;16:2545–55. doi: 10.1111/ajt.13778. [DOI] [PubMed] [Google Scholar]
- 2.Hameed AM, Hawthorne WJ, Pleass HC. Advances in organ preservation for transplantation. ANZ J Surg. 2016 doi: 10.1111/ans.13713. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 3.Pascual J, Zamora J, Pirsch JD. A systematic review of kidney transplantation from expanded criteria donors. Am J Kidney Dis. 2008;52:553–86. doi: 10.1053/j.ajkd.2008.06.005. [DOI] [PubMed] [Google Scholar]
- 4.MacKenzie HS, Azuma H, Rennke HG, et al. Renal mass as a determinant of late allograft outcome: Insights from experimental studies in rats. Kidney Int. 1995;52:S38–42. [PubMed] [Google Scholar]
- 5.Remuzzi G, Grinyò J, Ruggenenti P, et al. Early experience with dual kidney transplantation in adults using expanded donor criteria. Double Kidney Transplant Group (DKG) J Am Soc Nephrol. 1999;10:2591–98. doi: 10.1681/ASN.V10122591. [DOI] [PubMed] [Google Scholar]
- 6.O’Callaghan JM, Morgan RD, Knight SR, Morris PJ. Systematic review and meta-analysis of hypothermic machine perfusion versus static cold storage of kidney allografts on transplant outcomes. Br J Surg. 2013;100:991–1001. doi: 10.1002/bjs.9169. [DOI] [PubMed] [Google Scholar]
- 7.Tolstykh GP, Gelineau JF, Maier LM, Bunegin L. Novel portable hypothermic pulsatile perfusion preservation technology: Improved viability and function of rodent and canine kidneys. Ann Transplant. 2010;15:35–43. [PubMed] [Google Scholar]
- 8.Kwiatkowski A, Wszoła M, Kosieradzki M, et al. The early and long term function and survival of kidney allografts stored before transplantation by hypothermic pulsatile perfusion. A prospective randomized study. Ann Transplant. 2009;14:14–17. [PubMed] [Google Scholar]
- 9.Thuillier R, Allain G, Celhay O, et al. Benefits of active oxygenation during hypothermic machine perfusion of kidneys in a preclinical model of deceased after cardiac death donors. J Surg Res. 2013;184:1174–81. doi: 10.1016/j.jss.2013.04.071. [DOI] [PubMed] [Google Scholar]
- 10.Hosgood SA, Nicholson HF, Nicholson ML. Oxygenated kidney preservation techniques. Transplantation. 2012;93:455–59. doi: 10.1097/TP.0b013e3182412b34. [DOI] [PubMed] [Google Scholar]
- 11.Hosgood SA, Barlow AD, Dormer J, Nicholson ML. The use of ex-vivo normothermic perfusion for the resuscitation and assessment of human kidneys discarded because of inadequate in situ perfusion. J Transl Med. 2015;13:329. doi: 10.1186/s12967-015-0691-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hosgood SA, Saeb-Parsy K, Hamed MO, Nicholson ML. Successful transplantation of human kidneys deemed untransplantable but resuscitated by ex vivo normothermic machine perfusion. Am J Transplant. 2016;16:3282–85. doi: 10.1111/ajt.13906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Karpinski J, Lajoie G, Cattran D, et al. Outcome of kidney transplantation from high-risk donors is determined by both structure and function. Transplantation. 1999;67:1162–67. doi: 10.1097/00007890-199904270-00013. [DOI] [PubMed] [Google Scholar]
- 14.La Manna G, Comai G, Cappuccilli ML, et al. Prediction of three-year outcome of renal transplantation from optimal donors versus expanded criteria donors. Am J Nephrol. 2013;37:158–66. doi: 10.1159/000346257. [DOI] [PubMed] [Google Scholar]
- 15.Ravaioli M, De Pace V, Pinna AD. From six thousand transaminases level to three hundred after liver transplantation: A new era seems to be open. Updates Surg. 2017 doi: 10.1007/s13304-017-0466-4. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 16.Kidney Disease: Improving Global Outcomes (KDIGO) Transplant Work Group KDIGO clinical practice guideline for the care of kidney transplant recipients. Am J Transplant. 2009;9(Suppl. 3):S1–155. doi: 10.1111/j.1600-6143.2009.02834.x. [DOI] [PubMed] [Google Scholar]
- 17.Humar A, Ramcharan T, Kandaswamy R, et al. Risk factors for slow graft function after kidney transplants: A multivariate analysis. Clin Transplant. 2002;16:425–29. doi: 10.1034/j.1399-0012.2002.02055.x. [DOI] [PubMed] [Google Scholar]
- 18.Carta P, Zanazzi M, Caroti L, et al. Impact of the pre-transplant histological score on 3-year graft outcomes of kidneys from marginal donors: A single-centre study. Nephrol Dial Transplant. 2013;28:2637–44. doi: 10.1093/ndt/gft292. [DOI] [PubMed] [Google Scholar]
- 19.Sung RS, Christensen LL, Leichtman AB, et al. Determinants of discard of expanded criteria donor kidneys: impact of biopsy and machine perfusion. Am J Transplant. 2008;8:783–92. doi: 10.1111/j.1600-6143.2008.02157.x. [DOI] [PubMed] [Google Scholar]
- 20.Polyak MM, Arrington BO, Stubenbord WT, et al. The influence of pulsatile preservation on renal transplantation in the 1990s. Transplantation. 2000;69:249–58. doi: 10.1097/00007890-200001270-00010. [DOI] [PubMed] [Google Scholar]
- 21.Kron P, Schlegel A, de Rougemont O, et al. Short, cool, and well oxygenated – HOPE for kidney transplantation in a rodent model. Ann Surg. 2016;264:815–22. doi: 10.1097/SLA.0000000000001766. [DOI] [PubMed] [Google Scholar]
- 22.La Manna G, Ghinatti G, Tazzari PL, et al. Neutrophil gelatinase-associated lipocalin increases HLA-G(+)/FoxP3(+) T-regulatory cell population in an in vitro model of PBMC. PLoS One. 2014;9:e89497. doi: 10.1371/journal.pone.0089497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Parikh CR, Hall IE, Bhangoo RS, et al. Associations of perfusate biomarkers and pump parameters with delayed graft function and deceased donor kidney allograft function. Am J Transplant. 2016;16:1526–39. doi: 10.1111/ajt.13655. [DOI] [PMC free article] [PubMed] [Google Scholar]