Abstract
Background
The rising number of patients on waiting lists for kidney transplant and the shortage of available organs has intensified efforts to increase the number of potential donors.
Material/Methods
This study investigated changes in clinical parameters among potential deceased donors in the 15-year period between 1999 and 2013 and their impact on transplantation procedure and outcomes. A total of 1634 potential deceased donors were examined and divided into 2 groups: 707 of them identified from 1999 to 2005 (Group A), and 927 from 2006 to 2013 (Group B).
Results
The comparison between the potential donors in Group A vs. Group B revealed an increase over time in donor age (54.6±17.2 vs. 58.8±16.3, p<0.001), a reduction in the percentage of standard donors (52.3% vs. 39.8%, p<0.001), a broader utilization of organs from expanded criteria donors, and a greater number of comorbidities, particularly cardiovascular disease and dyslipidemia. However, renal function parameters and the bioptic scores did not change significantly over the years.
Conclusions
These results suggest the usefulness of strategies to increase the number of potential donors suitable for organ donation, especially among elderly and marginal donors.
MeSH Keywords: Kidney Failure, Chronic; Kidney Transplantation; Tissue and Organ Procurement
Background
Chronic kidney disease (CKD) has been recognized as a worldwide health problem, and the associated morbidity and mortality in patients reaching end-stage renal disease (ESRD) is steeply increasing [1]. Kidney transplantation is currently considered the best therapeutic option for ESRD patients, as it leads to a higher quality of life and longer survival than dialysis, together with considerable amelioration or resolution of the most common dialysis-related complications [2–5]. Furthermore, the cost-effectiveness of hemodialysis is significantly higher than transplantation [6]. The growing gap between organ demand and availability and the increasing mortality rate among patients on kidney transplant waiting lists has amplified the efforts to increase the number of potential donors [7,8]. Besides living donation, great attention has been devoted to the use of organs from incompatible AB0 donors [9], donation after cardiac death (DCD) [10], and expanded criteria donors (ECD) [11,12]. The need to consider alternative strategies to enlarge the donor pool has led to greater utilization of elderly donors and to a consequent increase in average donor age and comorbidity rate [13,14]. ECD are defined as any brain-dead donor aged >60 years or a donor aged >50 years with 2 of the following conditions: history of hypertension, terminal serum creatinine level ≥1.5 mg/dL, or death resulting from a cerebrovascular accident. This approach may be advantageous to overcome the organ shortage and to increase the chances to receive a transplant for some patients otherwise remaining under dialysis treatment [15].
The aim of this study was to investigate changes in clinical parameters among potential deceased donors in the 15 years from 1999 to 2013 in the Emilia-Romagna region of Italy, and to evaluate their impact on transplantation procedure.
Material and Methods
Study design
This was an observational retrospective data analysis performed in the Nephrology, Dialysis, and Renal Transplant Unit, St Orsola Hospital, University of Bologna, Italy. A total of 1634 subjects identified as potential donors by the Intensive Therapy Units in Emilia-Romagna between January 1999 and December 2013 were included in the analysis. Exclusion criteria were age below 18 years and interruption of the donation procedure due to lack of consensus or death. The study population was divided into 2 groups: patients identified as potential donors in the period 1999 to 2005 (Group A) and patients identified between 2006 and 2013 (Group B). Donors were further divided into 3 categories: standard criteria donors (SCD), ECD, and unsuitable donors. We defined as SCD patients with clinical and anamnestic characteristics that did not require bioptic analysis before transplantation. ECD donors required biopsy before transplantation because of anamnesis, comorbidities (cardiovascular disease, diabetes, or hypertension), or age (>60 years). The pre-implantation biopsies score resulted from a quantitative evaluation of lesions in different renal compartments, particularly vascular intimal sclerosis, tubular atrophy, interstitial fibrosis, and glomerular sclerosis as Karpinski’s histological score [16,17]. Unsuitable donors were considered not eligible for organ donation based on the following parameters: histology score (Karpinsky); comorbidities or severe renal dysfunction, anatomical, or vascular malformations that could affect surgical outcome of transplant; renal or other carcinomas; and increased risk of infections. Due to the retrospective nature of this study, registration or approval by the Ethics Committee was waived. The study was carried out in conformity with the Declaration of Helsinki.
General, biochemical, clinical, and transplant-related parameters
For each potential donor included in the analysis, the following anthropometric parameters were collected: age, sex, and body mass index (BMI). To evaluate renal function, serum creatinine and glomerular filtration rate (GFR) calculated with CKD-EPI formula and Cockroft-Gault formula were recorded. Smoking, systemic arterial hypertension, diabetes, dyslipidemia, and cardiovascular diseases were considered as risk factors. Traumatic brain injury, cerebral hemorrhage, ictus, post-anoxic encephalopathy, bullet wounds in the head, and suicide were included among the causes of death. We also computed cold ischemia intervals of each single kidney (K1 and K2).
Statistical analysis
All data were extracted from the database using FileMaker software and recorded in a Microsoft Excel datasheet. Continuous variables were compared with the Student t-test; categorical variables were analyzed with the chi-square test. To evaluate the sum of risk factors, we used the Mann-Whitney test. A p value lower than 0.05 was considered as statistically significant. All analyses were performed with the Statistical Package for the Social Sciences (SPSS).
Results
Patients
We identified 1845 potential donors in Emilia-Romagna region from 1999 to 2013. Among them, 211 did not complete the evaluation process and were not included in the analysis. Thus, 1634 were examined and divided into 2 groups based on the evaluation period: 707 potential donors were identified from 1999 to 2005 (Group A), and 927 from 2006 to 2013 (Group B). Donors in each group were further divided into 3 subgroups according to quality factors: there were 370 SCD in Group A and 369 in Group B; 173 ECD in Group A and 276 in Group B; and 164 unsuitable donors in Group A and 282 in Group B (Figure 1).
Figure 1.
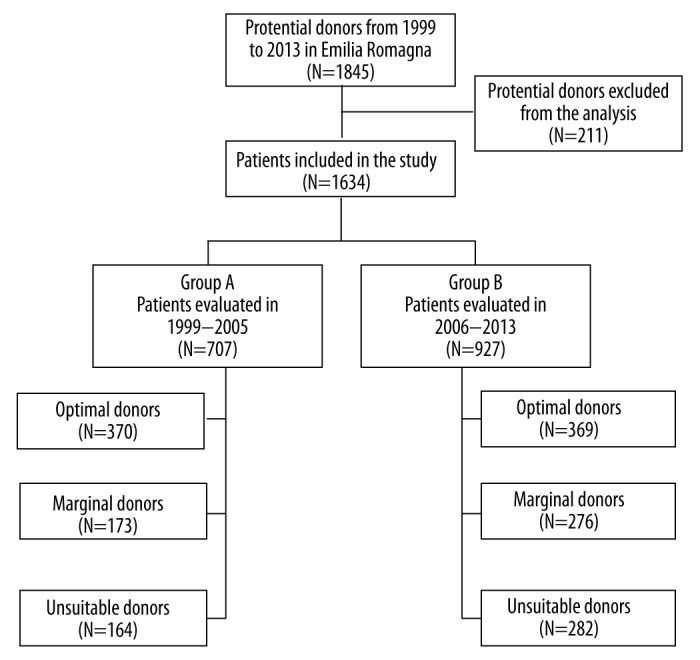
Scheme used for donor classification.
General, biochemical, clinical, and transplant-related features of the overall donor population
Donors characteristics evaluated in 1999–2005 (Group A) compared to those identified in 2006–2013 (Group B) are reported in Table 1. The age of donors significantly increased over the years, with a concomitant rise in the proportion of elderly donors (aged more than 65 years). Other parameters, including sex, BMI, creatinine, GFR, and bioptic scores, were similar between the groups. In Group B (corresponding to the most recent years) we found a significant reduction of cold ischemia time for both transplanted kidneys (Table 1).
Table 1.
General, biochemical, clinical and transplant-related characteristics of the donor population divided into Group A (years from 1999 to 2005) and Group B (years from 2006 to 2013). Continuous variable are presented as mean ± standard deviation (SD), categorical as number and percentages (%).
| Group A (N=707) | Group B (N=927) | p | |
|---|---|---|---|
| Sex (F) | 308 (43.6%) | 405 (43.7%) | n.s. |
| Age (>65 years) | 223 (31.5%) | 388 (41.9%) | <0.001 |
| Age (years) | 54.6±17.1 | 58.8±16.3 | <0.001 |
| BMI (kg/m2) | 25.4±3.9 | 25.7±3.8 | n.s. |
| Serum creatinine (mg/dL) | 0.98±0.54 | 0.94±0.49 | n.s. |
| GFR Cockcroft-Gault (mL/min) | 92.2±34.2 | 94.5±39.9 | n.s. |
| GFR CKD-EPI (mL/min) | 84.8±23.7 | 85.7±23.9 | n.s. |
| Cold ischemia time K1 (hours) | 15.6±4.6 | 13.5±4.4 | <0.001 |
| Cold ischemia time K2 (hours) | 17.5±5.5 | 14.6±4.8 | <0.001 |
| Bioptic score right kidney | 3.62±1.52 | 3.6±1.62 | n.s. |
| Bioptic score left kidney | 3.94±1.55 | 3.90±1.64 | n.s. |
BMI – body mass index; CKD – chronic kidney disease; GFR – glomerular filtration rate.
Table 2 depicts the distribution in the 2 groups by cause of death, type and number of risk factors, and type of donors. We found that over the years there was a decrease among the potential donors of the deaths for traumatic brain injury and a rise in deaths due to post-anoxic encephalopathy. Concerning the risk factors, cardiovascular disease and dyslipidemia were found to be significantly more frequent in Group B than in Group A, and there were more donors with 1 risk factor and with 3 or more risk factors in the period 2006–2013. As expected, there was an increase in the percentage of ECD and unsuitable donors in Group B (Table 2).
Table 2.
Distribution of cause of death, type and number of risk factors, and type of donors in the donor population divided into Group A (years from 1999 to 2005) and Group B (years from 2006 to 2013). Data (all categorical variables) are presented as number and percentages (%).
| Group A (N=707) | Group B (N=927) | p | |
|---|---|---|---|
| Cause of death | |||
| Cerebral hemorrhage | 407 (57.6%) | 501 (54.0%) | n.s. |
| Ictus | 52 (7.4%) | 89 (9.6%) | n.s. |
| Traumatic brain injury | 198 (27.0%) | 207 (22.3%) | 0.008 |
| Post-anoxic encefalopathy | 27 (3.8%) | 57 (6.1%) | 0.035 |
| Bullet wounds at the head | 11 (1.6%) | 10 (1.1%) | n.s. |
| Suicide | 3 (0.4%) | 7 (0.8%) | n.s. |
| Risk factors | |||
| Arterial hypertension | 241 (34.1%) | 348 (37.5%) | n.s. |
| Cardiovascular disease | 85 (12.0%) | 191 (20.6%) | <0.001 |
| Smoking | 143 (20.2%) | 172 (18.6%) | n.s. |
| Dyslipidemia | 18 (2.5%) | 91 (9.8%) | <0.001 |
| Diabetes | 50 (7.1%) | 72 (7.8%) | n.s. |
| Number of risk factors | |||
| 0 | 340 (48.1%) | 433 (46.7%) | n.s. |
| 1 | 231 (32.7%) | 228 (24.6%) | 0.008 |
| 2 | 108 (15.3%) | 180 (19.4%) | n.s. |
| 3 or more | 28 (4.0%) | 86 (9.3%) | <0.001 |
| Type of donors | |||
| SCD | 370 (52.3%) | 369 (39.8%) | 0.002 |
| ECD | 173 (24.5%) | 276 (29.8%) | 0.047 |
| Unsuitable | 164 (23.2%) | 282 (30.4%) | 0.016 |
ECD – expanded criteria donors; n.s. – not significant; SCD – standard criteria donors.
Considering the differences in the features of ECD alone in Group A vs. Group B, we noticed a significant rise in the proportion of elderly donors (aged >65 years) (64.9% vs. 71.5%, p=0.004). On the other hand, no significant differences were observed in the frequency of the other risks factors included in the criteria used to define ECD (serum creatinine >1.5 mg/dL: 5.1% vs. 5.6%, p=n.s.; arterial hypertension: 34.1% vs. 37.5%, p=n.s.; and cerebrovascular accident: 64.9% vs. 63.6%, p=n.s.).
Regarding the causes of the exclusion from donation of unsuitable donors, the comparison of Group A vs. Group B revealed that frequency of ineligibility due to bioptic score decreased over time (38.4% vs. 27.8%, p=0.044), while the rate of the other causes remained unchanged (nephrological unsuitability: 8.5% vs. 9.6%, p= n.s; surgical unsuitability: 2.4% vs. 6.8%, p=n.s.; other causes: 50.6% vs. 55.9%, p=n.s.).
Analysis of parameters after stratification by type of donor
As Tables 3 and 4 show, Group A (1999–2005) and Group B (2006–2013) were further analyzed according to the type of donor: SCD, ECD, and unsuitable donors.
Table 3.
General, biochemical, clinical and transplant-related characteristics of the donor population stratified by type of donors (SCD vs. ECD and unsuitable donors) in Group A (years from 1999 to 2005) and Group B (years from 2006 to 2013). Continuous variable are presented as mean ± standard deviation (SD), categorical as number and percentages (%).
| SCD (N=739) | ECD (N=449) | Unsuitable (N=446) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Group A (N=370) | Group B (N=369) | Group A (N=173) | Group B (N=276) | Group A (N=164) | Group B (N=282) | ||||
| Sex (F) | 155 (41.9%) | 160 (43.4%) | n.s. | 75 (43.3%) | 110 (39.9%) | n.s. | 66 (40.2%) | 140 (49.6%) | n.s. |
| Age (>65 years) | 111 (30.0%) | 149 (37.9%) | 0.048 | 47 (27.2%) | 118 (42.8%) | 0.027 | 50 (30.5%) | 112 (39.7%) | <0.001 |
| Age (years) | 44.9±15.7 | 46.6±13.6 | n.s. | 64.8±9.6 | 68.5±8.8 | <0.001 | 65.9±12.9 | 65.3±15.1 | n.s. |
| BMI (kg/m2) | 24.8± 3.8 | 25.2±3.9 | n.s. | 26.3±3.8 | 26.2±3.6 | n.s. | 25.7±3.9 | 25.9±3.8 | n.s. |
| Serum creatinine (mg/dL) | 0.91±0.35 | 0.87±0.33 | n.s. | 0.93±0.37 | 0.93±0.47 | n.s. | 1.23±0.90 | 1.08±0.71 | n.s. |
| GFR Cockcroft-Gault (mL/min) | 104.8±33.9 | 111.4±40.1 | 0.018 | 83.9±26.8 | 81.9±31.2 | n.s. | 70.5±29.0 | 79.2±38.4 | 0.024 |
| GFR CKD-EPI (mL/min) | 93.9±22.2 | 95.6±23.5 | n.s. | 78.1±18.1 | 78.0±20.7 | n.s. | 71.5±23.9 | 78.0±22.9 | <0.001 |
| Cold ischemia time K1 (hours) | 15.5±4.7 | 13.0±4.5 | <0.001 | 16.0±4.3 | 14.1±4.3 | <0.001 | / | / | / |
| Cold ischemia time K2 (hours) | 17.3±5.6 | 13.9±4.9 | <0.001 | 18.0±5.1 | 15.5±4.5 | <0.001 | / | / | / |
| Bioptic score right kidney | n.a. | n.a. | / | 3.03±1.18 | 3.09±1.35 | n.s. | 5.26±1.15 | 5.31±1.26 | n.s. |
| Bioptic score left kidney | n.a. | n.a. | / | 3.45±1.23 | 3.41±1.39 | n.s. | 5.40±1.38 | 5.55±1.34 | n.s. |
BMI – body mass index; CKD – chronic kidney disease; ECD – expanded criteria donors; GFR – glomerular filtration rate; n.a. – not applicable; n.s. – not significant; SCD – standard criteria donors.
Table 4.
Distribution of cause of death, type and number of risk factors, and type of donors in the donor population stratified by type of donors (SCD vs. ECD and unsuitable donors) in Group A (years from 1999 to 2005) and Group B (years from 2006 to 2013). Data (all categorical variables) are presented as number and percentages (%).
| SCD (N=739) | ECD (N=449) | Unsuitable (N=446) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Group A (N=370) | Group B (N=369) | Group A (N=173) | Group B (N=276) | Group A (N=164) | Group B (N=282) | ||||
| Cause of death | |||||||||
| Cerebral hemorrhage | 167 (45.1%) | 176 (47.7%) | n.s. | 123 (71.1%) | 170 (61.6%) | 0.042 | 117 (73.6%) | 155 (63.8%) | 0.001 |
| Ictus | 26 (7.0%) | 25 (6.8%) | n.s. | 22 (12.7%) | 42 (15.2%) | n.s. | 4 (2.5%) | 22 (9.1%) | 0.021 |
| Traumatic brain injury | 148 (40.0%) | 118 (32.0%) | 0.023 | 21 (12.1%) | 44 (15.9%) | n.s. | 29 (18.2%) | 45 (18.5%) | n.s. |
| Post-anoxic encefalopathy | 16 (4.3%) | 27 (7.3%) | n.s. | 4 (2.3%) | 12 (4.3%) | n.s. | 7 (4.4%) | 18 (7.8%) | n.s. |
| Bullet wounds at the head | 8 (2.2%) | 8 (2.2%) | n.s. | 2 (1.2%) | 1 (0.4%) | n.s. | 1 (1.2%) | 1 (0.4%) | n.s. |
| Suicide | 1 (0.3%) | 4 (1.1%) | n.s. | 1 (0.6%) | 2 (0.7%) | n.s. | 1 (0.6%) | 2 (0.4%) | n.s. |
| Risk factors | |||||||||
| Arterial hypertension | 64 (17.3%) | 79 (21.4%) | n.s. | 95 (54.9%) | 163 (59.1%) | n.s. | 82 (50.0%) | 106 (37.6%) | 0.012 |
| Cardiovascular disease | 13 (3.5%) | 20 (5.4%) | n.s. | 38 (22.0%) | 101 (36.6%) | 0.012 | 34 (20.7%) | 70 (24.8%) | n.s. |
| Smoking | 85 (23.0%) | 91 (24.7%) | n.s. | 36 (20.8%) | 39 (14.1%) | n.s. | 22 (13.4%) | 42 (14.9%) | n.s. |
| Dyslipidemia | 3 (0.8%) | 20 (5.4%) | <0.001 | 11 (6.4%) | 46 (16.7%) | 0.001 | 4 (2.4%) | 25 (8.9%) | 0.009 |
| Diabetes | 5 (1.4%) | 4 (1.1%) | n.s. | 21 (12.1%) | 32 (11.6%) | n.s. | 24 (14.6%) | 36 (12.8%) | n.s. |
ECD – expanded criteria donors; n.s. – not significant; SCD – standard criteria donors.
Considering the subgroup of SCD, no significant differences between the groups were observed in terms of sex, age, BMI, serum creatinine, or GFR calculated by CKD-EPI equation. The proportion of elderly donors grew in the latest period (Group B). Renal function estimated by Cockcroft-Gault formula was better in Group B than in Group A. The cold ischemia time was found to be significantly lower for both kidneys in Group B than in Group A (Table 3). The causes of death were fairly unchanged during the years, with the exception of brain traumatic injury, which was less common in the period 2006–2013. Among the risk factors, the frequency of dyslipidemia showed a highly significant rise in Group B (Table 4).
In the subgroup of ECD, we found that the ones in the Group B were older, and the percentage of donors aged more than 65 years grew over the years. No differences were observed in terms of sex distribution, BMI, serum creatinine levels, GFR, or bioptic scores. Again, the cold ischemia time was significantly decreased in 2006–2013 for both kidneys (Table 3). Death from cerebral hemorrhage was significantly more frequent in Group A vs. Group B, but the prevalences of cardiovascular diseases and dyslipidemia were lower (Table 4).
In unsuitable donors, sex distribution, age, BMI, serum creatinine levels, and bioptic score remained unchanged over the years; the frequency of elderly donors was greater in Group B, and GFR was also significantly increased (Table 3). Similar to the other subgroups, death from cerebral hemorrhage showed a significant decline over time, while the prevalence of stroke increased. Concerning risk factors, among unsuitable donors we observed an increase from the period 1999–2005 (Group A) to the period 2006–2013 (Group B) in the rate of arterial hypertension and dyslipidemia (Table 4).
Discussion
This observational retrospective study was undertaken to compare the main features of kidney transplant donors during the periods 1999–2005 and 2006–2013, with the aim to identify those parameters that have changed over the last 15 years. A total of 1634 patients were included in the analysis and clustered according to the evaluation period into Group A (period 1999–2005, 707 potential donors) and Group B (period 2006–2013, 927 potential donors). In line with the general trend, our donor population became older over the years, with an increasing proportion of donors older than 65 years, and presented more comorbidities; in particular, we found a growth in the rate of cardiovascular disease and dyslipidemia. Moreover, there was a decreased frequency of traumatic causes of death, and the procedure for organ management after procurement was progressively improved as indicated by the decreased cold ischemia interval for both kidneys, consistent with previous reports [18]. This finding is suggestive of a progressive improvement in organizational policies and procedures at our Regional Transplant Center, with acceleration of the alert phase when a potential donor becomes available, and the successive organ allocation. The reduction of cold ischemia time, together with the optimization of preservation techniques, represent essential strategies to limit the severity of tissue damage caused by hypothermic preservation, especially in expanded criteria donors [19,20]. There is a large body of evidence indicating that the cost-effectiveness associated with renal transplantation from ECD is consistently better than chronic dialysis treatment. In addition, the survival and quality of life of patients transplanted with marginal organs has improved. In the attempt to enlarge the pool of organs available for transplantation, many ECDs have been evaluated for donation; thus, unsurprisingly, the proportion of SCD has been decreasing in the last years, and this effect is partly due to the older average age of the general population [21,22]. In our analysis, we noticed that renal function parameters and bioptic scores did not change significantly over time when considering the overall donor population. However, after stratification by type of donors, eGFR was found to be improved in the donors of the latest period for SCD and unsuitable donors (nearly significant for SCD when using Cockcroft-Gault formula).
Taken together, these data suggest that the increasing age and presence of comorbidities might not be necessarily associated with impaired graft function or poor histological features. Radhawa et al. described a glomerulosclerosis rate ranging from 0% to 10% in patients aged 60–75 years and bioptic abnormalities in younger patients, showing that donor age alone may be a misleading criterion for organ classification and allocation [23]. In spite of the belief that the use of organs from elderly or marginal donors results in poorer post-transplant renal function and outcome [8,24,25], McGlynn et al. proposed that the performance of an organ is more strictly correlated to its level of senescence rather than donor age itself. Some organs from older donors have proven to function adequately for many years, similarly to those from younger donors, and the explication might lie in the fact that biological age of the organ is a more effective predictor of graft outcome than its chronological age. Hence, telomere shortening and the expression of biomarkers of cellular damage and senescence are likely to be more suitable criteria to assess the quality of an organ [26].
Among unsuitable donors, a particular finding of our study was the similar rates of discharge due to bioptic score between the potential donors of the less recent period (1999–2005) and the ones of the latest years (2006–2013). Despite the contrast with OPTN data, which report a higher rate of discard due to the degree of glomerulosclerosis [27], our finding might be explained by increased expertise of the transplant pathologists in our transplant center. Currently, there is no shared consensus regarding the policies for discard based on histology findings. Since our first kidney transplant in 1967, we have performed more than 2700 renal transplants, resulting in a higher percentage of patients on our waiting list needing retransplantation and longer average waiting times. A very recent study that focused on identification of the most relevant predictors in the selection of deceased donor kidneys in the USA reported a weak correlation between kidney biopsy performance and discard rate [28].
Our analysis has the limitation of considering only donors features to assess the effectiveness of potential donors based on the number of performed transplants, without examining transplant outcomes. Nevertheless, previous evidence has suggested that histology does not correlate with 1-year patient and allograft survival [29]. Further studies are needed to better address the contribution of ECD to long-term patient survival and graft outcomes in kidney transplantation.
Conclusions
The increasing organ shortage requires a great effort to promote and optimize novel strategies and specific allocation policies for ECD transplantation. Our analysis provides encouraging data that support enlarging criteria to broaden the pool of potential donors eligible for organ donation, especially among elderly and marginal donors. We confirmed that donor age alone may be not an independent and predictive factor in decision-making about organ classification and allocation. In particular, better organs should be allocated to patients with predicted longer life expectancy to prevent or minimize the risk of retransplantation in case of kidney allograft failure.
Abbreviations
- BMI
body mass index
- CKD
chronic kidney disease
- DCD
donation after circulatory death
- ECD
expanded criteria donors
- ESRD
end-stage renal disease
- GFR
glomerular filtration rate
- SCD
standard criteria donors
- SPSS
Statistical Package for Social Science
Footnotes
Conflict of interest statements
The authors have no financial conflicts of interest.
Source of support: Departmental sources
References
- 1.Radhakrishnan J, Remuzzi G, Saran R, et al. Taming the chronic kidney disease epidemic: A global view of surveillance efforts. Kidney Int. 2014;86(2):246–50. doi: 10.1038/ki.2014.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Garcia GG, Harden P, Chapman J, et al. The global role of kidney transplantation. Nephrol Dial Transplant. 2013;28(8):e1–5. doi: 10.1093/ndt/gfs013. [DOI] [PubMed] [Google Scholar]
- 3.Meier-Kriesche HU, Schold JD, Srinivas TR, et al. Kidney transplantation halts cardiovascular disease progression in patients with end-stage renal disease. Am J Transplant. 2004;4(10):1662–68. doi: 10.1111/j.1600-6143.2004.00573.x. [DOI] [PubMed] [Google Scholar]
- 4.Cianciolo G, La Manna G, Colì L, et al. 5-Methyltetrahydrofolate administration is associated with prolonged survival and reduced inflammation in ESRD patients. Am J Nephrol. 2008;28(6):941–48. doi: 10.1159/000142363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cianciolo G, Colì L, La Manna G, et al. Is beta2-microglobulin-related amyloidosis of hemodialysis patients a multifactorial disease? A new pathogenetic approach. Int J Artif Organs. 2007;30(10):864–78. doi: 10.1177/039139880703001003. [DOI] [PubMed] [Google Scholar]
- 6.Perovic S, Jankovic S. Renal transplantation vs. hemodialysis: Cost-effectiveness analysis. Vojnosanit Pregl. 2009;66(8):639–44. doi: 10.2298/vsp0908639p. [DOI] [PubMed] [Google Scholar]
- 7.Gandolfini I, Buzio C, Zanelli P, et al. The Kidney Donor Profile Index (KDPI) of marginal donors allocated by standardized pretransplant donor biopsy assessment: Distribution and association with graft outcomes. Am J Transplant. 2014;14(11):2515–25. doi: 10.1111/ajt.12928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Audard V, Matignon M, Dahan K, et al. Renal transplantation from extended criteria cadaveric donors: problems and perspectives overview. Transpl Int. 2008;21(1):11–17. doi: 10.1111/j.1432-2277.2007.00543.x. [DOI] [PubMed] [Google Scholar]
- 9.Muramatsu M, Gonzalez HD, Cacciola R, et al. ABO incompatible renal transplants: Good or bad? World J Transplant. 2014;4(1):18–29. doi: 10.5500/wjt.v4.i1.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hoogland ER, Snoeijs MG, Habets MA, et al. Improvements in kidney transplantation from donors after cardiac death. Clin Transplant. 2013;27(3):E295–301. doi: 10.1111/ctr.12107. [DOI] [PubMed] [Google Scholar]
- 11.Wadei HM, Heckman MG, Rawal B, et al. Comparison of kidney function between donation after cardiac death and donation after brain death kidney transplantation. Transplantation. 2013;96(3):274–81. doi: 10.1097/TP.0b013e31829807d1. [DOI] [PubMed] [Google Scholar]
- 12.Dominguez-Gil B, Haase-Kromwijk B, Van Leiden H, et al. Current situation of donation after circulatory death in European countries. Transpl Int. 2011;24(7):676–86. doi: 10.1111/j.1432-2277.2011.01257.x. [DOI] [PubMed] [Google Scholar]
- 13.Kute VB, Trivedi HL, Vanikar AV, et al. Deceased donor renal transplantation from older donors to increase the donor pool. Int J Artif Organs. 2012;35(9):663–70. doi: 10.5301/ijao.5000113. [DOI] [PubMed] [Google Scholar]
- 14.Cho YW. Expanded criteria donors. Clin Transpl. 1998:421–36. [PubMed] [Google Scholar]
- 15.Rosengard BR, Feng S, Alfrey EJ, et al. Report of the Crystal City meeting to maximize the use of organs recovered from the cadaver donor. Am J Transplant. 2002;2(8):701–11. doi: 10.1034/j.1600-6143.2002.20804.x. [DOI] [PubMed] [Google Scholar]
- 16.Kahu J, Kyllönen L, Räisänen-Sokolowski A, Salmela K. Donor risk score and baseline biopsy CADI value predict kidney graft outcome. Clin Transplant. 2011;25(3):E276–83. doi: 10.1111/j.1399-0012.2011.01401.x. [DOI] [PubMed] [Google Scholar]
- 17.Karpinski J, Lajoie G, Cattran D, et al. Outcome of transplantation. 1999;67(8):1162–67. doi: 10.1097/00007890-199904270-00013. [DOI] [PubMed] [Google Scholar]
- 18.Pérez-Sáez MJ, Arcos E, Comas J, et al. Catalan Renal Registry Committee. Survival Benefit from kidney transplantation using kidneys from deceased donors aged >75 years: A time-dependent analysis. Am J Transplant. 2016;16(9):2724–33. doi: 10.1111/ajt.13800. [DOI] [PubMed] [Google Scholar]
- 19.van der Vliet JA, Warlé MC. The need to reduce cold ischemia time in kidney transplantation. Curr Opin Organ Transplant. 2013;18(2):174–78. doi: 10.1097/MOT.0b013e32835e2a08. [DOI] [PubMed] [Google Scholar]
- 20.La Manna G, Conte D, Cappuccilli ML, et al. An in vivo autotransplant model of renal preservation: Cold storage versus machine perfusion in the prevention of ischemia/reperfusion injury. Artif Organs. 2009;33(7):565–70. doi: 10.1111/j.1525-1594.2009.00743.x. [DOI] [PubMed] [Google Scholar]
- 21.Rouchi AH, Mahdavi-Mazdeh M. When is transplantation with a “marginal kidney” justifiable? Ann Transplant. 2016;21:463–68. doi: 10.12659/aot.898405. [DOI] [PubMed] [Google Scholar]
- 22.Franchini M, Pieroni S, Fortunato L, et al. Integrated information for integrated care in the general practice setting in Italy: Using social network analysis to go beyond the diagnosis of frailty in the elderly. Clin Transl Med. 2016;5(1):24. doi: 10.1186/s40169-016-0105-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Randhawa PS, Minervini MI, Lombardero M, et al. Biopsy of marginal donor kidneys: Correlation of histologic findings with graft dysfunction. Transplantation. 2000;69(7):1352–57. doi: 10.1097/00007890-200004150-00024. [DOI] [PubMed] [Google Scholar]
- 24.Jacobi J, Beckmann S, Heller K, et al. Deceased donor kidney transplantation in the Eurotransplant Senior Program (ESP): A single-center experience from 2008 to 2013. Ann Transplant. 2016;21:94–104. doi: 10.12659/aot.895731. [DOI] [PubMed] [Google Scholar]
- 25.van Ittersum FJ, Hemke AC, Dekker FW, et al. Increased risk of graft failure and mortality in Dutch recipients receiving an Expanded Criteria Donor kidney transplant. Transpl Int. 2017;30(1):14–28. doi: 10.1111/tri.12863. [DOI] [PubMed] [Google Scholar]
- 26.McGlynn LM, Stevenson K, Lamb K, et al. Cellular senescence in pretransplant renal biopsies predicts postoperative organ function. Aging Cell. 2009;8(1):45–51. doi: 10.1111/j.1474-9726.2008.00447.x. [DOI] [PubMed] [Google Scholar]
- 27.Reese PP, Harhay MN, Abt PL, et al. New solutions to reduce discard of kidneys donated for transplantation. J Am Soc Nephrol. 2016;27(4):973–80. doi: 10.1681/ASN.2015010023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Marrero WJ, Naik AS, Friedewald JJ, et al. Predictors of deceased donor kidney discard in the United States. Transplantation. 2016 doi: 10.1097/TP.0000000000001238. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 29.Edwards EB, Posner MP, Maluf DG, et al. Reasons for non-use of recovered kidneys: the effect of donor glomerulosclerosis and creatinine clearance on graft survival. Transplantation. 2004;77(9):1411–15. doi: 10.1097/01.tp.0000123080.19145.59. [DOI] [PubMed] [Google Scholar]


