ABSTRACT
Reactive oxygen species (ROS) serve as a key signal messenger in plant cells. Plant NADPH oxidases, known as respiratory burst oxidase homologues (RBOHs), catalyze the production of superoxide, a type of ROS, and are involved in several essential processes in plants. In this review, we discuss recent studies about functional regulation of RBOHs by calcineurin B-like protein (CBL)-CIPKs (the CBL-interacting protein kinases), small GTPases, and lipids that integrate developmental cues and external stimuli.
KEYWORDS: Lipids, NADPH oxidases, reactive oxygen species, small GTPases
Introduction
Plants respond to diverse biotic and abiotic stresses as well as to endogenous developmental cues. The conserved signal messengers, reactive oxygen species (ROS), including singlet oxygen (1O2), superoxide (O2−) and hydrogen peroxide (H2O2), function in a plethora of developmental processes and reactions to environmental stimuli.1,2 The plant NADPH oxidases, which have been designated as respiratory burst oxidase homologs (RBOHs), are the most-studied ROS-producing enzymes. RBOHs play diverse roles in cell growth and development, plant defense, and cellular signal transduction.2 In this review, we summarize recent research into the fine regulation of RBOHs by differential cellular effectors, such as calcineurin B-like protein (CBL)-CIPKs (the CBL-interacting protein kinases), small GTPases, and membrane lipids.
CBL-CIPKs integrate calcium signaling into the ROS burst
Plant calcium sensors CBL-CIPKs regulate diverse signaling pathways by phosphorylating their target proteins.3-5 Studies of RBOHD from Arabidopsis provided evidence of NADPH oxidase synergistic regulation by phosphorylation and Ca2+ binding to the N-terminal EF-hand motif.6 Recent studies showed that CIPK26 served as a negative regulatory factor of RBOHF in Arabidopsis.7 Co-expression of CIPK26 decreased the ROS-producing activity of RBOHF in HEK293T cells, and colocalization of CIPK26 and RBOHF in the cell periphery was observed. However, how the CIPK26 is translocated to the plasma membrane where it phosphorylates RBOHF is still unknown. Moreover, in another report, CBL1/9 interacted with CIPK26 and strongly enhanced ROS production by RBOHF in HEK293T cells, suggesting a direct interconnection between CBL-CIPK-mediated Ca2+ signaling and ROS production in plants.8 Therefore, it will be most interesting to investigate the detailed mechanisms for the interrelations between Ca2+ and ROS signaling regulated by CBL-CIPK complex in plants (Fig. 1).
Figure 1.
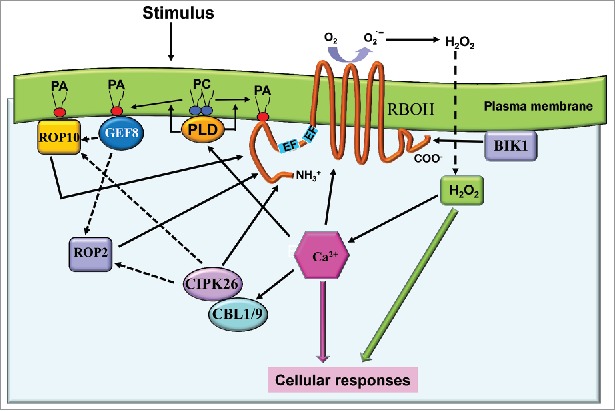
The RBOHs regulation network in cells. PC, phosphatidylcholine; PLD, phospholipase D; PA, phosphatidic acid.
In addition, Solanum tuberosum CDPK4/5 phosphorylated RBOHB at Ser82 and Ser97, and regulated the oxidative burst and plant innate immunity.9 During the plant immunity response, the plasma-membrane-associated kinase BIK1, a component of the FLS2 immune receptor complex, directly interacted with and phosphorylated RBOHD, thereby regulating RBOHD-mediated ROS burst and stomatal defense.10,11
Small GTPase regulation of RBOH activity
In phagocytes, the NADPH oxidase complex consists of gp91phox, p22phox, p47phox, p67phox, p40phox, and the small GTPase Rac2.12 However, only RBOH and Rac (also known as ROP) are found in the Arabidopsis and rice genomes.12 Several lines of evidence indicate Rac confers cellular ROS regulation. First, overexpression of a constitutively active (CA) form of OsRac1 resulted in elevation of ROS production, while overexpression of a dominant-negative form of OsRac1 suppressed ROS reduction.13,14 Secondly, GTPase GDP dissociation inhibitor, a negative regulator of Rho GTPase, inhibited RBOHC-mediated ROS production and lateral hair development.15 Rice Rac1 has been shown to directly interact with the N-terminal region of RBOH, mediating NADPH oxidase activity and ROS production during defense, abiotic stress, and development. The dynamic cytosolic calcium concentration might regulate the RAC-RBOH interaction.16
Studies of Arabidopsis ROP11 and RBOHF revealed that CA-ROP11 specifically interacts with RBOHF, and the amino acids Leu336 and Leu337 in RBOHF were key sites for its interaction with CA-ROP11.17 Mutated RBOHF that did not bind to CA-ROP11 partially reduced ROS production by root hairs in Arabidopsis plants overexpressing CA-ROP11, suggesting that ROP11 modulates ROS production by regulating RbohF activity during root hair development (Fig. 1).17
Lipid-mediated RBOH activity, ROS production, and stomatal movement
Membrane lipids are rich sources for sensing extracellular stimuli and generating intracellular messengers. Phospholipase D (PLD) and its product, phosphatidic acid (PA), are involved in regulating cell development, vesicle transport, cytoskeletal organization, and responses to stresses.18,19 PLDα1-derived PA binds to recombinant Arabidopsis RBOHD and RBOHF. The PA binding motifs in RBOHD were identified as Arg residues 149, 150, 156, and 157, and mutation of the 4 amino acids resulted in the loss of PA binding and the loss of PA-promoted activation of RBOHD.20
In mammals, PLD2-generated PA binds directly to the pleckstrin homology (PH) domain of guanine nucleotide-exchange factor Sos (Son of sevenless), a Ras GEF, and thus recruits Sos to the plasma membrane to catalyze the conversion of Ras-GDP to Ras-GTP.21 This suggests PA functionally regulates GEFs and GTPase activating proteins (GAPs).22 Interestingly, PLD2 had also been found to promote GTP/GDP exchange activity of RhoA that in turn mediates stress fiber formation.23 Further studies showed that PLD2 was a GEF for Rac2 with the PX being the major catalytic domain for its GEF activity.24 However, no plant PLDs with GTP/GDP exchange activity have been reported so far. In Arabidopsis, 14 members of the GEF family have a plant-specific catalytic domain, ROP nucleotide exchanger (PRONE), and they have no homology with RhoGEFs in animals.25 Using fat-western blot and isothermal titration calorimetry assays, PA was found to binding specifically to GEF8, and lysines 13 and 18 in GEF8 were key sites for its binding to PA.26 Moreover, Arabidopsis GEF8 activity toward ROP10 was inhibited by PA, but its activity toward ROP7 was stimulated by PA, suggesting PA influences the relative affinity of GEF for its different substrates (Fig. 1). Moreover, ROP11 has been found to bind to phosphatidylinositol 4,5-bisphosphate (PtdInsa[4,5]P2) and PA (our unpublished), suggesting the plasma membrane lipids might serve as a functional scaffold to regulate the interaction of GEFs and ROPs and to modulate RBOH function.
Conclusion
The Arabidopsis genome has 10 RBOH genes, most of which have been intensively studied, and like mammals, plants ROBHs function in different cellular contexts. RBOHs are localized in the plasma membrane and rapidly generate cellular ROS bursts in response to stimuli.Dynamic cellular changes in ROS level are thought to be essential for plants to grow and survive under stress conditions.27 However, some cross-liners or regulators between the plasma membrane and RBOHs are still limited. For example, the composition of the membrane often changes in response to external stimuli affecting the fluidity of the membrane which in turn may affect RBOH activity or the interaction between RBOH and its effectors. Additional questions yet to be addressed include: (1) whether RBOHs interact directly with plasma membrane proteins, or indirectly through a complex with other membrane components; (2) whether plasma membrane-RBOH association is important for physically generating ROS in response to diverse stimuli; and (3) how specific effectors regulate RBOH activity when plants are exposed to more than one environmental stress. Therefore, in future work, it will be necessary to further clarify RBOHs and effectors, including plasma membrane proteins and lipids, and other interacting proteins, to improve our understanding of intrinsic molecular properties of RBOHs in plants.
Acknowledgments
The work was supported by grants from the Natural Science Foundation of Jiangsu Province (BK20160720) for Y. Qu, and the National Natural Science Foundation of China (31470364) and the Fundamental Research Funds for the Central Universities (KYZ201423) to Q. Zhang.
References
- 1.Sagi M, Fluhr R. Production of reactive oxygen species by plant NADPH oxidases. Plant Physiol. 2006;141:336–40. doi: 10.1104/pp.106.078089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Marino D, Dunand C, Puppo A, Pauly N. A burst of plant NADPH oxidases. Trends Plant Sci. 2012;17:9–15. doi: 10.1016/j.tplants.2011.10.001. [DOI] [PubMed] [Google Scholar]
- 3.Pandey GK. Emergence of a novel calcium signaling pathway in plants: CBL-CIPK signaling network. Physiol Mol Biol Pla. 2008;14:51. doi: 10.1007/s12298-008-0005-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Luan S. The CBL–CIPK network in plant calcium signaling. Trends Plant Sci. 2009;14:37–42. doi: 10.1016/j.tplants.2008.10.005. [DOI] [PubMed] [Google Scholar]
- 5.Edel KH, Kudla J. Integration of calcium and ABA signaling. Curr Opin Plant Biol. 2016;33:83–91. doi: 10.1016/j.pbi.2016.06.010. [DOI] [PubMed] [Google Scholar]
- 6.Ogasawara Y, Kaya H, Hiraoka G, Yumoto F, Kimura S, Kadota Y, Hishinuma H, Senzaki E, Yamagoe S, Nagata K, et al.. Synergistic activation of the Arabidopsis NADPH oxidase AtrbohD by Ca2+ and phosphorylation. J Biol Chem. 2008;283:8885–92. doi: 10.1074/jbc.M708106200. [DOI] [PubMed] [Google Scholar]
- 7.Kimura S, Kawarazaki T, Nibori H, Michikawa M, Imai A, Kaya H, Kuchitsu K. The CBL-interacting protein kinase CIPK26 is a novel interactor of Arabidopsis NADPH oxidase AtRbohF that negatively modulates its ROS-producing activity in a heterologous expression system. J Biochem. 2012;153:191–5. doi: 10.1093/jb/mvs132. [DOI] [PubMed] [Google Scholar]
- 8.Drerup MM, Schlücking K, Hashimoto K, Manishankar P, Steinhorst L, Kuchitsu K, Kudla J. The calcineurin B-like calcium sensors CBL1 and CBL9 together with their interacting protein kinase CIPK26 regulate the Arabidopsis NADPH oxidase RBOHF. Mol Plant. 2013;6:559–69. doi: 10.1093/mp/sst009. [DOI] [PubMed] [Google Scholar]
- 9.Kobayashi M, Ohura I, Kawakita K, Yokota N, Fujiwara M, Shimamoto K, Doke N, Yoshioka H. Calcium-dependent protein kinases regulate the production of reactive oxygen species by potato NADPH oxidase. Plant Cell. 2007;19:1065–80. doi: 10.1105/tpc.106.048884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kadota Y, Sklenar J, Derbyshire P, Stransfeld L, Asai S, Ntoukakis V, Jones JD, Shirasu K, Menke F, Jones A, et al.. Direct regulation of the NADPH oxidase RBOHD by the PRR-associated kinase BIK1 during plant immunity. Mole Cell. 2014;54:43–55. doi: 10.1016/j.molcel.2014.02.021. [DOI] [PubMed] [Google Scholar]
- 11.Li L, Li M, Yu L, Zhou Z, Liang X, Liu Z, Cai G, Gao L, Zhang X, Wang Y, et al.. The FLS2-associated kinase BIK1 directly phosphorylates the NADPH oxidase RbohD to control plant immunity. Cell Host Microbe. 2014;15:329–38. doi: 10.1016/j.chom.2014.02.009. [DOI] [PubMed] [Google Scholar]
- 12.Gu Y, Wang Z, Yang Z. ROP/RAC GTPase: An old new master regulator for plant signaling. Curr Opin Plant Biol. 2004;7:527–36. doi: 10.1016/j.pbi.2004.07.006. [DOI] [PubMed] [Google Scholar]
- 13.Ono E, Wong HL, Kawasaki T, Hasegawa M, Kodama O, Shimamoto K. Essential role of the small GTPase Rac in disease resistance of rice. Proc Natl Acad Sci USA. 2001;98:759–64. doi: 10.1073/pnas.98.2.759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Suharsono U, Fujisawa Y, Kawasaki T, Iwasaki Y, Satoh H, Shimamoto K. The heterotrimeric G protein α subunit acts upstream of the small GTPase Rac in disease resistance of rice. Proc Natl Acad Sci USA. 2002;99:13307–12. doi: 10.1073/pnas.192244099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Carol RJ, Takeda S, Linstead P, Durrant MC, Kakesova H, Derbyshire P, Drea S, Zarsky V, Dolan L. A RhoGDP dissociation inhibitor spatially regulates growth in root hair cells. Nature. 2005;438:1013–6. doi: 10.1038/nature04198. [DOI] [PubMed] [Google Scholar]
- 16.Wong HL, Pinontoan R, Hayashi K, Tabata R, Yaeno T, Hasegawa K, Kojima C, Yoshioka H, Iba K, Kawasaki T, et al.. Regulation of rice NADPH oxidase by binding of Rac GTPase to its N-terminal extension. Plant Cell. 2007;19:4022–34. doi: 10.1105/tpc.107.055624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yan M, Jing W, Xu N, Shen L, Zhang Q, Zhang W. Arabidopsis thaliana constitutively active ROP11 interacts with the NADPH oxidase respiratory burst oxidase homologue F to regulate reactive oxygen species production in root hairs. Func Plant Biol. 2016;43:221–31. doi: 10.1071/FP15090 [DOI] [PubMed] [Google Scholar]
- 18.Wang X, Devaiah SP, Zhang W, Welti R. Signaling functions of phosphatidic acid. Prog Lipid Res. 2006;45:250–78. doi: 10.1016/j.plipres.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 19.Zhang Q, Qu Y, Jing W, Li L, Zhang W. Phospholipase Ds in plant response to hyperosmotic stresses. Phospholipases Plant Signaling. 2014;20:121–34. [Google Scholar]
- 20.Zhang Y, Zhu H, Zhang Q, Li M, Yan M, Wang R, Wang L, Welti R, Zhang W, Wang X. et al.. Phospholipase Dα1 and phosphatidic acid regulate NADPH oxidase activity and production of reactive oxygen species in ABA-mediated stomatal closure in Arabidopsis. Plant Cell. 2009;21:2357–77. doi: 10.1105/tpc.108.062992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhao C, Du G, Skowronek K, Frohman MA, Bar-Sagi D. Phospholipase D2-generated phosphatidic acid couples EGFR stimulation to Ras activation by Sos. Nat Cell Biol. 2007;9:706–12. doi: 10.1016/j.bbalip.2009.05.013. [DOI] [PubMed] [Google Scholar]
- 22.Zhang Y, Du G. Phosphatidic acid signaling regulation of Ras superfamily of small guanosine triphosphatases. Biochim Biophys Acta. 2009;1791:850–5. doi: 10.1016/j.bbalip.2009.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jeon H, Kwak D, Noh J, Lee MN, Lee CS, Suh PG, Ryu SH. Phospholipase D2 induces stress fiber formation through mediating nucleotide exchange for RhoA. Cell Signal. 2011;23:1320–6. doi: 10.1016/j.cellsig.2011.03.014. [DOI] [PubMed] [Google Scholar]
- 24.Mahankali M, Henkels KM, Alter G, Gomez-Cambronero J. Identification of the catalytic site of phospholipase D2 (PLD2) newly described guanine nucleotide exchange factor activity. J Biol Chem. 2012;287:41417–31. doi: 10.1074/jbc.M112.383596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Craddock C, Lavagi I, Yang Z. New insights into Rho signaling from plant ROP/Rac GTPases. Trends Cell Biol. 2012;22:492–501. doi: 10.1016/j.tcb.2012.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cao C, Wang P, Song H, Jing W, Shen L, Zhang Q, Zhang W. Phosphatidic acid binds to and regulates guanine nucleotide exchange factor 8 (GEF8) activity in Arabidopsis. Funct Plant Biol. 2017. doi: 10.1071/FP17113. [DOI] [PubMed] [Google Scholar]
- 27.Babior BM. NADPH oxidase. Curr Opin Immunol. 2004;16:42–7. doi: 10.1016/j.coi.2003.12.001. [DOI] [PubMed] [Google Scholar]


