ABSTRACT
Ethylene is gaseous plant hormone that controls a variety of physiologic activities. OsERS1 and OsETR2 are major ethylene receptors in rice that have been reported to have different regulatory functions. The GFP fused N-terminus of OsERS1 and OsETR2 showed differentially localization patterns when transiently expressed in onion epidermal cells. Base on these results, we suggested that OsERS1 could be localized to plasma membranes, whereas OsETR2 could be localized to the endoplasmic reticulum. Furthermore, instead of the constitutive expression profile of OsERS1, OsETR2 is differentially expressed in seedlings of light/dark-grown conditions, submergence or exogenous ethylene treatments. Our results and others support the notion that OsERS1 and OsETR2 could have different roles during rice plant submergence.
KEYWORDS: 1-MCP, ethylene receptor, rice, submergence, subcellular localization
Ethylene is gaseous plant hormone that controls a variety of physiologic activities. The biosynthesis of ethylene in higher plants via the methionine cycle has been extensively studied.1 The enzyme 1-aminocyclopropane-1-carboxylic acid (ACC) synthase is the key regulatory enzyme to convert S-adenosyl-L-methionine (AdoMet) to ACC in the methionine cycle.2 In tomato plants, ACC synthase is encoded by a multigene family whose members are differentially regulated by different developmental, hormonal, and environmental factors.3 ACC is then oxidized to ethylene by ACC oxidase. Ethylene signaling pathway components were first elucidated in the model plant Arabidopsis thaliana using a molecular genetics approach based on screening for ethylene-insensitive mutants that showed phenotypic changes from the normal “triple response”.4,5 The ETR1 gene was then identified and shown to be an ethylene receptor gene through an ethylene binding assay.6,7 Later, 4 more ethylene receptor genes in A. thaliana were isolated, namely ETR2,8 ERS1,9 EIN4, and ERS2.10 In tomato, a notable finding was the identification of a mutant that lacked fruit ripening called “Never ripe” or “nr,” referring to a mutation of the tomato ethylene receptor gene NR. Eventually, 6 ethylene receptor genes, LeETR1, LeETR2, NR,11 LeETR4,12 LeETR5, and LeETR613 were found in tomato. Other components of the ethylene signaling pathway, including CTR1, containing serine/threonine protein kinase activity, act downstream of ethylene receptors.14 EIN2 acts downstream of CTR1 and upstream of EIN3, and overexpression of EIN2 result in activation of part of the ethylene response.15 EIN3 is a nuclear protein and key transcription factor that mediates ethylene-regulated gene expression and morphological responses. The EIN3 protein level is related to signaling components and an ubiquitin/proteasome pathway mediated by EBF1 and EBF2.16,17 EIN3 consequently induces transcription of ERF1 and other ethylene-responsive genes.18 Many studies and discoveries on ethylene signaling based on these findings have been reported and reviewed by many authors.19,20
Most of the above findings are based on dicotyledonous species (dicots); studies on ethylene signaling in monocotyledonous plants (monocots) are relatively limited. Monocots, including rice, wheat, barley, maize, sorghum and other cereals are major human food sources. Understanding the ethylene signaling pathway and its related physiology may help to improve the quality and quantity of crops. However, variations in physiology and morphology between monocots and dicots limit the direct application of knowledge derived from dicots. The synthesis of ethylene in monocots is via the same ACC pathway as in dicots during different developmental stages.21 Recently, conserved ethylene signaling components were isolated and characterized based on screening of etiolated seedling “double response” mutants in rice.22-25 These studies showed divergent and conserved aspects of ethylene signaling in rice and some of their findings should have great potential for yield improvement in cereal crops. Previously, in this laboratory, we identified 5 ethylene receptor homologs in rice (Oryza sativa L. ssp. indica): OsERS1, OsERS2, OsETR2, OsETR3 and OsETR4. Within this gene family, there are differences in gene and protein structure as well as in expression profiles. OsERS1 shows strong and constitutive expression in different stages and treatments, whereas OsERS2, OsETR3 and OsETR4 only have very low expression levels. Expression of OsETR2 is highly inducible by application of exogenous auxin, ethylene and submergence.26 The differential expression of these ethylene receptor genes suggests that individual genes might have specific roles in physiology, as has been demonstrated in Arabidopsis10 and tomato.27
The subcellular localization of ethylene receptors has been studied in some plant species. Using a biochemical approach, ethylene receptor ETR1 in Arabidopsis was found to be located in the endoplasmic reticulum (ER).28 This finding was supported by the identification of the same localization of its downstream component, CTR1.29 These results suggested that the ethylene signal is initially perceived and transmitted in the endoplasmic reticulum. However, in tobacco, the ethylene receptor NT-HK1 equipped with kinase activity was shown to be located in the plasma membrane,30 which revealed the possibility of non-ER localization of ethylene receptors. Ma et al. found that melon CmERS1 was localized in the ER.31 Based on the similarity of the transmembrane domain sequences in all the ethylene receptors discovered, they proposed a common membrane topology of ethylene receptors and predicted that all ethylene receptors would localize in the ER. This prediction was substantiated by a study on Arabidopsis ethylene receptors,32 in which all 5 of its receptors were shown to be localized to the ER. The ER localization of ethylene receptors and their immediate downstream component CTR1 thereby forms a receptor complex responsible for early ethylene signaling in Arabidopsis.19 However, until now, there has been no report to show the subcellular localization of different members of an ethylene receptor multigene family in the same species. In this study, rice OsERS1 and OsETR2 were chosen as the candidates for subcellular localization identification. The expression profiles of the 2 genes are more active than those of other family members and they show significant variations in protein structure. Two different localization patterns have been observed after transient expression of ethylene receptor–GFP fusion proteins in onion cells, suggesting that they have different subcellular localizations and by implication separate roles in ethylene signal transduction. A recent report also suggested that Arabidopsis ETR1 and ETR2 have contrasting roles in seed germination during salt stress despite the fact that they co-localize to the ER.33
In this study, 2 members (OsERS1 and OsETR2) of the rice ethylene receptor multigene family were selected for a subcellular localization study. These 2 genes are regarded as the most significant members of the gene family based on their expression levels and profiles.26 In addition phenotypic changes have been demonstrated in their mutants, overexpression and RNAi lines.23,24,34 OsERS1 and OsETR2 also possess structural characteristics representative of subfamily I and subfamily II ethylene receptors, respectively. Partial sequences of the N-terminus (for OsERS1, 120 amino acids; for OsETR2, 160 amino acids) were linked with the fluorescence protein GFP sequence to form 2 fusion protein constructs. Full-length receptor–GFP constructs were not demonstrated in this study because the fluorescence signals were too weak and because aggregation occurred. It has been suggested that the N-terminus is the key region for proper protein localization for ethylene receptors.31,32 The transmembrane domain prediction program TMHMM server version 2.0 (http://www.cbs.dtu.dk/services/TMHMM-2.0), predicted that the 3 transmembrane domains are located at the first 120 and 160 amino acids N-terminus of OsERS1 and OsETR2, respectively. Similar studies in tobacco and melon have suggested that the N-terminal transmembrane domain is critical for the localization of ethylene receptors,30,31 and differential results were obtained; tobacco NTHK1 was found to be localized to the plasma membrane, and melon CmERS1 was found to be localized to the endoplasmic reticulum.
The 3 constructs (GFP, ERS1(N-120)–GFP and ETR2(N-160)–GFP) (Fig. 1a) were transiently expressed in onion cells successfully with sufficient fluorescence intensity for observation and the patterns from the 3 constructs were highly distinctive. The control (GFP) construct showed a typical pattern in which GFP signals were localized in most of the cell, including the cytoplasm and nucleus (Fig. 1b). By fusing the peptides from the N-terminus of OsERS1 and OsETR2 upstream of GFP, the fusion proteins were localized at restricted areas only. Most of the ERS1(N-120)–GFP fusion proteins were found surrounding the cells (Fig. 1c). This area is speculated to be the plasma membrane that matches the localization of protoplast-expressed tobacco NTHK1.30 The transformed cells were then treated with hypertonic solution (0.5 M CaCl2) to plasmolyze the cells. Under this condition, the fluorescence signals were separated along with the plasma membrane, suggesting that OsERS1 could be located in the plasma membrane but not in the cell wall (Fig. 1d). A large proportion of the ETR2(N-160)–GFP protein was located in the cytoplasm and exhibited a fluorescence pattern in a network extending from the nucleus (Fig. 1e). This network structure could have been the endoplasmic reticulum or simply the cytoskeleton. Therefore, rhodamine B hexylester, an endoplasmic reticulum staining dye, was applied to identify the actual location of the fusion protein. A large degree of overlap (yellow) between the dye signal (red) and the green fluorescence signal of ETR2(N-160)–GFP was observed, suggesting that OsETR2 could be located in the endoplasmic reticulum (Fig. 1f). In contrast, minimum overlap with ERS1(N-120)–GFP was observed (Fig. 1F) which further showed that the N-terminal part of OsERS1 and OsETR2 localized the C-terminal GFP to different subcellular locations.
Figure 1.

Subcellular localization of OsERS1 and OsETR2. (a) Schematic representation of the gene constructs for transient expression in onion cells. Expression is driven by the CaMV 35S promoter in the GFP control, at the 5′-end of OsERS1 cDNA (360 bp from start codon) and OsETR2 cDNA (480 bp from start codon) to fuse with GFP cDNA at the 3′ end, including the stop codon. (b) Expression of pGFP (control) in onion cells. (c) Expression of pERS1(N-120)–GFP in onion cells. (d) Expression of pERS1(N-120)–GFP in onion cells in hypertonic solution (0.5 M CaCl2). (e) Expression of pETR2(N-160)–GFP in onion cells. (f) Expression of pETR2(N-160)–GFP and pERS1(N-120)–GFP in onion cells with rhodamine B hexylester staining. (R) Filtered image specific for rhodamine B hexylester observation, (G) filtered image for GFP observation and (R+G) image with 2 filtered outputs, (R) and (G). White bars at the lower-right hand corners of each image represent the actual length as indicated.
This study adds one exception (OsERS1 and NTHK1 both localized to the plasma membrane) to other reported ethylene receptors localized to the endoplasmic reticulum and is the first report to show the differential localization of ethylene receptors in the same species. This finding reveals that ethylene perception in plants may take place in different locations within the cells and that the downstream components may also be variable, leading to different physiologic responses. These findings do not present a major contradiction to the present model of the ethylene signaling pathway if the C-terminal of these rice ethylene receptors is exposed to the cytosol and can still interact with their downstream components (CTR1), although the existence of a CTR1-independent ethylene signaling pathway cannot be excluded.35
Previous reports have suggested that submergence-related physiology is mainly initiated by ethylene, as extensively reviewed by Jackson.36 Plant responses to flooding stress are known to occur via the regulation of group VII ethylene response factors (ERFVIIs).37 It has been demonstrated that submergence induces the internal ethylene precursor ACC through the induction of ACC synthase genes.38 In rice, submergence induces the expression of the ACC synthase OsACS539 and in Rumex palustris the ethylene receptor homolog RP-ERS1 is also highly inducible by submergence.40
Among the 5 identified ethylene receptor homologs in rice, we found that OsETR2 is highly inducible by submergence, exogenous auxin and ethylene.26 Although it has been suggested that submergence could alter the OsERS1 transcription level,41 this effect is far less significant than in OsETR2. It has also been reported that for OsERL1 isolated from deep-water rice (identical to OsETR2), mRNA can be induced by submergence, ethylene and gibberellin treatments.42 When growth responses in submergence-tolerant and deep-water rice species were investigated, 2 groups of ERF proteins, encoded by SUBMERGENCE and SNORKEL loci, respectively, were found to be regulators of the submergence responses that help these rice cultivars either withstand prolonged submergence or escape from submergence, mainly by modulations of gibberellic acid synthesis and signaling.43-45 Most submergence-intolerant rice cultivars are still able to thrive in semi-aquatic environments, but the mechanisms to deal with flooding and temporary submergence conditions have not been fully elucidated.
We further examined the induction effect of submergence upon OsETR2 expression. The discovery of this specific property was accidental: when we were switching the auxin treatment method from liquid incubation to air-spray, we noticed that the transcription level in the water-incubated control was much higher than in the control in air.26 The expression levels of OsETR2 in different conditions are shown by Northern blot analysis in Fig. 2(a). In the controls (etiolated and light-grown seedlings) the expression was generally low. The mRNA transcripts of OsETR2 were highly induced by complete submergence in water for both light-grown and etiolated seedlings, although the level of induction in the light-grown seedlings was generally higher than in the etiolated seedlings. OsETR2 expression was also highly inducible by application of exogenous ethylene (5 ppm). The induction could be blocked by pretreatment of the ethylene antagonist 1-MCP (5 ppm), suggesting the induction was dependent on the ethylene perception. Similarly, OsETR2 transcripts were massively induced upon submergence but the induction could be completely inhibited in the light-grown seedlings that were pretreated with 1-MCP before submergence (Fig. 2(b)).
Figure 2.
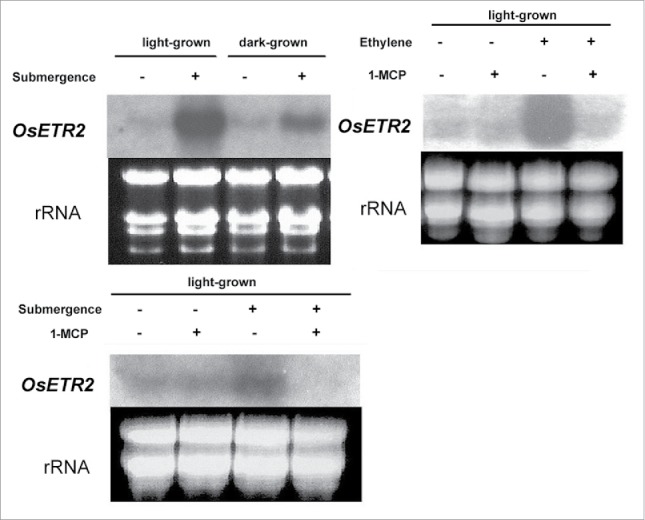
Expression of the OsETR2 gene in rice seedlings upon submergence, exogenous ethylene and 1-MCP treatments. Light-grown or dark-grown rice seedlings were completely submerged in water for 4 hours (Submergence), treated with exogenous ethylene (5 ppm) for 6 hours (Ethylene) or/and pre-treated with 1-MCP for overnight (5 ppm)(1-MCP). The equivalent loading of RNA is shown by the ethidium bromide stained rRNA. The expression of OsETR2 was detected by Northern blot using a specific 32P-labeled DNA probe.
The mRNA of receptor gene member OsERL1 (OsETR2, this study) isolated from deep-water rice by Watanabe et al.,42 was found to be induced by ethylene, gibberellic acid, and submergence. Clearly, OsETR2 mRNA induced by submergence is not specific to deep-water rice but is common to all rice cultivars. Vriezen et al.40 first reported that the ethylene receptor homolog RP-ERS1 was highly inducible in Rumex species by flooding, a high concentration of ethylene and carbon dioxide, and a low concentration of oxygen. This is very similar to OsETR2 in rice. Reports have shown that under submergence, plants will synthesize more ACC through the induction of ACC synthase; examples include LeACS7 in tomato38 and OsACS5 in rice.39 This raises the possibility that upon submergence, ethylene produced via the ACC pathway or trapped ethylene would increase to trigger the corresponding ethylene receptor gene expression. Here, we used the potent ethylene receptor antagonist 1-MCP to confirm that submergence-related OsETR2 induction is strictly ethylene-signaling–dependent. 1-MCP pretreatment at 5 ppm level is known to effectively block ethylene action and stabilize the receptor's repressive activity,9 and the effect can last for a few days in tissues that produce ethylene at high rates.46
Theoretically, if the induction of OsETR2 is through ethylene, blockage of ethylene perception by 1-MCP will stop the subsequent pathway and eventually inhibit the effect of submergence-related OsETR2 expression. The Northern blot shows that induction by submergence was completely inhibited in the samples that were pretreated with 1-MCP (Fig. 2(b), lane 4). However, we do not know why 1-MCP pretreated seedlings and 1-MCP pretreated green seedlings with subsequent ethylene treatment could still maintain a basal level of OsETR2 transcription. It is possible that when seedlings are submerged, the OsETR2 transcript turnover rate will be increased as new receptors are being made and transcripts are consumed. Our preliminary data showed that 1-MCP treated or submerged samples can significantly increase average ACC content (data not shown). This suggests that ACC/ethylene biosynthesis under submergence is not totally ethylene-signaling–dependent (or autocatalytic). OsACS5 induced upon submergence is also an ERF-dependent ACC synthase gene whose expression can be blocked by 1-MCP. Nonetheless, some other non–ethylene-signaling–dependent ACC synthase(s) such as OsACS1 can still function to produce ACC.47 We can also predict that ethylene receptors under the influence of 1-MCP exhibit a positive effect on ethylene biosynthesis. Thus, when the autocatalytic ethylene was blocked by 1-MCP, the ACC content increased instead of decreased. A recent report also showed that at a certain stage of maturation, 1-MCP will increase instead of decrease ethylene biosynthesis in apples and lead to fruit ripening.48 We believe that OsETR2 is one of the important components involved in the regulation of rice submergence responses by dynamically altering its cellular level, whereas OsERS1 should have differential roles in these responses.
Based on our present understanding, we propose a scheme of signaling related to OsERS1 and OsETRS2 when rice plants are submerged (Fig. 3). OsERS1 belongs to the subfamily I receptors and is constitutively expressed in most rice tissues in all stages of development. Its knockout mutant shows short roots in dark-grown seedlings and hypersensitivity to ethylene, and it is logical to assign root inhibition and shoot promotion responses to OsERS1 during submergence. OsETR2 belongs to subfamily II receptors, which express relatively low levels in normal conditions but increase dramatically when submerged. Its knockout mutants do not show phenotypic changes in the vegetative stage and its overexpression and RNAi lines show hyposensitivity and hypersensitivity to ethylene, respectively.23,24,34 We therefore assign a negative role to OsETR2 during submergence. The negative effect of OsETR2 is likely to act on an upstream component in the signaling pathway associated with OsERS122,25 as indicated. Attenuation of the signal initiated by sudden and temporary increase in ethylene trapped inside rice plant tissues when they are submerged is related to the species' adaptation to a semiaquatic environment, because overreaction to ethylene would lead to chlorosis, senescence, and cell death.47 The alternative view is that the induction of OsETR2 to desensitize its own signaling pathway when submerged is unlikely and inefficient because OsETR2 does not seem to directly control root and shoot growth. Unlike in other monocot seedlings, exogenous ethylene promotes instead of inhibits coleoptile elongation in etiolated rice seedlings. This specific “double response” in wild-type rice is thought to be related to its adaptation to paddy field submergence conditions during early stages of development. This escape mechanism is lost when etiolated rice seedlings develop into green stages. In most wild-type rice cultivars, which are not equipped with the submergence tolerance regulator SUBA1 or SNORKEL1 and SNORKEL2 in their genotypes,44,45,49 the rapid increase in OsETR2 expression may become a more important protective and adaptive measure when they encounter flooding and submergence. This is consistent with our observation that more OsETR2 mRNA was induced in light-grown seedlings than in dark-grown seedlings when submerged (Fig. 2a). It has been reported that overexpression of OsETR2 in rice could lead to accumulation of starch in stem internodes,34 which may also help rice to survive after submergence. However, how OsETR2 interacts with the downstream components and the actual regulatory mechanism during submergence remain to be investigated.
Figure 3.

Differential roles of OsERS1 and OsETR2 ethylene receptors when submerged. Submergence increases ethylene biosynthesis and triggers submergence responses such as inhibition of root growth and promotion of shoot elongation. These responses are speculated to occur via the signaling pathway associated with OsERS1 at the plasma-membrane (PM); whereas the rapid increase in OsETR2 expression at the endoplasmic reticulum (ER) is thought to act negatively in the early signaling pathway associated with OsERS1.
Supplementary Material
Acknowledgments
We thank Rohm and Hass, Japan for providing 1-MCP for the experiments.
Competing Interests
The authors declare that no competing interests exist.
References
- 1.Yang SF, Hoffman NE. Ethylene biosynthesis and its regulation in higher-plants. Annu Rev Plant Phys. 1984;35:155–189. doi: 10.1146/annurev.pp.35.060184.001103. [DOI] [Google Scholar]
- 2.Yu YB, Adams DO, Yang SF. 1-Aminocyclopropanecarboxylate synthase, a key enzyme in ethylene biosynthesis. Arch Biochem Biophys. 1979;198:280–286. doi: 10.1016/0003-9861(79)90420-X. [DOI] [PubMed] [Google Scholar]
- 3.Yip WK, Moore T, Yang SF. Differential accumulation of transcripts for four tomato 1-aminocyclopropane-1-carboxylate synthase homologs under various conditions. Proc Natl Acad Sci U S A. 1992;89:2475–2479. doi: 10.1073/pnas.89.6.2475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bleecker AB, Estelle MA, Somerville C, Kende H. Insensitivity to ethylene conferred by a dominant mutation in Arabidopsis thaliana. Science. 1988;241:1086–1089. doi: 10.1126/science.241.4869.1086. [DOI] [PubMed] [Google Scholar]
- 5.Guzman P, Ecker JR. Exploiting the triple response of Arabidopsis to identify ethylene-related mutants. Plant Cell. 1990;2:513–523. doi: 10.1105/tpc.2.6.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chang C, Kwok SF, Bleecker AB, Meyerowitz EM. Arabidopsis ethylene-response gene ETR1: similarity of product to two-component regulators. Science. 1993;262:539–544. doi: 10.1126/science.8211181. [DOI] [PubMed] [Google Scholar]
- 7.Schaller GE, Bleecker AB. Ethylene-binding sites generated in yeast expressing the Arabidopsis ETR1 gene. Science. 1995;270:1809–1811. doi: 10.1126/science.270.5243.1809. [DOI] [PubMed] [Google Scholar]
- 8.Sakai H, Hua J, Chen QG, Chang C, Medrano LJ, Bleecker AB, Meyerowitz EM. ETR2 is an ETR1-like gene involved in ethylene signaling in Arabidopsis. Proc Natl Acad Sci U S A. 1998;95:5812–5817. doi: 10.1073/pnas.95.10.5812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hall AE, Findell JL, Schaller GE, Sisler EC, Bleecker AB. Ethylene perception by the ERS1 protein in Arabidopsis. Plant Physiol. 2000;123:1449–1458. doi: 10.1104/pp.123.4.1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hua J, Sakai H, Nourizadeh S, Chen QG, Bleecker AB, Ecker JR, Meyerowitz EM. EIN4 and ERS2 are members of the putative ethylene receptor gene family in Arabidopsis. Plant Cell. 1998;10:1321–1332. doi: 10.1105/tpc.10.8.1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lashbrook CC, Gonzalez-Bosch C, Bennett AB. Two divergent endo-beta-1,4 glucanase genes exhibit overlapping expression in ripening fruit and abscising flowers. Plant Cell. 1994;6:1485–1493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tieman DM, Taylor MG, Ciardi JA, Klee HJ. The tomato ethylene receptors NR and LeETR4 are negative regulators of ethylene response and exhibit functional compensation within a multigene family. Proc Natl Acad Sci U S A. 2000;97:5663–5668. doi 10.1073/pnas.090550597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Klee HJ. Control of ethylene-mediated processes in tomato at the level of receptors. J Exp Bot. 2002;53:2057–2063. doi: 10.1093/jxb/erf062. [DOI] [PubMed] [Google Scholar]
- 14.Kieber JJ. The ethylene signal transduction pathway in Arabidopsis. J Exp Bot. 1997;48:211–218. doi: 10.1093/jxb/48.2.211. [DOI] [PubMed] [Google Scholar]
- 15.Wang KL, Li H, Ecker JR. Ethylene biosynthesis and signaling networks. Plant Cell. 2002;14:S131–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guo H, Ecker JR. Plant responses to ethylene gas are mediated by SCF(EBF1/EBF2)-dependent proteolysis of EIN3 transcription factor. Cell. 2003;115:667–677. doi: 10.1016/S0092-8674(03)00969-3. [DOI] [PubMed] [Google Scholar]
- 17.Potuschak T, Lechner E, Parmentier Y, Yanagisawa S, Grava S, Koncz C, Genschik P. EIN3-dependent regulation of plant ethylene hormone signaling by two arabidopsis F box proteins: EBF1 and EBF2. Cell. 2003;115:679–689. doi: 10.1016/S0092-8674(03)00968-1. [DOI] [PubMed] [Google Scholar]
- 18.Solano R, Stepanova A, Chao Q, Ecker JR. Nuclear events in ethylene signaling: a transcriptional cascade mediated by ETHYLENE-INSENSITIVE3 and ETHYLENE-RESPONSE-FACTOR1. Genes Dev. 1998;12:3703–3714. doi: 10.1101/gad.12.23.3703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ju C, Chang C. Advances in ethylene signalling: protein complexes at the endoplasmic reticulum membrane. AoB Plants. 2012;10:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shakeel SN, Wang X, Binder BM, Schaller GE. Mechanisms of signal transduction by ethylene: overlapping and non-overlapping signalling roles in a receptor family. AoB Plants. 2013;5:28. doi: 10.1093/aobpla/plt010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jiao XZ, Yip WK, Yang SF. The effect of light and phytochrome on 1-aminocyclopropane-1-carboxylic acid metabolism in etiolated wheat seedling leaves. Plant Physiol. 1987;85:643–647. doi: 10.1104/pp.85.3.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ma B, He SJ, Duan KX, Yin CC, Chen H, Yang C, Xiong Q, Song QX, Lu X, Chen HW, et al.. Identification of rice ethylene-response mutants and characterization of MHZ7/OsEIN2 in distinct ethylene response and yield trait regulation. Mol Plant. 2013;6:1830–1848. doi: 10.1093/mp/sst087. [DOI] [PubMed] [Google Scholar]
- 23.Ma B, Yin CC, He SJ, Lu X, Zhang WK, Lu TG, Chen SY, Zhang JS. Ethylene-induced inhibition of root growth requires abscisic acid function in rice (Oryza sativa L.) seedlings. PLoS Genet. 2014;10:e1004701. doi: 10.1371/journal.pgen.1004701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yang C, Lu X, Ma B, Chen SY, Zhang JS. Ethylene signaling in rice and Arabidopsis: conserved and diverged aspects. Mol Plant. 2015;8:495–505.. doi: 10.1016/j.molp.2015.01.003. [DOI] [PubMed] [Google Scholar]
- 25.Yang C, Ma B, He SJ, Xiong Q, Duan KX, Yin CC, Chen H, Lu X, Chen SY, Zhang JS. MAOHUZI6/ETHYLENE INSENSITIVE3-LIKE1 and ETHYLENE INSENSITIVE3-LIKE2 regulate ethylene response of roots and Coleoptiles and negatively affect salt tolerance in rice. Plant Physiol. 2015;169:148–165. doi: 10.1104/pp.15.00353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yau CP, Wang L, Yu M, Zee SY, Yip WK. Differential expression of three genes encoding an ethylene receptor in rice during development, and in response to indole-3-acetic acid and silver ions. J Exp Bot. 2004;55:547–556. doi: 10.1093/jxb/erh055. [DOI] [PubMed] [Google Scholar]
- 27.Lashbrook CC, Tieman DM, Klee HJ. Differential regulation of the tomato ETR gene family throughout plant development. Plant J. 1998;15:243–252. doi: 10.1046/j.1365-313X.1998.00202.x. [DOI] [PubMed] [Google Scholar]
- 28.Chen YF, Randlett MD, Findell JL, Schaller GE. Localization of the ethylene receptor ETR1 to the endoplasmic reticulum of Arabidopsis. J Biol Chem. 2002;277:19861–19866. doi: 10.1074/jbc.M201286200. [DOI] [PubMed] [Google Scholar]
- 29.Gao Z, Chen YF, Randlett MD, Zhao XC, Findell JL, Kieber JJ, Schaller GE. Localization of the Raf-like kinase CTR1 to the endoplasmic reticulum of Arabidopsis through participation in ethylene receptor signaling complexes. J Biol Chem. 2003;278:34725–34732. doi: 10.1074/jbc.M305548200. [DOI] [PubMed] [Google Scholar]
- 30.Xie C, Zhang JS, Zhou HL, Li J, Zhang ZG, Wang DW, Chen SY. Serine/threonine kinase activity in the putative histidine kinase-like ethylene receptor NTHK1 from tobacco. Plant J. 2003;33:385–393. doi: 10.1046/j.1365-313X.2003.01631.x. [DOI] [PubMed] [Google Scholar]
- 31.Ma B, Cui ML, Sun HJ, Takada K, Mori H, Kamada H, Ezura H. Subcellular localization and membrane topology of the melon ethylene receptor CmERS1. Plant Physiol. 2006;141:587–597. doi: 10.1104/pp.106.080523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Grefen C, Stadele K, Ruzicka K, Obrdlik P, Harter K, Horak J. Subcellular localization and in vivo interactions of the Arabidopsis thaliana ethylene receptor family members. Mol Plant. 2008;1:308–320. doi: 10.1093/mp/ssm015. [DOI] [PubMed] [Google Scholar]
- 33.Wilson RL, Kim H, Bakshi A, Binder BM. The Ethylene Receptors ETHYLENE RESPONSE1 and ETHYLENE RESPONSE2 have contrasting roles in seed germination of arabidopsis during salt stress. Plant Physiol. 2014;165:1353–1366. doi: 10.1104/pp.114.241695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wuriyanghan H, Zhang B, Cao WH, Ma B, Lei G, Liu YF, Wei W, Wu HJ, Chen LJ, Chen HW, et al.. The ethylene receptor ETR2 delays floral transition and affects starch accumulation in rice. Plant Cell. 2009;21:1473–1494. https://doi.org/ 10.1105/tpc.108.065391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shakeel SN, Gao Z, Amir M, Chen YF, Rai MI, Haq NU, Schaller GE. Ethylene regulates levels of ethylene receptor/CTR1 signaling complexes in arabidopsis thaliana. J Biol Chem. 2015;290:12415–12424. doi: 10.1074/jbc.M115.652503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jackson MB. Ethylene-promoted elongation: an adaptation to submergence stress. Ann Bot. 2008;101:229–48. doi: 10.1093/aob/mcm237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Loreti E, van Veen H, Perata P. Plant responses to flooding stress. Curr Opin Plant Biol. 2016;33:64–71. doi: 10.1016/j.pbi.2016.06.005. [DOI] [PubMed] [Google Scholar]
- 38.Grichko VP, Glick BR. Ethylene and flooding stress in plants. Plant Physiol Biochem. 2001;39:1–9. doi: 10.1016/S0981-9428(00)01213-4. [DOI] [Google Scholar]
- 39.Van Der Straeten D, Zhou Z, Prinsen E, Van Onckelen HA, Van Montagu MC. A comparative molecular-physiological study of submergence response in lowland and deepwater rice. Plant Physiol. 2001;125:955–968. doi: 10.1104/pp.125.2.955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vriezen WH, van Rijn CP, Voesenek LA, Mariani C. A homolog of the Arabidopsis thaliana ERS gene is actively regulated in Rumex palustris upon flooding. Plant J. 1997;11:1265–1271. doi: 10.1046/j.1365-313X.1997.11061265.x. [DOI] [PubMed] [Google Scholar]
- 41.Zhou Z, Vriezen W, Van Caeneghem W, Van Montagu M, Van Der Straeten D. Rapid induction of a novel ACC synthase gene in deepwater rice seedlings upon complete submergence. Euphytica. 2001;121:137–143. doi: 10.1023/A:1012059425624. [DOI] [Google Scholar]
- 42.Watanabe H, Saigusa M, Hase S, Hayakawa T, Satoh S. Cloning of a cDNA encoding an ETR2-like protein (Os-ERL1) from deep water rice (Oryza sativa L.) and increase in its mRNA level by submergence, ethylene, and gibberellin treatments. J Exp Bot. 2004;55:1145–1148. doi: 10.1093/jxb/erh110. [DOI] [PubMed] [Google Scholar]
- 43.Xu K, Xu X, Fukao T, Canlas P, Maghirang-Rodriguez R, Heuer S, Ismail AM, Bailey-Serres J, Ronald PC, Mackill DJ. Sub1A is an ethylene-response-factor-like gene that confers submergence tolerance to rice. Nature. 2006;442:705–708. doi: 10.1038/nature04920. [DOI] [PubMed] [Google Scholar]
- 44.Fukao T, Bailey-Serres J. Submergence tolerance conferred by Sub1A is mediated by SLR1 and SLRL1 restriction of gibberellin responses in rice. Proc Natl Acad Sci U S A. 2008;105:16814–16819. doi: 10.1073/pnas.0807821105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hattori Y, Nagai K, Furukawa S, Song XJ, Kawano R, Sakakibara H, Wu J, Matsumoto T, Yoshimura A, Kitano H, et al.. The ethylene response factors SNORKEL1 and SNORKEL2 allow rice to adapt to deep water. Nature. 2009;460:1026–1030. doi: 10.1038/nature08258. [DOI] [PubMed] [Google Scholar]
- 46.Sisler EC, Serek M. Compounds interacting with the ethylene receptor in plants. Plant Biol. 2003;5:473–480. doi: 10.1055/s-2003-44782. [DOI] [Google Scholar]
- 47.Rzewuski G, Sauter M. Ethylene biosynthesis and signaling in rice. Plant Sci. 2008;175:32–42. doi: 10.1016/j.plantsci.2008.01.012. [DOI] [Google Scholar]
- 48.Tadiello A, Longhi S, Moretto M, Ferrarini A, Tononi P, Farneti B, Busatto N, Vrhovsek U, Molin AD, Avanzato C, et al.. Interference with ethylene perception at receptor level sheds light on auxin and transcriptional circuits associated with the climacteric ripening of apple fruit (Malus x domestica Borkh.). Plant J. 2016;88:963–975. doi: 10.1111/tpj.13306. [DOI] [PubMed] [Google Scholar]
- 49.Fukao T, Xu K, Ronald PC, Bailey-Serres J. A variable cluster of ethylene response factor-like genes regulates metabolic and developmental acclimation responses to submergence in rice. Plant Cell. 2006;18:2021–2034. doi: 10.1105/tpc.106.043000. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


