Abstract
Study objective
Randomized trials suggest that nonoperative treatment of uncomplicated appendicitis with antibiotics-first is safe. No trial has evaluated outpatient treatment and no US randomized trial has been conducted, to our knowledge. This pilot study assessed feasibility of a multicenter US study comparing antibiotics-first, including outpatient management, with appendectomy.
Methods
Patients aged 5 years or older with uncomplicated appendicitis at 1 US hospital were randomized to appendectomy or intravenous ertapenem greater than or equal to 48 hours and oral cefdinir and metronidazole. Stable antibiotics-first-treated participants older than 13 years could be discharged after greater than or equal to 6-hour emergency department (ED) observation with next-day follow-up. Outcomes included 1-month major complication rate (primary) and hospital duration, pain, disability, quality of life, and hospital charges, and antibiotics-first appendectomy rate.
Results
Of 48 eligible patients, 30 (62.5%) consented, of whom 16 (53.3%) were randomized to antibiotics-first and 14 (46.7%) to appendectomy. Median age was 33 years (range 9 to 73 years), median WBC count was 15,000/μL (range 6,200 to 23,100/μL), and median computed tomography appendiceal diameter was 10 mm (range 7 to 18 mm). Of 15 antibiotic-treated adults, 14 (93.3%) were discharged from the ED and all had symptom resolution. At 1 month, major complications occurred in 2 appendectomy participants (14.3%; 95% confidence interval [CI] 1.8% to 42.8%) and 1 antibiotics-first participant (6.3%; 95% CI 0.2% to 30.2%). Antibiotics-first participants had less total hospital time than appendectomy participants, 16.2 versus 42.1 hours, respectively. Antibiotics-first-treated participants had less pain and disability. During median 12-month follow-up, 2 of 15 antibiotics-first-treated participants (13.3%; 95% CI 3.7% to 37.9%) developed appendicitis and 1 was treated successfully with antibiotics; 1 had appendectomy. No more major complications occurred in either group.
Conclusion
A multicenter US trial comparing antibiotics-first to appendectomy, including outpatient management, is feasible to evaluate efficacy and safety.
Introduction
Background
Standard management of acute uncomplicated appendicitis has been appendectomy, but 7 randomized controlled trials,1-7 nonrandomized pediatric trials,8-12 and case series13-16 suggest that an antibiotics-first approach is safe, with no increased risk of subsequent perforation and sepsis, and no reported deaths. In the largest randomized trial, the 1-year appendectomy rate among 257 adults with imaging-confirmed acute uncomplicated appendicitis treated with antibiotics-first was 27%.6 A meta-analysis found that antibiotic treatment was associated with fewer complications and less pain and disability than appendectomy.17
Patients undergoing appendectomy are typically hospitalized and discharged when they can tolerate fluids and achieve pain control. In the US, the trend is toward shorter hospital stays and, for appendicitis, length of postoperative hospitalization has decreased because more appendectomies are performed laparoscopically.18 All previous trials have required patients randomized to antibiotics to be hospitalized, and most appendectomies in these trials were performed by open technique. Primary antibiotic therapy may offer the opportunity to avoid hospitalization with discharge from the emergency department (ED) after treatment initiation, observation, and symptom control. This is the current management for acute uncomplicated diverticulitis,19 but it has yet to be studied in patients with uncomplicated appendicitis.
Importance
Approximately 300,000 people undergo appendectomy, resulting in nearly $2 billion in health care costs annually in the US.20 After appendectomy, patients miss a median of 10 to 14 days from work and resume normal activity in 7 to 21 days.21 Despite the results of European randomized controlled trials supporting the efficacy of antibiotics-first management, the actual application and effectiveness of nonoperative treatment have been questioned because of evidence gaps,22 and this approach has been infrequently used in the US according to the National Inpatient Sample database.23 To our knowledge, no US randomized trial comparing an antibiotics-first approach to urgent appendectomy among patients with uncomplicated appendicitis has been reported.
Goals of This Investigation
We present the methodology and results of a pilot randomized trial comparing antibiotics-first to appendectomy among patients receiving a diagnosis of uncomplicated appendicitis designed to determine the feasibility of conducting a large multicenter US trial. To adapt an antibiotics-first treatment strategy to US treatment patterns, we evaluated a protocol allowing outpatient antibiotic management. As opposed to previous trials that focused on the possible futility of antibiotic management and had a primary outcome of appendectomy rate in the antibiotics-first group,1-3,6,7 we evaluated a primary outcome that was independent of treatment strategy, the 1-month major complication rate. We evaluated patient-centered outcomes secondarily. Additionally, we report the frequency with which antibiotic-treated participants reached clinical stability in the ED, allowing successful outpatient management, and outcomes after 1-year follow-up.
Materials and Methods
Study Design and Setting
We conducted a pilot randomized trial comparing antibiotics-first to urgent appendectomy among patients with acute uncomplicated appendicitis. We enrolled patients from March to September 2015 at Olive View–UCLA Medical Center, a County of Los Angeles UCLA-affiliated hospital. The Olive View–UCLA institutional review board approved the study and protocol amendments. (All members of the Olive View–UCLA Appendicitis Study Group are listed in Appendix E1, available online at http://www.annemergmed.com.)
Selection of Participants
We included ED patients who had the presumptive diagnosis of acute appendicitis by the ED attending physician and who met the following criteria: (1) age 5 years or older; (2) radiographic diagnosis of uncomplicated appendicitis by computed tomography (CT) and/or ultrasonography as read by an attending radiologist and performed within 24 hours of consent; (3) clinical diagnosis of uncomplicated appendicitis by a surgical teaching service supervised by an attending surgeon; and (4) ability to provide written informed consent in English or Spanish (for participants ages 5 to 17 years, consent from parent/guardian and assent, when appropriate). Study coordinators screened ED patients between 7 AM and 9 PM, including those who presented overnight and were in the ED at 7 AM.
We excluded patients with the following: (1) inability to return or be contacted for follow-up visits; (2) evidence of severe sepsis or septic shock; (3) high-risk diabetes (eg, insulin dependent, diabetic ketoacidosis, hyperosmolar coma); (4) immunodeficiency (eg, absolute neutrophil count <500/μL, immunosuppressive drugs, chemotherapy, known AIDS [CD4 count <200/μL or AIDS-defining illness within the last year]) by patient history; (5) suspicion of acute coronary syndrome, congestive heart failure, or active chronic liver disease; (6) chronic renal insufficiency (serum creatinine level >2 mg/dL); (7) hepatic cirrhosis or failure; (8) acute inflammatory bowel disease or malignancy; (9) pregnant, nursing, or expectation of becoming pregnant within 10 days; (10) concurrent illness that would mandate hospitalization; (11) imaging findings suggesting a mass or mucocele; (12) severe allergy or reaction to study drugs or drugs similar to them; (13) receiving warfarin; (14) another infection requiring antibiotic treatment; (15) incarceration or police custody; (16) abdominal or pelvic surgery within the last month; (17) current long-term-care resident; (18) expected use of an investigational treatment; (19) intravenous drug use in the preceding month; (20) expected concurrent hemodialysis, peritoneal dialysis or indwelling peritoneal catheters or shunts, plasmapheresis, or hemoperfusion; (21) received parenteral antibiotics greater than or equal to 6 and less than or equal to 48 hours before screening; (22) received ertapenem within 24 hours before screening; or (23) previous study enrollment. Radiographic identification of appendicolith was initially an exclusion criterion but was later allowed (after 11 of 30 participants were enrolled) because of lack of consistent evidence for this being an antibiotic failure risk factor.
Methods of Measurement
On participant enrollment, we recorded demographics, history, pain severity, and physical examination findings. We obtained complete blood count, comprehensive metabolic panel, venous lactate level, and C-reactive protein tests. We assessed quality of life before appendicitis symptoms among adults by using the SF-12v2 Health Survey (acute version).24 All patients with suspected appendicitis had abdominal imaging, CT with intravenous contrast in adults and ultrasonography in persons younger than 18 years, followed by CT if indicated. An attending radiologist initially reviewed imaging studies during ED evaluation. The radiographic diagnosis of uncomplicated appendicitis was pragmatic and based on the radiologist's global impression, as is done in routine practice. Subsequently, findings were recorded with standardized methods (radiology methods [Appendix E2, available online at http://www.annemergmed.com]). Treatment of pain was at the discretion of the treating clinician for participants in both groups.
We conducted follow-up assessments on day 2 in person, days 3 to 5 by telephone (in person if the participant was hospitalized), and at 2 weeks (days 10 to 18) and 1 month (days 25 to 35) in person. We report results through approximately 1 year; however, participants will be followed for 2 years. All participants had a physical examination on day 2 and days 3 to 5 (the examination was conducted if the participant was hospitalized), at 2 weeks, and at 1 month. Antibiotics-first participants had complete blood count, comprehensive metabolic panel, serum lactate level, and C-reactive protein evaluated on day 2. We provided participants 24-hour, 7-day-a-week telephone access to a study coordinator and ED attending physician if they had questions or required reevaluation.
Interventions
After obtaining written informed consent, a study coordinator opened an opaque envelope with the preassigned treatment, either appendectomy with perioperative antibiotics or antibiotics alone (with subsequent appendectomy, if necessary). An author (W.R.M.) generated the randomization sequence. Using an exact 1:1 treatment distribution, we initially created 50 random assignments. We then used the first 30 assignments to create a nearly equal 1:1 distribution to both treatment strategies, thus making it impossible for clinicians to predict treatment for the last enrolled participants according to anticipation of equal treatment group distribution.
We administered a once-a-day intravenous antibiotic, ertapenem 1 g, to participants older than 13 years. After patients underwent ED triage and a minimum 6 hours of observation, the treating clinician had the option to discharge a participant older than 13 years if the following criteria were met: (1) systolic blood pressure greater than 90 mm Hg, pulse rate less than 100 beats/min, and temperature less than 38.5°C (101.3°F); (2) pain controlled with oral analgesics according to physician judgment; (3) participant tolerated oral fluids and medication; (4) participant able to return for further evaluation; (5) treating physician comfortable with participant going home; and (6) participant comfortable going home. We required all discharged participants to return to the ED for reevaluation the following day. If on reevaluation the participant demonstrated no worsening and his or her pain was controlled, then a second ertapenem dose was administered. The participant was then discharged with an 8-day supply of an oral antibiotic regimen of cefdinir (off-label) and metronidazole in prelabeled blister packs. The dose of cefdinir was as follows: for adults and children older than 13 years, 300-mg capsules twice daily; and for children aged 5 to 12 years, 7 mg/kg twice daily, maximum 300 mg/ dose. The dose of metronidazole was as follows: for adults and children older than 13 years, 500-mg tablets 3 times daily; and for children aged 5 to 12 years, 10 mg/kg 3 times daily, maximum 500 mg/dose. We dispensed oral antibiotics in a blister pack labeled with the specific date and time of dosing. Hospitalized participants in the antibiotics-first group who were older than 13 years received ertapenem 1 g intravenously every 24 hours, for a minimum of 2 doses, and children younger than 13 years received ertapenem intravenously every 12 hours (15 mg/kg per dose, maximum 1 g/day), for a minimum of 4 doses. We hospitalized participants younger than 13 years because ertapenem dosing for this age group is every 12 hours. The treating clinician could discharge a hospitalized participant older than 13 years and treated after 2 ertapenem doses with oral antibiotics by using the same criteria described above.
Criteria for transitioning an antibiotics-first participant to appendectomy were assessed by the surgeon site investigator (D.J.S.), in consultation with the participant's attending surgeon, and included (1) diffuse abdominal tenderness consistent with peritonitis; (2) severe sepsis or septic shock; or (3) no improvement in abdominal pain, temperature greater than or equal to 38.5°C (101.3°F), or WBC count less than 4,000 or greater than 15,000/μL after 48 hours of antibiotic treatment; and (4) participant consent.
We hospitalized all participants randomized to appendectomy. Urgent appendectomy was performed by an open or laparoscopic approach according to the surgeon's preference. Participants received 1 dose of ertapenem intravenously; additional preoperative antibiotic treatment was at the discretion of the treating clinicians. Treating clinicians discharged appendectomy participants by using the same criteria as above.
Outcome Measures
The primary outcome was the one-month major complication rate in a participant based on American College of Surgeons National Surgical Quality Improvement Program (ACS NSQIP) criteria25,26 modified to include major antibiotic-related complications. Major complications were defined as (1) organ/space infection, including peritonitis (not including uncomplicated appendicitis or complicated appendicitis found only at surgery); (2) wound dehiscence; (3) pneumonia; (4) unplanned intubation; (5) pulmonary embolism; (6) mechanical ventilation for more than 48 hours; (7) progressive renal insufficiency; (8) major urinary tract infection (eg, pyelonephritis); (9) malignant hyperthermia; (10) stroke/cerebral vascular accident; (11) coma for more than 24 hours; (12) cardiac arrest; (13) myocardial infarction; (14) bleeding requiring transfusion; (15) severe sepsis and septic shock; (16) deep venous thrombosis; (17) unexpected re-operation related to appendicitis; (18) dehydration requiring hospitalization; (19) unplanned hospitalization related to a complication of appendicitis or its treatment after initial hospitalization (not including suspicion of appendicitis or appendectomy in the antibiotics-first group); and (20) antibiotic-related adverse event, including colitis, requiring hospitalization.
Secondary outcomes included (1) appendectomy rate in the antibiotics-first group; (2) quality of life, measured by the SF-12v2 Health Survey24 (baseline) and the PedsQL Survey,27 both at 2 weeks and 1 month; (3) days unable to perform normal activities and work or school; (4) days of analgesic use; (5) pain scores at and 24 hours before each visit; (6) total hours in the ED and hospital at the initial visit from triage until ED or hospital discharge; (7) total hours in the hospital (including the ED) through 1 month; (8) Alvarado scores28 on day 1 for both groups and day 2 for antibiotics-first participants; and (9) hospital charges. We assessed medication adherence by inspecting blister packs for retained pills or, if lost, by reviewing a memory aid and participant interview. Adverse events were graded according to standard definitions.29 We collected baseline and outcome data on qualifying patients who declined participation by chart review.
Analysis
We managed study data using REDCap (Vanderbilt, Nashville, TN).30 We determined a sample size of 30 participants for this pilot investigation (4.5% of the projected sample; sample size calculation [Appendix E3, available online at http://www.annemergmed.com]) evaluating the feasibility of a large multicenter trial testing noninferiority of the antibiotics-first approach for major complication rate. Because this was a pilot study, we did not conduct formal hypothesis testing and present results as descriptive statistics in the intention-to-treat population.
Results
Characteristics of Study Participants
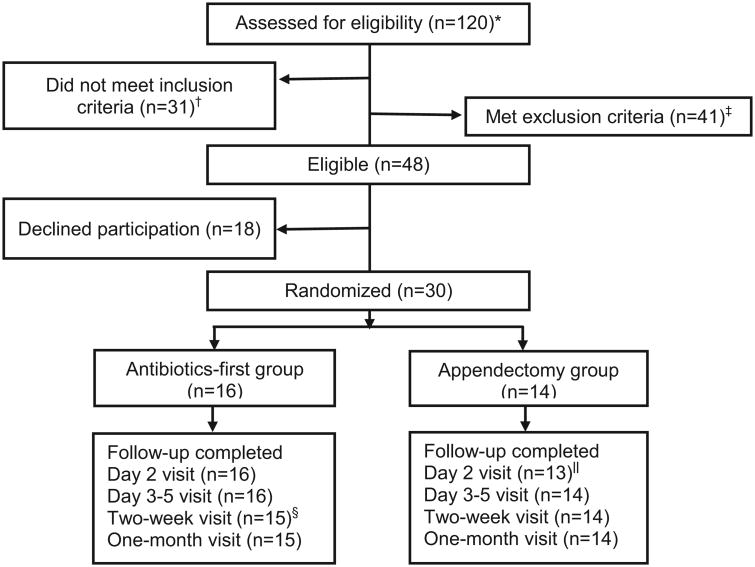
Of 48 eligible patients, 30 (62.5%) agreed to participate, of whom 16(53.3%) were randomized to antibiotics-first and 14 (46.7%) to appendectomy (Figure 1). At follow-up, all participants completed therapy in their assigned group and all except one (96.7%) were seen or contacted at each specified follow-up visit. One participant in the antibiotics-first group had a follow-up telephone call at day four and reported complete recovery but did not return subsequently.
Figure 1.
Screening, randomization, and follow-up of patients with the ED diagnosis of appendicitis.
*All patients with a presumptive emergency department diagnosis of appendicitis by an emergency medicine attending physician.
†Of the 31 patients who did not meet inclusion criteria, 11 had imaging that was interpreted as equivocal for appendicitis, 5 had imaging that was interpreted as equivocal for distinguishing complicated from uncomplicated appendicitis, 10 had imaging that was interpreted as complicated appendicitis, 4 had imaging suggesting acute uncomplicated appendicitis but did not have clinical confirmation by a surgeon, and 1 did not speak English or Spanish.
‡Of the 41 patients who met exclusion criteria, 23 had an appendicolith, 11 had intravenous antibiotics >6 hours prior to enrollment, 2 were prisoners, 2 had ulcerative colitis, 1 had high-risk diabetes, 1 was immunocompromised, and 1 had another infection that required antibiotic treatment. Radiographic identification of an appendicolith was initially an exclusion criterion but was later allowed (after 11 of 30 participants were enrolled) because of lack of consistent evidence of this being a risk factor for antibiotic failure.
§One antibiotics-first participant was lost to follow-up at the day 10-18 visit.
‖One appendectomy participant was intubated at the day 2 visit, so the visit was not completed.
Baseline characteristics were similar between treatment groups (Table 1 and Table E1 [available online at http://www.annemergmed.com]), and between qualifying enrolled and nonenrolled patients (Table E2, available online at http://www.annemergmed.com). For all participants, median age was 33 years (range 9 to 73 years old), 60.0% were male, 83.3% were white, and 86.7% were Hispanic. Right lower-quadrant pain was the most frequent symptom (27 participants [90%]). Participants had a median of 1 day of pain (range 0.5 to 5.0 days) with a median maximal pain level of 9 of 10 (range 4 to 10). Median triage temperature was 36.9°C (98.4°F) (range 36.4°C to 38.1°C [97.5°F to 100.6°F]). Median WBC count was 15,000/mL (range 6,200 to 23,100/mL). Each group's median Alvarado scores was 8 (range 4 to 10), including those who declined participation. All participants had CT imaging with intravenous contrast, except 1 child, who had ultrasonography and 1 adult who had an elevated creatinine level. Median appendiceal diameter was 10 mm (range 7 to 18 mm). An appendicolith was detected in 5 participants (17.2%).
Table 1.
Baseline characteristics of 30 participants with the diagnosis of acute uncomplicated appendicitis by treatment group.
| Characteristic | Appendectomy, n = 14 | Antibiotics-First, n = 16 |
|---|---|---|
| Age, median (IQR; range), y | 36 (33–46; 24–65) | 31 (25–40; 9–73) |
| Male sex, No. (%) | 9 (64.3) | 9 (56.3) |
| Race, No. (%) | ||
| White | 12 (85.7) | 13 (81.3) |
| Other | 2 (14.3) | 3 (18.8) |
| Hispanic ethnicity, No. (%) | 12 (85.7) | 14 (87.5) |
| Diabetes, No. (%) | 0 | 1 (6.3) |
| Body mass index, median (IQR; range), kg/m2 | 28.0 (24.6–29.8; 21.0–31.8) | 27.3 (25.1–33.0; 22.3–43.3) |
| Previous abdominal/pelvic surgery, No. (%) | 3 (21.4) | 3 (18.8) |
| Symptoms | ||
| Subjective fever, No. (%) | 4 (28.6) | 2 (12.5) |
| Nausea, No. (%) | 13 (92.9) | 12 (75.0) |
| Right lower quadrant pain, No. (%) | 13 (92.9) | 14 (87.5) |
| Duration of pain, median (IQR; range), days | 1.0 (0.5–3.0; 0.5–5.0) | 1.0 (1.0–2.5; 0.5–5.0) |
| Maximal pain before 24 h, median (IQR; range)* | 10 (8–10; 5–10) | 8 (8–10; 4–10) |
| Signs | ||
| Localized rebound tenderness, No. (%) | 10 (71.4) | 8 (50.0) |
| Localized guarding, No. (%) | 6 (42.9) | 6 (37.5) |
| Triage temperature, median (IQR; range), °C | 36.9 (36.6–36.7; 36.5–38.1) | 36.8 (36.7–37.2; 36.4–37.3) |
| CT findings† | ||
| Appendicolith, No. (%)‡ | 3 (21.4) | 2 (13.3) |
| Appendiceal diameter, median (IQR; range), mm | 9 (9–12; 7–8) | 10 (9–12; 7–14) |
| Periappendiceal stranding, No. (%) | 14 (100.0) | 13 (86.7) |
| Periappendiceal fluid, No. (%) | 3 (21.4) | 2 (13.3) |
| Laboratory results | ||
| WBC count, median (IQR; range), ×103/μL | 15.3 (11.0–18.4; 8.1–23.1) | 14.2 (11.3–17.0; 6.2–19.2) |
| Lactate, median (IQR; range), mmol/L§ | 1.0 (0.9–1.4; 0.6–1.6) | 1.1 (0.9–1.5; 0.7–2.3) |
| CRP, median (IQR; range), mg/L§ | 64.8 (42.6–101.6; 8.2–256.4) | 25.9 (10.8–64.8; 3.8–202.6) |
| Alvarado score, median (IQR; range)‖ | 8 (7–9; 4–10) | 8 (7–9; 4–10) |
| Appendix pathology findings, No. (%) | ||
| Normal | 1 (7.1) | |
| Acute uncomplicated | 9 (64.3) | NA |
| Suppurative or gangrenous | 4 (28.6) | |
| Quality-of-life measures | ||
| SF-12v2 Physical Component Score, median (IQR; range)¶ | 52.0 (47.4–57.0; 25.4–61.4) | 55.9 (54.4–57.1; 41.7–64.1) |
| SF-12v2 Mental Component Score, median (IQR; range)¶ | 57.0 (41.9–61.2; 31.6–68.4) | 49.4 (38.8–61.1; 35.5–62.1) |
IQR, Interquartile range; CRP, C-reactive protein; NA, not applicable.
Pain was rated on a scale of 0 to 10.
One pediatric participant randomized to antibiotics-first did not receive a CT scan, so results are presented only for the 15 adult participants who did.
Radiographic identification of an appendicolith was initially an exclusion criterion but was later allowed (after 11 of 30 participants were enrolled) because of lack of consistent evidence of this being a risk factor for antibiotic failure.
Two participants in the appendectomy group were missing results for lactate and CRP.
The Alvarado score28 consists of the following components (points): right lower quadrant tenderness (0/2), elevated temperature (≥37.3°C [99.1°F]) (0/1), rebound tenderness (0/1), migration of pain to the right lower quadrant (0/1), anorexia (0/1), nausea or vomiting (0/1), leukocytosis greater than 10,000 cells/μL (0/2), and polymorphonuclear cells greater than 75% (0/1).
SF-12v2 Health Survey Acute version24 (1-week recall) was used for adult (14 appendectomy and 15 antibiotic-first) participants to assess baseline quality of life before their appendicitis symptoms.
Among 15 antibiotics-first participants with complete follow-up, 14 (93.3%) completed 100% of oral antibiotic doses; 1 participant lost 1 tablet. Among 14 appendectomy participants, median time to operation was 8.0 hours (range 2.7 to 38.0 hours; 2 outliers >24 hours due to emergency surgeries interrupting scheduling). Appendectomy was performed laparoscopically in 9 (64.3%) participants and by open technique in 5 (35.7%) participants.
Main Results
Rates of hospitalization and ED discharge and time in hospital are summarized in Table 2. Of 15 adults treated with antibiotics-first, 14 (93.3%) met discharge criteria after a period of ED observation and were sent home with ED follow-up on day 2. One adult treated with antibiotics-first was hospitalized overnight on day 2 for observation and pain control after initial ED discharge. Per protocol, the one pediatric participant in the antibiotics-first group was hospitalized for twice-daily dosing of ertapenem. All appendectomy participants were admitted to the hospital. Median total hospital time (including ED time) during 1 month was 16.2 hours (range 10.9 to 106.6 hours) in the antibiotics-first group compared with 42.1 hours (range 28.0 to 128.8 hours) in the appendectomy group. Median hospital charges were $5,145 (range $3,885 to $38,337) in the antibiotics-first group and $12,447 (range $7,430 to $41,832) in the appendectomy group.
Table 2.
Disposition, appendectomy rate, and hospital duration through 1 month for 30 participants with the diagnosis of acute uncomplicated appendicitis by treatment group.
| Characteristic | Appendectomy, n = 14 | Antibiotics-First, n = 16 |
|---|---|---|
| Hospital admission at initial visit, No. (%) | 14 (100) | 2 (12.5)* |
| Received appendectomy, No. (%) | 14 (100) | 0 |
| Time in hospital for initial visit, median (IQR; range), h | 42.1 (28.8–51.0; 28.0–109.2) | 12.3 (10.4–14.8; 7.7–80.3) |
| Total time in hospital, median (IQR; range), h† | 42.1 (28.8–65.0; 28.0–128.8) | 16.2 (14.2–34.3; 10.9–106.6) |
Fourteen participants were discharged from the ED.
Total time in the hospital includes ED time and day 2 antibiotics-first group's evaluation and second intravenous dose of ertapenem and any subsequent hospital stay in both groups through the 1-month follow-up visit.
At 1 month, major complications occurred in 2 appendectomy participants (14.3%; 95% confidence interval [CI] 1.8% to 42.5%) and 1 antibiotics-first participant (6.3%; 95% CI 0.2% to 33.9%). Among appendectomy participants, 1 sustained a trochar-related retroperitoneal hemorrhage, and 1, who was found to have cecal inflammation and a normal appendix at appendectomy, developed a postoperative intra-abdominal abscess on day 5. All antibiotic-treated participants had symptom resolution. One antibiotic-treated participant (without appendicolith) had recurrent appendicitis complicated by a phlegmon seen on CT scan on day 18 that was successfully treated with antibiotics (all major complications are described in Figure E1, available online at http://www.annemergmed.com). During median 12-month follow-up (range 9 to 18 months), 2 of 15 antibiotics-first participants (13.3%; 95% CI 3.7% to 37.8%) developed recurrent appendicitis; 1 had appendectomy for pathology-confirmed uncomplicated appendicitis at 9 months. No more major complications occurred in the 15 antibiotics-first and 14 surgery participants who followed-up during this period.
Table 3 and Table E3 (available online at htttp://www.annemergmed.com) show pain levels, analgesic use, activity, and quality-of-life outcomes by visit. Antibiotics-first participants were pain free and returned to normal activities sooner and had higher physical SF-12v2 Health Survey® scores than appendectomy participants. Figure 2 depicts extent of improvement of antibiotics-first participants over the first day based on serial Alvarado scores; most improved substantially and none worsened. Antibiotic-treated participants experienced more, mostly mild, adverse events, including diarrhea, headache, and nausea (Table E4, available online at http://www.annemergmed.com). We observed no wound infections.
Table 3.
Pain, analgesic use, activity, and quality-of-life outcomes of 30 participants with the diagnosis of acute uncomplicated appendicitis by treatment group.
| Characteristic | Appendectomy, n = 14 | Antibiotics-First, n = 16 |
|---|---|---|
| Number of participants pain free, No. (%) | ||
| At day 2* | 0 | 5 (31.3) |
| Days 3–5 | 1 (7.1) | 10 (62.5) |
| 2 wk | 2 (14.3) | 12 (75.0) |
| 1 mo | 9 (64.3) | 14 (87.5) |
| Total days receiving analgesics, median (IQR; range) | ||
| Through day 2 | 1.0 (0.0–1.0; 0.0–1.0) | 0.5 (0.0–1.0; 0.0–1.0) |
| Days 3–5 | 2.0 (1.0–2.0; 0.0–3.0) | 1.0 (0.0–1.0; 0.0–3.0) |
| 2 wk | 4.0 (2.0–6.0; 1.0–10.0) | 1.0 (0.0–1.0; 0.0–12) |
| 1 mo | 4.5 (3.0–8.0; 1.0–24.0) | 1.0 (0.0–1.5; 0.0–12) |
| Unable to perform normal activities, No. (%) | ||
| At day 2 | 14/14 (100.0) | 10/16 (62.5) |
| Day 2 to 3–5 | 12/14 (85.7) | 7/16 (43.8) |
| Days 3–5 to 2 wk | 6/14 (42.9) | 1/15 (6.7) |
| 2 wk–1 mo | 2/13 (15.4) | 0/15 |
| Quality-of-life measures | ||
| SF-12v2 Physical Component score, median (IQR; range)† | ||
| 2 wk | 44 (36–51; 31–56) | 54 (52–58; 38–63) |
| 1 mo | 47 (40–53; 32–55) | 56 (47–57; 33–62) |
| SF-12v2 Mental Component score, median (IQR; range)† | ||
| 2 wk | 58 (48–61; 17–68) | 55 (53–59; 38–61) |
| 1 mo | 56 (43–58; 37–68) | 55 (49–57; 36–63) |
Follow-up visits occurred at day 2, days 3 to 5, 2 weeks (days 10 to 18), and 1 month (days 25 to 35) after enrollment (day 1).
SF-12v2 Health Survey Acute version24 (1-week recall) was used for adult (14 appendectomy and 15 antibiotic-first) participants at 2 weeks and the 4-week recall version at 1 month.
Figure 2.
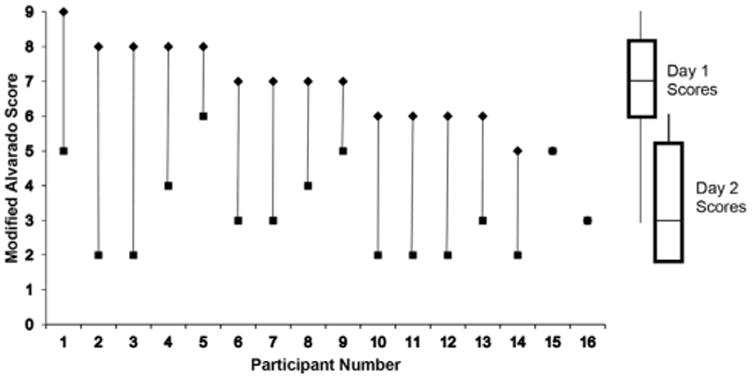
Modified Alvarado scores* at day 1 (◆) and day 2 (■) for 16 participants with the diagnosis of acute uncomplicated appendicitis randomized to antibiotics-first treatment. *The modified Alvarado score28 consists of the following components (points): right lower quadrant tenderness (0/2); elevated temperature (≥37.3°C [99.1°F]) (0/1); rebound tenderness (0/1); anorexia (0/1); nausea or vomiting (0/1); leukocytosis >10,000 cells/mL (0/2); polymorphonuclear cells >75% (0/1). The modified score does not include migration of pain to the right lower quadrant since this variable would not be applicable for comparison of serial scores among a cohort of patients with imaging-confirmed appendicitis. The maximum modified Alvarado score is 9 instead of 10 for the original score.
There was no change between day 1 and day 2 scores for participant numbers 15 and 16.
Limitations
This study has a number of limitations. First, because a sham surgery would be unethical, these investigations are unblinded and management of participants and outcome assessments could be biased. Second, the small number of participants, and only 1 child, preclude full assessment of comparative effectiveness and could account for no case of initial antibiotic failure and the low recurrence rate. Third, this was a single-center study of mostly Hispanic patients treated at 1 Los Angeles–area public teaching hospital and the findings may not be generalizable. Notable, however, among this frequently non-English-speaking population, medication adherence and follow-up were sufficient to allow 14 of 15 adults to be managed successfully as outpatients after ED discharge. As opposed to past studies in which crossover from the assigned to alternate treatment strategy occurred in up to 53%,4 we had none, which may be due to providing adequate education, pain control, and access to follow-up care. Finally, clinical examination and imaging misclassify some cases as uncomplicated appendicitis that are actually nonappendicitis or complicated disease. We had 1 appendectomy case (7.2%) in which appendicitis was not found on pathologic review; we did not categorize this as a major complication of surgery. Similarly, we did not categorize the day 2 overnight hospitalization of one antibiotic-treated participant for observation and pain control as a major complication because it was part of the treatment strategy. Complicated appendicitis can be missed preoperatively and only discovered at surgery, as we observed in 4 participants (28.6%).5-7,31,32 Because finding complicated appendicitis only at surgery, and not according to clinical examination and imaging, would be more common among patients undergoing routine as opposed to rescue surgery, we did not include it as a major complication in general or consider it as a major complication only in the antibiotics-first group, which has been a criticism of one trial that concluded that the antibiotics-first approach was not noninferior to appendectomy.5,33 Consequently, we may have missed cases of complicated disease that developed between randomization and operation. However, this is unlikely, given the generally short time to surgery and evidence that surgery delays for patients receiving antibiotics do not appear to be associated with an increased perforation rate.34
Discussion
In this pilot randomized trial comparing antibiotics-first and appendectomy, we describe a novel, safe, and effective strategy that allows outpatient antibiotic management of imaging-confirmed uncomplicated appendicitis. All but 1 of the 15 adult patients randomized to antibiotics-first were successfully managed as outpatients after a minimum 6-hour ED observation period after triage. Of 15 antibiotics-first participants followed over approximately 1 year, only 2 (13.3%) had recurrence of appendicitis; 1 underwent appendectomy and the other was successfully treated medically. Unlike in past trials, in which approximately one quarter of patients had appendectomy during 1 year of follow-up, only 1 (6.7%) participant in this pilot did. The ability to manage appendicitis with antibiotics and without hospitalization could significantly reduce costs and could be an important consideration for a patient choosing between surgical and medical management. To our knowledge, this pilot study is also the first randomized trial comparing antibiotics-first and appendectomy to be conducted in the US. Although concerns have been raised that patients would refuse to participate in randomized trials of appendicitis if they could not choose their treatment,8 approximately two thirds of eligible patients provided consent, no participants crossed over to the alternate treatment strategy, and nearly universal follow-up was achieved. These trial performance measures and excellent outcomes support the feasibility of a large multicenter US randomized trial.
We included patients similar to those enrolled in most published trials (ie, patients with clinical- and CT-confirmed uncomplicated appendicitis). We excluded patients with severe sepsis, immunocompromise, or with acute comorbidities, but not the elderly and those with most stable comorbidities. Initially, we excluded patients with appendicolith because the results of some9,14 but not all studies4,5,7,10-12,15 found an association with antibiotic failure. Given our early success with antibiotic therapy, we later elected to include patients with appendicolith to explore the feasibility of including this large subset in future trials.
The hospital stays we report are shorter than those reported from previous randomized trials, likely due in part to differences between European and US health care practices. Among the 7 European trials comparing an antibiotics-first approach to urgent appendectomy, duration of hospitalization has been similar between treatment groups, approximately 3 days.1-7 In these trials, patients randomized to antibiotics-first were required to be hospitalized up to 3 days, and most appendectomies were performed by open technique. The general trend in US healthcare is toward shorter hospital stays. The US Nationwide Inpatient Sample of adults who underwent appendectomy from 2003 to 2011 revealed that mean stay decreased from 3.2 to 2.6 days while laparoscopically performed procedures increased from 40.7% to 80.1%.18 Same-day laparoscopic appendectomy, using discharge criteria similar to ours, has been described, although it is not widely practiced.35
Unlike past randomized trials, we applied symmetrical discharge criteria for participants in each group, ie, clinical stability, tolerating fluids, and pain control with oral medications. Participants older than 13 years randomized to antibiotics-first were evaluated, treated, and observed in the ED and were discharged if these criteria were met. Participants then returned for a next-day ED follow-up evaluation. To ensure effective antibiotic coverage, we administered a Food and Drug Administration–approved once-a-day parenteral antibiotic recommended for intra-abdominal infections, ertapenem,36 at enrollment and the next day, followed by oral antibiotics to complete a 10-day course. Our rationale for using a long-acting parenteral agent was that, early in appendicitis, nausea and vomiting could interfere with full adherence with an oral antibiotic regimen. All appendectomy participants were hospitalized as opposed to most adult antibiotics-first-treated participants, who were discharged from the ED. Consequently, antibiotics-first participants had substantially reduced total hospital time, including ED time (median 16.2 hours compared with 42.1 hours through 1 month). Other off-label once-daily parenteral antibiotic strategies could be used, also allowing similar management in young children.
Previous randomized trials had various criteria for antibiotic failure and reported that about 10% of antibiotics-first-treated participants required appendectomy during their initial hospitalization.2,4-7 One trial found that the presence of an appendicolith was a significant risk factor for initial antibiotic failure.14 None of our antibiotic-first participants had initial treatment failure, including 2 with appendicolith. Because almost all of our antibiotics-first-treated participants were discharged from the ED, lack of initial antibiotic failure may be due to fewer opportunities for surgeons to observe variations in pain control and suggest operative intervention and patient inertia to continue outpatient care once home. Knowledge of the association of appendicolith and complicated disease may also decrease a provider's threshold to consider a case a treatment failure. The appendicitis recurrence rate after antibiotics may be as low as 15% over the first year following the initial attack of appendicitis with a more extended antibiotic trial. One of the 2 antibiotic-treated participants who had recurrence chose retreatment with antibiotics, an option that would further decrease subsequent appendectomy rate.
As opposed to previous trials in which the primary outcome was appendectomy rate in the antibiotic group,1-3,6,7 we chose an outcome independent of treatment strategy, ie, major complications defined by ACS NSQIP criteria modified to include serious antibiotic-related adverse events.25,26 Before conducting this trial, we surveyed persons with and without previous appendectomy and found they prioritized clinical outcomes over quality-of-life and pain outcomes.37 Clinical outcomes are also likely more important to physicians whom patients trust for direction of their care. We observed the rate of major complications (all of which occurred in the first month) among participants in the antibiotics-first and appendectomy groups to be 6.3% and 14.3%, respectively. The high rate in the appendectomy group likely represents a sampling error. Rates of major complications after appendectomy at our hospital have historically been consistent with reported rates of less than or equal to 5%,25,26,38 and no qualifying but nonenrolled patient receiving appendectomy experienced a major complication.
Secondary outcomes, which included pain scores, analgesic use, disability time, and quality-of-life measures, favored the antibiotics-first group. Remarkably, approximately one third of antibiotic-treated participants were pain free and resumed their normal activities after approximately 24 hours. Few data exist describing the rate and variability of early response to antibiotics.1 Although the Alvarado score28 was developed as a clinical tool to diagnose appendicitis, it contains components that clinicians might use to follow the progress of an antibiotic-treated patient, eg, fever, tenderness, nausea, leukocytosis. In most antibiotics-first participants, as reflected by serial scores, these findings substantially improved during only 1 day. A few participants had lower scores that stayed constant, but ultimately their symptoms resolved, suggesting that, even with persistent symptoms at 24 hours, it is reasonable to continue antibiotics. Next-day contact by telephone may be sufficient for most patients. Participants treated with antibiotics-first experienced more mild adverse events, such as diarrhea, nausea, and headache, likely related to greater antibiotic exposure. However, these symptoms did not interfere with good antibiotic adherence.
Antibiotic management of uncomplicated appendicitis remains an uncommon practice in the US.23,39 We believe that clinical and patient-centered outcomes require comparison in a large US-based multicenter randomized trial among a diverse population in which imaging is routinely used, most appendectomies are performed laparoscopically, early discharge is promoted, including from the ED for antibiotics-first patients, and patients are offered antibiotic retreatment and are followed for at least 2 years. In accordance with our successful experience with this pilot study, we believe that such a trial is necessary and feasible.
Supplementary Material
Figure E1. Summary of major complications.
Table E1. Baseline characteristics of 30 participants with the diagnosis of acute uncomplicated appendicitis by treatment group.*
Table E2. Baseline characteristics of qualifying enrolled and nonenrolled patients with the diagnosis of acute uncomplicated appendicitis.*
Table E3. Pain, analgesic use, activity, and quality-of-life outcomes of 30 participants with the diagnosis of acute uncomplicated appendicitis by treatment group.*
Table E4. Number of adverse events by severity* and treatment group through 1 month for 30 participants with the diagnosis of acute uncomplicated appendicitis.
Editor's Capsule Summary.
What is already known on this topic
There is limited evidence suggesting that uncomplicated appendicitis can be treated with antibiotics instead of surgery.
What question this study addressed
Is an outpatient strategy of antibiotics as safe and effective as hospitalization and appendectomy?
What this study adds to our knowledge
In this randomized controlled trial of computed tomography–proven appendicitis, the 15 evaluable patients receiving antibiotics all recovered. Two, however, had recurrence; one treated with appendectomy and one that resolved with further antibiotics.
How this is relevant to clinical practice
This pilot study cannot establish safety or efficacy but does suggest feasibility for a large-scale trial of nonoperative management.
Acknowledgments
The authors acknowledge the residents of the Olive View–UCLA Department of Emergency Medicine and Department of Surgery, part-time Department of Emergency Medicine faculty, and the Division of Digestive Disease and Nutrition National Institute of Diabetes and Digestive and Kidney Diseases for their assistance and support of the study.
Funding and support: By Annals policy, all authors are required to disclose any and all commercial, financial, and other relationships in any way related to the subject of this article as per ICMJE conflict of interest guidelines (see www.icmje.org). This study was supported by a grant from the National Institutes of Health (NIH)/National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) (1R21DK102048-01A1, to Drs. Talan and Moran). Dr. Talan reports serving on a speakers bureau for Allergan; providing consultation, serving on an advisory board, and conducting research for Allergan and Cempra; and creating an educational video for Merck. Dr. Moran reports conducting research for Cempra, Allergan, Cerexa, and Cubist Astra Zeneca.
Footnotes
Author contributions: DAT, DJS, WRM, DAD, JXW, and GJM developed the study methods. DAT, DJS, AK, RA, and GJM supervised study implementation. DAT drafted the article. WRM and AK conducted data analysis. AK served as project director. CMJ developed radiology methods. DAD, JXW, and KP assisted with study implementation. KP supervised the study coordinators. AM conducted chart reviews, entered data, and ensured data quality and integrity. DAT takes responsibility for the paper as a whole.
All authors attest to meeting the four ICMJE.org authorship criteria: (1) Substantial contributions to the conception or design of the work; or the acquisition, analysis, or interpretation of data for the work; AND (2) Drafting the work or revising it critically for important intellectual content; AND (3) Final approval of the version to be published; AND (4) Agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Trial registration number: NCT02447224
The NIH/NIDDK had no involvement in study design; in the collection, analysis, and interpretation of data; in the writing of the report; or in the decision to submit the article for publication.
References
- 1.Eriksson S, Granstrom L. Randomized controlled trial of appendicectomy versus antibiotic therapy for acute appendicitis. Br J Surg. 1995;82:166–169. doi: 10.1002/bjs.1800820207. [DOI] [PubMed] [Google Scholar]
- 2.Styrud J, Eriksson S, Nilsson I, et al. Appendectomy versus antibiotic treatment in acute appendicitis. a prospective multicenter randomized controlled trial. World J Surg. 2006;30:1033–1037. doi: 10.1007/s00268-005-0304-6. [DOI] [PubMed] [Google Scholar]
- 3.Turhan AN, Kapan S, Kutukcu E, et al. Comparison of operative and non operative management of acute appendicitis. Ulus Travma Acil Cerrahi Derg. 2009;15:459–462. [PubMed] [Google Scholar]
- 4.Hansson J, Körner U, Khorram-Manesh A, et al. Randomized clinical trial of antibiotic therapy versus appendicectomy as primary treatment of acute appendicitis in unselected patients. Br J Surg. 2009;96:473–481. doi: 10.1002/bjs.6482. [DOI] [PubMed] [Google Scholar]
- 5.Vons C, Barry C, Maitre S, et al. Amoxicillin plus clavulanic acid versus appendicectomy for treatment of acute uncomplicated appendicitis: an open-label, non-inferiority, randomised controlled trial. Lancet. 2011;377:1573–1579. doi: 10.1016/S0140-6736(11)60410-8. [DOI] [PubMed] [Google Scholar]
- 6.Salminen P, Paajanen H, Rautio T, et al. Antibiotic therapy vs appendectomy for treatment of uncomplicated acute appendicitis: the APPAC randomized clinical trial. JAMA. 2015;313:2340–2348. doi: 10.1001/jama.2015.6154. [DOI] [PubMed] [Google Scholar]
- 7.Svensson JF, Patkova B, Almström M, et al. Nonoperative treatment with antibiotics versus surgery for acute nonperforated appendicitis in children: a pilot randomized controlled trial. Ann Surg. 2015;261:67–71. doi: 10.1097/SLA.0000000000000835. [DOI] [PubMed] [Google Scholar]
- 8.Minneci PC, Mahida JB, Lodwick DL, et al. Effectiveness of patient choice in nonoperative vs surgical management of pediatric uncomplicated acute appendicitis. JAMA Surg. 2016;151:408–415. doi: 10.1001/jamasurg.2015.4534. [DOI] [PubMed] [Google Scholar]
- 9.Tanaka Y, Uchida H, Kawashima H, et al. Long-term outcomes of operative versus nonoperative treatment for uncomplicated appendicitis. J Pediatr Surg. 2015;50:1893–1897. doi: 10.1016/j.jpedsurg.2015.07.008. [DOI] [PubMed] [Google Scholar]
- 10.Steiner Z, Buklan G, Stackievicz R, et al. A role for conservative antibiotic treatment in early appendicitis in children. J Pediatr Surg. 2015;50:1566–1568. doi: 10.1016/j.jpedsurg.2015.04.008. [DOI] [PubMed] [Google Scholar]
- 11.Hartwich J, Luks FI, Watson-Smith D, et al. Nonoperative treatment of acute appendicitis in children: a feasibility study. J Pediatr Surg. 2016;51:111–116. doi: 10.1016/j.jpedsurg.2015.10.024. [DOI] [PubMed] [Google Scholar]
- 12.Armstrong J, Merritt N, Jones S, et al. Non-operative management of early, acute appendicitis in children: is it safe and effective? J Pediatr Surg. 2014;49:782–785. doi: 10.1016/j.jpedsurg.2014.02.071. [DOI] [PubMed] [Google Scholar]
- 13.Coldrey E. Five years of conservative treatment of acute appendicitis. J Int Coll Surg. 1959;32:255–261. [Google Scholar]
- 14.Shindoh J, Niwa H, Kawai K, et al. Predictive factors for negative outcomes in initial non-operative management of suspected appendicitis. J Gastrointest Surg. 2010;14:309–314. doi: 10.1007/s11605-009-1094-1. [DOI] [PubMed] [Google Scholar]
- 15.Di Saverio S, Sibilio A, Giorgini E, et al. The NOTA Study (Non Operative Treatment for Acute Appendicitis): prospective study on the efficacy and safety of antibiotics (amoxicillin and clavulanic acid) for treating patients with right lower quadrant abdominal pain and long-term follow-up of conservatively treated suspected appendicitis. Ann Surg. 2014;260:109–117. doi: 10.1097/SLA.0000000000000560. [DOI] [PubMed] [Google Scholar]
- 16.Campbell MR, Johnston SL, 3rd, Marshburn T, et al. Nonoperative treatment of suspected appendicitis in remote medical care environments: implications for future spaceflight medical care. J Am Coll Surg. 2004;198:822–830. doi: 10.1016/j.jamcollsurg.2004.01.009. [DOI] [PubMed] [Google Scholar]
- 17.Mason RJ, Moazzez A, Sohn H, et al. Meta-analysis of randomized trials comparing antibiotic therapy with appendectomy for acute uncomplicated (no abscess or phlegmon) appendicitis. Surg Infect (Larchmt) 2012;13:74–84. doi: 10.1089/sur.2011.058. [DOI] [PubMed] [Google Scholar]
- 18.Bliss LA, Yang CJ, Kent TS, et al. Appendicitis in the modern era: universal problem and variable treatment. Surg Endosc. 2015;29:1897–1902. doi: 10.1007/s00464-014-3882-2. [DOI] [PubMed] [Google Scholar]
- 19.Stollman N, Smalley W, Hirano I, et al. American Gastroenterological Association Institute guideline on the management of acute diverticulitis. Gastroenterology. 2015;149:1944–1949. doi: 10.1053/j.gastro.2015.10.003. [DOI] [PubMed] [Google Scholar]
- 20.Chang DC, Shiozawa A, Nguyen LL, et al. Cost of inpatient care and its association with hospital competition. J Am Coll Surg. 2011;212:12–19. doi: 10.1016/j.jamcollsurg.2010.09.014. [DOI] [PubMed] [Google Scholar]
- 21.Sauerland S, Jaschinski T, Neugebauer EA. Laparoscopic versus open surgery for suspected appendicitis. Cochrane Database Syst Rev. 2010;10:CD001546. doi: 10.1002/14651858.CD001546.pub3. [DOI] [PubMed] [Google Scholar]
- 22.Ehlers AP, Talan DA, Moran GJ, et al. Evidence for an antibiotics-first strategy for uncomplicated appendicitis in adults: a systematic review and gap analysis. J Am Coll Surg. 2016;222:309–314. doi: 10.1016/j.jamcollsurg.2015.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Khalil M, Rhee P, Jokar TO, et al. Antibiotics for appendicitis! Not so fast. J Trauma Acute Care Surg. 2016;80:923–932. doi: 10.1097/TA.0000000000001030. [DOI] [PubMed] [Google Scholar]
- 24.Maruish M. User's Manual for the SF-12v2 Health Survey. Lincoln, RI: QualityMetric; 2012. [Google Scholar]
- 25.Ingraham AM, Cohen ME, Bilimoria KY, et al. Comparison of outcomes after laparoscopic versus open appendectomy for acute appendicitis at 222 ACS NSQIP hospitals. Surgery. 2010;148:625–637. doi: 10.1016/j.surg.2010.07.025. [DOI] [PubMed] [Google Scholar]
- 26.Mason RJ, Moazzez A, Moroney JR, et al. Laparoscopic vs. open appendectomy in obese patients: outcomes using the American College of Surgeons National Surgical Quality Improvement Program Database. J Am Coll Surg. 2012;215:88–99. doi: 10.1016/j.jamcollsurg.2012.03.012. [DOI] [PubMed] [Google Scholar]
- 27.Varni JW, Seid M, Rode CA. The PedsQL™: measurement model for the Pediatric Quality of Life Inventory. Med Care. 1999;37:126–139. doi: 10.1097/00005650-199902000-00003. [DOI] [PubMed] [Google Scholar]
- 28.Alvarado A. A practical score for the early diagnosis of acute appendicitis. Ann Emerg Med. 1986;15:557–564. doi: 10.1016/s0196-0644(86)80993-3. [DOI] [PubMed] [Google Scholar]
- 29.US Department of Health and Human Services, Food and Drug Administration. Guidance for industry E6 good clinical practice: consolidated guidance. [Accessed March 15, 2016]; Available at: http://www.fda.gov/downloads/Drugs/…/Guidances/ucm073122.pdf.
- 30.Harris PA, Taylor R, Thielke R, et al. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bixby SD, Lucey BC, Soto JA, et al. Perforated versus nonperforated acute appendicitis: accuracy of multidetector CT detection. Radiology. 2006;241:780–786. doi: 10.1148/radiol.2413051896. [DOI] [PubMed] [Google Scholar]
- 32.Foley TA, Earnest F, IV, Nathan MA, et al. Differentiation of nonperforated from perforated appendicitis: accuracy of CT diagnosis and relationship of CT findings to length of hospital stay. Radiology. 2005;235:89–96. doi: 10.1148/radiol.2351040310. [DOI] [PubMed] [Google Scholar]
- 33.Mason RJ. Appendicitis: is surgery the best option? Lancet. 2011;377:1545–1546. doi: 10.1016/S0140-6736(11)60623-5. [DOI] [PubMed] [Google Scholar]
- 34.Drake FT, Mottey NE, Farrokhi ET, et al. Time to appendectomy and risk of perforation in acute appendicitis. JAMA Surg. 2014;149:837–844. doi: 10.1001/jamasurg.2014.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cash CL, Frazee RC, Abernathy SW, et al. A prospective treatment protocol for outpatient laparoscopic appendectomy for acute appendicitis. J Am Coll Surg. 2012;215:101–105. doi: 10.1016/j.jamcollsurg.2012.02.024. [DOI] [PubMed] [Google Scholar]
- 36.Solomkin JS, Mazuski JE, Bradley JS. Diagnosis and management of complicated intra-abdominal infection in adults and children: guidelines by the Surgical Infection Society and the Infectious Diseases Society of America. Clin Infect Dis. 2010;50:133–164. doi: 10.1086/649554. [DOI] [PubMed] [Google Scholar]
- 37.Kadera SP, Mower WR, Krishnadasan A, et al. Patient perspectives on antibiotics for appendicitis at one hospital. J Surg Res. 2016;201:253–257. doi: 10.1016/j.jss.2015.11.016. [DOI] [PubMed] [Google Scholar]
- 38.Tiwari MM, Reynoso JF, Tsang AW, et al. Comparison of outcomes of laparoscopic and open appendectomy in management of uncomplicated and complicated appendicitis. Ann Surg. 2011;254:927–932. doi: 10.1097/SLA.0b013e31822aa8ea. [DOI] [PubMed] [Google Scholar]
- 39.Flum DR. Acute appendicitis—appendectomy or the “antibiotics first” strategy. N Engl J Med. 2015;372:1937–1943. doi: 10.1056/NEJMcp1215006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure E1. Summary of major complications.
Table E1. Baseline characteristics of 30 participants with the diagnosis of acute uncomplicated appendicitis by treatment group.*
Table E2. Baseline characteristics of qualifying enrolled and nonenrolled patients with the diagnosis of acute uncomplicated appendicitis.*
Table E3. Pain, analgesic use, activity, and quality-of-life outcomes of 30 participants with the diagnosis of acute uncomplicated appendicitis by treatment group.*
Table E4. Number of adverse events by severity* and treatment group through 1 month for 30 participants with the diagnosis of acute uncomplicated appendicitis.