Abstract
Background
The short term superiority of video-assisted thoracoscopic surgery (VATS) lobectomy compared to open lobectomy for early stage lung cancer has been suggested by single institution studies. Lack of equipoise limits the feasibility of a randomized study to confirm this. The hypothesis of this study (CALGB 31001) was that VATS lobectomy results in shorter length of hospital stay and fewer complications compared to open lobectomy in stages I and II non-small cell lung cancer in a multi-institutional setting.
Methods
519 patients whose tumors had been collected as part of CALGB 140202 (lung cancer tissue bank) were eligible. Propensity-scoring using age, race, gender, performance status, comorbidities, histology, tumor stage and size as independent variables was used to create a 1:1 matched group of 175 pairs of patients. McNemar’s test for binary and Wilcoxon signed-rank test for continuous variables were used to assess differences in length of hospital stay, complications, and discharge dispositions between the groups. Comparison of disease-free and overall survival between the two approaches was done using the log-rank test. P-values < 0.05 were considered significant.
Results
The matched data on length of hospital stay, complications and discharge dispositions significantly favored the VATS group. There was no statistically significant difference in survival between the two approaches.
Conclusion
This multi-institutional study supports the assertion that thoracoscopic lobectomy results in shorter hospital length of stay, fewer peri-operative complications and greater likelihood of independent home discharge compared to open lobectomy for early stage lung cancer. Survival was comparable between the two groups.
Keywords: Lobectomy, Lung cancer surgery, Outcomes, Thoracoscopy/VATS, Thoracotomy
Introduction
Surgery remains the best option for cure in the treatment of non-small cell lung cancer (NSCLC) and lobectomy continues to be the gold standard in early stage NSCLC. The demonstration of a survival benefit from screening high risk patients for NSCLC (1) is expected to increase the proportion of patients who have resectable early stage disease. It is imperative that the thoracic surgical community dissects the strengths and limitations of the procedures to treat these patients. This will in turn maximize their survival while minimizing their morbidity. Currently, lobectomy can be performed via multiple variations of a VATS approach or via thoracotomy. Proponents of the VATS approach have touted several potential advantages compared with a thoracotomy: less morbidity, shorter convalescence, and superior survival rates. Critics of the VATS approach have argued that it may not be an equivalent oncologic operation. Unfortunately, the evidence in the literature to support one or the other view is largely limited to single institution case series and small observational studies. A systematic review of the literature demonstrated that compared with thoracotomy, VATS lobectomy was associated with shorter chest tube duration, shorter length of hospital stay, and improved survival (2). It has also been shown to be associated with lower morbidity (3–7) and overall cost-savings (8, 9). There is also increasing evidence to support the hypothesis that reduced perioperative immunosuppression associated with VATS versus open lobectomy may contribute to improved outcomes (2, 10). To date, large, well-designed, prospective multi-institutional randomized trials have not been successfully performed to compare the two approaches.
Cancer and Leukemia Group B (CALGB) 39802 (VATS Lobectomy: A Feasibility Study) demonstrated the feasibility and safety of this surgical procedure in a multi-institution setting (11). It also showed 78% disease-free survival at 36 months for patients with stage I NSCLC, suggesting that the VATS operation yields results equal to those observed using a thoracotomy approach. However, it seems that a randomized definitive trial may never be performed, given a lack of equipoise on the part of surgeons and patients alike. In CALGB 31001, we proposed to use data from CALGB 140202 (the lung cancer tissue bank) to compare the outcome of VATS versus open lobectomies for stages I and II NSCLC. This is a large tissue bank of surgically resected NSCLC associated with comprehensive clinical, treatment and outcome information. Our hypothesis was that VATS Lobectomy results in shorter lengths of hospital stay and fewer peri-operative complications compared to open lobectomy in stages I and II NSCLC. Our primary endpoint was length of hospital stay; secondary endpoints included discharge disposition, peri-operative complications and disease-free survival.
Patients and Methods
Relevant data were rigorously abstracted by surgeon members of the study team from the operative and pathology reports of patients in the CALGB 140202 lung cancer tissue bank study who had a lobectomy (VATS or open) for stages I and II NSCLC. Additional clinical information was obtained from history/physical examination documents and discharge summaries on all study participants. Patients with prior treatments for NSCLC, active second malignancies (other than non-melanoma cancers), who were younger than 18 years of age or were converted from VATS to thoracotomy were excluded. Each participant signed an IRB-approved, protocol-specific informed consent in accordance with federal and institutional guidelines. Data collection and statistical analyses were conducted by the Alliance Statistics and Data Center.
Five hundred and nineteen patients (282 VATS and 237 open) enrolled between October 2004 and June 2010 were identified to be eligible. The data was downloaded and locked on May 5, 2013. We first performed overall unmatched analysis for all 519 patients using Fisher’s exact tests for categorical variables and Wilcoxon rank-sum tests for continuous variables. We then fit a logistic regression model with VATS and open lobectomy as dependent variables and patients’ characteristics such as age, race, gender, Eastern Cooperative Oncology Group (ECOG) performance status, histology, pathologic stage, tumor size, and co-morbidity as independent variables to calculate the propensity score (12) for the probability of a particular patient receiving open lobectomy. Presence of co-morbidities was defined as occurrence of any of the following: hypertension, hypercholesterolemia, coronary artery disease, diabetes, chronic obstructive pulmonary disease, congestive heart failure or renal failure. Three hundred and fifty patients (350/519=67.4%) were then matched by the propensity score using a greedy 1:1 matching algorithm (13). For matched patients, McNemar’s and Wilcoxon signed-rank tests were used. For multivariate analysis, generalized estimating equation (GEE) models (14) were used to model both continuous (length of hospital stay) and binary (peri-operative complications and discharge to home/others) outcomes. Matched survival analysis was performed using the Cox proportional hazard model for cluster data (15). Covariate adjustments in multivariate GEE and Cox proportional hazard models were operative approach (VATS versus Open), age, race, sex, ECOG performance status, histology, pathologic stage, tumor size, and medical co-morbidity.
Results
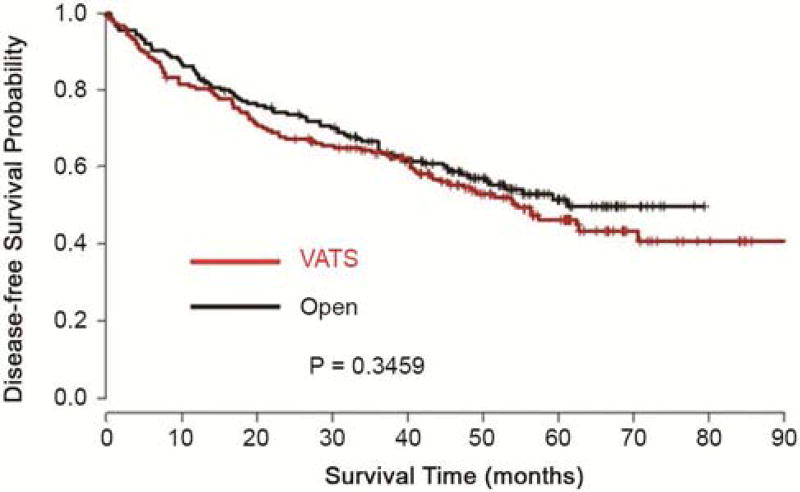
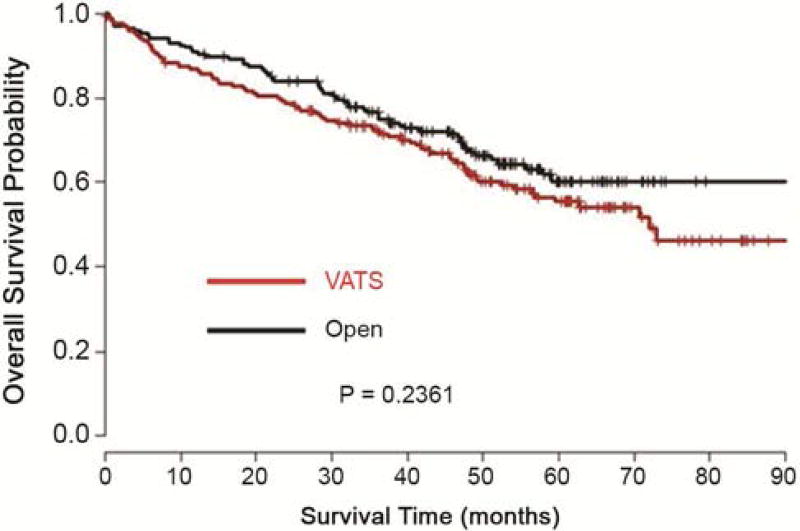
Forty-seven surgeons from 15 institutions contributed the 519 cases. Based on the cases submitted to the tumor bank, five institutions performed predominantly open lobectomy (80% or more of total cases within their institutions) and two institutions performed predominantly VATS lobectomy. These latter institutions contributed 46% of the open and 50% of the VATS cases, respectively. The median follow-up time for this cohort of patients was 60 months. The unmatched patient characteristics are outlined in Table 1. There was imbalance between the VATS and open lobectomy groups in terms of histology, pathologic stage, and tumor size (p < 0.05). This justified the use of matched analysis. The characteristics of the matched groups are outlined in Table 2. In the matched samples, none of the patient characteristics are significantly different between VATS and open groups. Further analyses were performed on the matched groups. Table 3 shows the summary of the number of lymph node stations and the total number of lymph nodes sampled in the two groups. The VATS lobectomy group showed shorter length of hospital stay, less incidence of peri-operative complications and a greater likelihood of home discharge than the open lobectomy group (Table 4). The most common complications were supraventricular arrhythmia/atrial fibrillation (9.4%), need for blood transfusion (4%), Pneumonia (2.9%) and atelectasis (1.7%). There was no difference between the groups for individual complications, but on aggregate the open lobectomy group had significantly more complications than the VATS lobectomy group (p<0.0001). The 30-day post-operative mortality rate in both groups was identical – 1.7%. There was no statistically significant difference in disease-free or overall survival between the two operative approaches (Figures 1 and 2). From the Cox proportional hazards regression model, the hazard ratio for disease-free survival of VATS versus Open lobectomy matched cases was 1.15 (95% confidence limits: 0.85, 1.55), p-value 0.36. The hazard ratio for overall survival of VATS versus Open lobectomy matched cases was 1.27 (95% confidence limits: 0.92, 1.76), p-value 0.15.
Table 1.
Patients Characteristics – Unmatched Data
| Open (N=237) |
VATS (N=282) |
Total (N=519) |
p-value | |
|---|---|---|---|---|
| Age | 0.40 | |||
| Mean (SD) | 67.5 (9.5) | 68.1 (10.4) | 67.9 (10.0) | |
| Median | 68 | 68.5 | 68 | |
| Range | (43–87) | (33–95) | (33–95) | |
| Race | 0.60 | |||
| White | 228 (96.2%) | 255 (90.4%) | 483 (93.1%) | |
| Non-white | 9 (3.8%) | 27 (9.6%) | 36 (6.9%) | |
| Gender | 0.11 | |||
| Male | 127 (53.6%) | 131 (46.5%) | 258 (49.7%) | |
| Female | 110 (46.4%) | 151 (53.5%) | 261 (50.3%) | |
| ECOG Performance Status | 0.11 | |||
| 0 | 166 (70%) | 216 (76.6%) | 382 (73.6%) | |
| 1/2/3 | 71 (30%) | 66 (23.4%) | 137 (26.4%) | |
| Histology | 0.01 | |||
| Adenocarcinoma | 103 (43.5%) | 163 (57.8%) | 266 (51.3%) | |
| Squamous cell carcinoma | 86 (36.3%) | 79 (28%) | 165 (31.8%) | |
| Other | 48 (20.3%) | 40 (14.2%) | 88 (17%) | |
| Pathologic Stage | 0.0002 | |||
| I | 174 (73.4%) | 244 (86.5%) | 418 (80.5%) | |
| II | 63 (26.6%) | 38 (13.5%) | 101 (19.5%) | |
| Tumor Size (cm) | <0.0001 | |||
| Mean (SD) | 4.3 (2.6) | 3.0 (1.4) | 3.6 (2.2) | |
| Median | 3.7 | 2.7 | 3.0 | |
| Range | (0.9–19) | (1.0–8) | (0.9–19) | |
| Any Medical Co-morbidities | 0.65 | |||
| Yes | 155 (65.4%) | 178 (63.1%) | 333 (64.2%) | |
| No | 82 (34.6%) | 104 (36.9%) | 186 (35.8%) |
SD = standard deviation
Table 2.
Patients Characteristics – Matched Data
| Open (N=175) |
VATS (N=175) |
Total (N=350) |
p-value | |
|---|---|---|---|---|
| Age | 0.10 | |||
| Mean (SD) | 67.5 (9.6) | 69.3 (9.7) | 68.4 (9.7) | |
| Median | 68.0 | 70.0 | 69.0 | |
| Range | (43–84) | (41–95) | (41–95) | |
| Race | 0.60 | |||
| White | 169 (96.6%) | 166 (94.9%) | 335 (95.7%) | |
| Non-white | 6 (3.4%) | 9 (5.1%) | 15 (4.3%) | |
| Gender | 0.75 | |||
| Male | 88 (50.3%) | 84 (48.0%) | 172 (49.1%) | |
| Female | 87 (49.7%) | 91 (52.0%) | 178 (50.9%) | |
| ECOG Performance Status | 0.64 | |||
| 0 | 128 (73.1%) | 123 (70.3%) | 251 (71.7%) | |
| 1/2/3 | 47 (26.9%) | 52 (29.7%) | 99 (28.3%) | |
| Histology | 0.86 | |||
| Adenocarcinoma | 85 (48.6%) | 82 (46.9%) | 167 (47.7%) | |
| Squamous cell carcinoma | 57 (32.6%) | 62 (35.4%) | 119 (34%) | |
| Others | 33 (18.9%) | 31 (17.7%) | 64 (18.3%) | |
| Pathologic Stage | 0.79 | |||
| I | 139 (79.4%) | 142 (81.1%) | 281 (80.3%) | |
| II | 36 (20.6%) | 33 (18.9%) | 69 (19.7%) | |
| Tumor Size (cm) | 0.30 | |||
| Mean (SD) | 3.4 (1.7) | 3.5 (1.5) | 3.4 (1.6) | |
| Median | 3.0 | 3.1 | 3.0 | |
| Range | (0.9–9.5) | (1.1–8.0) | (0.9–9.5) | |
| Any Medical Co-morbidities | 0.82 | |||
| Yes | 110 (62.9%) | 113 (64.6%) | 223 (63.7%) | |
| No | 65 (37.1%) | 62 (35.4%) | 127 (36.3%) |
SD = standard deviation
Table 3.
Summary of Lymph Nodes (LNs) Sampled
| Open (N=117) |
VATS (N=156) |
Total (N=273) |
||
|---|---|---|---|---|
| Number of LN stations sampled | 0.07 | |||
| Mean (SD) | 2.8 (2.2) | 2.3 (2.3) | 2.6 (2.3) | |
| Median | 3.0 | 2.0 | 3.0 | |
| Range | (0–10) | (0–8) | (0–10) | |
| Total number of LNs sampled | 0.33 | |||
| Mean (SD) | 8.3 (6.7) | 7.4 (5.6) | 7.8 (6.1) | |
| Median | 6 | 5 | 6 | |
| Range | (1.0–40) | (1.0–30) | (1.0–40) |
LNs= Lymph Nodes; SD= standard deviation
Table 4.
Endpoints – Matched Data
| Open (N=175) |
VATS (N=175) |
Total (N=350) |
p value | |
|---|---|---|---|---|
| Length of Hospital Stay | <0.0001 | |||
| Mean (SD) | 8.0 (6.0) | 5.4 (4.7) | 6.7 (5.5) | |
| Median | 6 | 4 | 5 | |
| Range | (3.0–44.0) | (1.0–34.0) | (1.0–44.0) | |
| Number of patients with prolonged hospital stay (>14 days) | 15 (8.6%) | 11 (6.3%) | 26 (7.5%) | <0.0001 |
| Chest tube duration | <0.0001 | |||
| Mean (SD) | 5.0 (2.5) | 3.3 (1.7) | 4.1 (2.3) | |
| Median | 4 | 3 | 4 | |
| Range | (1–19) | (1–11) | (1–19) | |
| Any Surgical Procedure Complication | <0.0001 | |||
| Yes | 44 (25.1%) | 26 (14.9%) | 70 (20%) | |
| No | 131 (74.9%) | 149 (85.1%) | 280 (80%) | |
| Discharge Disposition | <0.0001 | |||
| Home | 158 (90.3%) | 164 (93.7%) | 322 (92%) | |
| Other | 17 (9.7%) | 11 (6.3%) | 28 (8%) |
SD = standard deviation
Figure 1.
Kaplan-Meier Plot of Disease-free Survival from Matched Data
Figure 2.
Kaplan-Meier Plot of Overall Survival from Matched Data
Comment
In our multi-institution study, there was a wide variety of practice patterns in the selection of the surgical approach to lobectomies for NSCLC. Almost half of the institutions performed predominantly VATS or open lobectomies while the other half had a combination of both. A statistically equivalent number of lymph nodes and lymph node stations were sampled in the two groups. However, there was a trend towards more lymph nodes stations being sampled in the open lobectomy group. The length of hospital stay, incidence of peri-operative complications and likelihood of home discharge all favored the VATS lobectomy group over the open lobectomy group. These short-term clinical benefits would be expected to translate to a significant economic benefit. In this era of diminishing health care resources, this would be attractive to patients, clinicians, hospitals and payers alike. The disease-free and overall survival was equivalent between the two groups. Although propensity-matching is a very useful tool in the absence of randomization, it often does not completely eliminate differences between the groups. One of the institutions that performed open lobectomy exclusively was an outlier in terms of survival from all other institutions after controlling for confounding factors. This also contributed to the statistically insignificant difference in survival curves. With this single outlier eliminated, the slight separation in the survival curves was completely eliminated (p-value for overall survival = 0.94).
The variety of procedures that can be performed using minimally invasive thoracic surgical approaches is expanding. Surgeons began using video-assisted thoracoscopic surgery (VATS) to perform lobectomies in the 1990s. An ever-increasing body of literature supports the assertion that VATS lobectomy provides clear ‘quality-of-life’ advantages over open lobectomy for early stage NSCLC patients (3–6). These benefits include less pain, reduced peri-operative complications, improved cosmesis, independent home discharge, and earlier return to pre-hospitalization activities. The major questions about VATS lobectomy have been centered on the oncologic validity and procedural safety of performing major lung cancer resections using minimally invasive techniques. Ideally, a large, multi-institutional, randomized study in both academic and community settings would control for potential confounding variables such as patient selection and answer these questions. However, neither surgeons nor patients would support such a study at this time, given what is currently known about these procedures. The single institution case series, national database studies, and meta-analyses that have addressed these safety and oncologic quality issues have increasingly demonstrated the equivalence or superiority of VATS lobectomy over open lobectomy for peripheral, small to moderate sized lung lesions (4, 16–19). This study was done to add an additional multi-institutional perspective to the literature.
Despite the reported benefits of VATS lobectomy, and extensive efforts to provide training to practicing surgeons on VATS lobectomy techniques, the national adoption rate has been low. Only 32% of lobectomies included in the Society of Thoracic Surgeons (STS) national database in 2006 were performed thoracoscopically (20). The Nationwide Inpatient Sample (NIS) database had only 15% of lobectomies performed by VATS in 2007–2008 (6). The difference in the proportion of VATS cases reported in the STS versus NIS databases likely arises from the fact that the former is a voluntary database that represents a predominance of academic centers while the latter is a non-voluntary database representing a broader spectrum of practice settings. The low VATS lobectomy adoption rate is probably due to the lack of familiarity or confidence with the VATS lobectomy technique amongst thoracic surgeons who did not receive this training during their residency. A transition from open to VATS lobectomy requires concerted effort, time and financial expense. As evidence mounts favoring VATS over lobectomy via thoracotomy, more surgeons may invest the time and effort to learn the procedure and incorporate it into their practices. Thoracic surgeons have traditionally emphasized high-quality patient care. Thus, we have an individual and collective obligation to incorporate evidence-based improvements in surgical techniques into our practices. We believe that this study adds to the mounting evidence that VATS lobectomy should become the most common surgical approach to the management of early stage NSCLC in the United States and wherever the resources are available to offer this specialized technique to patients. The lack of randomization in our study lends itself to patient selection bias. This was indeed illustrated in the imbalance in patient characteristics in the unmatched groups in Table 1. A 1:1 propensity-score based matching algorithm was used in an attempt to offset this inherent selection bias. This study was also conducted as an ancillary analysis of a lung cancer tissue banking study. Thus, the data was not originally collected for the purpose of this type of comparison and supplemental information was obtained from the study sites to meet our endpoints. This resulted in missing information in a sizeable number of patients. For instance, lymph nodes sampling forms were submitted on only 273 of 350 patients −117(69%) Open & 156(89%) VATS. This information was collected retrospectively so the lymph node sampling data is incomplete. More patients may have undergone systematic lymph node sampling or dissection, but we used only the cases for which documentation was available for table 3. Greater attention to lymph node dissection or sampling in general still seems to be necessary. Pulmonary function test results were not available for the patients. The voluntary nature of the enrolment of patients in the tissue banking study also resulted in a partial representation of the cases from each institution. The different surgical preferences and post-operative clinical algorithms employed in the various institutions could also have impacted our results. A small number of institutions contributed a large number of patients and this could serve as a source of bias in the results. About half of the cases were performed in institutions with a strong preference for VATS or open lobectomies. This is a reflection of the training and experience of the specific surgeons involved in this study and is consistent with the determinants of what approach is selected for patients in clinical practice.
In conclusion, this multi-institutional study supports the assertion that thoracoscopic lobectomy results in shorter length of hospital stay, fewer peri-operative complications and greater likelihood of independent home discharge compared to open lobectomy for early stage NSCLC. Disease-free and overall survival was comparable between the VATS and open lobectomy groups.
Acknowledgments
The research for CALGB 31001 and 140202 (Alliance) was supported, in part, by grants from the National Cancer Institute (CA31946) to the Alliance for Clinical Trials in Oncology (Monica M. Bertagnolli, M.D., Chair) and to the Alliance Statistics and Data Center (Daniel J. Sargent, Ph.D., CA33601). The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Presented at the 50th Annual Meeting of the Society of Thoracic Surgeons, Orlando, FL, January 25–29, 2014
References
- 1.National Lung Screening Trial Research Team. Aberle DR, Adams AM, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med. 2011;365:395–409. doi: 10.1056/NEJMoa1102873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Whitson BA, Groth SS, Duval SJ, Swanson SJ, Maddaus MA. Surgery for early-stage non-small cell lung cancer: A systematic review of the video-assisted thoracoscopic surgery versus thoracotomy approaches to lobectomy. Ann Thorac Surg. 2008;86 doi: 10.1016/j.athoracsur.2008.07.009. 2008, 16; discussion 2016-8. [DOI] [PubMed] [Google Scholar]
- 3.Villamizar NR, Darrabie MD, Burfeind WR, et al. Thoracoscopic lobectomy is associated with lower morbidity compared with thoracotomy. J Thorac Cardiovasc Surg. 2009;138:419–25. doi: 10.1016/j.jtcvs.2009.04.026. [DOI] [PubMed] [Google Scholar]
- 4.Cai YX, Fu XN, Xu QZ, Sun W, Zhang N. Thoracoscopic lobectomy versus open lobectomy in stage I non-small cell lung cancer: A meta-analysis. PLoS One. 2013;8:e82366. doi: 10.1371/journal.pone.0082366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jeon JH, Kang CH, Kim HS, et al. Video-assisted thoracoscopic lobectomy in non-small-cell lung cancer patients with chronic obstructive pulmonary disease is associated with lower pulmonary complications than open lobectomy: A propensity score-matched analysis. Eur J Cardiothorac Surg. 2013 doi: 10.1093/ejcts/ezt460. [DOI] [PubMed] [Google Scholar]
- 6.Paul S, Sedrakyan A, Chiu YL, et al. Outcomes after lobectomy using thoracoscopy vs thoracotomy: A comparative effectiveness analysis utilizing the nationwide inpatient sample database. Eur J Cardiothorac Surg. 2013;43:813–7. doi: 10.1093/ejcts/ezs428. [DOI] [PubMed] [Google Scholar]
- 7.Demmy TL, Nwogu C. Is video-assisted thoracic surgery lobectomy better? quality of life considerations. Ann Thorac Surg. 2008;85:S719–28. doi: 10.1016/j.athoracsur.2007.09.056. [DOI] [PubMed] [Google Scholar]
- 8.Casali G, Walker WS. Video-assisted thoracic surgery lobectomy: Can we afford it? Eur J Cardiothorac Surg. 2009;35:423–8. doi: 10.1016/j.ejcts.2008.11.008. [DOI] [PubMed] [Google Scholar]
- 9.Swanson SJ, Meyers BF, Gunnarsson CL, et al. Video-assisted thoracoscopic lobectomy is less costly and morbid than open lobectomy: A retrospective multiinstitutional database analysis. Ann Thorac Surg. 2012;93:1027–32. doi: 10.1016/j.athoracsur.2011.06.007. [DOI] [PubMed] [Google Scholar]
- 10.Whitson BA, D'Cunha J, Maddaus MA. Minimally invasive cancer surgery improves patient survival rates through less perioperative immunosuppression. Med Hypotheses. 2007;68:1328–32. doi: 10.1016/j.mehy.2006.09.063. [DOI] [PubMed] [Google Scholar]
- 11.Swanson SJ, Herndon JE, 2nd, D'Amico TA, et al. Video-assisted thoracic surgery lobectomy: Report of CALGB 39802--a prospective, multi-institution feasibility study. J Clin Oncol. 2007;25:4993–7. doi: 10.1200/JCO.2007.12.6649. [DOI] [PubMed] [Google Scholar]
- 12.Rosenbaum PR, Rubin DB. The central role of the propensity score in observational studies for causal effects. Biometrika. 1983;70:41–55. [Google Scholar]
- 13.Parson LS. Reducing bias in a propensity score matched-pair sample using greedy matching techniques. 2001:214–6. [Google Scholar]
- 14.Zeger SL, Liang KY. Longitudinal data analysis for discrete and continuous outcomes. Biometrics. 1986;42:121–30. [PubMed] [Google Scholar]
- 15.Wei LJ, Lin DY, Weissfeld L. Regression analysis of multvariate incomplete failure time data by using the marginal distributions. Journal of American Statistical Association. 1989;84:1065–73. [Google Scholar]
- 16.Su S, Scott WJ, Allen MS, et al. Patterns of survival and recurrence after surgical treatment of early stage non-small cell lung carcinoma in the ACOSOG Z0030 (ALLIANCE) trial. J Thorac Cardiovasc Surg. 2014;147:747–53. doi: 10.1016/j.jtcvs.2013.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cao C, Zhu ZH, Yan TD, et al. Video-assisted thoracic surgery versus open thoracotomy for non-small-cell lung cancer: A propensity score analysis based on a multi-institutional registry. Eur J Cardiothorac Surg. 2013;44:849–54. doi: 10.1093/ejcts/ezt406. [DOI] [PubMed] [Google Scholar]
- 18.Lee PC, Nasar A, Port JL, et al. Long-term survival after lobectomy for non-small cell lung cancer by video-assisted thoracic surgery versus thoracotomy. Ann Thorac Surg. 2013;96:951. doi: 10.1016/j.athoracsur.2013.04.104. 60; discussion 960-1. [DOI] [PubMed] [Google Scholar]
- 19.Hanna WC, de Valence M, Atenafu EG, et al. Is video-assisted lobectomy for non-small-cell lung cancer oncologically equivalent to open lobectomy? Eur J Cardiothorac Surg. 2013;43:1121–5. doi: 10.1093/ejcts/ezs623. [DOI] [PubMed] [Google Scholar]
- 20.Boffa DJ, Allen MS, Grab JD, Gaissert HA, Harpole DH, Wright CD. Data from the society of thoracic surgeons general thoracic surgery database: The surgical management of primary lung tumors. J Thorac Cardiovasc Surg. 2008;135:247–54. doi: 10.1016/j.jtcvs.2007.07.060. [DOI] [PubMed] [Google Scholar]