Abstract
Background
The non-small cell lung cancer (NSCLC) TNM classification system uses only the anatomic extent of lymph node (LN) metastases to define the N category. The number of LNs resected and the ratio of positive LNs to total examined LNs are prognostic in other solid tumors. We used the Surveillance, Epidemiology and End Results (SEER) database to investigate the impact of these parameters on the overall survival of NSCLC.
Methods
All patients with NSCLC in the SEER database from 1988–2007 who had curative resections and had at least one lymph node examined were included. The prognostic value of age, race, sex, tumor size, histologic grade, number of examined LNs and ratio of positive LNs to total examined nodes was assessed using a multivariate Cox proportional hazards model for overall survival. The number of nodes examined was categorized into four levels. The percent LN positive was stratified into three levels.
Results
Among patients with localized disease, fewer nodes examined corresponded with a worse prognosis. Prognosis improved as more LNs were examined. For patients with regional disease, the differences were significant only at the extremes. Older patients, males and those with higher grade or larger tumors did worse. Patients with low or moderate ratios of positive to total LNs had better prognoses than those with high ratios.
Conclusions
More LNs resected and lower ratios of positive LNs to total examined LNs are associated with better patient survival after NSCLC resection independent of age, sex, grade, tumor size and stage of disease.
Keywords: Lung cancer, lymph node metastases
Introduction
Lung cancer is the leading cause of cancer death in the United States accounting for 157,000 deaths annually (1). Non-small cell lung cancer (NSCLC) comprises about 80% of all cases. Unfortunately, only 20% of patients present with potentially surgically curable loco-regional disease (2). For these patients, lymph node metastasis is the most important prognostic factor. Survival is also influenced by age, sex, socioeconomic status, tumor size, histology, tumor grade and type of treatment (3).
In the current lung cancer tumor-node-metastasis (TNM) staging system, the anatomic extent of lymph node metastases is the only factor used to define the N category of TNM (4). However, the TNM classification system for breast, gastric, and colorectal cancer has been updated from the traditional system to include number of metastatic lymph nodes (MLNs) in the N staging. In these cancers, the number of MLNs has been shown to be a more effective prognostic indicator than the anatomic location of MLNs (5). It has been suggested that the ratio of metastatic lymph nodes to total number of lymph nodes examined (lymph node ratio - LNR) in breast, bladder, gastric, colon and rectal cancers is a better prognostic indicator than the number of MLNs (6–10). For NSCLC, it has been reported that the number of MLNs can give a better N category prognosis than the anatomic location of metastatic lymph nodes, which is currently used (11).
Therefore, we used the Surveillance, Epidemiology and End Results (SEER) database to explore the prognostic value of the number of lymph nodes examined (LNE) and the ratio of metastatic lymph nodes to total number of lymph nodes examined (LNR). Our hypothesis was that a higher number of lymph nodes examined and a lower lymph node ratio would both be associated with better overall survival and disease specific survival in all stages of resectable NSCLC.
Material and Methods
Population-based data were obtained from the SEER program. Data on resected lung cancer cases were obtained from the SEER 9 registry for the years 1988–1992 and from the SEER 13 registry for the years 1993–2007. 1988 was selected because the extent of lymph node evaluation was not uniformly available in this database until then. The details about the data collection and database are provided in the National Cancer Institute SEER Cancer Statistics Review. Because we used existing data without individual subject identification, our institutional review board waived individual informed consent. The lung cancers included ICD codes C33.0 through C34.9 and C39.0 through C 39.9. Small cell lung cancers were excluded. The study sample was restricted to patients undergoing curative resections (lobectomy, bilobectomy and pneumonectomy) who had at least one lymph node examined. This included patients with both localized and regional disease (Stages I, II and III). Localized disease was defined as a single tumor confined to the lung. Regional disease included disease that extended to the chest wall, diaphragm, and mediastinal structures or involved ipsilateral regional lymph nodes. It also included separate tumor nodules in the same lobe. American Joint Committee on Cancer (AJCC) tumor-node-metastasis (TNM) staging information was available for patients diagnosed in 2004 or later. Patients who received radiation therapy were excluded because such treatment may have been administered in the adjuvant setting as a result of positive or close surgical margins.
Based on the distribution of patients in our cohort (quartiles), the number of lymph nodes examined was categorized into four groups: 1–3, 4–6, 7–9 and 10 or more. The percent LN positive was stratified into three levels: Low: 0.01% to 24%, Moderate: 25% to 49% and High: 50% or higher. This was calculated simply by dividing the number of lymph nodes that were reported as positive in the database by the total number of lymph nodes examined. These are similar to the groups used in other studies in the literature (12–16).
Prognostic value of a given variable (either the number of LN examined or the percentage of LN that were positive) was assessed using the associated hazard ratio and 95% confidence interval from a multivariate Cox proportional hazards model for overall and disease specific survival. Potential confounding variables associated with survival were included in the model to demonstrate that the prognostic effect persists after accounting for the effect of these variables. These included age, race, sex, tumor size and histologic grade of the tumor. Proportional hazard results were supplemented with Kaplan Meier survival curves.
Results
25,887 patients met the eligibility criteria; 15,978 had localized disease while 9,909 had regional disease. Demographic, surgical treatment and histopathologic characteristics of the entire cohort (including distribution of the lymph node categories) are listed in Table 1. The mean tumor size was 34 mm. The median follow-up time for the entire cohort was 48 months. A small proportion of the whole cohort (3,568 patients) had TNM staging information and their median follow-up time was 20 months.
Table 1.
Clinical and Pathologic Patient Characteristics
| Variables | No. of Patients (%) | |
|---|---|---|
| Overall Cohort | N | 25,887 (100) |
| Age | ≤ 70 | 16,080 (62.1) |
| >70 | 9,807 (37.9) | |
| Race | White | 22,210 (85.8) |
| Black | 2,029 (7.8) | |
| Other | 1,648 (6.4) | |
| Gender | Male | 13,883 (53.6) |
| Female | 12,004 (46.4) | |
| Stage | Localized | 15,978 (61.7) |
| Regional | 9,909 (38.3) | |
| Grade | I | 3,335 (12.9) |
| II | 10,359 (40.0) | |
| III | 10,759 (41.6) | |
| IV | 1,434 (5.5) | |
| Histology | Squamous Cell Carcinoma | 7,701 (29.8) |
| Bronchiolo-alveolar Carcinoma | 2,596 (10.1) | |
| Adenocarcinoma | 11,254 (43.6) | |
| Other | 4,252 (16.5) | |
| Surgery | Lobectomy | 24,521 (95.1) |
| Pneumonectomy | 1,277 (4.9) | |
| Nodal stage (where available) | N0 | 2,891 (81.6) |
| N1 | 531 (15.0) | |
| N2 | 120 (3.4) | |
| Nodes Examined | 1–3 | 6,764 (26.1) |
| 4–6 | 7,144 (27.6) | |
| 7–9 | 4,782 (18.5) | |
| 10=< | 7,197 (27.8) | |
| Node Positivity Ratio | 0.01% to 24% | 2355 (47) |
| 25% to 49% | 1231 (24.6) | |
| 50% or higher | 1426 (28.4) | |
| Survival Status | Alive | 10,661 (41.2) |
| Dead | 15,226 (58.8) | |
| Follow-up (months) | Median (Min/Max) | 48.00 (0.00/239.00) |
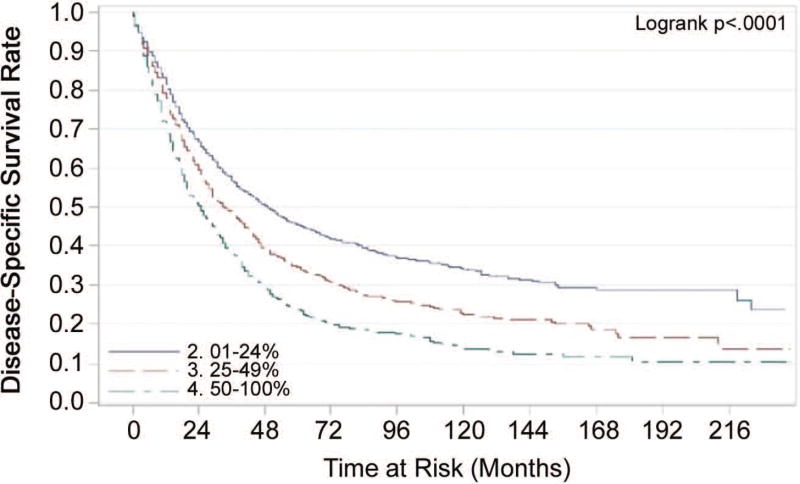
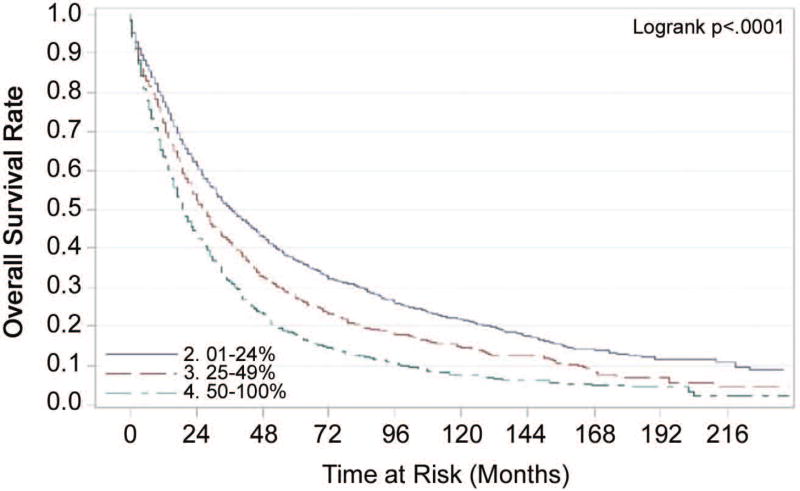
The number of lymph nodes examined (LNE) had greater prognostic value for disease specific and overall survival in patients with localized disease than in those with regional disease (Tables 2 and 3). Fewer lymph nodes examined corresponded with a worse prognosis. The median number of nodes examined was six. Prognosis improved as more lymph nodes were examined. In patients with positive lymph nodes, the ratio of metastatic lymph nodes to total number of lymph nodes examined (LNR) was associated with disease specific and overall survival. Patients with low (0.01% to 24%) or moderate (25% to 49%) ratios of positive to total LNs had better prognoses than those with high (50% or higher) ratios (Figures 1 and 2). This statistically significant association between LNR and overall survival was maintained even when unadjusted Kaplan Meier curves were analyzed for the different ‘lymph node examined’ groupings (1–3, 4–7, 7–9 and 10+).
Table 2.
Cox proportional hazards model for overall survival
| Localized Disease (n=15,978) HR (95% CI) |
p-value | Regional Disease (n=9,909) HR (95% CI) |
p-value | |
|---|---|---|---|---|
| Nodes Examined | ||||
| 1–3 | 1.20 (1.13, 1.27) | p= <.001 | 1.08 (1.01, 1.15) | p= 0.03 |
| 4–6 | 1.09 (1.03, 1.16) | p= 0.004 | 1.06 (0.99, 1.13) | p= 0.08 |
| 7–9 | 1.06 (1.00, 1.14) | p= 0.07 | 1.03 (0.96, 1.10) | p= 0.43 |
| 10 + | 1.0 | 1.0 | ||
| % Nodes Positive | ||||
| 0.01–24% (n=2355) | 0.51 (0.46, 0.55) | p= <.001 | ||
| 25–49% (n=1231) | 0.68 (0.63, 0.75) | p= <.001 | ||
| 50–100% (n=1426) | 1.0 | |||
| Age at Diagnosis (per year) | 1.04 (1.04, 1.04) | p= <.001 | 1.03 (1.02, 1.03) | p= <.001 |
| Race | ||||
| White | 1.0 | 1.0 | ||
| Black | 1.13 (1.05, 1.23) | p= 0.002 | 1.04 (0.95, 1.14) | p= 0.39 |
| Other | 0.77 (0.70, 0.85) | p= <.001 | 0.95 (0.86, 1.05) | p= 0.29 |
| Sex (Female vs. Male) | 0.70 (0.67, 0.73) | p= <.001 | 0.74 (0.71, 0.78) | p= <.001 |
| Histologic Grade | ||||
| Grade I | 1.0 | 1.0 | ||
| Grade II | 1.32 (1.23, 1.42) | p= <.001 | 1.28 (1.16, 1.41) | p= <.001 |
| Grade III | 1.57 (1.46, 1.68) | p= <.001 | 1.49 (1.36, 1.64) | p= <.001 |
| Grade IV | 1.58 (1.42, 1.76) | p= <.001 | 1.57 (1.39, 1.79) | p= <.001 |
| Tumor size (mm) | 1.00 (1.01, 1.01) | p= <.001 | 1.01 (1.01, 1.01) | p= <.001 |
CI = Confidence Interval
HR = Hazard ratio
Table 3.
Cox proportional hazards model for disease specific survival
| Localized Disease (n=15,978) HR (95% CI) |
p-value | Regional Disease (n=9,909) HR (95% CI) |
p-value | |
|---|---|---|---|---|
| Nodes Examined | ||||
| 1–3 | 1.25 (1.16, 1.35) | p= <.001 | 1.02 (0.94, 1.10) | p= 0.65 |
| 4–6 | 1.11 (1.03, 1.20) | p= 0.01 | 1.06 (0.99, 1.15) | p= 0.10 |
| 7–9 | 1.10 (1.00, 1.20) | p= 0.04 | 1.02 (0.94, 1.11) | p= 0.57 |
| 10 + | 1.0 | 1.0 | ||
| % Nodes Positive | ||||
| 0.01–24% (n=2355) | 0.47 (0.43, 0.52) | p= <.001 | ||
| 25–49% (n=1231) | 0.67 (0.61, 0.74) | p= <.001 | ||
| 50–100% (n=1426) | 1.0 | |||
| Age at Diagnosis (per year) | 1.02 (1.02, 1.03) | p= <.001 | 1.02 (1.01, 1.02) | p= <.001 |
| Race | ||||
| White | 1.0 | 1.0 | ||
| Black | 1.21 (1.10, 1.34) | p= <.001 | 1.03 (0.92, 1.14) | p= 0.64 |
| Other | 0.77 (0.68, 0.88) | p= <.001 | 0.96 (0.85, 1.07 | p= 0.45 |
| Sex (Female vs. Male) | 0.77 (0.73, 0.82) | p= <.001 | 0.82 (0.77, 0.87) | p= <.001 |
| Histologic Grade | ||||
| Grade I | 1.0 | 1.0 | ||
| Grade II | 1.37 (1.24, 1.51) | p= <.001 | 1.30 (1.16, 1.46) | p= <.001 |
| Grade III | 1.72 (1.56, 1.89) | p= <.001 | 1.62 (1.44, 1.82) | p= <.001 |
| Grade IV | 1.82 (1.58, 2.09) | p= <.001 | 1.57 (1.39, 1.79) | p= <.001 |
| Tumor size (mm) | 1.00 (1.00, 1.00) | p= 0.009 | 1.00 (1.00, 1.01) | p= <.001 |
CI = Confidence Interval
HR = Hazard ratio
Figure 1.
Unadjusted Kaplan-Meier Estimates
Figure 2.
Unadjusted Kaplan-Meier Estimates
In the subset of patients with AJCC nodal staging information, the number of lymph nodes examined was not prognostic, but LNR was prognostic for both disease specific and overall survival in patients with N1 and N2 disease (Table 4).
Table 4.
Cox proportional hazards model for disease specific and overall survival in patients with AJCC nodal staging data
| HR (95% CI) for Disease Specific Survival |
p-value | HR (95% CI) for Overall Survival |
p-value | |
|---|---|---|---|---|
| Nodes Examined | ||||
| 1–3 | 0.94 (0.74, 1.18) | p= 0.577 | 1.02 (0.83, 1.25) | p= 0.838 |
| 4–6 | 0.85 (0.66, 1.08) | p= 0.181 | 0.91 (0.73, 1.12) | p= 0.364 |
| 7–9 | 0.79 (0.60, 1.05) | p= 0.104 | 0.83 (0.65, 1.05) | p= 0.125 |
| 10 + | 1.0 | 1.0 | ||
| % Nodes Positive | ||||
| 0.01–24% | 0.38 (0.26, 0.56) | p= <.001 | 0.42 (0.29, 0.60) | p= <.001 |
| 25–49% | 0.56 (0.38, 0.82) | p= 0.003 | 0.58 (0.41, 0.82) | p= 0.002 |
| 50–100% | 1.0 | 1.0 | ||
| Age at Diagnosis (per year) | 1.03 (1.02, 1.03) | p= <.001 | 1.03 (1.02, 1.04) | p= <.001 |
| Race | ||||
| White | 1.0 | 1.0 | ||
| Black | 1.05 (0.74, 1.50) | p= 0.780 | 1.01 (0.74, 1.36) | p= 0.973 |
| Other | 0.91 (0.61, 1.37) | p= 0.657 | 0.80 (0.55, 1.14) | p= 0.215 |
| Sex (Female vs. Male) | 0.69 (0.57, 0.83) | p= <.001 | 0.66 (0.56, 0.77) | p= <.001 |
| Histologic Grade | ||||
| Grade I | 1.0 | 1.0 | ||
| Grade II | 1.18 (0.87, 1.60) | p= 0.284 | 1.18 (0.92, 1.52) | p= 0.192 |
| Grade III | 1.83 (1.36, 2.46) | p= <.001 | 1.69 (1.32, 2.17) | p= <.001 |
| Grade IV | 1.59 (0.97, 2.62) | p= 0.066 | 1.78 (1.19, 2.65) | p= 0.005 |
| Tumor size (mm) | 1.01 (1.00, 1.01) | p= <.001 | 1.01 (1.01, 1.01) | p= <.001 |
The odds of having at least one malignant LN increased with the number of lymph nodes examined (LNE). Compared to patients with 1–3 LNE, the odds ratio for 4–6, 7–9 and 10 or more LNE were 1.57 (1.42, 1.73), 2.02 (1.82, 2.23), and 2.81 (2.57, 3.07), respectively. The p-values were all <0.001.
Younger age, lower grade disease, smaller tumor size and female sex were associated with better disease specific and overall survival (Tables 2, 3 and 4). Race was not a consistent independent predictor of survival.
Comment
In this study, we analyzed data from the Surveillance, Epidemiology and End Results (SEER) database to determine the influence of number of lymph nodes examined and the ratio of metastatic to total resected lymph nodes on the survival of all patients with resectable NSCLC. The case ascertainment rate of the SEER registries has been reported to be 97.5% and it is felt to accurately represent the entire American population (17). SEER currently collects and publishes cancer incidence and survival data from population-based cancer registries covering approximately 28 percent of the US population (18). It has been shown that the number of lymph nodes evaluated following resection for Stage I NSCLC is associated with patient survival (13, 17, 19). This study examined this association in both localized and regional disease (Stages I to III). We also sought to corroborate the findings of others about the prognostic value of lymph node ratio in resectable NSCLC patients (11).
The extent of lymphadenectomy has remained controversial for quite some time, but at a minimum, systematic lymph node sampling is considered vital for adequate staging (17, 20). Unfortunately, a large number of patients are inadequately staged (19, 21). This may negatively impact survival by depriving understaged patients of the potential benefits of adjuvant therapy. The National Comprehensive Cancer Network® Guidelines Version 2.2012 for treatment of Non-small Cell Lung cancer includes recommendations for the sampling of at least three N2 stations or complete mediastinal lymph node dissection. Formal ipsilateral mediastinal lymph node dissection for patients undergoing resection for stage IIIA (N2) disease is also recommended (22).
Our study results support the hypothesis that a lower number of lymph nodes examined and a higher ratio of metastatic to total lymph nodes is associated with poorer overall survival from non-small cell lung cancer. There are several possible explanations for our findings. Firstly, stage migration can certainly occur as more lymph nodes are harvested and pathologically examined, resulting in improved staging accuracy. Some patients who would have otherwise been erroneously included among stage I patients are upstaged (12).The patients that remain in stage I would then have better survival figures. Conversely, patients that migrate to stages II and III with less burden of disease improve the survival for those stages. Secondly, there is the possibility that a more robust immunologic response in the regional lymph nodes may result in both greater ease of identification/examination of these nodes and improved survival of such patients (23). Thirdly, there is the potential for therapeutic benefit of systematic lymphadenectomy in a subset of patients that have minimal disease in the lymph nodes without systemic disease.
The favorable impact of younger age, lower grade disease, smaller tumor size and female sex is consistent with established knowledge (3). There were some unexpected findings from our study. The number of lymph nodes examined (LNE) was more prognostic in patients with localized disease than in those with regional disease. This suggests that the differences in survival may be more attributable to stage migration rather than to a therapeutic effect. As more lymph nodes are examined in patients with localized disease, the staging accuracy improves, but once metastatic lymph nodes are detected, the benefit of examining more nodes could be diminished. Lymph node ratio (LNR) was consistently prognostic for overall and disease-specific survival, even in the small subset of patients with TNM staging information. LNR may thus be more attractive than LNE as a new variable to include in the next revision of the staging system. Only 14% of the cohort had TNM staging information because this was not required for SEER database entry until 2004. The number of lymph nodes examined (LNE) was not prognostic in this group of patients. However, the median follow up for these patients was only 20 months. Sufficient maturity of the data with longer follow-up may be necessary to permit adequate assessment of the value of LNE.
A major strength of SEER data is that the large sample size allows the detection of moderate associations and permits complex multivariate analysis (13). It is also more generalizable to the community but it lacks granular detail such as smoking history, performance status, pre-operative staging methods (such as PET scans), lymph node level dissected, completeness of resection (R0, R1 or R2), lymphatic and/or vascular invasion, recurrence patterns and the use of chemotherapy (17). Also, the database does not differentiate between intact lymph nodes and nodal fragments. Thus, the number of lymph nodes examined may have been overestimated in our study. As reported by Ludwig et al (13), if such misclassification is random (i.e. not related to survival), this would bias the results toward null. Thus, the true association may be somewhat stronger than what we reported in our study.
The American College of Surgeons Oncology Group (ACOSOG) conducted a large, prospective randomized multicenter study of N0 and non-hilar N1 resectable lung cancer patients (Z0030) (24). One group had systematic lymph node sampling while the other had complete lymphadenectomy. For this subset of patients, there was no difference in survival between the two groups. However, it is must be emphasized that systematic lymph node sampling entails rigorous identification, resection and examination of several nodes from a combination of hilar and mediastinal lymph node stations. This ACOSOG study cannot be used as justification to remove an insufficient number of lymph nodes, which would compromise accurate staging of the disease.
As the International Association for the Study of Lung Cancer (IASLC) continues to collect prospective data beyond the set used for the recent update in the Lung cancer staging system (25), it may become apparent that the addition of number of resected lymph nodes or the ratio of metastatic to total lymph nodes will improve our prognostication for NSCLC patients. The IASLC database includes patients from several countries and provides more lung cancer specific detail than the SEER database. Thus, it can serve as a great resource to study these lymph node variables further. This may also facilitate the designation of a minimum number of lymph nodes which must be sampled for the lymph node staging to be considered adequate.
In summary, our study shows that lower number of lymph nodes examined and higher metastatic lymph node ratio are both associated with poorer disease specific and overall survival. This emphasizes the need for ongoing education in the broad surgery and pathology communities about the value of adequate lymph node assessment.
Acknowledgments
This work was supported by Roswell Park Cancer Institute and National Cancer Institute (NCI) grant #P30 CA016056.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Presented at the Southern Thoracic Surgical Association Annual Meeting, San Antonio, TX, November 9 – 12, 2011
References
- 1.Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 2.Park BJ, Rusch VW. Lung cancer workup and staging. In: Sellke FW, del Nido PJ, Swanson SJ, editors. Surgery of the Chest. 7. Philadelphia, PA: Elsevier; 2005. pp. 241–251. [Google Scholar]
- 3.Ou SH, Zell JA, Ziogas A, Anton-Culver H. Prognostic factors for survival of stage I nonsmall cell lung cancer patients : A population-based analysis of 19,702 stage I patients in the california cancer registry from 1989 to 2003. Cancer. 2007;110:1532–41. doi: 10.1002/cncr.22938. [DOI] [PubMed] [Google Scholar]
- 4.Detterbeck FCFCCP, Boffa DJ, Tanoue LTFCCP. The new lung cancer staging system. Chest. 2009;136:260–71. doi: 10.1378/chest.08-0978. [DOI] [PubMed] [Google Scholar]
- 5.Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A, editors. AJCC Cancer Staging Manual. Seventh. New York: Springer; 2010. [Google Scholar]
- 6.Peschaud F, Benoist S, Julie C, et al. The ratio of metastatic to examined lymph nodes is a powerful independent prognostic factor in rectal cancer. Ann Surg. 2008;248:1067–73. doi: 10.1097/SLA.0b013e31818842ec. [DOI] [PubMed] [Google Scholar]
- 7.Marchet A, Mocellin S, Ambrosi A, et al. The ratio between metastatic and examined lymph nodes (N ratio) is an independent prognostic factor in gastric cancer regardless of the type of lymphadenectomy: Results from an italian multicentric study in 1853 patients. Ann Surg. 2007;245:543–52. doi: 10.1097/01.sla.0000250423.43436.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vinh-Hung V, Verschraegen C, Promish DI, et al. Ratios of involved nodes in early breast cancer. Breast Cancer Res. 2004;6:R680–8. doi: 10.1186/bcr934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Herr HW. Superiority of ratio based lymph node staging for bladder cancer. J Urol. 2003;169:943–5. doi: 10.1097/01.ju.0000032474.22093.06. [DOI] [PubMed] [Google Scholar]
- 10.Berger AC, Sigurdson ER, LeVoyer T, et al. Colon cancer survival is associated with decreasing ratio of metastatic to examined lymph nodes. J Clin Oncol. 2005;23:8706–12. doi: 10.1200/JCO.2005.02.8852. [DOI] [PubMed] [Google Scholar]
- 11.Bria E, Milella M, Sperduti I, et al. A novel clinical prognostic score incorporating the number of resected lymph-nodes to predict recurrence and survival in non-small-cell lung cancer. Lung Cancer. 2009;66:365–71. doi: 10.1016/j.lungcan.2009.02.024. [DOI] [PubMed] [Google Scholar]
- 12.Gajra A, Newman N, Gamble GP, Kohman LJ, Graziano SL. Effect of number of lymph nodes sampled on outcome in patients with stage I non-small-cell lung cancer. J Clin Oncol. 2003;21:1029–34. doi: 10.1200/JCO.2003.07.010. [DOI] [PubMed] [Google Scholar]
- 13.Ludwig MS, Goodman M, Miller DL, Johnstone PA. Postoperative survival and the number of lymph nodes sampled during resection of node-negative non-small cell lung cancer. Chest. 2005;128:1545–50. doi: 10.1378/chest.128.3.1545. [DOI] [PubMed] [Google Scholar]
- 14.Fukui T, Mori S, Yokoi K, Mitsudomi T. Significance of the number of positive lymph nodes in resected non-small cell lung cancer. J Thorac Oncol. 2006;1:120–5. [PubMed] [Google Scholar]
- 15.Lee JG, Lee CY, Park IK, et al. Number of metastatic lymph nodes in resected non-small cell lung cancer predicts patient survival. Ann Thorac Surg. 2008;85:211–5. doi: 10.1016/j.athoracsur.2007.08.020. [DOI] [PubMed] [Google Scholar]
- 16.Wisnivesky JP, Arciniega J, Mhango G, Mandeli J, Halm EA. Lymph node ratio as a prognostic factor in elderly patients with pathological N1 non-small cell lung cancer. Thorax. 2011;66:287–93. doi: 10.1136/thx.2010.148601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Varlotto JM, Recht A, Nikolov M, Flickinger JC, Decamp MM. Extent of lymphadenectomy and outcome for patients with stage I nonsmall cell lung cancer. Cancer. 2009;115:851–8. doi: 10.1002/cncr.23985. [DOI] [PubMed] [Google Scholar]
- 18. [Accessed January 11, 2011];Overview of the SEER program. Available at: http://seer.cancer.gov/about/overview.html.
- 19.Osarogiagbon RU, Allen JW, Farooq A, Berry A, O'Brien T. Pathologic lymph node staging practice and stage-predicted survival after resection of lung cancer. Ann Thorac Surg. 2011 doi: 10.1016/j.athoracsur.2010.11.065. [DOI] [PubMed] [Google Scholar]
- 20.Rami-Porta R, Wittekind C, Goldstraw P International Association for the Study of Lung Cancer (IASLC) Staging Committee. Complete resection in lung cancer surgery: Proposed definition. Lung Cancer. 2005;49:25–33. doi: 10.1016/j.lungcan.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 21.Little AG, Rusch VW, Bonner JA, et al. Patterns of surgical care of lung cancer patients. Ann Thorac Surg. 2005;80:2051. doi: 10.1016/j.athoracsur.2005.06.071. 6; discussion 2056. [DOI] [PubMed] [Google Scholar]
- 22.Ettinger DS, Akerley W, Bepler G, et al. Non-small cell lung cancer. J Natl Compr Canc Netw. 2010;8:740–801. doi: 10.6004/jnccn.2010.0056. [DOI] [PubMed] [Google Scholar]
- 23.Takenoyama M, Yasumoto K, Harada M, Sugimachi K, Nomoto K. Antitumor response of regional lymph node lymphocytes in human lung cancer. Cancer Immunol Immunother. 1998;47:213–20. doi: 10.1007/s002620050523. [DOI] [PMC free article] [PubMed] [Google Scholar]