Abstract
Individual differences in temperament are associated with psychopathology in humans. Moreover, the relationship between temperament and anxiety-, depression-, PTSD- and addiction-related behaviors can be modeled in animals. This review will highlight these relationships with a focus on individual differences in the response to stressors, fear conditioning and drugs of abuse using animals that differ in their response to a novel environment. We will discuss behavioral and neurobiological commonalities amongst these behaviors with a focus on the hippocampus and, in particular, growth factors as promising novel targets for therapeutic intervention.
Keywords: anxiety, addiction, PTSD, depression
Introduction
Temperament represents stable features in an individual’s reactivity to the environment and biases towards particular coping strategies following severe stress. Temperament is innate, highly heritable and emerges early in life. Moreover, temperamental tendencies predict the types of psychopathology that an individual might develop. Thus, some individuals gravitate towards novelty, risk and excitement, and they are more prone to “Externalizing Disorders,” such as substance abuse and risk-taking behaviors. At the other extreme are individuals who are timid, avoid novelty and prefer predictable conditions, and they are more prone to “Internalizing Disorders,” such as clinical depression and anxiety disorders. Indeed, recent evidence shows that nearly half of children with a highly inhibited temperament will develop an anxiety disorder, resulting in a heightened risk for depression [1]. Moreover, the quantity and quality of stress experienced may determine whether that individual will develop an “internalizing disorder”, as well as the type of disorder (i.e. major depressive disorder (MDD) or post-traumatic stress disorder (PTSD)). Since temperament is the expression of the brain’s response to the environment, we hypothesize that temperament is what determines the response to stressors and whether the individual will shift to psychopathology or not. Thus, understanding temperament can help understand the mechanisms that might lead to psychopathology.
Animal models are essential to disentangle brain responses related to temperament from those related to adaptation to stressors. Individual variation in stress responsiveness is evident in rodents, and we can exploit these differences to investigate the neural mechanisms underlying natural variation in emotionality and the associated psychopathology. For example, rats that exhibit high levels of locomotor activity in response to a novel environment (HRs) also exhibit low levels of depression- and anxiety-like behaviors; whereas rats with lower levels of locomotor activity in response to a novel environment (LRs) typically exhibit higher levels of anxiety-like behavior [2]. We are not suggesting that rodents exhibit psychopathology per se. However, the locomotor response to a novel environment is an index of temperament, and this is associated with traits such as anxiety-like behavior. These traits can be selected for through genetic breeding, and in doing so, we have found that selectively bred high-responders (bHRs) provide an excellent model of vulnerability to a range of behavioral features of externalizing disorders; whereas the selectively bred low-responders (bLRs) provide a model of vulnerability to the behavioral features of internalizing disorders [3,4]. bHRs are representative of individuals prone to externalizing disorders or suffering from “behavioral disinhibition”. That is, relative to bLRs, bHRs are more impulsive, more aggressive and more likely to exhibit addiction-like behavior [5]. A common theme throughout this review will be how HRs/bHRs and LRs/bLRs respond to stressors, fear conditioning and drugs of abuse. We will focus on the neurobiology of temperament in humans, and stress reactivity in animal models, drawing on parallels in the literature from the past three years.
Behavioral and neurobiological parallels between temperament in humans and stress responsiveness in rodents
The neural circuitry and some of the molecular players underlying features of personality and temperament in humans have emerged in recent years. This circuitry involves neural systems implicated in both reward and stress responsiveness, including the prefrontal cortex and its monoaminergic innervation, the nucleus accumbens, the amygdala and the hippocampus. Genetic studies of personality and temperament in humans have recently identified roles for the opioidergic system, the serotonergic system and the corticotropin-releasing hormone (CRH) system [1,6]. Interestingly, psychosocial stress can result in a blunted dopamine response in the nigrostriatal system [7,8]. A major controller of stress responsiveness is the hippocampus, which responds to threatening stimuli and senses circulating glucocorticoids from the hypothalamic-pituitary-adrenal (HPA) axis. For example, there are individual differences in hippocampal activation in response to threatening stimuli such that individuals with a history of past deprivation or neglect, show increased in activation in response to emotional faces [9]. It is important to note that past neglect and abuse are associated with increased risk for psychopathology in youth [10]. When the emotional faces are threatening in nature, the increased response is an indicator of temperament and by extension associated with increased levels of anxiety and depression.
Animal models can explore the neurobiological bases of individual differences in the response to stressors in greater detail. The recent human findings described above are consistent with findings in rodent models of stress where serotonin and CRH levels in the nucleus accumbens have been related to individual differences in depression-like behavior [11], and blockade of the kappa opioid receptor in the same brain region prevented depression-like behavior [12]. The prefrontal cortex also plays a role in individual differences in response to stressors [13]. Following acute stress, rats with a high GABA to glutamate ratio in the prefrontal cortex also had blunted activation and an exaggerated stress response relative to rats with a low GABA to glutamate ratio.
In contrast to the prefrontal cortex, the hippocampus may be the region that most determines the response to a novel environment, especially early in life. In rats that were selectively bred to differ in their response to a novel environment, the hippocampus showed the most divergence in gene expression between bHRs and bLRs during postnatal brain development [14]. Similarly, the hippocampus was the region with the most synchronized anatomical differences in mice susceptible to stress [15]. In this later study, the volume of the hippocampus was correlated with the volume of the hypothalamus, the cingulate cortex and the ventral tegmental area.
The hippocampus is enriched with glucocorticoid receptors (GR), which bind glucocorticoids (cortisol in humans, corticosterone in rodents) and mediate negative feedback to attenuate the stress response [16]. Thus, when a negative regulator of GR, FK506 binding protein 51 (FKBP51), was knocked out, mice had higher functional GR tone, reduced HPA axis activity, and a blunted response to acute stress [17]. However, GR plays a dual and somewhat contradictory role, not only as a mediator of negative feedback but also as an initial detector of threat and mediator of anxiety [18]. Interestingly, the less anxious HRs also exhibit less GR expression in the hippocampus than LRs. Even though both bred and outbred HRs show elevated levels of circulating glucocorticoids, this does not deter them from exploring novel environments or seeking drugs of abuse. Whether these behaviors are mediated by GR in the hippocampus or other regions deserves further investigation. Perhaps HRs find glucocorticoids more rewarding than LRs, and this leads them to seek out rewarding stimuli [19]? This represents one example of the complex role of molecular players in mediating individual differences in various phases of the stress response-- initial reactivity, duration of the response, and its impact in shaping behavior.
Equally important is the long-term role of stress in shaping emotionality, and the interaction of this modulation with temperamental tendencies. Chronic stress, or chronically elevated levels of glucocorticoids, cause damage to the hippocampus [20]. Yet, chronic mild stress during adolescence in bLRs can promote resilience to depression-like behavior in adulthood [21]. This is in contrast to the high resiliency of bHRs to early life stress and perinatal stress [22,23]. Thus, sensitivity to stress can change over the lifespan and even subtle stressors can switch a phenotype from being prone to depression, to resilient [3]. In mice, genetic deletion of metabotropic glutamate receptor, mGluR2, leads to increased stress susceptibility [24]. This provides an avenue whereby glucocorticoids acting through the mineralocorticoid receptor in the hippocampus can decrease resilience to stress by downregulating mGluR2. This is achieved by epigenetic modifications of histones which regulate the expression of mGluR2. It is possible that early life stress epigenetically modifies the MR promoter, but this is still under investigation.
Growth factors also play a role in individual differences in stress responsiveness. Duclot & Kabbaj [25] have implicated hippocampal brain-derived neurotrophic factor (BDNF) expression in differential responsiveness to social stress in outbred animals. In our own work with selectively bred animals, administration of fibroblast growth factor 2 (FGF2), which we have previously shown to decrease anxiety-like behavior only in LRs [26], increased social interaction following social defeat in both HRs and LRs (see Figure 1). This suggests that HRs can also benefit from early life FGF2 under conditions of severe stress. Thus, BDNF and FGF2 may each impart resilience to stress, but FGF2 can impart resilience to both bHRs and bLRs.
Figure 1. Early life FGF2 is protective of social stress in both bHRs and bLRs.
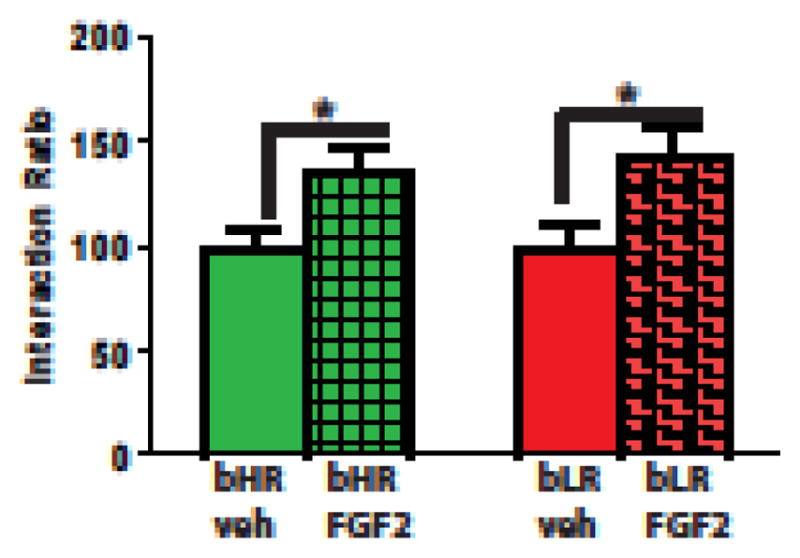
FGF2 was administered (20ng/g, s.c. in 50 μl 0.1M PBS with 0.1% BSA) the day after birth. The animals were tested for social interaction in adulthood following 10 days of repeated social defeat stress. FGF2 increased time in the interaction zone in both bHRs and bLRs compared to vehicle-injected controls. *p < 0.05. This study followed the National Institutes of Health guide for the care and use of laboratory animals.
Relationship between stress responsiveness in rodents and anxiety disorders
As is the case with the response to stressors, individual differences in temperament can shape the neurobiological response to fear and fear memories. Therefore, personality traits such as anxiety can modulate the response to trauma [27]. This is likely because high anxiety can impair discriminatory learning and enable fear overgeneralization [28]. These individual differences are also gender-dependent. Females exhibit greater renewal of fear and PTSD symptoms [27]. Additionally, relative to males, females show greater variations in stress genes that predict brain activation in response to fearful faces [29].
Neuroimaging studies in humans suggest that individual differences in fear conditioning involve the amygdala and its connections with the prefrontal cortex [30]. There is evidence that hyperactivity in the amygdala is associated with the extinction process, particularly in the basolateral and central amygdala [31]. The basolateral amygdala (BLA) can integrate sensory and context information and lead to the expression of fear via central amygdala projections to the periaqueductal gray. Given the bi-directional connections of the basolateral amygdala to the prefrontal cortex and hippocampus, these regions can drive the expression of fear and fear extinction, respectively [32]. The amygdala and prefrontal cortex are also involved in the stress response via their connections with the hippocampus. Moreover, many of the stress-related alterations in the amygdala and prefrontal cortex recapitulate findings in extinction-impaired rodents. Thus, stress sensed by GR in the hippocampus has the ability to decrease fear extinction. Understanding the extinction process following fear conditioning in animal models is necessary to understand PTSD and potential therapeutic interventions.
Differential responsiveness to fear learning and extinction, and its linkage to differences in trait anxiety, can readily be modeled in animals. For example, outbred HR rats extinguish their conditioned fear response faster than LR rats [33]. However, our unpublished studies (Prater et al., unpublished) suggest more dramatic differences between the selectively bred rats that differ in anxiety, with bLRs having reduced extinction learning and retention when compared to outbred animals. In various animal models of anxiety, differences in neurotransmitter systems have been identified following fear conditioning. These studies focused on the serotonin 1a receptor (5HT1A), the GABA-A receptor and the dopamine D1 receptor (DRD1) [34–36]. For example, animals with high anxiety had increased D1 receptor responsivity following fear conditioning in the prefrontal cortex [34]. Differences in GABA-A receptors have also been found between high-anxiety and low-anxiety rats in the prefrontal cortex [37]. Here, high-anxiety rats had reduced GABA-A receptor density. This is consistent with compensation in the GABA system in response to stressors. The HPA axis also plays a significant role in determining susceptibility to trauma. In a rodent model of individual differences in trauma exposure using a rodent-model of PTSD, a recent comprehensive gene expression study found that GR signaling was the most significant pathway identified using upstream transcription factors to predict behavior postexposure, across the hippocampus and amygdala and between sexes [38]. Moreover, corticosterone treatment following the predator-stress-scent paradigm prevented the characteristic behavioral responses.
The role of growth factors in the amygdala is opposite to that seen in the hippocampus. In the hippocampus, growth factors tend to be protective against fear learning, and rats with high context- or cue-induced conditioned fear responses exhibit lower FGF2 levels [39]. Moreover, the difference in FGF2 levels is sustained long after fear conditioning, suggesting differences in hippocampal plasticity and/or function [40]. By contrast, higher levels of growth factors in the basolateral amygdala are associated with reactivity to fear conditioning, as stress susceptible animals had increased BDNF levels [41]. Thus, individual differences in fear processing are not simply due to a common dysregulation in underlying plasticity, as BDNF is upregulated in the amygdala and FGF2 is downregulated in the hippocampus in rats with increased fear conditioning. BDNF is located on neurons, whereas FGF2 is located primarily on astrocytes in the hippocampus. This suggests that the neurotrophic tone in the hippocampus, and hence fear processing, may be driven by neuron-glia interactions. It is possible that growth factor signaling can control both fear learning and fear extinction through different cell types, and this balance can be shifted following stress. Moreover, the neurotrophic tone in the hippocampus may influence the activity of the amygdala through direct connections.
Relationship between stress responsiveness in rodents and risk for addiction
A strong literature has linked traits such as impulsivity and sensation-seeking to drug taking behavior in the clinical population. Studies have related behavioral differences in impulsivity and anxiety to differences in serotonin receptor genotype (5HT2C) and reduced volume or connectivity in the mesolimbic system [42–46]. As with many psychiatric disorders, individual differences in addiction liability are evident in the brain prior to the first experience with a drug of abuse. This predisposition to drug self-administration can be modeled in animals.
The bHRs, which are selected based on their propensity for “sensation-seeking” acquire cocaine self-administration more rapidly than bLRs [47], and the same is true in outbred HR/LR rats [48]. However, when exposed to psychosocial stress, drug-taking behavior is enhanced in the more anxious LRs [48] Thus, this model of temperament instantiates two different paths to drug seeking--- sensation-seeking and stress-induced drug-taking behavior. However, it should be mentioned that our data demonstrating that bRHs are more prone to addiction-like behavior relative to bLRs is discordant with a series of papers suggesting that the locomotor response to novelty trait is not relevant to the transition to addiction or compulsive drug use, but associated only with the initial propensity to take drugs [49,50]. However, it should also be noted that the bHRs are different from outbred rats characterized based on locomotor response to novelty in that the bHRs have been co-selected for a number of traits that are not apparent in outbred HRs. For example, in bHRs, locomotor response to novelty is correlated with measures of impulsive action and the propensity to sign-track, two traits that are not related to “sensation-seeking” in outbred rats. Thus, we have created a line of rats that exhibit a constellation of traits relevant to addiction, rather than a single trait, and this constellation of traits is what likely renders them more susceptible to compulsive drug use.
Beyond the initial likelihood to take drugs, temperamental differences play a role in the conversion to addiction. We have recently shown that following a prolonged cocaine self-administration paradigm, bHRs, but not bLRs, develop addiction-like behaviors, including drug-seeking behavior when the drug is no longer available and a greater propensity for relapse following abstinence [5]. Thus, the a priori knowledge that bHRs are more susceptible to ‘addiction’ than bLRs, allows us to investigate any pre-existing neurobiological differences that are associated with addiction liability. Indeed, we have shown that under “baseline” conditions bHRs have lower levels of dopamine receptor D2 mRNA in the core of the nucleus accumbens, but more D2high receptors, the functionally active state of the receptor. In addition, there are differences in the epigenetic regulation of the D2 receptor under basal conditions, with a greater association of the repressive mark on histones (H3K9me3) at D2 in bHRs relative to bLRs [5]. Interestingly, these phenotypic differences in D2 expression and its epigenetic regulation are no longer apparent following prolonged cocaine self-administration and abstinence. However, the epigenetic regulation of D2 does seem to play a role in susceptibility to reinstatement, or relapse [5].
CRH may be another mediator of individual difference in the response to drugs of abuse. In LR mice, CRH overexpression in the brain was associated with stronger rewarding effects of cocaine [51]. Thus, CRH and D2 receptors may be key players in mediating individual differences in the response to drugs of abuse.
Due to the role of context in drug-seeking behaviors and relapse, the hippocampus has gained increasing attention in addiction research [52,53]. Drug-taking and drug sensitization can be modulated by FGF2 with corresponding changes in hippocampal gene expression [54]. In response to cocaine abstinence, FGF2 expression decreased in the dentate gyrus of bLRs and increased in the nucleus accumbens of bHRs, suggesting that different circuits may be mediating the individual differences [55]. In agreement, neonatal FGF2 in bLRs increased cocaine sensitization and FGF2 expression in the nucleus accumbens compared to bHRs [56]. Given the diverse and widespread effects of FGF2, this growth factor may mediate susceptibility to substance abuse via different pathways in different individuals.
FGF2 is also a target and a trigger of epigenetic mechanisms [57]. Relative to bLRs, bHRs have lower FGF2 expression and increased association of the repressive mark on histones (H3K9me3) at FGF2 in the nucleus accumbens core [5]. Remarkably, and unlike D2, these differences persist long after cocaine self-administration and prolonged abstinence. Thus, low levels of FGF2 may serve as a protective factor against addiction liability. Future studies should assess how epigenetic regulation of these molecules influences the development of temperament.
Conclusions
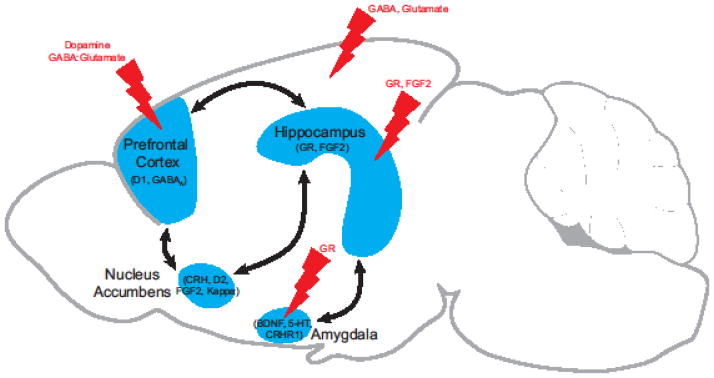
Temperament is a highly stable trait that influences emotional responsiveness including the type of psychopathology that an individual might express. Individual differences in temperament regulate the response to stressors, fear conditioning and drugs of abuse in animals. GR, the serotonergic and the dopaminergic systems are involved in the response to stressors, fear conditioning and drugs of abuse; however, different players are implicated in different regions. The hippocampus is involved in the development of temperament and is known to mediate the response to context, which can drive anxiety, depression, PTSD and addiction. As illustrated in Figure 2, stress can impact different brain regions. These brain regions are interconnected and exhibit differences in gene expression in HR-like and LR-like brains, paving the way for novel therapeutics.
Figure 2. Individual differences in the response to stressors, fear conditioning and drugs of abuse.
The effects of stress on the brains of animals that differ in their response to stressors are illustrated by lightning bolts. The regions are interconnected and can influence the response to fearful stimuli and drugs of abuse. Gene expression differences in the brains of HR-like and LR-like animals are listed below each region.
Highlights.
Temperament in humans can be studied as individual differences in animal models
The hippocampus integrates the brain’s response to stress, fear and drugs of abuse
FGF2 plays a role in anxiety, depression, PTSD and addiction-related behaviors
Acknowledgments
This work was supported by NIMH Grant R01 MH104261 (HA), the Office of Naval Research (ONR) Grants N00014-12-1-0366 (HA), the Hope for Depression Research Foundation (HA) and the Pritzker Neuropsychiatric Disorders Research Consortium Fund LLC (CAT, PB, SJW, HA). SB is supported by the following NIDA Grants: R01-DA-038599; P50-DA-037844 and P01-DA-031656. This work was supported by the Pritzker Neuropsychiatric Disorders Research Fund L.L.C. The authors are members of the Pritzker Neuropsychiatric Disorders Research Consortium, which is supported by the Pritzker Neuropsychiatric Disorders Research Fund L.L.C. A shared intellectual property agreement exists between this philanthropic fund and the University of Michigan, Stanford University, the Weill Medical College of Cornell University, the University of California at Irvine, and the HudsonAlpha Institute for Biotechnology to encourage the development of appropriate findings for research and clinical applications. We would also like to thank Dr. Elyse L. Aurbach for assistance with figure preparation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1*.Clauss JA, Avery SN, Blackford JU. The nature of individual differences in inhibited temperament and risk for psychiatric disease: A review and meta-analysis. Prog Neurobiol. 2015;127–128:23–45. doi: 10.1016/j.pneurobio.2015.03.001. This review is the most recent one to highlight individual differences in temperament and psychiatric risk in humans. The role of inhibited temperament is discussed in terms of neuroimaging and genetic studies. Evidence was found for changes in the fronto-limbic-basal ganglia circuit and point to genes that regulate stress and affect as neural substrates for changes in temperament. Moreover, the hippocampus plays a stronger role than the amygdala in rodent models of inhibited temperament. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stead JD, Clinton S, Neal C, Schneider J, Jama A, Miller S, Vazquez DM, Watson SJ, Akil H. Selective breeding for divergence in novelty-seeking traits: heritability and enrichment in spontaneous anxiety-related behaviors. Behav Genet. 2006;36:697–712. doi: 10.1007/s10519-006-9058-7. [DOI] [PubMed] [Google Scholar]
- 3.Aydin C, Frohmader K, Akil H. Revealing a latent variable: individual differences in affective response to repeated injections. Behav Neurosci. 2015;129:679–682. doi: 10.1037/bne0000084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Flagel SB, Waselus M, Clinton SM, Watson SJ, Akil H. Antecedents and consequences of drug abuse in rats selectively bred for high and low response to novelty. Neuropharmacology. 2014;76(Pt B):425–436. doi: 10.1016/j.neuropharm.2013.04.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5**.Flagel SB, Chaudhury S, Waselus M, Kelly R, Sewani S, Clinton SM, Thompson RC, Watson SJ, Jr, Akil H. Genetic background and epigenetic modifications in the core of the nucleus accumbens predict addiction-like behavior in a rat model. Proc Natl Acad Sci U S A. 2016;113:E2861–2870. doi: 10.1073/pnas.1520491113. An animal model that differs in temperament was utilized to uncover differences in addiction-related behaviors following prolonged cocaine self-administration. The authors focused on the core of the nucleus accumbens and examined gene expression and epigenetic regulation of DRD2 and FGF2, two key players in substance abuse that are also known to differ in the bHR/bLR animal model. Perhaps most importantly, the epigenetic regulation of DRD2 may underlie relapse, whereas low FGF2 expression and its epigenetic regulation may be prevent addiction-related behavior. Interestingly, the epigenetic findings with FGF2 persisted following prolonged cocaine self-administration and abstinence; whereas the DRD2 findings were no longer evident at this time point. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lovallo WR, Enoch MA, Acheson A, Cohoon AJ, Sorocco KH, Hodgkinson CA, Vincent AS, Glahn DC, Goldman D. Cortisol Stress Response in Men and Women Modulated Differentially by the Mu-Opioid Receptor Gene Polymorphism OPRM1 A118G. Neuropsychopharmacology. 2015;40:2546–2554. doi: 10.1038/npp.2015.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mocan O, Stanciu O, Visu-Petra L. Relating individual differences in internalizing symptoms to emotional attention set-shifting in children. Anxiety Stress Coping. 2014;27:509–526. doi: 10.1080/10615806.2014.888419. [DOI] [PubMed] [Google Scholar]
- 8.Suridjan I, Boileau I, Bagby M, Rusjan PM, Wilson AA, Houle S, Mizrahi R. Dopamine response to psychosocial stress in humans and its relationship to individual differences in personality traits. J Psychiatr Res. 2012;46:890–897. doi: 10.1016/j.jpsychires.2012.03.009. [DOI] [PubMed] [Google Scholar]
- 9.Maheu FS, Dozier M, Guyer AE, Mandell D, Peloso E, Poeth K, Jenness J, Lau JY, Ackerman JP, Pine DS, et al. A preliminary study of medial temporal lobe function in youths with a history of caregiver deprivation and emotional neglect. Cogn Affect Behav Neurosci. 2010;10:34–49. doi: 10.3758/CABN.10.1.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kaufman J, Plotsky PM, Nemeroff CB, Charney DS. Effects of early adverse experiences on brain structure and function: clinical implications. Biol Psychiatry. 2000;48:778–790. doi: 10.1016/s0006-3223(00)00998-7. [DOI] [PubMed] [Google Scholar]
- 11.Sequeira-Cordero A, Mora-Gallegos A, Cuenca-Berger P, Fornaguera-Trias J. Individual differences in the forced swimming test and the effect of environmental enrichment: searching for an interaction. Neuroscience. 2014;265:95–107. doi: 10.1016/j.neuroscience.2014.01.047. [DOI] [PubMed] [Google Scholar]
- 12.Zan GY, Wang Q, Wang YJ, Liu Y, Hang A, Shu XH, Liu JG. Antagonism of kappa opioid receptor in the nucleus accumbens prevents the depressive-like behaviors following prolonged morphine abstinence. Behav Brain Res. 2015;291:334–341. doi: 10.1016/j.bbr.2015.05.053. [DOI] [PubMed] [Google Scholar]
- 13.Drouet JB, Fauvelle F, Maunoir-Regimbal S, Fidier N, Maury R, Peinnequin A, Denis J, Buguet A, Canini F. Differences in prefrontal cortex GABA/glutamate ratio after acute restraint stress in rats are associated with specific behavioral and neurobiological patterns. Neuroscience. 2015;285:155–165. doi: 10.1016/j.neuroscience.2014.10.058. [DOI] [PubMed] [Google Scholar]
- 14.Clinton SM, Stead JD, Miller S, Watson SJ, Akil H. Developmental underpinnings of differences in rodent novelty-seeking and emotional reactivity. European Journal of Neuroscience. 2011;34:994–1005. doi: 10.1111/j.1460-9568.2011.07811.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15*.Anacker C, Scholz J, O’Donnell KJ, Allemang-Grand R, Diorio J, Bagot RC, Nestler EJ, Hen R, Lerch JP, Meaney MJ. Neuroanatomic Differences Associated With Stress Susceptibility and Resilience. Biol Psychiatry. 2016;79:840–849. doi: 10.1016/j.biopsych.2015.08.009. This study is the first of its kind to complete a system level analysis of region volume correlations with stress susceptibility and resilience in an animal model. Using structural magnetic resonance imaging and diffusion tensor imaging, social avoidance was positive and negative correlated with various brain regions following stress. Perhaps most importantly, a role for the hippocampus was supported, as it was significantly correlated with volume changes in the cingulate cortex, the ventral tegmental area and the hypothalamus. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Akil H. Stressed and depressed. Nat Med. 2005;11:116–118. doi: 10.1038/nm0205-116. [DOI] [PubMed] [Google Scholar]
- 17.Hoeijmakers L, Harbich D, Schmid B, Lucassen PJ, Wagner KV, Schmidt MV, Hartmann J. Depletion of FKBP51 in female mice shapes HPA axis activity. PLoS One. 2014;9:e95796. doi: 10.1371/journal.pone.0095796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18**.Turner CA, Watson SJ, Akil H. Fibroblast growth factor 2 sits at the interface of stress and anxiety. Biological Psychiatry. 2016;80:419–421. doi: 10.1016/j.biopsych.2016.07.010. This commentary touches on the interaction between GR and FGF2. There is a complex interplay between these molecules depending on the activation level of the stress axis and the anxiety phenotype of the animal. This interplay allows GR to have different functions as a detector of threat leading to anxiety or as a braking mechanism that limits the deleterious effects of glucocorticoids. This commentary suggests that these two systems may be working simultaneously and coordinately to modulate affect. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Piazza PV, Deroche V, Deminiere JM, Maccari S, Le Moal M, Simon H. Corticosterone in the range of stress-induced levels possesses reinforcing properties: implications for sensation-seeking behaviors. Proc Natl Acad Sci U S A. 1993;90:11738–11742. doi: 10.1073/pnas.90.24.11738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McEwen BS. Glucocorticoids, depression, and mood disorders: structural remodeling in the brain. Metabolism. 2005;54:20–23. doi: 10.1016/j.metabol.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 21.Rana S, Nam H, Glover ME, Akil H, Watson SJ, Clinton SM, Kerman IA. Protective effects of chronic mild stress during adolescence in the low-novelty responder rat. Stress. 2016;19:133–138. doi: 10.3109/10253890.2015.1108304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Clinton SM, Watson SJ, Akil H. High novelty-seeking rats are resilient to negative physiological effects of the early life stress. Stress. 2014;17:97–107. doi: 10.3109/10253890.2013.850670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Glover ME, Pugh PC, Jackson NL, Cohen JL, Fant AD, Akil H, Clinton SM. Early-life exposure to the SSRI paroxetine exacerbates depression-like behavior in anxiety/depression-prone rats. Neuroscience. 2015;284:775–797. doi: 10.1016/j.neuroscience.2014.10.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24**.Nasca C, Bigio B, Zelli D, Nicoletti F, McEwen BS. Mind the gap: glucocorticoids modulate hippocampal glutamate tone underlying individual differences in stress susceptibility. Mol Psychiatry. 2015;20:755–763. doi: 10.1038/mp.2014.96. This study was the first to demonstrate that mGluR2 plays a specific role in susceptibility to stress. Knockout of the receptor yielded increased depression-like behavior. Moreover, susceptible mice following various stressors had decreased levels of mGluR2, especially in the hippocampus. The novel concept is that the mineralocorticoid receptor may mediate the epigenetic control of mGluR2 modeling epigenetic allostasis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Duclot F, Kabbaj M. Individual differences in novelty seeking predict subsequent vulnerability to social defeat through a differential epigenetic regulation of brain-derived neurotrophic factor expression. J Neurosci. 2013;33:11048–11060. doi: 10.1523/JNEUROSCI.0199-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Turner CA, Clinton SM, Thompson RC, Watson SJ, Jr, Akil H. Fibroblast growth factor-2 (FGF2) augmentation early in life alters hippocampal development and rescues the anxiety phenotype in vulnerable animals. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:8021–8025. doi: 10.1073/pnas.1103732108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Holmes A, Singewald N. Individual differences in recovery from traumatic fear. Trends Neurosci. 2013;36:23–31. doi: 10.1016/j.tins.2012.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cohen Kadosh K, Haddad AD, Heathcote LC, Murphy RA, Pine DS, Lau JY. High trait anxiety during adolescence interferes with discriminatory context learning. Neurobiol Learn Mem. 2015;123:50–57. doi: 10.1016/j.nlm.2015.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pagliaccio D, Luby JL, Bogdan R, Agrawal A, Gaffrey MS, Belden AC, Botteron KN, Harms MP, Barch DM. HPA axis genetic variation, pubertal status, and sex interact to predict amygdala and hippocampus responses to negative emotional faces in school-age children. Neuroimage. 2015;109:1–11. doi: 10.1016/j.neuroimage.2015.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Feng P, Feng T, Chen Z, Lei X. Memory consolidation of fear conditioning: bi-stable amygdala connectivity with dorsal anterior cingulate and medial prefrontal cortex. Soc Cogn Affect Neurosci. 2014;9:1730–1737. doi: 10.1093/scan/nst170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Goode TD, Maren S. Animal models of fear relapse. ILAR J. 2014;55:246–258. doi: 10.1093/ilar/ilu008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bouton ME, Westbrook RF, Corcoran KA, Maren S. Contextual and temporal modulation of extinction: behavioral and biological mechanisms. Biol Psychiatry. 2006;60:352–360. doi: 10.1016/j.biopsych.2005.12.015. [DOI] [PubMed] [Google Scholar]
- 33.Duclot F, Perez-Taboada I, Wright KN, Kabbaj M. Prediction of individual differences in fear response by novelty seeking, and disruption of contextual fear memory reconsolidation by ketamine. Neuropharmacology. 2016;109:293–305. doi: 10.1016/j.neuropharm.2016.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.An XL, Zheng XG, Liang J, Bai YJ. Corticosterone combined with intramedial prefrontal cortex infusion of SCH 23390 impairs the strong fear response in high-fear-reactivity rats. Psych J. 2013;2:1–10. doi: 10.1002/pchj.5. [DOI] [PubMed] [Google Scholar]
- 35.Ferreira R, Nobre MJ. Conditioned fear in low- and high-anxious rats is differentially regulated by cortical subcortical and midbrain 5-HT(1A) receptors. Neuroscience. 2014;268:159–168. doi: 10.1016/j.neuroscience.2014.03.005. [DOI] [PubMed] [Google Scholar]
- 36.Wislowska-Stanek A, Lehner M, Skorzewska A, Krzascik P, Maciejak P, Szyndler J, Ziemba A, Plaznik A. Changes in the brain expression of alpha-2 subunits of the GABA-A receptor after chronic restraint stress in low- and high-anxiety rats. Behav Brain Res. 2013;253:337–345. doi: 10.1016/j.bbr.2013.07.042. [DOI] [PubMed] [Google Scholar]
- 37.Skorzewska A, Lehner M, Wislowska-Stanek A, Krzascik P, Ziemba A, Plaznik A. The effect of chronic administration of corticosterone on anxiety- and depression-like behavior and the expression of GABA-A receptor alpha-2 subunits in brain structures of low- and high-anxiety rats. Horm Behav. 2014;65:6–13. doi: 10.1016/j.yhbeh.2013.10.011. [DOI] [PubMed] [Google Scholar]
- 38.Daskalakis NP, Cohen H, Cai G, Buxbaum JD, Yehuda R. Expression profiling associates blood and brain glucocorticoid receptor signaling with trauma-related individual differences in both sexes. Proc Natl Acad Sci U S A. 2014;111:13529–13534. doi: 10.1073/pnas.1401660111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Graham BM, Richardson R. Fibroblast Growth Factor 2 as a New Approach to Fighting Fear. JAMA Psychiatry. 2015;72:959–960. doi: 10.1001/jamapsychiatry.2015.1187. [DOI] [PubMed] [Google Scholar]
- 40.Walters E, Richardson R, Graham BM. Individual differences in conditioned fear expression are associated with enduring differences in endogenous Fibroblast Growth Factor-2 and hippocampal-mediated memory performance. Neurobiol Learn Mem. 2016 doi: 10.1016/j.nlm.2016.07.021. [DOI] [PubMed] [Google Scholar]
- 41.Chou D, Huang CC, Hsu KS. Brain-derived neurotrophic factor in the amygdala mediates susceptibility to fear conditioning. Exp Neurol. 2014;255:19–29. doi: 10.1016/j.expneurol.2014.02.016. [DOI] [PubMed] [Google Scholar]
- 42.Anastasio NC, Liu S, Maili L, Swinford SE, Lane SD, Fox RG, Hamon SC, Nielsen DA, Cunningham KA, Moeller FG. Variation within the serotonin (5-HT) 5-HT(2)C receptor system aligns with vulnerability to cocaine cue reactivity. Transl Psychiatry. 2014;4:e369. doi: 10.1038/tp.2013.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cheetham A, Allen NB, Whittle S, Simmons J, Yucel M, Lubman DI. Volumetric differences in the anterior cingulate cortex prospectively predict alcohol-related problems in adolescence. Psychopharmacology (Berl) 2014;231:1731–1742. doi: 10.1007/s00213-014-3483-8. [DOI] [PubMed] [Google Scholar]
- 44.Kirkpatrick MG, Goldenson NI, Kapadia N, Kahler CW, de Wit H, Swift RM, McGeary JE, Sussman S, Leventhal AM. Emotional traits predict individual differences in amphetamine-induced positive mood in healthy volunteers. Psychopharmacology (Berl) 2016;233:89–97. doi: 10.1007/s00213-015-4091-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mei S, Xu J, Carroll KM, Potenza MN. Self-reported impulsivity is negatively correlated with amygdalar volumes in cocaine dependence. Psychiatry Res. 2015;233:212–217. doi: 10.1016/j.pscychresns.2015.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Motzkin JC, Baskin-Sommers A, Newman JP, Kiehl KA, Koenigs M. Neural correlates of substance abuse: reduced functional connectivity between areas underlying reward and cognitive control. Hum Brain Mapp. 2014;35:4282–4292. doi: 10.1002/hbm.22474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Davis BA, Clinton SM, Akil H, Becker JB. The effects of novelty-seeking phenotypes and sex differences on acquisition of cocaine self-administration in selectively bred High-Responder and Low-Responder rats. Pharmacol Biochem Behav. 2008;90:331–338. doi: 10.1016/j.pbb.2008.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kabbaj M, Norton CS, Kollack-Walker S, Watson SJ, Robinson TE, Akil H. Social defeat alters the acquisition of cocaine self-administration in rats: role of individual differences in cocaine-taking behavior. Psychopharmacology (Berlin) 2001;158:382–387. doi: 10.1007/s002130100918. [DOI] [PubMed] [Google Scholar]
- 49.Deroche-Gamonet V, Piazza PV. Psychobiology of cocaine addiction: Contribution of a multi-symptomatic animal model of loss of control. Neuropharmacology. 2014;76(Pt B):437–449. doi: 10.1016/j.neuropharm.2013.07.014. [DOI] [PubMed] [Google Scholar]
- 50.Molander AC, Mar A, Norbury A, Steventon S, Moreno M, Caprioli D, Theobald DE, Belin D, Everitt BJ, Robbins TW, et al. High impulsivity predicting vulnerability to cocaine addiction in rats: some relationship with novelty preference but not novelty reactivity, anxiety or stress. Psychopharmacology (Berl) 2011;215:721–731. doi: 10.1007/s00213-011-2167-x. [DOI] [PubMed] [Google Scholar]
- 51.Kasahara M, Groenink L, Bijlsma EY, Olivier B, Sarnyai Z. Lifelong, central corticotropin-releasing factor (CRF) overexpression is associated with individual differences in cocaine-induced conditioned place preference. Eur J Pharmacol. 2015;753:151–157. doi: 10.1016/j.ejphar.2014.07.050. [DOI] [PubMed] [Google Scholar]
- 52.Noonan MA, Bulin SE, Fuller DC, Eisch AJ. Reduction of adult hippocampal neurogenesis confers vulnerability in an animal model of cocaine addiction. J Neurosci. 2010;30:304–315. doi: 10.1523/JNEUROSCI.4256-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Noonan MA, Choi KH, Self DW, Eisch AJ. Withdrawal from cocaine self-administration normalizes deficits in proliferation and enhances maturity of adult-generated hippocampal neurons. J Neurosci. 2008;28:2516–2526. doi: 10.1523/JNEUROSCI.4661-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Turner CA, Watson SJ, Akil H. The fibroblast growth factor family: neuromodulation of affective behavior. Neuron. 2012;76:160–174. doi: 10.1016/j.neuron.2012.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Waselus M, Flagel SB, Jedynak JP, Akil H, Robinson TE, Watson SJ., Jr Long-term effects of cocaine experience on neuroplasticity in the nucleus accumbens core of addiction-prone rats. Neuroscience. 2013;248:571–584. doi: 10.1016/j.neuroscience.2013.06.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Clinton SM, Turner CA, Flagel SB, Simpson DN, Watson SJ, Akil H. Neonatal fibroblast growth factor treatment enhances cocaine sensitization. Pharmacol Biochem Behav. 2012;103:6–17. doi: 10.1016/j.pbb.2012.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chaudhury S, Aurbach EL, Sharma V, Blandino P, Jr, Turner CA, Watson SJ, Akil H. FGF2 is a target and a trigger of epigenetic mechanisms associated with differences in emotionality: partnership with H3K9me3. Proc Natl Acad Sci U S A. 2014;111:11834–11839. doi: 10.1073/pnas.1411618111. [DOI] [PMC free article] [PubMed] [Google Scholar]