Abstract
Objectives
Management of the axilla in stage II/III breast cancer undergoing neoadjuvant systemic therapy (NST) is controversial. To understand current patterns of care, we collected axillary data from two NST trials: HER2-positive (CALGB40601) and triple-negative (CALGB40603).
Methods
Axillary evaluation pre-/post-NST was per the treating surgeon and could include sentinel node biopsy (SNB). Post-NST, node-positive patients were recommended to undergo axillary lymph node dissection (ALND). We report: (1) pre-NST histopathologic nodal evaluation, (2) post-NST axillary surgical procedures with correlation to clinical and pathologic nodal status.
Results
742 treated patients, 704 had complete nodal data pre-NST and post-NST. Pre-NST, 422 (60%) of 704 patients underwent at least one procedure for axillary node evaluation, (total of 468 procedures): fine needle aspiration (N=234, 74%-positive), core needle biopsy (N=138, 72%-positive) and SNB (N=96, 33%-positive). Pre-NST, 304 patients were considered node positive. Post-NST, 304 of 704 patients (43%) underwent SNB; 44 were positive and 259 negative (29 and 36 patients, respectively, had subsequent ALND). 391(56%) patients went directly to post-NST ALND and 9 (1%) pre-NST node positive patients had no post-NST axillary procedure. Post-NST, 170 (24%) of the 704 patients had residual axillary disease. Agreement between post-NST clinical and radiologic staging and post-NST histologic staging was strongest for node-negative (81%) and weaker for node-positive (N1 31%, N2 29%) with more than half of the clinically node-positive patients found to be pathologic negative (p<0.001).
Conclusions
Our results suggest there is no widely accepted standard for axillary nodal evaluation pre-NST. Post-NST staging was highly concordant in patients with N0 disease, but poorly so in node-positive disease. Accurate methods are needed to identify post-NST patients without residual axillary disease to potentially spare ALND.
INTRODUCTION
Neoadjuvant systemic therapy (NST) has been used to convert some patients with stage II-III breast cancer initially deemed to require a mastectomy to candidates for breast-conserving therapy (BCT) by downsizing the volume of disease. (1–4) Reducing the size of a breast tumor is often used to justify administration of systemic therapy in the neoadjuvant rather than the adjuvant setting, especially in patients with human epidermal growth factor receptor-2-positive breast cancer (HER2+BC) and triple-negative breast cancer (TNBC) where the need for adjuvant therapy is not in question. Importantly, BCT after NST has been found to have equivalent local, regional and distant recurrence rates and overall survival as compared to BCT performed prior to systemic adjuvant therapy.(1–4)
While many NST trials have shown the ability of this approach to reduce the extent of breast surgery, data on the optimal approach and management of the axilla after NST are unclear. The presence or absence of axillary nodal metastases after NST remains an important prognostic factor to patient long-term outcome. While multiple studies (5–7) have demonstrated that NST can down-stage node-positive axillae to node-negative in up to 40% of patients, the persistence of axillary nodal involvement after NST remains an important prognostic factor. However, the value of pre-NST confirmation of nodal involvement by fine needle aspiration (FNA) or core needle biopsy (CNB), the timing of sentinel node biopsy (SNB) and the role of axillary lymph node dissection (ALND) relative to clinical and pathologic nodal status prior to and following NST remain areas of controversy.
We sought to elucidate patterns of axillary management for patients with HER2+ BC and TNBC enrolled in two large cooperative group prospective randomized trials. To address these questions, we incorporated identical practice-oriented surgical studies into two NST trials: CALGB 40601, a randomized phase III trial which compared lapatinib (L) or the combination of L and trastuzumab (T) to T given in combination with wP (weekly Paclitaxel) in HER2+BC and CALGB 40603, a randomized phase II trial which tested the addition of carboplatin (Cb) and bevacizumab (B) to a standard neoadjuvant chemotherapy regimen wP followed by dose-dense doxorubicin and cyclophosphamide (ddAC) in TNBC. The primary endpoints of these trials was pCR and have been previously been published; CALGB 40601, which included 295 patients, demonstrated a 46–56% pCR rate in the breast whereas 443 patients in 40603 had a 53% pCR rate in the breast.(8, 9) For both studies, there was no study-mandated axillary management. For patients with node-positive disease either pre- or post-NST, level I/II ALND was recommended. We captured pre-NST percutaneous lymph node biopsy data, utilization/timing of SNB and post-NST axillary management. This is a planned analysis of the combined trials with identical data acquisition to identify axillary practice patterns and assess the relationship between clinical and pathologic staging.
METHODS
Patient Eligibility
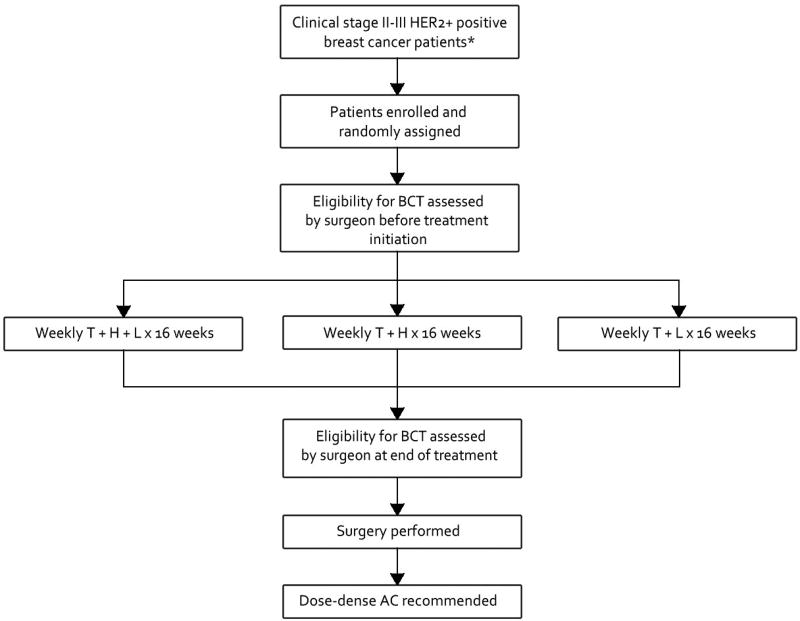
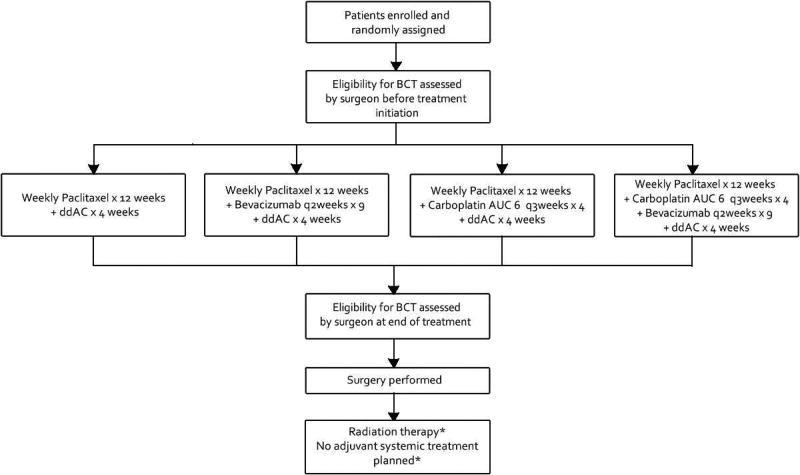
Patients with stage II or III HER2+BC or TNBC patients with operable, biopsy confirmed, previously untreated, non-inflammatory disease were eligible. For CALGB 40601, HER2 positivity was defined as IHC 3+ or fluorescent-in-situ-hybridization (FISH) amplified (ratio ≥2.0) and for CALGB 40603 TNBC was defined as ER and PR expression <10% and HER2 negativity as immunohistochemical (IHC) staining of 0 to 1+ or FISH ratio of < 2.0. The neoadjuvant systemic therapy regimens on both trials have been previously published.(8, 9) The schematics are provided in figures 1 and 2.
Figure 1.
C40601 schema. AC, adjuvant chemotherapy; BCT, breast conserving therapy; T, weekly paclitaxel; H, trastuzumab; L, lapatinib. *Excluding clinical T4d tumors.
Figure 2.
C40603 Schema. AUC – Area under the curve; BCT, breast conserving therapy; ddAC, dose-dense doxorubicin and cyclophosphamide; *Treatment decision made at the discretion of breast oncology team.
Study Procedures
Baseline breast imaging including mammography with or without focused breast ultrasound was required for all patients. Magnetic resonance imaging was suggested, but not mandatory as baseline imaging. In patients with clinically positive axillae, histologic confirmation was encouraged by fine needle aspiration and/or core biopsy. Patients with clinically negative axillae at study entry could undergo a pre- or post-treatment sentinel lymph node (SLN) procedure or ALND after NST; in patients with a percutaneous positive axillae or SLN-positive pre-NST, a complete ALND post-NST was recommended even in patients who were converted to clinically node-negative following NST. Surgical therapy on CALGB 40601 occurred within 6 weeks of the last dose of wP. Surgical therapy on CALGB 40603 occurred 4 to 8 weeks after the last cycle of ddAC, and at least 6 weeks after the last dose of B. Genetic testing for BRCA mutations was not required in either study.
Data Collection and Analysis
Results for the primary clinical study endpoint (pCR) (8, 9) and results for the primary surgical outcome, conversion from pre-NST BCT ineligibility to post-NST BCT eligibility, have been reported previously.(10, 11) A second surgical objective, which is the focus of the current study, was to document axillary management, including the relationship between pre-NST/post-NST clinical staging and pathologic staging in patients with N0, N1 or N2 disease. Possible pre-NST nodal evaluation that was documented included: fine-needle aspiration (positive/negative), core needle biopsy (positive/negative), SNB (positive/negative). A patient could have received more than one pre-NST nodal evaluation modality. Possible post-NST nodal evaluation included: SNB (positive/negative), ALND (positive/negative). Pathologic complete response (pCR) in the axilla was defined as no evidence of hematoxylin and eosin detected metastases in the axillary lymph nodes. Use of immunohistochemical stains was as per institutional standards. Patients with ypN0(i+) were considered node-negative, ypN0.
For all patients on the two trials, we sought to determine the agreement between the pre-NST/post-NST clinical staging and pathology in patients with N0, N1 or N2 disease. As the interest was in documentation, statistics are largely descriptive, in particular using proportions of patients with the total sample as those treated on either of the NST clinical trials with complete surgical information. Comparisons of two or more proportions used the chi square test.
Data were collected and stored at the CALGB (now Alliance) Statistics and Data Center (Durham, NC) with quality ensured through data review performed by the Data Center, the study chairpersons (WMS and LAC), and surgical co-chairs (MG and DWO). Statistical analyses were performed by CALGB statisticians using SAS 9.2 (Cary, NC). The data cutoff for this report was November 2015.
RESULTS
The two trials registered a total of 759 patients between 2009 and 2012. Of these, 742 received NST treatment. A total of 704 of the treated patients (296 from CALGB 40601 and 408 from CALGB 40603) had complete pre and post-NST nodal evaluation and axillary management data available. Patient demographics and tumor characteristics from both studies are displayed in Table 1. Two-thirds of patients (68%) were clinical stage II and 32% were clinical stage III. The median age was 49, 57% were premenopausal, 72% had high-grade disease, and 91% had ductal histology (Table 1). Pre-NST clinical and radiographic nodal status was as follows: N0 311 (44%), N1 277 (39%), N2 50 (7%), NX 66 (9%). All NX patients had pathologic nodal evaluation prior to NST.
Table 1.
Patient and Tumor Characteristics
| Characteristic | 40601 | 40603 | Total |
|---|---|---|---|
| Assessable, N (%) | 296 (100) | 408 (100) | 704 (100) |
| Target population | HER2-positive | Triple negative | |
| Stratification | |||
| Clinical stage, n (%) | |||
| II | 203 (68) | 279 (68) | 482 (68) |
| III | 93 (32) | 129 (32) | 222 (32) |
| Baseline variable | |||
| Patient age, y, n (%) | |||
| 20 to 29 | 6 (2) | 10 (2) | 16 (2) |
| 30 to 39 | 51 (17) | 83 (21) | 134 (19) |
| 40 to 49 | 102 (34) | 124 (30) | 226 (32) |
| 50 to 59 | 84 (28) | 123 (30) | 207 (29) |
| 60 to 69 | 43 (15) | 59 (15) | 102 (15) |
| 70 to 79 | 10 (3) | 9 (2) | 19 (3) |
| Median age, y, (IQR) | 49 (41 – 56) | 49 (40 – 56) | 49 (41 – 56) |
| Minimum age, maximum age, y | 24, 75 | 26, 79 | 24, 79 |
| Menopausal status, n (%) | |||
| Pre-menopausal | 175 (59) | 224 (55) | 399 (57) |
| Post-menopausal | 121 (41) | 184 (45) | 305 (43) |
| Tumor histology, n (%) | |||
| Ductal | 278 (94) | 366 (90) | 644 (91) |
| Lobular | 4 (1) | 3 (1) | 7 (1) |
| Mixed ductal and lobular | 0 (0) | 16 (4) | 16 (2) |
| Invasive, NOS | 7 (2) | 8 (2) | 15 (2) |
| Other | 6 (2) | 11 (3) | 17 (2) |
| Unknown/missing | 1 (<1) | 4 (1) | 5 (1) |
NOS – Not otherwise specified
Prior to NST, 282 (40%) patients had no attempt at axillary pathologic evaluation while 422 (60%) of patients underwent at least one axillary node histopathologic evaluation: 234 fine needle aspiration (FNA) (74% positive), 138 core needle biopsies (72% positive), 96 SNB (33% positive) (Table 2), while 282 (40%) patients had clinical axillary status only. Of the patients who underwent an axillary nodal procedure 118 (17%) had pathologic node-negative axillae and 304 (43%) had pathologic proven node-positive axillae.
Table 2.
Pre-Neoadjuvant Systemic Therapy Axillary Nodal Evaluation
| Histologic method |
Negative result |
Positive result | Total (N=422) |
|---|---|---|---|
| Fine needle aspiration, n (%) | 61 (26) | 173 (74) | 234 (33) |
| Core biopsy, n (%) | 39 (28) | 99 (72) | 138 (20) |
| Sentinel node biopsy, n (%) | 64 (67) | 32 (33) | 96 (14) |
Note: Patients could undergo more than one axillary procedure
Post-NST, no axillary FNA or core needle biopsies were performed. Three hundred and four (43%) patients underwent a post-NST SNB; 44 (14%) were positive, but only 29 (66%) underwent completion ALND despite this additional procedure being recommended by protocol. Two hundred sixty (86%) were negative, of whom 36 (13%) patients underwent a completion ALND. 391 (56%) patients went directly to a post-NST ALND and 9 (1%) pre-NST patients had no post-NST axillary procedure. Overall, post-NST 148 (21%) patients had residual histologically-proven axillary disease.
The agreement between post-NST clinical staging and post-NST histologic staging was strongest for node-negative disease and considerably weaker for node-positive disease (p<0.001). Of patients clinically and/or radiographically N0 post-NST (ycN0), 81% were histologically node-negative (including patients with only isolated tumor cells (ypN0(i+)) post-NST and 19% had pathologic axillary involvement, including 23 (5%) patients with N2 or N3 disease. Conversely, of those patients thought clinically and radiographically to have persistent nodal involvement (ycN1 or ycN2), the majority (54%) were in fact node-negative pathologically, including 3 of 7 patients judged as having ycN2 disease. (Table 3)
Table 3.
Correlation of Post-Neoadjuvant Systemic Therapy Clinical and Radiologic Staging with Pathology
| Post-NST pathology |
Post-NST clinical and radiologic stage | |||
|---|---|---|---|---|
| Not done | N0 | N1 | N2 | |
| N | 129 | 507 | 61 | 7 |
| ypN0, n (%) | 94 (73) | 403 (79) | 34 (56) | 3 (43) |
| ypN0(i+), n (%) | 1 (1) | 12 (2) | 0 (0) | 0 (0) |
| ypN1mic, n (%) | 4 (3) | 8 (2) | 0 (0) | 1 (14) |
| ypN1, n (%) | 18 (14) | 54 (11) | 19 (31) | 0 (0) |
| ypN2, n (%) | 7 (5) | 18 (4) | 5 (8) | 2 (29) |
| ypN3, n (%) | 3 (2) | 5 (1) | 3 (5) | 1 (14) |
| ypNX, n (%) | 5 (2) | 4 (1) | 0 (0) | 0 (0) |
NST, neoadjuvant systemic therapy
DISCUSSION
We present the surgical results for the axillary management of two large randomized prospective NST studies for HER2+ BC and TNBC. When these NST trials were designed, the management of the axilla was largely left to the discretion of the treating surgeon. Surgeons could perform sentinel node biopsy prior to/or after NST. Surgeons were also able to evaluate the axilla prior to NST with axillary FNA or core biopsy. Post-NST ALND was strongly suggested for patients with histologically proven node positive prior to NST, and for patients in whom no pre-NST axillary evaluation was performed. Our results indicate a wide range of practice patterns across a broad spectrum of institutions that constituted CALGB 40601/40603 ranging from quaternary academic centers to private practice community based sites in the United States. Sixty percent of patients had one or more pre-NST axillary procedures performed with highest rate of positivity in those undergoing FNA/core biopsy as opposed to SNB. Of concern, post-NST 43% of women underwent SNB but only 66% of those with a positive sentinel node underwent completion ALND, and conversely 13% with a node negative SNB (12) underwent completion ALND. Collectively, this highlights the wide variation in the management of the axilla in NST patients. While in patients who proceed to surgery without NST, trials such as American College of Surgeons Oncology Group ACOSOG Z0011,(13) International Breast Cancer Study Group (IBCSG)(12) and After Mapping of the Axilla, Radiotherapy or Surgery? (AMAROS) (14) have helped refine the approach to the axilla in those with positive sentinel nodes, our current findings demonstrate that the management of the axilla in those undergoing NST remains highly variable.
Modern management of the axilla for those who were node positive pre-NST are guided by three prospective clinical trials have investigated the accuracy of SNB after NST: The American College of Surgeons Oncology Group (ACOSOG) Z1071 trial,(15) Sentinel lymph node biopsy in patients with breast cancer before and after neoadjuvant chemotherapy (SENTINA)(16) and Sentinel Node Biopsy After Neoadjuvant Chemotherapy in Biopsy-Proven Node-Positive Breast Cancer: The SN FNAC Study.(17) All three trials evaluated whether the SNB accurately predicted the axillary nodal status following NST in women who presented with pathologic confirmed N1/N2 disease. In total, these three trials enrolled 2,591 women who underwent NST. Unfortunately, the false-negative rate for the SN using hematoxylin and eosin stains ranged from 12.6–14.2%, and therefore the use of SNB after NST could not be recommended in this setting. The investigators on these trials have continued to look at subset analyses to see if the false-negative rate can be lowered to acceptable levels. Factors identified include removing ≥3 sentinel nodes,(15) use of dual tracer (15) and/or routine use of immunohistochemical stains (17) and suggests that only within these parameters is this false-negative rate reduced to acceptable levels.
Our current study highlights the discordance between post-NST clinical exam and radiologic studies and the post-NST axillary pathologic examination. Post-NST, 19% of our clinical and radiographic N0 patients had residual axillary disease including 23 patients (5%) with ypN2 or ypN3 disease. Conversely, 54% of our patients with clinical and/or radiographic N1 or N2 nodes post-NST actually had a complete pathologic response in the axilla, ypN0. Others have reported similar findings and currently, radiologic imaging options post-NST do not have the sensitivity and specificity to be deemed acceptable.(18, 19)
Novel post-NST surgical options to the pre-NST N1 or N2 nodes seem to hold some promise. Straver et al.,(20) have investigated pre-NST placement of radioactive seeds in the axillary nodes (MARI Procedure). Caudle et al,(21) have investigated pre-NST clip placement in the pathologic axillary nodes and post-NST targeted axillary dissection (TAD) of the clipped node. These new surgical techniques to the axilla should undergo prospective multi-institutional clinical trials for validation, just as the surgical community did for ACOSOG 1071, SENTINA and SN FNAC.(15–17)
In conclusion, our data demonstrates a wide variation in the surgical community in the management of the axilla in NST setting, both pre- and post-NST. With a potential 40% pCR of the axilla after NST, these are the patients that may benefit from novel imaging and/or surgical axillary approaches to determine who is truly node-negative after NST and avoid the morbidity of an ALND. Until we accurately determine this subgroup, diminishing surgical therapy in those with significant axillary burden post-NST should be approached with caution.
Acknowledgments
Support: This study was supported by Alliance Grant U10CA180821.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure Information: Nothing to disclose.
Disclosures outside the scope of this work: Dr Sikov receives payment as a board member to AbbVie, receives payment for lectures from Elsai, Inc, and receives payment for travel from AstraZeneca.
Presented at the Southern Surgical Association 128th Annual Meeting, Palm Beach, FL, December 2016.
References
- 1.Barry PA, Schiavon G. Primary Systemic Treatment in the Management of Operable Breast Cancer: Best Surgical Approach for Diagnosis, Biological Evaluation, and Research. J Natl Cancer Inst Monogr. 2015 May;2015(51):4–8. doi: 10.1093/jncimonographs/lgv008. [DOI] [PubMed] [Google Scholar]
- 2.Mauri D, Pavlidis N, Ioannidis JP. Neoadjuvant versus adjuvant systemic treatment in breast cancer: a meta-analysis. J Natl Cancer Inst. 2005 Feb 2;97(3):188–94. doi: 10.1093/jnci/dji021. [DOI] [PubMed] [Google Scholar]
- 3.Rastogi P, Anderson SJ, Bear HD, et al. Preoperative chemotherapy: updates of National Surgical Adjuvant Breast and Bowel Project Protocols B-18 and B-27. J Clin Oncol. 2008 Feb 10;26(5):778–85. doi: 10.1200/JCO.2007.15.0235. [DOI] [PubMed] [Google Scholar]
- 4.van der Hage JA, van de Velde CJ, Julien JP, et al. Preoperative chemotherapy in primary operable breast cancer: results from the European Organization for Research and Treatment of Cancer trial 10902. J Clin Oncol. 2001 Nov 15;19(22):4224–37. doi: 10.1200/JCO.2001.19.22.4224. [DOI] [PubMed] [Google Scholar]
- 5.Fisher B, Brown A, Mamounas E, et al. Effect of preoperative chemotherapy on local-regional disease in women with operable breast cancer: findings from National Surgical Adjuvant Breast and Bowel Project B-18. [see comments] J Clin Oncol. 1997;15(7):2483–93. doi: 10.1200/JCO.1997.15.7.2483. [DOI] [PubMed] [Google Scholar]
- 6.Kuerer HM, Sahin AA, Hunt KK, et al. Incidence and impact of documented eradication of breast cancer axillary lymph node metastases before surgery in patients treated with neoadjuvant chemotherapy. Ann Surg. 1999;230(1):72–8. doi: 10.1097/00000658-199907000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gianni L, Pienkowski T, Im YH, et al. Efficacy and safety of neoadjuvant pertuzumab and trastuzumab in women with locally advanced, inflammatory, or early HER2-positive breast cancer (NeoSphere): a randomised multicentre, open-label, phase 2 trial. Lancet Oncol. 2012 Jan;13(1):25–32. doi: 10.1016/S1470-2045(11)70336-9. [DOI] [PubMed] [Google Scholar]
- 8.Carey LA, Berry DA, Cirrincione CT, et al. Molecular Heterogeneity and Response to Neoadjuvant Human Epidermal Growth Factor Receptor 2 Targeting in CALGB 40601, a Randomized Phase III Trial of Paclitaxel Plus Trastuzumab With or Without Lapatinib. J Clin Oncol. 2015 Nov 2; doi: 10.1200/JCO.2015.62.1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sikov WM, Berry DA, Perou CM, et al. Impact of the addition of carboplatin and/or bevacizumab to neoadjuvant once-per-week paclitaxel followed by dose-dense doxorubicin and cyclophosphamide on pathologic complete response rates in stage II to III triple-negative breast cancer: CALGB 40603 (Alliance) J Clin Oncol. 2015 Jan 1;33(1):13–21. doi: 10.1200/JCO.2014.57.0572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Golshan M, Cirrincione CT, Sikov WM, et al. Impact of neoadjuvant chemotherapy in stage II–III triple negative breast cancer on eligibility for breast-conserving surgery and breast conservation rates: surgical results from CALGB 40603 (Alliance) Ann Surg. 2015 Sep;262(3):434–9. doi: 10.1097/SLA.0000000000001417. discussion 8–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Golshan M, Cirrincione CT, Sikov WM, et al. Impact of neoadjuvant therapy on eligibility for and frequency of breast conservation in stage II-III HER2-positive breast cancer: surgical results of CALGB 40601 (Alliance) Breast Cancer Res Treat. 2016 Nov;160(2):297–304. doi: 10.1007/s10549-016-4006-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Galimberti V, Cole BF, Zurrida S, et al. Axillary dissection versus no axillary dissection in patients with sentinel-node micrometastases (IBCSG 23-01): a phase 3 randomised controlled trial. Lancet Oncol. 2013 Apr;14(4):297–305. doi: 10.1016/S1470-2045(13)70035-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Giuliano AE, Hunt KK, Ballman KV, et al. Axillary dissection vs no axillary dissection in women with invasive breast cancer and sentinel node metastasis: a randomized clinical trial. JAMA. 2011 Feb 09;305(6):569–75. doi: 10.1001/jama.2011.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Donker M, van Tienhoven G, Straver ME, et al. Radiotherapy or surgery of the axilla after a positive sentinel node in breast cancer (EORTC 10981-22023 AMAROS): a randomised, multicentre, open-label, phase 3 non-inferiority trial. Lancet Oncol. 2014 Nov;15(12):1303–10. doi: 10.1016/S1470-2045(14)70460-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Boughey JC, Suman VJ, Mittendorf EA, et al. Sentinel lymph node surgery after neoadjuvant chemotherapy in patients with node-positive breast cancer: the ACOSOG Z1071 (Alliance) clinical trial. JAMA. 2013 Oct 9;310(14):1455–61. doi: 10.1001/jama.2013.278932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kuehn T, Bauerfeind I, Fehm T, et al. Sentinel-lymph-node biopsy in patients with breast cancer before and after neoadjuvant chemotherapy (SENTINA): a prospective, multicentre cohort study. Lancet Oncol. 2013 Jun;14(7):609–18. doi: 10.1016/S1470-2045(13)70166-9. [DOI] [PubMed] [Google Scholar]
- 17.Boileau JF, Poirier B, Basik M, et al. Sentinel node biopsy after neoadjuvant chemotherapy in biopsy-proven node-positive breast cancer: the SN FNAC study. J Clin Oncol. 2015 Jan 20;33(3):258–64. doi: 10.1200/JCO.2014.55.7827. [DOI] [PubMed] [Google Scholar]
- 18.Schipper RJ, Moossdorff M, Beets-Tan RG, et al. Noninvasive nodal restaging in clinically node positive breast cancer patients after neoadjuvant systemic therapy: a systematic review. Eur J Radiol. 2015 Jan;84(1):41–7. doi: 10.1016/j.ejrad.2014.09.020. [DOI] [PubMed] [Google Scholar]
- 19.Hieken TJ, Boughey JC, Jones KN, et al. Imaging response and residual metastatic axillary lymph node disease after neoadjuvant chemotherapy for primary breast cancer. Ann Surg Oncol. 2013 Oct;20(10):3199–204. doi: 10.1245/s10434-013-3118-z. [DOI] [PubMed] [Google Scholar]
- 20.Straver ME, Loo CE, Alderliesten T, et al. Marking the axilla with radioactive iodine seeds (MARI procedure) may reduce the need for axillary dissection after neoadjuvant chemotherapy for breast cancer. Br J Surg. 2010 Aug;97(8):1226–31. doi: 10.1002/bjs.7073. [DOI] [PubMed] [Google Scholar]
- 21.Caudle AS, Yang WT, Mittendorf EA, et al. Selective surgical localization of axillary lymph nodes containing metastases in patients with breast cancer: a prospective feasibility trial. JAMA Surg. 2015 Feb;150(2):137–43. doi: 10.1001/jamasurg.2014.1086. [DOI] [PMC free article] [PubMed] [Google Scholar]