Abstract
Hydrogenated curcuminoids are the major metabolites of the curcumin and ‘CuroWhite’ is a unique blend of hydrogenated curcuminoids encapsulated with β-cyclodextrin. There is no particular scientific evidence for the toxicology regarding the hydrogenated curcuminoids, so the present work reports the results of the studies investigating the acute (single dose) and subchronic (repeatedly 90 days) oral toxicity of the CuroWhite in Sprague Dawley rats. For acute oral toxicity testing a sighting study was conducted on female rats in a sequential manner to allow selection of the appropriate starting dose for the main study. In acute toxicity, the dosage was 2000 mg/kg body weight for four female rats. In the sub-chronic study, rats of both sexes divided into three groups and each group were orally treated with CuroWhite daily at 200, 400 and 800 mg/kg for 90 days consecutively. No evidence of treatment related toxicity was detected during the study. Thus, data analysis of mortality, body weight gain, feed consumption, clinical observations, hematology, organ weights and histopathological findings did not show significant differences between control and treated groups. It is concluded that CuroWhite orally administered to rats was safe and no drug-related toxicity was detected even at the highest doses investigated in both acute (2000 mg/kg) and subchronic toxicity (200, 400 and 800 mg/kg) studies. Based on the study, the no-observed-adverse-effect level (NOAEL) value could be considered as 800 mg/kg per day in both the sexes. These results indicate that CuroWhite can be generally regarded as safe for use as a food additive.
Keywords: CuroWhite, Acute oral toxicity, Subchronic oral toxicity, Sprague Dawley rats
1. Introduction
Turmeric has a long history of use in Ayurvedic medicine for the treatment of inflammatory conditions [1] and a wide variety of diseases including those of the skin, pulmonary, and gastrointestinal systems, aches, pains, wounds, sprains, and liver disorders [2]. Curcumin, extracted from the dried turmeric root of plant Curcuma longa plays the major role in health care and in food ingredients. Curcumin is responsible for medicinal properties and the main constituents are Curcumin (C1), Demethoxycurcumin (C2), and Bisdemethoxycurcumin (C3) [3]. The characteristic yellow color of the curcumin is mainly due to the two double bonds conjugated with beta-diketones [4].
Hydrogenation of curcuminoids can be done by a catalyst [5,6] and the obtained product is light yellow to brown having more bio-availability than the curcuminoids when encapsulated with β-cyclodextrin [4]. The resultant product is the mixture of hydrogenated curcumioids namely, Tetrahydrocurcumin, (THC), Hexahydrocurcumin (HHC) and Octahydrocurcumin (OHC) or Hexahydrocurcuminol and the structures are as in Fig. 1. These are the major metabolites of curcumin [7], [8] and they inhibited various Lipopolysaccharide (LPS) induced responses in vitro, including excess NO production, increased iNOS and COX-2 protein expression as well as LPS-induced degradation of IκB-α and overexpression of nuclear p65 [9]. THC, one of the major metabolites of curcumin showed good antioxidative property and greater inhibitory effect than curcumin on the lipid peroxidation of erythrocyte membrane [10], [11] in vitro. Its role in protection against lipid peroxidation proved by the significant increase in the activities of superoxide dismutase, catalase, glutathione peroxidase, glutathione-S-transferase, reduced glutathione, vitamin C and vitamin E in liver and kidney of diabetic rats [12], [13]. Cytotoxicity of HHC to colorectal cancer cells SW480 is significant [14] and the treatment of human platelet-rich plasma with HHC resulted in an inhibitory effect on platelet aggregation, suggests the potential of this compound as an anti-atherosclerogenic agent in humans [15].
Fig. 1.
Structures of THC, HHC and OHC (R1 and R2 = OMe or H).
Aurea Biolabs developed a unique formulation of the hydrogenated curcuminoids encapsulated with β-cyclodextrin branded as ‘CuroWhite, and by this method the hydrogenated molecules can be entrapped in cyclodextrin lipohilic cage, hence the cyclodextrin matrix have more bio-availability, solubility and stability [4]. CuroWhite contains THC (17–20%), HHC (2.5–5%) and OHC (0.70–1.25%).
Even though, apart from the pharmacological properties there is no particular scientific evidence of the toxicology regarding the hydrogenated curcuminoids. Herein we first report, to the best of our knowledge, a study of the acute and subchronic oral toxicity of the hydrogenated curcumin formulation, CuroWhite.
2. Materials and methods
CuroWhite was prepared according to the method of Sreeraj et al. and the total hydrogenated curcuminoids was 25% by HPLC [4]. Required quantity of test item was weighed as per the dose and suspended in distilled water to get the desired concentration. Formulation of the test item was prepared shortly before dosing. The homogeneity of the test formulation was maintained by continuous stirring on the magnetic stirrer during dosing. The test item was administered by oral gavage to each rat as a single dose, using gavaging needle. The dosage volume administered to individual rat was adjusted according to its body weight recorded on the day of dosing and the dose volume was 10 mL/kg.
Adult male and female Sprague Dawley rats (7–8 weeks old) were used for acute and 90 day subchronic toxicity tests and all the rats bred in Liveon Biolabs (P) LTD, Karnataka, India. The animals were acclimatized for a minimum of 5 days period to standard laboratory conditions and were housed in centralized air-conditioned rooms with adequate fresh air supply (Air changes of 12–15 h−1). The room temperature was 21.2–24.8 °C and relative humidity was 44–69% with 12 h light and 12 h dark cycle. All the animals were approved by the Institutional Animals Ethics Committee (IAEC) and the animal feed ad libitum manufactured by Pranav Agro Industries Limited, India throughout the acclimatization, treatment and recovery periods.
2.1. Acute oral toxicity study
For acute oral toxicity testing a sighting study was planned to allow selection of the appropriate starting dose for the subchronic study. In the sighting study, an effect of CuroWhite (300 mg/kg) was investigated in a sequential manner by administering to a single female animal having 130–150 g body weight. The animal was observed for the toxicity and then another dose (2000 mg/kg) administrated in another female rat. After observing the clinical signs of toxicity and mortality for 24 h, the main acute toxicity study was conducted in another set of 4 female rats by administering a dose of 2000 mg/kg body weight of CuroWhite through oral gavage as a single dose.
2.2. Subchronic 90-day oral toxicity study
For Sub chronic toxicity testing, grouping of animals having 140–169 g body weight was done on the last day of acclimatization by body weight randomization and stratification method. Grouping of animals was done such that body weight variation of animals did not exceed ±20% of the mean body weight of each sex. The details of the classification of the animals are given in Table 1. A recovery group is a non-dosing group that follows the main dosing phase of a study; it is included in nonclinical toxicity studies to understand whether toxicities observed at the end of the dosing phase are partially or completely reversible. Also information on the persistence or reversibility of effects will be obtained from recovery groups, comprising the controls and the high-dose group [16]. G1R refers to Control recovery group and G4R refers to high dose recovery group.
Table 1.
The details of the classification of the animals.
| Group | Treatment | Dose (mg/kg) |
Average body weight (g) |
No. of Animals |
Animal numbers |
|||
|---|---|---|---|---|---|---|---|---|
| Male | Female | Male | Female | Male | Female | |||
| G1 | Control | – | 175.78 ± 10.61 | 161.52 ± 7.38 | 10 | 10 | 1–10 | 51–60 |
| G2 | Low dose | 200 | 173.75 ± 11.20 | 160.90 ± 9.62 | 10 | 10 | 11–20 | 61–70 |
| G3 | Mid dose | 400 | 175.73 ± 11.65 | 159.80 ± 9.72 | 10 | 10 | 21–30 | 71–80 |
| G4 | High dose | 800 | 175.27 ± 12.07 | 159.91 ± 8.16 | 10 | 10 | 31–40 | 81–90 |
| G1R | Control Recovery | – | 175.88 ± 9.99 | 160.36 ± 14.1 | 05 | 05 | 41–45 | 91–95 |
| G4R | High dose Recovery | 800 | 177.80 ± 16.71 | 161.25 ± 15.2 | 05 | 05 | 46–50 | 96–100 |
Average body weight in Mean ± Standard Deviation.
The duration of the administration was for a period of 90 days for both main and recovery groups. The animals were fasted overnight before test item administration. Food was offered 3–4 h following dosing. All animals were observed twice daily for clinical signs of toxicity, mortality and morbidity.
Blood samples were collected from main groups (G1–G4) and recovery groups (G1R and G4R) on day 90 and 120, respectively before sacrificing the animals. One batch was collected in tubes containing Dipotassium ethylene di-amide tetra acetic acid (K2-EDTA) anticoagulant for hematology analysis and another without anticoagulant for clinical chemistry analysis. The blood samples collected for clinical chemistry were centrifuged at 3000 rpm for 10 min to obtain clear serum. Blood samples were collected humanely from retro-orbital plexus puncture method under carbon-dioxide anaesthesia with the help of a fine capillary tube.
The hematology parameters estimated were hemoglobin (HGB), hematocrit (HCT), erythrocyte count (RBC), total leukocyte count (WBC), mean corpuscular volume (MCV), mean corpuscular hemoglobin (MCH), mean corpuscular hemoglobin concentration (MCHC) and platelet counts. Blood smears were prepared with Leishman’s stain and differential leucocyte count (DLC) was carried out by standard microscopy of counting 100 cells such as neutrophils (N), lymphocytes (L), eosinophils (E), monocytes (M) and basophils (B). The clinical chemistry parameters such as total protein, glucose, albumin, alanine aminotransferase (ALT), aspartate aminotransferase (AST), blood urea nitrogen, total cholesterol, triglycerides, urea, creatinine, Na and K also measured.
At the completion of the experimental period, all animals fasted for 12 h prior to the sacrifice and animals were humanely sacrificed by exposing them to excess carbon dioxide in gas chamber and subjected to the external and internal gross necropsy examinations. In subchronic testing all the surviving animals from main groups (G1–G4) and recovery groups (G1R and G4R) were sacrificed on day 91/92 and 119/120, respectively.
During the necropsy, the organs from all animals were collected and trimmed off any adherent tissue, as appropriate and weighed as soon as possible to avoid drying. These included liver, kidney, testis, epididymis, adrenals, uterus, ovaries, heart, spleen, brain and thymus. Detailed gross pathological examination was performed and recorded. The organs showing gross lesions (if any) were collected for histological evaluation.
2.3. Statistical analysis
The obtained raw data were subjected to statistical analysis using graph pad prism software. The data on body weight, body weight gain, feed consumption, organ weights, hematological and clinical chemistry estimations were analyzed statistically using One-Way ANOVA (Analysis of Variance) with Dunnett’s posttest for different treatment groups comparing with vehicle control group data. All analyses and comparisons were evaluated at 95% level of confidence (P < 0.05). The statistically significant changes obtained from the above mentioned tests were designated with the superscripts in summarized tables of the study.
3. Results
During sighting study, both the female animals (300 and 2000 mg/kg doses) did not show any clinical signs of toxicity and mortality for 24 h. There were no significant changes in clinical signs, weight gain, gross pathological changes and mortality in any of the female rats during acute oral toxicity test. (Table 2, Table 3). These results suggested the necessity of the subchronic study.
Table 2.
Clinical signs and mortality of female rats given acute oral dose.
| Study Type | Dose (mg/kg) | No. of Animals | Sex | Clinical signs | Mortality |
|---|---|---|---|---|---|
| Sighting Study—Step-I | 300 | 1 | Female | Normal | 0 |
| Sighting Study—Step-II | 2000 | 1 | Female | Normal | 0 |
| Main Study | 2000 | 4 | Female | Normal | 0 |
Table 3.
Body weight (g) and body weight gain (%) of female rats given acute oral dose (Values are in Mean ± SD.
| Study Type | Dose (mg/kg) |
No. of Animals | Sex | Body weight on days |
% Body weight gain |
||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 8 | 15 | 1–8 | 8–15 | |||||
| Sighting Study—Step-I | 300 | 1 | Female | 138.42 | 148.74 | 151.59 | 7.46 | 1.92 | |
| Sighting Study—Step-II | 2000 | 1 | Female | 129.92 | 155.37 | 168.24 | 19.59 | 8.28 | |
| Main Study | 2000 | 4 | Female | Mean | 146.68 | 171.61 | 179.65 | 17.45 | 4.66 |
| SD | ±8.67 | ±14.13 | ±15.65 | ±13.83 | ±1.24 | ||||
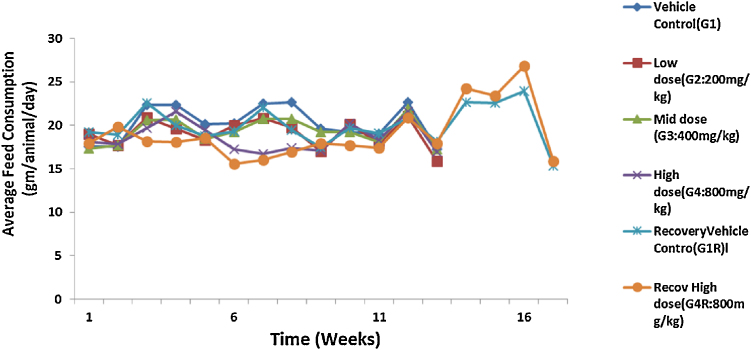
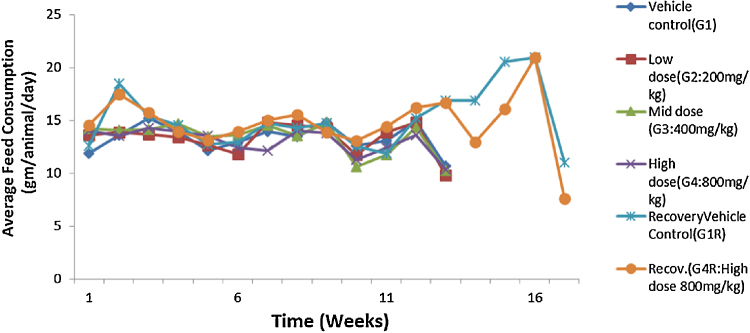
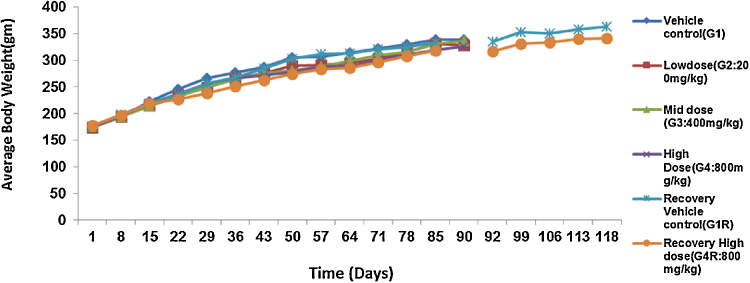
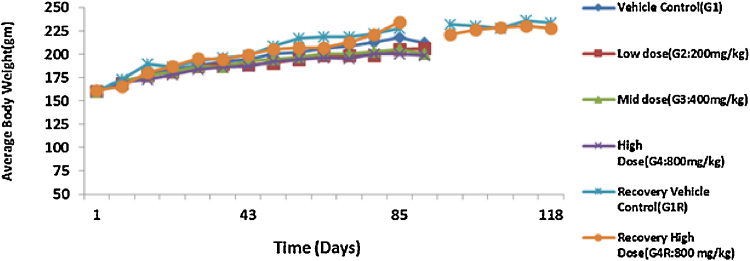
The administration of acute and repeated 90 day subchronic oral dose of CuroWhite produced neither drug related deaths nor treatment related signs of toxicity in any of the animals during the study. In the subchronic oral toxicity test there were no biologically significant treatment related adverse effects and abnormality on food consumption (Fig. 2, Fig. 3) and body weights (Fig. 4, Fig. 5) as depicted. Hematology (Table 4, Table 5), clinical biochemistry (Table 6, Table 7) and absolute organ weights of all internal organs (Table 8, Table 9) at necropsy in animals are given.
Fig. 2.
Effect of repeated dose treatment on feed consumption of male rats.
Fig. 3.
Effect of repeated dose treatment on feed consumption of female rats.
Fig. 4.
Effect of repeated dose treatment on average body weight of male rats.
Fig. 5.
Effect of repeated dose treatment on average body weight of female rats.
Table 4.
Haematology of male rats given subchronic oral dose of CuroWhite.
| Group | WBC | RBC | HGB | HCT | MCV | MCH | MCHC | Platelet count | Differential Leucocyte Count |
||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (103 cells/μL) | (106 cells/μL) | (g/dL) | (%) | (fL) | (pg) | (g/dL) | (103 cells/μL) | Neutrophils (%) | Lymphocytes (%) | Monocytes (%) | Eosinophils (%) | Basophils (%) | |
| Main Groups | |||||||||||||
| G1 Control |
11.58 | 7.36 | 12.73 | 39.61 | 53.90 | 17.33 | 32.16 | 749.60 | 18.80 | 78.70 | 2.00 | 0.10 | 0.40 |
| ±3.19 | ±0.51 | ±0.55 | ±1.94 | ±1.72 | ±0.89 | ±0.86 | ±97.82 | ±2.66 | ±2.91 | ±1.05 | ±0.32 | ±0.52 | |
| G2 200mg/kg |
10.16 | 7.41 | 13.16 | 40.27 | 54.36 | 17.78 | 32.66 | 825.10 | 17.00 | 80.60 | 2.10 | – | 0.30 |
| ±4.26 | ±0.53 | ±1.32 | ±3.55 | ±2.99 | ±1.42 | ±1.05 | ±187.60 | ±3.02 | ±3.63 | ±1.10 | – | ±0.48 | |
| G3 400 mg/kg |
10.99 | 7.64 | 13.22 | 40.63 | 53.25 | 17.33 | 32.55 | 742.00 | 18.00 | 79.40 | 2.00 | 0.10 | 0.50 |
| ±2.12 | ±0.54 | ±0.94 | ±2.66 | ±1.91 | ±0.90 | ±0.58 | ±102.74 | ±2.58 | ±2.84 | ±1.33 | ±0.32 | ±0.53 | |
| G4 800 mg/kg |
12.59 | 7.22 | 12.84 | 38.91 | 53.92 | 17.82 | 33.01 | 806.11 | 18.56 | 79.33 | 1.67 | 0.11 | 0.33 |
| ±4.60 | ±0.40 | ±0.87 | ±2.65 | ±2.02 | ±0.86 | ±0.68 | ±161.20 | ±3.57 | ±3.28 | ±1.00 | ±0.33 | ±0.50 | |
| Recovery Groups | |||||||||||||
| G1R Control Recovery | 8.92 | 8.44 | 13.70 | 44.60 | 52.80 | 16.22 | 30.72 | 814.20 | 18.40 | 79.80 | 1.40 | 0.20 | 0.20 |
| ±1.35 | ±0.39 | ±0.78 | ±2.75 | ±1.01 | ±0.31 | ±0.37 | ±140.79 | ±3.36 | ±2.59 | ±0.89 | ±0.45 | ±0.45 | |
| G4R High dose Recovery 800 | 12.02 | 8.20 | 13.54 | 44.22 | 53.98 | 16.52 | 30.62 | 891.40 | 19.20 | 78.40 | 2.00 | – | 0.40 |
| ±6.25 | ±0.59 | ±0.85 | ±2.74 | ±1.70 | ±0.56 | 0.57 | ±146.77 | ±2.17 | ±1.14 | ±1.00 | – | ±0.55 | |
Values are in Mean ± Standard Deviation.
Table 5.
Haematology of female rats given subchronic oral dose of CuroWhite.
| Group | WBC | RBC | HGB | HCT | MCV | MCH | MCHC | Platelet count | Differential Leucocyte Count |
||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (103 cells/μL) | (106 cells/μL) | (g/dL) | (%) | (fL) | (pg) | (g/dL) | (103 cells/μL) | Neutrophils (%) | Lymphocytes (%) | Monocytes (%) | Eosinophils (%) | Basophils (%) | |
| Main Groups | |||||||||||||
| G1 Control |
8.29 | 6.05 | 11.18 | 33.42 | 55.42 | 18.51 | 33.45 | 740.70 | 19.00 | 78.70 | 1.80 | 0.10 | 0.40 |
| ±3.22 | ±0.59 | ±1.13 | ±3.16 | ±5.10 | ±1.36 | ±1.39 | ±143.00 | ±2.21 | ±2.11 | ±0.92 | ±0.32 | ±0.52 | |
| G2 200 mg/kg |
9.79 | 5.77 | 11.04 | 31.65 | 54.89 | 19.16 | 34.91** | 647.70 | 18.70 | 78.20 | 2.30 | 0.20 | 0.60 |
| ±1.99 | ±0.41 | ±0.89 | ±2.58 | ±2.98 | ±1.16 | ±0.66 | ±61.55 | ±2.91 | ±2.86 | ±0.82 | ±0.42 | ±0.70 | |
| G3 400 mg/kg |
10.20 | 6.19 | 11.48 | 33.50 | 54.32 | 18.64 | 34.32 | 745.10 | 18.40 | 79.00 | 2.00 | 0.20 | 0.40 |
| ±2.02 | ±0.88 | ±1.27 | ±4.11 | ±2.33 | ±1.06 | ±0.62 | ±168.59 | ±2.41 | ±2.40 | ±1.05 | ±0.42 | ±0.70 | |
| G4 800 mg/kg |
8.84 | 5.93 | 11.10 | 32.14 | 54.21 | 18.76 | 34.56* | 689.80 | 18.30 | 79.30 | 1.70 | 0.10 | 0.60 |
| ±2.41 | ±0.58 | ±1.07 | ±2.93 | ±1.11 | ±0.58 | ±0.71 | ±115.44 | ±2.75 | ±2.91 | ±0.67 | ±0.32 | ±0.70 | |
| Recovery Groups | |||||||||||||
| G1R Control Recovery | 5.94*** | 7.31 | 12.96 | 41.92 | 57.34 | 17.74 | 30.94 | 712.80 | 18.60 | 79.40 | 1.40 | – | 0.60 |
| ±1.15 | ±0.16 | ±0.61 | ±2.46 | ±3.56 | ±1.03 | ±0.56 | ±88.50 | ±2.19 | ±1.52 | ±0.55 | – | ±0.89 | |
| G4R High dose Recovery 800 | 5.38** | 7.44 | 12.90 | 41.25 | 55.43 | 17.35 | 31.28 | 816.50 | 20.00 | 78.00 | 1.50 | – | 0.50 |
| ±0.43 | ±0.26 | ±0.27 | ±1.42 | ±1.16 | ±0.34 | ±0.51 | ±170.20 | ±2.45 | ±1.83 | ±0.58 | – | ±0.58 | |
Values are in Mean ± Standard Deviation.
P < 0.05.
P < 0.01.
P < 0.001.
Table 6.
Clinical biochemistry of male rats given subchronic oral dose of CuroWhite.
| Group | Total Protein (g/dl) | Glucose (mg/dl) | Albumin (g/dl) | Alanine Aminotransferase (ALT) (IU/L) | Aspartateaminotransferase (AST) (IU/L) | Blood Urea Nitrogen (mg/dL) | Total Cholesterol (mg/dl) | Triglycerides (mg/dl) | Urea (mg/dl) | Creatinine (mg/dl) | Na (mmol/L) | K (mmol/L) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Main Groups | ||||||||||||
| G1 Control |
6.99 | 66.38 | 3.21 | 52.16 | 147.46 | 23.57 | 51.92 | 62.38 | 38.43 | 0.79 | 140.56 | 96.47 |
| ±0.68 | ±15.68 | ±0.47 | ±12.56 | ±24.51 | ±3.27 | ±9.98 | ±15.37 | ±8.11 | ±0.18 | ±6.16 | ±7.35 | |
| G2 200 mg/kg |
6.59 | 65.31 | 3.28 | 52.16 | 159.12 | 27.04 | 52.36 | 56.29 | 46.38* | 0.64* | 144.54 | 99.35 |
| ±0.50 | ±8.68 | ±0.15 | ±10.32 | ±18.17 | ±3.44 | ±9.82 | ±15.14 | ±9.03 | ±0.09 | ±5.83 | ±3.77 | |
| G3 400 mg/kg |
6.51 | 94.39* | 3.32 | 48.44 | 147.11 | 27.47 | 52.94 | 40.75* | 51.78*** | 0.64* | 148.68** | 92.63 |
| ±0.77 | ±37.90 | ±0.48 | ±6.63 | ±29.65 | ±5.06 | ±11.93 | ±12.19 | ±5.31 | ±0.10 | ±5.85 | ±6.84 | |
| G4 800 mg/kg |
7.04 | 85.37 | 3.40 | 49.50 | 146.76 | 23.44 | 58.61 | 57.67 | 42.76 | 0.60** | 144.50 | 101.74 |
| ±0.65 | ±12.00 | ±0.24 | ±15.18 | ±40.56 | ±2.88 | ±8.59 | ±17.32 | ±2.40 | ±0.13 | ±5.27 | ±7.94 | |
| Recovery Groups | ||||||||||||
| G1R Control Recovery | 8.65 | 76.41 | 3.24 | 50.92 | 186.00 | 18.30 | 44.21 | 82.15 | 46.24 | 0.16 | 155.04 | 83.82 |
| ±0.64 | ±14.62 | ±0.17 | ±6.30 | ±34.95 | ±4.42 | ±10.29 | ±10.62 | ±7.67 | ±0.05 | ±28.97 | ±9.53 | |
| G4R Highdose Recovery 800 mg/kg | 8.49 | 70.55 | 3.27 | 65.06** | 184.22 | 20.01 | 40.57 | 73.77 | 53.96 | 0.17 | 124.56 | 77.41 |
| ±1.20 | ±8.57 | ±0.17 | ±5.06 | ±64.96 | ±1.94 | ±8.89 | ±27.87 | ±11.60 | ±0.03 | ±13.84 | ±4.47 | |
Values are in Mean ± Standard Deviation.
P < 0.05.
P < 0.01.
P < 0.001.
Table 7.
Clinical biochemistry of female rats given subchronic oral dose of CuroWhite.
| Group | Total Protein (g/dl) | Glucose (mg/dl) | Albumin (g/dl) | Alanine Aminotransferase (ALT) (IU/L) | Aspartateaminotransferase (AST) (IU/L) | Blood Urea Nitrogen (mg/dL) | Total Cholesterol (mg/dl) | Triglycerides (mg/dl) | Urea (mg/dl) |
Creatinine (mg/dl) | Na (mmol/L) | K (mmol/L) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Main Groups | ||||||||||||
| G1 Control |
6.45 | 68.00 | 3.20 | 42.26 | 173.44 | 23.95 | 55.37 | 55.35 | 50.70 | 0.75 | 143.31 | 91.43 |
| ±1.22 | ±16.75 | ±0.40 | ±6.48 | ±28.61 | ±3.50 | ±18.00 | ±20.03 | ±6.90 | ±0.14 | ±10.40 | ±7.51 | |
| G2 200 mg/kg |
6.86 | 68.91 | 2.99 | 50.56 | 174.16 | 26.21 | 54.41 | 52.66 | 49.27 | 0.69 | 146.79 | 90.14 |
| ±0.78 | ±19.32 | ±0.40 | ±9.94 | ±37.94 | ±4.71 | ±12.70 | ±23.03 | ±6.61 | ±0.14 | ±6.17 | ±4.64 | |
| G3 400 mg/kg |
7.09 | 59.61 | 3.21 | 46.85 | 176.98 | 25.06 | 47.01 | 37.27* | 49.46 | 0.64 | 147.37 | 91.09 |
| ±1.00 | ±16.43 | ±0.40 | ±10.68 | ±37.16 | ±4.13 | ±5.06 | ±5.06 | ±7.14 | ±0.08 | ±9.87 | ±6.20 | |
| G4 800 mg/kg |
6.44 | 65.16 | 3.07 | 51.27 | 158.94 | 25.55 | 55.16 | |||||
| ±0.37 | ±16.32 | ±0.42 | ±8.04 | ±31.76 | ±3.02 | ±10.34 | 41.17 | 44.65 | 0.60* | 145.26 | 90.93 | |
| Recovery Groups | ||||||||||||
| G1R Control Recovery | 8.44 | 115.90 | 4.05 | 40.67 | 140.72 | 17.97 | 46.94 | 70.59 | 53.89 | 0.79 | 192.54 | 99.33 |
| ±0.25 | ±20.89 | ±0.50 | ±6.00 | ±26.05 | ±4.22 | ±9.50 | ±23.91 | ±11.02 | ±0.20 | ±59.07 | ±4.37 | |
| G4R Highdose Recovery 800 mg/kg | 7.93* | 119.10 | 3.90 | 47.74 | 123.78 | 15.57 | 39.01 | 59.63 | 42.21 | 0.81 | 143.50 | 96.58 |
| ±0.27 | ±34.09 | ±0.37 | ±4.33 | ±11.83 | ±2.82 | ±15.22 | ±42.00 | ±4.47 | ±0.08 | ±27.56 | ±4.34 | |
n = 10 (Main groups), n = 5 (Recovery groups); Values are Mean ± Standard Deviation.
P < 0.05.
Table 8.
Absolute organ weights of male rats given subchronic oral dose of CuroWhite.
| Group | Treatment and Dose | Adrenals | Spleen | Thymus | Testes | Brain | Heart | Epididymis | Kidneys | Liver |
|---|---|---|---|---|---|---|---|---|---|---|
| Main Groups | ||||||||||
| G1 | Control | 0.05 | 1.05 | 0.33 | 3.04 | 1.82 | 1.01 | 1.27 | 1.94 | 8.46 |
| ±0.01 | ±0.23 | ±0.04 | ±0.27 | ±0.12 | ±0.12 | ±0.14 | ±0.23 | ±1.34 | ||
| G2 | Low dose—200(mg/kg) | 0.05 | 0.97 | 0.28 | 3.13 | 1.81 | 0.97 | 1.28 | 2.06 | 9.18 |
| ±0.01 | ±0.15 | ±0.07 | ±0.28 | ±0.10 | ±0.14 | ±0.09 | ±0.26 | ±1.26 | ||
| G3 | Mid dose—400(mg/kg) | 0.05 | 1.01 | 0.35 | 3.01 | 1.86 | 1.05 | 1.25 | 2.06 | 9.18 |
| ±0.01 | ±0.26 | ±0.13 | ±0.31 | ±0.16 | ±0.21 | ±0.17 | ±0.40 | ±1.12 | ||
| G4 | High dose—800(mg/kg) | 0.05 | 1.20 | 0.31 | 3.28 | 1.73 | 0.99 | 1.29 | 2.15 | 10.77 |
| ±0.01 | ±0.48 | ±0.08 | ±0.49 | ±0.27 | ±0.09 | ±0.19 | ±0.35 | ±2.13 | ||
| Recovery Groups | ||||||||||
| G1R | Control Recovery | 0.12 | 1.02 | 0.33 | 3.32 | 1.79 | 1.16 | 1.43 | 2.05 | 8.30 |
| ±0.17 | ±0.21 | ±0.06 | ±0.41 | ±0.18 | ±0.14 | ±0.25 | ±0.35 | ±0.95 | ||
| G4R | High dose Recovery 800 mg/kg |
0.05 | 0.92 | 0.24 | 3.12 | 1.76 | 1.05 | 1.33 | 1.91 | 8.64 |
| ±0.01 | ±0.23 | ±0.08 | ±0.26 | ±0.12 | ±0.09 | ±0.10 | ±0.26 | ±1.87 | ||
Note: Values are Mean ± Standard Deviation.
Table 9.
Absolute organ weights of female rats given subchronic oral dose of CuroWhite.
| Group | Treatment and Dose | Adrenals | Spleen | Thymus | Testes | Brain | Heart | Epididymis | Kidneys | Liver |
|---|---|---|---|---|---|---|---|---|---|---|
| Main Groups | ||||||||||
| G1 | Control | 0.06 | 0.87 | 0.28 | 0.10 | 1.79 | 0.73 | 0.55 | 1.42 | 6.19 |
| ±0.01 | ±0.18 | ±0.10 | ±0.03 | ±0.15 | ±0.10 | ±0.21 | ±0.20 | ±1.09 | ||
| G2 | Low dose—200(mg/kg) | 0.05 | 0.73 | 0.24 | 0.10 | 1.63 | 0.64 | 0.50 | 1.28 | 5.90 |
| ±0.01 | ±0.10 | ±0.07 | ±0.03 | ±0.15 | ±0.08 | ±0.08 | ±0.18 | ±1.03 | ||
| G3 | Mid dose—400(mg/kg) | 0.05 | 0.78 | 0.25 | 0.09 | 1.69 | 0.70 | 0.63 | 1.47 | 6.20 |
| ±0.03 | ±0.16 | ±0.11 | ±0.03 | ±0.12 | ±0.08 | ±0.22 | ±0.40 | ±1.23 | ||
| G4 | High dose—800(mg/kg) | 0.06 | 0.75 | 0.25 | 0.11 | 1.67 | 0.64 | 0.60 | 1.36 | 6.41 |
| ±0.02 | ±0.17 | ±0.16 | ±0.04 | ±0.16 | ±0.06 | ±0.19 | ±0.16 | ±0.80 | ||
| Recovery Groups | ||||||||||
| G1R | Control Recovery | 0.05 | 0.88 | 0.22 | 0.10 | 1.78 | 0.73 | 0.84 | 1.39 | 6.52 |
| ±0.01 | ±0.18 | ±0.03 | ±0.02 | ±0.09 | ±0.09 | ±0.34 | ±0.20 | ±0.58 | ||
| G4R | High dose Recovery 800 mg/kg | 0.05 | 0.54 | 0.25* | 0.08 | 1.72 | 0.72 | 0.81 | 1.31 | 5.50 |
| ±0.01 | ±0.18 | ±0.08 | ±0.02 | ±0.16 | ±0.14 | ±0.27 | ±0.14 | ±0.95 | ||
Note: Values are Mean ± Standard Deviation.
P < 0.05.
4. Discussion
Two mortalities, one male of G4 (800 mg/kg, high dose, animal no. 31) and one female of G4R treatment group (800 mg/kg recovery group, animal no. 99) were noticed on day 63. However, upon necropsy based gross pathological examinations by pathologist, none of the changes were found at organ level that may potentially relate the high dose toxicity. The dead animals had moderate to marked degree of autolytic changes in liver, kidneys, stomach, intestine, genital organs, brain, sciatic nerve spleen, thymus, spinal cord on microscopic examination. The lungs from animal number 31 revealed bronchopneumonia with congestion, edema of alveoli whereas animal number 99 revealed congestion, edema of alveoli. Lungs from both animals also had minimal degree of autolytic changes. These two deaths had no dose-dependent adverse changes attributable to treatment effects. The history of animal during dosing showed that these animals had an accidental gavaging error. Also on gross pathological examination the changes noticed were related to dosing error and related to treatment effect. All these points are suggestive of deaths due to gavaging method [17]. The microscopic lesions noticed in lungs from both the animals were suggestive of accidental and or gavaging error. Lungs of affected animals resulted in inflammation of bronchi and associated alveolar structures. The accidental entry of small dose of test formulation must have acted as foreign to the lungs and resulted in infiltration of the alveoli by inflammatory cells, congestion and or hemorrhage. The cause of apoptosis could be predominantly due to entry of small dose of formulations and later resulted in bronchopneumonia but without bacterial contamination at microscopic level. The cause of moderate to marked degree of autolytic changes in the organs evaluated could be due to the post mortem changes [18].
There were no treatment related histopathological changes at G4 (high dose, 800 mg/kg) in both the sexes observed. All the other lesions noticed at high dose and vehicle control group were considered as spontaneous and incidental in nature for this particular species and strain of rats. There was no considerable difference in food consumption between the groups of female and male rats (Fig. 2, Fig. 3). But after 90 days there were a slight increase in food consumption for control recovery (G1R) and high dose recovery (G4R) in both sexes then decreased in the last week. The food consumption of the high dose recovery group was little higher than that of the control recovery group in males and reversed in the case of the females.
Similarly, no statistically significant as well as biological differences in the body weights and body weight gains (Fig. 4, Fig. 5). There was a gradual increase in body weight for all the groups during the study indicates there was no dosage related toxicity. During the recovery period, the average body mass of the high dose recovery group was lower than that of the control recovery group in both the sexes. Table 4, Table 5 indicate that there were no significant alterations in hematological parameters between control and different treatment groups in both male and female groups. The total Leukocyte Count (WBC) was decreased for both G1 and G4R (5.94 and 5.38 × 103 cells/μL respectively) in female rats.
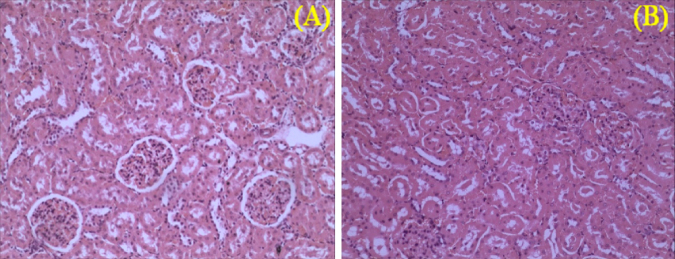
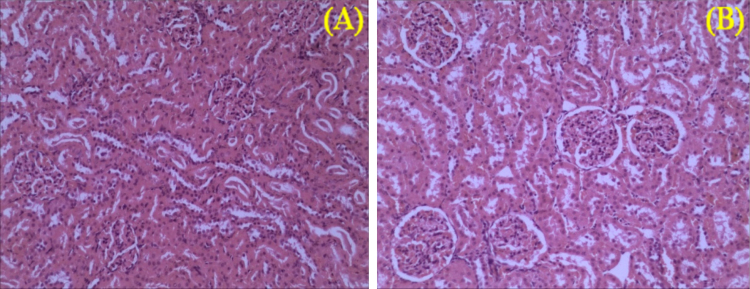
In addition, there were no abrupt differences in clinical biochemistry values (Table 6, Table 7) in both sexes. The values of creatinine decreased in both the male recovery groups as in Table 6. In G2 and G3 male groups, urea was significantly increased and creatinine was significantly decreased compared to the controls. Similarly, in G3 sodium was significantly increased. The creatinine was 0.79 ± 0.18 mg/dL in the control group, however in 200, 400, 800 mg/kg dose groups creatinine was significantly decreased. While in both control recovery and 800 mg/kg dose groups it was 0.16 ± 0.05 and 0.17 ± 0.03 mg/dL, respectively. Though the values are statistically significant and decreased, the decrease in creatinine values [19], [20] indicates the result of nephroprotective effect of CuroWhite to improve creatinine clearance [21]. On histopathology of G1R and G4R group animals, the kidneys did not show any abnormal changes correlative to kidney toxicity and specific to reduction in the urea or creatinine values [18]. Hence it cannot be attributed to kidney toxicity. The microphotographs of the kidney for histopathological changes which are all within the normal histological limits as shown in Fig. 6, Fig. 7.
Fig. 6.
Kidney sections of male rats of control (A) and high dose (B) of CuroWhite groups after 90 days with intact parenchyma and glomeruli. H&E × 10.
Fig. 7.
Kidney sections of female rats of control (A) and high dose (B) of CuroWhite groups after 90 days with intact parenchyma and glomeruli. H&E × 10.
The AST values are higher in male rats for both G1R and G4R (186 and 184.22 IU/L respectively) as compared with G1 (147.76 IU/L). Similarly, the glucose level was slightly high in both G1R and G4R (115.90 and 119.10 mg/dl respectively) as compared with vehicle dose (68 mg/dl) in female rats, but for all other groups there was no significant difference. Even though the values of AST and glucose are higher, there are no significant changes at high dose recovery (G4R) when compared to the control recovery (G1R) groups which is a concurrent control group. Moreover, these values are fall within the in-house historical control data and also are within physiological range.
Absolute organ weights were similar in all the groups as depicted in Table 8, Table 9. However, in males, absolute organ weight of liver was statistically increased at high dose group (800 mg) and there were associated correlative changes observed under microscopic examination. CuroWhite is the hydrogenated formulation of the curcuminoids and generally considered as the metabolites of the curcumin [22], hence it is safe as functional food and food additives. In addition, this study data may also be used as a basis for the dose selection and also for establishing safety criteria for human exposure.
The data derived would allow the characterization of the CuroWhite toxicity with an indication of the dose response relationship and determination of the NOAEL and to classify the test item according to Globally Harmonized System (GHS) in rats. NOAEL could be considered as 800 mg/kg body weight in both the sexes. Therefore, CuroWhite can be labeled as unclassified in the hazard category according to GHS.
5. Conclusion
In conclusion, CuroWhite is the unique blend of hydrogenated curcumin which are the metabolites of the curcumin and at the oral doses tested, CuroWhite can be considered safe as it did not cause either any lethality or adverse changes in the general behavior in both the acute toxicity studies and the subchronic toxicity studies in rats. Based on the results from the current study the NOAEL for could be considered as 800 mg/kg body weight in both the sexes. Therefore, CuroWhite can be labeled as unclassified in the hazard category according to GHS.
Conflict of interest
The authors have nothing to declare.
References
- 1.Jurenka J.S. Anti-inflammatory properties of curcumin, a major constituent of Curcuma longa: a review of pre-clinical and clinical research. Altern. Med. Rev. 2009;14(2):141–153. [PubMed] [Google Scholar]
- 2.Aggarwal B.B., Sundaram C., Malani N., Ichikawa H. Curcumin: the Indian solid gold. Adv. Exp. Med. Biol. 2007;595:1–75. doi: 10.1007/978-0-387-46401-5_1. [DOI] [PubMed] [Google Scholar]
- 3.Rout K.K., Parida S., Mishra S.K. Standardization of the ayurvedic formulation Haridra Khanda using high-performance thin-layer chromatography-densitometry. J. AOAC Int. 2008;91(5):1162–1168. [PubMed] [Google Scholar]
- 4.Sreeraj G., Jacob J., George R., Sreeraj T.R. A unique formulation of hydrogenated curcuminoids with higher bio availability and the application in food matrices. J. Nutr. Food Sci. 2016;6(2):1–4. [Google Scholar]
- 5.Roughley P.J., Whiting D.A. Experiments in the biosynthesis of curcumin. J. Chem. Soc. Perkin Trans. 1973;1(20):2379–2388. [Google Scholar]
- 7.Ireson C., Orr S., Jones D.J., Verschoyle R., Lim C.K., Luo J.L., Howells L., Plummer S., Jukes R., Williams M., Steward W.P., Gescher A. Characterization of metabolites of the chemopreventive agent curcumin in human and rat hepatocytes and in the rat in vivo, and evaluation of their ability to inhibit phorbol ester-induced prostaglandin E2 production. Cancer Res. 2001;61(3):1058–1064. [PubMed] [Google Scholar]
- 8.Dempe J.S., Pfeiffer E., Grimm A.S., Metzler M. Metabolism of curcumin and induction of mitotic catastrophe in human cancer cells. Mol. Nutr. Food Res. 2008;52(9):1074–1081. doi: 10.1002/mnfr.200800029. [DOI] [PubMed] [Google Scholar]
- 9.Zhao F., Gong Y., Hu Y., Lu M., Wang J., Dong J., Chen D., Chen L., Fu F., Qiu F. Curcumin and its major metabolites inhibit the inflammatory response induced by lipopolysaccharide: translocation of nuclear factor-κB as potential target. Mol. Med. Rep. 2015;4:3087–3093. doi: 10.3892/mmr.2014.3079. [DOI] [PubMed] [Google Scholar]
- 10.Sugiyama Y., Kawakishi S., Osawa T. Involvement of the β-diketone moiety in the antioxidative mechanism of tetrahydrocurcumin. Biochem. Pharmacol. 1996;52(4):519–525. doi: 10.1016/0006-2952(96)00302-4. [DOI] [PubMed] [Google Scholar]
- 11.Osawa T., Sugiyama Y., Inayoshi M., Kawakishi S. Antioxidative activity of tetrahydrocurcuminoids. Biosci. Biotechnol. Biochem. 1995;59(9):1609–1612. doi: 10.1271/bbb.59.1609. [DOI] [PubMed] [Google Scholar]
- 12.Murugan P., Pari L. Antioxidant effect of tetrahydrocurcumin in streptozotocin-nicotinamide induced diabetic rats. Life Sci. 2006;79(18):1720–1728. doi: 10.1016/j.lfs.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 13.Murugan P., Pari L., Rao C.A. Effect of tetrahydrocurcumin on insulin receptor status in type 2 diabetic rats: studies on insulin binding to erythrocytes. J. Biosci. 2008;33(1):63–72. doi: 10.1007/s12038-008-0022-y. [DOI] [PubMed] [Google Scholar]
- 14.Chen C.Y., Yang W.L., Kuo S.Y. Cytotoxic activity and cell cycle analysis of hexahydrocurcumin on SW 480 human colorectal cancer cells. Nat. Prod. Commun. 2011;6(11):1671–1672. [PubMed] [Google Scholar]
- 15.Dong H.P., Yang R.C., Chunag I.C., Huang L.J., Li H.T., Chen H.L., Chen C.Y. Inhibitory effect of hexahydrocurcumin on human platelet aggregation. Nat. Prod. Commun. 2012;7(7):883–884. [PubMed] [Google Scholar]
- 16.Pandher K., Leach M.W., Burns-Naas L.A. Appropriate use of recovery groups in nonclinical toxicity studies: value in a science-driven case-by-case approach. Vet. Pathol. 2012;49(2):357–361. doi: 10.1177/0300985811415701. [DOI] [PubMed] [Google Scholar]
- 17.Eichenbaum G., Damsch S., Looszova A., Vandenberghe J., Van den Bulck K., Roels K., Megens A., Knight E., Hillsamer V., Feyen B., Kelley M.F., Tonelli A., Lammens L. Impact of gavage dosing procedure and gastric content on adverse respiratory effects and mortality in rat toxicity studies. J. Appl. Toxicol. 2011;31(4):342–354. doi: 10.1002/jat.1592. [DOI] [PubMed] [Google Scholar]
- 18.Tsokos M. vol. 3. Humana Press Inc.; Totowa, NJ: 2005. (Forensic Pathology Reviews). (5) [Google Scholar]
- 19.Zhong-Ze H., Hong-De X., Kwang-Ho K., Tae-Hwan A., Jin-Sook B., Ji-Young L., Ki-Hyun G., Joo-Young L., Su-Jung W., Hyun-Jung Y., Hyun-Kul L., Kap-Ho K., Chan-Koo P., Hu-Song Z., Si-Whan S. Reference data of the main physiological parameters in control Sprague-Dawley rats from pre-clinical toxicity studies. Lab. Anim. Res. 2010;26(2):153–164. [Google Scholar]
- 20.Reddy V.C., Amulya V., Lakshmi C.A., Reddy D.B.P.K., Pratima D., Thirupathi A.T., Kumar K.P., Sengottuvelu S. Effect of simvastatin in gentamicin induced nephrotoxicity in albino rats. Asian J. Pharm. Clin. Res. 2012;5(1):36–40. [Google Scholar]
- 21.Osawa T. Nephroprotective and hepatoprotective effects of curcuminoids. Adv. Exp. Med. Biol. 2007;595:407–423. doi: 10.1007/978-0-387-46401-5_18. [DOI] [PubMed] [Google Scholar]
- 22.Pan M.H., Huang T.M., Lin J.K. Biotransformation of curcumin through reduction and glucuronidation in mice. Drug Metab. Dispos. 1999;27(4):486–494. [PubMed] [Google Scholar]