Abstract
Toxicological evaluations of two N-alkyl benzamide umami flavour compounds, N-(heptan-4-yl)benzo[d][1,3]dioxole-5-carboxamide (S807, CAS 745047-51-2) and (R)-N-(1-methoxy-4-methylpentan-2-yl)-3,4-dimethylbenzamide (S9229, CAS 851669-60-8), were completed for the purpose of assessing their safety for use in food and beverage applications. Both S807 and S9229 undergo rapid oxidative metabolism by both rat and human liver microsomes in vitro. In pharmacokinetic studies in rats, the systemic exposure to S9229 on oral administration is very low at all doses (% F < 1%), while that of S807 demonstrated a non-linear dose dependence. In metabolism studies in rats, hydroxylation of the C-4 aryl methyl group was found to be the dominant metabolic pathway for S9229. The dominant metabolic pathway for S807 in the rat involved oxidative scission of the methylenedioxy moiety to produce the corresponding 3,4-dihydroxybenamide which is further converted by Phase II metabolic enzymes to the 3- and 4-O-methyl ethers as well as their corresponding glucuronides. Both S807 and S9229 were not found to be mutagenic or clastogenic in vitro, and did not induce micronuclei in polychromatic erythrocytes in vivo. In a subchronic oral toxicity study in rats, the no-observed-effect-level (NOEL) for S807 was 20 mg/kg bw/day when administered in the diet for 13 weeks. The no-observed-adverse-effect-level (NOAEL) for S9229 in rats was 100 mg/kg bw/day (highest dose tested) when administered in the diet for 28 consecutive days.
Abbreviations: amu, atomic mass units; AUC, area under the curve; CL, plasma clearance; Cmax, peak plasma concentration; COMT, catechol-O-methyltransferase; FDA, Food and Drug Administration; FEMA, Flavour and Extract Manufacturers Association of the United States; FL-no, FLAVIS number; GLP, good laboratory practices; GMP, good manufacturing practices; HPBL, human peripheral blood lymphocytes; LC/MS, liquid chromatography with mass spectrometry; MC, methylcellulose; MRM, multiple-reaction monitoring; MSG, monosodium glutamate; MTD, maximum tolerated dose; NOAEL, no-observed-adverse-effect-level; NOEL, no-observed-effect-level; OECD, Organization for Economic Cooperation and Development; PK, pharmacokinetics; RCG, relative cell growth; RMI, relative mitotic index; t1/2, half-life; Tmax, time to reach Cmax; TK, toxicokinetics; Vss, volume of distribution at steady-state
Keywords: S807, S9229, FEMA GRAS, Subchronic toxicological evaluation, Genetic toxicological evaluation
1. Introduction
Umami, the savory taste of the amino acid l-glutamate, is one of the five basic taste qualities detected by humans. Monosodium glutamate (MSG) is the prototypical umami substance, commonly added to many food and beverage compositions, often at concentrations of 0.1–0.8% (1000–8000 ppm) by weight, to improve their overall fullness and savory flavour. In addition, it is known that naturally occurring purine ribonucleotides such as inosine-5′-monophosphate and guanosine-5′-monophosphate which elicit no umami taste on their own, can synergistically potentiate the umami taste of glutamate, thereby requiring less MSG for a given flavouring application. While these purine ribonucleotides can be present along with glutamate in certain food ingredients such as autolyzed yeast extracts, they are expensive to isolate from natural sources or to synthesize. Until recently, little progress has been made in identifying high potency artificial substitutes for MSG or potentiators of the effectiveness of naturally occurring glutamate already present in food products.
Umami substances are detected by a specific subset of taste receptor cells localized in the taste bud and characterized by the expression of members of the hTAS1R family of class C G-protein-coupled receptors (GPCRs), which are distantly related to calcium sensing receptor, V2R pheromone receptors, and metabotropic glutamate receptors [18], [19], [38]. Co-expression of both hTAS1R1 and hTAS1R3 in heterologous cells results in a functional, heteromeric receptor which is highly selective for umami stimuli, responding only to glutamate, aspartate, and l-2-amino-4-phosphonobutrate, and is also strongly potentiated by purine ribonucleotides such as inosine-5′-monophosphate and guanosine-5′-monophosphate, which is a key characteristic of the human receptor for umami taste. This functional assay for hTAS1R1/hTAS1R3 has been adapted for high-throughput screening of natural extract and synthetic libraries leading to the discovery of several classes of novel umami agonists including a series of highly potent oxalamide compounds typified by N1-(2,4-dimethoxybenzyl)-N2-(2-(pyridin-2-yl)ethyl)oxalamide (S336, CAS 745047-53-4, FEMA 4233, FL-no. 16.099, Savorymyx® UM33).
We previously reported the results of a toxicological evaluation of 2-(((3-(2,3-dimethoxyphenyl)-1H-1,2,4-triazol-5-yl)thio)methyl)pyridine (S3643, CAS 902136-79-2, FEMA 4798), which differs from the oxalamide-based umami agonists mainly by the replacement of the oxalamide moiety by a 1,2,4-triazole ring [14]. Concurrent with the discovery of the oxalamide umami agonists, workers at Senomyx also identified a series of N-alkyl benzamide analogs including N-(heptan-4-yl)benzo[d][1,3]dioxole-5-carboxamide (S807, CAS 745047-51-2, Savorymyx® UM80) and (R)-N-(1-methoxy-4-methylpentan-2-yl)-3,4-dimethylbenzamide (S9229, CAS 851669-60-8), which are also potent agonists of the human umami receptor. These N-alkyl benzamides, like the aforementioned oxalamide and 1,2,4-triazole derivatives, can provide an umami flavour effect in product applications equivalent to that of MSG at a 1000-fold lower concentration [31], [32], [33], [34], [35]. The structures of S807 and S9229 are shown in Fig. 1.
Fig. 1.
Structures of S807 and S9229.
Both S807 and S9229 have been reviewed by the Expert Panel of the Flavour and Extract Manufacturers Association of the United States (FEMA) and determined to be generally recognized as safe (GRAS) under conditions of intended use as flavour ingredients [30], [23], [11] and therefore are available for use in human food in the United States as “FEMA GRAS” flavour ingredients. S807 was assigned FEMA GRAS Number 4232 in 2005 [30], and S9229 was assigned FEMA GRAS Number 4751 in 2011 [23]. In addition, S807 was also determined to be safe at the current levels of intake by the Joint FAO/WHO Expert Committee on Food Additives [12]; assigned JECFA No. 1767) and the European Union [8]; assigned FL-no: 16.098). Other jurisdictions permit the use of S807 including China, Korea, Indonesia, and Mexico.
The purpose of this publication is to summarize the results obtained from in vitro/in vivo metabolism and pharmacokinetic (PK) studies, general toxicology studies in rodents, and genotoxicity studies conducted with both S807 and S9229. Additional supporting data obtained in these studies is included in a Supplementary data section in the online publication.
2. Materials and methods
The batch of S807 used for the in vitro/in vivo metabolism and pharmacokinetic studies, (Batch no. 061001T01L, purity 99.9%), was synthesized at Derivados Quimicos Fine Chemicals, Murcia, Spain using the procedure described in US Patent No. 7,476,399 B2; 8,8124121 B2; and 8,895050 B2 [31], [32], [33]. The batch of S807 used for the in vitro/in vivo genotoxicity, 21-day range-finding toxicity, and 90-day subchronic toxicity studies (Lot no. 4KL0071A, purity > 99.95%) was synthesized at Albany Molecular Research, Inc., Albany, NY using the same synthetic method. The batch of S9229 used for the in vitro/in vivo metabolism, the pharmacokinetic, the in vitro/in vivo genotoxicity, and 28-day toxicity studies (Batch ID 50764226, chemical purity > 98.8%, optical purity > 99.8%) was synthesized at Senomyx, San Diego, CA using the procedure described in the same US Patents noted above for S807. The batch of S9229 used for the in vitro metabolism study conducted by Senomyx (Batch ID 58490390, chemical purity > 97%) was also synthesized at Senomyx using the same procedure.
All genetic toxicology studies were conducted in compliance with the FDA Good Laboratory Practices (GLP) regulations 21 CFR Part 58 [9] and OECD guidelines [27]. The experimental design for these studies followed the OECD Guidelines for the Testing of Chemicals − 471, 473, and 474 [25], [26], [28]. The 28-day and 90-day toxicology studies in rats were conducted in compliance with the United States Food and Drug Administration (FDA) Guidelines [10] Toxicological Principles for the Safety of Food Ingredients and FDA Good Laboratory Practice (GLP) Regulations, 21 CFR Part 58.
The in vitro microsomal metabolism studies on S9229 were carried out by PharmOptima, Portage, MI. The microsomal metabolism studies utilized male and female rat liver microsomes (Lot no. 1010122 and 0710104, respectively) and mixed gender human microsomes (Lot no. H0910255) obtained from XenoTech, Lenexa, KS. Additional in vitro microsomal metabolism studies, as well as pharmacokinetic and in vivo metabolism studies on both S807 and S9229, were conducted at Senomyx, San Diego, CA using male and female rat liver microsomes (Lot no. 1410271 and 1310205, respectively) and mixed gender human microsomes (Lot no. 1410013) obtained from XenoTech, Lenexa, KS. The analytical methods used for the in vitro metabolism, pharmacokinetic and in vivo metabolism studies can be found in the Supplementary data section published online.
The in vitro and in vivo genotoxicity studies for S807 were conducted at Nucro-Technics, Scarborough, Ontario, Canada. The strains of S. typhimurium and E. coli, as well as rat liver S9 (9000 x g supernatant fraction of liver homogenate from Sprague-Dawley rats treated with Aroclor™ 1254) used in the reverse bacterial mutation assay were obtained from Molecular Toxicology Inc., Boone, NC. Chinese hamster ovary cell line WBL used for the in vitro chromosome aberration test of S807 was obtained from the Department of Pathobiology, University of Guelph (Guelph, ON, Canada). Rat liver S9 (9000 × g supernatant fraction of liver homogenate from Sprague-Dawley rats treated with phenobarbital and 5,6-benzoflavone) used in the chromosome aberration test was obtained from Molecular Toxicology Inc., Boone, NC.
The in vitro and in vivo genotoxicity studies for S9229 were conducted at BioReliance, Rockville, MD. The S. typhimurium tester strains were from Dr. Bruce Ames’ Master cultures, and the E. coli tester strains were from the National Collection of Industrial and Marine Bacteria, Aberdeen, Scotland. Tester strains TA100, TA1535 and TA1537 were obtained from Molecular Toxicology Inc., Boone, NC, using cultures derived from the above sources. Peripheral blood lymphocytes used for both the preliminary toxicity test and chromosome aberration assay were obtained from a healthy, non-smoking, 31-year-old adult female. The donor had no recent history of radiotherapy, viral infection or the administration of drugs. The rat liver S9 (9000 × g supernatant fraction of liver homogenate from Sprague-Dawley rats treated with Aroclor™ 1254) used in the reverse bacterial mutation and chromosome aberration assays was obtained from Molecular Toxicology Inc., Boone, NC.
The 21-day range-finder and 90-day subchronic toxicity studies on S807 were conducted at Covance Laboratories Inc., Vienna, VA; the 28-day toxicity study on S9229 was conducted at MPI Research, Mattawan, WI. A description of the study designs is included in the individual study sections below. Detailed data tables for the genotoxicity, 21-day, 28 day, and 90-day toxicity studies on S807 and S9229 can be found in the Supplementary data section published online.
3. Results and study designs
3.1. Absorption, distribution, metabolism, excretion
The in vitro metabolism of both S807 and S9229 was studied using rat and human liver microsomes. A study of the pharmacokinetics and in vivo metabolism of both compounds was also carried out in male and female Sprague-Dawley rats.
3.1.1. In vitro metabolism of S807 and S9229 by rat and human liver microsomes
The potential of S807 and S9229 to undergo oxidative metabolism was investigated using Sprague-Dawley rat and human liver microsomes in order to determine the similarity of the metabolic profile across these species and to assess the suitability of the rat as a species for toxicology studies. Reference standards were synthesized for two potential oxidative metabolites of S9229 that could be produced by mono-hydroxylation of the 3,4-dimethylphenyl moiety, as well as their corresponding O-demethylated analogs. A reference standard for the metabolite resulting from demethylenation of 1,3-benzodioxole moiety of S807 was also synthesized.
Solutions of either S807 (10 μM) or S9229 (10 μM) were incubated with mixed gender, pooled liver microsomes (0.5 mg/mL) from both rat and human (XenoTech, Lenexa, KS) in the presence of NADPH at 37 °C for 10, 20, or 60 min prior to quenching the samples with acetonitrile. Control samples included time zero and 60 min incubates without NADPH. Buspirone and loperamide were tested in parallel with the test compounds to confirm the functionality of the microsomes. Samples were centrifuged to separate the precipitated microsomes from the supernatant containing the parent compound and its metabolites. The supernatants were analyzed by LC-QTOF/MS (Agilent iFunnel 6550A MS QTOF, positive mode) equipped with an Agilent 1290 Infinity Binary pump and an Agilent 1290 Infinity autosampler using a Waters CSH C18 column (50 × 2.1 mm, 1.7 μm) with 0.1% formic acid/water (v/v) and acetonitrile gradient system to evaluate the metabolism of both S807 and S9229. Details of the experimental and analytical methods can be found in the Supplementary data section.
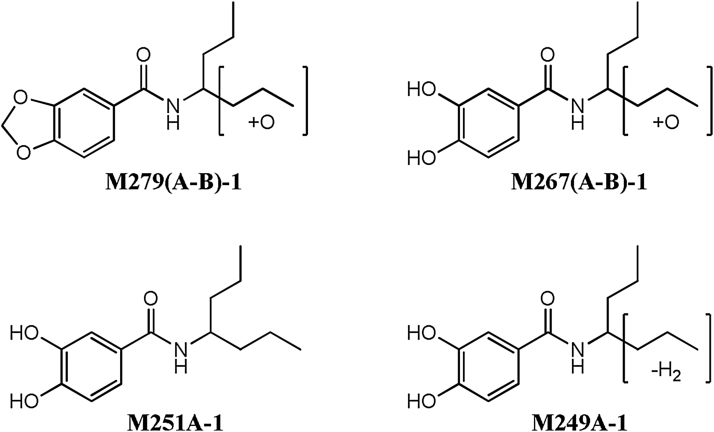
Both S807 and S9229 were metabolized significantly faster by the rat than by the human microsomes. In the case of S807, roughly 0.47% (rat) and 65% (human) of the parent was remaining at the end of the 60 min microsomal incubation period. Six potential Phase I metabolites were observed at levels ≥0.1% of the S807 extracted ion chromatogram (EIC) peak area at time zero. The structures and relative abundance of these metabolites are shown in Fig. 2 and Table 1, respectively. Note that all statements of scale (quantitative) assume that the relative response factors for S807 and all of its metabolites in the mass spectra data are equivalent. The major metabolite observed in both the rat and human microsomal incubations was the corresponding demethylenated compound M251A-1, representing roughly 34.4% (rat) and 15.2% (human) of the initial S807 EIC peak area at the 60 min time point. The identity of M251A-1 was confirmed by direct comparison to a synthetic sample by LC–MS/MS. Minor metabolites observed in both the rat and human microsomal incubations consisted of two pairs of compounds resulting from mono-hydroxylation of the 4-heptamine side chain of the parent compound S807 (i.e., M279A-1 and M279B-1) and of the corresponding demethylenated metabolite M251A-1 (i.e., M267A-1 and M267B-1). The position of the hydroxylation of the 4-heptamine side chain in these four metabolites was not determined. A sixth metabolite (M249A-1) observed in the rat microsomal incubations was an olefin which results from the loss of water from the hydroxylated metabolite(s) M267A-1 and/or M267B-1. No dihydroxylated metabolites were observed in either the rat or human microsomal incubations.
Fig. 2.
Major Metabolites of S807 in Rat and Human Microsomal Incubations.
Table 1.
Major Metabolites of S807 in Rat and Human Microsomal Incubations.
| Metabolite | m/z (M + H) | Formula | RT (min) | % MS (EIC) Peak Areaa |
|||||
|---|---|---|---|---|---|---|---|---|---|
| Rat (min) |
Human (min) |
||||||||
| 10 | 20 | 60 | 10 | 20 | 60 | ||||
| S807 | 264.1594 | C15H22NO3+ | 6.92 | 70.0 | 34.0 | 0.47 | 89.4 | 80.8 | 65.0 |
| M279A-1 | 280.1543 | C15H22NO4+ | 4.16 | 0.5 | 0.6 | 0.2 | 0.1 | 0.3 | 0.4 |
| M279B-1 | 280.1543 | C15H22NO4+ | 4.58 | 0.2 | 0.2 | 0.1 | 0.1 | 0.2 | 0.2 |
| M267A-1 | 268.1543 | C14H22NO4+ | 2.62 | 0.1 | 0.3 | 2.1 | – | – | 0.0 |
| M267B-1 | 268.1543 | C14H22NO4+ | 2.93 | 0.1 | 0.1 | 0.5 | – | – | 0.1 |
| M251A-1 | 252.1594 | C14H22NO3+ | 5.02 | 15.2 | 29.9 | 34.4 | 3.1 | 7.8 | 15.2 |
| M249A-1 | 250.1438 | C14H20NO3+ | 4.41 | – | 0.0 | 0.1 | – | – | – |
% MS peak area relative to S807 at time = 0.
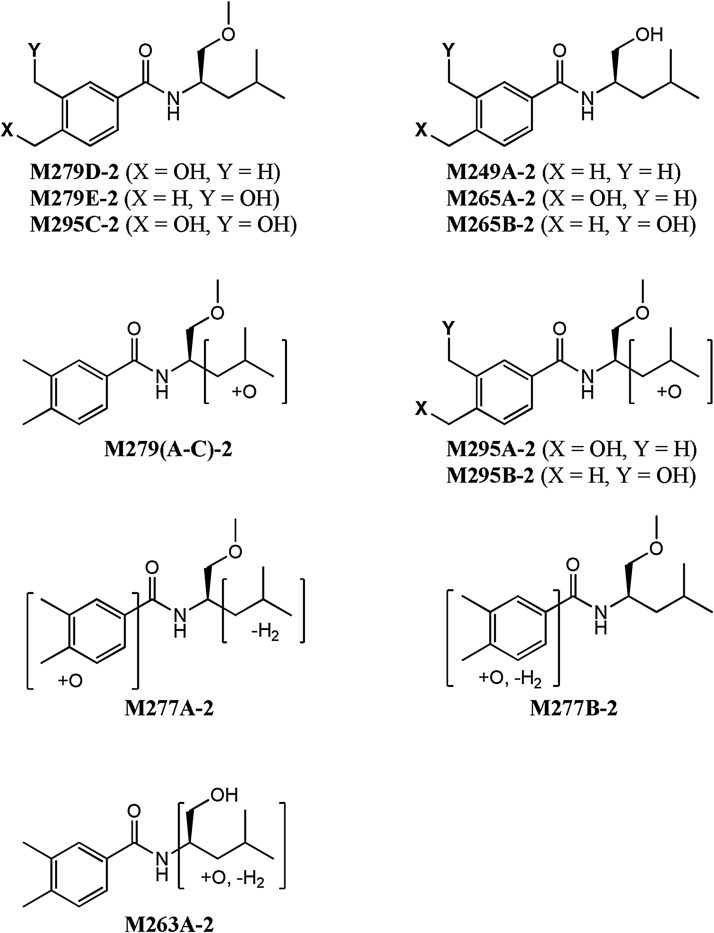
In the case of S9229, roughly 0.33% (rat) and 22.6% (human) of the parent was remaining at the end of the 60 min microsomal incubation period. Fourteen potential Phase I metabolites were observed at levels ≥0.1% of the S9229 EIC peak area at time zero. The metabolic transformation of S9229 involved hydroxylation of the aryl methyl groups, hydroxylation of the isobutyl side chain, and demethylation of the side chain methyl ether. The structures and relative abundance of these metabolites are shown in Fig. 3 and Table 2, respectively. The major metabolite observed in both the rat and human microsomal incubations was the corresponding C-4 hydroxymethyl compound (M279D-2), representing roughly 20.2% (rat) and 21.2% (human) of the initial S9229 EIC peak area at the 60 min time point. The corresponding C-3 hydroxymethyl (M279E-2) and C-3,4 dihydroxymethyl (M295C-2) metabolites were also observed in both the rat and human microsomal incubations, albeit at significantly lower concentrations. An aryl aldehyde (M277B-2) resulting from the further oxidation of either M279D-2 or M279E-2 was also observed as a minor metabolite. The corresponding demethylated analogs of both the parent and the two aryl methyl hydroxylated compounds (i.e., M249A-2, M265A-2 and M265B-2) were also observed in the microsomal incubations of both species. The identity of metabolites M279D-2, M279E-2, M249A-2, M265A-2, and M265B-2 was confirmed by direct comparison to synthetic samples by LC–MS/MS.
Fig. 3.
Major Metabolites of S9229 in Rat and Human Microsomal Incubations.
Table 2.
Major Metabolites of S9229 in Rat and Human Microsomal Incubations.
| Metabolite |
m/z (M + H) |
Formula | RT (min) |
% MS (EIC) Peak Areaa |
|||||
|---|---|---|---|---|---|---|---|---|---|
| Rat (min) |
Human (min) |
||||||||
| 10 | 20 | 60 | 10 | 20 | 60 | ||||
| S9229 | 264.1958 | C16H26NO2+ | 6.96 | 36.4 | 1.93 | 0.33 | 86.9 | 70.6 | 22.6 |
| M279A-2 | 280.1907 | C16H26NO3+ | 4.20 | 0.6 | 0.6 | 0.2 | 0.2 | 0.3 | 0.8 |
| M279B-2 | 280.1907 | C16H26NO3+ | 4.34 | 0.4 | 0.4 | 0.2 | 0.6 | 1.2 | 2.3 |
| M279C-2 | 280.1907 | C16H26NO3+ | 4.45 | 0.5 | 0.5 | 0.0 | 0.1 | 0.3 | 0.7 |
| M279D-2 | 280.1907 | C16H26NO3+ | 4.57 | 28.5 | 35.7 | 20.2 | 3.9 | 3.6 | 21.2 |
| M279E-2 | 280.1907 | C16H26NO3+ | 4.70 | 1.0 | 0.7 | – | 0.8 | 1.8 | 5.2 |
| M265A-2 | 266.1751 | C15H24NO3+ | 3.45 | 0.8 | 2.7 | 8.5 | 0.0 | 0.1 | 0.8 |
| M265B-2 | 266.1751 | C15H24NO3+ | 3.68 | 0.2 | 0.5 | 0.5 | – | – | 0.1 |
| M295A-2 | 296.1856 | C16H26NO4+ | 2.28 | 0.1 | 0.2 | 0.9 | – | – | 0.2 |
| M295B-2 | 296.1856 | C16H26NO4+ | 2.36 | 0.0 | 0.1 | 0.3 | – | – | – |
| M295C-2 | 296.1856 | C16H26NO4+ | 3.42 | – | 0.1 | 0.1 | 0.2 | 0.4 | 1.1 |
| M277A-2 | 278.1751 | C15H24NO4+ | 4.03 | – | 0.1 | 0.1 | – | – | – |
| M277B-2 | 278.1751 | C15H24NO4+ | 5.77 | 0.2 | 0.9 | 2.3 | 0.1 | 0.2 | 0.3 |
| M263A-2 | 264.1594 | C15H22NO3+ | 4.45 | – | 0.0 | 0.7 | – | – | – |
| M249A-2 | 250.1802 | C15H24NO2+ | 5.55 | 2.5 | 1.2 | – | 0.5 | 1.0 | 1.8 |
% MS peak area relative to S9229 at time = 0.
Other minor metabolites observed in both the rat and human microsomal incubations of S9229 consisted of compounds derived by mono-hydroxylation of the isobutyl group of either the parent compound (i.e., M279A-2, M279B-2, M279C-2) or the two aryl methyl hydroxylated compounds (i.e., M295A-2 and M295B-2). A metabolite (M277A-2) resulting from loss of water from the side chain of either M295A-2 or M295B-2, and a metabolite (M263A-2) resulting from further oxidation of a hydroxylated metabolite of M249A-2 were also seen as a minor components in the rat microsomal incubations.
The metabolic profiles of both S807 and S9229 in rat and human liver microsomes were qualitatively very similar across species, each producing the same set of oxidative metabolites in either species, confirming that the rat was an appropriate species for evaluating the potential toxicity of S807 and S9229.
3.1.2. Pharmacokinetics and in vivo metabolism of S807 in rats
The PK parameters and oral bioavailability of S807 in plasma was evaluated following either a single intravenous or oral administration in male and female Sprague-Dawley rats. Plasma samples were also analyzed for the presence of the metabolites observed in incubations of S807 with rat liver microsomes. For intravenous administration, 4 male and 4 female Sprague-Dawley rats (Charles River Laboratories, Hollister, CA) were bolus injected with S807 at 1 mg/kg bw in 20% PEG400/10% ethanol/2% DMSO/68% sterile saline (0.9% NaCl). Blood samples were collected from an implanted jugular cannulae of each rat at pre-dose and at approximately 2, 5, 10, 30 min, 1, 2, 4, and 8 h post-dose. For oral administration, 4 male and 4 female Sprague-Dawley rats per group were given a single dose of S807 at either 20, 50, or 200 mg/kg bw in 1% methyl cellulose (MC) in deionized water by oral gavage. Blood samples were taken from an implanted jugular cannulae of each rat at pre-dose and at approximately 15, 30 min, 1, 2, 4, 8, and 24 h post-dose. Plasma samples spiked with an internal standard [(R)-N-(1-methoxy-4-methylpentan-2-yl)-3,4-dimethylbenzamide; S9229] were analyzed for S807 and its metabolites by LC–MS/MS using a Waters XSelect™ CSH C18 column, 130 Å (2.1 mm × 50 mm, 3.5 μm) with 0.1% formic acid/water and 0.1% formic acid/acetonitrile gradient system and a API 3200 Q-Trap mass spectrometer operated in positive ionization mode equipped with an Agilent 1100 binary pump with a CTC PAL injector. The parent compound and internal standard (IS) were detected using a source which was configured with turboionspray ionization in the positive mode using multiple-reaction monitoring (MRM) of mass transition pairs at m/z of 264.2/123.1 (S807) and 264.2/133.1 (IS, S9229) amu. The plasma concentration-time data were analyzed by non-compartmental methods using Phoenix WinNonlin version 6.2 (Pharsight/Certara company). Mass transition pairs targeted for the metabolite exposure analysis were chosen based on results obtained from a second LC–MS/MS method which was designed to determine the identity of the in vivo metabolites by measuring the exact mass of the parent ion as well as its fragment ions. For this purpose, the pooled plasma samples from all male and female rats dosed orally at 200 mg/kg were analyzed by LC-QTOF/MS (Agilent iFunnel 6550A MS QTOF, positive mode) equipped with an Agilent 1290 Infinity Binary pump and an Agilent 1290 Infinity autosampler using a Waters CSH C18 column (50 × 2.1 mm, 1.7 μm) with 0.1% formic acid/water and acetonitrile gradient system. Agilent MassHunter software was used in the data processing. Details of the analytical methods can be found in the Supplementary data section. Test article formulations prepared for this study were analyzed for concentration by HPLC-UV (240 nm). The pharmacokinetic parameters for S807 are shown in Table 3.
Table 3.
Pharmacokinetics of S807 in Male and Female Sprague-Dawley Rats.
| Route | Dose (mg/kg bw) | Sex | Cmax (ng/mL) | AUClast (ng·hr/mL) | Tmax (hr) | t1/2 (hr) | Cmax Ratioa | AUClast Ratiob | %F |
|---|---|---|---|---|---|---|---|---|---|
| iv | 1 | M | 1110 ± 237 | 491 ± 33.3 | 0.033 | 3.02 ± 0.24 | – | – | – |
| F | 949 ± 222 | 342 ± 81.4 | 0.033 | 3.02 ± 0.92 | – | – | – | ||
| oral gavage | 20 | M | 174 ± 95.1 | 182 ± 70.5 | 0.313 | 1.14 ± 0.35 | 1 | 1 | 1.85% |
| F | 187 ± 87.6 | 166 ± 72.2 | 0.25 | 1.07 ± 0.67 | 1 | 1 | 2.43% | ||
| 50 | M | 1270 ± 1170 | 4890 ± 4960 | 1.50 | 1.83 ± 0.61 | 7.30 | 26.9 | 19.9% | |
| F | 529 ± 379 | 1410 ± 1020 | 1.0 | 1.17 ± 0.16 | 2.83 | 8.49 | 8.25% | ||
| 200 | M | 10500 ± 937 | 101000 ± 19500 | 4.0 | 2.49 ± 0.07 | 60.3 | 555 | 102.9% | |
| F | 9150 ± 2410 | 108000 ± 27900 | 3.0 | 2.62 ± 0.20 | 48.9 | 651 | 157.9% |
Male rat: CL = 30.7 mL/min/kg; Vss = 4630 mL/kg.
Female rat: CL = 47.2 mL/min/kg; Vss = 5610 mL/kg.
CL = clearance; Vss = steady-state volume of distribution; %F = bioavailability.
Cmax Ratio = Cmax/Cmax at 20 mg/kg bw dose.
AUClast Ratio = AUClast/AUClast at 20 mg/kg bw dose.
For intravenous administration, the values for terminal half-life for S807 in plasma were 3.02 ± 0.24 h in male rats and 3.02 ± 0.92 h in female rats. Mean plasma clearance (CL) in rats averaged 30.7 mL/min/kg for males (55.6% of hepatic blood flow, [6] and 47.2 mL/min/kg for females (85.5% of hepatic blood flow), and the volume of distribution at steady-state (Vss) averaged 4630 and 5610 mL/kg (6.93 and 8.40-times total body water volume) for males and females, respectively.
For oral administration, the half-life values for S807 in plasma tended to increase with increasing dose ranging from 1.14 ± 0.35 to 2.49 ± 0.07 h in male rats, and 1.07 ± 0.67 to 2.62 ± 0.20 h in female rats. Consistent with increased plasma half-life, both Cmax and AUClast increased in a significantly greater than dose-proportional manner throughout the oral dose range. For example, increasing the dose from 20 to 200 mg/kg bw resulted in a 555 and 651-fold increase in systemic exposure (AUClast) in male and female rats, respectively. Oral bioavailability (%F) ranged from 1.85 to 2.43% at the 20 mg/kg bw dose to 102.9–157.9% at the 200 mg/kg bw dose. Although Cmax and AUClast values were very similar for male and female rats at both the 20 and 200 mg/kg bw doses, there was a significant gender difference in both the rate and extent of exposure at the 50 mg/kg bw oral dose (female/male Cmax ratio: 0.42; female/male AUClast ratio: 0.29). Taken together, the data suggests that the dramatic increase in bioavailability at the higher doses is likely due to saturation of first-pass metabolism and/or tissue distribution.
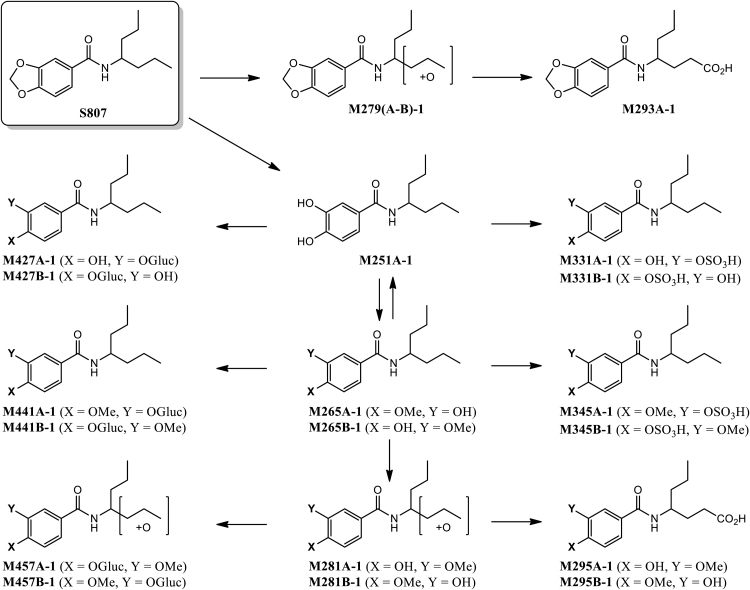
Table 4 shows the metabolite peak areas obtained from the extracted ion chromatograms (EIC) of the +MS scans from the LC-QTOF/MS analysis of the male and female pooled plasma samples at 1 h post dose (200 mg/kg bw). The metabolic pathway of S807 in rats is shown in Fig. 4. A total of four Phase I and thirteen Phase II metabolites were found with a peak area greater than 0.1% of the total peak area. At the 1 h time point, the parent compound S807 represents 43.84% of the total peak area. Based on the peak areas from the +MS scans, the parent compound is rapidly demethylenated to form the corresponding 3,4-dihydroxybenzamide M251A-1, which undergoes further methylation and/or sulfation and/or glucuronidation to form the observed Phase II metabolites. The structure of M251A-1 was confirmed by direct comparison to a synthetic standard by LC–MS/MS. Synthetic standards were also available for the two mono-methyl ether metabolites M265A-1 and M265B-1, as well as the two glucuronide, mono-methyl ethers M441A-1 and M441B-1. The mono-methyl ether derivatives M265(A–B)-1 and glucuronide mono-methyl ethers M441(A–B)-1 were the dominant biotransformations representing 24.34% and 18.44% of the total metabolite peak area, respectively. The two mono-methyl ether derivatives M265(A–B)-1 were not readily separable under the HPLC conditions used in this analysis. However, under HPLC conditions where they were separable (Halo RP-Amide column (150 × 3.0 mm, 2.7 μm); 0.1% formic acid/water-acetonitrile gradient), the slower eluting isomer M265B-1 was found to be the major mono-methyl ether metabolite by a ratio of approximately 100:1 at the one hour time point (see Supplemental data for details). Based on EIC peak areas, the concentration of M265B-1 was 60- to 170-fold higher than that of M265A-1 over the entire 24 h observation period. The structure of the major mono-methyl ether metabolite M265B-1 was found to be the 3-methoxy, 4-hydroxy-regioisomer by direct comparison to a synthetic standard by LC–MS/MS (see Fig. 4). Both glucuronide, mono-methyl ethers M441(A–B)-1 were also observed, but the slower eluting isomer M441B-1 was formed in to a significantly greater extent. The structure of the major glucuronide, mono-methyl ether metabolite M441B-1 was found to be the 3-methoxy, 4-glucuronyloxy-regioisomer by direct comparison to a synthetic standard by LC–MS/MS, which is likely to be formed by glucuronidation of the major catechol mono-methyl ether M265B-1 by uridine diphosphate glucuronosyltransferases.
Table 4.
Summary of Metabolites of S807 Observed in Male and Female Pooled Rat Plasma Samples at 1 h Post Dose (200 mg/kg bw).
| Metabolite | m/z (M + H) | Formula | RT (min) | MS (EIC) Peak Area (cps) | % MS Peak Area |
|---|---|---|---|---|---|
| S807 | 264.1594 | C15H22NO3+ | 6.90 | 29,000,000 | 43.84 |
| M265A-1 | 266.1751 | C15H24NO3+ | 5.81 | 16,100,000a | 24.34a |
| M265B-1 | 266.1751 | C15H24NO3+ | |||
| M441A-1 | 442.2072 | C21H32NO9+ | 4.56 | 698,000 | 1.06 |
| M441B-1 | 442.2072 | C21H32NO9+ | 4.83 | 11,500,000 | 17.38 |
| M427A-1 | 428.1915 | C20H30NO9+ | 4.46 | 648,000 | 0.98 |
| M427B-1 | 428.1915 | C20H30NO9+ | 4.59 | 1,520,000 | 2.30 |
| M293A-1 | 294.1336 | C15H20NO5+ | 4.31 | 1,350,000 | 2.04 |
| M279A-1 | 280.1544 | C15H22NO4+ | 4.16 | 379,000 | 0.57 |
| M279B-1 | 280.1544 | C15H22NO4+ | 4.57 | 1,260,000 | 1.90 |
| M345B-1 | 346.1319 | C15H24NO6S+ | 7.15 | 1,140,000 | 1.72 |
| M281A-1 | 282.1700 | C15H24NO4+ | 3.31 | 691,000 | 1.04 |
| M281B-1 | 282.1700 | C15H24NO4+ | 3.65 | 141,000 | 0.21 |
| M251A-1 | 252.1594 | C14H22NO3+ | 5.00 | 653,000 | 0.99 |
| M331A-1 | 332.1163 | C14H22NO6S+ | 6.97 | 515,000 | 0.78 |
| M457A-1 | 458.2021 | C21H32NO10+ | 2.73 | 274,000 | 0.41 |
| M457B-1 | 458.2021 | C21H32NO10+ | 2.93 | 109,000 | 0.16 |
| M295A-1 | 296.1493 | C15H22NO5+ | 3.44 | 172,000 | 0.26 |
Combined peak area of M265A-1 and M265B-1; ratio of M265B-1/M265A-1 is approximately 100:1 at the 1 h timepoint.
Fig. 4.
Metabolic Pathway of S807 in Rat.
Two mono-glucuronides M427(A–B)-1 derived from M251A-1 were also identified in the EICs of the plasma samples. These likely represent the two possible mono-glucuronide regioisomers and account for 3.28% of the total metabolite peak area at the one hour time point. Both M251A-1 and mono-methyl ethers M265(A–B)-1 also undergo sulfation to produce M331A-1 and M345(A–B)-1; only one dihydroxy, mono-sulfate (M331A-1) of unknown regiochemistry is seen in the EICs of the plasma samples at the one hour time point. Two sulfate, mono-methyl ethers M345(A–B)-1 were seen at the one hour time point, but the isomer with the shorter retention time M345A-1 was only formed in trace amounts. By analogy to glucuronide, mono-methyl ether M441B-1, the major sulfate, mono-methyl ether is assumed to be the 3-methoxy regioisomer M345B-1, likely formed by sulfation of the major catechol mono-methyl ether M265B-1 by sulfotransferases.
Minor metabolic pathways included hydroxylation of the 4-heptamine moiety of either the parent S807 or of the corresponding mono-methyl ether metabolites to produce M279(A–B)-1, M281A-1 and M281B-1. The position of the hydroxyl group on the 4-heptyl group was not determined. The C-terminal hydroxylated analogs of these metabolites undergoes further oxidation to produce carboxylic acids M293A-1 and M295(A–B)-1. Mono-methyl ethers M281(A-B)-1 also undergo further Phase II metabolism to produce glucuronide mono-methyl ethers M457(A–B)-1.
In order to determine if saturation of a metabolic clearance pathway was responsible for the dramatic increase in the bioavailability of S807 at the higher doses, the relative exposures (Cmax and AUClast) of eight of the major metabolites observed at the one hour time point were determined in both male and female rats as a function of administered dose. The results are shown in Table 5. The results are expressed as a ratio of the Cmax and AUClast for each metabolite at the 50 and 200 mg/kg bw dose to that of the same metabolite at the 20 mg/kg bw dose. The relative exposure for each metabolite is also shown as a percent of the total AUClast for the eight metabolites designated in Table at a given dose.
Table 5.
Relative Exposure to the Major Metabolites of S807 as a Function of Administered Dose in Male and Female Rats.
| Metabolite | Dose (mg/kg bw) | Males |
Females |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Tmax (hr) | Cmax Ratioa |
AUClast Ratiob | % Total Metabolite AUClastc | Tmax (hr) | Cmax Ratioa |
AUClast Ratiob |
% Total Metabolite AUClastc | ||
| M265(A-B)-1 | 20 | 0.31 | 1.0 | 1.0 | 44.17 | 0.25 | 1.0 | 1.0 | 41.70 |
| 50 | 0.75 | 1.94 | 3.37 | 45.94 | 0.38 | 1.48 | 2.60 | 46.58 | |
| 200 | 3.0 | 4.32 | 17.7 | 50.06 | 0.50 | 2.66 | 11.6 | 48.54 | |
| M441B-1 | 20 | 4.5 | 1.0 | 1.0 | 29.26 | 2.2 | 1.0 | 1.0 | 35.74 |
| 50 | 6.0 | 2.10 | 2.17 | 19.55 | 2.8 | 1.83 | 2.14 | 32.82 | |
| 200 | 8.0 | 3.80 | 3.97 | 7.42 | 8.0 | 4.24 | 5.49 | 19.64 | |
| M427B-1 | 20 | 7.0 | 1.0 | 1.0 | 5.40 | 5.0 | 1.0 | 1.0 | 10.43 |
| 50 | 15 | 1.55 | 1.86 | 3.10 | 4.3 | 1.33 | 1.27 | 5.68 | |
| 200 | 24 | 3.98 | 3.20 | 1.10 | 8.3 | 3.05 | 3.33 | 3.47 | |
| M293A-1 | 20 | 0.50 | 1.0 | 1.0 | 7.34 | 0.44 | 1.0 | 1.0 | 7.25 |
| 50 | 1.75 | 2.34 | 3.26 | 7.37 | 1.0 | 1.28 | 2.59 | 8.06 | |
| 200 | 1.5 | 4.63 | 8.95 | 4.20 | 1.4 | 3.48 | 11.8 | 8.55 | |
| M279B-1 | 20 | 0.56 | 1.0 | 1.0 | 1.83 | 0.38 | 1.0 | 1.0 | 2.00 |
| 50 | 2.5 | 5.59 | 13.6 | 7.65 | 0.75 | 2.30 | 4.94 | 4.24 | |
| 200 | 6.0 | 51.1 | 156 | 18.19 | 8.0 | 17.7 | 87.1 | 17.43 | |
| M345B-1 | 20 | 1.0 | 1.0 | 1.0 | 6.65 | 0.50 | 1.0 | 1.0 | 0.80 |
| 50 | 2.0 | 2.19 | 4.37 | 8.96 | 0.88 | 1.49 | 2.43 | 0.84 | |
| 200 | 5.0 | 3.96 | 12.8 | 5.44 | 0.88 | 2.84 | 6.10 | 0.49 | |
| M281A-1 | 20 | 0.38 | 1.0 | 1.0 | 5.25 | 0.25 | 1.0 | 1.0 | 1.89 |
| 50 | 0.75 | 1.73 | 4.33 | 7.02 | 0.38 | 1.23 | 1.84 | 1.49 | |
| 200 | 3.0 | 6.48 | 39.2 | 13.16 | 0.50 | 1.73 | 5.61 | 1.06 | |
| M251A-1 | 20 | 0.31 | 1.0 | 1.0 | 0.09 | 0.25 | 1.0 | 1.0 | 0.19 |
| 50 | 0.44 | 2.63 | 13.8 | 0.39 | 0.31 | 1.63 | 3.47 | 0.28 | |
| 200 | 0.50 | 14.3 | 73.7 | 0.44 | 0.31 | 12.1 | 43.7 | 0.82 | |
Cmax Ratio = Cmax/Cmax at 20 mg/kg bw dose.
AUC Ratio = AUClast/AUClast at the 20 mg/kg bw dose.
% Total Metabolite AUClast =% peak area for a given metabolite as a% of the total peak area of the eight designated metabolites at that dose.
In general, the Tmax for most of the metabolites increased with increasing dose. Exposure to methyl ethers M265(A–B)-1 expressed as a percentage of the total metabolite AUClast did not change significantly at the higher doses in either male or female rats. However, at 50 and 200 mg/kg bw dose, the relative exposure to glucuronides M441B-1 and M427B-1 significantly decreased as a function of dose in both genders. In contrast, the relative exposure to the hydroxylated metabolite M279B-1 significantly increased from 1.83 to 2.00% at the 20 mg/kg bw dose, to 17.43–18.19% at the 200 mg/kg bw dose. Like the parent compound S807, both of its initial CYP-mediated oxidation products M251A-1 and M279B-1 also showed a greater than dose-proportional increase in AUClast at the 50 and 200 mg/kg bw doses relative to the 20 mg/kg bw dose in both male and female rats, although not to the same extent seen with S807. The product of oxidation of the 4-heptamine moiety of M265(A–B)-1 (i.e., M281A-1) is formed in a greater than dose-proportional manner in male rats, but less than dose-proportional in female rats, suggesting this metabolic pathway may be becoming saturated at higher doses in female rats. This was also reflected in an increase of the percent total metabolite AUClast for M281A-1 in male rats, but a slight decrease in female rats.
All of these findings taken together suggests that glucuronidation of M251A-1 and its mono-methyl ethers M265(A–B)-1 to produce M427B-1 and M441B-1 becomes rate-limiting at higher doses in both male and female rats, and oxidation of the 4-heptamine moiety of S807 and M265(A–B)-1 to produce M279B-1 (males and females) and M281A-1 (males only) becomes a more dominant metabolic pathway. The observation that the mono-methyl ether metabolites M265(A–B)-1 showed a roughly dose-proportional increase in AUClast despite a greater than dose-proportion increase in S807 plasma concentrations, is likely due to saturation of the pathway forming M251A-1 from S807, together with the saturation of the pathway producing M441B-1 from M265B-1 at higher doses.
3.1.3. Pharmacokinetics and in vivo metabolism of S9229 in rats
The PK parameters and oral bioavailability of S9229 in plasma was evaluated following either a single intravenous or oral administration in male and female Sprague-Dawley rats. Plasma samples were also analyzed for the presence of the metabolites observed in incubations of S9229 with rat liver microsomes. For intravenous administration, 4 male and 4 female Sprague-Dawley rats (Harlan Laboratories, Frederick, MD) were bolus injected with S9229 at 1 mg/kg bw in 1% ethanol in sterile saline (0.9% NaCl). Blood samples were collected from a tail vein at approximately 2, 5, 10, 30 min, 1, 2, 4, and 8 h post-dose. For oral administration, 3 male and 3 female Sprague-Dawley rats per group were given a single dose of S9229 at either 10, 30, or 100 mg/kg bw in 1% methyl cellulose (MC) in deionized water by oral gavage. Blood samples were taken from a tail vein at approximately 15, 30 min, 1, 2, 4, 8, and 24 h post-dose. Plasma samples spiked with an internal standard [N-(heptan-4-yl)benzo[d][1,3]dioxole-5-carboxamide; S807] were analyzed for S9229 by LC–MS/MS using a Waters SunFire C18 column (50 × 2.1 mm, 3.5 μm) with 0.1% formic acid/water and acetonitrile gradient system and an API 3200 Q-Trap mass spectrometer operated in positive ionization mode equipped with an Agilent 1100 binary pump with a CTC PAL injector. The pooled plasma samples from all of male and female rats dosed orally at 100 mg/kg were analyzed for S9229 metabolites using a similar method using a shallower 0.1% formic acid/water and acetonitrile gradient. A second chromatographic method using an Agilent Zorbax Eclipse XDB-C18 column (150 mm × 4.6 mm, 5 μm) with 0.1% formic acid/water and acetonitrile gradient was also used in the analysis for S9229 metabolites. The parent compound and internal standard (IS) were detected using a source which was configured with turboionspray ionization in positive mode using multiple-reaction monitoring (MRM) of mass transition pairs at m/z of 264.2/133.1 (S9229) and 264.2/166.1 (IS, S807) amu. The plasma concentration-time data were analyzed by non-compartmental methods using Phoenix WinNonlin version 1.1 (Pharsight/Certara company). Information Dependent Acquisition (IDA) from the Analyst software was used to create analytical methods for the S9229 metabolite analysis. Four modes of IDA survey (Enhanced MS, Precursor Ion, Neutral Loss, and Multiple Reaction Monitoring) and Enhanced Product Ion were used for data acquisition. Metabolite ID software was used for data processing. Ions selected for the Precursor Ion Neutral Loss Scans were based on the results of microsomal metabolism studies. Details of the analytical methods can be found in the Supplementary data section. Test article formulations prepared for this study were analyzed for concentration by HPLC-UV (240 nm). The pharmacokinetic parameters for S9229 are shown in Table 6.
Table 6.
Pharmacokinetics of S9229 in Male and Female Sprague-Dawley Rats.
| Route | Dose (mg/kg bw) | Sex | Cmax (ng/mL) | AUC0-24hr (ng·hr/mL) | Tmax (hr) | t1/2 (hr) | Cmax Ratioa | AUC0-24hr Ratiob | %F |
|---|---|---|---|---|---|---|---|---|---|
| iv | 1 | M | 3650 ± 1180 | 1694 ± 416 | 0.03 | 5.38 ± 2.16 | – | – | – |
| F | 3935 ± 1070 | 1733 ± 376 | 0.03 | 4.33 ± 1.65 | – | – | – | ||
| oral gavage | 10 | M | 19.4 ± 24 | 11.3 ± 10 | 0.25 | 1.38 ± 1.28 | 1 | 1 | 0.07% |
| F | 10.5 ± 13.9 | 15.2 ± 11.2 | 0.25 | 0.96 ± 0.30 | 1 | 1 | 0.09% | ||
| 30 | M | 11.9 ± 0.60 | 20.6 ± 3.7 | 0.25 | 1.78 ± 0.69 | 0.61 | 1.82 | 0.04% | |
| F | 55.4 ± 43.9 | 49.5 ± 31.7 | 0.25 | 1.82 ± 2.12 | 5.28 | 3.26 | 0.10% | ||
| 100 | M | 52.3 ± 12 | 175.4 ± 57.8 | 0.83 | 2.24 ± 1.09 | 2.70 | 15.5 | 0.10% | |
| F | 101.6 ± 124 | 175.4 ± 169 | 1.67 | 1.03 ± 0.32 | 9.68 | 11.5 | 0.10% |
Male rat: CL = 7.65 mL/min/kg; Vss = 3473 mL/kg.
Female rat: CL = 7.90 mL/min/kg; Vss = 2823 mL/kg.
CL = clearance; Vss = steady-state volume of distribution; %F = bioavailability.
Cmax Ratio = Cmax/Cmax at 10 mg/kg dose.
AUC0-24hr Ratio = AUC0-24hr/AUC0-24hr at 10 mg/kg dose.
For intravenous administration, the values of terminal half-life for S9229 in plasma were 5.38 ± 2.16 h in male rats and 4.33 ± 1.65 h in female rats. Mean plasma clearance (CL) in rats averaged 7.65 mL/min/kg for males (13.9% of hepatic blood flow) and 7.90 mL/min/kg for females (14.4% of hepatic blood flow), and the volume of distribution at steady-state (Vss) averaged 3473 and 2823 mL/kg (5.20 and 4.23-times total body water volume) for males and females, respectively.
For oral administration, the half-life values for S9229 in plasma ranged from 1.38 ± 1.28 to 2.24 ± 1.09 h in male rats and 0.96 ± 0.30 to 1.82 ± 2.12 h in female rats. With oral administration, AUC0-24hr was roughly proportional to dose in female rats, but was lower in males than in females at the 30 mg/kg bw dose. However, the absolute bioavailability and systemic exposure (AUC0-24hr) in female rats were not significantly different from those in male rats at the 100 mg/kg bw dose level.
The exposure to S9229 (Cmax and AUC0-24hr) in plasma was used for comparison of gender differences. For intravenous administration, the ratios of female/male AUC0-24hr and Cmax were 1.02 and 1.08, respectively. For oral administration, the ratios of female/male AUClast ranged from 1.00 to 2.41 and female/male Cmax ranged from 0.54 to 4.67. The absolute bioavailability (%F) of S9229 was very low and ranged from 0.09% to 0.10% in female rats and 0.04% to 0.10% in male rats.
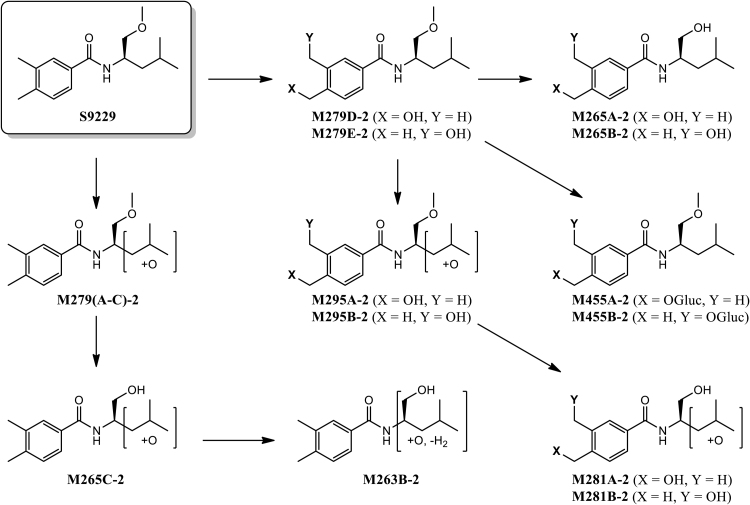
Table 7 shows the metabolite peak areas obtained from the extracted ion chromatograms (EIC) of the +MRM scans of the male and female pooled plasma samples at 1 h post dose (100 mg/kg bw). The nine Phase I metabolites shown are those with a peak area greater than 1% of the total peak area. Also included is a glucuronide (M455A/B-2) which was the only observed Phase II metabolite. At the 1 h time point, the parent compound S9229 represents less than 1% of the total peak area. The metabolic biotransformation of S9229 involved the hydroxylation, dihydroxylation, demethylation, and glucuronidation. Based on the peak areas from the Extracted Ion Chromatograms (EIC) of the Multiple Reaction Monitoring (MRM) scans, the hydroxylation of the C-4 aryl methyl group (M279D-2) was the dominant biotransformation representing 54% of the total metabolite peak area. The corresponding C-3 hydroxymethyl metabolite M279E-2 was formed to a much lesser extent, (<1%),which is also consistent with the results of the in vitro rat microsomal incubations. A glucuronide M455A/B-2 derived from either M279D-2 or M279E-2 was observed as a minor Phase II metabolite; the position of the glucuronide was not determined. Other major metabolic pathways involved the oxidative demethylation of the methyl ether moiety of either the C-3 or C-4 hydroxymethyl metabolites to produce the corresponding alcohols M265A-2 and M265B-2. Minor metabolic pathways included hydroxylation of the isobutyl moiety of either the parent S9229 or of the corresponding hydroxymethyl and/or demethylated primary metabolites to produce M279(A–C)-2, M295A-2, M281A-2 and M281B-2. The position of the hydroxyl group on the isobutyl moiety was not determined. The MRM scans suggest that the isobutyl hydroxylated metabolites are a mixture of isomeric compounds. The structures of the 3- and 4-hydroxymethyl (M279D-2 and M279E-2), as well as the 3- and 4- hydroxymethyl, O-demethyl metabolites (M265A-2 and M265B-2) were confirmed by comparison to synthetic standards by LC–MS/MS. The metabolic pathway of S9229 in rats is shown in Fig. 5. Taken together, the results of the in vivo metabolism study indicate that rapid oxidative metabolism by CYP enzymes is likely to be responsible for the poor oral bioavailability observed for the parent compound S9229.
Table 7.
Summary of Metabolites of S9229 Observed in Rat Plasma from a Sample at 1 h Post Dose (100 mg/kg bw).
| Metabolite | MRM (Q1/Q3) Ion Pair | Retention Time (min) | Peak Area (cps) | % of Total Metabolites |
|---|---|---|---|---|
| M279D-2 | 280.2/149.1 | 8.07 | 9190000 | 54.14 |
| M265A-2 | 266.2/149.1 | 6.24 | 3240000 | 19.09 |
| M265B-2 | 266.2/149.1 | 6.54 | 998000 | 5.88 |
| M295A-2 | 296.2/149.1 | 4.58 | 1180000 | 6.95 |
| M281A-2 | 282.2/149.1 | 3.42 | 441000 | 2.60 |
| M281B-2 | 282.2/149.1 | 3.97 | 362000 | 2.13 |
| M263A-2 | 264.2/133.1 | 9.26 | 414000 | 2.44 |
| M279(A-C)-2 | 280.2/133.1 | 7.79 | 308000 | 1.81 |
| M265C-2 | 266.2/133.1 | 6.34 | 250000 | 1.47 |
| M455(A or B)-2 | 456.2/280.2 | 6.50 | 76700 | 0.45 |
Fig. 5.
Metabolic Pathway of S9229 in Rat.
3.2. Genotoxicity and mutagenicity studies
Both S807 and S9229 were evaluated for their genotoxic potential through a standard (5-strain) Ames, chromosome aberration, and in vivo micronucleus test (see Table 8). All genetic toxicology studies were conducted in compliance with the FDA GLP regulations 21 CFR Part 58 [9] and [27].The data tables for the genotoxicity studies can be found in the Supplemental Material.
Table 8.
Summary of Genotoxicity Studies Conducted on S807 and S9229.
| End-Point | Test System | Cmpd No. | Concentration/Dose | Result |
|---|---|---|---|---|
| Reverse mutation (in vitro) | S. typhimurium strains TA98, TA100, TA1535, TA1537 and E. coli strain WP2 uvrA | S807 | 21–5000 μg/plate, plate incorporation and pre-incubation, ±S9a | Negative |
| S9229 | 50–5000 μg/plate, plate incorporation, ±S9a | Negative | ||
| Chromosome aberration (in vitro) | Chinese hamster ovary cells (CHO-WBL) | S807 | 21–5000 μg/mL, 3 h exposure −S9 21–5000 μg/mL, 3 h exposure +S9b 21–5000 μg/mL, 18 h exposure −S9 |
Negative |
| Primary human lymphocytes | S9229 | 225–322 μg/mL, 4 h exposure −S9 225–322 μg/mL, 4 h exposure +S9a 55–158 μg/mL, 20 h exposure −S9 |
Negative | |
| Micronucleus formation (in vivo) | Male Swiss albino (CD-1) mice, bone marrow PCEs | S807 | 175, 350, 700 mg/kg bw (ip) | Negative |
| Male and Female Hsd:ICR (CD-1) mice, bone marrow PCEs | S9229 | 500, 624, 1352, 2000 mg/kg bw (oral) | Negative |
S9 from rat liver homogenate from male Sprague-Dawley rats treated with Aroclor-1254.
S9 from rat liver homogenate from male Sprague-Dawley rats treated with phenobarbital/5,6-benzoflavone.
3.2.1. Bacterial reverse mutation test (5-strain Ames)
S807 and S9229 were evaluated for the potential to induce point mutations in S. typhimurium strains TA98, TA100, TA1535, TA1537 and E. coli strain WP2 uvrA in the presence and absence of metabolic activation with rat liver S9 from rats induced with Aroclor™ 1254. The assay was designed to meet the current OECD Guideline for Testing of Chemicals No. 471, Bacterial Reverse Mutation Test [25].
The concentrations of S807 investigated for both a plate incorporation and pre-incubation test ranged from 21 to 5000 μg per plate. In the case of S9229, the concentrations investigated for both an initial toxicity-mutation and confirmatory mutation test ranged from 50 to 5000 μg per plate. No toxicity was observed with either S807 or S9229 at any concentration, both the absence and presence of S9 mix, as evident by a normal background lawn and colony counts similar to the concurrent negative controls. No precipitate was observed at any concentration of S9229. In the case of S807, at 5000 and 1670 μg/plate both the absence and presence of S9 mix, the precipitate in some plates interfered with colony counting so that not all plates at these concentrations were analyzable. Therefore, the maximum analyzable concentrations of S807 were either 1670 or 5000 μg/plate depending on the extent of precipitation. Neither S807 nor S9229 increased the number of revertant colonies in either test with any of the tester strains both in the presence and absence of metabolic activation with rat liver S9. The concurrent positive controls demonstrated the sensitivity of the assay and the metabolizing activity of the liver preparations. Thus, it was concluded that both S807 and S9229 were not mutagenic to S. typhimurium strains TA98, TA100, TA1535, TA1537 and E. coli strain, WP2 uvrA in the absence and presence of metabolic activation under the test conditions employed.
3.2.2. In vitro chromosome aberration test
S807 and S9229 were investigated for their potential to induce structural and numerical chromosome aberrations in mammalian cells, both in the presence and absence of a supplemental rat liver fraction (S9). The experimental design followed the OECD Guideline for the Testing of Chemicals − 473, In Vitro Mammalian Chromosome Aberration Test [26].
In the case of S807, cultures of Chinese hamster ovary cells (CHO-WBL) were treated for 3 and 18 h in the non‐activated test system, and for 3 h in the presence of S9 from rats induced with phenobarbital and 5,6-benzoflavone. Solvent and positive control (mitomycin C, −S9; cyclophosphamide, +S9) cultures were also included. Concentrations of S807 evaluated in all test conditions ranged from 21 to 5000 μg/mL. Varying degrees of precipitate were seen in the cell cultures under all conditions at test article concentrations of 62 μg/mL or higher. Exposure to S807 resulted in a “U” shaped curve of Relative Cell Growth (RCG). The lowest RCG was observed at 560 μg/mL, not at 5000 μg/mL, and ranged from 7 to 37% for all three conditions. Concentrations of 0, 21, 190, 1670, 5000 μg/mL in the 3 and 18 h cultures in the absence of S9 had a RCG of ≥19% with a Relative Mitotic Index (RMI) of ≥28% and were chosen for chromosome analysis. For the 3 h exposure in the presence of S9, concentrations of 0, 21, 560, 1670, 5000 μg/mL had a RCG of ≥37% with a Relative Mitotic Index (RMI) of ≥100% and were chosen for chromosome analysis. Under these test conditions, no structural or numerical chromosome aberrations were observed in the S807 treated cultures beyond those seen in the concurrent solvent controls. The positive control agents induced chromosome aberrations as expected. It was therefore concluded that S807 did not induce chromosomal aberrations in cultured CHO-WBL cells when tested in accordance with regulatory guidelines.
In the case of S9229, cultures of human peripheral blood lymphocytes were treated for 4 and 20 h in the non‐activated test system, and for 4 h in the presence of S9 from rats induced with Aroclor™ 1254. A preliminary toxicity test was performed to establish the dose range for testing in the cytogenetic test. Substantial toxicity (at least 50% reduction in mitotic index relative to the solvent control) was observed at doses ≥789 μg/mL in both non-activated and S9-activated 4-h exposure groups, and at dose levels ≥263 μg/mL in the non-activated 20-h exposure group. Based on these findings, the doses chosen for the chromosome aberration assay ranged from 158 to 800 μg/mL for both the non-activated and the S9-activated 4-h exposure groups, and from 25 to 280 μg/mL for the non-activated 20-h exposure group. Solvent and positive control (mitomycin C, −S9; cyclophosphamide, +S9) cultures were also included in the definitive assay.
Visible precipitate was observed in the treatment medium at dose levels ≥ 322 μg/mL, while dose levels ≤ 280 μg/mL were soluble in the treatment medium at the beginning of the treatment period. At the conclusion of the treatment period, in the non‐activated and S9-activated 4-h exposure groups, visible precipitate was observed in the treatment medium at dose levels ≥ 460 μg/mL, while dose levels ≤ 322 μg/mL were soluble in the treatment medium. In the non-activated 20-h exposure group, all dose levels were soluble in the treatment medium at the conclusion of the treatment period. Selection of doses for microscopic analysis was based on mitotic inhibition (the lowest dose with at least 50% reduction in mitotic index, relative to the solvent control and two lower doses) in all harvests. Dose levels of 225, 280, and 322 μg/mL were analyzed for the non-activated and the S9-activated 4-h exposure groups and dose levels of 55, 110, 158 μg/mL were analyzed for the non-activated 20-h exposure group.
Under these test conditions, no structural or numerical chromosome aberrations were observed in the S9229 treated cultures beyond those seen in the concurrent solvent controls at any dose level (p > 0.05, Fisher’s Exact Test). The positive control agents induced chromosome aberrations as expected (p ≤ 0.01, Fisher’s Exact Test). It was concluded that exposure to S9229 did not induce chromosome aberrations in the in vitro mammalian chromosome aberration test using human peripheral blood lymphocytes in both the absence and presence of rat liver S9, when tested in accordance with regulatory guidelines.
3.2.3. In vivo micronucleus assay in mice
S807 and S9229 were evaluated for potential in vivo clastogenic activity and/or disruption of the mitotic apparatus, as measured by their ability to increase the incidence of micronucleated polychromatic erythrocytes (mnPCEs) in the bone marrow of CD-1 mice. The studies were designed to meet the current OECD Guideline for the Testing of Chemicals No. 474, Mammalian Erythrocyte Micronucleus Test [28]. Dose-range finding studies were performed to assess test articles toxicity and determine the maximum tolerated dose (MTD) or maximum feasible dose (MFD) for the definitive assay.
For the dose range finding phase of the study with S807, three groups of Swiss Albino (CD-1) mice (3 animals/sex/group; Charles River, Canada) were treated with either 500, 1000, or 2000 mg/kg bw of S807 as a solution in DMSO (132 mg/mL) by an intraperitoneal injection. A fourth group of 3 male and 3 female mice receiving 800 mg/kg bw S807, and a fifth group of three male mice receiving 700 mg/kg bw S807 were added after the initial dosing and received the test article by the same route of administration. Since deaths were seen in all dose groups ≥800 mg/kg bw, within 48 h of treatment, a 700 mg/kg bw dose level was considered to be the maximum tolerated dose (MTD) level of S807 given as a DMSO solution by intraperitoneal injection to CD-1 mice. Based on the results of the preliminary study, dose levels of 175, 350, and 700 mg/kg bw (21 animals/group; dose volume 5.3 mL/kg bw), were used for the definitive study with S807. Since no substantial differences in toxicity were observed between the sexes, the main test was performed using male animals only. In the definitive phase of the study, DMSO (5.8 mL/kg bw) was used as the vehicle (negative) control and cyclophosphamide, at a dose of 40 mg/kg bw (dose volume 2 mL/kg bw), was used as the positive control article. Animals were observed for signs of toxicity during the course of these studies.
In the definitive assay, 7 animals from each of group were euthanized 24, 36, or 48 h after dosing. Immediately following euthanasia, femoral bone marrow was collected from each animal. Bone marrow slides were prepared, fixed and stained (May-Grunwald/Giemsa) and polychromatic erythrocytes (PCEs, 2000/animal) were examined microscopically for the presence of micronuclei (mnPCEs). The ratio of PCEs to total erythrocytes (TE) in the test article groups relative to the vehicle control groups was also evaluated to reflect the test article’s cytotoxicity.
All animals survived for the duration of the test. Transient lethargy, passivity, and piloerection were observed in all dose groups treated with S807 during the first 4 h post-dose, after which all animals appeared to be normal. No appreciable reductions in the PCE/TE ratio in the S807 treated groups compared to the vehicle control group were observed indicating that the test article did not inhibit erythropoiesis. No statistically significant increase in the incidence of mnPCEs in the S807 treated groups was observed relative to the negative control group. The positive control (cyclophosphamide) induced statistically significant increases in the incidence of mnPCEs when compared to both the negative control groups and the test article treated groups at all three dose levels (p < 0.05).
In the in vivo micronucleus study of S9229, ICR [Hsd:ICR (CD-1)] mice (Harlan, Fredrick, MD) were treated with S9229 suspended in vehicle (1% methylcellulose (MC) in purified water) and administered at a volume of 20 mL/kg body weight by oral gavage for both the dose range finding and definitive phases of the study. In the definitive phase of the study, 1% MC was used as the vehicle (negative) control and cyclophosphamide, at a dose of 50 mg/kg bw, was used as the positive control article. Animal were observed for signs of toxicity during the course of the study.
The preliminary dose range finding study indicated that S9229 was well tolerated at the highest dose tested (2000 mg/kg bw) in both male and female mice. On the basis of the preliminary test, dose levels of 0, 500, 624, 1352, and 2000 mg/kg bw were used for the definitive micronucleus test. In the definitive study, both male and female animals (5 animals/sex/group) were treated with either S9229 or positive control and were euthanized by carbon dioxide asphyxiation 24 h post dosing. An additional three groups of animals (5 animals/sex/group) were treated either with vehicle or with S9229 at the two highest dose levels (1352 and 2000 mg/kg bw) and euthanized by carbon dioxide asphyxiation 48 h after dosing. An additional 5 animals/sex/group were also dosed at the two highest doses to be used as replacement animals in the event of mortality at these doses. At the time of euthanasia at either 24 and 48 h post-dose, femoral bone marrow was collected from 5 animals/sex/group; bone marrow smears (slides) were prepared and stained with Acridine orange stain. The proportion of PCEs to total erythrocytes (PCE/TE ratio) was determined as a measure of bone marrow toxicity. The polychromatic erythrocytes (2000 PCEs/animal) were microscopically evaluated and the incidence of mnPCE was determined.
No mortality was observed in the definitive studies of S9229. No appreciable reductions in the PCE/TE ratio in the S9229 treated groups relative to vehicle control groups were observed, suggesting that S9229 did not markedly inhibit erythropoiesis. No statistically significant increase in the incidence of micronucleated polychromatic erythrocytes in the male and female test article groups to the respective vehicle control groups was observed at 24 or 48 h after dose administration. Cyclophosphamide, the positive control, induced a statistically significant increase in the incidence of mnPCEs in both the male and female groups relative to vehicle controls (p < 0.05).
Under the condition tested, neither a single intraperitoneal injection of S807 at doses up to and including 700 mg/kg bw, nor a single oral dose of S9229 at doses up to and including 2000 mg/kg bw induced a significant increase in the incidence of mnPCEs in the bone marrow of CD-1 mice. Therefore, both S807 and S9229 were neither clastogenic nor aneugenic in the in vivo mouse micronucleus assay.
3.3. In vivo toxicological studies
S807 was evaluated in 21-day dose-range finding and 90-day subchronic toxicology studies in rats in compliance with the United States Food and Drug Administration (FDA) Guidelines [10] Toxicological Principles for the Safety of Food Ingredients. S9229 was evaluated in a 28-day subacute toxicology study in rats (see Table 9). Summary data tables for the 28-day toxicology study for S9229, and for the 21- and 90-day toxicology studies for S807 can be found in the Supplemental Material.
Table 9.
Summary of In Vivo Toxicity Studies Conducted on S807 and S9229.
| Study | Cmpd No. | Species/Gender (N value) | Dose | Findings |
|---|---|---|---|---|
| 21-day Dose Range Finding Toxicity Study | S807 | Male & Female Sprague-Dawley Rats – 5 animals/sex/group |
50, 100, 200 mg/kg bw/day (food ad-mix) |
Lower bw gain in females at 200 mg/kg bw/day; increased liver weight in females at 100 and 200 mg/kg bw/day; histomorphological changes in livers of both male and females at all doses; NOEL < 50 mg/kg bw/day |
| 13 Week Sub-Chronic Toxicity Study | S807 | Male & Female Sprague-Dawley Rats – 20 animals/sex/group |
2, 10, 20 mg/kg bw/day (food ad-mix) |
No test-article related findings; NOEL = 20 mg/kg bw/day |
| 28-day Sub-Acute Toxicity Study | S9229 | Male & Female Sprague-Dawley Rats – 10 animals/sex/group |
10, 30, 100 mg/kg bw/day (oral gavage) |
No test-article related findings; NOAEL = 100 mg/kg bw/day |
3.3.1. 21-day dose-range finding toxicity study on S807
The purpose of this study was to evaluate the potential systemic toxicity of S807 in rats after dietary administration for 21 days in order to select doses for a 90-day subchronic toxicity study in rats. Three treatment groups of male and female Crl:CD®(SD)IGS BR rats (n = 5/sex/group, Charles River Laboratories, Raleigh, NC) were administered S807 in the diet at dose levels of 50, 100, or 200 mg/kg bw/day. One additional group of five animals/sex served as the control and received the vehicle diet. The test substance was administered continuously via the diet throughout the treatment period. Dietary concentrations (ppm) of S807 for each group were adjusted each week based on bodyweight and food consumption data, in order to achieve constant doses in terms of mg/kg body weight/day. At the conclusion of the study, animals were anaesthetized with sodium pentobarbital, exsanguinated, and necropsied.
Survival, clinical observations, body weight, food consumption, hematology, clinical chemistry, organ weights, and macroscopic evaluations of all animals were used to assess potential toxicity. The liver from each animal was processed and examined microscopically; the kidneys from the control and high-dose (200 mg/kg bw/day) animals were processed and examined microscopically. Macroscopic lesions were examined from each animal.
All animals survived until scheduled euthanasia on Day 23. There were no clinical observations that were considered test article-related. Over the 21 days, mean body weight gain in females treated at 200 mg/kg bw/day was significantly less (−38%, p ≤ 0.05) compared to the controls (see Table 10). With the exception of mean food consumption for Days 7–10, mean absolute body weight and food consumption values were not significantly different from the concurrent control values. Numerically, however, both parameters were decreased 9.7% and 14.1%, respectively, by the end of the study. Mean absolute body weights, body weight change, and food consumption for males treated at 200 mg/kg bw/day and males and females treated at 50 and 100 mg/kg bw/day were not significantly different from respective control values.
Table 10.
Body and Liver Weight Changes in Rats Treated with S807 for 21 days Compared to Controls.
| S807 Dose: | 50 mg/kg bw/day | 100 mg/kg bw/day | 200 mg/kg bw/day |
|---|---|---|---|
| Males | |||
| Body weight (%) | ↓ 1.16% | ↑ 1.74% | ↑ 0.87% |
| Body weight gain (%, Day 21 vs Day 1) | ↑ 0.78% | ↑ 8.59% | ↑ 5.47% |
| Food consumption (%) | ↓ 6.09% | ↓ 1.25% | ↓ 2.33% |
| Liver weight (%) | ↑ 1.99% | ↑ 17.2% | ↑ 10.9% |
| Liver/body weight (%) | ↑ 4.10% | ↑ 15.2% | ↑ 9.44% |
| Liver/brain weight ratio (%) | ↓ 0.44% | ↑ 14.0% | ↑ 6.92% |
| Females | |||
| Body weight (%) | ↓ 0.40% | ↓ 3.23% | ↓ 9.68% |
| Body weight gain (%, Day 21 vs Day 1) | ↓ 7.04% | ↓ 14.1% | ↓ 38.0%* |
| Food consumption (%) | ↓ 4.99% | ↓ 5.90% | ↓ 14.1% |
| Liver weight (%) | ↑ 8.59% | ↑ 14.7% | ↑ 15.6% |
| Liver/body weight (%) | ↑ 8.94% | ↑ 19.1%* | ↑ 23.5%* |
| Liver/brain weight ratio (%) | ↑ 4.07% | ↑ 7.83% | ↑ 9.75% |
↑ = Increased; ↓ = Decreased;
Clinical pathology alterations were limited to slightly lower erythrocyte counts and hemoglobin and hematocrit values in males, and slightly higher cholesterol values in males treated at 100 or 200 mg/kg bw/day when compared to concurrent controls (p ≤ 0.05). Cholesterol values in females at all three treatment groups were also higher than that of controls, but only reached statistical significance at the highest dose. Globulin values were slightly higher than controls in males at 100 and 200 mg/kg bw/day, with resultant lower A/G ratios in these dose groups (p ≤ 0.05). These observations did not have a dose response effect and the hematological changes observed in males did not reach statistical significance. However, hemoglobin and hematocrit values for all males at 100 and 200 mg/kg bw/day were below the lowest control values, and the lack of statistical significance was considered to be related to the small group sizes, relatively small magnitude of difference between controls and dose-treated rats, and relatively large standard deviations. There were no meaningful alterations in the urinalysis parameters. Several other clinical pathology values were significantly different from controls; however, these were considered to be incidental and not related to test article administration.
Relative liver weights as a percent of body weight were increased in the 100 and 200 mg/kg bw/day females (p ≤ 0.05). These changes were due to a combination of decreased body weights and slightly increased liver weights in these groups since the increase in liver/brain weight ratio did not reach statistical significance (see Table 10). In one male treated at 200 mg/kg bw/day, the decrease in relative testicular weight was due to bilateral testicular atrophy and hypospermia observed microscopically and is not considered test article-related.
No macroscopic findings were attributed to test article administration. Test article-related histomorphologic changes were observed in the livers of 50, 100, and 200 mg/kg bw/day males and females. These changes were characterized by vacuolization of centrilobular hepatocytes in males and vacuolization of scattered random hepatocytes in females. Vacuoles were most commonly multiple and clear suggesting that they may be consistent with intracytoplasmic lipid accumulation. This is further supported by the clinical chemistry findings of increased plasma cholesterol levels observed in the 100 and 200 mg/kg bw/day dose groups. However, since this diagnosis is based strictly on hematoxylin and eosin-stained liver sections, cytoplasmic vacuolation due to phospholipidosis could not be ruled out [36]. The severity of changes was dose-dependent, but no hepatocellular necrosis was seen microscopically, and no increase in liver enzymes was seen in the clinical chemistry evaluations. All other microscopic findings of the liver or kidney or lesions were considerd incidential and not related to test-article administration.
In conclusion, S807 administered in the diet of rats for 21 days was considered palatable at 50, 100 and 200 mg/kg bw/day for all animals except females treated at 200 mg/kg bw/day since there were indications of non-palatability and altered body weight gains. Primarily due to the increased relative liver weights in females treated at 100 and 200 mg/kg bw/day and the histomorphological changes in the livers of both males and females in all treatment groups, a NOEL for S807 could not be established.
3.3.2. 13-Week subchronic toxicity study on S807
The purpose of this study was to evaluate the potential subchronic toxicity of S807 in rats after administration for 13 weeks. Test article was administered in the diet to four groups of twenty male and twenty female Crl:CD®(SD)IGS BR rats (Charles River Laboratories, Raleigh, NC) at dose levels of 0 (control), 2, 10, or 20 mg/kg bw/day for 93 consecutive days. The test substance was administered continuously via the diet throughout the treatment period. Dietary concentrations (ppm) of S807 for each group were adjusted each week based on body weight and food consumption data, in order to achieve constant doses in terms of mg/kg body weight/day.
Survival, clinical observations, body weight gain, food consumption, hematology, clinical chemistry, urinalysis, organ weights, macroscopic examination, and histopathologic evaluations were performed to assess potential toxicity. Once-daily cageside observations were made for each rat. Detailed clinical observations were done once prior to initiation of treatment, weekly during the study, and on the day of scheduled sacrifice. These weekly observations were made outside the home cage and included, but were not limited to, changes in skin, fur, eyes, and mucous membranes; occurrences of secretions and excretions; and autonomic activity (e.g., lacrimation, piloerection, pupil size, unusual respiratory pattern). Changes in posture and reactivity to handling and the presence of clonic or tonic movements, stereotypies (e.g., excessive grooming, circling), or bizarre behaviour (e.g., self-mutilation, walking backwards) were also recorded weekly. Changes in gait were assessed weekly by allowing the animal to walk freely for evaluation. Expanded clinical observations were conducted on 10 rats/sex/group once prior to treatment and on the same 10 rats/sex/group during Week 13 to screen for neurotoxic effects. Each rat was evaluated during handling (Hand-Held Observations) in an open field (Open Field Observations) and assessed for sensory reactivity to stimuli (Elicited Behaviors). These observations were made on a day other than that scheduled for weekly examination.
Opthalmoscopic examinations were conducted prior to initiation of treatment and on Week 13 of test article administration for animals in the control and high dose groups. Samples for hematology, coagulation, clinical chemistry and urinalysis were collected from all animals at scheduled sacrifice. Urine was collected overnight (approximately 16 h; timed sample collection) before blood collection. Body weights were taken prior to initiation of treatment, on the first day of treatment, on Day 4, and weekly thereafter. Food consumption was measured on Day 4 (from Day 1) and weekly thereafter. After at least 90 days of treatment, rats were anaesthetized with sodium pentobarbital, exsanguinated, and necropsied. A total of 12 protocol-specified organ weights were recorded for all animals at scheduled sacrifice.
A total of 44 protocol-specified tissues from each animal in the control and high-dose groups and any animal that died or was sacrificed at an unscheduled interval were embedded in paraffin, sectioned, stained with hematoxylin and eosin, and examined microscopically. At study termination, after weighing, the liver from each rat in all groups was divided into two portions (left and right lobes). One lobe was flash frozen in OCT (optimum cutting temperature formulation) and subsequently sectioned and stained with Oil Red O for microscopic examination to visualize potential fat deposits. The remaining lobe underwent preservation for histopathological testing in the normal manner. Portions of the lymph node (mandibular and mesenteric), Peyer’s patches, spleen, and thymus were also preserved in OCT and stored at −60 to −80 °C, for possible future immunohistochemical examination.
There were no test article-related deaths during the study. One control female was found dead on study Day 72. The cause of death was not evident upon microscopic examination. Mean weekly body weights for males and females given 2, 10, or 20 mg/kg bw/day and mean weekly body weight changes and food consumption for females given 2, 10, or 20 mg/kg bw/day were not significantly different from the respective controls (see Fig. 6, Fig. 7). Mean body weight changes during Week 6 in males given 20 mg/kg bw/day and during Week 11 in males given 2, 10, and 20 mg/kg bw/day were significantly higher compared to the controls (p ≤ 0.05). During Week 12, mean body weight changes were significantly lower in males given 2, 10, and 20 mg/kg bw/day (p ≤ 0.05). The aforementioned findings appeared related to the significant changes in food consumption for these groups. Based on their infrequency and inconsistency and given the fact that the body weights were not similarly affected, these changes are not considered adverse or necessarily related to S807 treatment.
Fig. 6.
Mean body weights of male Sprague-Dawley rats receiving S807 for 13 weeks (n = 20).
Fig. 7.
Mean body weights of female Sprague-Dawley rats receiving S807 for 13 weeks (n = 20).
There were no test article-related clinical signs observed during the study and there were no treatment-related ophthalmic lesions. Red eye discharge was generally noted at all dose levels in both males and females. Based on the occurrence in controls and lack of a dose-response, red eye discharge was not necessarily considered test article-related. Lens opacity was observed in one control male. Chromodacryorrhea was detected in two females given 20 mg/kg bw/day, which corresponds to the red eye discharge noted in the clinical observations. This finding was not considered adverse or necessarily treatment-related. The appearance and behaviour of the animals, sensory reactivity, nociceptive reflex, and grip strength were unaffected by treatment.
There were no test article-related effects among hematology parameters, coagulation times, clinical chemistry analytes, or urinalysis parameters in either sex at any dose level. Any observed differences were minor and as the majority of individual values were within the background range, were not considered toxicologically significant. There were no test article-related organ weight, macroscopic or microscopic changes noted at any dose level. All inter-group differences from controls were minor, seen in one sex only and were therefore attributed to normal biological variation. In the liver, bile duct hyperplasia, mononuclear infiltrates, and mild vacuolization of hepatocytes were seen with similar frequency in both males and females in all groups including controls.
In conclusion, once daily oral administration of S807 for 93 days was well tolerated in rats at dose levels up to 20 mg/kg bw/day. No test article-related mortality or evidence of any systemic toxicity was observed and no target organs were identified. Based on the findings in this study the no-observed-effect-level (NOEL) was considered to be 20 mg/kg bw/day for both male and female rats. See Supplementary data for summary of the 13-week subchronic toxicity study data for S807.
3.3.3. 28-Day subacute toxicity study on S9229
The purpose of this study was to evaluate the potential toxicity of S9229 in rats after administration for 28 consecutive days. Three treatment groups of ten male and ten female CD® [Crl:CD®(SD)] rats (Charles River Laboratories, Portage, MI) were administered S9229 as a suspension in 1% MC (10 mL/kg bw dose volume) by oral gavage at dose levels of 10, 30, or 100 mg/kg bw/day. One additional group of ten animals/sex served as the control and received the vehicle, 1% MC in deionized water. Additionally, one group of three animals/sex and three groups of six animals/sex/group served as toxicokinetic (TK) animals and received the vehicle or test article in the same manner and at the same dose levels as the main study groups.
Survival, clinical observations, body weight gain, food consumption, hematology, clinical chemistry, urinalysis, organ weights, macroscopic examination, and histopathologic evaluations were performed to assess potential toxicity. Cageside clinical observations were conducted on main study animals daily. Detailed observations for clinical signs were made outside the home cage in a standard area and were conducted on main study animals weekly, beginning in Week 1. A sensory reactivity and motor activity assessment (including, but not limited to, evaluation of motor activity, arousal, auditory startle response, righting reflex, tail pinch response, grip strength, pupil response, and respiration) was conducted on all main study animals during the 4th week of test article administration. Opthalmoscopic examinations were conducted pre-test and on all main study animals on the day prior to terminal necropsy. Blood samples for hematology, coagulation, and clinical chemistry evaluations were collected from all main study animals after an overnight fast prior to terminal necropsy. Samples for urinalysis evaluations were collected from all main study animals for at least 12 h prior to terminal necropsy. Blood samples (approximately 0.5 mL) were collected from TK animals via the sublingual vein for determination of the plasma concentrations of the test article. Samples were collected from control animals at 1 h post-dose on Days 1 and 28, and from two cohorts of three treated animals/sex/group each, at alternating time points at 0.5, 1, 3, 6, 12 and 24 h post-dose on Day 1 and at 0, 1, 3, 6, 12, and 24 h post-dose on Day 28. The animals were not fasted prior to blood collection. Samples were placed in tubes containing K2EDTA anticoagulant. At study termination, all main study animals were euthanized by carbon dioxide asphyxiation followed by exsanguination via the abdominal vena cava, necropsy examinations were performed, and organ weights were measured and recorded. Microscopic examination of fixed hematoxylin and eosin-stained paraffin sections were performed on sections of tissues of all animals in the control and high-dose (100 mg/kg bw/day) groups.
The plasma half-life values for S9229 ranged from 0.90 ± 0.23 to 1.16 ± 0.46 h on Day 1 and from 0.98 ± 0.10 to 2.16 ± 1.30 h on Day 28. Exposure to S9229 in plasma (based on AUC0-24hrs and Cmax) increased in a greater than dose proportional manner with increasing dose. The low values observed for both Cmax and AUC0-24hrs are likely to be related to poor oral absorption and/or rapid metabolism. In general, the exposure to S9229 in plasma was not significantly different between genders on both Day 1 and Day 28. Exposure to S9229 (AUC0-24hrs and Cmax) appeared to be lower on Day 28 day than on Day 1. However, this is largely due to differences in sampling times on Days 1 and 28. Note that on Day 1, Tmax occurred at the first time point (0.5 h), while the earliest time points collected on Day 28 were 0 (pre-dose) and 1.0 h. Therefore, exposure on Day 28 was likely to have been underestimated (see Table 11). Using AUC1-24hrs to assess possible accumulation of S9229 following repeat dosing, no significant accumulation of S9229 occurred after 28 days of dosing. The ratios of Day 28 AUC1-24hrs/Day 1 AUC1-24hrs ranged from 0.55 to 1.53 for female rats and 0.56 to 1.08 for male rats.
Table 11.
Toxicokinetics of S9229 in Male and Female Sprague-Dawley Rats (oral gavage).
| Time Point | Dose (mg/kg bw) | Sex | Cmax (ng/mL) | AUC0-24hr (ng ·hr/mL) | Tmax (hr) | t1/2 (hr) | Cmax Ratioa | AUC0-24hr Ratiob |
|---|---|---|---|---|---|---|---|---|
| Day 1 | 10 | M | 3.2 ± 1.3 | 3.9 ± 0.8 | 0.50 | 0.90 ± 0.23 | 1 | 1 |
| F | 15.6 ± 17.0 | 11.4 ± 11.1 | 0.50 | NC | 1 | 1 | ||
| 30 | M | 22.6 ± 10.5 | 25.8 ± 4.3 | 0.50 | 1.16 ± 0.46 | 7.06 | 6.62 | |
| F | 8.6 ± 2.6 | 12.6 ± 2.8 | 0.50 | 0.95 ± 0.48 | 0.55 | 1.11 | ||
| 100 | M | 197.7 ± 98.5 | 188.3 ± 78.8 | 0.50 | 1.04 ± 0.04 | 61.8 | 48.3 | |
| F | 356.1 ± 255.9 | 266.8 ± 173.7 | 0.50 | 0.97 ± 0.36 | 22.8 | 23.4 | ||
| Day 28 | 10 | M | 1.2 ± 0.3 | 1.5 ± 0.6 | 1.67 | NC | 1 | 1 |
| F | 1.3 ± 1.3 | 2.1 ± 1.9 | 1.0 | NC | 1 | 1 | ||
| 30 | M | 6.8 ± 2.3 | 15.6 ± 2.7 | 1.0 | 1.42 ± 0.28 | 5.67 | 10.4 | |
| F | 6.3 ± 3.2 | 14.5 ± 4.3 | 1.0 | 2.16 ± 1.30 | 4.85 | 6.90 | ||
| 100 | M | 40.3 ± 20.5 | 106.6 ± 41.6 | 1.0 | 1.39 ± 0.28 | 33.6 | 71.1 | |
| F | 74.4 ± 31.7 | 144.0 ± 45.1 | 1.0 | 0.98 ± 0.10 | 57.2 | 68.6 |
NC = not calculated.
Cmax Ratio = Cmax/Cmax at 10 mg/kg dose.
AUC0-24hr Ratio = AUC0-24hr/AUC0-24hr at 10 mg/kg dose.
No unscheduled deaths occurred during the course of the study and no clinical observations of toxicity were noted. High dose (100 mg/kg bw/day) males had a significantly higher body weight gain (16.7%, p < 0.05) relative to controls which was associated with higher food consumption. In females, body weight gain tended to be slightly lower in all treatment groups relative to controls but did not show a dose relationship and did not reach statistical significance (see Fig. 8, Fig. 9, and Table 12). The appearance, behaviour, sensory reactivity findings, grip strength values and motor activity scores of the animals were unaffected by treatment. There were no ophthalmic lesions in Week 4 that were considered to be associated with treatment. There were no test article-related effects on coagulation or urinalysis parameters. There were no test article-related macroscopic or microscopic changes noted at any dose level of S9229. All macroscopic and microscopic observations were considered incidental/spontaneous, of the nature commonly observed in this strain and age of rats, and/or were of similar incidence and severity in control and treated animals.
Fig. 8.
Mean body weights of male Sprague-Dawley rats receiving S9229 for 4 weeks (n = 10).
Fig. 9.
Mean body weights of female Sprague-Dawley rats receiving S9229 for 4 weeks (n = 10).
Table 12.
Body, Thymus, and Liver Weight Changes in Rats Treated with S9229 for 28 days Compared to Controls.
| S9229 Dose: | 10 mg/kg bw/day | 30 mg/kg bw/day | 100 mg/kg bw/day |
|---|---|---|---|
| Males | |||
| Body weight (%) | ↑ 3.38% | ↑ 2.74% | ↑ 6.94%* |
| Body weight gain (%, Day 28 vs Day −1) | ↑ 8.50% | ↑ 6.11% | ↑ 16.7%* |
| Food consumption (%) | ↓ 0.55% | ↑ 2.77% | ↑ 5.35% |
| Thymus weight (%) | ↑ 24.1% | ↑ 20.2% | ↑ 26.3%* |
| Thymus/body weight (%) | ↑ 17.9% | ↑ 16.6% | ↑ 17.5% |
| Thymus/brain weight ratio (%) | ↑ 22.9% | ↑ 23.5% | ↑ 26.1%* |
| Liver weight (%) | ↑ 4.89% | ↑ 4.12% | ↑ 16.8%** |
| Liver/body weight (%) | ↑ 0.51% | ↑ 1.08% | ↑ 8.63%** |
| Liver/brain weight ratio (%) | ↑ 3.66% | ↑ 6.93% | ↑ 16.2%** |
| Females | |||
| Body weight (%) | ↓ 4.11% | ↓ 2.53% | ↓ 5.72% |
| Body weight gain (%, Day 28 vs Day −1) | ↓ 12.8% | ↓ 8.28% | ↓ 17.3% |
| Food consumption (%) | ↓ 6.50% | ↓ 1.58% | ↓ 4.36% |
| Thymus weight (%) | ↑ 10.7% | ↓ 4.50% | ↓ 12.0% |
| Thymus/body weight (%) | ↑ 14.3% | ↓ 1.69% | ↓ 6.96% |
| Thymus/brain weight ratio (%) | ↑ 8.71% | ↓ 6.90% | ↓ 12.5% |
| Liver weight (%) | ↓ 5.78% | ↓ 2.86% | ↓ 6.93% |
| Liver/body weight (%) | ↓ 1.57% | ↑ 0.12% | ↓ 1.44% |
| Liver/brain weight ratio (%) | ↓ 7.08% | ↓ 5.13% | ↓ 7.27% |
↑ = Increased; ↓ = Decreased.
Significantly different from control (p < 0.05).
Significantly different from control (p < 0.01).
Non-adverse hematology changes included decreased red cell mass (erythrocytes, hemoglobin, and hematocrit) in males at 30 and 100 mg/kg bw/day (p < 0.05–0.01), but individual values remained within expected ranges and there was no similar change in the females. Non-adverse clinical chemistry changes included minimally increased albumin among females at 100 mg/kg bw/day (p < 0.05).
Thymus absolute weights and thymus to brain weight ratio tended to be higher compared to controls in all treated males, although only reaching statistical significance at the 100 mg/kg bw/day dose (26.1% increase in thymus to brain weight ratio at 100 mg/kg bw/day, p < 0.05). Liver absolute weight, liver to body weight percentage, and liver to brain weight ratio were all significantly increased in 100 mg/kg bw/day males (16.8%, 8.63%, and 16.2%, respectively; p < 0.01) over controls (see Table 12). However, these changes were not considered adverse as they only occurred in one sex and no corresponding microscopic findings were associated with these organ weight changes.
In conclusion, once daily oral administration of S9229 for 28 days was well tolerated in rats at dose levels up to 100 mg/kg bw/day. No test article-related mortality or evidence of any systemic toxicity was observed and no target organs were identified. Based on these results, the No Observed Adverse Effect Level (NOAEL) was considered to be 100 mg/kg bw/day, the highest dose level tested, in male and female rats. See Supplementary data for summary of the 28-day subacute toxicity study data for S9229.
4. Discussion and conclusions
Toxicological evaluations of two N-alkyl benzamide umami flavour compounds, N-(heptan-4-yl)benzo[d][1,3]dioxole-5-carboxamide (S807) and (R)-N-(1-methoxy-4-methylpentan-2-yl)-3,4-dimethylbenzamide (S9229), were completed for the purpose of assessing their safety for use in food and beverage applications. S807 and S9229 are members of a novel series of N-alkyl benzamide agonists of the human umami receptor hTAS1R1/hTAS1R3 which can provide an umami flavour effect in product applications equivalent to that of MSG at a 1000-fold lower concentration.
The metabolic profile of S807 in both rat and human liver microsomes was qualitatively very similar producing the same set of oxidative metabolites in either species, suggesting that the rat was an appropriate species for evaluating the potential toxicity of S807. The major metabolite of S807 produced by the microsomes of both species was the catechol M251A-1. Oxidative demethylenation is known to be a dominant metabolic pathway for methylenedioxyphenyl derivatives, especially in the case of highly lipophilic compounds [16], [13]. On oral dosing in rats, S807 exhibited marked non-linear pharmacokinetics; oral bioavailability (%F) of S807 ranged from 1.85 to 2.43% at the 20 mg/kg bw dose to 102.9–157.9% at the 200 mg/kg bw dose. There was also a significant gender difference in both the rate and extent of exposure at the 50 mg/kg bw oral dose of S807 in rats which was not seen at the 20 and 200 mg/kg bw doses. Taken together, the data suggests that the dramatic increase in the bioavailability of S807 at the higher doses is likely due to saturation of first-pass metabolism and/or tissue distribution. The gender difference seen at the 50 mg/kg bw dose suggests that the clearance pathway for S807 becomes saturated at a lower concentration in males than in females. Gender-dependent metabolism of xenobiotics and sexual dimorphisms in response to inducing agents are well known phenomena in rats that has been attributed to differences in the profile of CYP isozymes found in male and female rat liver [15], [24].
In vivo, the initially formed catechol M251A-1 observed as the major metabolite formed in the microsomal incubations is rapidly conjugated, either directly or after subsequent O-methylation, and then further oxidized to form a total of thirteen Phase II metabolites. At the 20 mg/kg bw dose, the mono-methyl ethers M265(A-B)-1 and glucuronide mono-methyl ether M441B-1 metabolites were the dominant biotransformations representing 41.70–44.17% and 29.26–35.74% of the total metabolite AUClast, respectively. The 3-methoxy isomer M265B-1 was by far the dominant O-methyl regiosiomer formed from M251A-1 by the action of catechol-O-methyltransferase (COMT). In vitro, the COMT enzymes from both rat and human are known to favour the 3-O-methylation of numerous catechol substrates with side chains containing anionic or cationic functional groups including 3,4-dihydroxybenzoic acid, 3,4-dihydroxyphenylacetic acid, 3,4-dihydroxyphenylalanine, as well as various catecholamines such as dopamine, α-methyldopamine, and norepinephrine. The reported 3-O-methyl/4-O-methyl ratios for these substrates using rat COMT ranges from 5.5 to 19.8 [4], [5], [20]. In contrast, catechol substrates with neutral or non-polar side chains such as 4-ethyl catechol, 3,4-dihydroxybenzyl alcohol, ethyl 3,4-dihydroxybenzoate, 3,4-dihydroxybenzonitrile, 3,4-dihydroxyacetophenone, and N-acetyl dopamine show little or no preference for 3-O-methylation over 4-O-methylation. The reported 3-O-methyl/4-O-methyl ratios for these substrates using rat COMT ranges from 0.46 to 2.0. Different meta/para ratios of substituted catechols have been attributed to their relative ability to bind in two dissimilar orientations in the active site of COMT [17], [37], [22].
Based on its lack of a preference for 3-O-methylation over 4-O-methylation for catechols with non-polar, lipophilic side chains, COMT would be expected to produce both mono-methyl ethers M265A-1 (4-O-methyl) and M265B-1 (3-O-methyl) and from catechol M251A-1 (calculated logP value of 2.99) in more or less equal amounts. Indeed, when catechol M251A-1 was incubated in vitro with native porcine COMT in the presence of S-adenosyl-l-methionine, M265B-1 and M265A-1 were produced in a ratio of 1.9 to 1 [2]. However, after an oral dose of S807 in rats, M265B-1 was found to be present at concentrations 60- to 170-fold higher than that of M265A-1 over the entire 24 h observation period. Also, in the case of the glucuronide, mono-methyl ethers M441(A-B)-1, the 3-O-methyl regioisomer M441B-1 is again present at concentrations that are significantly higher than the 4-O-methyl regioisomer M441A-1, indicating that the lower concentration of M265A-1 relative to M265B-1 is not due to rapid conversion to the corresponding glucuronide M441A-1.
This paradoxical in vivo regioselectivity for 3-O-methylation of catechols has been reported for other substrates and has been attributed to selective demethylation of the putative 4-O-methyl derivatives by the microsomal enzyme system to regenerate the parent catechol derivative [29], [1]. The net result is an accumulation of the 3-O-methyl metabolite over the 4-O-methyl regioisomer. To see if this might explain the apparent in vivo regioselectivity for 3-O-methylation for catechol M251A-1, synthesized samples of both mono-methyl regioisomers M265A-1 and M265B-1 were incubated with mixed gender, rat liver microsomes using the same protocol described in Section 3.1.1 for S807. In a parallel experiment designed to assess whether one regioisomer could affect the rate of demethylation of the other, an equimolar mixture of M265A-1 and M265B-1 was also incubated with rat liver microsomes. Samples were analyzed for the loss of the parent compound and production of M251A-1 (demethylated product) and M281(A-B)-1 (side chain oxidation product) at 0, 5, 10, 20 and 60 min (see Table 13). The 4-O-methyl regioisomer M265A-1 was found to be metabolized by rat microsomes only slightly faster than its 3-O-methyl regioisomer M265B-1. At the end of the 60 min incubation period, 47.7% of M265A-1 remained versus 55.7% of M265B-1. The co-incubation experiment produced similar results. Both regioisomers produced both catechol M251A-1 and hydroxylation products M281(A–B)-1, but these products only accounted for a small percentage of the parent compound loss assuming that the MS response factor for M265(A–B)-1 and its oxidation products M251A-1 and M281(A–B)-1 are comparable. However, more of these products were produced from M265A-1 than M265B-1. In addition, a significant loss of parent compound was observed with both compounds in the absence of NADPH consistent with metabolism by a CYP-independent pathway. Assuming that the two O-methyl regioisomers are initially produced in more or less similar quantities by the action of rat COMT in vivo, an alternative explanation for the apparent in vivo regioselectivity for 3-O-methylation may be that the 4-O-methyl isomer M265A-1 is selectively removed by an alternative metabolic or elimination pathway.
Table 13.
Metabolism of M265A-1 and M265B-1 by Rat Liver Microsomes.
| Test Articlea | Incubation Time (min) | % M265A-1 Remainingb | % M265B-1 Remainingb | % M251A-1 Producedb | % M281(A-B)-1 Producedb |
|---|---|---|---|---|---|
| M265A-1 | 0 | 100.0 | N/A | ||
| 5 | 87.0 | 0.67 | 0.40 | ||
| 10 | 85.0 | 1.13 | 0.65 | ||
| 20 | 68.4 | 1.14 | 1.22 | ||
| 60 | 47.7 | 4.26 | 3.83 | ||
| 60 (w/o NADPH) | 94.9 | ||||
| M265B-1 | 0 | N/A | 100.0 | ||
| 5 | 105.6 | 0.48 | 0.03 | ||
| 10 | 95.0 | 0.64 | 0.07 | ||
| 20 | 80.8 | 0.79 | 0.26 | ||
| 60 | 55.7 | 1.84 | 1.12 | ||
| 60 (w/o NADPH) | 81.1 | ||||
| M265A-1 and M265B-1 | 0 | 100.0 | 100.0 | ||
| 5 | 106.2 | 106.9 | 1.17 | 0.54 | |
| 10 | 101.9 | 105.7 | 1.72 | 0.81 | |
| 20 | 73.0 | 77.9 | 1.76 | 1.18 | |
| 60 | 42.8 | 54.0 | 5.80 | 3.65 | |
| 60 (w/o NADPH) | 71.9 | 74.2 |
Test article concentrations were 1.0 μM.
All data normalized to test article MS peak area at time = 0.
An evaluation of the relative exposures of the eight major metabolites of S807 as a function of increasing dose indicated that glucuronidation of catechol M251A-1 and its mono-methyl ethers M265(A–B)-1 becomes rate limiting at higher doses and oxidation of the 4-heptamine moiety of S807 and M265(A–B)-1 becomes a more dominant metabolic pathway. The M265B-1 exposure data also suggests the oxidative demethylenation of S807 to form M251A-1 may also be rate limiting at higher doses, but this is offset by the saturation of the pathway producing glucuronide M441B from mono-methyl ether M265B-1. Therefore, saturation of the oxidative demethylenation pathway for S807 is likely to be at least partially responsible for the dramatic increase in exposure of S807 with increasing dose.
In the case of S9229, the metabolite profile produced by both rat and human liver microsomes was also qualitatively similar for both species and involved hydroxylation of the aryl methyl groups, hydroxylation of the isobutyl side chain, and demethylation of the side chain methyl ether. The major metabolite produced by the microsomes of both species was the corresponding C-4 hydroxymethyl compound M279D-2. On oral dosing in rats, the oral bioavailability (%F) of S9229 was very low and ranged from 0.04% to 0.10% in male and female rats. Consistent with the results of the microsomal metabolism study, the C-4 hydroxymethyl metabolite M279D-2 was the dominant in vivo metabolite in the rat. Other metabolic pathways involved the oxidative demethylation of the methyl ether moiety and/or hydroxylation of the isobutyl moiety of S9229 or its C-3 or C-4 hydroxymethyl metabolites. The results of the in vivo metabolism study indicate that rapid oxidative metabolism by CYP enzymes is likely to be responsible for the poor oral bioavailability observed for the parent compound S9229.
Both S807 and S9229 were evaluated for their genotoxic potential through a standard battery of in vitro genotoxicity assays which included a bacterial reverse mutation assay (S. typhimurium strains TA98, TA100, TA1535, TA1537 and E. coli strain WP2 uvrA), and a chromosome aberration test in either CHO-WBL cells or HPBL. Both compounds were found to be neither mutagenic or clastogenic in these in vitro genotoxicity assays. S807 and S9229 were also evaluated in an in vivo mouse micronucleus assay. Intraperitoneal administration of S807 at doses up to 700 mg/kg bw (MTD) to male CD-1 mice, or oral administration of S9229 at doses up to 2000 mg/kg bw (limit dose) to male and female CD-1 mice, was well tolerated and did not induce clastogenicity nor aneugenicity in bone marrow erythrocytes. No appreciable reductions in the PCE/TE ratio in the test article groups compared to the concurrent vehicle control groups were observed with either compound indicating neither compound was cytotoxic to the bone marrow or inhibited erythropoiesis.
In the 21-day dose range-finding toxicity study of S807, the most significant findings were increased relative liver weights in females treated at 100 and 200 mg/kg bw/day and the histomorphological changes in the livers of both males and females in all treatment groups consistent with intracytoplasmic lipid accumulation, although vacuolation due to phospholipidosis could not be ruled out. As a result of these findings, the NOEL in the 21 day range finding study was considered to be <50 mg/kg bw/day. The doses chosen for the definitive 13-week subchronic toxicology study of S807 were 2, 10, or 20 mg/kg bw/day. These doses were designed to define a NOEL for S807 while still providing an adequate margin of safety under conditions of intended use as a flavouring agent. Because of the finding of cytoplasmic vacuolation in the livers of rats at the higher doses utilized in the range-finding study, one lobe of the liver of all animals was flash frozen in OCT and subsequently sectioned and stained with Oil Red O for microscopic examination to visualize potential lipid deposits and rule out vacuolation due to phospholipidosis. Dietary administration of S807 rats for 13-weeks at doses of up to 20 mg/kg bw/day was generally well tolerated. In contrast to the liver findings in the range-finding study conducted at higher doses of S807, only a few animals were seen with cytoplasmic vacuolization of hepatocytes, and this occurred with similar frequency in both males and females in all groups including controls. Due to the low frequency and mild severity of this finding, staining the frozen sections with Oil Red O did not conclusively identify these vacuoles as lipid containing. There were no increases in liver weights seen in any of the dose groups. Based on the findings in this study the NOEL was considered to be 20 mg/kg bw/day for both male and female rats.
The Joint FAO/WHO Expert Committee on Food Additives (JECFA) and ESFA Panel on Food Contact Material, Enzymes, Flavouring and Processing Aids (CEF) employed the maximized survey-derived intake (MSDI) method as a measure of dietary exposure to a flavouring agent for use in their safety evaluation of S807 [12], [8]. The MSDI is based on the reported amount of a flavouring agent introduced into the food supply per year in specific regions of the world and provides a per capita estimate of the exposure to the flavouring agent, assuming that 10% of the relevant population would consume foods containing the flavouring agent. In the case of S807, the JECFA included intake estimates based on the MSDI approach derived from both European and USA production figures at the time of its evaluation. The FEMA Expert Panel recently updated this exposure calculation based on the most recent reported annual volume of use (1000 kg). Based on this most recent surveyed volume, the per capita intake of S807 from use as a flavour ingredient was calculated to be 294 μg/person/day [3]. Therefore, the no-observed-effect level (NOEL) of 20 mg/kg bw/day from the 13-week study in rats was judged to provide an adequate margin of safety (>4000-fold) in relation to the currently estimated use as a flavouring agent (4.9 μg/kg bw/day).
However, in many cases the MSDI is believed to underestimate the dietary exposure to some flavouring agents especial in cases where the annual production values were reported to be small. In connection with their safety evaluation of S807, the EFSA Panel also performed an estimate of the intake per person using a Modified Theoretical Added Maximum Daily Intake (mTAMDI) approach, which is based on the normal use levels of the flavouring agent in various food categories. Using this more conservative approach, the mTAMDI for S807 was estimated to be 470 μg/person/day or 7.8 μg/kg bw/day [8]. Based on the mTAMDI, a NOEL of 20 mg/kg bw/day would still be 2560 times the estimated dietary exposure to S807 when used as a flavouring agent. Applying a margin of safety of 1000-fold in extrapolating animal data to humans to account for species differences in susceptibility, numerical differences in population ranges between the test animals and the human population, the greater variety of complicating disease processes in the human population, and the possibility of synergistic action among food additives, is generally believed to be an adequate margin of safety for most substances proposed for use in food [21], [7].
The doses of S9229 selected for the 28-day short term toxicology study (10, 30, 100 mg/kg bw/day) were designed to provide a high margin of safety rather than define a maximum tolerated dose (MTD) in rats. No unscheduled deaths occurred during the course of the study and no clinical observations of toxicity were noted. Consistent with the results of the single dose pharmacokinetic study of S9229, the toxicokinetic study conducted in conjunction with the 28-day toxicology study on S9229 demonstrated that the compound is poorly absorbed and/or rapidly metabolized resulting in low systemic levels of the parent compound. No significant accumulation of S9229 following repeated dosing was observed in either male or female rats.
High dose males had a significantly higher body weight gain (16.7%) relative to controls which was associated with higher food consumption and was not considered adverse. Absolute thymus and liver weights, as well as thymus and liver to brain weight ratios were significantly increased at the 100 mg/kg bw/day dose in male rats. However, these changes were not considered adverse since they only occurred in one sex and had no histopathological correlates. There were no other test article-related organ weight, macroscopic or microscopic changes noted at any dose level in either sex. Based on the findings in this study the no-observed-adverse-effect level (NOAEL) for S9229 was 100 mg/kg bw/day in both sexes.
In conclusion, both S807 and S9229 demonstrated a lack of genotoxicity with or without metabolic activation in vitro at concentrations that greatly exceed those observed in rat plasma following oral administration at doses of 100–200 mg/kg bw. In addition, neither compound showed any evidence of clastogenicity or aneugenicity in a standard in vivo mouse micronucleus test. The results of a 90-day subchronic toxicity study with S807 and a 28 day short term toxicity study with S9229 established NOEL for of 20 mg/kg bw/day for S807, and a NOAEL of 100 mg/kg bw/day (the highest dose evaluated) for S9229. Assuming that the systemic exposure of these compounds after oral administration to humans is comparable to that observed at an equivalent dose in the rat, these NOAELs are orders of magnitude higher than the expected human exposure for both compounds under the conditions of intended use.
Conflict of interest
All the studies described herein were funded by Senomyx, Inc.
Acknowledgements
The authors wish to thank Michael Saganich and Ginger Toschiaddi of Senomyx, Inc., for carrying out the in-life phase of the pharmacokinetic studies on S807 and S9229. The authors would also like to thank Joseph Fotsing, Sara Werner, and Timothy Davis for preparing synthetic samples of the potential metabolites of S807 and S9229.
Footnotes
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.toxrep.2016.10.008.
Appendix A. Supplementary data
The following is Supplementary data to this article:
References
- 1.Chen Z., Chen M., Pan H., Sun S., Li L., Zeng S., Jiang H. Role of catechol-O-methyltransferase in the disposition of luteolin in rats. Drug Metab. Disp. 2011;39(4):667–674. doi: 10.1124/dmd.110.037333. [DOI] [PubMed] [Google Scholar]
- 2.Chi, B., 2016. Unpublished results. Briefly, the COMT reaction mixture contained the following components (final concentrations): catechol M251A-1 (0.01 mM), S-(5(-adenosyl)-l-methionine iodide (0.10 mM), magnesium chloride (0.12 mM), dithiothreitol (0.40 mM), N-tris(hydroxymethyl)-methyl-2-aminoethane buffer, pH 7.6 (4.0 mM), native porcine catechol O-methyl transferease (100 units, Creative Enzymes, Shirley, NY; catalog number NATE-0148). The final volume was 250 μL. The reaction was started by the addition of the enzyme, incubated at 37 °C for 60 min, and terminated by the addition of cold acetonitrile (250 μL). The mixture was centrifuged, and the supernatant analyzed by UHPLC-QTOF/MS.
- 3.Cohen S.M., Fukushima S., Gooderham N.J., Hecht S.S., Marnett L.J., Rietjens I.M.C.M., Smith R.L. GRAS Flavouring Substances 27: the 27th publication by the Expert Panel of the Flavour and Extract Manufacturers Association provides an update on recent progress in the consideration of flavouring ingredients generally recognized as safe under the Food Additive Amendment. Food Technol. 2015;69(8):40–59. Key findings of the FEMA Expert Panel GRAS determinations for each substance on the GRAS 27 list are available online: www.femaflavor.org. [Google Scholar]
- 4.Creveling G., Dalgard N., Shimizu N., Daly J. Catechol-O-methyltransferase: III. m- and p-O-methylation of catecholamines and their metabolites. Mol. Pharmacol. 1970;6:691–696. [PubMed] [Google Scholar]
- 5.Creveling G., Morris N., Shimizu N., Ong H.H., Daly J. Catechol-O-methyltransferase. IV. factors affecting m- and p-methylation of substituted catechols. Mol. Pharmacol. 1972;8:398–409. [PubMed] [Google Scholar]
- 6.Davies B., Morris T. Physiological parameters in animals and humans. Pharmaceut. Res. 1993;10:1093–1095. doi: 10.1023/a:1018943613122. [DOI] [PubMed] [Google Scholar]
- 7.EFSA EFSA panel on food contact materials, enzymes, flavourings and processing aids. 2010. draft guidance on the data required for the risk assessment of flavourings. EFSA J. 2010;8(6):1623. www.efsa.europa.eu Available online: [Google Scholar]
- 8.EFSA Scientific Opinion on Flavouring Group Evaluation 94, Revision 2 (FGE.94Rev2): Consideration of aliphatic amines and amides evaluated in an addendum to the group of aliphatic and aromatic amines and amides evaluated by the JECFA (68th meeting) EFSA J. 2014;12(4):3622. [Google Scholar]
- 9.FDA, 2006. Good Laboratory Practice for Nonclinical Laboratory Studies, 21 CFR Part 58, National Archives and Records Administration.
- 10.FDA, 2010. Guidance for Industry and Other Stakeholders. Toxicological Principles for the Safety Assessment of Food Ingredients. Redbook 2000.
- 11.Hallagan J.B., Hall R.L. Under the conditions of intended use – new developments in the FEMA GRAS program and the safety assessment of flavour ingredients. Food Chem. Toxicol. 2009;47:267–278. doi: 10.1016/j.fct.2008.11.011. [DOI] [PubMed] [Google Scholar]
- 12.JECFA (Joint FAO/WHO Expert Committee on Food Additives), 2008. Safety evaluation of certain food additives and contaminants. Sixty-eight Meeting of the Joint FAO/WHO Expert Committee on Food Additives, WHO Food Additives Series: 59. IPCS, WHO, Geneva 2008. http://whqlibdoc.who.int/publications/2008/9789241660594_eng.pdf (May 2008).
- 13.Kamienski F.X., Casida J.E. Importance of demethylenation in the metabolism In vitro and In vivo of methylenedioxyphenyl synergists and related compounds in mammals. Biochem. Pharmacol. 1970;19:91–112. doi: 10.1016/0006-2952(70)90331-x. [DOI] [PubMed] [Google Scholar]
- 14.Karanewsky D.S., Arthur A.J., Liu H., Chi B., Ida L., Markison S. Toxicological evaluation of a novel umami flavour compound: 2-(((3-(2,3-dimethoxyphenyl)-1H-1,2,4-triazol-5-yl)thio)methyl)pyridine. Toxicol. Rep. 2016;3:501–512. doi: 10.1016/j.toxrep.2016.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kato R., Yamazoe Y. Sex-specific cytochrome P450 as a cause of sex- and species-related differences in drug toxicity. Toxicol. Lett. 1992;64/65:661–667. doi: 10.1016/0378-4274(92)90245-f. [DOI] [PubMed] [Google Scholar]
- 16.Klungsoyr J., Scheline R.R. Metabolism of piperonal and piperonyl alcohol in the rat with special reference to the scission of the methylenedioxy group. Acta Pharm. Suec. 1984;21:67–72. [PubMed] [Google Scholar]
- 17.Lau E.Y., Bruice T.C. Importance of correlated motions in forming highly reactive near attack conformations in catechol-O-methyltransferase. J. Amer. Chem. Soc. 1998;120(48):12387–12394. [Google Scholar]
- 18.Li X., Staszewski L., Xu H., Durick K., Zoller M., Alder E.A. Human receptors for sweet and umami taste. PNAS. 2002;99(7):4692–4696. doi: 10.1073/pnas.072090199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li X. T1R receptors mediate sweet and umami taste. Am. J. Clin. Nutr. 2009;90(suppl):733S–737S. doi: 10.3945/ajcn.2009.27462G. [DOI] [PubMed] [Google Scholar]
- 20.Lotta T., Vidgren J., Tilgmann C., Ulmanen I., Melen K., Jukunen I., Taskinen J. Kinetics of human soluble and membrane-bound catechol-O-methyltransferase: a revised mechanism and description of the thermolabile variant of the enzyme. Biochemistry. 1995;34(13):4202–4210. doi: 10.1021/bi00013a008. [DOI] [PubMed] [Google Scholar]
- 21.Magnuson B., Munro I., Abbot P., Baldwin N., Lopez-Garcia R., Ly K., McGirr L., Roberts A., Socolovsky S. Review of the regulation and safety assessment of food substances in various countries and jurisdictions. Food Addit. Contam. 2013;30(7):1147–1220. doi: 10.1080/19440049.2013.795293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mannisto P.T., Kaakkola S. Catechol-O-methyltransferase (COMT): biochemistry, molecular biology, pharmacology, and clinical efficacy of the new selective COMT inhibitors. Pharmacol. Rev. 1999;51(4):594–628. [PubMed] [Google Scholar]
- 23.Marnett L.J., Cohen S.M., Fukushima S., Gooderham N.J., Hecht S.S., Rietjens I.M.C.M., Smith R.L., Adams T.B., Hallagan J.B., Harman C., McGowen M.M., Taylor S.V. GRAS flavouring substances 26: the 26th publication by the Expert Panel of the Flavour and Extract Manufacturers Association provides an update on recent progress in the consideration of flavouring ingredients generally recognized as safe under the Food Additive Amendment. Food Technol. 2013;67(8):38–56. [Google Scholar]
- 24.Mugford C.A., Kedderis G.L. Sex-dependent metabolism of xenobiotics. Drug Metab. Rev. 1998;30(3):441–498. doi: 10.3109/03602539808996322. [DOI] [PubMed] [Google Scholar]
- 25.OECD, 1997a. Guideline test no. 471: Bacterial reverse mutation test. In: OECD Guidelines for the Testing of Chemicals. Organisation for Economic Co-operation and Development, Paris.
- 26.OECD, 1997b. Guideline test no. 473: In Vitro mammalian chromosome aberration test. In: OECD Guidelines for the Testing of Chemicals. Organisation for Economic Co-operation and Development, Paris.
- 27.OECD, 1998. Principles on Good Laboratory Practice (as revised in 1997). In: OECD Series on Principles of Good Laboratory Practice and Compliance Monitoring, Number 1, OECD Environmental Health and Safety Publications, Environment Directorate, Organisation for Economic Co-operation and Development, Paris.
- 28.OECD, 1997c. Guideline test no. 474: Mammalian Erythrocyte Micronucleus Test. In: OECD Guidelines for the Testing of Chemicals. Organisation for Economic Co-operation and Development, Paris.
- 29.Palma P.N., Bonifacio M.J., Loureiro A.I., Wright L.C., Learmonth D.A., Soares-Da-Siliva P. Molecular modeling and metabolic studies of the interaction of catechol-O-methyltrasnsferase and a new nitrocatechol inhibitor. Drug Metab. Disp. 2003;31(3):250–258. doi: 10.1124/dmd.31.3.250. [DOI] [PubMed] [Google Scholar]
- 30.Smith R.L., Cohen S.M., Doull J., Feron V.J., Goodman J.I., Marnett L.J., Portoghese P.S., Waddell W.J., Wagner B.M., Adams T.B. GRAS Flavouring Substances 22: the 22nd publication by the FEMA Expert Panel presents provides safety and usage data on 185 new generally recognized as safe flavoring ingredients and describes an approach to assessing the safety of natural flavour complexes. Food Technol. 2005;59(8):24–62. [Google Scholar]
- 31.Tachdjian, C., Li, X., Qi, M., Rinnova, M., Servant, G., and Zoller, M., 2009. Flavors, Flavor Modifiers, Tastants, Taste Enhancers, Umami or Sweet Tastants, and/or Enhancers and Use Thereof. US Patent No. 7,476,399 B2, issued January 13, 2009.
- 32.Tachdjian, C., Patron, A.P., Adamski-Werner, S.L., Bakir, F., Chen, Q., Darmohusodo, Hobsen, S.T., Li, X., Qi, M., Rogers, D.H., Rinnova, M., Servant, G., Tang, X.-Q., Zoller, M., Wallace, D., Xing, A., and Gubernator, K., 2012. Flavors, Flavor Modifiers, Tastants, Taste Enhancers, Umami or Sweet Tastants, and/or Enhancers and Use Thereof. US Patent No. 8,124,121 B2, issued February 28, 2012.
- 33.Tachdjian, C., Patron, A.P., Adamski-Werner, S.L., Bakir, F., Chen, Q., Darmohusodo, Hobsen, S.T., Li, X., Qi, M., Rogers, D.H., Rinnova, M., Servant, G., Tang, X.-Q., Zoller, M., Wallace, D., Xing, A., and Gubernator, K., 2014a. Flavors, Flavor Modifiers, Tastants, Taste Enhancers, Umami or Sweet Tastants, and/or Enhancers and Use Thereof. US Patent No. 8,895,050 B2, issued November 25, 2014.
- 34.Tachdjian, C., Lebl-Rinnova, M., and Wallace, D., 2014b. Compounds Comprising Linked Heteroaryl Moieties and Their Use as Novel Umami Flavor Modifiers, Tastants and Taste Enhancers for Comestible Compositions. US Patent No. 8,784,782 B2, issued July 22, 2014.
- 35.Tachdjian, C., Lebl-Rinnova, M., and Wallace, D., 2015. Compounds Comprising Linked Heteroaryl Moieties and Their Use as Novel Umami Flavor Modifiers, Tastants and Taste Enhancers for Comestible Compositions. US Patent No. 8,968,708 B2, issued March 3, 2015.
- 36.Thoolen B., Maronpot R.R., Harada T., Nyska A., Rousseax C., Nolte T., Malarkey D.E., Kaufmann W., Kuttler K., Deschl U., Nakae D., Gregson R., Vinlove M.P., Brix A.E., Singh B., Belpoggi F., Ward J.M. Proliferative and nonproliferative lesions of the rat and mouse hepatobiliary system. Toxicol. Pathol. 2010;38:5S–81S. doi: 10.1177/0192623310386499. [DOI] [PubMed] [Google Scholar]
- 37.Vidgren J., Ovaska M., Tenhunen J., Tilgmann C., Lotta T., Mannisto P.T. Catechol-O-methyltransferase. In: Cheng X., Blumenthal R.M., editors. Structure and Function of AdoMet-Dependent Methyltransferases. World Scientific; Singapore: 1999. pp. 55–91. [Google Scholar]
- 38.Zhang F., Klebansky B., Fine R.M., Xu H., Pronin A., Liu H., Tachdjian C., Li X. Molecular mechanism for umami taste synergism. PNAS. 2008;106(52):20930–20934. doi: 10.1073/pnas.0810174106. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.