Abstract
The murine lacrimal gland (LG), which produces crucial components of the ocular tear film, contains a population of natural killer (NK) cells. LG NK cells appear to belong to the conventional NK cell lineage, based on their cell surface receptor and transcription factor expression, absence in NFIL3−/− mice, and lack of RORγt expression during development. LG NK cells produce IFN-γ during the early stages of systemic murine cytomegalovirus (MCMV) infection. This effector response occurs in the absence of noticeable MCMV replication in the LG, indicating that LG NK cells are being activated by soluble factors. However, the magnitude of LG NK cell IFN-γ production during MCMV infection is significantly lower than spleen and liver NK cells. Adoptive transfer experiments in lymphopenic mice revealed that this hyporesponsive phenotype is tissue-specific, which indicates that that LG NK cells can produce a robust effector response.
Introduction
Innate lymphoid cells (ILCs) comprise diverse cell types that combat infectious microorganisms and cancer cells, and help to maintain tissue homeostasis (1). The different subsets of ILCs are broadly classified as ILC1s, ILC2s, or ILC3s based on their developmental pathways and the cytokines they produce at maturity (2). Conventional natural killer (cNK) cells are the prototypical ILC1s (3), which function mainly to induce apoptosis of virally infected cells and tumor cells (4). cNK cells develop from the common lymphoid progenitor in the bone marrow (5, 6), and mature before entering the circulation and traveling to lymphoid and non-lymphoid tissues (7).
In recent years, unique populations of NK cells have been identified in many different tissues. Some are cNK cells that take on altered phenotypes due to signals within the tissue environment (8), while others appear to be completely distinct ILC1 lineages. For instance, thymic NK cells have a unique phenotype and developmental pathway compared to cNK cells (9). Recently, populations of “tissue-resident” NK (trNK) NK cells have been identified in the liver, skin (10), kidney (11), uterus (12), and salivary gland (13, 14). These trNK cells represent distinct populations of ILC1s, which have unique phenotypes and developmental requirements from cNK cells. cNK cells require the transcription factor NFIL3 for development (15–17), and express Eomes. trNK cells in most tissues are Eomes negative, and develop at least partially independently of NFIL3 (18–20). This is in contrast to evidence that NFIL3 is required for the development of all ILCs (21–24).
The populations of cNK cells, trNK cells, and other ILCs in lymphoid and mucosal tissues have been well characterized (8, 19, 25, 26). Mucosal tissues are varied in structure and function, and their exposure to the external environment results in colonization by a wide variety of commensal and pathogenic microorganisms (27). NK cells and other ILCs are important for maintaining the composition and integrity of mucosal tissues, particularly in response to microbial colonization (28). However, the presence of ILCs in exocrine glands, such as the lacrimal gland (LG), has been relatively understudied. Exocrine glands secrete factors that help to maintain the integrity of mucosal and epithelial surfaces. The LG is essential for eye health, since it is responsible for producing both the mucin and aqueous layers of the tear coating. The tear coating is necessary for normal eye function and protection from pathogens, as it supplies the eye with anti-microbial enzymes and protective immunoglobulins. Excessive inflammation can damage the LG, which can result in reduced tear production and damage to the ocular surface (29, 30).
The LG is known to contain populations of T cells and B cells (31). Here we report that the LG also has a population of NK cells. LG NK cells express Eomes and T-bet, and are mostly absent in NFIL3−/− mice. This suggests that they develop from the conventional NK cell lineage. In support of this, we found that LG NK cells do not express RORγt during development, which indicates that they are not ex-ILC3s. Although we could not detect viral replication in this organ, LG NK cells mount an effector response during systemic MCMV infection. However, this response is low in magnitude compared to splenic and liver NK cells. This weak response was found to be tissue-specific, as LG NK cells produce similar IFN-γ levels as splenic NK cells after acclimating to the spleen and liver following adoptive transfer into lymphopenic mice.
Materials and Methods
Mice
C57BL/6 and B6.SJL mice were purchased from Taconic Biosciences (Germantown, NY). A breeding pair of Rag2−/−IL-2Rγ−/− mice was purchased from Taconic Biosciences, and these mice were subsequently bred in-house. Rorc.cre and R26R-EYFP mice were purchased from The Jackson Laboratory (Bar Harbor, ME). Rorc.cre mice were bred with R26R-EYFP mice in-house to produce RORγt fate mapping mice. NFIL3−/− mice were a generous gift from Dr. Hugh JM Brady (15). NFIL3+/+, NFIL3+/−, and NFIL3−/− mice were subsequently bred in-house. All mice were maintained in pathogen free facilities at Brown University. Mice of both sexes were included and no differences were observed.
Infection and NK cell depletion protocols
Mice were infected i.p. with 5×104 PFU MCMV (strain: RVG102), as previously described (32). In experiments with NK cell depletion, mice were initially injected i.p. with 100 μg of anti-NK1.1 (clone: PK136) 24 hours prior to MCMV infection, and again every 7 days until takedown. NK cell IFN-γ was measured directly ex vivo without culture following MCMV infection.
Isolation of murine lymphocytes
Mice were sacrificed with isoflurane, and cardiac puncture was performed prior to organ removal. Spleens were processed with a GentleMACS Dissociator (Miltenyi Biotec), filtered through nylon mesh, and layered onto a Lympholyte-M gradient (Cedarlane Laboratories Ltd., Canada). Lymphocytes were harvested from the gradient interface, and washed once in PBS supplemented with 1% FBS (1% PBS-serum). Alternatively, spleens were processed with ammonium chloride to lyse red blood cells and enrich for lymphocytes. Livers were perfused with 1% PBS-serum before removal, processed in 1% PBS-serum with the GentleMACS, and filtered through nylon mesh. Samples were washed 3 times with 1% PBS-serum, suspended in 40% Percoll and layered on 70% Percoll. Lymphocytes were harvested from the gradient interface and washed once with 1% PBS-serum. Extraorbital lacrimal glands were processed in Collagenase IV (Sigma-Aldrich) or Liberase-DL (Sigma-Aldrich) with the GentleMACS, incubated at 37 °C for 10 minutes, filtered through nylon mesh, and washed once with 1% PBS-serum before being layered on a Lympholyte-M gradient. In some experiments, 6 to12 lacrimal glands from 3 to 6 animals were pooled. Lymphocytes were harvested from the gradient interface and washed once in 1% PBS-serum.
Flow cytometry antibodies, reagents, and analysis
Lymphocyte samples were incubated in 1% PBS-serum with the blocking monoclonal antibody (mAb) 2.4G2 and stained with specific mAbs for 20 minutes at 4 °C. For intracellular cytokine staining, cells were first stained with extracellular mAbs, then fixed with Cytofix/Cytoperm (BD Biosciences) for 20 minutes, and stained with intracellular mAbs in 1X PermWash (BD Biosciences) for 20 minutes. For intranuclear transcription factor staining, cells were stained with intracellular antibodies using FoxP3 transcription factor staining reagents (BD Biosciences). Events were collected on a FACSAria III (BD Biosciences), and the data were analyzed using FlowJo (FlowJo LLC). Alexa Fluor 488-AsGM1, FITC-CD27, PE-TRAIL, PE-IFN-γ, PE-T-bet, Per-CPCy5.5-NK1.1, PerCP-Cy5.5-TCRβ, PE-Cy5-DX5, PE-Cy7-NKp46, allophycocyanin-CD3, allophycocyanin-CD19, allophycocyanin-Ly49H, allophycocyanin-KLRG1, allophycocyanin-eFluor780-CD45, allophycocyanin-eFluor780-CD45.2, allophycocyanin-eFluor780-CD117, eFluor450-CD3, eFluor450-CD11b, eFluor450-CD45.1, and eFluor450-Eomes were purchased from eBioscience (Thermo Fisher Scientific). PE-CD49a, allophycocyanin-CD49a, BV421-CD127, BV510-TCRβ, BV570-CD45, BV605-CD3, and BV785-NK1.1 were purchased from Biolegend. FITC-Ly49C/I was purchased from BD Pharmingen. FITC-DX5 was purchased from Miltenyi Biotec.
Adoptive transfer of NK cells
NK cells were sorted under sterile conditions from the spleens of C57BL/6 (CD45.2+) mice and the lacrimal glands of B6.SJL (CD45.1+) congenic mice. Donor NK cells were injected 1:1 into Rag2−/−IL-2Rγ−/− recipient mice. Recipient mice were allowed to reconstitute for 7 days before being infected i.p. with 5×104 PFU MCMV. At 38 hours post-infection, the recipient mice were sacrificed.
Plaque assays
Standard plaque assays were carried out, as previously described (33).
In vitro stimulation assays
Lymphocytes from naïve C57BL/6 spleen, liver, and LG were incubated for 4 hours in either RPMI complete media, RPMI with IL-12 (10 ng/ml) and IL-18 (10 ng/ml), or RPMI with PMA (20 ng/ml) and Ionomycin (1 μg/ml). GolgiStop (BD Biosciences) was added at the beginning of the incubation. Cells were washed twice with 1% PBS-serum before antibody staining.
Statistical Analysis
All statistical analyses were performed with Prism Version 7.0 (GraphPad Software). Unpaired two-tailed Student’s t-tests were used to compare cell populations from different mice. Paired two-tailed Student’s t-tests were used for experiments involving adoptive transfer. ****p < 0.0001, ***p = 0.0001–0.001, **p = 0.001–0.01, *p = 0.01–0.05.
Results
The lacrimal gland contains a population of CD3−NK1.1+NKp46+ cells
The LG is an exocrine gland that is similar in structure and function to the submandibular salivary gland (SMG)(29), which contains well-characterized populations of ILC1s (13, 14, 32, 34). We isolated lymphocytes from the extraorbital LG of naïve C57BL/6 mice, and found a population of CD3−NK1.1+NKp46+ cells (Figure 1A). The maturity of circulating NK cells is a four-stage developmental process, distinguished by expression of CD11b and CD27. NK cell maturity progresses from CD11blowCD27low, to CD11blowCD27high, CD11bhighCD27high, and finally CD11bhighCD27low (35). In comparison to spleen and liver NK cells, LG NK cells are relatively immature, having a very low frequency of CD11bhighCD27low cells (Figure 1B) and low expression of KLRG1 (Figure 1C). KLRG1 is generally expressed on fully mature CD11bhighCD27low NK cells (36), which further supports the classification of LG NK cells as relatively immature.
Figure 1. The lacrimal gland contains CD3−NK1.1+NKp46+ lymphocytes.

(A) Representative staining of spleen, liver, and LG NK cells. (B) Representative staining of CD11b and CD27 expression on spleen, liver, and LG NK cells. (C) Representative staining of KLRG1 expression on spleen, liver, and LG NK cells. Lymphocytes from three C57BL/6 mice were pooled in each experiment. Data are representative of three independent experiments.
LG NK cells are mostly conventional in development
In organs such as the liver, skin, uterus (10, 12, 37), and kidney (11), cNK cells can be distinguished from trNK cells based on surface expression of DX5 and CD49a. cNK cells are identified as DX5+CD49a−, whereas trNK cells are DX5−CD49a+. Liver trNK cells also express high levels of TNF-related apoptosis-inducing ligand (TRAIL) at baseline (10). We found that LG NK cells are mainly DX5+, and some are also CD49a+. However, they cannot be easily defined as DX5+CD49a− and DX5−CD49a+ subsets, like the NK cells of the liver (Figure 2A). Much like splenic NK cells and liver cNK cells, LG NK cells are mainly Eomes+T-bet+ (Figure 2B), and TRAIL− (Figure 2C). Liver and kidney trNK cells were also recently shown to be largely negative for the surface receptor Asialo-GM1 (AsGM1), which was once used as a marker to identify all NK cells (11). We observed that the majority of LG NK cells are AsGM1+ (Supplementary Figure 1A) and CD127− (Supplementary Figure 1B). However, differential expression of Eomes, DX5, CD49a, and other surface markers is not sufficient to distinguish cNK from trNK cells in all organs. For instance, SMG NK cells are mostly DX5+CD49a+, as well as Eomes+T-bet+ (13, 14). The majority of SMG NK cells are cNK cells, however there is also a trNK cell population. These populations are distinguished based on differential requirements for the transcription factor NFIL3 during development (14). cNK cells are generally dependent on NFIL3 for development, whereas liver, skin, uterus, kidney, and SMG trNK cells develop somewhat independently of this transcription factor (10–12, 20, 38). In the LG, we observed a significant decrease in NK cell frequency in NFIL3−/− mice compared to wild type littermate controls (Figure 3A and 3B). Together, these data support the conclusion that the vast majority of LG NK cells belong to the conventional lineage.
Figure 2. Lacrimal gland NK cells appear to be conventional in phenotype.

(A) Representative staining of CD49a and DX5 expression on spleen, liver, and LG NK cells. (B) Representative staining of Eomes and T-bet expression in spleen, liver, and LG NK cells. (C) Representative staining of TRAIL expression on spleen, liver, and LG NK cells. Lymphocytes from three C57BL/6 mice were pooled in each experiment. Data are representative of three independent experiments.
Figure 3. Lacrimal gland NK cells are mostly NFIL3-dependent.
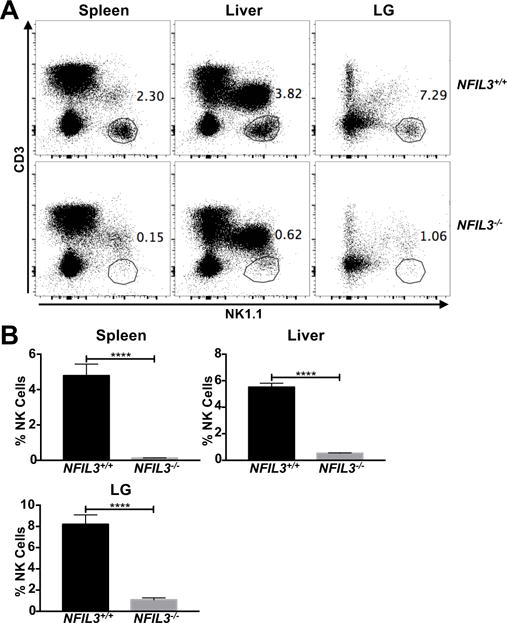
(A) Representative staining of spleen, liver, and LG NK cells from NFIL3+/+ and NFIL3−/− mice. Lymphocytes from individual mice were stained. (B) Frequency of spleen, liver, and LG NK cells in NFIL3+/+ (n = 7) and NFIL3−/− (n = 9) mice. Data are pooled from four independent experiments and error bars indicate SEM. ****p < 0.0001
LG NK cells are not ex-ILC3s
Recent research has shown that some NKp46+ ILC3s down-regulate RORγt expression, increase T-bet expression, and gain the ability to produce IFN-γ, effectively taking on an ILC1 phenotype (39, 40). This phenotypic plasticity has made it difficult to classify these “ex-ILC3s” as either ILC1s or ILC3s (28, 41). We investigated whether any of the LG NK cells were ex-ILC3s based on past RORγt expression by generating RORγt fate mapping mice. Mice that expressed cre recombinase under the control of the Rorc gene were crossed with those carrying the ROSA26-floxstop-YFP allele. In agreement with previous findings (39), we found that the resulting F1 mice had YFP expression in cells that had expressed RORγt during development (Supplementary Figure 1C).
Using the RORγt fate mapping mice, we found that LG NK cells, as well as spleen NK and liver cNK cells, were mainly YFP− (Figure 4A and 4B). This rules out the presence of ILC3s or ex-ILC3s within the LG CD3−NK1.1+NKp46+ population. Interestingly, nearly 20% of liver trNK cells were positive for YFP (Figure 4B). It is currently unknown whether these cells are ex-ILC3s, or if some liver trNK cells express RORγt as part of a yet-unknown developmental pathway.
Figure 4. Lacrimal gland NK cells are not ex-ILC3s.

(A) Representative expression of YFP on spleen NK, liver cNK and trNK, and LG NK cells from RORγT.cre.ROSA.YFP mice. Lymphocytes from individual mice were stained. (B) Frequency of YFP+ spleen NK, liver cNK and trNK, and LG NK cells from RORγT.cre.ROSA.YFP mice (n = 5). Data are pooled from two independent experiment and error bars indicate SEM.
LG NK cells respond weakly to systemic MCMV infection
NK cells are crucial for the early control of many viral infections, including cytomegalovirus (CMV)(42–44). The CMV family members all have strict species tropism, thus MCMV is often used as a model system to study the pathogenesis and immune response to human CMV. To investigate the effector response of LG NK cells, C57BL/6 mice were infected with MCMV, and NK cell IFN-γ production was assessed at 38 hours, Day 7, and Day 14 post-infection. Previous studies have shown that the effector response of spleen NK cells peaks during the second day of MCMV infection (32). Spleen NK cells, liver cNK cells, and liver trNK cells produced a robust IFN-γ response at 38 hours post-infection (Figure 5A). LG NK cells also produced IFN-γ at 38 hours post-infection, but at a significantly lower magnitude (Figure 5A and 5B). This effector response only occurs during early MCMV infection, as LG NK cell IFN-γ production drops by Day 7, and returns to baseline by Day 14 post-infection (Supplementary Figure 1D). Interestingly, we also found that the frequency of LG NK cells increases dramatically at 38 hours post-infection, before dropping at Day 7, as T cells infiltrate the organ. This is in contrast to the spleen, where the NK cell frequency decreases at 38 hours post-infection (Supplementary Figure 1E). However, the effector response of LG NK cells is less robust than the NK cell populations in the spleen and liver.
Figure 5. Lacrimal gland NK cells respond weakly to systemic MCMV infection.
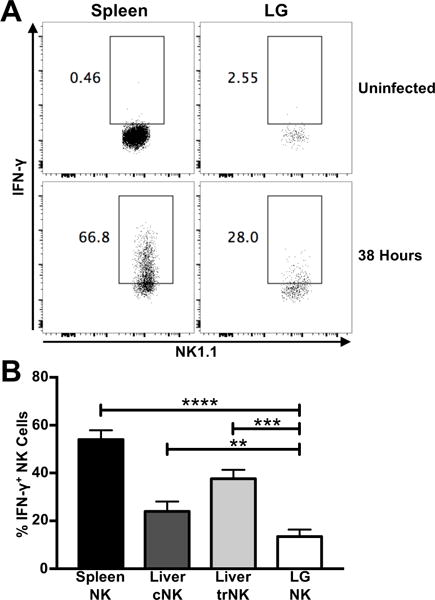
(A) Representative IFN-γ production by spleen NK and LG NK cells from C57BL/6 mice 38 hours post-MCMV infection. Lymphocytes from individual mice were stained. (B) Frequency of IFN-γ+ spleen NK, liver cNK and trNK, and LG NK cells from C57BL/6 mice 38 hours post-MCMV infection (n = 8). Data are pooled from three independent experiments and error bars indicate SEM. ****p < 0.0001, ***p = 0.0001–0.001, **p = 0.001–0.01
LG NK cell hyporesponsive phenotype is tissue-specific
We previously reported that SMG NK cells are hyporesponsive to MCMV infection (32). However, we also showed that this phenotype is tissue-specific and reversible (14). Thus, we investigated whether the weak effector response of LG NK cells to MCMV is also tissue-specific. CD3−NK1.1+NKp46+ lymphocytes were sorted from the spleen of C57BL/6 mice (CD45.2+) and the LG of B6.SJL mice (CD45.1+) and injected into recipient Rag2−/−IL-2Rγ−/− mice, which lack B cells, T cells, and ILCs. After 7 days, the recipient mice were infected with MCMV. 38 hours after infection, IFN-γ production was assessed in NK cells recovered from the recipient spleen and liver.
Although donor spleen and LG NK cells were found in the recipient spleen and liver, neither of the donor populations traveled to the LG (Figure 6A). This result indicates that circulating NK cells are not recruited to the LG during MCMV infection. We also found that donor spleen and LG NK cells produced comparable levels of IFN-γ, in both the recipient spleen and liver (Figure 6B and 6C). This result indicates that IFN-γ production by LG NK cells during systemic MCMV infection is limited by tissue-specific factors present in the LG, but not the spleen or liver. This is further supported by the low magnitude of IFN-γ produced by naïve LG NK cells in vitro during IL-12 + IL-18 stimulation (Supplementary Figure 2A). Naïve LG NK cells stimulated with PMA + Ionomycin produced similar levels of IFN-γ as spleen and liver NK cells (Supplementary Figure 2B).
Figure 6. Lacrimal gland NK cell hyporesponsiveness is a tissue-specific phenotype.

(A) Representative staining of donor NK cells in the spleen, liver, and LG of Rag2−/−IL-2Rγ−/− adoptive transfer recipients. Lymphocytes from individual mice were stained. (B) Representative IFN-γ production by donor spleen and LG NK cells in the spleen and liver of Rag2−/−IL-2Rγ−/− adoptive transfer recipients, 38 hours after MCMV infection. (C) Frequency of IFN-γ+ donor spleen and LG NK cells in the spleen and liver of Rag2−/−IL-2Rγ−/− adoptive transfer recipients (n = 6), 38 hours after MCMV infection. Data are pooled from three independent experiments and error bars indicate SEM. **p = 0.001–0.01
MCMV is not detectable in the lacrimal gland during systemic infection
In order to determine if MCMV replicates within the LG, C57BL/6 mice were infected with MCMV, and standard plaque assays were performed on homogenates of the spleen, SMG, and LG at 38 hours, Day 7, Day 14, and Day 21 post-infection. In agreement with previous reports, MCMV was detected in the spleen at 38 hours post-infection ((45) and Supplementary Figure 2C), and in the SMG starting at Day 7 through to Day 21 ((46, 47) and Supplementary Figure 2D). However, our analysis did not reveal viral plaques in the LG at any of the time points (data not shown). Since NK cells are a crucial component of the early immune response to MCMV, we also depleted C57BL/6 mice of NK cells. This treatment resulted in higher levels of viral replication in the spleen (Supplementary Figure 2C) and SMG (Supplementary Figure 2D), however we still did not detect virus in the LG (data not shown).
Discussion
For decades after their discovery, cNK cells were the only known innate lymphocytes. Within the last few years however, many different subsets of ILCs have been identified and characterized, in lymphoid tissues, mucosal tissues, and elsewhere (8, 19, 48). The various ILC subsets are broadly classified as ILC1s, ILC2s, and ILC3s. ILC1s are united in their constitutive expression of the transcription factor T-bet, as well as production of type 1 cytokines, such as IFN-γ and TNF-α (49, 50). cNK cells are included in the ILC1 group, along with other subsets of “helper-like ILC1s.” cNK cells are cytotoxic effector cells. Helper-like ILC1s produce type 1 cytokines, but are generally considered non-cytotoxic. cNK cells also diverge early from helper-like ILC1s in development (51–53).
The growing diversity of ILC1s has called into question the dichotomous categorization of the various ILC1 subsets as either cytotoxic or helper-like. For instance, the trNK cells found in the liver are more closely related to other helper-like ILC1s than cNK cells. However, a recent study demonstrated that unlike mucosal helper-like ILC1s, liver trNK cells have high cytotoxic potential (38). Intraepithelial (ie)ILC1s have been identified in mucosal tissues and appear to be distinct from cNK cells, trNK cells, and other helper-like ILC1 subsets (54). These findings indicate that ILC1s cannot be simply classified as cytotoxic cNK and non-cytotoxic/helper-like ILC1, but that ILC1 subsets exist along a continuum of diverse phenotypes.
In this study, we identified and characterized a population of ILC1s in the murine lacrimal gland. LG NK cells appear to be relatively immature, and display an unusual expression pattern of DX5 and CD49a. However, as we reported previously in the SMG (14), CD49a is not a definitive marker of trNK cells in all organs. LG NK cells mostly express T-bet and Eomes, and are almost completely absent in NFIL3−/− mice. These findings indicate that LG NK cells are not NFIL3−independent ILC1s. Fate mapping experiments also showed that LG NK cells do not express the transcription factor RORγt during development, which indicates that they do not develop from an ILC3 lineage. Based on these data, we found it prudent to identify LG NK cells as conventional−like NK cells.
cNK cells are homogenous in terms of development, but they are not phenotypically uniform at maturity. Rather, they can take on unique phenotypes after they exit the bone marrow and acclimate to different tissues. For instance, the NK cells of the lung appear to be derived from the conventional lineage, but are more mature than splenic NK cells (8). They also express higher levels of inhibitory receptors, lower levels of activating receptors, and lower levels of migratory and adhesion molecules than splenic NK cells (55). Based on our findings, the LG also contains a population of NK cells. These LG NK cells appear to be conventional in development, with a unique phenotype shaped by the tissue environment.
NK cells are crucial for the early defense against viral infections, particularly herpesviruses such as CMV. Similar to the NK cells of the murine SMG (32), LG NK cells produce a weak effector response to systemic MCMV infection. However, this response is likely mediated by inflammatory cytokines, as we could not detect MCMV in this organ. When LG NK cells were removed from their native environment and allowed to proliferate in the spleen and liver of Rag2−/−IL-2Rγ−/− mice, they produced a similar magnitude of IFN-γ as donor splenic NK cells (Figure 6). Thus, LG NK cells are fully equipped to produce a robust effector response to MCMV infection. Much like SMG NK cells (14), the effector response of LG NK cells is suppressed by factors in their native environment. It is also possible that the weak effector response of LG NK cells in situ is due to the lack of viral replication in the LG during infection. However, we consider this unlikely as Ly49H+ and Ly49H− NK cells of both the spleen and LG produce similar levels of IFN-γ during early MCMV infection ((56) and Supplementary Figure 2E). This indicates that direct contact with m157-expressing cells is not necessary for an NK cell IFN-γ response at this early time point in either organ.
In addition to killing virally infected cells and producing pro-inflammatory cytokines, NK cells can help limit inflammation (57). This is especially important in secretory tissues such as the SMG and LG, where extensive inflammation results in a phenotype resembling human Sjögren’s syndrome, an autoimmune disease characterized by a lack of saliva and tear production (58). The LG is a crucial exocrine gland that can be easily damaged by inflammation, and LG NK cells are capable of producing a potent pro-inflammatory immune response. It is possible that the effector response of LG NK cells is self-modulated, or suppressed by other factors within the tissue, in order to prevent inflammatory damage and the resulting lack of tear production. Further work will be necessary to determine the mechanisms behind this.
Supplementary Material
Acknowledgments
We thank Kevin Carlson for cell sorting, Céline Fugère for tail vein injections, Courtney K. Anderson for critical reading of the manuscript, and Dr. Hugh JM Brady for providing NFIL3−/− mice.
This work was supported by National Institutes of Health Research Grants AI46709 and AI122217 (to LB) and 1F31DE024360 (to TKE).
Footnotes
Authorship
T.K.E. conceived, performed, and analyzed the experiments and wrote the paper. L.G. conceived, performed, and analyzed the experiments. L.B. conceived and analyzed the experiments, and wrote the paper.
References
- 1.McKenzie AN, Spits H, Eberl G. Innate lymphoid cells in inflammation and immunity. Immunity. 2014;41:366–374. doi: 10.1016/j.immuni.2014.09.006. [DOI] [PubMed] [Google Scholar]
- 2.Robinette ML, Fuchs A, Cortez VS, Lee JS, Wang Y, Durum SK, Gilfillan S, Colonna M, C. Immunological Genome Transcriptional programs define molecular characteristics of innate lymphoid cell classes and subsets. Nat Immunol. 2015;16:306–317. doi: 10.1038/ni.3094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vosshenrich CA, Di Santo JP. Developmental programming of natural killer and innate lymphoid cells. Curr Opin Immunol. 2013;25:130–138. doi: 10.1016/j.coi.2013.02.002. [DOI] [PubMed] [Google Scholar]
- 4.Lanier LL, Phillips JH, Hackett J, Jr, Tutt M, Kumar V. Natural killer cells: definition of a cell type rather than a function. J Immunol. 1986;137:2735–2739. [PubMed] [Google Scholar]
- 5.Carotta S, Pang SH, Nutt SL, Belz GT. Identification of the earliest NK-cell precursor in the mouse BM. Blood. 2011;117:5449–5452. doi: 10.1182/blood-2010-11-318956. [DOI] [PubMed] [Google Scholar]
- 6.Fathman JW, Bhattacharya D, Inlay MA, Seita J, Karsunky H, Weissman IL. Identification of the earliest natural killer cell-committed progenitor in murine bone marrow. Blood. 2011;118:5439–5447. doi: 10.1182/blood-2011-04-348912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huntington ND, Vosshenrich CA, Di Santo JP. Developmental pathways that generate natural-killer-cell diversity in mice and humans. Nat Rev Immunol. 2007;7:703–714. doi: 10.1038/nri2154. [DOI] [PubMed] [Google Scholar]
- 8.Erick TK, Brossay L. Phenotype and functions of conventional and non-conventional NK cells. Curr Opin Immunol. 2016;38:67–74. doi: 10.1016/j.coi.2015.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Di Santo JP, Vosshenrich CA. Bone marrow versus thymic pathways of natural killer cell development. Immunol Rev. 2006;214:35–46. doi: 10.1111/j.1600-065X.2006.00461.x. [DOI] [PubMed] [Google Scholar]
- 10.Sojka DK, Plougastel-Douglas B, Yang L, Pak-Wittel MA, Artyomov MN, Ivanova Y, Zhong C, Chase JM, Rothman PB, Yu J, Riley JK, Zhu J, Tian Z, Yokoyama WM. Tissue-resident natural killer (NK) cells are cell lineages distinct from thymic and conventional splenic NK cells. Elife. 2014;3:e01659. doi: 10.7554/eLife.01659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Victorino F, Sojka DK, Brodsky KS, McNamee EN, Masterson JC, Homann D, Yokoyama WM, Eltzschig HK, Clambey ET. Tissue-Resident NK Cells Mediate Ischemic Kidney Injury and Are Not Depleted by Anti-Asialo-GM1 Antibody. J Immunol. 2015;195:4973–4985. doi: 10.4049/jimmunol.1500651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Doisne JM, Balmas E, Boulenouar S, Gaynor LM, Kieckbusch J, Gardner L, Hawkes DA, Barbara CF, Sharkey AM, Brady HJ, Brosens JJ, Moffett A, Colucci F. Composition, Development, and Function of Uterine Innate Lymphoid Cells. J Immunol. 2015;195:3937–3945. doi: 10.4049/jimmunol.1500689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cortez VS, Fuchs A, Cella M, Gilfillan S, Colonna M. Cutting edge: Salivary gland NK cells develop independently of Nfil3 in steady-state. J Immunol. 2014;192:4487–4491. doi: 10.4049/jimmunol.1303469. [DOI] [PubMed] [Google Scholar]
- 14.Erick TK, Anderson CK, Reilly EC, Wands JR, Brossay L. NFIL3 Expression Distinguishes Tissue-Resident NK Cells and Conventional NK-like Cells in the Mouse Submandibular Glands. J Immunol. 2016;197:2485–2491. doi: 10.4049/jimmunol.1601099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gascoyne DM, Long E, Veiga-Fernandes H, de Boer J, Williams O, Seddon B, Coles M, Kioussis D, Brady HJ. The basic leucine zipper transcription factor E4BP4 is essential for natural killer cell development. Nat Immunol. 2009;10:1118–1124. doi: 10.1038/ni.1787. [DOI] [PubMed] [Google Scholar]
- 16.Male V, Nisoli I, Kostrzewski T, Allan DS, Carlyle JR, Lord GM, Wack A, Brady HJ. The transcription factor E4bp4/Nfil3 controls commitment to the NK lineage and directly regulates Eomes and Id2 expression. J Exp Med. 2014;211:635–642. doi: 10.1084/jem.20132398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kamizono S, Duncan GS, Seidel MG, Morimoto A, Hamada K, Grosveld G, Akashi K, Lind EF, Haight JP, Ohashi PS, Look AT, Mak TW. Nfil3/E4bp4 is required for the development and maturation of NK cells in vivo. J Exp Med. 2009;206:2977–2986. doi: 10.1084/jem.20092176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Daussy C, Faure F, Mayol K, Viel S, Gasteiger G, Charrier E, Bienvenu J, Henry T, Debien E, Hasan UA, Marvel J, Yoh K, Takahashi S, Prinz I, de Bernard S, Buffat L, Walzer T. T-bet and Eomes instruct the development of two distinct natural killer cell lineages in the liver and in the bone marrow. J Exp Med. 2014;211:563–577. doi: 10.1084/jem.20131560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sojka DK, Tian Z, Yokoyama WM. Tissue-resident natural killer cells and their potential diversity. Semin Immunol. 2014;26:127–131. doi: 10.1016/j.smim.2014.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Crotta S, Gkioka A, Male V, Duarte JH, Davidson S, Nisoli I, Brady HJ, Wack A. The transcription factor E4BP4 is not required for extramedullary pathways of NK cell development. J Immunol. 2014;192:2677–2688. doi: 10.4049/jimmunol.1302765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yu X, Wang Y, Deng M, Li Y, Ruhn KA, Zhang CC, Hooper LV. The basic leucine zipper transcription factor NFIL3 directs the development of a common innate lymphoid cell precursor. Elife. 2014;3 doi: 10.7554/eLife.04406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Seillet C, Rankin LC, Groom JR, Mielke LA, Tellier J, Chopin M, Huntington ND, Belz GT, Carotta S. Nfil3 is required for the development of all innate lymphoid cell subsets. J Exp Med. 2014;211:1733–1740. doi: 10.1084/jem.20140145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Geiger TL, Abt MC, Gasteiger G, Firth MA, O’Connor MH, Geary CD, O’Sullivan TE, van den Brink MR, Pamer EG, Hanash AM, Sun JC. Nfil3 is crucial for development of innate lymphoid cells and host protection against intestinal pathogens. J Exp Med. 2014;211:1723–1731. doi: 10.1084/jem.20140212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xu W, Domingues RG, Fonseca-Pereira D, Ferreira M, Ribeiro H, Lopez-Lastra S, Motomura Y, Moreira-Santos L, Bihl F, Braud V, Kee B, Brady H, Coles MC, Vosshenrich C, Kubo M, Di Santo JP, Veiga-Fernandes H. NFIL3 orchestrates the emergence of common helper innate lymphoid cell precursors. Cell Rep. 2015;10:2043–2054. doi: 10.1016/j.celrep.2015.02.057. [DOI] [PubMed] [Google Scholar]
- 25.Artis D, Spits H. The biology of innate lymphoid cells. Nature. 2015;517:293–301. doi: 10.1038/nature14189. [DOI] [PubMed] [Google Scholar]
- 26.Yokoyama WM, Sojka DK, Peng H, Tian Z. Tissue-resident natural killer cells. Cold Spring Harb Symp Quant Biol. 2013;78:149–156. doi: 10.1101/sqb.2013.78.020354. [DOI] [PubMed] [Google Scholar]
- 27.McGhee JR, Fujihashi K. Inside the mucosal immune system. PLoS Biol. 2012;10:e1001397. doi: 10.1371/journal.pbio.1001397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sonnenberg GF, Artis D. Innate lymphoid cells in the initiation, regulation and resolution of inflammation. Nat Med. 2015;21:698–708. doi: 10.1038/nm.3892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Conrady CD, Joos ZP, Patel BC. Review: The Lacrimal Gland and Its Role in Dry Eye. J Ophthalmol. 2016;2016:7542929. doi: 10.1155/2016/7542929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Knop E, Knop N. The role of eye-associated lymphoid tissue in corneal immune protection. J Anat. 2005;206:271–285. doi: 10.1111/j.1469-7580.2005.00394.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Saitoh-Inagawa W, Hiroi T, Yanagita M, Iijima H, Uchio E, Ohno S, Aoki K, Kiyono H. Unique characteristics of lacrimal glands as a part of mucosal immune network: high frequency of IgA-committed B-1 cells and NK1.1+ alphabeta T cells. Invest Ophthalmol Vis Sci. 2000;41:138–144. [PubMed] [Google Scholar]
- 32.Tessmer MS, Reilly EC, Brossay L. Salivary gland NK cells are phenotypically and functionally unique. PLoS Pathog. 2011;7:e1001254. doi: 10.1371/journal.ppat.1001254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Reilly EC, Thompson EA, Aspeslagh S, Wands JR, Elewaut D, Brossay L. Activated iNKT cells promote memory CD8+ T cell differentiation during viral infection. PLoS One. 2012;7:e37991. doi: 10.1371/journal.pone.0037991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cortez VS, Cervantes-Barragan L, Robinette ML, Bando JK, Wang Y, Geiger TL, Gilfillan S, Fuchs A, Vivier E, Sun JC, Cella M, Colonna M. Transforming Growth Factor-beta Signaling Guides the Differentiation of Innate Lymphoid Cells in Salivary Glands. Immunity. 2016;44:1127–1139. doi: 10.1016/j.immuni.2016.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chiossone L, Chaix J, Fuseri N, Roth C, Vivier E, Walzer T. Maturation of mouse NK cells is a 4-stage developmental program. Blood. 2009;113:5488–5496. doi: 10.1182/blood-2008-10-187179. [DOI] [PubMed] [Google Scholar]
- 36.Huntington ND, Tabarias H, Fairfax K, Brady J, Hayakawa Y, Degli-Esposti MA, Smyth MJ, Tarlinton DM, Nutt SL. NK cell maturation and peripheral homeostasis is associated with KLRG1 up-regulation. J Immunol. 2007;178:4764–4770. doi: 10.4049/jimmunol.178.8.4764. [DOI] [PubMed] [Google Scholar]
- 37.Peng H, Jiang X, Chen Y, Sojka DK, Wei H, Gao X, Sun R, Yokoyama WM, Tian Z. Liver-resident NK cells confer adaptive immunity in skin-contact inflammation. J Clin Invest. 2013;123:1444–1456. doi: 10.1172/JCI66381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tang L, Peng H, Zhou J, Chen Y, Wei H, Sun R, Yokoyama WM, Tian Z. Differential phenotypic and functional properties of liver-resident NK cells and mucosal ILC1s. J Autoimmun. 2016;67:29–35. doi: 10.1016/j.jaut.2015.09.004. [DOI] [PubMed] [Google Scholar]
- 39.Vonarbourg C, Mortha A, Bui VL, Hernandez PP, Kiss EA, Hoyler T, Flach M, Bengsch B, Thimme R, Holscher C, Honig M, Pannicke U, Schwarz K, Ware CF, Finke D, Diefenbach A. Regulated expression of nuclear receptor RORgammat confers distinct functional fates to NK cell receptor-expressing RORgammat(+) innate lymphocytes. Immunity. 2010;33:736–751. doi: 10.1016/j.immuni.2010.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Klose CS, Kiss EA, Schwierzeck V, Ebert K, Hoyler T, d’Hargues Y, Goppert N, Croxford AL, Waisman A, Tanriver Y, Diefenbach A. A T-bet gradient controls the fate and function of CCR6-RORgammat+ innate lymphoid cells. Nature. 2013;494:261–265. doi: 10.1038/nature11813. [DOI] [PubMed] [Google Scholar]
- 41.Spits H, Bernink JH, Lanier L. NK cells and type 1 innate lymphoid cells: partners in host defense. Nat Immunol. 2016;17:758–764. doi: 10.1038/ni.3482. [DOI] [PubMed] [Google Scholar]
- 42.Welsh RM, Brubaker JO, Vargas-Cortes M, O’Donnell CL. Natural killer (NK) cell response to virus infections in mice with severe combined immunodeficiency. The stimulation of NK cells and the NK cell-dependent control of virus infections occur independently of T and B cell function. J Exp Med. 1991;173:1053–1063. doi: 10.1084/jem.173.5.1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.McIntyre KW, Welsh RM. Accumulation of natural killer and cytotoxic T large granular lymphocytes in the liver during virus infection. J Exp Med. 1986;164:1667–1681. doi: 10.1084/jem.164.5.1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Loh J, Chu DT, O’Guin AK, Yokoyama WM, Virgin HWt. Natural killer cells utilize both perforin and gamma interferon to regulate murine cytomegalovirus infection in the spleen and liver. J Virol. 2005;79:661–667. doi: 10.1128/JVI.79.1.661-667.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Allan JE, Shellam GR. Genetic control of murine cytomegalovirus infection: virus titres in resistant and susceptible strains of mice. Arch Virol. 1984;81:139–150. doi: 10.1007/BF01309303. [DOI] [PubMed] [Google Scholar]
- 46.Henson D, Strano AJ. Mouse cytomegalovirus. Necrosis of infected and morphologically normal submaxillary gland acinar cells during termination of chronic infection. Am J Pathol. 1972;68:183–202. [PMC free article] [PubMed] [Google Scholar]
- 47.Lucin P, Pavic I, Polic B, Jonjic S, Koszinowski UH. Gamma interferon-dependent clearance of cytomegalovirus infection in salivary glands. J Virol. 1992;66:1977–1984. doi: 10.1128/jvi.66.4.1977-1984.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Diefenbach A, Colonna M, Koyasu S. Development, differentiation, and diversity of innate lymphoid cells. Immunity. 2014;41:354–365. doi: 10.1016/j.immuni.2014.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Spits H, Artis D, Colonna M, Diefenbach A, Di Santo JP, Eberl G, Koyasu S, Locksley RM, McKenzie AN, Mebius RE, Powrie F, Vivier E. Innate lymphoid cells–a proposal for uniform nomenclature. Nat Rev Immunol. 2013;13:145–149. doi: 10.1038/nri3365. [DOI] [PubMed] [Google Scholar]
- 50.Walker JA, Barlow JL, McKenzie AN. Innate lymphoid cells–how did we miss them? Nat Rev Immunol. 2013;13:75–87. doi: 10.1038/nri3349. [DOI] [PubMed] [Google Scholar]
- 51.Constantinides MG, McDonald BD, Verhoef PA, Bendelac A. A committed precursor to innate lymphoid cells. Nature. 2014;508:397–401. doi: 10.1038/nature13047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Constantinides MG, Gudjonson H, McDonald BD, Ishizuka IE, Verhoef PA, Dinner AR, Bendelac A. PLZF expression maps the early stages of ILC1 lineage development. Proc Natl Acad Sci U S A. 2015;112:5123–5128. doi: 10.1073/pnas.1423244112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Klose CS, Flach M, Mohle L, Rogell L, Hoyler T, Ebert K, Fabiunke C, Pfeifer D, Sexl V, Fonseca-Pereira D, Domingues RG, Veiga-Fernandes H, Arnold SJ, Busslinger M, Dunay IR, Tanriver Y, Diefenbach A. Differentiation of type 1 ILCs from a common progenitor to all helper-like innate lymphoid cell lineages. Cell. 2014;157:340–356. doi: 10.1016/j.cell.2014.03.030. [DOI] [PubMed] [Google Scholar]
- 54.Fuchs A, Vermi W, Lee JS, Lonardi S, Gilfillan S, Newberry RD, Cella M, Colonna M. Intraepithelial type 1 innate lymphoid cells are a unique subset of IL-12- and IL-15-responsive IFN-gamma-producing cells. Immunity. 2013;38:769–781. doi: 10.1016/j.immuni.2013.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wang J, Li F, Zheng M, Sun R, Wei H, Tian Z. Lung natural killer cells in mice: phenotype and response to respiratory infection. Immunology. 2012;137:37–47. doi: 10.1111/j.1365-2567.2012.03607.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Geurs TL, Zhao YM, Hill EB, French AR. Ly49H engagement compensates for the absence of type I interferon signaling in stimulating NK cell proliferation during murine cytomegalovirus infection. J Immunol. 2009;183:5830–5836. doi: 10.4049/jimmunol.0901520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lunemann A, Lunemann JD, Munz C. Regulatory NK-cell functions in inflammation and autoimmunity. Mol Med. 2009;15:352–358. doi: 10.2119/molmed.2009.00035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ohyama Y, Carroll VA, Deshmukh U, Gaskin F, Brown MG, Fu SM. Severe focal sialadenitis and dacryoadenitis in NZM2328 mice induced by MCMV: a novel model for human Sjogren’s syndrome. J Immunol. 2006;177:7391–7397. doi: 10.4049/jimmunol.177.10.7391. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


