Abstract
Disparities in clinical care have been described for patients with limited insurance coverage or social support. We hypothesized that patients with relapsed Hodgkin lymphoma (HL), non-Hodgkin lymphoma (NHL), or multiple myeloma (MM) treated at an urban county hospital serving indigent and under-insured patients would face barriers for referral to a private academic transplant center for autologous stem cell transplantation (ASCT). Charts of patients with HL, NHL, or MM treated at Grady Memorial Hospital between 2007 and 2013 were reviewed, and 215 patients with diagnosis of HD (n=40), NHL (n=96), and MM (n=79). 55 patients were referred for ASCT consults and 160 patients were not referred. Reasons for transplant non-referral included established clinical criteria (64% of cases), poor performance status (13%), refusal (4%), moved/lost-to-follow-up (4%), medical non-compliance (3%), death (3%), or referral to another hospital (1%). Non-referral based upon socio-economic criteria included: lack of legal immigration status/insurance (2%), and lack of social support/substance abuse (2%). Among the 55 referred patients, 27 patients (49%) underwent ASCT. Median follow-up for all referred patients from the time of diagnosis was 3.9 [0.7–22.7] years. 5-year survival from the date of diagnosis for patients who received ASCT was 80.2% versus 65.7% for non-transplanted patients (log-rank test, p-value=0.11). While the referral process did not demonstrate significant barriers based upon insurance or social status, further evaluation is needed to identify modifiable factors that can improve referral and assess the impact of the Affordable Care Act on access to ASCT.
Keywords: Autologous stem cell transplantation, Health disparity, Referral, Lymphoma, Myeloma
Introduction
Autologous hematopoietic stem cell transplantation (ASCT) provides a potentially curative treatment for patients with various hematological malignancies including relapsed Hodgkin lymphoma (HL) [1,2] and relapsed non-Hodgkin lymphoma (NHL), [3,4] and prolongs survival of patients with multiple myeloma [5,6]. The number of ASCT has continued to increase since 2000, making the proportion of ASCT approximately 58% of total hematopoietic stem cell transplants performed [6,7].
Autologous hematopoietic stem cell transplantation (ASCT) requires highly specialized and multidisciplinary care. It has been suggested that health disparity by patients’ age, gender, and race influence outcomes of ASCT [8]. Since ASCT is an extremely costly procedure, patients’ socioeconomic resources including appropriate coverage from health insurance is often a pre-requisite for treatment, and lack of insurance is a major barrier to access to ASCT [8–10]. Significant proportions of patients undergoing ASCT are referred from county hospitals, where the majority of patients have low socioeconomic status or financial hardships, to tertiary care centers such as university-based cancer centers. The Affordable Care Act (ACA) was activated on January 1, 2014 to improve health insurance coverage in the US population by decreasing the proportion of citizens without healthcare insurance.
We hypothesized that, in the era prior to the implementation of the ACA, patients treated at an urban county hospital with relapsed HL, NHL, or MM for whom ASCT might be indicated would face barriers for referral to a private academic transplant center, and would have inferior survival compared with similar patients treated primarily at the transplant center. We analyzed a series of patients with HL, NHL, or MM treated at an urban county hospital in the southeastern United States and determined referral rates, frequency of ASCT, and overall survival rates compared to similar patients treated primarily at the academic transplant center.
Methods
Hospitals
Grady Memorial Hospital is the largest hospital in the state of Georgia and the fifth-largest public hospital in the United States. Grady serves the residents of two urban counties in Atlanta with a large proportion of low-income and under-insured patients. Emory University Hospital is an academic tertiary care facility located in the suburbs of Atlanta. The Bone Marrow and Stem Cell Transplant Center of Emory University Hospital and the Winship Cancer Institute performs more than 350 marrow or stem cell transplants/year for a patient catchment area that includes Georgia and neighboring states in the southeastern United States.
Study population and identification of the patients
We utilized published methods [11,12] to identify patients diagnosed with DLBCL and MM based on prior pathology review and by the World Health Organization classification of Tumours of Haematopoietic and Lymphoid Tissues [13]. Patients with MM (including plasma cell dyscrasia, amyloidosis and plasmacytoma), HL and NHL subtypes who received care at Grady Memorial Hospital between January 01, 1991 and December 31, 2013 were included in the study. Cases were included if there was a diagnosis confirmed by record review.
Data collection
Following IRB approval, a retrospective chart review was performed using the Grady Medical record system. For this retrospective analysis, a Comprehensive Case Report Form was used to extract data from the medical record detailing demographics, whether the patient was referred for transplant and if not, why not. A face-to-face interview with the primary Hematologist/Oncologist who evaluated potential candidates for ASCT (L.B.) was performed for quality control of reasons for non-referral. For patients who were referred to the Winship Cancer Institute, an electronic record system (PowerChart) was utilized for comprehensive chart review. Abstracted data included clinical indication for ASCT by histology, reasons for non-referral, decision whether to offer ASCT at the academic center, and survival following referral and ASCT.
Medical reasons for non-referral included: KPS of less than equal 70%, documented dementia that impaired self-care, poor pulmonary function with DLCO of less than 40%, poor cardiac function with EF of less than 45%, or other significant comorbidities that affected KPS or organ function. Patients with a diagnosis of NHL for which a SCT is not indicated.
Statistical analysis
The principle objectives of this study were to examine determined referral rate and to compare outcomes for patients receiving ASCT following referral from an urban county hospital with those of contemporary patients who did not receive referral and counterparts undergoing ASCT following referral from other centers. Data were analyzed using the Mann Whitney U test for numerical covariates for numerical covariates and the chi-squared test or Fisher’s exact for categorical covariates where appropriate. Survival was analyzed using Cox proportional hazards models and log rank tests. Patients were censored at the time of last follow-up. Kaplan-Meier survival plots were generated for subsets of referred patients.
Results
Patient characteristics
A total of 215 patients with diagnosis of HL (n=40), NHL (n=96), MM (n=79) were identified. 169 patients (78.6%) were African Americans, 16 (7.4%) white, 14 (6.5%) Hispanic, and 117 (54.4%) were male. The median age of patients was 54 (19–89) years. Out of 62 potential transplant candidates, 55 patients were referred for ASCT consults and 160 patients were not referred (Figure 1). Among patients referred, 37 patients (67.3%) were male and 51 (92.7%) were African American. The median age was 51 (19–68) (Table 1).
Figure 1.
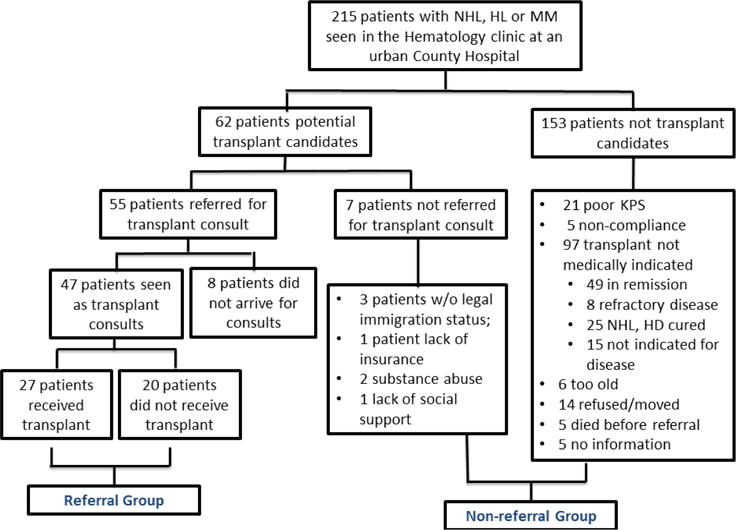
Consort diagram for the analysis of referral 215 patients with NHL, HL, MM seen at an urban County Hospital were identified. Amongst 62 patients who were potential transplant candidates, 7 patients were not referred due to socioeconomic/ psychosocial reasons, 8 patients did not show up at the transplant center. 47 patients were seen as transplant consults.
Table 1.
Summary of study subjects.
| Non-referral (N=160) |
Referral (N=55) |
Total (N=215) |
p-values | |
|---|---|---|---|---|
| Gender (male) | 80 (50%) | 37 (67.3%) | 117 (54.4%) | 0.027 |
| Race | 0.128 | |||
| White | 13 (8.1%) | 3 (5.5%) | 16 (7.4%) | |
| Black | 118 (73.8%) | 51 (92.7%) | 169 (78.6%) | |
| Hispanic | 13 (8.1%) | 1(1.8%) | 14 (6.5%) | |
| Age | 55(20–89) | 51 (19–68) | 54(19–89) | 0.052 |
| Diagnosis | <0.001 | |||
| HL | 32 (20%) | 8 (14.5%) | 40 (18.6%) | |
| NHL | 82 (51.2%) | 14 (25.5%) | 96 (44.7%) | |
| MM | 46 (28.8%) | 33 (60%) | 79 (36.7%) | |
| Disease Status(R/R) | 25 (15.6%) | 20 (36.4%) | 45 (20.9%) | 0.001 |
| Disease Response (CR+PR) | 85 (53.1%) | 29 (52.7%) | 114 (53.0%) | 0.959 |
| CR | 60 (37.5%) | 8 (14.5%) | 68 (31.6%) | 0.002 |
Reason for non-referral
Patients not referred for ASCT included: 21/160 (13%) with Karnofsky performance status (KPS) <60%; 74 (46%) in remission/ cure; 15 (9%) with a NHL histology for which ASCT was not indicated according to the management plan of the treatment physician including newly diagnosed follicular lymphoma, marginal zone lymphoma, peripheral T-cell lymphoma (PTCL), and chronic lymphocytic leukemia; 8 (5%) with refractory disease; 14 (9%) who refused referral, were lost to follow up, or were referred to a VA hospital; 5 (3%) who died before referral; 6 (4%) with age >70 years; 1 (1%) with lack of insurance; 3 (2%) who were illegal immigrants; 5 (3%) noncompliant to medical regimens; 2 (1%) with substance abuse; and 1 (1%) lacking adequate social support for ASCT (Table 2).
Table 2.
Reasons against referral.
| Reason against referral n=160 | N (%) | Non-Hodgkins | Hodgkins Lymphoma | Myeloma |
|---|---|---|---|---|
| 1. Uninsured | 1 (1 | 1 | ||
| 1a Illegal | 3 (2) | 2 | 1 | |
| 2. Lack of social support | 1 (1) | 1 | ||
| 3. Substance abuse | 2 (1) | 2 | ||
| 4. Poor performance status -total | 21 (13) | |||
| a. Alzheimers/dementia | 5 | 1 | 4 | |
| b. Pulmonary (Corrected DLCO <40) | 4 | 4 | ||
| c. Heart (LV EF <45%) | 6 | 6 | ||
| d. Other co-morbidities | 6 | 2 | 1 | 3 |
| 5. Medical non-compliance | 5 (3) | 1 | 2 | 2 |
| 6. Transplant not indicated at this time – total | 103 (64) | |||
| a. In remission | 49 | 38 | 9 | 2 |
| b. Not indicated for this disease | 15 | 12 | 3* | |
| c. Refractory disease | 8 | 2 | 1 | 5 |
| d. Cured | 25 | 12 | 13 | |
| e. age precludes transplant | 6 | 1 | 5 | |
| 7. No information | 5 (3) | 3 | 1 | 1 |
| 8. Refuses treatment | 7 (4) | 2 | 1 | 4 |
| 9. Patient no longer available total | 6 (4) | |||
| a. Patient moves | 2 | 1 | 1 | |
| b. LTF | 4 | 1 | 2 | 1 |
| 10. Patient dies before referral | 5 (3) | 4 | 1 | |
| 11. Referred to VA | 1 (1) | 1 |
Of three patients with myeloma, two had “smoldering myeloma” and one patient had an incorrect diagnosis.
Reason for non-transplantation after referral
Among the 55 referred patients, 27 patients underwent ASCT and 28 did not. Among patients undergoing ASCT, 19 patients had MM, 5 had NHL, and 3 had HL. Eight out of 28 patients did not report to the transplant center and were lost to follow-up. Amongst 20 patients seen at transplant center, reasons that referred patients did not undergo ASCT included: recommendation to continue conventional maintenance therapy for MM [n=9 (45%)]; comorbid conditions and KPS <60% [n=4 (25%)]; refractory/progressive diseases [n=4 (25%)]; disease not indicated for ASCT [n=2 (13%)] including PTCL, good response to the current chemotherapy in HL; and noncompliance with treatment [n=1 (6%)].
Analysis of survival
Two patients in the transplant group were excluded in the analysis of survival due to lack of data on the date of diagnosis. The median followup time for the transplant group is 3.9 years (range: 1.0–22.7 years). The median follow-up time for the non-transplant group is 5.5 years (range: 0.7–8.0 years). The median follow-up time for all referred patients is 4.7 years (range: 0.7–22.7 years). 5-year survival of transplanted patients from the date of diagnosis was 80.2% versus 65.7% for patients not transplanted. (Log-rank test, p-value=0.11) (Figure 2). From the date of ASCT, 5-year survival of transplanted patients was 77%. Among transplanted patients, 5-year survival from the date of ASCT was 69% for MM patients with no deaths were noted to-date in patients with NHL or HL who underwent ASCT (Log-rank test, p-value=0.26). The data are comparable or better than the historical 5-year overall survival rates for all patients with myeloma [14] (50% 5-year OS) or NHL (60% 5-year OS) transplanted at the center.
Figure 2.
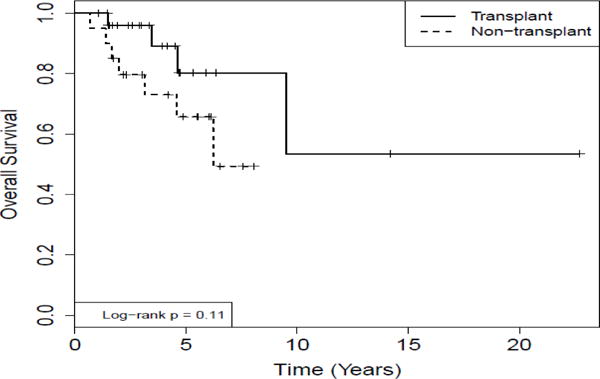
Comparison of survival analysis for MM and Lymphoma patients who underwent auto-transplantation and those referred to the transplant center but not transplanted.
Kaplan Meir analysis of survival from the date of diagnosis was calculated for both groups. 5 year-survival for the transplanted group (n=25) was compared to the non-transplanted group (n=20) was 80.2% vs. 65.7%, respectively (Log-rank, p=0.11).
Discussion
Although <20% of patients were referred for ASCT, contrary to our a priori hypothesis, in the era immediately prior to implementation of the Affordable Care Act (ACA), the process of referral of patients with MM, NHL, or HL from a county hospital to an academic tertiary care center for ASCT did not demonstrate significant barriers based upon patients’ insurance, socio-economic status, or social support. Previous reports indicated that AA patients are less likely to undergo ASCT for lymphoma and myeloma [15,16]. However, the settings for these previous reports differ substantially from ours. In the current study, the role of race/ethnicity could not be evaluated in the referral process, as 92.7% of referred patients in our cohort were African American. The majority of patients who were not referred to the transplant center appeared to arise for medical reasons rather than purely socio-economic reasons. 7/160 (4%) of patients were not referred due to non-medical reasons, and only 4/160 (2.5%) of patients not referred were illegal immigrants, lacked of insurance coverage and were ineligible for public assistance for other reasons contributing to lack of referral. Post-referral decisions whether or not to proceed with ASCT among referred patients also appeared to be based upon application of established criteria. However, these medical reasons for lack of referral and ineligibility for ASCT such as poor performance status and comorbid diseases may be secondary effects arising from lower socioeconomic status and reduced access to primary and other specialty care.
Our study has several limitations. First, our cohort consists of heterogeneous disease groups including various types of lymphomas and myeloma. However, because the large proportion of patients had MM, we were able to directly compare the cohort of referred and transplanted patients with previously published data from our transplant group [17]. Second, the majority of patients in our study cohort were African American limiting our ability to examine the relationship between referral bias and race. Hence, our study results may not be generalizable to patients of other ethnic groups. Third, the small sample size limited our ability to perform statistical analyses of outcomes stratified by referral versus non-referral status. For example, it was not possible to perform an analysis of outcomes for a discrete population of patients for whom transplant is clearly indicated, such as patients with relapsed DLBCL that remains chemo sensitive. However, this study of referrals from an inner city county hospital to a tertiary-care academic center may provide a baseline for future studies in this referral setting following introduction of the ACA.
27 of the 55 patients (49%) that were referred for transplant evaluation from Grady underwent transplant during the study period. These numbers are comparable to the regular transplant selection process at the transplant center. A total of 700 patients were evaluated for transplant eligibility, of which approximately 375 patients (54%) underwent transplant in the year 2014. These findings suggest that socio-economic reasons may not primarily influence the providers to offer transplant as a therapeutic approach.
A finding of general interest is that post-ASCT survivals among referred patients from a county hospital were comparable or superior to those of patients in published series [1–4]. For example, 68.9% of overall survival at 8 years in patients with MM transplanted in this series of referred patients are comparable to 79.7% 3-year overall survival (OS) in myeloma patients undergoing ASCT following bortezomib-based induction chemotherapy, and 74.7% post-ASCT 3-year OS in nonbortezomib-treated group reported in a meta-analysis of phase III randomized, controlled trials [18]. The 100% OS among a small number of referred patients with HL or NHL undergoing ASCT is not inferior to published reports of OS for a larger group of similar patients transplanted at Emory University Hospital [19]. These results lend support for our current practice of offering aggressive treatment, including ASCT, for patients with hematological malignancies in the catchment area of the county hospital.
To our knowledge, this is the first descriptive study with a long-term follow up documenting the initial referral process, process of evaluation for the medical appropriateness of ASCT, and overall survival after ASCT in a group of patients with lymphoma and myeloma referred from an inner city county hospital to a suburban academic tertiary care center. Further evaluations are needed to identify modifiable factors that can improve referral and assess the impact of the ACA on access to ASCT.
Acknowledgments
None of the authors have a financial relationship to report. Research reported in this publication was supported in part by the Biostatistics and Bioinformatics Shared Resource of Winship Cancer Institute of Emory University and NIH/NCI under award number P30CA138292. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Abbreviations
- ASCT
Autologous Stem Cell Transplantation
- ACA
Affordable Care Act
- HL
Hodgkin Lymphoma
- NHL
Non-Hodgkin Lymphoma
- MM
Multiple Myeloma
References
- 1.Schmitz N, Pfistner B, Sextro M, Sieber M, Carella AM, et al. Aggressive conventional chemotherapy compared with high-dose chemotherapy with autologous haemopoietic stem-cell transplantation for relapsed chemosensitive Hodgkin’s disease: a randomised trial. Lancet. 2002;359:2065–2071. doi: 10.1016/S0140-6736(02)08938-9. [DOI] [PubMed] [Google Scholar]
- 2.Linch DC, Winfield D, Goldstone AH, Moir D, Hancock B, et al. Dose intensification with autologous bone-marrow transplantation in relapsed and resistant Hodgkin’s disease: results of a BNLI randomised trial. Lancet. 1993;341:1051–1054. doi: 10.1016/0140-6736(93)92411-l. [DOI] [PubMed] [Google Scholar]
- 3.Hagenbeek A, Philip T, Bron D, Guglielmi C, Coiffier B, et al. The Parma international randomized study in relapsed non Hodgkin lymphoma: 1st interim analysis of 128 patients (as 15 January 1991: 153 patients) Bone Marrow Transplant. 1991;7:142. [PubMed] [Google Scholar]
- 4.Philip T, Chauvin F, Armitage J, Bron D, Hagenbeek A, et al. Parma international protocol: pilot study of DHAP followed by involved-field radiotherapy and BEAC with autologous bone marrow transplantation. Blood. 1991;77:1587–1592. [PubMed] [Google Scholar]
- 5.Attal M, Harousseau JL, Stoppa AM, Sotto JJ, Fuzibet JG, et al. A prospective, randomized trial of autologous bone marrow transplantation and chemotherapy in multiple myeloma. Intergroupe Francais du Myelome. N Engl J Med. 1996;335:91–97. doi: 10.1056/NEJM199607113350204. [DOI] [PubMed] [Google Scholar]
- 6.McCarthy PL, Jr, Hahn T, Hassebroek A, Bredeson C, Gajewski J, et al. Trends in use of and survival after autologous hematopoietic cell transplantation in North America, 1995-2005: significant improvement in survival for lymphoma and myeloma during a period of increasing recipient age. Biol Blood Marrow Transplant. 2013;19:1116–1123. doi: 10.1016/j.bbmt.2013.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Branson RD, DAvis KJ, Butler KL. African Americans participation in clinical research ; importance, barriers and solutions. Am J Surg. 2007;193:32–39. doi: 10.1016/j.amjsurg.2005.11.007. [DOI] [PubMed] [Google Scholar]
- 8.Majhail NS, Omondi NA, Denzen E, Murphy EA, Rizzo JD. Access to hematopoietic cell transplantation in the United States. Biol Blood Marrow Transplant. 2010;16:1070–1075. doi: 10.1016/j.bbmt.2009.12.529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Majhail NS, Murphy EA, Denzen EM, Ferguson SS, Anasetti C, et al. The National Marrow Donor Program’s Symposium on Hematopoietic Cell Transplantation in 2020: a health care resource and infrastructure assessment. Biol Blood Marrow Transplant. 2012;18:172–182. doi: 10.1016/j.bbmt.2011.10.004. [DOI] [PubMed] [Google Scholar]
- 10.Maziarz RT, Farnia S, Martin P, Komanduri KV. Optimal benefits for hematopoietic stem cell transplantation: a consensus opinion. Biol Blood Marrow Transplant. 2014;20:1671–1676. doi: 10.1016/j.bbmt.2014.07.007. [DOI] [PubMed] [Google Scholar]
- 11.Graiser M, Moore SG, Victor R, Hilliard A, Hill L, et al. Development of query strategies to identify a histologic lymphoma subtype in a large linked database system. Cancer inform. 2007;3:149–158. [PMC free article] [PubMed] [Google Scholar]
- 12.Huang T, Shenoy PJ, Sinha R, Graiser M, Bumpers KW, et al. Development of the Lymphoma Enterprise Architecture Database: a caBIG Silver level compliant system. Cancer Inform. 2009;8:45–64. doi: 10.4137/cin.s940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jaffe ES. The 2008 WHO classification of lymphomas: implications for clinical practice and translational research. Hematology Am Soc Hematol Educ Program. 2009;2009:523–531. doi: 10.1182/asheducation-2009.1.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nooka AK, Johnson HR, Kaufman JL, Flowers CR, Langston A, et al. Pharmacoeconomic analysis of palifermin to prevent mucositis among patients undergoing autologous hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2014;20:852–857. doi: 10.1016/j.bbmt.2014.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mitchell JM, Meehan KR, Kong J, Schulman KA. Access to bone marrow transplantation for leukemia and lymphoma: the role of sociodemographic factors. J Clin Oncol. 1997;15:2644–2651. doi: 10.1200/JCO.1997.15.7.2644. [DOI] [PubMed] [Google Scholar]
- 16.Joshua TV, Rizzo JD, Zhang MJ, Hari PN, Kurian S, et al. Access to hematopoietic stem cell transplantation: effect of race and sex. Cancer. 2010;116:3469–3476. doi: 10.1002/cncr.25297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pooja C, Nooka AK, Chatwal MS, Kim S, Chen Z, et al. Outcomes for Myeloma in the Era of Lenalidomide Maintenance Are Not Different Among Ethnic Groups Following Autologous Transplant. American Society of Hematology 2014 [Google Scholar]
- 18.Sonneveld P, Goldschmidt H, Rosinol L, Bladé J, Lahuerta JJ, et al. Bortezomib-based versus nonbortezomib-based induction treatment before autologous stem-cell transplantation in patients with previously untreated multiple myeloma: a meta-analysis of phase III randomized, controlled trials. J Clin Oncol. 2013;31:3279–3287. doi: 10.1200/JCO.2012.48.4626. [DOI] [PubMed] [Google Scholar]
- 19.Zhang H, Graiser M, Hutcherson DA, Dada MO, McMillan S, et al. Pharmacokinetic-directed high-dose busulfan combined with cyclophosphamide and etoposide results in predictable drug levels and durable long-term survival in lymphoma patients undergoing autologous stem cell transplantation. Biol Blood Marrow Transplant. 2012;18:1287–1294. doi: 10.1016/j.bbmt.2012.02.006. [DOI] [PubMed] [Google Scholar]


