Abstract
Immunotherapy is revolutionizing cancer care across disciplines. The original success of immune checkpoint blockade in melanoma has already been translated to Food and Drug Administration–approved therapies in a number of other cancers, and a large number of clinical trials are underway in many other disease types, including breast cancer. Here, we review the basic requirements for a successful antitumor immune response, with a focus on the metabolic and physical barriers encountered by lymphocytes entering breast tumors. We also review recent clinical trials of immunotherapy in breast cancer and provide a number of interesting questions that will need to be answered for successful breast cancer immunotherapy.
Keywords: Breast cancer, immunotherapy, immune evasion, tumor metabolism, tumor infiltrating lymphocytes
Antitumor Immunity
Basic mechanisms of a successful antitumor immune response
Immunotherapy has dramatically expanded patient’s lives in melanoma1 and has given hope for a new generation of patients to dramatically improve survival without going through the severe side effects of traditional chemotherapy. The initial success observed in melanoma has been translated to non–small-cell lung cancer,2 renal cell carcinoma,3 and recently bladder cancer,4 and clinical trials are underway to determine whether immunotherapy provides a clinical benefit in breast cancer (BC). In this review, we will attempt to give a brief overview on the basics of antitumor immunity, the effects of the breast tumor microenvironment (TME) on infiltrating lymphocytes, and current clinical trials for immunotherapy in BC.
A successful antitumor immune response requires many steps involving many components of the immune system. The adaptive immune system is highly complex and relies on educating cytotoxic lymphocytes (CTLs) to recognize modified or mutated antigens and eliminating malignant cells that express them. This process begins with antigen-presenting cells (APCs; typically dendritic cells [DCs] or macrophages) which can internalize dead tumor cells, digest them into small peptides, and present these at the cell surface in either class II major histocompatibility complex (MHC-II) (“classical” antigen presentation) or class I MHC (“cross-priming”).5,6 T cells continuously interact with APCs (typically within the spleen or peripheral lymph nodes) and scan peptides bound to MHC-I or MHC-II in a highly sequence-specific manner. Successful engagement of a T-cell receptor (TCR) with its specific/cognate peptide-MHC complex on an APC leads to a biochemical signaling cascade that culminates in T cells undergoing an activation/differentiation and proliferation/expansion program. This leads to a large number of antigen-specific activated CD4+ helper T (TH) cells and CD8+ cytotoxic T cells primed for effector functions that survey virtually all cells within the organism for expression of the specific antigen.
All nucleated cells process their intracellular protein contents through the proteasome system, and MHC-I complex presents the degraded peptide fragments (epitopes) on the cell surface. In this manner, all nucleated cells in the human body present their intracellular contents to surveying lymphocytes. Thus, if a circulating activated T cell recognizes an antigen within a peptide-MHC-I complex, it will either kill the target cell or produce inflammatory cytokines, depending on the type of lymphocyte.7 After recognizing an antigen on a malignant cell, CD4+ TH cells can secrete pro-inflammatory cytokines to recruit other immune cell types and mount an immune response, and CD8+ cytotoxic T cells can directly kill tumor cells by secretion of cytotoxic molecules such as perforin and granzymes, which lead to apoptosis of the target cell.
Thus, a successful antitumor immune response requires a few key steps: (1) capturing of tumor antigens by APCs, (2) presentation of tumor antigens to lymphocytes (typically in spleen and/or tumor-draining lymph nodes), (3) activation and expansion of CD4+ and/or CD8+ lymphocytes, (4) direct cell-cell contacts between activated lymphocytes and tumor cells (at primary or metastatic tumor sites), and (5) production of inflammatory cytokines (CD4+) and killing of tumor cells (CD8+), as well as other mechanisms involving B cells, natural killer (NK) cells, and macrophages, reviewed elsewhere.8–10
Immune responses to tumor-associated antigens in BC
The study of antitumor immune responses in BC began in the early 1990s with the discovery that CTLs obtained from tumor-draining lymph nodes of patients with BC could specifically recognize and kill breast tumor cell lines in culture (but not normal breast epithelial cell lines). This response was mediated through the immune recognition of the glycoprotein mucin (MUC-1). Although MUC-1 was expressed in both BC and normal epithelial cell lines, this study showed that it was underglycosylated in tumor cells, leading to the exposure of a hidden epitope that could be recognized by CTLs.11 Thus, a “self-antigen” was shown to be aberrantly expressed and modified to trigger an immune response. Later studies showed that very low levels of MUC-1–specific T cells could be detected in peripheral blood and bone marrow of both patients with BC and healthy subjects, but with a significantly higher percentage of these T cells in patients with BC.12,13 Along similar lines, flow cytometric analysis of tumor cells and tumor-infiltrating lymphocytes (TILs) obtained after surgical resection of breast tumors in 31 patients showed that the expression of MHC-I on tumor cells was strongly associated with infiltration of both CD4+ and CD8+ cells into the tumors.14 Furthermore, by studying the levels of MUC-1–specific IgG antibodies (which are typically secreted by effector B cells) in the serum of patients with nonmetastatic BC, 2 separate studies (a combined total of 442 patients) showed that increased MUC-1–specific antibodies were significantly correlated with improved disease-free and overall survival.15,16
Similar to MUC-1, HER2 was also found to be immunogenic, although its overexpression in tumor cells seems to be responsible for its immunogenicity rather than uncovering of hidden epitopes. In a study of 104 patients with variable expression of HER2 on primary tumors, HER2 expression was significantly correlated with both HER2-specific IgG antibodies in serum, as well as with HER2-specific T cells in blood.17 Furthermore, in a study analyzing the serum samples of more than 500 patients with BC, those with HER2-specific antibodies above the median had a significantly improved recurrence-free survival after surgery compared with patients with low antibody levels.18
TILs in BC
The above studies established that immune responses (B–cell and T–cell-mediated) are sometimes successful in mounting an antitumor immune response. An important factor determining immune control of tumors is the ability of T lymphocytes to infiltrate tumors. The importance of TILs in BC was noted as early as 1922 when lymphocytic infiltration was correlated with improved survival in a cohort of 218 patients with BC.19 More recent studies have confirmed these findings in many cancer types, including BC.20 A study of more than 1000 patients found that lymphocytic infiltration (as measured by immunohistochemical [IHC] staining of lymphocytes, as well as quantification of messenger RNA [mRNA] abundance of immune-related transcripts by reverse transcription-polymerase chain reaction) showed a clear and significant correlation between immune infiltration and pathologic complete response (pCR) to neoadjuvant chemotherapy (NAC) (40% vs 7% pCR in highly immune-infiltrated tumor vs tumors without immune infiltration).21 This trend was also observed in a study of 180 patients, where high TIL scores were associated with a better pCR. Interestingly, the difference in pCR between TIL high vs low was only significant in hormone receptor (HR)-negative patients (triple-negative BC [TNBC] or HER2+), but not HR+ patients (ER+ or PR+).22 In a meta-analysis of 13 studies including 3251 patients, Mao et al23 found that the presence of TILs in pretreatment biopsies was associated with a better pCR rate; interestingly, there was an approximately 4-fold better pCR specifically in TNBC and HER2+, but not in HR+ patients (confirming the above observations). Focusing specifically on ~500 patients with TNBC, Adams et al24 found that the percentage of stromal TILs had a significant effect on overall survival, with the 10-year overall survival probability of ~90% for patients with the highest stromal TIL score vs ~65% for those with no stromal TIL. Similar results were obtained in a 2013 study of 2000 pretreatment breast tumor samples, where the authors found that lymphocytic infiltration improved survival in TNBC, with a 5-year survival rate of 92% for patients with high TIL scores vs 71% for those with low TIL scores.25 Thus, a picture emerges in BC where, despite being more aggressive, HR-negative tumors are typically more immune-infiltrated, and the subset of patients with high TILs within this subtype respond better to NAC. Because immune infiltration is associated with improved response to NAC and overall survival in HR-negative patients with BC, new avenues of treatments that boost immune infiltration for these highly aggressive tumors should be explored. Namely, to study the mechanisms that restrict TIL entry into TNBC and HER2+ breast tumors that show little or no immune reactivity.
Barriers to Lymphocytic Infiltration
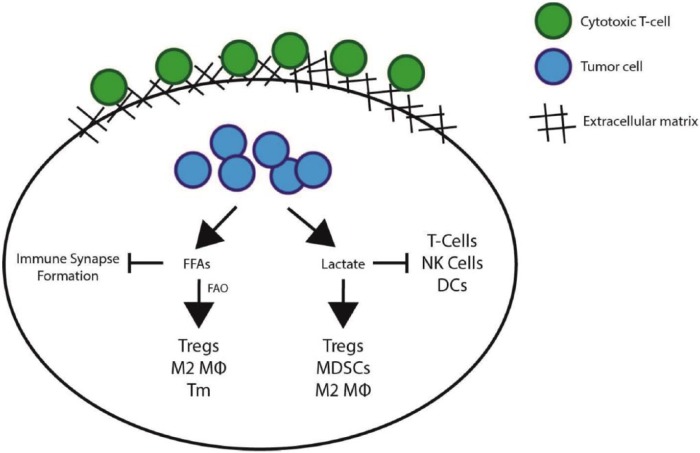
Although the presence of TILs in breast tumors leads to improved prognosis in patients, immune cells must overcome many challenges before they are able to directly interact with tumor cells and exert their antitumor effector function. Tumors are known to exhibit numerous mechanisms that lead to an immunosuppressive microenvironment, where immune cells are restricted from entering the tumor, become inactivated within the tumor, or even promote further immunosuppression.26 This article will focus on the metabolic challenges encountered by tumor-infiltrating immune cells, as well as the role of the extracellular matrix (ECM) on modulating immune infiltration (Figure 1).
Figure 1.
Mechanisms of immune suppression within the tumor microenvironment (TME). Immune cells that reach the tumor bed are faced with numerous challenges. The extracellular matrix (ECM) acts as a sink for immune cells and prevents cell-cell contacts between cytotoxic T cells and tumor cells, which inhibits infiltration of T cells into the body of the tumor. Once antitumorigenic immune cells reach the tumor epithelium, they are faced with a highly hostile microenvironment. A key component is lactic acid, produced mostly by tumor cells, which has been shown to inhibit antitumorigenic immune cells (such as cytotoxic T cells, natural killer cells [NK cells], and dendritic cells [DCs]) and to promote the survival and formation of immunosuppressive immune cells (such as regulatory T cells, myeloid-derived suppressor cells [MDSCs], and M2 macrophages [Mϕ]). Furthermore, free fatty acids (FFAs) are also abundant within the TME and have been shown to affect the plasma membrane fluidity of cytotoxic T cells, severely inhibiting their ability to form functional immune synapses with target cells, thereby inhibiting the function of the few infiltrating cytotoxic T cells that are able to come in contact with tumor cells (see main text for details). FAO indicates fatty acid oxidation.
Lactic acid regulates immune cell function within the TME
Tumors have been observed to have increased rates of glycolysis since 1927 when Otto Warburg published his original seminal study.27 Since then, and especially within the past 2 decades, the importance of tumor metabolism for fueling rapid cell proliferation has been increasingly appreciated. It is now recognized that both glucose and other nutrients (lipids, amino acids) are critical not only for energy (adenosine triphosphate) production but also, more importantly, for providing the molecular building blocks required for cell proliferation, such as proteins, nucleotides, and plasma membranes.28 Not surprisingly, oncogenic pathways involved in malignant transformation have been shown to upregulate expression of glycolytic genes to fuel cell growth. For example, Myc was shown to upregulate LDHA (lactate dehydrogenase A) in 1997 and has been shown to upregulate many other glycolytic genes since then.29,30 The PI3K/Akt/mTOR pathway was known to be involved in physiological glucose homeostasis,31 and in 2004, it was shown that Akt could directly upregulate glucose uptake in cancer cells,32 an effect that was later found to rely on upregulation of glucose transporter and several other glycolytic enzymes.33,34 Mutation or loss of p53 was also shown to lead to decreased oxidative phosphorylation and increased lactate production through glycolysis,35 an effect thought to be mediated mostly via TIGAR.36 Similarly, BRAF was recently shown to upregulate expression of a number of glycolytic genes, and glycolysis was found to be critical for the resistance to BRAF inhibition in a melanoma cell line.37 Furthermore, hypoxia in the TME can lead to stabilization of hypoxia-inducible factors, which have been shown to upregulate expression of glycolytic genes.38,39 Thus, pathways that promote cell proliferation and malignant transformation are tightly associated with increased rates of glycolysis and thus secretion of lactic acid within the TME, leading to an acidic microenvironment with extracellular pH (pHe) ranging from 6.5 to 7.25 in human breast tumors.40,41 Importantly, lactic acid accumulation within primary tumors has been linked to poor prognosis in a variety of cancer types.42–45
Tumor-derived lactic acid has been shown to have many protumorigenic effects, including promotion of angiogenesis,46,47 increased cancer cell migration,48 fueling oxidative cancer cells (“reverse Warburg” effect),49,50 and contributes to an overall immunosuppressive TME. Many excellent reviews have been written on the effects of lactic acid on tumor cells and infiltrating immune cells51,52; the following is a brief composite overview of the immunosuppressive effects of lactate.
As mentioned above, antitumor immune responses require many steps to be successful, and lactate has been shown to inhibit many of these key steps. Lactate was shown to inhibit tumor necrosis factor α production from human monocytes, and although pHe contributed to this effect, pHe alone did not fully recapitulate the effects of lactic acid.53 Furthermore, a study on DCs found that lactate inhibited endocytosis (necessary for antigen capture), cytokine production, T-cell activation potential, as well as expression of the DC-specific markers CD1a and CD209 in a dose-dependent manner.54 Similar studies on DCs showed that lactic acid dramatically reduced CD1a expression during DC differentiation, whereas acidic pHe (6.0) had a minimal effect. In addition, conditioned media from 3-dimensional (3D) melanoma cell cultures were found to significantly inhibit DC-specific CD1a expression that could be rescued by addition of oxamate (a lactate dehydrogenase [LDH] inhibitor) to the 3D tumor cell cultures.55 An important cell type mediating immune suppression is myeloid-derived suppressive cells (MDSCs), which are a very heterogeneous population of myeloid cells that include myeloid progenitor cells, immature DCs, and granulocytes.56 Lactate was shown to enhance generation of MDSCs from the bone marrow of mice, and these MDSCs were then shown to potently inhibit CD4+, CD8+, and NK cell proliferation and cytotoxic capacity. Lactate enhanced not only the number of MDSCs formed but also their immunosuppressive capacity.57 Lactate was also recently found to polarize tumor-associated macrophages (TAMs) toward the protumorigenic M2 phenotype. Lactate was shown to stabilize HIF1a within TAMs and led to increased vascular endothelial growth factor (VEGF) and arginase-1 production, contributing to an immunosuppressive TME.58 Recent studies also showed an inhibitory effect of lactate on NK cells, where addition of lactic acid inhibited NK activation markers, cytokine production, and cytotoxic capacity.57,59 Furthermore, lactic acid has been shown to inhibit T-cell activation, cytokine production, and cytotoxic capacity and to induce cell death in a manner that was not fully recapitulated merely by acidic pH.60 In 2011, the same group demonstrated that stimulation of TCR signaling led to a rapid (1-15 minutes) activation of the MAPK, ERK, and Akt pathways and found that the presence of lactic acid specifically prevented the phosphorylation of key members of the MAPK pathway, but not those of the ERK or Akt pathway.61 These observations point to the importance of these signaling pathways for T-cell activation and illuminate the mechanism responsible for lactate’s immune inhibitory effects. Importantly, these rapid inhibitory effects of lactic acid were not observed with an acidic pH alone. Along similar lines, Haas et al62 showed that lactic acid could directly inhibit chemokine-dependent T-cell motility, mediated through the lactate transporters SLC16A1 (MCT1) and SLC25A2 (SMCT2). Interestingly, clinical trials of immune checkpoint inhibition in melanoma63 and BC64 show that increased serum LDH levels correlate with lack of response. Whether increased serum LDH is merely a measure of tumor burden, or whether it reflects the metabolic activity of tumors and the resulting levels of lactic acid within the TME, is something that will need to be studied in more detail. Thus, modulating tumor metabolism may be an important adjuvant approach to improve responses to immunotherapy.
High rates of glycolysis by tumor cells can lead to the accumulation of lactic acid and contribute to creating an immunosuppressive TME, as described above. However, high rates of tumor glycolysis and lactate production also reflect high rates of glucose consumption, as shown by fludeoxyglucose F 18-positron emission tomography scans. Glycolysis has been known to be a critical step during T-cell activation, and recently, competition for glucose within the TME was described as a key factor in promoting T-cell exhaustion.65 Furthermore, the glycolytic metabolite phosphoenolpyruvate was recently described to be critical for Ca2+ influx and nuclear factor of activated T cell (NFAT) nuclear translocation during T-cell stimulation in both CD4+ and CD8+ T cells, providing a mechanism for the requirement of glycolysis for T-cell activation.66 An additional role for glycolysis is also emerging in controlling epigenetic mechanisms of tumor and immune cell phenotypes, which is thought to be mediated by production of acetyl-CoA from glucose-derived pyruvate. Acetyl-CoA can be used by histone acetyltransferases to modify histones throughout the genome and modify cancer and immune cell gene expression.67,68 Thus, glycolysis has been clearly established to be a critical regulator within the TME that can affect both tumor and immune cell phenotypes.
Immunosuppressive role of free fatty acids within the TME
A clinically relevant immunosuppressive role of fatty acids was observed as early as 1977.69 Similar immunosuppressive effects were found when quantifying the inhibitory effects of serum from pediatric patients with acute lymphoblastic leukemia on phytohemagglutinin-induced lymphocyte proliferation.70 More recently, a statistically significant negative correlation between serum free fatty acid (FFA) concentration and calcium response following TCR stimulation was observed (r = −.6).71 A similar effect was observed in BC suggesting that tumor cell secretion of unsaturated FFAs may be a mechanism of immune evasion.72 Following the initial in vivo data regarding the immune inhibitory role of FFAs, a series of articles by Richieri et al in 1989 to 1990 shed a great light into the mechanisms behind FFA immunosuppression. These studies showed that addition of oleic acid (but not steric acid, which is the saturated form of oleic acid [OA]) led to inhibition of intracellular calcium accumulation following stimulation with either target cells or concanavalin A (ConA), and this effect was observed within seconds to minutes of addition of the fatty acids. Importantly, within this time frame, the authors show that OA was bound to the T-cell plasma membrane and was not significantly metabolized (esterified), suggesting that membrane composition may strongly affect the capacity of T cells to kill target cells. In all, the authors show that OA rapidly and robustly inhibited the target cell–induced or ConA-induced intracellular calcium increase, CTL degranulation, and CTL-mediated lysis of target cells within seconds to minutes of OA addition. The authors suggest that “the perturbative effects must be due to a physical perturbation, most likely of membrane lipid structure.”73–76
The mechanism behind the observed inhibitory effects of FFAs on immune function has been more clearly elucidated in the past 2 to 3 decades. Detailed analysis of the phosphorylation cascade following TCR stimulation (with anti-CD3 + other co-stimulatory molecules [CD28, CD59]) revealed that while control primary T cells exhibited strong activation of many RTK pathways (MAPK, ERK, PI3K) within minutes, pretreatment of T cells with unsaturated fatty acids (UFAs) for 1 to 3 days strongly inhibited the CD3-induced phosphorylation of JNK and c-Jun, whereas pretreatment with saturated fatty acids had no effect. Of note, TCR stimulation–induced NFAT activation, interleukin 2 (IL-2) secretion, and expression of the IL-2 receptor (CD25) were also inhibited in primary T cells by UFA pretreatment.77
The importance of T-cell membrane fluidity/rigidity for effector T-cell function has been studied in great detail. Following a short (3-7 minutes) stimulation with activating anti-CD3 beads or DCs, the lipid density at the site of contact with the beads or DCs significantly increased.78 Jurkat cells treated with either anti-CD3 beads or antigen-loaded B cells showed similar increased membrane density at the site of contact.79 Furthermore, the authors found that increasing amounts of 7-ketocholesterol (7KC) in the plasma membrane inhibited the stimulation-dependent increase in lipid density at the site of contact between T cells and beads or APCs, with a resulting inhibition of IL-2 production. Detailed analysis of the molecular composition of the contact sites showed that 7KC inhibited the recruitment of CD3, Zap70, and LAT to the TCR signaling domains following TCR stimulation.
The above studies suggest that stimulation of the TCR leads to condensation of the plasma membrane at the site of contact followed by downstream signaling, and that the addition of UFAs (whose double bond(s) cause a kink in their hydrophobic tails, causing membranes to become more fluid) prevented plasma membrane condensation and downstream signaling. In agreement with this interpretation of the above results, a lipidomics approach was taken to study biochemically isolated plasma membrane fractions containing the TCR machinery (“TCR activation domains”) following stimulation with anti-CD3 beads. T-cell receptor activation domains were enriched in cholesterol, sphingomyelin, and saturated forms of phosphatidylcholine after just 3 minutes of stimulation. Pretreatment of T cells with polyunsaturated fatty acids (PUFAs) prevented the condensation of T-cell plasma membrane regions in contact with anti-CD3 beads and caused an increase in the amount of polyunsaturated lipids within TCR domains, providing a mechanism for the inhibitory role of PUFAs in TCR signaling.80 Membrane lipid order (density) was also found to be critical for CD4+ T cells. Human CD4+ T cells were isolated from healthy volunteers, stained with a fluorescent lipid dye, and subpopulations of T cells with low, intermediate, and high lipid order were observed. The authors found that T cells with high lipid order had increased cholesterol levels in the plasma membrane, formed larger and more stable immune synapses (ISs) with anti-CD3–coated coverslips or APCs, and led to higher T-cell proliferation and cytokine production compared with intermediate-order T cells.81 A 2015 genetic and lipidomic study showed that the lipid profile of various macrophage cell lines correlated with their functional response to various toll-like receptor (TLR) ligands as measured by their ligand-induced cytokine production (IL-6). Furthermore, the authors showed that the lipid profiles of fibroblasts derived from patients with sphingolipid storage disorders were sufficient to accurately predict their hyperresponsiveness or hyporesponsiveness to TLR stimulation, underscoring the importance of membrane lipid dynamics in immune function.82 Cholesterol was also found to greatly affect the functional level of mouse CD8+ T cells. Following stimulation, T cells exhibited increased plasma membrane and intracellular levels of cholesterol and increased expression of cholesterol biosynthesis and transport genes. The gene for Acat1, which esterifies free cholesterol into cholesteryl esters for storage, was also dramatically upregulated following CD3/CD28 stimulation. Inhibiting ACAT1 during CD3/CD28 stimulation led to increased cytokine production and granule secretion, as well as increased cytotoxicity against Ovalbumin (OVA)-expressing target cells. Furthermore, T–cell-specific knockout of Acat1 led to a significantly improved antitumor immune response, as evidenced by decreased tumor growth and increased survival in melanoma and lung carcinoma mouse models.83
Free fatty acids have clearly been shown to inhibit immune function. Free fatty acids have been shown to insert into plasma membranes and decrease membrane fluidity, precluding lipid raft and IS formation and inhibiting downstream signaling. Besides this direct effect, recent advances in immunometabolism are showing that uptake and metabolism of FFAs by immune cells can also affect their functional state, especially FFA β-oxidation. This has been extensively reviewed,84 but briefly, fatty acid β-oxidation is characteristic of anti-inflammatory M2 macrophages, regulatory T cells (Tregs), and is required for the transition from effector to memory T cells.85–87 Furthermore, the FFA-induced increase in membrane fluidity has been associated with increased cancer cell proliferation and survival through epidermal growth factor receptor and PI3K pathway activation.88,89 Thus, increased serum FFAs may be an important protumorigenic factor in patients with cancer which requires further study.
The ECM as a physical barrier to immune infiltration
Although recognized as an important factor in promoting tumor growth, survival, and migration/invasion of tumor cells for decades,90 the role of the ECM in regulating TIL function has gained traction in the past few years, mostly due to technological advances in microscopic imaging of TILs. The ECM is the noncellular component of tissues and organs and can be divided into 2 main classes: a fibrillar fraction, which consists of arrays of fibrillar collagen bundles, fibronectin fibers, and elastin, and the nonfibrillar fraction, which is the soluble fraction consisting mainly of glycosaminoglycans and proteoglycans. These sugars are large and charged and can thus bind water and fill the space between the insoluble fibers within the ECM. Thus, the ECM plays mostly a structural role and defines the shape and stiffness of organs (and tumors).91,92
The initial observation that the ECM may contribute to tumor development came from a 2001 analysis of 9 clinical studies regarding breast mammography, which calculated a 4-fold to 6-fold greater risk of developing BC in women with high mammographic densities compared with those of low densities, and this was the most significant BC risk factor besides age and mutations in BRCA1/2.93 Importantly, a later study found that in excision biopsies following breast mammograms, regions of high mammographic density showed significantly higher collagen density and expression of proteoglycans, strengthening the link between a mammographic density, abundant ECM, and tumorigenesis.91 Detailed studies on lymphocyte biology and trafficking in the 1990s by Friedl et al94 showed that in an in vitro 3D collagen matrix assay, isolated human T cells traveled using highly transient and low-affinity interactions with collagen fibers. Later studies by the Friedl group showed that in similar 3D collagen matrices, T cells migrated along the matrix by ameboid shape changes and contact guidance that follow preexisting ECM scaffolds, where T cells filled the gaps within the fibers and followed the path of least resistance.95 Another study showed that tumor-associated fibroblasts could remodel a collagen gel in vitro, making it more dense and preventing T-cell trafficking into the gel.96
These early in vitro studies were followed by in vivo time-lapse imaging of fluorescently labeled T cells, which yielded more detailed information of the movements and dynamics of TILs in situ. In 2006, Mrass et al. used transgenic mice with T–cell-restricted green fluorescent protein (GFP) expression and implanted a mouse lung tumor cell line (TC1) which regresses following vaccination against an antigen expressed by this cell line (E7), even after tumors become established. The authors implanted TC1 cells subcutaneously into mice, vaccinated them, and monitored both progressing (nonvaccinated) and regressing (vaccinated) tumors by live-cell fluorescence microscopy. This study showed that very few GFP+ T cells were found in progressing tumors, and those that successfully infiltrated had very limited mobility. In contrast, following vaccination, regressing tumors were richly infiltrated with GFP+ T cells which were highly motile. Interestingly, when second harmonic generation (SHG) methods were used to visualize collagen fibers, the authors noted that tumor-infiltrating T cells tended to crawl along collagen fibers, “suggesting that these structures may be used as guidance cues through the interstitial space,” strengthening the role of the ECM in lymphocyte migration in tumors in vivo.97 The role of the stroma and ECM in regulating lymphocytic infiltration within tumors was definitively and clearly established in 2012 by Salmon et al. The authors obtained fresh human lung tumors, embedded them in a transparent agarose gel, and cut thick slices that were placed on a microscope insert under controlled environmental conditions. Then, in vitro–activated T cells from healthy donors, or freshly isolated TILs from the corresponding patients, were fluorescently labeled and overlaid on top of the freshly cut tumors. After a 60-minute rest period, the preparation was imaged and T cells were found to localize preferentially to the tumor stroma, where the T-cell concentration was 5-fold higher compared with localization to epithelial tumor islets. The authors confirmed these findings by fluorescence staining of endogenous T cells in the explanted tumor slices by CD3 immunofluorescence. This study confirmed that the “overlaid” T cells had similar localization patterns compared with endogenous T cells. Detailed examination of fluorescent images of 6 fixed human lung tumor sections showed that T cells preferentially localized to collagen-low or fibronectin-low regions within the stroma compartment. In fact, stromal regions with high density of collagen or fibronectin were nearly devoid of T cells, and those T cells that were present tended to reside within the gaps between the ECM fibers. Fibronectin was found to be particularly dense near the tumor-stroma interface, suggesting that the dense fibronectin could be responsible for excluding T cells from the tumor islets. To test the causal relationship between ECM density and T-cell localization, the authors treated the explanted human lung tumors with collagenase for 30 minutes prior to addition of fluorescently labeled T cells. Collagenase treatment was found to greatly reduce collagen density around tumor islets, and a significant increase in the number of T cells was observed at the tumor-stroma interface and those in direct contact with peripheral tumor cells.98 Importantly, similar patterns of T-cell localization were observed in a study of fresh ovarian tumors by the same group in 2015, with the additional benefit that in this study, the authors only analyzed endogenous T cells instead of “overlaid” T cells using a modified labeling protocol.99
The above studies using fluorescence microscopy illuminated the precise mechanisms by which a dense ECM could serve a physical barrier to prevent immune infiltration and promote immune escape. These observations were validated by Ohno et al, in a study of 84 fixed human gastric tumor samples. The authors found that stromal collagen staining was associated with more aggressive disease, as evidenced by an almost 4-fold higher staining intensity in patients with stage T3 disease compared with those with stage T2, as well as a more than 5-fold decrease in intratumoral CD8+ T cells in collagen-high tumors. Furthermore, low stromal collagen was robustly associated with improved disease-free survival after curative surgery, with an 8-year survival probability of ~85% vs ~35% for patients with low vs high stromal collagen, respectively.100 Further evidence to support the protumorigenic role of a dense ECM was recently reported in pancreatic ductal adenocarcinoma (PDAC). Pancreatic ductal adenocarcinoma is a highly aggressive disease with very poor 5-year survival rates that is characterized by strong desmoplasia and a very abundant stromal fraction which often exceeds the epithelial fraction of tumors. In a study of 100 PDAC tissue samples, the authors found that about half showed little or no CD3+ T-cell infiltration, and of those tumors with a lymphocytic infiltrate, most lymphocytes were found within the stroma. Surprisingly, although tumors expressed very high levels of chemokines and their cognate receptors, there was a lack of correlation between chemokine concentrations and T-cell infiltration. Furthermore, although T cells migrated along a chemokine gradient in an in vitro transwell migration assay, this directed movement was abrogated when chambers were coated with collagen, supporting the notion that collagen may pose a physical barrier to chemotaxis within tumors. Thus, the authors performed immunofluorescence and SHG analysis (for CD3+ T cells and collagen fibers, respectively) of human PDAC tumors and found a significantly lower collagen density in regions of high T-cell infiltration.101
Although chimeric antigen receptor (CAR) T-cell therapy has shown good results in liquid cancers, solid tumors are more resistant to this type of therapy.102 To study the potential effects of long-term in vitro activation of T cells (which is a necessary step during the clinical manufacturing process of CAR T cells from patients), Caruana et al isolated and activated peripheral blood T cells. The authors found that expression of heparanase (HPSE), an ECM-degrading enzyme, was significantly lower in long-term ex vivo isolated T cells (LTE T cells) compared with either freshly isolated or briefly activated T cells. Of note, the time frame for the ex vivo culture of the LTE T cells (12-14 days) is similar to the one used in the clinic for expansion of CAR T cells prior to infusion back into patients. To determine whether loss of HPSE expression affected the ability of CAR T cells to target solid tumors, the authors engineered CAR T cells to express HPSE. They showed, in 4 different mouse tumor models (3 neuroblastoma and 1 melanoma), that HPSE-expressing CAR T cells significantly improved T-cell infiltration into solid tumors and overall survival compared with control CAR T cells.103 These results underscore the importance of the ECM in blocking T-cell infiltration and suggest that targeting the ECM may be important to improve immunotherapies in solid tumors, along with other strategies.
Recent clinical trials in melanoma have shown that increased expression of genes involved in cell adhesion and ECM organization are one of the most significant factors that negatively correlate with response to anti-PD-1 or anti-CTLA4 therapy.104 In addition, the mesenchymal subtype of TNBC (with extensive connective tissue and ECM proteins, as discussed below) is the least immune-infiltrated TNBC subtype, underscoring the potential importance of the breast tumor stroma in regulating immune infiltration and response to immunotherapy.
Anti-inflammatory immune cell types
The immune system’s ability to turn itself off after a bacterial or viral threat has been neutralized is a critical component of an immune response to pathogens. As is often the case, tumors co-opt the natural negative feedback loops to their own advantage. For example, this can lead to the accumulation of immunosuppressive cell types such as MDSCs and Tregs. Myeloid-derived suppressive cells originate from immature myeloid cells and exhibit a block in their differentiation (to granulocytes, macrophages, and DCs) and remain in an immunosuppressive immature stage (reviewed in Gabrilovich and Nagaraj105). Similarly, Tregs normally function to suppress T-cell responses following resolution of an infection and are critical for preventing autoimmune disease. However, tumors can co-opt this feedback mechanism and promote increased numbers of Tregs within tumors to suppress cytotoxic T cells.106 Interestingly, the accumulation of lactic acid within the TME has been recently shown to promote increased numbers of Tregs within tumors.107
Blood flow and angiogenesis
Besides providing a system for nutrient delivery and waste disposal, angiogenesis has also been shown to directly promote immune suppression. This effect is due to angiogenic factors released by tumor cells such as VEGF, which can inhibit DC maturation, promote Treg infiltration, and inhibit pro-inflammatory cytokine production. Furthermore, although tumors contain higher numbers of blood vessels, they are functionally compromised, and they often do not express receptors required for T-cell extravasation into tissues, such as ICAM-1 and VCAM-1, precluding effective cytotoxic T-cell tumor infiltration.108
Current Advances in BC Care
TNBC subtypes
In recent years, it has become clear to the BC clinical community that TNBC tumors represent a highly diverse subtype in terms of histology, response to chemotherapy, and overall survival. Two recent studies from 2 different groups used transcriptomic analysis to identify 4 subtypes within TNBC to address this heterogeneity, and although some minor differences exist between the 2 studies, both arrive at similar conclusions.109,110 These studies performed unsupervised clustering of TNBC tumors based on gene expression and identified 4 main subtypes of TNBC: luminal androgen receptor (LAR), mesenchymal-like (MES), and 2 subtypes of basal-like tumors (basal-like 1/2 [BL1, BL2] in Lehmann 2016 or basal-like immune activated/suppressed [BLIA/BLIS] in Burstein 2015). The LAR subtype, although ER negative by IHC, showed enrichment of androgen and estrogen metabolism and other hormonally regulated pathways by gene expression. Androgen receptor (AR) mRNA and protein levels were ~10-fold higher in the LAR subtype compared with the other subtypes. Patients with LAR tumors tended to be older at diagnosis, have a low pCR after NAC, and exhibited a high rate of bone metastasis compared with other metastatic sites. These findings have led to a number of clinical trials with androgen antagonists, including a phase 2 trial which showed a clinical benefit rate (as measured by CR [complete response], PR [partial response], or SD [stable disease] for >24 weeks) of 29% in tumors positive for AR by IHC, including 2 CRs and 5 PRs of 75 evaluable patients.111,112 The MES subtype is characterized by enrichment in pathways involved in cell motility, ECM organization, and cell differentiation pathways (such as Wnt, ALK, transforming growth factor β). Immune infiltration was lowest in this subtype as measured by expression of immune-related genes as well as pathological scoring of mononuclear cells in tumor sections. Clinically, these patients had the lowest rate of lymph node involvement and a prevalence of lung metastasis. The basal-like subtypes are characterized by expression of cell cycle genes, including Ki67 mRNA and protein overexpression. BLIA/BL1 tumors in addition show increased expression levels of immune genes as well as STAT and STAT-regulated genes. Patients with this subtype of TNBC show the best overall survival and pCR compared with the other TNBC subtypes. In contrast, the BLIS/BL2 subtype is characterized by decreased expression of immune-related and antigen presentation genes and exhibits high levels of multiple SOX family transcription factors. It has the worst overall survival and tends to have low pCR compared with the other TNBC subtypes.
TILs in BC subtypes
Historically, only a few BC antigens have been described, and BC has not been considered to be a particularly immunogenic tumor type. However, some breast tumors do show immune infiltration, and the location of TILs (tumor parenchyma vs stroma surrounding the tumor mass) is an important distinction. Furthermore, programmed cell death protein 1 (PD-1), its cognate ligands (PD-L1 and PD-L2), and cytotoxic T lymphocyte–associated protein 4 (CTLA-4) have been shown to negatively regulate immune responses.113 It should be noted that the presence of TILs frequently co-exist with PD-1 and PD-L1 expression in tumors due to feedback mechanisms.114 In BC, a recent study showed that while PD-1 only expressed in breast TILs, PD-L1 could be expressed in both tumor cells and TILs (mostly of macrophage linage).115 A 2014 analysis of PD-L1 mRNA expression by fluorescence in situ hybridization analyzed 636 breast tumors and showed that more than half of the tumors had positive PD-L1 expression. Tumor-infiltrating lymphocyte counts showed that most tumors had low levels of lymphocyte infiltration, whereas only ~15% had modest/strong TIL counts. Importantly, PD-L1 mRNA-positive tumors had increased levels of CD3 and CD20 signal by immunofluorescence staining and had increased relapse-free survival compared with PD-L1 mRNA-negative patients.116 This is in agreement with a recent report on 45 primary breast tumors that showed increased levels of tumor cell PD-L1 expression were associated with higher TIL as well as a trend toward improved overall survival.117 Furthermore, to study mechanisms of immune escape during metastatic progression, a number of recent studies have analyzed immune infiltration in primary and matched metastatic samples.117–121 All these studies reported that metastatic tumors had significantly less CD3−, CD4−, CD8−, or CD20-expressing TILs compared with the matched primary tumors.
As described above, studies over the past decade have shown that BC is a highly heterogeneous disease, with different subtypes showing different degrees of immune infiltration. Hormone receptor–positive tumors were shown to have very limited immune infiltration, whereas a small portion of HER2 and TNBC tumors are richly infiltrated. A tumor microarray of ~3400 patients with BC stained for CD8 found that intratumoral CD8+ TILs were associated with ER negativity. Patients whose tumors had at least 1 intratumoral CD8+ TIL had a survival advantage over those without any intratumoral CD8+ TIL in ER-negative patients, but not in ER-positive cases.122 These results are in agreement with recent studies. Cimino-Matthews et al117 found that basal-like tumors had significantly higher number of T cells per square millimeter of tumor compared with luminal cancers, and Buisseret et al115 similarly found that high TIL density was significantly associated with hormone receptor negativity. Interestingly, the latter study also showed that TILs had an antigen-experienced effector/memory phenotype rather than an antigen-naïve phenotype, underscoring the immunogenicity of some breast tumors. Furthermore, a study of gene expression in more than 2100 patients with BC found that an immune gene signature (lymphocyte-specific kinase metagene) was significantly associated with improved relapse-free survival in TNBC and HER2 disease, but not in ER/PR-positive cancers.123 These studies indicate that HR-positive tumors are mostly immunologically silent, whereas a small portion of TNBC and HER2 tumors shows significant immune infiltration. Immune infiltration in TNBC and HER2 tumors is associated with increased patient survival compared with the survival of most of the patients bearing HR-negative tumors with little or no immune infiltration.
Recent and ongoing clinical trials of immunotherapy in BC
The excitement of immunotherapy has translated to BC in recent years, and a number of clinical trials have recently reported encouraging results. Most of these studies focused on the PD-L1–positive population, as PD-L1 positivity has been associated with improved response to immune checkpoint blockade in other cancers.124 In contrast to the very limited or modest responses in HR-positive disease,125,126 clinical trials in HR-negative patients show some promising initial results. A study of 111 patients with TNBC reported that 59% of patients were PD-L1+ by IHC; of these PD-L1+ patients with TNBC, 32 patients with relapsed or metastatic disease were enrolled in a phase 1b study of pembrolizumab (anti PD-1). Of these, 27 were eligible for efficacy analysis (observed response rate [ORR]) and an 18.5% response rate was observed.64 In another phase 1 trial of atezolizumab (an anti-PD-L1 antibody), 21 PD-L1–positive metastatic TNBC were tested and reported a similar ORR of 19%.127
Given the limited success of single-agent immunotherapies in BC, there are many ongoing attempts to create a more immune friendly microenvironment with other therapies, in combination with checkpoint blockade. These include studies testing combinations of exemestane + anti-CTLA4,128 cryoablation of primary tumors + anti-CTLA4,129,130 and HER2-directed vaccines + immune checkpoint blockade.131,132 Furthermore, since patients with high TIL have better responses to conventional therapies in BC (discussed above), combinations of immune checkpoint blockade with conventional chemotherapies are also being investigated. The I-SPY 2 trial is testing the addition of pembrolizumab to standard NAC regimens in HER2-negative locally advanced BC. Recently reported results showed that the combination of pembrolizumab + chemotherapy led to an impressive almost 3-fold increase in response rates (pCR) compared with chemotherapy alone in TNBC and HR-positive/HER2-negative patients.133
Conclusions and Future Directions
Although many patients with HR-positive BC do well with current therapies, a significant portion of patients with HR-negative disease die as a result of progressive disease and their metastases. Nevertheless, recent studies have shown that a subset of HR-negative tumors are immune reactive, with patients showing improved responses to chemotherapy and better survival. Recent clinical trials show that approximately 20% to 25% of patients with advanced HR-negative disease may benefit from immune checkpoint blockade. Although an important milestone in BC, single-agent immune checkpoint blockade still leaves most of the HR-negative patients with limited treatment options and combinations with other therapies are being investigated.
Given the clear evidence that immune infiltration provides benefits in terms of response to therapy and survival,134 it will be critical to develop a deeper understanding of the mechanisms driving immune exclusion in BC. The metabolic profile of tumors has been shown to dramatically affect the phenotypic profile of infiltrating lymphocytes and their functional capacity, both due to accumulation of lactic acid as well as the depletion of glucose from the TME. Historically, targeting tumor metabolism has been hindered by the requirement of glycolysis for normal tissue homeostasis, but new small molecules are being developed to target different parts of the glycolytic pathway that may show more specificity against tumors.52,135 Our understanding of the protumorigenic role of FFAs has been greatly enhanced in recent years. Their role in directly inhibiting IS formation, in modulating immune cells toward a protumorigenic phenotype, and in providing a fuel for tumor cells is clearly established.136,137 However, the source of FFAs within the TME remains unclear, with the possibility that FFAs may originate from tumor cell necrosis, increased lipolysis within the TME, or they may be released from neighboring adipocytes. Furthermore, while a dense ECM has been shown to prevent immune infiltration, a loose ECM has been associated with increased intravasation and metastasis formation.138 Clearly, the TME and the ECM have a major impact on the quantity and quality of the immune system, and whether they can be manipulated for improving BC immunotherapy is a very active area of current research.
Moving forward, a better understanding of the molecular players involved in immune infiltration and exclusion will be necessary to develop therapies that increase tumor lymphocytic infiltration. Recent studies have shown that the number of somatic mutations or neoantigens does not correlate with the degree of immune infiltration in melanoma and BC,139,140 suggesting that other biological mechanisms are driving immune evasion. Genomic and transcriptomic data from clinical trials involving immunotherapy will be a key asset in understanding the molecular basis of immune infiltration, immune exclusion, and in predicting response to immunotherapy.
Footnotes
Peer review:Two peer reviewers contributed to the peer review report. Reviewers’ reports totaled 425 words, excluding any confidential comments to the academic editor.
Funding:The author(s) received no financial support for the research, authorship, and/or publication of this article.
Declaration of conflicting interests:The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Author Contributions: IJC and RGB conceived of, researched data for, and edited the manuscript.
References
- 1. Postow MA, Chesney J, Pavlick AC, et al. Nivolumab and ipilimumab versus ipilimumab in untreated melanoma. N Engl J Med. 2015;372:2006–2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Reck M, Rodríguez-Abreu D, Robinson AG, et al. Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl J Med. 2016;375:1823–1833. [DOI] [PubMed] [Google Scholar]
- 3. Motzer RJ, Escudier B, McDermott DF, et al. Nivolumab versus everolimus in advanced renal-cell carcinoma. N Engl J Med. 2015;373:1803–1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bellmunt J, de Wit R, Vaughn DJ, et al. Pembrolizumab as second-line therapy for advanced urothelial carcinoma. N Engl J Med. 2017;376:1015–1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Joffre OP, Segura E, Savina A, Amigorena S. Cross-presentation by dendritic cells. Nat Rev Immunol. 2012;12:557–569. [DOI] [PubMed] [Google Scholar]
- 6. Fehres CM, Unger WWJ, Garcia-Vallejo JJ, van Kooyk Y. Understanding the biology of antigen cross-presentation for the design of vaccines against cancer. Front Immunol. 2014;5:149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hadrup S, Donia M, Thor Straten P. Effector CD4 and CD8 T cells and their role in the tumor microenvironment. Cancer Microenviron. 2013;6:123–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zhang Y, Schreiber TH, Rosenblatt JD. The role of B cells in shaping the antitumor immune response. In: Rosenblatt JD, Podack ER, Barber GN, Ochoa A, eds. Advances in Tumor Immunology and Immunotherapy. New York, NY: Springer; 2014:19–35. [Google Scholar]
- 9. Marcus A, Gowen BG, Thompson TW, et al. Recognition of tumors by the innate immune system and natural killer cells. Adv Immunol. 2014;122:91–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kumar V, Patel S, Tcyganov E, Gabrilovich DI. The nature of myeloid-derived suppressor cells in the tumor microenvironment. Trends Immunol. 2016;37:208–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Jerome KR, Barnd DL, Bendt KM, et al. Cytotoxic T-lymphocytes derived from patients with breast adenocarcinoma recognize an epitope present on the protein core of a mucin molecule preferentially expressed by malignant cells. Cancer Res. 1991;51:2908–2916. [PubMed] [Google Scholar]
- 12. Beckhove P, Feuerer M, Dolenc M, et al. Specifically activated memory T cell subsets from cancer patients recognize and reject xenotransplanted autologous tumors. J Clin Invest. 2004;114:67–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Feuerer M, Rocha M, Bai L, et al. Enrichment of memory T cells and other profound immunological changes in the bone marrow from untreated breast cancer patients. Int J Cancer. 2001;92:96–105. [PubMed] [Google Scholar]
- 14. Whitford P, George WD, Campbell AM. Flow cytometric analysis of tumour infiltrating lymphocyte activation and tumour cell MHC class I and II expression in breast cancer patients. Cancer Lett. 1992;61:157–164. [DOI] [PubMed] [Google Scholar]
- 15. Mensdorff-Pouilly Sv, Verstraeten AA, Kenemans P, et al. Survival in early breast cancer patients is favorably influenced by a natural humoral immune response to polymorphic epithelial mucin. J Clin Oncol. 2000;18:574–583. [DOI] [PubMed] [Google Scholar]
- 16. Fremd C, Stefanovic S, Beckhove P, et al. Mucin 1-specific B cell immune responses and their impact on overall survival in breast cancer patients. Oncoimmunology. 2016;5:e1057387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Goodell V, Waisman J, Salazar LG, et al. Level of HER-2/neu protein expression in breast cancer may affect the development of endogenous HER-2/neu-specific immunity. Mol Cancer Ther. 2008;7:449–454. [DOI] [PubMed] [Google Scholar]
- 18. Tabuchi Y, Shimoda M, Kagara N, et al. Protective effect of naturally occurring anti-HER2 autoantibodies on breast cancer. Breast Cancer Res Treat. 2016;157:55–63. [DOI] [PubMed] [Google Scholar]
- 19. Sistrunk WE, MacCarty WC. Life expectancy following radical amputation for carcinoma of the breast: a clinical and pathologic study of 218 cases. Ann Surg. 1922;75:61–69. [PMC free article] [PubMed] [Google Scholar]
- 20. Naito Y, Saito K, Shiiba K, et al. CD8+ T cells infiltrated within cancer cell nests as a prognostic factor in human colorectal cancer. Cancer Res. 1998;58:3491–3494. [PubMed] [Google Scholar]
- 21. Denkert C, Loibl S, Noske A, Roller M, Muller BM, Komor M. Tumor-associated lymphocytes as an independent predictor of response to neoadjuvant chemotherapy in breast cancer. J Clin Oncol. 2010;28:105–113. [DOI] [PubMed] [Google Scholar]
- 22. Ono M, Tsuda H, Shimizu C, et al. Tumor-infiltrating lymphocytes are correlated with response to neoadjuvant chemotherapy in triple-negative breast cancer. Breast Cancer Res Treat. 2012;132:793–805. [DOI] [PubMed] [Google Scholar]
- 23. Mao Y, Qu Q, Zhang Y, Liu J, Chen X, Shen K. The value of tumor infiltrating lymphocytes (TILs) for predicting response to neoadjuvant chemotherapy in breast cancer: a systematic review and meta-analysis. PLoS ONE. 2014;9:e115103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Adams S, Gray RJ, Demaria S, Goldstein LJ, Perez EA, Shulman LN. Prognostic value of tumor-infiltrating lymphocytes (TILs) in triple negative breast cancers (TNBC) from two phase III randomized adjuvant breast cancer trials: ECOG 2197 and ECOG 1199. J Clin Oncol. 2014;32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Loi S, Sirtaine N, Piette F, et al. Prognostic and predictive value of tumor-infiltrating lymphocytes in a phase III randomized adjuvant breast cancer trial in node-positive breast cancer comparing the addition of docetaxel to doxorubicin with doxorubicin-based chemotherapy: BIG 02-98. J Clin Oncol. 2013;31:860–867. [DOI] [PubMed] [Google Scholar]
- 26. Muenst S, Läubli H, Soysal SD, Zippelius A, Tzankov A, Hoeller S. The immune system and cancer evasion strategies: therapeutic concepts. J Intern Med. 2016;279:541–562. [DOI] [PubMed] [Google Scholar]
- 27. Warburg O. The metabolism of carcinoma cells. J Cancer Res. 1925;9:148–163. [Google Scholar]
- 28. Ward PS, Thompson CB. Metabolic reprogramming: a cancer hallmark even Warburg did not anticipate. Cancer Cell. 2012;21:297–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Shim H, Dolde C, Lewis BC, et al. c-Myc transactivation of LDH-A: implications for tumor metabolism and growth. Proc Natl Acad Sci U S A. 1997;94:6658–6663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Dang CV. MYC, metabolism, cell growth, and tumorigenesis. Cold Spring Harb Perspect Med. 2013;3:a014217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Saltiel AR, Kahn CR. Insulin signalling and the regulation of glucose and lipid metabolism. Nature. 2001;414:799–806. [DOI] [PubMed] [Google Scholar]
- 32. Elstrom RL, Bauer DE, Buzzai M, et al. Akt stimulates aerobic glycolysis in cancer cells. Cancer Res. 2004;64:3892–3899. [DOI] [PubMed] [Google Scholar]
- 33. Zeigerer A, McBrayer MK, McGraw TE. Insulin stimulation of GLUT4 exocytosis, but not its inhibition of endocytosis, is dependent on RabGAP AS160. Mol Biol Cell. 2004;15:4406–4415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Simons A, Orcutt K, Madsen J, Scarbrough P, Spitz D. The role of Akt pathway signaling in glucose metabolism and metabolic oxidative stress. In: Spitz DR, Dornfeld KJ, Krishnan K, Gius D, eds. Oxidative Stress in Cancer Biology and Therapy. Totowa, NJ: Humana Press; 2012:21–46. [Google Scholar]
- 35. Matoba S, Kang J-G, Patino WD, et al. p53 regulates mitochondrial respiration. Science. 2006;312:1650–1653. [DOI] [PubMed] [Google Scholar]
- 36. Bensaad K, Tsuruta A, Selak MA, et al. TIGAR, a p53-inducible regulator of glycolysis and apoptosis. Cell. 2006;126:107–120. [DOI] [PubMed] [Google Scholar]
- 37. Hall A, Meyle KD, Lange MK, et al. Dysfunctional oxidative phosphorylation makes malignant melanoma cells addicted to glycolysis driven by the (V600E)BRAF oncogene. Oncotarget. 2013;4:584–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Brahimi-Horn MC, Pouysségur J. HIF at a glance. J Cell Science. 2009;122:1055–1057. [DOI] [PubMed] [Google Scholar]
- 39. Soga T. Cancer metabolism: key players in metabolic reprogramming. Cancer Sci. 2013;104:275–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Yang N, Liu C, Peck AR, et al. Prolactin-Stat5 signaling in breast cancer is potently disrupted by acidosis within the tumor microenvironment. Breast Cancer Res. 2013;15:R73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Wike-Hooley JL, van den Berg AP, van der Zee J, Reinhold HS. Human tumour pH and its variation. Eur J Cancer Clin Oncol. 1985;21:785–791. [DOI] [PubMed] [Google Scholar]
- 42. Ziebart T, Walenta S, Kunkel M, Reichert TE, Wagner W, Mueller-Klieser W. Metabolic and proteomic differentials in head and neck squamous cell carcinomas and normal gingival tissue. J Cancer Res Clin Oncol. 2011;137:193–199. [DOI] [PubMed] [Google Scholar]
- 43. Brizel DM, Schroeder T, Scher RL, et al. Elevated tumor lactate concentrations predict for an increased risk of metastases in head-and-neck cancer. Int J Radiat Oncol Biol Phys. 2001;51:349–353. [DOI] [PubMed] [Google Scholar]
- 44. Walenta S, Wetterling M, Lehrke M, et al. High lactate levels predict likelihood of metastases, tumor recurrence, and restricted patient survival in human cervical cancers. Cancer Res. 2000;60:916–921. [PubMed] [Google Scholar]
- 45. Saraswathy S, Crawford FW, Lamborn KR, et al. Evaluation of MR markers that predict survival in patients with newly diagnosed GBM prior to adjuvant therapy. J Neurooncol. 2009;91:69–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Végran F, Boidot R, Michiels C, Sonveaux P, Feron O. Lactate influx through the endothelial cell monocarboxylate transporter MCT1 supports an NF-κB/IL-8 pathway that drives tumor angiogenesis. Cancer Res. 2011;71:2550–2560. [DOI] [PubMed] [Google Scholar]
- 47. Porporato PE, Payen VL, De Saedeleer CJ, et al. Lactate stimulates angiogenesis and accelerates the healing of superficial and ischemic wounds in mice. Angiogenesis. 2012;15:581–592. [DOI] [PubMed] [Google Scholar]
- 48. Goetze K, Walenta S, Ksiazkiewicz M, Kunz-Schughart LA, Mueller-Klieser W. Lactate enhances motility of tumor cells and inhibits monocyte migration and cytokine release. Int J Oncol. 2011;39:453–463. [DOI] [PubMed] [Google Scholar]
- 49. Migneco G, Whitaker-Menezes D, Chiavarina B, et al. Glycolytic cancer associated fibroblasts promote breast cancer tumor growth, without a measurable increase in angiogenesis: evidence for stromal-epithelial metabolic coupling. Cell Cycle. 2010;9:2412–2422. [DOI] [PubMed] [Google Scholar]
- 50. Pavlides S, Whitaker-Menezes D, Castello-Cros R, et al. The reverse Warburg effect: aerobic glycolysis in cancer associated fibroblasts and the tumor stroma. Cell Cycle. 2009;8:3984–4001. [DOI] [PubMed] [Google Scholar]
- 51. Buck MD, Sowell RT, Kaech SM, Pearce EL. Metabolic instruction of immunity. Cell. 169:570–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Marchiq I, Pouyssegur J. Hypoxia, cancer metabolism and the therapeutic benefit of targeting lactate/H(+) symporters. J Mol Med. 2016;94:155–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Dietl K, Renner K, Dettmer K, et al. Lactic acid and acidification inhibit TNF secretion and glycolysis of human monocytes. J Immunol. 2010;184:1200–1209. [DOI] [PubMed] [Google Scholar]
- 54. Puig-Kroger A, Pello OM, Selgas R, et al. Peritoneal dialysis solutions inhibit the differentiation and maturation of human monocyte-derived dendritic cells: effect of lactate and glucose-degradation products. J Leukoc Biol. 2003;73:482–492. [DOI] [PubMed] [Google Scholar]
- 55. Gottfried E, Kunz-Schughart LA, Ebner S, et al. Tumor-derived lactic acid modulates dendritic cell activation and antigen expression. Blood. 2006;107:2013–2021. [DOI] [PubMed] [Google Scholar]
- 56. Markowitz J, Wesolowski R, Papenfuss T, Brooks TR, Carson WE. Myeloid-derived suppressor cells in breast cancer. Breast Cancer Res Treat. 2013;140:13–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Husain Z, Huang Y, Seth P, Sukhatme VP. Tumor-derived lactate modifies antitumor immune response: effect on myeloid-derived suppressor cells and NK cells. J Immunol. 2013;191:1486–1495. [DOI] [PubMed] [Google Scholar]
- 58. Colegio OR, Chu NQ, Szabo AL, et al. Functional polarization of tumour-associated macrophages by tumour-derived lactic acid. Nature. 2014;513:559–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Brand A, Singer K, Koehl Gudrun E, et al. LDHA-associated lactic acid production blunts tumor immunosurveillance by T and NK cells. Cell Metab. 2016;24:657–671. [DOI] [PubMed] [Google Scholar]
- 60. Fischer K, Hoffmann P, Voelkl S, et al. Inhibitory effect of tumor cell-derived lactic acid on human T cells. Blood. 2007;109:3812–3819. [DOI] [PubMed] [Google Scholar]
- 61. Mendler AN, Hu B, Prinz PU, Kreutz M, Gottfried E, Noessner E. Tumor lactic acidosis suppresses CTL function by inhibition of p38 and JNK/c-Jun activation. Int J Cancer. 2012;131:633–640. [DOI] [PubMed] [Google Scholar]
- 62. Haas R, Smith J, Rocher-Ros V, et al. Lactate regulates metabolic and pro-inflammatory circuits in control of T cell migration and effector functions. PLoS Biol. 2015;13:e1002202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Diem S, Kasenda B, Spain L, et al. Serum lactate dehydrogenase as an early marker for outcome in patients treated with anti-PD-1 therapy in metastatic melanoma. Br J Cancer. 2016;114:256–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Nanda R, Chow LQM, Dees EC, et al. Pembrolizumab in patients with advanced triple-negative breast cancer: phase Ib KEYNOTE-012 study. J Clin Oncol. 2016;34:2460–2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Chang CH, Qiu J, O’Sullivan D, et al. Metabolic competition in the tumor microenvironment is a driver of cancer progression. Cell. 2015;162:1229–1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Ho PC, Bihuniak JD, Macintyre AN, et al. Phosphoenolpyruvate is a metabolic checkpoint of anti-tumor T cell responses. Cell. 2015;162:1217–1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Keating ST, El-Osta A. Epigenetics and metabolism. Circ Res. 2015;116:715–736. [DOI] [PubMed] [Google Scholar]
- 68. Wong CC, Qian Y, Yu J. Interplay between epigenetics and metabolism in oncogenesis: mechanisms and therapeutic approaches. Oncogene. 2017;36:3359–3374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. McHugh MI, Wilkinson R, Elliott RW, et al. Immunosuppression with polyunsaturated fatty acids in renal transplantation. Transplantation. 1977;24:263–267. [DOI] [PubMed] [Google Scholar]
- 70. Brown RE, Steele RW, Marmer DJ, Hudson JL, Brewster MA. Fatty acids and the inhibition of mitogen-induced lymphocyte transformation by leukemic serum. J Immunol. 1983;131:1011–1016. [PubMed] [Google Scholar]
- 71. Stulnig TM, Berger M, Roden M, Stingl H, Raederstorff D, Waldhäusl W. Elevated serum free fatty acid concentrations inhibit T lymphocyte signaling. FASEB J. 2000;14:939–947. [DOI] [PubMed] [Google Scholar]
- 72. Kleinfeld AM, Okada C. Free fatty acid release from human breast cancer tissue inhibits cytotoxic T-lymphocyte-mediated killing. J Lipid Res. 2005;46:1983–1990. [DOI] [PubMed] [Google Scholar]
- 73. Richieri GV, Kleinfeld AM. Free fatty acid perturbation of transmembrane signaling in cytotoxic T lymphocytes. J Immunol. 1989;143:2302–2310. [PubMed] [Google Scholar]
- 74. Richieri GV, Kleinfeld AM. Free fatty acids inhibit cytotoxic T lymphocyte-mediated lysis of allogeneic target cells. J Immunol. 1990;145:1074–1077. [PubMed] [Google Scholar]
- 75. Richieri GV, Mescher MF, Kleinfeld AM. Short term exposure to cis unsaturated free fatty acids inhibits degranulation of cytotoxic T lymphocytes. J Immunol. 1990;144:671–677. [PubMed] [Google Scholar]
- 76. Richieri GV, Anel A, Kleinfeld AM. Interactions of long-chain fatty acids and albumin: determination of free fatty acid levels using the fluorescent probe ADIFAB. Biochemistry. 1993;32:7574–7580. [DOI] [PubMed] [Google Scholar]
- 77. Zeyda M, Szekeres AB, Säemann MD, et al. Suppression of T cell signaling by polyunsaturated fatty acids: selectivity in inhibition of mitogen-activated protein kinase and nuclear factor activation. J Immunol. 2003;170:6033–6039. [DOI] [PubMed] [Google Scholar]
- 78. Gaus K, Chklovskaia E, Fazekas de St. Groth B, Jessup W, Harder T. Condensation of the plasma membrane at the site of T lymphocyte activation. J Cell Biol. 2005;171:121–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Rentero C, Zech T, Quinn CM, et al. Functional implications of plasma membrane condensation for T cell activation. PLoS ONE. 2008;3:e2262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Zech T, Ejsing CS, Gaus K, et al. Accumulation of raft lipids in T-cell plasma membrane domains engaged in TCR signalling. EMBO J. 2009;28:466–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Miguel L, Owen DM, Lim C, et al. Primary human CD4+ T cells have diverse levels of membrane lipid order that correlate with their function. J Immunol. 2011;186:3505–3516. [DOI] [PubMed] [Google Scholar]
- 82. Köberlin MS, Heinz LX, Superti-Furga G. Functional crosstalk between membrane lipids and TLR biology. Curr Opin Cell Biol. 2016;39:28–36. [DOI] [PubMed] [Google Scholar]
- 83. Yang W, Bai Y, Xiong Y, et al. Potentiating the antitumour response of CD8(+) T cells by modulating cholesterol metabolism. Nature. 2016;531:651–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Ho P-C, Liu P-S. Metabolic communication in tumors: a new layer of immunoregulation for immune evasion. J Immunother Cancer. 2016;4:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Huang SC-C, Everts B, Ivanova Y, et al. Cell-intrinsic lysosomal lipolysis is essential for alternative activation of macrophages. Nat Immunol. 2014;15:846–855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Michalek RD, Gerriets VA, Jacobs SR, et al. Cutting edge: distinct glycolytic and lipid oxidative metabolic programs are essential for effector and regulatory CD4+ T cell subsets. J Immunol. 2011;186:3299–3303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Pearce EL, Walsh MC, Cejas PJ, et al. Enhancing CD8 T-cell memory by modulating fatty acid metabolism. Nature. 2009;460:103–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Vacaresse N, Lajoie-Mazenc I, Augé N, et al. Activation of epithelial growth factor receptor pathway by unsaturated fatty acids. Circ Res. 1999;85:892–899. [DOI] [PubMed] [Google Scholar]
- 89. Hardy S, Langelier Y, Prentki M. Oleate activates phosphatidylinositol 3-kinase and promotes proliferation and reduces apoptosis of MDA-MB-231 breast cancer cells, whereas palmitate has opposite effects. Cancer Res. 2000;60:6353–6358. [PubMed] [Google Scholar]
- 90. Lu P, Weaver VM, Werb Z. The extracellular matrix: a dynamic niche in cancer progression. J Cell Biol. 2012;196:395–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Alowami S, Troup S, Al-Haddad S, Kirkpatrick I, Watson PH. Mammographic density is related to stroma and stromal proteoglycan expression. Breast Cancer Res. 2003;5:R129–R135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Peranzoni E, Rivas-Caicedo A, Bougherara H, Salmon H, Donnadieu E. Positive and negative influence of the matrix architecture on antitumor immune surveillance. Cell Mol Life Sci. 2013;70:4431–4448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Boyd NF, Martin LJ, Stone J, Greenberg C, Minkin S, Yaffe MJ. Mammographic densities as a marker of human breast cancer risk and their use in chemoprevention. Curr Oncol Rep. 2001;3:314–321. [DOI] [PubMed] [Google Scholar]
- 94. Friedl P, Entschladen F, Conrad C, Niggemann B, Zanker KS. CD4+ T lymphocytes migrating in three-dimensional collagen lattices lack focal adhesions and utilize beta1 integrin-independent strategies for polarization, interaction with collagen fibers and locomotion. Eur J Immunol. 1998;28:2331–2343. [DOI] [PubMed] [Google Scholar]
- 95. Wolf K, Müller R, Borgmann S, Bröcker E-B, Friedl P. Amoeboid shape change and contact guidance: T-lymphocyte crawling through fibrillar collagen is independent of matrix remodeling by MMPs and other proteases. Blood. 2003;102:3262–3269. [DOI] [PubMed] [Google Scholar]
- 96. Lieubeau B, Heymann M-F, Henry F, Barbieux I, Meflah K, Grégoire M. Immunomodulatory effects of tumor-associated fibroblasts in colorectal-tumor development. Int J Cancer. 1999;81:629–636. [DOI] [PubMed] [Google Scholar]
- 97. Mrass P, Takano H, Ng LG, et al. Random migration precedes stable target cell interactions of tumor-infiltrating T cells. J Exp Med. 2006;203:2749–2761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Salmon H, Franciszkiewicz K, Damotte D, et al. Matrix architecture defines the preferential localization and migration of T cells into the stroma of human lung tumors. J Clin Invest. 2012;122:899–910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Bougherara H, Mansuet-Lupo A, Alifano M, et al. Real-time imaging of resident T cells in human lung and ovarian carcinomas reveals how different tumor microenvironments control T lymphocyte migration. Front Immunol. 2015;6:500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Ohno S, Tachibana M, Fujii T, Ueda S, Kubota H, Nagasue N. Role of stromal collagen in immunomodulation and prognosis of advanced gastric carcinoma. Int J Cancer. 2002;97:770–774. [DOI] [PubMed] [Google Scholar]
- 101. Hartmann N, Giese NA, Giese T, et al. Prevailing role of contact guidance in intrastromal T-cell trapping in human pancreatic cancer. Clin Cancer Res. 2014;20:3422–3433. [DOI] [PubMed] [Google Scholar]
- 102. Scarfò I, Maus MV. Current approaches to increase CAR T cell potency in solid tumors: targeting the tumor microenvironment. J Immunother Cancer. 2017;5:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Caruana I, Savoldo B, Hoyos V, et al. Heparanase promotes tumor infiltration and antitumor activity of CAR-redirected T lymphocytes. Nature Med. 2015;21:524–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Hugo W, Zaretsky JM, Sun L, et al. Genomic and transcriptomic features of response to anti-PD-1 therapy in metastatic melanoma. Cell. 2016;165:35–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol. 2009;9:162–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Tanaka A, Sakaguchi S. Regulatory T cells in cancer immunotherapy. Cell Res. 2017;27:109–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Angelin A, Gil-de-Gómez L, Dahiya S, et al. Foxp3 reprograms T cell metabolism to function in low-glucose, high-lactate environments. Cell Metab. 2017;25:1282.e7-1293e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Motz GT, Coukos G. The parallel lives of angiogenesis and immunosuppression: cancer and other tales. Nat Rev Immunol. 2011;11:702–711. [DOI] [PubMed] [Google Scholar]
- 109. Lehmann BD, Jovanović B, Chen X, et al. Refinement of triple-negative breast cancer molecular subtypes: implications for neoadjuvant chemotherapy selection. PLoS ONE. 2016;11:e0157368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Burstein MD, Tsimelzon A, Poage GM, et al. Comprehensive genomic analysis identifies novel subtypes and targets of triple-negative breast cancer. Clin Cancer Res. 2015;21:1688–1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Gucalp A, Traina TA. Androgen receptor-positive, triple-negative breast cancer. Cancer. 2017;123:1686–1688. [DOI] [PubMed] [Google Scholar]
- 112. Traina TA. Results from a phase 2 study of enzalutamide (ENZA), an androgen receptor (AR) inhibitor, in advanced AR triple-negative breast cancer (TNBC). J Clin Oncol. 2015;33(Suppl; Abstr 1003). [Google Scholar]
- 113. Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12:252–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Spranger S, Spaapen RM, Zha Y, et al. Up-regulation of PD-L1, IDO, and T(regs) in the melanoma tumor microenvironment is driven by CD8(+) T cells. Sci Transl Med. 2013;5:200ra116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Buisseret L, Garaud S, de Wind A, et al. Tumor-infiltrating lymphocyte composition, organization and PD-1/PD-L1 expression are linked in breast cancer. Oncoimmunology. 2017;6:e1257452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Schalper KA, Velcheti V, Carvajal D, Wimberly H, Brown J, Pusztai L. In situ tumor PD-L1 mRNA expression is associated with increased TILs and better outcome in breast carcinomas. Clin Cancer Res. 2014;20:2773–2782. [DOI] [PubMed] [Google Scholar]
- 117. Cimino-Mathews A, Thompson E, et al. PD-L1 (B7-H1) expression and the immune tumor microenvironment in primary and metastatic breast carcinomas. Hum Pathol. 2016;47:52–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Cimino-Mathews A, Ye X, Meeker A, Argani P, Emens LA. Metastatic triple-negative breast cancers at first relapse have fewer tumor-infiltrating lymphocytes than their matched primary breast tumors: a pilot study. Hum Pathol. 2013;44:2055–2063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Sobottka B, Pestalozzi B, Fink D, Moch H, Varga Z. Similar lymphocytic infiltration pattern in primary breast cancer and their corresponding distant metastases. Oncoimmunology. 2016;5:e1153208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Solinas C, Boisson A, Brown D, et al. Tumor infiltrating lymphocytes and tertiary lymphoid structures in paired primary tumors and metastases from breast cancer patients. Ann Oncol. 2016;27:545–551.26659397 [Google Scholar]
- 121. Ogiya R, Niikura N, Kumaki N, et al. Comparison of tumor-infiltrating lymphocytes between primary and metastatic tumors in breast cancer patients. Cancer Sci. 2016;107:1730–1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Liu S, Lachapelle J, Leung S, Gao D, Foulkes WD, Nielsen TO. CD8+ lymphocyte infiltration is an independent favorable prognostic indicator in basal-like breast cancer. Breast Cancer Res. 2012;14:R48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Rody A, Holtrich U, Pusztai L, et al. T-cell metagene predicts a favorable prognosis in estrogen receptor-negative and HER2-positive breast cancers. Breast Cancer Res. 2009;11:R15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Patel SP, Kurzrock R. PD-L1 expression as a predictive biomarker in cancer immunotherapy. Mol Cancer Ther. 2015;14:847–856. [DOI] [PubMed] [Google Scholar]
- 125. Vonderheide RH, LoRusso PM, Khalil M, et al. Tremelimumab in combination with exemestane in patients with advanced breast cancer and treatment-associated modulation of inducible costimulator expression on patient T cells. Clin Cancer Res. 2010;16:3485–3494. [DOI] [PubMed] [Google Scholar]
- 126. Rugo H, Delord J-P, Im S-A, et al. Abstract S5-07: preliminary efficacy and safety of pembrolizumab (MK-3475) in patients with PD-L1-positive, estrogen receptor-positive (ER+)/HER2-negative advanced breast cancer enrolled in KEYNOTE-028. Cancer Res. 2016;76:S5. [Google Scholar]
- 127. Emens LA, Braiteh FS, Cassier P, et al. Abstract PD1-6: inhibition of PD-L1 by MPDL3280A leads to clinical activity in patients with metastatic triple-negative breast cancer. Cancer Res. 2015;75:PD1-6-PD1-6. [Google Scholar]
- 128. Vonderheide RH, LoRusso PM, Khalil M, et al. Tremelimumab in combination with exemestane in patients with advanced breast cancer and treatment-associated modulation of inducible costimulator expression on patient T cells. Clin Cancer Res. 2010;16:3485–3494. [DOI] [PubMed] [Google Scholar]
- 129. McArthur HL, Page DB. Immunotherapy for the treatment of breast cancer: checkpoint blockade, cancer vaccines, and future directions in combination immunotherapy. Clin Adv Hematol Oncol. 2016;14:922–933. [PubMed] [Google Scholar]
- 130. McArthur HL, Diab A, Page DB, et al. A pilot study of preoperative single-dose ipilimumab and/or cryoablation in women with early-stage breast cancer with comprehensive immune profiling. Clin Cancer Res. 2016;22:5729–5737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. de la Cruz-Merino L, Chiesa M, Caballero R, et al. Chapter 1. Breast cancer immunology and immunotherapy: current status and future perspectives. In: Lorenzo G, ed. International Review of Cell and Molecular Biology. New York, NY: Academic Press; 2017:1–53. [DOI] [PubMed] [Google Scholar]
- 132. Disis ML, Stanton SE. Immunotherapy in breast cancer: an introduction. The Breast. http://doi.org/10.1016/j.breast.2017.01.013. [DOI] [PubMed]
- 133. Nanda R, Liu MC, Yau C, et al. Pembrolizumab plus standard neoadjuvant therapy for high-risk breast cancer (BC): Results from I-SPY 2. J Clin Oncol. 2017;35(15)(suppl; abstr 506):506-506. DOI: 10.1200/JCO.2017.35.15_suppl.506 [DOI] [Google Scholar]
- 134. Fridman WH, Zitvogel L, Sautes-Fridman C, Kroemer G. The immune contexture in cancer prognosis and treatment [published online ahead of print July 25, 2017]. Nat Rev Clin Oncol. doi:10.1038/nrclinonc.2017.101. http://www.nature.com/nrclinonc/journal/vaop/ncurrent/abs/nrclinonc.2017.101.html#supplementary-information. [DOI] [PubMed]
- 135. Louie SM, Grossman EA, Crawford LA, et al. GSTP1 is a driver of triple-negative breast cancer cell metabolism and pathogenicity. Cell Chem Biol. 2016;23:567–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Ho P-C, Kaech SM. Reenergizing T cell anti-tumor immunity by harnessing immunometabolic checkpoints and machineries. Curr Opin Immunol. 2017;46:38–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Beloribi-Djefaflia S, Vasseur S, Guillaumond F. Lipid metabolic reprogramming in cancer cells. Oncogenesis. 2016;5:e189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Harisi R, Jeney A. Extracellular matrix as target for antitumor therapy. Onco Targets Ther. 2015;8:1387–1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Spranger S, Luke JJ, Bao R, et al. Density of immunogenic antigens does not explain the presence or absence of the T-cell-inflamed tumor microenvironment in melanoma. Proc Natl Acad Sci U S A. 2016;113:E7759–E7768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Safonov A, Jiang T, Bianchini G, et al. Immune gene expression is associated with genomic aberrations in breast cancer. Cancer Res. 2017;77:3317–3324. [DOI] [PubMed] [Google Scholar]