Abstract
Trichotillomania is a functionally impairing, often overlooked disorder with no Food and Drug Administration-approved medications indicated for its treatment. The ability of clinical trials to detect the beneficial effects of pharmacologic treatment in trichotillomania has been hampered by the high placebo response rate. Very little is known about baseline demographic and clinical characteristics that may be predictive of placebo response in such patients. Overall, 104 participants assigned to placebo were pooled from five double-blind trials conducted at three sites in the USA and Canada. Participants were classified as placebo responders or nonresponders on the basis of a cutoff of a 35% reduction in symptom severity on the Massachusetts General Hospital Hair Pulling Scale. Baseline group differences were characterized using t-tests and equivalent nonparametric tests as appropriate. Thirty-one percent of individuals assigned to placebo treatment showed a significant clinical response to placebo. Placebo responders (n=32) and nonresponders (n=72) did not differ significantly on any demographic or clinical variable. Predictors of placebo response for trichotillomania remain elusive and do not appear to be similar to those reported for other mental health disorders.
Keywords: clinical trial, pharmacotherapy, placebo, trichotillomania
Introduction
Trichotillomania (TTM) is a potentially disabling, under-recognized condition in which individuals repeatedly pull out their hair, leading to hair loss. Psychosocial problems are common among individuals with TTM and may include significantly reduced quality of life, lowered self-esteem, and impaired social functioning (Diefenbach et al., 2005; Houghton et al., 2016; Grant and Chamberlain, 2016). Although TTM has been described for almost two centuries, it remains poorly understood, with limited data on pathophysiology and treatment (Christenson and Mansueto, 1999; Chamberlain et al., 2009; Grant and Chamberlain, 2016).
Most of the double-blind, placebo-controlled pharmacological studies of TTM have failed to separate symptomatic changes significantly from placebo. Interestingly, our clinical experience suggests that in many cases, this lack of effectiveness seems less to do with the medication failing to produce results and more to do with the high placebo response rates. For example, in a double-blind study of inositol, 37% of the placebo group (using a last observation carried forward approach) responded (Leppink et al., 2017). Understanding the complexity of the placebo response in these disorders is challenging because of the limited sizes of the research samples (e.g. sample sizes of <25 taking placebo in any single study). The present study seeks to overcome this limitation by using a relatively large dataset that combines participants from five double-blind, placebo-controlled pharmacological trials in TTM conducted in the USA and Canada (Dougherty et al., 2006; Grant et al., 2009, 2014; Van Ameringen et al., 2010; Leppink et al., 2017).
Understanding the factors associated with a placebo response in TTM may allow for a more efficient examination of potentially beneficial pharmacological treatments for this disabling disorder. Here, we pooled data from studies in which all participants fulfilled the diagnostic criteria for TTM, took placebo pills, and were seen regularly by a medical professional. Many factors have been suggested to contribute toward the high placebo response rates of clinical trials in mental health. In the case of major depressive disorder, interpersonal interactions, the strength of the therapeutic alliance with research personnel (Leuchter et al., 2014), or lower levels of depression severity (Khan et al., 2002, 2005) may result in a placebo response. Data from trials of bipolar depression suggest that baseline illness severity and trial duration predict placebo response (Nierenberg et al., 2015). The case of obsessive-compulsive disorder (OCD), however, has yielded no clear clinical variables associated with the placebo response (Mataix-Cols, et al., 1999). On the basis of the (admittedly limited) extant mental health literature and our clinical experience, we hypothesized that the placebo effect in TTM would be associated with milder illness severity at baseline.
Participants and methods
Participants
Data from participants in TTM treatment studies at the University of Chicago, University of Minnesota, McMaster University, and Massachusetts General Hospital (MGH)/Harvard Medical School who were assigned to placebo during the clinical trial were included in this study (one exception was the sertraline trial, which had a 2-week single-blind placebo phase before treatment assignment in the double-blind portion of the study and only two were randomized to placebo treatment in this study arm). All participants had a primary diagnosis of TTM on the basis of expert clinical assessment. As is customary in TTM research, before May 2013, the diagnosis was made on the basis of Diagnostic and Statistical Manual of Mental Disorders, 4th ed. (DSM-IV) criteria with or without the endorsement of increasing and decreasing tension associated with pulling (criteria B and C). After the release of the DSM-V in May 2013, all patients fulfilled the DSM-V criteria for TTM. Other inclusion criteria included age 18 years or older, the ability to be interviewed in person, and able to provide written informed consent. Participants from MGH were excluded if they fulfilled the criteria for a lifetime diagnosis of psychosis, autism, or mental retardation. Participants at the University of Chicago and the University of Minnesota were excluded if they were pregnant, fulfilled the lifetime criteria for bipolar disorder or a psychotic disorder, or had an organic mental disorder. Participants taking any psychotropic medications were included as long as the dose of medication had been stable for at least 3 months before study entry. Participants taking part at McMaster University were excluded if they had comorbid primary mental disorders; were less than moderately ill at baseline; had received olanzapine without success in the past; had comorbid OCD, depression, substance use disorder; or had a lifetime history of schizophrenia, bipolar disorder, dementia, or other neurologic disorders.
All study procedures were carried out in accordance with the latest version of the Declaration of Helsinki. Study approvals were received from the Institutional Review Boards of all relevant institutions before study initiation. Detailed methodologies of the various clinical trials have been published previously (Dougherty et al., 2006; Grant et al., 2009, 2014; Van Ameringen et al., 2010; Leppink et al., 2017). Data were de-identified according to the Safe Harbor method for deidentification before data sharing [section 164.514(b)] (U.S. Department of Health & Human Services, 2012). After all the procedures were explained, all participants provided written informed consent.
All participants in the trials completed a full psychiatric assessment using the Structured Clinical Interview for DSM-IV (SCID-I) (First et al., 1995). Participants also completed general demographic questionnaires, and self-report and clinician-administered severity measures. In addition, each participant underwent a semistructured interview to examine psychiatric disorders in first-degree relatives (except for the sertraline study). No relatives were interviewed directly.
Assessments
The Massachusetts General Hospital Hair Pulling Scale (MGH-HPS) (Keuthen et al., 1995) was used to assess the severity of TTM symptoms. The MGH-HPS is a valid and reliable, seven-item, self-report scale that rates urges to pull hair, actual amount of pulling, perceived control over behavior, and distress associated with hair pulling over the preceding 7 days. Analysis of the MGH-HPS has shown two separate factors with acceptable reliability for both: ‘severity’ and ‘resistance and control’ (Keuthen et al., 2007).
Psychosocial functioning was assessed using the Sheehan Disability Scale (Sheehan, 1983). The Sheehan Disability Scale is a valid and reliable, three-item, self-report scale that assesses psychosocial functioning in work, social or leisure activities, and home/family life. Scores on the scale range from 0 to 30, with higher scores indicating better perceived psychosocial functioning.
Depression and anxiety symptoms over the past month were assessed using a clinician-administered Hamilton Depression Rating Scale (Hamilton, 1960) and Hamilton Anxiety Rating Scale (Hamilton, 1959), respectively. Scores on these two measures were not a basis for inclusion/exclusion.
Data analysis
Baseline characteristics of the placebo participants pooled from all of the studies were presented in terms of means and SDs for continuous variables and frequencies and percentages for categorical variables.
Patients were grouped as placebo responders (>35% reduction in MGH-HPS total scores from the baseline to the end-point) or nonresponders. The two groups were compared on pertinent demographic and clinical measures using independent-sample t-tests or equivalent nonparametric tests as indicated in the text. This being an exploratory study, statistical significance was defined as P less than 0.05 uncorrected, one tailed.
As a secondary analysis, we also pooled all data from the same studies from active treatment responders and compared these data with those of placebo responders.
Results
Data from 104 participants with primary TTM [91 (87.5%) women, mean age 32.6±11.0 years] who were assigned placebo were included in the analysis. In the pooled analysis, 31.4% of participants assigned to placebo improved at least 35% on the MGH-HPS during placebo treatment.
In terms of the individual studies, the sample sizes for those receiving placebo, and N (%) of patients responding to placebo, were as follows: Inositol N=19, seven (36.8%) were placebo responders; N-acetylcysteine N=25, six (24.0%) were placebo responders; Naltrexone N=30, nine (30.0%) were placebo responders; Olanzapine N=12, three (25.0%) were placebo responders; Sertraline N=18, seven (39.9%) were placebo responders. The studies did not differ significantly on the placebo response rate (Likelihood ratio=1.619, d.f.=4, P=0.805).
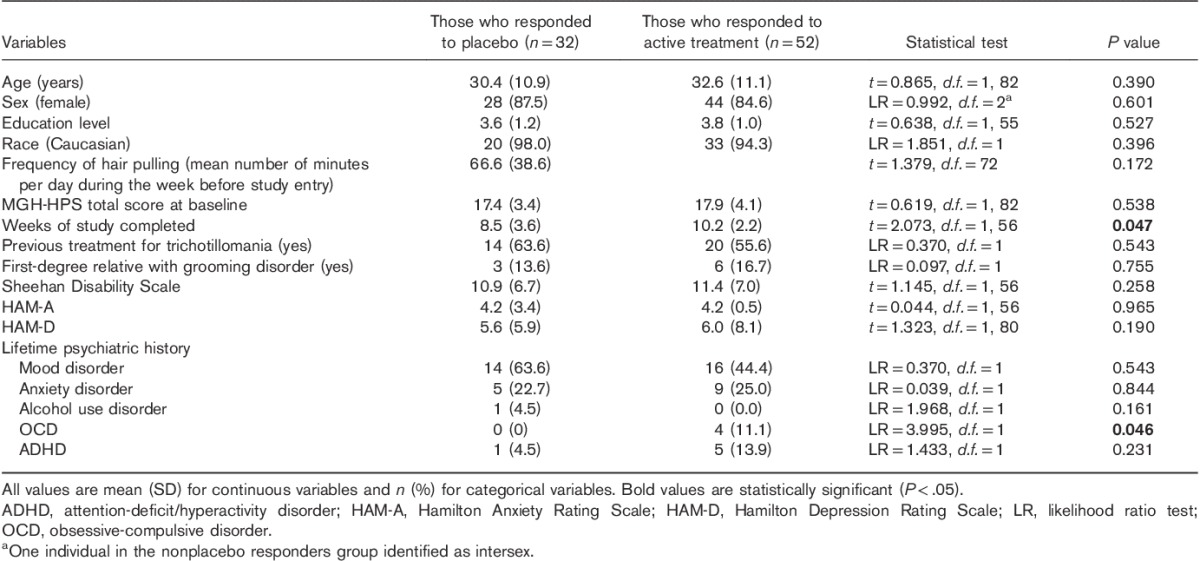
Clinical variables of responders and nonresponders are presented in Table 1, where it can be seen that the groups did not differ from each other in terms of demographic variables or clinical characteristics. Clinical variables of placebo responders are compared with reference data for active treatment responders in Table 2. Active treatment responders completed significantly more study weeks than placebo responders and had a marginally higher rate of OCD (although OCD was uncommon in both groups).
Table 1.
Clinical variables of participants with trichotillomania who did and did not respond to placebo
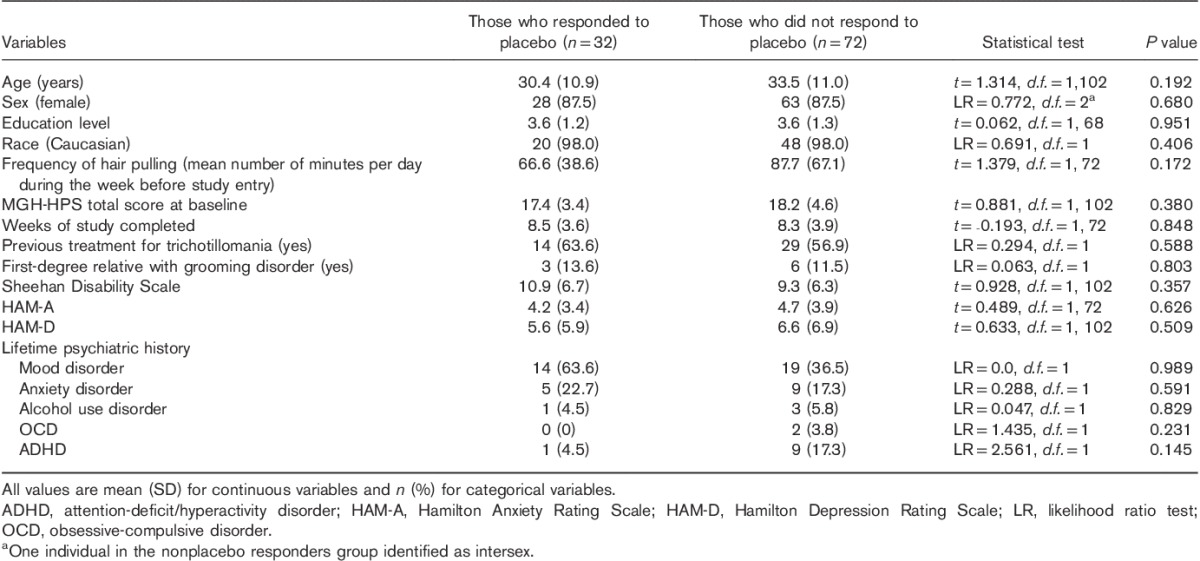
Table 2.
Clinical variables of participants with trichotillomania who were placebo responders compared with active treatment responders
Discussion
To our knowledge, this is the first study that has examined clinical variables associated with the placebo response in the pharmacological treatment of TTM. Given that the pooled placebo response in these studies was 31%, and that there is as of yet no Food and Drug Administration-approved medication indicated for the treatment of TTM, determining predictors of placebo response is crucial for the timely and cost-effective development of pharmacological interventions. Knowledge of variables associated with placebo response might also be useful for sample enrichment in clinical trials. In addition, a placebo response rate of 31% suggests that larger numbers will be needed in future placebo-controlled efficacy studies of TTM than have been previously considered necessary.
Some research suggests that the placebo effect in clinical drug trials generally may influence as many as 49% of treated patients, that the effect may be related to symptom severity, and that its duration may vary from minutes to years (Breidert and Hofbauer, 2009). Interestingly, the placebo response rate in our sample is much higher than found previously in OCD treatment trials, a disorder with possible biological links to TTM (<20%; Greist et al., 1995; Stein et al., 1995; Pigott and Seay, 1999; Ackerman and Greenland, 2002; Stein et al., 2006). Whether this difference is reflective of methodological issues or more substantial biological differences between TTM and OCD, however, remains unclear.
This study found no differences between those who did and did not respond to placebo.
Contrary to our expectations, baseline symptom severity did not differ between placebo responders and nonresponders. The differences between our results and studies of other mental health conditions such as major depressive disorder in which baseline symptom severity was a meaningful predictor of placebo response (Stein et al., 2006; Nierenberg et al., 2015) could reflect the particular characteristics of our patient population or of the disorder itself. Surprisingly, in view of the contribution of placebo response toward clinical outcomes in trials, relatively few studies have explored predictors of placebo response, especially so in obsessive-compulsive and related disorders. Our findings of a lack of predictive variables are in broad agreement with several previous papers in OCD, which reported in statistically significant predictors (DeVeaugh-Geiss et al., 1990; Mataix-Cols et al., 1999).
Our comparison of baseline characteristics between placebo responders and active treatment responders (data pooled from the same source studies) was similarly negative, except for two findings. Active treatment responders remained in the trials for a longer period of time and had a marginally higher occurrence of OCD than placebo responders. The former result probably stems from greater treatment benefit that participants may experience with at least some of the active treatments reported in the literature, compared with placebo, even if placebo participants respond somewhat to placebo. The latter result is likely a chance finding as the actual numbers of patients with OCD were low in both groups, and data were generally from randomized trials.
One possible explanation for the high placebo response in TTM studies could be the phenotypic variation encountered in the disorder. For example, some individuals pull only from their eyebrows or eyelashes. In these cases, it is quite common to pull all of the hair and then report no pulling for several weeks until the hair regrows. This is quite distinct from individuals who pull from their heads as that variation tends to be more chronic. Of course, a complication is that many individuals pull from several areas as well. Having said that, future studies may aim to enroll only those who pull from their heads and therefore have a chronic and predictable course so that change in behavior could more reliably be attributed to the intervention and not the lack of hair or need for hair to regrow.
This study suggests that few (if any) typically collected baseline clinical characteristics in TTM distinguish placebo responders from nonresponders, but there exist several limitations to the studies included in the pooled analysis. Some studies unrelated to TTM suggest that expectancy (i.e. an individual’s beliefs about whether he or she will improve because of the treatment) may play a large role in a placebo response (Brown, 1994; Linden, 2017). Expectancy was not measured in the studies analyzed here. Although the MGH-HPS scoring has shown strong validity and reliability in previous trials as reflecting a response to medication, the ideal threshold for response remains somewhat in doubt (Houghton et al., 2015). We chose a 35% reduction as being clinically meaningful, but some authors suggest that a 45% reduction may be more optimal for TTM (Houghton et al., 2015). In response to this suggestion, we also examined the current measures using a 45% definition in a post-hoc analysis (Grant JE, Redden SA, Chamberlain SR, unpublished data), with a similar lack of significant results. Some clinical measures were available only for a subset of individuals in the pooled dataset. This study did not examine baseline cognition or brain function. Such types of baseline measures would merit scrutiny in future work. This may in the future be a useful means of distinguishing placebo responders from nonresponders before treatment, especially given that the placebo response can be linked to changes in brain functioning in other contexts (Leuchter et al., 2002). The studies included in the present paper had some restrictions on comorbidity in the protocols, which might explain the low rates of comorbid OCD or alcohol use disorder. If the studies had broader inclusion criteria allowing for comorbidity, it is possible that certain co-occurring disorders may have contributed toward the placebo response. The duration of treatment by week is reported and thus it would be important to analyze the placebo for each week of the studies. Given the collective data, this was not possible across all studies and should be noted as a limitation. Finally, although this study represents the largest sample of participants in treatment trials for TTM, the sample size is still relatively small and thus had only modest power to detect moderate effect size. The current sample size, however, had adequate power (power=∼0.80) to detect a group difference on a given measure of interest with medium (Cohen’s D≥0.6) effect size and it had very high power (power=∼0.96) to detect a group difference with large effect size (Cohen’s D=0.8).
Placebo-controlled studies are the gold standard for the examination of pharmacological interventions. Individuals with TTM who respond to placebo appear no different clinically from those who do not respond to placebo on the basis of the types of measure typically collected in existing clinical trials. Given the fairly high estimated prevalence of TTM (Christenson et al., 1991; Odlaug and Grant, 2010) and the associated reduced quality of life in those who struggle with this disorder (Odlaug et al., 2010; Tung et al., 2014; Houghton et al., 2016), further exploration of placebo response will be crucial for developing better pharmacological interventions. Of course, it is not possible to discuss meaningfully treatment resistance in TTM as there is no licensed treatment and only a limited evidence base of efficacy for any treatment.
Acknowledgements
Conflicts of interest
Dr. Grant has received research grant support from TLC Foundation for BFRBs, NIDA, NIAAA, National Center for Responsible Gaming, Brainsway, Psyadon, and Takeda Pharmaceuticals. He is on the scientific advisory board of the TLC Foundation for BFRBs. He receives yearly compensation from Springer Publishing for acting as Editor-in-Chief of the Journal of Gambling Studies and has received royalties from Oxford University Press, American Psychiatric Publishing, Inc., Norton Press, and McGraw Hill. Dr. Chamberlain consults for Cambridge Cognition and Shire. Dr. Chamberlain’s involvement in this project was funded by a Wellcome Trust Clinical Fellowship (110049/Z/15/Z). Dr. Odlaug has received research funding from the TLC Foundation for BFRBs and receives royalties from Oxford University Press. He has consulted for and is currently employed by H. Lundbeck A/S. H. Lundbeck A/S had no part in any of the studies mentioned in this paper and did not contribute to this paper in any form. Dr Keuthen has received research support from the TLC Foundation for BFRBs and Forest Laboratories. She receives royalties from New Harbinger Inc. She is on the scientific advisory boards of the TLC Foundation for BFRBs and the International OCD Foundation. For the remaining authors, there are no conflicts of interest.
References
- Ackerman DL, Greenland S. (2002). Multivariate meta-analysis of controlled drug studies for obsessive-compulsive disorder. J Clin Psychopharmacol 22:309–317. [DOI] [PubMed] [Google Scholar]
- Breidert M, Hofbauer K. (2009). Placebo: misunderstandings and prejudices. Dtsch Arztebl Int 106:751–755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown WA. (1994). Placebo as a treatment for depression. Neuropsychopharmacol 10:265–269. [DOI] [PubMed] [Google Scholar]
- Chamberlain SR, Odlaug BL, Boulougouris V, Fineberg NA, Grant JE. (2009). Trichotillomania: neurobiology and treatment. Neurosci Biobehav Rev 33:831–842. [DOI] [PubMed] [Google Scholar]
- Christenson GA, Mansueto CS.Stein DJ, Christianson GA, Hollander E. (1999). Trichotillomania: descriptive characteristics and phenomenology. Trichotillomania. Washington, DC: American Psychiatric Press; 1–41. [Google Scholar]
- Christenson GA, Pyle RL, Mitchell JE. (1991). Estimated lifetime prevalence of trichotillomania in college students. J Clin Psychiatry 52:415.e7. [PubMed] [Google Scholar]
- DeVeaugh-Geiss J, Katz R, Landau P, Goodman W, Rasmussen S. (1990). Clinical predictors of treatment response in obsessive compulsive disorder: exploratory analyses from multicenter trials of clomipramine. Psychopharmacol Bull 26:54–59. [PubMed] [Google Scholar]
- Diefenbach GJ, Tolin DF, Hannan S, Crocetto J, Worhunsky P. (2005). Trichotillomania: impact on psychosocial functioning and quality of life. Behav Res Ther 43:869–884. [DOI] [PubMed] [Google Scholar]
- Dougherty DD, Loh R, Jenike MA, Keuthen NJ. (2006). Single modality versus dual modality treatment for trichotillomania: sertraline, behavioral therapy, or both? J Clin Psychiatry 67:1086–1092. [DOI] [PubMed] [Google Scholar]
- Dunlop BW, Thase ME, Wun CC, Fayyad R, Guico-Pabia CJ, Musgnung J, Ninan PT. (2012). A meta-analysis of factors impacting detection of antidepressant efficacy in clinical trials: the importance of academic sites. Neuropsychopharmacology 37:2830–2836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. (1995). Structured Clinical Interview for DSM-IV-Patient Edition (SCID-I/P, Version 20). New York: Biometrics Research Department, New York State Psychiatric Institute. [Google Scholar]
- Grant JE, Chamberlain SR. (2016). Trichotillomania. Am J Psychiatry 173:868–874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant JE, Odlaug BL, Kim SW. (2009). N-acetylcysteine, a glutamate modulator, in the treatment of trichotillomania: a double-blind, placebo-controlled study. Arch Gen Psychiatry 66:756–763. [DOI] [PubMed] [Google Scholar]
- Grant JE, Odlaug BL, Schreiber LR, Kim SW. (2014). The opiate antagonist, naltrexone, in the treatment of trichotillomania: results of a double-blind, placebo-controlled study. J Clin Psychopharmacol 34:134–138. [DOI] [PubMed] [Google Scholar]
- Greist JH, Jefferson JW, Kobak KA, Katzelnick DJ, Serlin RC. (1995). Efficacy and tolerability of serotonin transport inhibitors in obsessive compulsive disorder: a meta-analysis. Arch Gen Psychiatry 52:53–60. [DOI] [PubMed] [Google Scholar]
- Hamilton M. (1959). The assessment of anxiety states by rating. Br J Med Psychol 32:50–55. [DOI] [PubMed] [Google Scholar]
- Hamilton M. (1960). A rating scale for depression. J Neurol Neurosurg Psychiatr 23:56–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houghton DC, Capriotti MR, De Nadai AS, Compton SN, Twohig MP, Neal-Barnett AM, et al. (2015). Defining treatment response in trichotillomania: a signal detection analysis. J Anxiety Disord 36:44–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houghton DC, Maas J, Twohig MP, Saunders SM, Compton SN, Neal-Barnett AM, et al. (2016). Comorbidity and quality of life in adults with hair pulling disorder. Psychiatry Res 12–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keuthen NJ, O'Sullivan RL, Ricciardi JN, Shera D, Savage CR, Borgmann AS, et al. (1995). The Massachusetts General Hospital (MGH) Hairpulling Scale: 1. Development and factor analyses. Psychother Psychosom 64: 141–145. [DOI] [PubMed] [Google Scholar]
- Keuthen NJ, Flessner CA, Woods DW, Franklin ME, Stein DJ, Cashin SE. (2007). Trichotillomania Learning Center Scientific Advisory Board. Factor analysis of the Massachusetts General Hospital Hairpulling Scale. J Psychosom Res 62:707–709. [DOI] [PubMed] [Google Scholar]
- Khan A, Leventhal RM, Khan SR, Brown WA. (2002). Severity of depression and response to antidepressants and placebo: an analysis of the Food and Drug Administration database. J Clin Psychopharmacol 22:40–45. [DOI] [PubMed] [Google Scholar]
- Khan A, Brodhead AE, Kolts RL, Brown WA. (2005). Severity of depressive symptoms and response to antidepressants and placebo in antidepressant trials. J Psychiatr Res 39:145–150. [DOI] [PubMed] [Google Scholar]
- Leppink EW, Redden SA, Grant JE. (2017). A double-blind, placebo-controlled study of inositol in trichotillomania. Int Clin Psychopharmacol 32:107–114. [DOI] [PubMed] [Google Scholar]
- Leuchter AF, Cook IA, Witte EA, Morgan M, Abrams M. (2002). Changes in brain function of depressed subjects during treatment with placebo. Am J Psychiatry 159:122–129. [DOI] [PubMed] [Google Scholar]
- Leuchter AF, Hunter AM, Tartter M, Cook IA. (2014). Role of pill-taking, expectation and therapeutic alliance in the placebo response in clinical trials for major depression. Br J Psychiatry 205:443–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linden M. (2017). Placebo: unsolved problems for science, and simple conclusions for clinical practice. Am J Psychiatry 174:91–92. [DOI] [PubMed] [Google Scholar]
- Mataix-Cols D, Rauch SL, Manzo PA, Jenike MA, Baer L. (1999). Use of factor-analyzed symptom dimensions to predict outcome with serotonin reuptake inhibitors and placebo in the treatment of obsessive-compulsive disorder. Am J Psychiatry 156:1409–1416. [DOI] [PubMed] [Google Scholar]
- Nierenberg AA, Østergaard SD, Iovieno N, Walker RS, Fava M, Papakostas GI. (2015). Predictors of placebo response in bipolar depression. Int Clin Psychopharmacol 30:59–66. [DOI] [PubMed] [Google Scholar]
- Odlaug BL, Grant JE. (2010). Impulse-control disorders in a college sample: results from the self-administered Minnesota Impulse Disorders Interview (MIDI). Prim Care Companion J Clin Psychiatry 12:PCC.09m00842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odlaug BL, Kim SW, Grant JE. (2010). Quality of life and clinical severity in pathological skin picking and trichotillomania. J Anxiety Disord 24:823–829. [DOI] [PubMed] [Google Scholar]
- Pigott TA, Seay SM. (1999). A review of the efficacy of selective serotonin reuptake inhibitors in obsessive-compulsive disorder. J Clin Psychiatry 60:101–106. [DOI] [PubMed] [Google Scholar]
- Sheehan DV. (1983). The anxiety disease. New York, NY: Scribner. [Google Scholar]
- Stein DJ, Spadaccini E, Hollander E. (1995). Meta-analysis of pharmacotherapy trials for obsessive-compulsive disorder. Int Clin Psychopharmacol 10:11–18. [DOI] [PubMed] [Google Scholar]
- Stein DJ, Baldwin DS, Dolberg OT, Despiegel N, Bandelow B. (2006). Which factors predict placebo response in anxiety disorders and major depression? An analysis of placebo-controlled studies of escitalopram. J Clin Psychiatry 67:1741–1746. [DOI] [PubMed] [Google Scholar]
- Tung ES, Tung MG, Altenburger EM, Pauls DL, Keuthen NJ. (2014). The relationship between hair pulling style and quality of life. Ann Clin Psychiatry 26:193–198. [PubMed] [Google Scholar]
- U.S. Department of Health & Safety (2012). Guidance Regarding Methods for De-identification of Protected Health Information in Accordance with the Health Insurance Portability and Accountability Act (HIPAA) Privacy Rule. Available at: https://www.hhs.gov/hipaa/for-professionals/privacy/special-topics/de-identification/. [Google Scholar]
- Van Ameringen M, Mancini C, Patterson B, Bennett M, Oakman J. (2010). A randomized, double-blind, placebo-controlled trial of olanzapine in the treatment of trichotillomania. J Clin Psychiatry 71:1336–1343. [DOI] [PubMed] [Google Scholar]


