Abstract
Following preventive chemotherapy covering the entire population in the two endemic regions in Cambodia, the prevalence of schistosomiasis dropped from 77% in 1995 to 0.5% in 2003. The study presented here reports on the running cost of the control programme, and evaluates its cost-effectiveness and cost-benefit.
Financial costs were assessed using data taken from the annual reports of the National Center for Malaria Control, the Cambodian institution responsible for the control activities. Other data were collected from interviews with provincial and district staff. The analysis was conducted from the point of views of the Cambodian Ministry of Health and that of the society, and the comparison was undertaken using the "do-nothing" option.
The cost to treat an individual for the 9 years period of the implementation phase was 29.23 PPP Int $ (3.23 per year), the cost for each severe infection avoided was 195 PPP Int $ and 20,708 PPP Int $ for each death avoided. The drug cost corresponds on average to 17.34% of the programme’s implementation cost. The cost of bringing one severely infected individual of productive age to complete productivity, was estimated at 361.46 PPP Int $ and for 1 PPP Int $ invested in the programme the return in increased productivity, for the economic system, was estimated to be 3.85 PPP Int $.
The control programme demonstrated significant economical advantages. However, its costs are too high to be entirely supported by the Cambodian Ministry of Health.
Keywords: Health Technology Assessment, cost-effectiveness analysis, cost-benefit analysis, schistosomiasis, full costing approach
Introduction
Schistosomiasis is one of the most prevalent parasitic infections in the world. It is endemic in 76 countries and continues to be a public health problem in the developing world (Engels, 2002, Steinmann et al, Lancet Infect Dis). In Cambodia, the parasite responsible for the disease is Schistosoma mekongi.
The parasite is transmitted by contact with contaminated water (WHO, 2002, Gryseels et al, Lancet). At an early stage of infection, lesions of the liver can be detected (Hatz, 2001). At a late stage symptoms and signs associated with S. mekongi infection include cachexia, hepatosplenomegaly, stunting and retardation of puberty, portal hypertension, ascites and rupture of oesophageal varices (Biays et al. 1999). Pathology associated with the infection consists of periportal thickening and portal vein enlargement (Hatz et al., 2001). Severely infected individuals experience a significant reduction of working capacity and productivity (King et al., 2005). In individuals continuously exposed to infection without treatment, the disease progresses into different degrees of severity (infection of light, moderate and high intensity) and can lead to death (Warren et al., 1993).
Large scale preventive chemotherapy is the public health strategy recommended by World Health Organization (WHO) for the control of helminthic diseases such as schistosomiasis (WHO, 2006). The control strategy consists of the annual administration of praziquantel to the population at risk in the endemic areas (WHO, 2002).
The treatment, administered at regular intervals, eliminates most of the infecting schistosomes and prevent the development of severe morbidity. Since no infective stages of the parasite are contaminating water, reinfection takes place, however most of the infected individuals present “light intensity” infection because the annual interval between two treatments allows only few schistosomes to complete the cycle in the host.
Praziquantel has rare side effects which include nausea and abdominal pain. These do not require treatment (Stelma et al., 1995). The frequency of side effects is related to the intensity of the infection in the population and therefore is higher during the first year of the control programme after which it progressively decreases over the following years (N’Goran et al., 2003).
The first case of schistosomiasis in Cambodia was diagnosed in 1968 (Biays, 1999; Ohmae et al., 2004). The activities used in the collection of data about this pathology were interrupted due to the presence of the Khmer Rouge regime. From the last months of 1994, with the fall of the political regime, the monitoring activities started again and several severe cases of schistosomiasis were diagnosed in 20 villages in Kratie Province (Stich et al., 1999).
In the last weeks of 1994 the Cambodian Ministry of Health (MoH), started regular preventive chemotherapy in the North-East Provinces of Cambodia. The responsibility for the control activities was given to the National Center for Malaria Control (CNM), with the technical support from Medecins sans Frontières (MSF) and WHO. As of 1995, the area of intervention increased from the 20 villages where schistosomiasis was originally reported, to include all new areas that were progressively identified; this brought the total to approximately 110 villages.
The programme was based on a Mass Drug Administration (MDA) carried out by CNM staff, who reached the villages using boats and local volunteers. The control programme presented a decreasing number of difficulties each successive year: the mass distribution of praziquantel were increasingly accepted by the local communities.
Materials and Methods
The primary objective of the study was to provide information about the effectiveness and the cost of the programme, through the evaluation of the incremental cost-effectiveness of the public health intervention for schistosomiasis control, taking the MoH viewpoint. The comparison was undertaken with the “do-nothing” option, in which the MoH does not implement any control activity, as before 1995.
To achieve this primary objective, a Health Technology Assessment (HTA) approach, based on the Guidelines of the Canadian Agency for Drugs and Technologies in Health (2006), was used in order to evaluate the most important aspects to calculate the cost and the effectiveness of the programme.
HTA is a systematic review of existing evidence that provides an evaluation of the effectiveness, cost-effectiveness and impact both on patient health and on health care systems (Fox-Rushby, 2005). We calculated the cost for each averted death, for each severe infection averted and for each infection averted. The organizational impact, the budget impact, the equity (Goodman, 1998) and the generalizability (Sculpher et al., 2004) of the programme implementation were also assessed.
Epidemiological data
Epidemiological data were collected with periodical parasitological surveys: every year, an average of 2000 stool samples from individuals in randomly selected villages were analyzed with the Kato-Katz method. Annual surveys were also conducted in primary schools of five sentinel villages (Sinuon et al., 2007)
There was active search and follow up of cases of severe morbidity due to schistosomiasis during the mass treatments and the parasitological surveys and the age and sex of the cases identified were recorded.
The data were analysed and internal reports and scientific papers were regularly published (Urbani, 1997; Stich, 1999; Urbani, 2002; Ohmae, 2004; Sinuon, 2007). A linear model (Davison, 2003) was used to estimate the effectiveness of the programme, based on the prevalence data collected by the operators (Sinuon et al., 2007).
Economic data
Most of the economic data were taken from the “Annual Progress Report of the National Center for Parasitology Entomology and Malaria Control ” (CNM, 2006). Additional data were collected from documents published by the National Institute of Statistics of the Cambodian Ministry of Planning (2005), and by the WHO. Further information was taken from interviews administered to experts involved in the programme.
Cost data were recorded in Cambodian Riel and than converted into PPP Int $ with an exchange rate of 0.00077, Purchasing Power Parity rate of 2006 (International Monetary Fund, 2009) and referred to 2006. Purchasing Power Parity data were derived by the International Comparison Program of the World Bank, updated in December 2007.
Four different categories of cost were taken into consideration, both direct and indirect (Finkler, 1999): initial investment, coordination & overhead, distribution and survey costs, see Table 1.
Table 1.
Cost categories of the schistosomiasis control programme in Cambodia
| Cost category | Cost element | Source |
|---|---|---|
| Initial investment | Staff salaries, transports, materials, questionnaire distribution and questionnaire analysis. | Interview, CNM documents |
| Coordination & overhead |
Salaries and per diem: CNM officers, MoH provincial officers, volunteersa Vehicle: purchase, maintenance, insurance, national taxes, amortization (7 years); Utilitiesb: CNM cost for electricity, telephone, water, building maintenance, administration of the programme and guards. |
Annual report of CNM, interview |
| Distribution cost | Activities planning, drugs procurement (delivery at Phnom Penh), staff per diem, transport (cars fuel and boats renting), welcome costs (province and villages lunches), social marketing (T-shirt, posters). | Interview, International Drug Price Indicator Guide, annual report of CNM |
| Survey cost | Staff per diem, transport (cars fuel and boats renting), equipment kits for Kato-Katz test and scale. | Interview, annual report of CNM |
The salary of staff involved in the procurement and in the administration of the programme and of the CNM drivers are valued as distribution costs
No analytical accounting scheme was available to calculate the utility costs in the CNM office (Phnom Penh). The costs were extrapolated for the schistosomiasis programme, assuming that the expenditures in each of the five institutional programmes of CNM, are proportional to the number of people and number of cars available for implementing each the programme.
The time spent by Village Health Workers (VHW) was valued at the same level as the government salary 175.02 PPP Int $/months, cost to the company) and the required number of VHW was 1 per 400 inhabitants.
We calculated the Incremental Cost-Effectiveness Ratio (ICER) (Tan-Torres et al. 2003) comparing the programme implementation scenario, as the new technology, with the “do-nothing” option, as the already existing technology.
The secondary objective of the study was to estimate the cost-benefit of the intervention, from the perspective of the society, evaluating the increases in productivity that were not taken into consideration from the MoH perspective, to investigate the sustainability of the programme.
Impact on productivity
The impact of preventive chemotherapy on productivity was measured taking into account the decrease in the number of severe cases of schistosomiasis and deaths. The proportion of severely infected and the number of deaths was considered to decrease linearly, throughout the implementation phase, to be zero in 2003. The number of severely infected individuals of productive age (15-59 years), was extrapolated combining the total number of severely infected individuals and the age structure of the population in Cambodia. The number of severely infected individuals was considered to be distributed equally in all age groups, this was a conservative assumption since, from the available data on morbidity indicate that the majority of the cases of severe schistosomiasis was in the productive age. The decrease in productivity of each individual with severe schistosomiasis was estimated to be 50% (Guyatt 2000). The productivity of one life year was considered to be 488.64 PPP Int $ in 2006, equal to the pro-capita Cambodian GDP in the agriculture and fishing sectors, as these are the main economic activities in the two provinces. We assumed a situation of full employment in the area.
Additional analyses were performed to evaluate the robustness of the results, using two different methodologies: sensitivity analysis, assuming a ±30% change in the per diem salary, a ±50% change in fuel cost and a ±10% change in the cost of utilities (Saltelli et al., 2000), and “Monte Carlo” method, applied to salaries, with 100 iterations (Doubilet et al., 1985). For both approaches, the temporal horizon was fixed at 5 years, the value of annual volatility was fixed at plus or minus 30%. The variation was considered to elapse at the end of each year. The cost categories used for the sensitivity analysis were chosen because they are the ones most exposed to market variations, time and the most relevant for the total cost of the health programme.
Phases of the control programme
To facilitate the analysis and the processing of the economic data, the schistosomiasis control programme was divided into three phases: planning & start up, implementation and maintenance, as suggested in literature (Johns et al., 2003). The cost incurred in the planning & start-up phase (including studies and surveys conducted for the identification of the infected areas) were amortized to the 9 years of the implementation phase. The length of the implementation phase was 9 years: from 1995 (first drug distribution) up to and including 2003 (when no schistosomiasis cases were identified by parasitological surveys). For the maintenance phase, expenditure for 3 years (2004 -2006) was analyzed and some suggestions on how to contain the cost of this phase were provided. This phase was conducted after reaching the “no more cases” situation.
Results
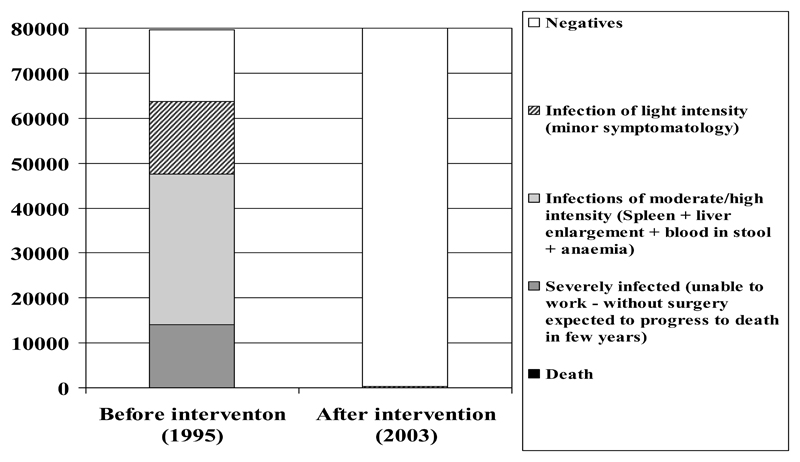
The activities during the 9 years of the implementation phase reduced the schistosomiasis prevalence from an estimated 77% (61,600 individuals infected) in 1995 to an estimated 0.5% in 2003 (Figure 1). No cases were found with the parasitological survey carried out from 2003 to 2006 (Sinuon et al., 2007). However, due to the low sensitivity of the Kato-Katz method at low intensity of infection (Yu et al. 2007) and the interval of confidence of the results of the survey, the presence of 400 cases of low intensity was estimated. The effectiveness of the 9 years programme in reducing the number of infected individuals was calculated to be 99.35%. This should be considered a conservative hypothesis.
Figure 1.
Estimated population at different intensity of infection with S. mekongi in 1995, before the initiation of the control activities and in 2006 after 9 years of intervention
Before intervention the total number of severe infections was estimated to be 12,000/year requiring approximately 250 hospital admissions each year. The residual classes of infection (moderate and light intensity infections) were estimated to be 49,600, requiring approximately 1,000 consultations each year. The yearly number of deaths due to schistosomiasis was estimated to be 25/year. Figure 1 graphically presents the morbidity due to schistosomiasis in Cambodia before and after the control intervention. We assumed that, without control intervention, the epidemiological situation would not have changed.
The cost of the "do-nothing" option was estimated to be 63,414.10 PPP Int $ per year (the evaluation is based on 2006 costs data), taking into account three consequences of the infection: outpatient consultations (1,000 consultations at 6.34 PPP Int $/each), hospital admissions (200 admissions at 158.54 PPP Int $/each) and surgical interventions (50 interventions at 507.31 PPP Int $/each), death and reduced productivity were not valuated because, despite representing a significant loss for a family, they do not constitute a cost for the MoH. This economic information was taken from interviews administered to CNM experts and Provincial Hospital staff. The total costs of the “do-nothing” option, for the 9 years of the implementation phase (1995-2003) are equal to 570,727 PPP Int $.
In the intervention option: the total cost of the implementation phase (1995-2003) is evaluated at 2,086,267 PPP Int $, details on the different cost categories are presented in Table 2. The additional cost of implementation phase due to the treatments still to be provided to infected individuals was estimated at 253,656 PPP Int $ bringing the total cost of this option to 2,339,923 PPP Int $. During this phase the estimated annual cost per treated person is 3.23 PPP Int $. Using this approach, the drug cost corresponds to 17.34% of the programme’s implementation cost.
Table 2.
Details of costs of Schistosomiasis control programme in Cambodia by cost category.
| Start-up & Implementation activities (USD) | Maintainance (USD) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1995 | 1996 | 1997 | 1999 | 2000 | 2001 | 2002 | 2003 | Total | 2004 | 2005 | 2006 | |
| Initial investment | 786 | 786 | 1571 | 1571 | 1571 | 1571 | 1571 | 1571 | 11000 | - | - | - |
| Coordination and overhead | 10747 | 10747 | 20760 | 19295 | 18563 | 17830 | 17097 | 16365 | 131403 | 15632 | 13068 | 12335 |
| Distribution cost | 34653 | 34653 | 67918 | 65142 | 63753 | 62356 | 60997 | 59589 | 449051 | 58201 | 56812 | 55424 |
| Survey cost | 4752 | 4752 | 9504 | 9504 | 9504 | 9504 | 9504 | 9504 | 66528 | 9504 | 4752 | 4752 |
| Total | 50937 | 50937 | 99754 | 95512 | 93391 | 91271 | 89150 | 87029 | 657982 | 83337 | 74632 | 72551 |
From 2004 to 2006 the annual cost of the maintenance phase was on average 243,596 PPP Int $. Drug distribution cost (drug procurement and administration) represent 73.95% of the total cost.
The yearly cost of the programme is gradually diminishing over the years from 316,290 PPP Int $ in 1997, to 229,911 PPP Int $ in 2006 (all the amount were calculated at 2006 constant price).
The results from the variability analysis show that the changes in salaries ( ± 30%), fuel (± 50%) and utilities (± 10%) would not have high impact on the total programme cost (± 11,32% increasing and decreasing all the variables). The “Monte Carlo” Analysis applied to the most relevant costs in percentage and with the major probability to increase salaries does not show any unexpected variation, reinforcing the robustness of the results.
The cost of treating an individual for the 9 years of the intervention phase was 29.23 PPP Int $. The yearly cost per infection avoided is 4.22 PPP Int $. The cost per each case of severe infection avoided is 195 PPP Int $ and the cost per each death avoided is 20,708 PPP Int $. During the 9 years of the implementation phase, the total number of avoided deaths was estimated to be 113, decreasing linearly from 25 in the first year to be zero in 2003.
The Cost-Effectiveness Value (ratio between the cost and the effectiveness of the programme implementation) per treated person for the 9 years implementation phase of the control programme is equal to 29.46 PPP Int $ per person.
The ICER (representing the cost to increase the effectiveness of one unit) resulted to be 22.26 PPP Int $ per capita. ICER represents the cost to increase the effectiveness of one unit.
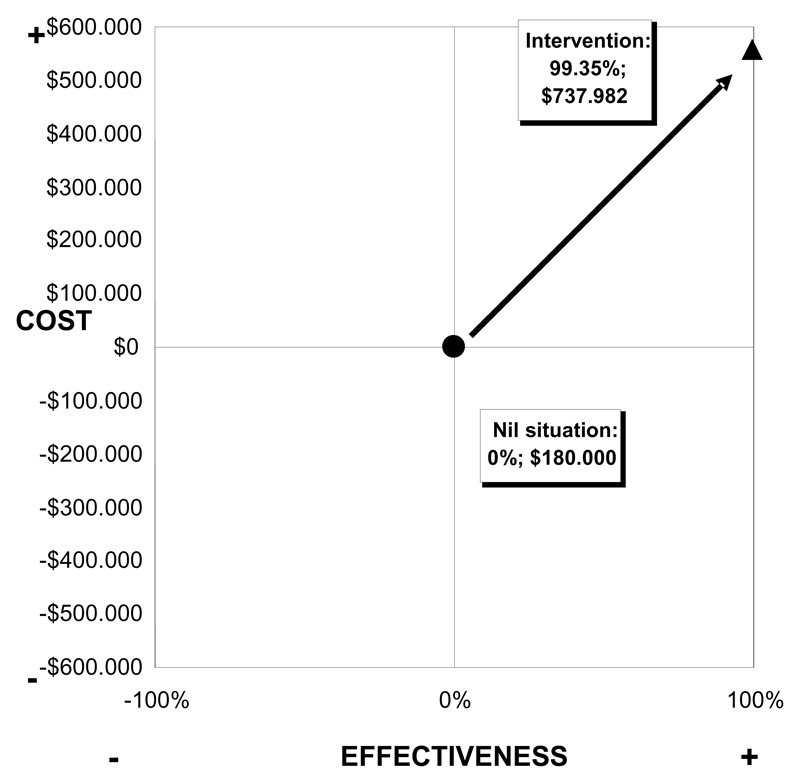
The incremental cost-effectiveness plan (Figure 2) compares the "do-nothing" option with the implementation phase results and shows the complete rationality of the choice to treat the whole population through an MDA. The cost increase is accompanied by a greater increase in effectiveness. This result suggests a dominance for the choice to implement the programme with respect to the "do-nothing" option (Tan-Torres et al. 2003).
Figure 2.
Incremental cost-effectiveness plan, comparing the “do-nothing” option and intervention programme, based on costs per protected individual.
From the point of view of society, it is important to underline that the schistosomiasis control programme increased the societal productivity, giving to 6,479 severely infected individuals between 15 and 59 years of age the possibility to return to full productivity. The cost of bringing one severely infected individual of productive age to complete productivity was estimated to be 361.17 PPP Int $ (2,339,923 PPP Int $ / 6,479 severely infected individuals).
809 individuals, of the 6,479 severely infected in 1995, are estimated to recover to complete productivity every year in which mass drug administration is conducted. This, considering the productivity of severely infected individuals before the implementation of the control programme to be 50%, (Guyatt, 2000), correspond to a productivity gain of 14,562 year, to which the productivity gained from the averted deaths (3,546 years) should be added for a total of 18,108 full year of productivity gained. The total financial gain in productivity is estimated to be equal to 8,848,242 PPP Int $ (18,108 years X 488.64 PPP Int $).
The necessary investment within the programme to increase societal productivity by 1 PPP Int $, is 0.26 PPP Int $ (2,339,923 PPP Int $ / 8,848,242 PPP Int $). For 1 PPP Int $ invested in the programme, the return in increased productivity is 3.84 PPP Int $.
Taking the MoH viewpoint, the Cost-Benefit Value (CBV) is 0.24. The benefits were estimated to be 570,727 PPP Int $ (saved costs), while the cost of the programme was 2,339,923 PPP Int $.
The yearly cost per capita of the maintenance phase (3.04 PPP Int $) is representing 15.74% of the total government per capita expenditures on health (WHO, 2007).
If we exclude the drug cost, on the hypothesis that this cost will be covered by an external agency, the per capita expenditure is 2.41 PPP Int $, representing 12.46% of the total government per capita expenditure on health.
In terms of organizational impact analysis of the control programme, the number of people involved was estimated as 14 health staff, with an average commitment of 3 months each year and 10 public servants with an average commitment of 1 months each year, this corresponds to less than 1% of the staff of the MoH that includes over 10,000 midwives and nurses (WHO, 2000).
No ethical or equity problems have emerged from the study. Rather, the more marginalized and poor population groups benefit to a larger extent from the intervention since drugs are administered free of charge, for those who present themselves at the distribution point, in each endemic village. This also allows access to treatment for the more disadvantaged groups of the population.
Discussion
This is the first study that applies an HTA approach to a successful schistosomiasis control programme that lasts over 12 years. In all the available economic evaluations, the sole direct costs of control programmes were taken into consideration, or a shorter programme implementation was considered (see Kirigia et al., 2000; Zhou et al., 2005 and Brooker et al., 2008).
The progressive reduction of the costs of the programme, was due to an increase in the efficiency of the personnel involved, to a reduction of the number of surveys, to an increase in participation of the target population, in addition to a reduction of the number of side effects from praziquantel requiring follow up.
The cost per death avoided calculated for this intervention (20,708 PPP Int $ in 9 years) is in the order of magnitude of the ones obtained by insecticide-impregnated bed nets used for malaria control (Hanson Kikumbih et al., 2003) and vaccination campaign for measles (Uzicanin et al., 2004) that are considered extremely cost-effective intervention.. However, mortality is a relatively rare outcome of schistosomiasis and the most important effects are on morbidity that was eliminated by preventive chemotherapy at very low cost.
In the review of the relevant literature performed, the yearly costs per infection avoided are within a range of 9.83 PPP Int $ (Gryseels, 1989) and 39.41 PPP Int $ (Guyatt et al., 1995), but these studies consider only the direct costs of the programmes.
The study demonstrated a lower cost of the "do-nothing" option from a MoH perspective; however, the low cost of this option is a consequence of the unclear symptomatology of schistosomiasis, that results in a very limited number of individuals presenting themselves to health units in order to receive treatment. This is also the reason why the WHO recommends periodical chemotherapy as public health intervention for the control of schistosomiasis: in absence of mass drugs administration the majority of the schistosomiasis cases are untreated, and the morbidity due to the disease could reach high levels.
Taking into consideration the perspective of the society and including the increase in productivity, the programme has an extremely favourable cost-effectiveness: over 8.8 million PPP Int $ of increase in productivity at a cost of less than 2.4 million PPP Int $ with a return of investment that is greater than the one obtained by vaccination programmes (Andre et al., 2008).
All these economical advantages do not take into consideration the additional benefits due to the increase in school attendance and performances that are well recognized following schistosomiasis control (Nokes et al., 1999 and Jukes et al., 2002). These last effects were difficult to be quantified in Cambodia, because of the poor maintenance of school records.
Despite the very good cost-effectiveness obtained and the relatively low annual cost of the programme, the cost of the programme per beneficiary (3.23 PPP Int $) represents a relevant part of the per capita expenditure on health of the Cambodian government (19.34 PPP Int $). The same situation can also be observed in the case where drugs are provided by an external donors. This cost is considered to be too high, to be sustained by the MoH, especially in the maintenance phase, when the number of schistosomiasis cases is very low.
Using the HTA approach, it is clear how the programme implementation scenario should be preferred to the “do-nothing” option.
Considering the organizational impact, the staff and the public servants needed to implement the programme are less than 1% of the total MoH staff, which includes over 10,000 midwives and nurses (WHO, 2000). Therefore, the work load of the MoH personnel will not vary considerably due to the schistosomiasis control programme implementation and the MoH could easily identify the staff who will participate in the programme.
In terms of generalizability, the programme is simple and easy to be managed and implemented; it is performed during few months every year. The drugs used are available on the international market and their cost has constantly decreased in the last decade (in 2006 it corresponds to 22% of the total cost of the programme). These factors demonstrated a good generalizability of the programme for other countries.
However, in accordance with the CNM staff responsible for the implementation of the programme, the small dimension of the endemic area, the high profile of the health staff involved and the very accurate planning are characteristics seldom present in other endemic countries. Furthermore, the particular morphology of the endemic area of the study should be considered as a distinguishing factor. The area shows a low population density, that implies high costs to reach the target population. This factor led to an increase of transportation costs and determined a decrease of the generalizability.
Finally, it shall be considered that schistosomiasis in Cambodia is present in only two intensively infected provinces and this fact facilitated the CNM to focus the control intervention and to contain costs.
There is a risk that the decision makers could interrupt the control programme to invest in other more prevalent diseases. The experience from Lao People's Democratic Republic demonstrated that if the intervention is interrupted, within 10 years the benefits of the control programme could be completely lost and therefore the epidemiological situation of schistosomiasis could return to the pre-intervention level. Hence the necessity to find a way to reduce the cost (for example, organizing drugs distribution every two years) and not to interrupt the intervention. The high impact of the control programme on the MoH expenditure could be an indication of the need for the donor community to support endemic countries in NTD control.
Future scenarios should consider the hypothesis to continue the maintainance phase, in order to reduce the cost related to schistosomiasis within the country. The possibility to measure the social impact of the implementation phase of similar programmes will probably show additional advantages and a higher cost saving in comparison to the one estimated for this study.
References
- Andre FE, Booy R, Bock HL, Clemens J, Datta SK, John TJ, Lee BW, Lolekha S, Peltola H, Ruff TA, Santosham M, et al. Vaccination greatly reduces disease, disability, death and inequity worldwide. Bull World Health Organ. 2008;86:140–146. doi: 10.2471/BLT.07.040089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biays S, Stich AHR, Odermatt P, Chan L, Yersin C, Chan M, Chaem S, Lormand JD. Foyer de bilharziose a` Schistosoma mekongi rede´couvert au Nord du Cambodge: I. Perception culturelle de la maladie, description et suivi de 20 cas cliniques graves. Trop Med Int Health. 1999;4:662–673. doi: 10.1046/j.1365-3156.1999.00472.x. [DOI] [PubMed] [Google Scholar]
- Brooker S, Kabatereine NB, Fleming F, Devlin N. Cost and cost-effectiveness of nationwide school-based helminth control in Uganda: intra-country variation and effects of scaling-up. Health Policy Plan. 2008;23:24–35. doi: 10.1093/heapol/czm041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canadian Agency for Drugs and Technologies in Health. Guidelines for the Economic Evaluation of the Health Technologies. Canada: 2006. [Google Scholar]
- CNM. Annual Progress Report of the National for Parasitology Entomology and Malaria Control. 2006 Internal document. [Google Scholar]
- Davison AC. Statistical models. Cambridge University Press; 2003. [Google Scholar]
- Doubilet P, Begg CB, Weinstein MC, Braun P, McNeil BJ. Probabilistic Sensitivity Analysis Using Monte Carlo Simulation. A practical approach Med Decis Making. 1985;5:157–177. doi: 10.1177/0272989X8500500205. [DOI] [PubMed] [Google Scholar]
- Engels D, Chitsulo L, Montresor A, Savioli L. The global epidemiological situation of schistosomiasis and new approaches to control and research. Acta Tropica. 2002;82:139–146. doi: 10.1016/s0001-706x(02)00045-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkler SA, Ward DM. Essentials of Cost Accounting for Health Care Organizations. Jones & Bartlett Publishers; 1999. [Google Scholar]
- Fox-Rushby J, Cairns J. Economic evaluation. Open University press; Maidenhead UK: 2005. [Google Scholar]
- Goodman CS. Healthcare Technology Assessment: methods, framework and role in policy making. Am J Manag Care. 1998;4:200–214. [PubMed] [Google Scholar]
- Gryseels B. The relevance of Schistosomiasis for Public Health. Tropical Medicine Parassitology. 1989;40:134–142. [PubMed] [Google Scholar]
- Guyatt H, Evans D. Desirable characeristics of a schistomiasis vaccine: some implications of a cost-effectiveness analysis. Acta Tropica. 1995;59:197–209. doi: 10.1016/0001-706x(95)91938-5. [DOI] [PubMed] [Google Scholar]
- Guyatt H. Do intestinal nematodes affect productivity in adulthood? Parasitol Today. 2000;16:153–8. doi: 10.1016/s0169-4758(99)01634-8. [DOI] [PubMed] [Google Scholar]
- Hanson Kikumbih N, Armstrong Schellenberg J, Mponda H, Nathan R, Lake S, Mills A, Tanner M, Lengeler C. Cost effectiveness of social marketing of insecticide-treated nets for malaria control in the United Republic of Tanzania. Bull World Health Organ. 2003;81:269. [PMC free article] [PubMed] [Google Scholar]
- Hatz C. The use of ultrasounds in schistosomiasis. Adv Parasitol. 2001;48:225–284. doi: 10.1016/s0065-308x(01)48007-9. [DOI] [PubMed] [Google Scholar]
- International Monetary Fund. World Economic Outlook Database. 2009 Apr [Google Scholar]
- Johns B, Baltussen R, Hutubessy R. Programme costs in the economic evaluation of health interventions, Cost Effectiveness and Resource Allocation. 2003 doi: 10.1186/1478-7547-1-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jukes MC, Nokes CA, Alcock KJ, Lambo JK, Kihamia C, Ngorosho N, Mbise A, Lorri W, Yona E, Mwanri L, Baddeley AD, et al. Partnership for Child Development. Heavy schistosomiasis associated with poor short-term memory and slower reaction times in Tanzanian schoolchildren. Trop Med Int Health. 2002;7:104–117. doi: 10.1046/j.1365-3156.2002.00843.x. [DOI] [PubMed] [Google Scholar]
- King CH, Dickman K, Tisch DJ. Reassessment of the cost of chronic helminthic infection: 10 ameta-analysis of disability-related outcomes in endemic schistosomiasis. Lancet. 2005;11:1561–69. doi: 10.1016/S0140-6736(05)66457-4. [DOI] [PubMed] [Google Scholar]
- Kirigia JM, Sambo LG, Kainyu LH. A cost-benefit analysis of preventive schistosomiasis interventions in Kenya. Afr J Health Sci. 2000;7:5–11. [PubMed] [Google Scholar]
- Miolo Vitali P, Cinquini L, Frucci G, Giannetti R, Marelli A, Pitzalis A, Tenucci A. Strumenti per l'analisi dei costi Vol.II - Approfondimenti di Cost Accounting. G. Giappichelli Editore; Torino: 2004. [Google Scholar]
- National Institutes of Statistic. Cambodia life tables. Phnom Penh: 2005. [Google Scholar]
- N’Goran EK, Gnaka HN, Tanner M, Utzinger J. Efficacy and side-effects of two praziquantel treatments against Schistosoma haematobium infection, among schoolchildren from Côte d'Ivoire. Ann Trop Med Parasitol. 2003;97:37–51. doi: 10.1179/000349803125002553. [DOI] [PubMed] [Google Scholar]
- Nokes C, McGarvey ST, Shiue L, Wu G, Wu H, Bundy DA, Olds GR. Evidence for an improvement in cognitive function following treatment of Schistosoma japonicum infection in Chinese primary schoolchildren. Am J Trop Med Hyg. 1999;60:556–565. doi: 10.4269/ajtmh.1999.60.556. [DOI] [PubMed] [Google Scholar]
- Ohmae H, Sinuon M, Kirinoki M, Matsumoto J, Chigusa Y, Socheat D, Matsuda H. Schistosoma mekongi: from discovery to control. Parasitol Intern. 2004;53:135–142. doi: 10.1016/j.parint.2004.01.004. [DOI] [PubMed] [Google Scholar]
- Saltelli A, Chan K, Scott EM. Sensitivity Analysis. Wiley series in Probability and Statistic; 2000. [Google Scholar]
- Sculpher MJ, Pang FS, Manca A, Drummond MF, Golder S, Urdahl H, Davies LM, Eastwood A. Generalisability in economic evaluation studies in healthcare: a review and case studies. Health Technology Assessment. 2004;8:1–192. doi: 10.3310/hta8490. [DOI] [PubMed] [Google Scholar]
- Sinuon M, Tsuyuoka R, Socheat D, Montresor A, Palmer K. Financial costs of deworming children in all primary schools in Cambodia. Trans R Soc Trop Med Hyg. 2005;99:664–668. doi: 10.1016/j.trstmh.2004.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinuon M, Tsuyuoka R, Socheat D, Odermatt P, Ohmae H, Matsuda H, Montresor A, Palmer K. Control of Schistosoma mekongi in Cambodia. Results of eight years of control activities in the two endemic provinces. Trans R Soc Trop Med Hyg. 2007;101:34–39. doi: 10.1016/j.trstmh.2006.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stelma FF, Talla I, Sow S, Kongs A, Niang M, Polman K, Deelder AM, Gryssels B. Efficacy and side effect of Praziquantel in an Epidemic focus of Schistosoma Mansoni. American Journal of Tropical Medicine and Hygiene. 1995;53:167–170. doi: 10.4269/ajtmh.1995.53.167. [DOI] [PubMed] [Google Scholar]
- Stich AHR, Biays S, Odermatt P, Chan M, Cheam S, Kiev S, Chuong SL, Legros P, Philips M, Lormand JD, Tanner M. Foci of schistosomiasis mekongi, Northern Cambodia: II. 20 Distribution of infection and morbidity. Trop Med Int Health. 1999;4:674–685. doi: 10.1046/j.1365-3156.1999.00474.x. [DOI] [PubMed] [Google Scholar]
- Tan-Torres Edejer T, Baltussen R, Adam T, Hutubessy R, Acharya A, Evans DB, Murray CJL. WHO guide to cost-effectiveness analysis. World Health Organization; Geneva: 2003. [Google Scholar]
- Urbani C, Sinoun M, Socheat D, Pholsena K, Strandgaard H, Odermatt P, Hatz C. Epidemilogy and control of mekongi schistosomiasis. Acta Tropica. 2002;82:157–168. doi: 10.1016/s0001-706x(02)00047-5. [DOI] [PubMed] [Google Scholar]
- Urbani C, Socheat D. Schistosomiasis control project: activity report. Phnom Penh: Mèdecins Sans Frontieres report; 1997. [Google Scholar]
- Uzicanin A, Zhou F, Eggers R, Webb E, Strebel P. Economic analysis of the 1996-1997 mass measles immunization campaigns in South Africa. Vaccine. 2004;22:3419–26. doi: 10.1016/j.vaccine.2004.02.042. [DOI] [PubMed] [Google Scholar]
- Warren KS, Bundy DAP, Anderson RM, Davis AR, Henderson DA, Jamison DT, Prescott N, Senft A. Helminth infection. In: Jamison DT, Mosley WH, Measham AR, Bobadilla JL, editors. Disease control priorities in developing countries. Oxford University Press; Oxford: 1993. pp. 131–160. [Google Scholar]
- WHO. Global Health Atlas. World Health Organization; Geneva: 2000. [Google Scholar]
- WHO. Report of a WHO Expert Committee. World Health Organization; Geneva: 2002. Prevention and control of schistosomiasis and soil-transmitted helminthiasis. [PubMed] [Google Scholar]
- WHO. Preventive Chemotherapy in human helminthiasis. World Health Organization; Geneva: 2006. [Google Scholar]
- WHO. World Health Report. World Health Organization; Geneva: 2007. [Google Scholar]
- Yu JM, de Vlas SJ, Jiang QW, Gryseels B. Comparison of the Kato-Katz technique, hatching test and indirect hemagglutination assay (IHA) for the diagnosis of Schistosoma japonicum infection in China. Parasitol Int. 2007;56:45–49. doi: 10.1016/j.parint.2006.11.002. [DOI] [PubMed] [Google Scholar]
- Zhou XN, Wang LY, Chen MG, Wang TP, Guo JG, Wu XH, Jiang QW, Zheng J, Chen XY. An economic evaluation of the national schistosomiasis control programme in China from 1992 to 2000. Acta Tropica. 2005;96:255–65. doi: 10.1016/j.actatropica.2005.07.026. [DOI] [PubMed] [Google Scholar]