Abstract
Objective
To determine whether sex modifies the relationship between fitness and mortality.
Patients and Methods
We included 57,284 patients without coronary artery disease or heart failure who completed a routine treadmill exercise test between 1991 and 2009. We determined metabolic equivalents of task (METs) and linked patient records with mortality data via the Social Security Death Index. Multivariable Cox regression was used to determine the association between sex, fitness, and all-cause mortality.
Results
There were 29,470 (51.5%) men and 27,814 (48.6%) women with mean ages of 53 and 54 years, respectively. Overall, men achieved 2 METs higher than women (P<.001). Over a median of 10 years follow-up, there were 6,402 deaths. The mortality rate for men in each MET group was similar to that of women who achieved an average 2.6 METs lower (P=.03). Fitness was inversely associated with mortality in both men (HR 0.84 per 1 MET; 95% CI: 0.83, 0.85) and women (HR 0.83 per 1 MET; 95% CI: 0.81, 0.84). This relationship did not plateau at high or low METs values.
Conclusions
While men demonstrated 2 METs higher than women, their survival was equivalent to women demonstrating 2.6 METs lower. Further, higher METs were associated with lower mortality for both men and women across the range of MET values. These findings are useful for tailoring prognostic information and lifestyle guidance to men and women undergoing stress testing.
Keywords: fitness, death, cohort, sex, cardiovascular disease
Cardiorespiratory fitness is an important measure of human health1 that represents both cumulative physical activity as well as underlying genetic composition.2,3 However, there are known differences in fitness levels between the sexes.4 While some of these differences in fitness are physiologic (e.g., skeletal muscle mass, heart and lung size),5,6 there are also a number of social and behavioral differences between sexes.7 For example, in a 2003-2004 survey of United States adults, men reported being more physically active than women.8,9 Whether or not these sex-specific differences in fitness translate into a difference in mortality risk is unknown, as many of the existing cohort studies on fitness have been limited to either men10–12 or women13,14 rather than both sexes.15 Furthermore, current recommendations to improve individual fitness through physical activity do not account for differences in fitness between men and women.16
The purpose of this study was to: (1) compare the population distribution of fitness levels between men and women; (2) determine what levels of fitness are associated with the same absolute risk of all-cause mortality in men and women; and (3) assess whether sex modifies the relative association between fitness and mortality. We hypothesized that while men would have higher levels of fitness than women, these levels would be associated with different absolute risks across sexes.
Patients and Methods
Study Population
The Henry Ford ExercIse Testing Project (The FIT Project) is a retrospectively defined registry of 69,885 consecutive patients who underwent physician-referred treadmill stress testing in the Henry Ford Health System in metropolitan Detroit, Michigan, between January 1st, 1991 and May 28th, 2009. Details of the study are published elsewhere.17 In brief, patients were excluded from the registry if they were less than 18 years old at the time of stress testing or if they underwent pharmacologic stress testing or exercise stress testing using non-Bruce protocols. In this analysis, we further excluded patients with known coronary artery disease (N = 10,190), congestive heart failure (N = 877), and patients with missing relevant variables (N = 1,534). The final sample included 57,284 patients. The FIT project was approved by the Institutional Review Board of Henry Ford Health System.
Treadmill Stress Testing and Cardiorespiratory Fitness
The reason for ordering an exercise stress test was based on physician referrals in the electronic medical record (EMR), and organized by the most common indications (chest pain, shortness of breath, “rule out” ischemia, or other). All patients underwent routine clinical treadmill stress testing using the standard Bruce protocol for various clinical indications between January 1st, 1991 and May 28th, 2009. The day the treadmill test was performed was the baseline for this study. Participants were encouraged to exercise as long as possible independent of achieved heart rate. The treadmill test was stopped if the patient had exercise-limiting chest pain, shortness of breath, or other limiting symptoms as assessed by the supervising clinician. In addition, testing could be terminated early at the discretion of the supervising clinician for significant arrhythmias, abnormal hemodynamic responses, diagnostic ST-segment changes, or other clinical reasons such as patient fatigue.
Resting heart rate and blood pressure were measured in the seated position prior to exercise testing by trained clinical personnel. In addition to continuous heart rate monitoring, blood pressure was measured every three minutes during the test. Cardiorespiratory fitness, expressed in metabolic equivalents of task (METs), was calculated by the Quinton treadmill controller based on maximal speed and grade achieved. METs achieved were examined as a continuous variable as well as in 4 groups based on distribution of the data, consistent with prior publications of these data: <6, 6-9, 10-11, ≥12 METs.18
Primary Outcome: All-cause Mortality
Participants were followed from the date of their baseline exercise test through May 28, 2009. Patient information was linked with vital status using the Social Security Death Index (SSDI) Death Master File (DMF), a national registry of nearly all deaths that have occurred within the United States. A complete algorithmic search of the SSDI DMF was completed in 100% of patients. Patients who were not identified as dead in the death registry were administratively censored on the date of their last electronic record encounter.
Mortality Risk Factors
Nurses and/or clinical exercise physiologists collected cardiovascular risk factor data immediately prior to the stress test. Age at the time of stress testing was derived from participants' date of birth. Sex and race were self-reported. Risk factors were also defined and gathered prospectively by self-report, and then augmented by a retrospective search of the electronic medical record (EMR). A history of smoking was based on self-reported current cigarette smoking. Diabetes mellitus was defined as a prior diagnosis of diabetes, use of hypoglycemic medications including insulin, or EMR problem list-based diagnosis of diabetes. Hypertension was defined as a prior diagnosis of hypertension, use of anti-hypertensive medications, or EMR problem list-based diagnosis of diabetes. Dyslipidemia was defined by prior diagnosis of any major lipid abnormality, use of lipid-lowering medications, or EMR problem list-based diagnosis of hypercholesterolemia or dyslipidemia. Obesity was identified by clinical staff at time of testing or as a problem listed in the EMR. Family history of coronary artery disease was defined as compatible history in a first degree relative. Physical activity status (active vs. sedentary) was informally assessed by a non-standard question, asking patients if they regularly exercised (yes or no). Baseline Framingham risk score and the atherosclerotic cardiovascular diseases (ASCVD) risk score at 10-years were determined using published algorithms.19,20
In all cases, complete medication use history was collected by staff prior to the exercise stress test. Medication use was then retrospectively verified and supplemented using the EMR as well as pharmacy claims files from enrollees in the Health System's integrated health insurance plan. Medications were categorized as beta-blockers, lipid-lowering medications, medications for lung disease, or medications for depression.
Statistical Analysis
Means and proportions of characteristics of the study population were determined for both men and women. We also determined the percentile distribution of METs overall and by sex for 1st, 5th, 10th, 25th, 50th, 75th, 90th 95th, and 99th percentiles. Percentiles were compared between men and women via quantile regression adjusted for age and race.
Absolute risk was characterized by mortality rates with age as the time axis. Mortality rates were determined for subpopulations of men and women achieving different MET thresholds (defined by achieving < MET values between 3 and 18). METs thresholds below 3 were not used due to small numbers. Linear regression was used to generate an average comparison across sexes (YMETS = XSEX + XMR), where MR represents the mortality rate per 1,000 person-years at risk. We also compared the incidence rate in groups of 2 METs, i.e. 1-2, 3-4, 5-6, etc, to address the fact that some METs values were represented disproportionately in our data.
With regard to relative risk, we used Cox proportional hazard models to examine the association between baseline METs (categories or as a continuous variable) and all-cause mortality by strata of men and women. All models were adjusted for age, race, resting systolic blood pressure, resting diastolic blood pressure, history of hypertension, beta blocker medication use, history of diabetes, history of hyperlipidemia, lipid-lowering medication use, history of obesity, family history of coronary heart disease, current smoking status, pulmonary medication use, depression medication use, physical activity, and indication for stress testing. Interaction terms were utilized to determine whether sex modified the relationship between METs and mortality. We plotted a restricted cubic spline model to characterize the continuous relationship between METs and mortality after adjustment for covariates. We also performed a sensitivity analysis examining the association between METs and death, limited to deaths occurring at least 6 months after stress testing.
Results
Baseline Characteristics
There were 29,470 men and 27,814 women included in our study population (N = 57,284) (Table 1). Overall 46,176 (81%) achieved a target heart rate ≥85 beats per minute. Men had a mean age of 53 years and were 24% black with a mean MET value of 10.1 (SD 3.0). Women had a mean age of 54 years, were 34% black, and achieved a mean MET value of 8.2 (SD, 2.6). Family history of coronary heart disease, depression medication use, and regular exercise were more prevalent among women compared with men. The proportion with a history of smoking and with an ASCVD risk score above 7.5% was greater in men compared with women. The most common indications for stress testing were chest pain (47% and 56% for men and women, respectively) followed by “rule out” ischemia (12% and 10%), and shortness of breath (10% and 8%).
T1. Baseline population characteristics by sex, mean (SD) or N (%).
| Men (N = 29,470) | Women (N = 27,814) | |
|---|---|---|
| Age, yr | 52.8 (12.7) | 54.0 (12.4) |
| Race, % | ||
| White | 20,034 (68.0) | 16,724 (60.1) |
| Black | 7,056 (23.9) | 9,380 (33.7) |
| Other | 2,380 (8.1) | 1,710 (6.1) |
| METs categories | ||
| <6 | 2,442 (8.3) | 4,918 (17.7) |
| 6-10 | 5,675 (19.3) | 9,778 (35.2) |
| 10-12 | 10,880 (36.9) | 10,288 (37.0) |
| >12 | 10,473 (35.5) | 2,830 (10.2) |
| METs achieved, units | 10.1 (3.0) | 8.2 (2.6) |
| Achieved a heart rate of 85% | 23,886 (81.1) | 22,290 (80.1) |
| % heart rate achieved, mean % | 90.9 (9.9) | 90.4 (10.0) |
| Resting systolic blood pressure, mm Hg | 132.2 (18.0) | 129.6 (19.6) |
| Resting diastolic blood pressure, mm Hg | 82.6 (10.2) | 79.5 (10.4) |
| History of hypertension, % | 17,525 (59.5) | 17,650 (63.5) |
| History of diabetes, % | 5,224 (17.7) | 5,094 (18.3) |
| History of hyperlipidemia, % | 12,846 (43.6) | 11,773 (42.3) |
| History of obesity, % | 5,887 (20.0) | 7,366 (26.5) |
| Family history of coronary heart disease, % | 13,855 (47.0) | 15,616 (56.1) |
| ASCVD risk score, % | ||
| <5% | 8,623 (29.3) | 14,505 (52.1) |
| 5-<7.5% | 3,439 (11.7) | 2,937 (10.6) |
| ≥7.5% | 17,408 (59.1) | 10,372 (37.3) |
| 10-year Framingham risk score, % | ||
| <10% | 11,721 (42.1) | 19,728 (72.6) |
| 10-<20% | 9,014 (32.4) | 5,939 (21.8) |
| ≥20% | 7,081 (25.5) | 1,521 (5.6) |
| Beta-blocker medication use, % | 4,597 (15.6) | 4,975 (17.9) |
| Aspirin use, % | 5,533 (18.8) | 4,325 (15.5) |
| Statin use, % | 5,471 (18.6) | 4,984 (17.9) |
| Lipid-lowering medications, % | 6,077 (20.6) | 5,387 (19.4) |
| Lung disease medication use, % | 2,088 (7.1) | 3,032 (10.9) |
| Depression medication use, % | 1,400 (4.8) | 3,138 (11.3) |
| History of smoking, % | 13,482 (45.7) | 10,266 (36.9) |
| Regular exercise habit, % | 7,789 (26.4) | 9,028 (32.5) |
| Reason for stress test, % | ||
| Chest Pain | 13,838 (47.0) | 15,427 (55.5) |
| Shortness of Breath | 2,944 (10.0) | 2,138 (7.7) |
| Rule Out Ischemia | 3,483 (11.8) | 2,833 (10.2) |
| Other | 9,205 (31.2) | 7,416 (26.7) |
Population distribution of fitness by sex
A comparison of the distribution of METs between men and women may be found in Table 2. Overall the median MET value achieved by men was 10 versus 7 for women. After adjusting for age and race, the difference in fitness between men and women at the median value was 1.7 METs (95% CI: 1.64, 1.74; P<.001) or 17% (i.e. 1.7/10 METs, the median value for men).
Table 2. Distribution of metabolic equivalents achieved (METs) by sex.
| Overall | Men | Women | Difference (METs, 95% CI)* | |
|---|---|---|---|---|
| N = 57,284 | N = 29,470 | N = 27, 814 | ||
| Percentile | ||||
| 1st | 2 | 2 | 2 | 0.39 (0.08, 0.70) |
| 5th | 5 | 5 | 4 | 1.07 (0.97, 1.16) |
| 10th | 5 | 7 | 5 | 1.15 (1.08, 1.22) |
| 25th | 7 | 7 | 7 | 1.56 (1.51, 1.62) |
| 50th | 10 | 10 | 7 | 1.69 (1.64, 1.74) |
| 75th | 10 | 13 | 10 | 1.95 (1.90, 2.00) |
| 90th | 13 | 13 | 12 | 1.79 (1.73, 1.86) |
| 95th | 13 | 15 | 13 | 1.58 (1.50, 1.66) |
| 99th | 15 | 15 | 13 | 1.54 (1.44, 1.63) |
Determined via quantile regression adjusted for age and race. All P-values <.001.
Comparison of absolute risk by sex
During a median of 10 years of follow-up (range: 3 days to 22 years), there were 6,402 deaths (11.2%). Mortality rates, using age as the time axis, were determined for thresholds of METs (Figure 1; Supplemental Table S1). Overall, fitness thresholds were 2.6 METs greater for men compared to women (difference = 2.60, 95% CI: 0.91, 4.3; P=.004) given the same mortality rates. We also examined mortality rates in increments of 2 METS (Table 3), which was generally consistent with a higher mortality in men than women given similar MET achievement.
Figure 1.
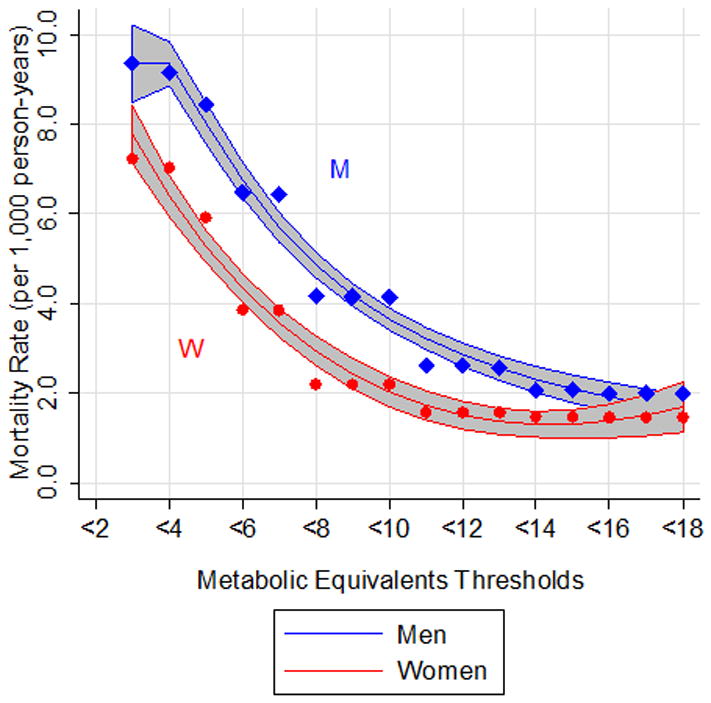
Mortality rate per 1,000 person-years corresponding to metabolic equivalent (METs) thresholds (i.e. achieving a METs value < each number between 3 and 18) The time axis is age. Fractional polynomials are used to fit a curve for both men (blue) and women (red). Gray shade represents 95% confidence interval. Points represent mortality rate (per 1,000 person-years) at each threshold (circles for women; diamonds for men).
Table 3. Mortality rate by metabolic equivalent (MET) achieved.
| Metabolic Equivalents Achieved | Mortality Rate per 1,000 person years | |
|---|---|---|
|
| ||
| Men | Women | |
| 1-2 | 9.36 | 7.23 |
| 3-4 | 7.65 | 4.81 |
| 5-6 | 5.23 | 2.78 |
| 7-8 | 3.07 | 1.27 |
| 9-10 | 1.34 | 0.57 |
| 11-12 | 0.41 | 0.31 |
| 13-14 | 0.74 | 0.28 |
| 15-16 | 0.40 | 0.40 |
| 17-18 | 0.25 | 0.00 |
Comparison of relative risk by sex
After adjustment for mortality-related risk factors, compared with men achieving a MET value <6, men with a MET value ≥12 had a hazard ratio of 0.20 (95% CI: 0.17, 0.23; P<.001) (Table 4). Similarly, women with a MET value ≥12 versus a MET value <6 demonstrated a hazard ratio of 0.20 (95% CI: 0.15, 0.28; P<.001). Moreover, there were significant trends across categories of METs in both men and women with higher METs being inversely associated with all-cause mortality (P-trends <.001 for both men and women). Comparison of the effect of sex on the association between MET and mortality revealed a significant interaction (P=.002); although the magnitude of the association was quite similar (Table 4). In particular, women achieving 6-9 METs had a lower risk of mortality (HR: 0.50; 95% CI: 0.45, 0.54) than men achieving 6-9 METs (HR: 0.58; 95% CI: 0.53, 0.63). Furthermore, examination of the continuous relationship between MET levels and mortality according to sex revealed roughly linear associations for both men and women with no evidence of a plateau in effect (Figure 2). In a sensitivity analysis, restricting to events occurring after 6 months of follow-up did not change our findings (Supplement Table S2).
Table 4. Association of metabolic equivalents (METs) and all-cause mortality by strata of sex.
| Hazard Ratio (95% CI) | |||
|---|---|---|---|
|
|
|
||
| Men, N = 29,470 | Women, N = 27,814 | P-interaction | |
| Categories of metabolic equivalents | |||
| <6 | 1.0 (reference) | 1.0 (reference) | |
| 6-9 | 0.58 (0.53, 0.63) | 0.50 (0.45, 0.54) | |
| 10-11 | 0.32 (0.29, 0.35) | 0.31 (0.27, 0.36) | |
| ≥12 | 0.20 (0.17, 0.23) | 0.20 (0.15, 0.28) | |
| P trend across categories as ordinal variable | <.001 | <.001 | .006 |
| METS per 1 unit | 0.84 (0.83, 0.85) | 0.83 (0.81, 0.84) | |
| P value | <.001 | <.001 | .002 |
All models adjusted for age, race, resting systolic blood pressure, resting diastolic blood pressure, history of hypertension, beta blocker medication use, history of diabetes, history of hyperlipidemia, lipid-lowering medication use, history of obesity, family history of coronary heart disease, current smoking status, pulmonary medication use, depression medication use, physical activity, and indication for stress testing
Figure 2.
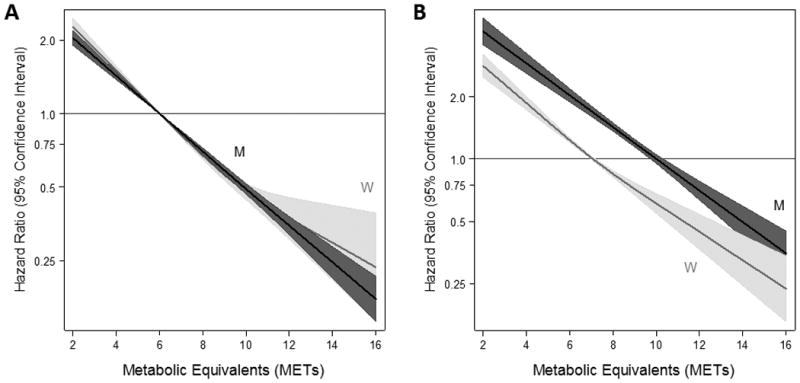
Adjusted hazard ratios (solid line) from restricted cubic spline models for mortality stratified by men (M) and women (W) using categories of metabolic equivalents (METs). Men are depicted in black; women are depicted in gray. Shading and dashed lines are the 95% confidence intervals. The models were expressed in the following fashion: (A) relative the median values for men and women (10 for men, 7 for women) with three knots based on the population distribution (5, 7, 10 for women; 5, 10, 13 for men); and (B) relative to a METs value of 6 with knots specified at values of 6, 10, and 12. Models were adjusted for age, race, resting systolic blood pressure, resting diastolic blood pressure, history of hypertension, history of diabetes, history of hyperlipidemia, history of obesity, family history of coronary heart disease, current smoking status, beta blocker medication use, lipid-lowering medication use, pulmonary medication use, depression medication use, and indication for stress testing. The hazard ratios are shown on a natural log scale.
Discussion
This study represents one of the largest longitudinal studies on the association between fitness and mortality in men and women. In this population of intermediate and high risk adults, we found that men have higher cardiorespiratory fitness compared to women by about 1-2 METs (i.e. 20%). However, the absolute risk associated with METs levels also varies across sexes, with women achieving equivalent survival at 2.6 lower METs than men. Regardless of sex, fitness showed a strong inverse relationship with mortality with no evidence of a threshold.
In this study, we show a significant difference in fitness levels between men and women. This confirms prior work conducted in 20-49 year-old US adults, which showed that maximal oxygen uptake, another measure of fitness, was higher in US men versus women.4 The reason for this difference is in part behavioral in that women generally report lower physical activity levels than men;7–9,21 although, this was not in case in our cohort. However, there are a number of physiologic differences between men and women that are also likely contributory including heart rate,22 heart size,6 lung volume,23 pulmonary function,24,25 and body mass composition.26 Determining the cause of the observed difference in fitness between men and women is beyond the scope of this study, however.
Despite the differences in fitness achievement between men and women, we found that lower MET levels were associated with higher absolute risk in men versus women. These findings are supported by studies showing higher mortality rates among men despite lower morbidity compared with women.27–30 We can speculate that part of the reason why lower fitness does not translate into equal or higher risk among women is due to physiologic differences between men and women (e.g. heart rate, body mass composition, etc). It is also possible that there are differences in the underlying conditions contributing to lower fitness. For example, one study demonstrated that women are more likely to carry diagnoses of arthritis and depression while men are more likely to carry diagnoses of heart disease.31 This was to some extent reflected in our study in that depression medication use was higher in women compared with men. While each of these conditions may be reflected by lower fitness, heart disease may have a higher association with premature death. Understanding the underlying causes of the different prognosis associated with fitness across sex remains an important topic of future research.
Our study demonstrated strong, linear associations between fitness and mortality in men and women, which did not plateau. While some prior studies suggest there is a threshold of 5-6 METs to realize mortality benefit,32 we show that having a higher fitness level is associated with lower mortality risk even among persons with an achieved METs level below 6. This reflects studies on physical activity, which have shown a strong, linear relationship between physical activity level and mortality.33 We also found that the magnitude of the association was similar for both men and women. This is consistent with prior studies showing an association between fitness in men,10–12 women,13,14 and both men and women.15 Similar to these studies, our data does not justify sex-specific fitness targets.
This study has important clinical implications. At present there is no sex-specific standard for determining a desirable fitness level. However, our study showed that not only do women generally demonstrate lower fitness levels than men, but that these lower levels of fitness were associated with a lower risk of mortality than men. Furthermore, there was the same, inverse, linear relationship between fitness and mortality in both men and women. Physicians conducting stress tests in men and women should recognize these sex-specific differences in fitness when interpreting their results. While these data do not currently support a specific cut point by which to define fit versus unfit patients, they are supportive of other trials demonstrating a causal role for fitness in reducing mortality.15
This study has a number of limitations that warrant discussion. First, our assessment of fitness was based on a single measurement so we could not determine changes in fitness over time or issues related to within-person variability. Furthermore, there is evidence of “end-digit preference”34 in that some MET values were disproportionately represented in our patient population. This limited our ability to evaluate the absolute risk associated with individual MET levels. Second, treadmill machines were different across clinical sites without standard utilization by patients (e.g. some participants used handrails). Moreover, we did not assess for musculoskeletal deficits prior to stress testing that would influence use of handrails and overall performance. These differences in equipment and baseline function increase variability in METs achieved, attenuating our findings. Third, our data were collected from electronic records retrospectively. As a result, we do not have direct laboratory and anthropometric measures on patients, but instead rely on reporting and documentation from health providers. While on the one hand this confers the advantage of having our population data vetted by a health professional, this introduces measurement error and may contribute to residual confounding. Finally, our study population was comprised of people referred for stress testing, which undoubtedly carries a higher burden of cardiovascular disease risk factors at baseline than the general population. This had the unintended effect of enriching our study with many clinical events and afforded the opportunity to examine fitness in a unique study population (contrary to many prior studies in ambulatory, general populations). However, our results may not be generalizable to all clinical settings.
This study also has a number of important strengths. It comprised a large sample size of a population at risk for cardiovascular disease, often the target population of fitness interventions. Further, the study population was diverse and representative of a typical, albeit less healthy, US community. As a result, our findings are directly applicable to the large body of patients referred for stress testing and are useful for result interpretation and risk counselling. Finally, the study included substantial follow-up time and multiple, well-ascertained clinical events, allowing for rigorous adjustment in regression models.
Conclusions
In conclusion, in this population of patients referred for stress testing, women demonstrate lower fitness levels than men by about 2 MET levels, but have a similar prognosis at 2.6 MET levels lower than men. Both men and women have lower relative risk of mortality with higher MET levels without any observed plateau, and this relationship is similar across sexes. Future studies should determine sex-specific minimum fitness levels for men and women for risk stratification as well as identify the underlying conditions behind the observed differences in prognosis between men and women.
Supplementary Material
Acknowledgments
The authors thank the staff and patients of the FIT project for their important contributions.
SPJ is supported by a NIH/NIDDK T32DK007732-20 Renal Disease Epidemiology Training Grant.
Abbreviations
- METS
metabolic equivalents
- CI
confidence interval
Footnotes
Parts of this study were presented as an oral presentation in the 63rd annual meeting of the American College of Cardiology, Washington DC, March 29-31, 2014.
Disclosures: The authors declare that there is no conflict of interest associated with this manuscript.
References
- 1.Sui X, LaMonte MJ, Laditka JN, et al. Cardiorespiratory fitness and adiposity as mortality predictors in older adults. JAMA. 2007;298(21):2507–2516. doi: 10.1001/jama.298.21.2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shiroma EJ, Lee IM. Physical activity and cardiovascular health: lessons learned from epidemiological studies across age, gender, and race/ethnicity. Circulation. 2010;122(7):743–752. doi: 10.1161/CIRCULATIONAHA.109.914721. [DOI] [PubMed] [Google Scholar]
- 3.Bouchard C, An P, Rice T, et al. Familial aggregation of VO(2max) response to exercise training: results from the HERITAGE Family Study. J Appl Physiol. 1999;87(3):1003–1008. doi: 10.1152/jappl.1999.87.3.1003. [DOI] [PubMed] [Google Scholar]
- 4.Wang CY, Haskell WL, Farrell SW, et al. Cardiorespiratory fitness levels among US adults 20-49 years of age: findings from the 1999-2004 National Health and Nutrition Examination Survey. Am J Epidemiol. 2010;171(4):426–435. doi: 10.1093/aje/kwp412. [DOI] [PubMed] [Google Scholar]
- 5.Weltman A, Weltman JY, Hartman ML, et al. Relationship between age, percentage body fat, fitness, and 24-hour growth hormone release in healthy young adults: effects of gender. J Clin Endocrinol Metab. 1994;78(3):543–548. doi: 10.1210/jcem.78.3.8126124. [DOI] [PubMed] [Google Scholar]
- 6.Brooks GA, Fahey TD. Exercise Physiology: Human Bioenergetics and Its Applications. Newy York: Wiley & Sons; 1984. [Google Scholar]
- 7.Thomas JR, Nelson JK, Church G. A Developmental Analysis of Gender Differences in Health Related Physical Fitness. PEdiatric Exercise Science. 1991;3:28–42. [Google Scholar]
- 8.Troiano RP, Berrigan D, Dodd KW, Mâsse LC, Tilert T, McDowell M. Physical activity in the United States measured by accelerometer. Med Sci Sports Exerc. 2008;40(1):181–188. doi: 10.1249/mss.0b013e31815a51b3. [DOI] [PubMed] [Google Scholar]
- 9.Centers for Disease Control and Prevention (CDC) Prevalence of regular physical activity among adults--United States, 2001 and 2005. MMWR Morb Mortal Wkly Rep. 2007;56(46):1209–1212. [PubMed] [Google Scholar]
- 10.Kokkinos P, Myers J, Faselis C, et al. Exercise capacity and mortality in older men: a 20-year follow-up study. Circulation. 2010;122(8):790–797. doi: 10.1161/CIRCULATIONAHA.110.938852. [DOI] [PubMed] [Google Scholar]
- 11.Myers J, Prakash M, Froelicher V, Do D, Partington S, Atwood JE. Exercise capacity and mortality among men referred for exercise testing. N Engl J Med. 2002;346(11):793–801. doi: 10.1056/NEJMoa011858. [DOI] [PubMed] [Google Scholar]
- 12.Sandvik L, Erikssen J, Thaulow E, Erikssen G, Mundal R, Rodahl K. Physical fitness as a predictor of mortality among healthy, middle-aged Norwegian men. N Engl J Med. 1993;328(8):533–537. doi: 10.1056/NEJM199302253280803. [DOI] [PubMed] [Google Scholar]
- 13.Gulati M, Pandey DK, Arnsdorf MF, et al. Exercise capacity and the risk of death in women: the St James Women Take Heart Project. Circulation. 2003;108(13):1554–1559. doi: 10.1161/01.CIR.0000091080.57509.E9. [DOI] [PubMed] [Google Scholar]
- 14.Mora S, Redberg RF, Cui Y, et al. Ability of exercise testing to predict cardiovascular and all-cause death in asymptomatic women: a 20-year follow-up of the lipid research clinics prevalence study. JAMA. 2003;290(12):1600–1607. doi: 10.1001/jama.290.12.1600. [DOI] [PubMed] [Google Scholar]
- 15.Blair SN, Kohl HW, Barlow CE, Paffenbarger RS, Gibbons LW, Macera CA. Changes in physical fitness and all-cause mortality. A prospective study of healthy and unhealthy men. JAMA. 1995;273(14):1093–1098. [PubMed] [Google Scholar]
- 16.US Department of Health and Human Services. 2008 physical activity guidelines for Americans. [Accessed May 15, 2015]; http://www.health.gov/paguidelines/. Published 2008.
- 17.Al-Mallah MH, Keteyian SJ, Brawner CA, Whelton S, Blaha MJ. Rationale and Design of the Henry Ford ExercIse Testing Project (The FIT Project) Clin Cardiol. 2014;37(8):456–461. doi: 10.1002/clc.22302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Juraschek SP, Blaha MJ, Whelton SP, et al. Physical fitness and hypertension in a population at risk for cardiovascular disease: the Henry Ford ExercIse Testing (FIT) Project. J Am Heart Assoc. 2014;3(6):e001268. doi: 10.1161/JAHA.114.001268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goff DC, Lloyd-Jones DM, Bennett G, et al. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63(25 Pt B):2935–2959. doi: 10.1016/j.jacc.2013.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 2002;106(25):3143–3421. [PubMed] [Google Scholar]
- 21.Rockwood K, Howlett SE, MacKnight C, et al. Prevalence, attributes, and outcomes of fitness and frailty in community-dwelling older adults: report from the Canadian study of health and aging. J Gerontol A Biol Sci Med Sci. 2004;59(12):1310–1317. doi: 10.1093/gerona/59.12.1310. [DOI] [PubMed] [Google Scholar]
- 22.Gulati M, Shaw LJ, Thisted RA, Black HR, Bairey Merz CN, Arnsdorf MF. Heart rate response to exercise stress testing in asymptomatic women: the st. James women take heart project. Circulation. 2010;122(2):130–137. doi: 10.1161/CIRCULATIONAHA.110.939249. [DOI] [PubMed] [Google Scholar]
- 23.Schwartz J, Katz SA, Fegley RW, Tockman MS. Sex and race differences in the development of lung function. Am Rev Respir Dis. 1988;138(6):1415–1421. doi: 10.1164/ajrccm/138.6.1415. [DOI] [PubMed] [Google Scholar]
- 24.Harms CA. Does gender affect pulmonary function and exercise capacity? Respir Physiol Neurobiol. 2006;151(2-3):124–131. doi: 10.1016/j.resp.2005.10.010. [DOI] [PubMed] [Google Scholar]
- 25.Hopkins SR, Barker RC, Brutsaert TD, et al. Pulmonary gas exchange during exercise in women: effects of exercise type and work increment. J Appl Physiol. 2000;89(2):721–730. doi: 10.1152/jappl.2000.89.2.721. [DOI] [PubMed] [Google Scholar]
- 26.Lewis DA, Kamon E, Hodgson JL. Physiological differences between genders. Implications for sports conditioning. Sports Med. 1986;3(5):357–369. doi: 10.2165/00007256-198603050-00005. [DOI] [PubMed] [Google Scholar]
- 27.Singh-Manoux A, Guéguen A, Ferrie J, et al. Gender differences in the association between morbidity and mortality among middle-aged men and women. Am J Public Health. 2008;98(12):2251–2257. doi: 10.2105/AJPH.2006.107912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Verbrugge LM, Wingard DL. Sex differentials in health and mortality. Health Matrix. 1987;5(2):3–19. [PubMed] [Google Scholar]
- 29.Belon AP, Lima MG, Barros MBA. Gender differences in healthy life expectancy among Brazilian elderly. Health Qual Life Outcomes. 2014;12:88. doi: 10.1186/1477-7525-12-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nusselder WJ, Looman CWN, Van Oyen H, Robine JM, Jagger C. Gender differences in health of EU10 and EU15 populations: the double burden of EU10 men. Eur J Ageing. 2010;7(4):219–227. doi: 10.1007/s10433-010-0169-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Crimmins EM, Kim JK, Solé-Auró A. Gender differences in health: results from SHARE, ELSA and HRS. Eur J Public Health. 2011;21(1):81–91. doi: 10.1093/eurpub/ckq022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Drygas W, Jegler A, Kunski H. Study on threshold dose of physical activity in coronary heart disease prevention. Part I. Relationship between leisure time physical activity and coronary risk factors. Int J Sports Med. 1988;9(4):275–278. doi: 10.1055/s-2007-1025021. [DOI] [PubMed] [Google Scholar]
- 33.Lee IM, Skerrett PJ. Physical activity and all-cause mortality: what is the dose-response relation? Med Sci Sports Exerc. 2001;33(6 Suppl):S459–S471. doi: 10.1097/00005768-200106001-00016. discussion S493-S494. [DOI] [PubMed] [Google Scholar]
- 34.Stratford PW, Wainwright AV, Kennedy DM. An example of end-digit preference in physiotherapy practice. Physiother Can. 2013;65(3):276–278. doi: 10.3138/ptc.2012-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


