Abstract
Background
Aggressive behaviors are common in individuals diagnosed with autism spectrum disorder (ASD) and may be phenotypic indicators of different subtypes within ASD. In current research literature for non-ASD samples, aggression has been linked to several brain structures associated with emotion and behavioral control. However, few if any studies exist investigating brain volume differences in individuals with ASD who have comorbid aggression as indicated by standardized diagnostic and behavioral measures.
Method
We examined neuroimaging data from individuals rigorously diagnosed with ASD versus typically developing (TD) controls. We began with data from brain volume regions of interest (ROI) taken from previous literature on aggression including the brainstem, amygdala, orbitofrontal cortex, anterior cingulate cortex, and dorsolateral prefrontal cortex. We defined aggression status using the Irritability subscale of the Aberrant Behavior Checklist and used lasso logistic regression to select among these predictor variables. Brainstem volume was the only variable shown to be a predictor of aggression status.
Results
We found that smaller brainstem volumes are associated with higher odds of being in the high aggression group.
Conclusions
Understanding brain differences in individuals with ASD who engage in aggressive behavior from those with ASD who do not can inform treatment approaches. Future research should investigate brainstem structure and function in ASD to identify possible mechanisms related to arousal and aggression.
Keywords: Autism, aggression, imaging, brainstem, structural MRI
Introduction
Aggressive behavior is a common symptom of autism spectrum disorders (ASD) that can be particularly difficult for families to manage (Dominick, Davis, Lainhart, Tager-Flusberg, & Folstein, 2007; Farmer, et al., 2014; Horner, Carr, Strain, Todd, & Reed, 2002; Kanne & Mazurek, 2011; Mazurek, Kanne, & Wodka, 2013). In particular, parents frequently report that aggression in their child is more distressing than poor adaptive skills (Lecavalier, Leone, & Wiltz, 2006).
In typically developing (TD) children, there is mounting evidence that increased aggression is associated with brain functioning in regions of emotional or behavioral control (Lamm, Granic, Zelazo, & Lewis, 2011; Lozier, Cardinale, VanMeter, & Marsh, 2014; Paus, 2005; Sterzer & Stadler, 2009). Particular regions identified include the amygdala, brainstem, orbitofrontal cortex (OFC), dorsolateral prefrontal cortex (DLPFC), and anterior cingulate cortex (ACC) (Coccaro, McCloskey, Fitzgerald, & Phan, 2007; Ducharme, et al., 2011; Kolla, et al., 2015; Rylands, et al., 2012; Saxbe, Del Piero, Immordino-Yang, Kaplan, & Margolin, 2016; Siegel & Victoroff, 2009; Visser, et al., 2014). These regions might also be associated with increased aggression in children with ASD. Because brain function is linked in some degree to brain structure (Meier et al., 2016; Ponten, Daffertshofer, Hillebrand, & Stam, 2010; Stam et al., 2016), we reasoned that these findings from functional scans would provide a useful starting place for examining structural integrity.
To date, few if any studies exist investigating brain volume differences in individuals with ASD who have comorbid aggression. Because aggression is likely related to brain function in regions of emotional control and occurs in some children with autism but not others, it may be useful to use aggression as an indicator of different subcategories of ASD. This approach may increase the likelihood that researchers will find reliable associations between symptoms and brain regions (Chaste, et al., 2015). Improved understanding of brain correlates with behavioral outcomes could go a long way towards identifying effective interventions (South, Wolf, & Herlihy, 2012). Understanding brain differences in individuals with ASD who engage in aggressive behavior from those with ASD who do not have frequent aggressive behaviors can inform treatment approaches at all levels. The aim of this exploratory study was to determine the nature of relationships between brain structure volumes and parent-re ported symptoms of aggression in children with ASD.
Methods
Participants
The data for this project were collected as part of a multifaceted, joint longitudinal project by researchers at Brigham Young University and the University of Utah. The study was approved by Institutional Review Boards at both universities and appropriate signed informed consent was obtained for all procedures. This work was carried out in accordance with the ethical standards of both universities and with the Declaration of Helsinki as revised in 2000. All participants were recruited via community autism support, health care, and educational service providers. In order to enhance homogeneity for this exploratory project, we selected data from a larger sample of over 150 children and adults enrolled in the longitudinal study (Travers, et al., 2015) including 45 male children with ASD who had available scores on the Aberrant Behavior Checklist and imaging data. The participants with available data ranged from 3-13 years old, and so we used comparison data (from the same dataset) of 18 typically developing (TD) male children of the same age range.
For the ASD sample, diagnosis was verified through extensive assessment according to DSM-IV (American Psychiatric Association, 1994) and ICD-10 (World Health Organization, 1992) criteria based on data collected from the Autism Diagnostic Observation Schedule (ADOS) (Lord, et al., 2000), Autism Diagnostic Interview-Revised (ADI-R) (Lord & Rutter, 1994), and clinical judgment. Binary aggression status was determined from the Irritability subscale of the Aberrant Behavior Checklist, where a cut score ≥18 indicates high-aggression (ASDHA) and < 18 for low-aggression (ASDLA) status (Aman, et al., 2010; Aman, Singh, & Stewart, 1985; Brown, Aman, & Havercamp, 2002; Carroll, et al., 2014; Marcus, et al., 2009; Marshburn & Aman, 1992; McCracken, et al., 2002). According to this cutoff, 14 ASD participants were in the “high aggression” group, 31 ASD participants were in the “low aggression” group, and all 18 TD participants were in the “low aggression” group.
TD participants were evaluated using ADOS, IQ, language tests, and parent interview to confirm that all had age appropriate verbal skills and no history of learning, developmental, cognitive, neurological, or neuropsychiatric problems (Alexander, et al., 2007; Allen-Brady, et al., 2010; Jantz, et al., 2015; Prigge, et al., 2013; Travers, et al., 2014). See Table 1 for more detailed descriptions of participant recruitment and characteristics.
Table 1. Participant Characteristics.
| ASDHA | ASDLA | TD | |
|---|---|---|---|
|
|
|||
| TOTAL | 14 | 31 | 18 |
| Race | |||
| # Caucasian | 13 | 31 | 18 |
| # African American | 1 | - | - |
| Language | |||
| # Verbal | 10 | 28 | 18 |
| # Minimally Verbal | 3 | 1 | - |
| # No language data | 1 | 2 | - |
| ASDHA M (SD) | ASDLA M (SD) | TD M (SD) | |
|
|
|||
| ABC Irritability | 23.86 (5.17) | 7.10 (4.84) | 1.00 (1.57) |
| (min-max) | (18-33) | (0-17) | (0-5) |
| BASC Anxiety | 52.40 (13.30) | 51.50 (11.92) | -- |
| (min-max) | (36-71) | (34-80) | |
| ADOS | 14.57 (3.80) | 15.22 (3.95) | 1.50 (1.38) |
| (min-max) | (7-20) | (6-23) | (0-4) |
| Full Scale IQ | 97.43 (27.61) | 94.13(20.85) | 118.57 (18.11) |
| (min-max) | (48-136) | (58-137) | (93-144) |
| Scan age in years | 7.93 (3.34) | 8.77 (3.22) | 9.22 (2.32) |
| (min-max) | (3-13) | (3-13) | (5-13) |
| TICV, mm3 | 1,575,136 (159,480) | 1,610,711 (183,651) | 1,513,308 (249,142) |
| (min-max) | (1,383,898-2,010,302) | (1,179,262-1,942,259) | (1,000273-2,151,003) |
Note. All participants were male. Language category (verbal versus minimally verbal) was determined using quesiton 30 on the ADI-R. Aggression status was determined using Irritability subscale score from the Aberrant Behavior Checklist (ABC). Following McCracken et al. (2002) among others, scores <18 are Low Aggression (ASDLA) and scores ≥18 are High Aggression (ASDHA). BASC Anxiety = Anxiety subscale from the Behavioral Assessment Scales for Children. The Composite Intelligence Estimate was calculated from various IQ tests given to different participants following the procedures described below. TICV = Total Intracranial Brain Volume, in mm3.
Behavioral Measures
ABC-Irritability
The Irritability subscale of the Aberrant Behavior Checklist (ABC) (Aman, et al., 1985) is commonly used as a measure of problematic behavior focused on aggression. It has been used in many studies assessing the effects of various interventions on the maladaptive and aggressive behaviors of children with autism (Aman, et al., 2010; Aman, et al., 2009; Bearss, et al., 2015; McCracken, et al., 2002; Research Units on Pediatric Psychopharmacology (RUPP) Autism Network, 2005). This scale consists of 15 parent-reported Likert-type items rated on a scale of 0 – 3. Items include questions about intentional self-injury; aggression to others; noisy, rough behavior; temper tantrums/outbursts; irritable and whiny behavior; disobedience or difficulty to control; uncooperative behavior; insistence that demands be met immediately; disruptive behavior; stamping or banging; and temper outbursts or tantrums when the individual does not get his/her own way. A score of 18 and above has been used to indicate high-aggression status (Aman, et al., 2010; Aman, Singh, & Stewart, 1985; Brown, Aman, & Havercamp, 2002; Carroll, et al., 2014; Marcus, et al., 2009; Marshburn & Aman, 1992; McCracken, et al., 2002).
Wechsler Intelligence Scales for Children-Third Edition
IQ was assessed at the initial study visit, using the Differential Abilities Scale (Elliott, 1990) or the Wechsler Intelligence Scales for Children-Third Edition (Wechsler, 1991). As in previous papers from this study (Alexander, et al., 2007; Allen-Brady, et al., 2010; Dominick, et al., 2007; Jantz, et al., 2015; Prigge, et al., 2013; Travers, et al., 2014), we used the most comprehensive IQ score from each measure (i.e., Wechsler Full Scale IQ, DAS General Cognitive Ability score). While IQ scores are reported, we did not use IQ as a covariate in the model. This is because covarying IQ takes away important and meaningful variance that is part-and-parcel of neurodevelopmental disorders (Dennis, et al., 2009).
ADOS
We verified autism diagnoses using the Autism Diagnostic Observation Schedule, administered by research reliable clinicians (Lord, et al., 2000). Although the ADOS Modules are determined by language level, the ADOS was used in our study only as a factor in determining case status, not language ability.
ADI-R
The Autism Diagnostic Interview-Revised was also used to verify case status. In addition, Question 30, which asks “How much speech does ____ have now?” was used to identify verbal language levels. The research reliable interviewer determines the appropriate code based on language abilities including parts of speech used, number of words used, and if other people understand the language of the individual being evaluated. We distinguished a minimally verbal group on the basis of a code fewer than five words total, or does not use speech on a daily basis, from this item.
Imaging procedures
Neuroimaging data were selected for each individual based on the first time point in the longitudinal study where both MRI and behavioral data were available. Participants were scanned using a Siemens Trio 3.0T scanner and an 8-channel, receive-only, RF head coil (Alexander, et al., 2007). Structural scans were sagittal 3D MPRAGE T1-weighted images with 1 × 1 × 1 mm isotropic resolution (inversion time = 1100ms, echo time = 2.93 ms, repetition time = 1800 ms, flip angle = 12°, field of view = 56mm, matrix = 256 × 256 × 160, slice thickness = 1.0mm, 160 slices) (Zielinski, et al., 2014).
Imaging and statistical data analysis
MRI scans were processed using FreeSurfer v5.1.0 (http://surfer.nmr.mgh.harvard.edu/) including automated cortical parcellation and region of interest (ROI) definition using the Desikan-Killany Atlas (Desikan, et al., 2006) resulting in 34 cortical parcellations per hemisphere (Zielinski, et al., 2014). The technical details of the automated parcellation process have been described previously (Fischl et al. 2002). These parcellations were used to examine differences in the mean brain structure ROI of the ASDHA, ASDLA, and TD groups. Age across the three groups (shown in Table 1) was not significantly different (p = 0.48). Likewise, mean total intracranial brain volumes were not significantly different across groups (p = 0.27). The FreeSurfer-based bilateral ROIs we investigated for contributions to aggression were the amygdala, brainstem (defined from the base of the skull to superior colliculus, excluding the cerebellum), orbitofrontal cortex (OFC), dorsolateral prefrontal cortex (DLPFC), and anterior cingulate cortex (ACC), see Table 2.
Table 2. Average brain volumes by region of interest and participant group.
| ASDHA M (SD) | ASDLA M (SD) | TD M (SD) | |
|---|---|---|---|
|
|
|||
| Amygdala | 3,325 (492) | 3,418 (472) | 3,262 (450) |
| Brainstem | 19,869 (2,904) | 21,546 (2,953) | 21,357 (2,396) |
| OFC | 35,293 (3,918) | 35,464 (3,519) | 34,783 (4,469) |
| DLPFC | 126,122 (17,312) | 129,705 (13,772) | 128,103 (13,697) |
| ACC | 11,214 (2,077) | 11,171 (2,121) | 10,961 (2,184) |
We used logistic regression, and in particular elastic net regularization (Lockhart, Taylor, Tibshirani, & Tibshirani, 2014; Zou & Hastie, 2005), in conjunction with logistic regression, to simultaneously select informative ROIs and estimate a model for predicting aggression status based on the ROIs. (Tibshirani, 1996) The elastic net is a variation of lasso (least absolute shrinkage and selection operator) (Tibshirani, 1996), both of which are regularization methods that shrink some of the regression coefficients to zero. Remaining non-zero coefficients correspond to variables that are important for prediction. Elastic net regularization is recommended over lasso when the predictor variables are correlated (Zou & Hastie, 2005). The predictor variables included in the analysis were the volumes for the five ROIs, total brain volume, and age at scan (Zou & Hastie, 2005). We selected the appropriate elastic net regularization parameter using cross-validation based on minimizing the binomial deviance. All analyses were done using R version 3.2.3 (R Core Team, 2015), also using the “glmnet” package for elastic net logistic regression (Friedman, Hastie, & Tibshirani, 2010) and the “covTest” package for computing the p-values (Lockhart, Taylor, Tibshirani, & Tibshirani, 2013).
Results
ASD group only
In order to address concerns of possible confounding between ASD diagnosis and aggression, we first ran the elastic net logistic regression using only the 45 ASD participants. Using the regularization parameter that minimized the binomial deviance, the only predictor that remained with a non-zero coefficient was brainstem volume, which was significant at the 0.05 level (p-value=0.0453). The regression coefficient corresponding to the brainstem was -0.0001. The negative coefficient means that the odds of being in the high aggression group decreases as brainstem volume increases. The coefficient is small because it is the average change in log odds for a 1-cubic millimeter increase in brainstem volume. However, the magnitude of the coefficient is not important, just that its significance indicates that the brainstem can possibly be used as one predictor for aggression. It is unlikely the brainstem would be the only predictor, but our findings suggest a hopeful connection worth future study.
ASD and TD
We obtained very similar results when predicting aggression status based on both the ASD group and the typically developing group. In this later case, we classified all of the TD participants into the low aggression group (since none of the TD participants had an ABC irritability score higher than 18). The elastic net logistic regression again selected only the brainstem as a meaningful predictor (p-value=0.0427). In the logistic regression model, the brainstem coefficient is again -0.0001. These results are suggestive that the brainstem is important in classifying aggression status, although it is unlikely the brainstem would be the only predictor.
Discussion and Implications
Although symptoms of aggression add substantially to the burden of care for individuals affected by ASD, there has been little research regarding the neural mechanisms that underlie this behavior. To our knowledge, this is the first study to directly explore brain volumes as related to aggression in ASD. Our most striking finding is that reduced brainstem volume is associated with the likelihood of being in the high versus low aggression group. We also acknowledge that progress also needs to be made in finding other useful predictors.
Although the brainstem develops from three distinct embryological neural tube vesicles, our finding suggests that at least one of the structures of the brainstem is associated with aggression in ASD. However, our findings with aggression could be explained by functional connectivity (temporal correlation of brain activity) between the brainstem and other regions of the brain. Abnormalities in functional connectivity of the brainstem have been observed in autism (for a review, see Bressler and Menon (2010) and Kirsch, et al. (2005)). The latter study is discussed below. Structural connectivity via white matter tracts are also possible, however, these studies rarely include the brainstem (see Kleinhans, et al. (2012) for an exception). To explore the possibility of brainstem involvement in aggression in autism, there is a need for more specific research that includes the brainstem.
We note that our findings cannot be due simply to differences in intelligence scores. As a reminder, we did not use IQ scores as a covariate. This is because covarying IQ takes away important and meaningful variance that is part-and-parcel of neurodevelopmental disorders (Dennis, et al., 2009). Additionally, the high aggression ASD group did not have lower composite IQ scores than the low aggression ASD group. More participants in the low-aggression group were verbal (91% vs. 73%) and language skills may logically play a role in ability to express feelings without acting out. However, there were not enough available formal language measures at this point to perform additional analyses, and Maskey, Warnell, Parr, Le Couteur, and McConachie (2013) suggest that language may not play as large a factor in aggression as assumed. Future research should include additional communication measures to explore possible associations with acting out in aggressive children with ASD.
In addition, our study is not the first to identify a potential biological marker for aggression and autism. Anckarsäter (2006) reviews several findings concerning autism and aggression including abnormalities in limbic circuitry and increased dopaminergic relative to serotonergic neurotransmission. Arrested development of serotonergic neurons has been associated with increased aggression in mice (Hendricks, et al., 2003) and this has been proposed as a model for autism (Schaefer, Vorhees, & Williams, 2009). The present study is also consistent with some earlier findings that suggest associations between the brainstem and aggression or between the brainstem and autism. For example, Kirsch, et al. (2005) found that oxytocin (which modulates aggression) (Bosch, Meddle, Beiderbeck, Douglas, & Neumann, 2005) acts on the amygdala, which has both tracts that project to the brainstem (LeDoux, 2000) and functional connectivity with the upper brainstem (including the periaqueductal gray area and the reticular formation). Another region of the brainstem (locus coeruleus) has also been found to be associated with autism (Mehler & Purpura, 2009). In addition, ASD has been associated with reduced volume in the brainstem (Hashimoto, et al., 1992) and with the HOXA1 gene, which is involved in brainstem development (Weidenheim, 2001). While connections between the brainstem and aggression or between the brainstem and autism have been made, our contribution is to connect aggression in ASD with brainstem volume.
This line of research has the potential to improve proactive approaches to aggression in ASD. Understanding what is happening in the brain shortly before an aggressive episode could lead to better interventions. For example, if we knew that it was general physiological arousal that led to the apparent association between the brainstem and aggression (the association that we report in this paper), that could inform better treatments. Some treatments might be in regard to baseline physiological arousal levels and/or modulation of physiological arousal in response to threatening or frustrating situations. For example, individuals with better regulation of physiological arousal in reaction to environmental stimuli may be slower to “default” to aggressive behavior. Improved regulation (by psychopharmacology and/or behavioral methods) may provide a window of opportunity to ameliorate distress before aggressive behavior escalates.
Our findings warrant replication and refinement using larger samples, investigating potential sex differences (our study used only males), exploring variations in development and age, and including other imaging modalities. More fine-grained imaging approaches with larger samples may target structural measures, including volumetrics, and connectivity measures as well as functional activity in the region as a putative area of interest for predicting aggression associated with autism symptoms. Better procedures for a matched control group are also necessary to address issues such as the contribution of IQ to our findings. This exploratory study suggests further work regarding the brainstem and aggression in ASD in order to improve understanding of neural mechanisms and more specific targets for intervention in this important symptom domain.
Figure 1.
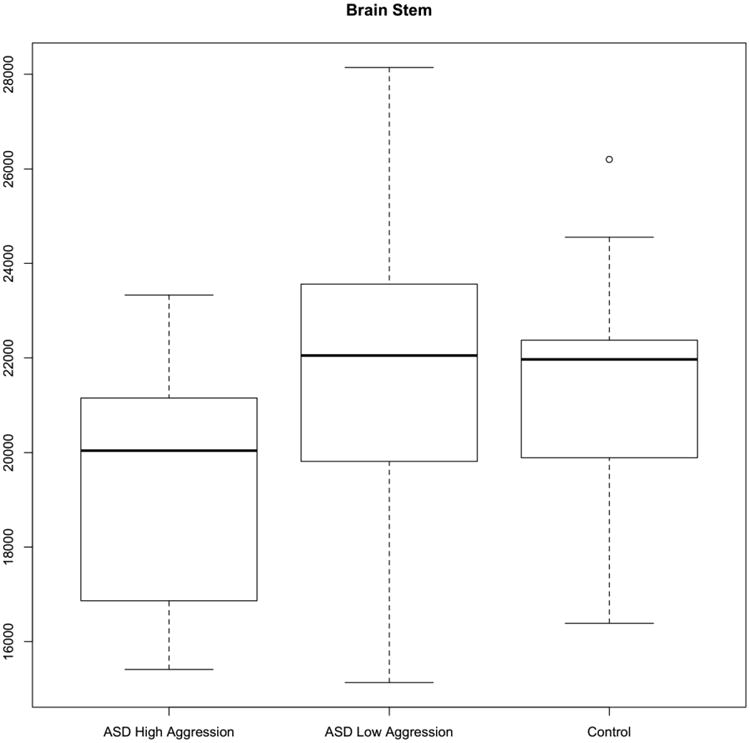
Brainstem volume (in mm3) by group. The boxes represent the median and the first and third quartiles for each group. The whiskers represent the minimum and maximum of all data in each group. The low aggression ASD group is more similar to the control group on brainstem volume.
Highlights.
Used structural MRI data from 63 male children, 45 with autism spectrum disorder (ASD).
Examined brain volume in individuals with ASD with and without comorbid aggression.
Smaller brainstem volume was associated with odds of higher aggression.
Acknowledgments
We gratefully acknowledge Hilary Coon for providing access to additional behavioral data to allow for this analysis. The project described was supported by Award Number RO1MH080826 (JEL, EDB) from the National Institutes of Mental Health. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Mental Health or the National Institutes of Health. We gratefully acknowledge support from the Poelman Foundation to Brigham Young University and BYU's McKay School of Education.
Footnotes
Brain volumes associated with high levels of aggression in male children diagnosed with autism spectrum disorder
Conflict of Interest: The authors state that they have no financial, personal, or other relationships with individuals who or organizations that could inappropriately influence, or be perceived to influence, this work.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Rebecca A Lundwall, Brigham Young University, Provo, UT 84602, USA.
Kevin G Stephenson, Brigham Young University, Provo, UT 84602, USA.
E Shannon Neeley-Tass, Brigham Young University, Provo, UT 84602, USA.
Jonathan C Cox, Brigham Young University, Provo, UT 84602, USA.
Mikle South, Brigham Young University, Provo, UT 84602, USA.
Erin D Bigler, Brigham Young University, Provo, UT 84602, USA.
Emily Anderberg, Brigham Young University, Provo, UT 84602, USA.
Molly D Prigge, University of Utah, 201 Presidents Circle, SLC, UT 84112, USA.
Blake D Hansen, University of Utah, 201 Presidents Circle, SLC, UT 84112, USA.
Janet E Lainhart, University of Wisconsin-Madison, 500 Lincoln Drive, Madison, WI 53706, USA.
Ryan O Kellems, University of Wisconsin-Madison, 500 Lincoln Drive, Madison, WI 53706, USA.
Jo Ann Petrie, University of Wisconsin-Madison, 500 Lincoln Drive, Madison, WI 53706, USA.
Terisa P Gabrielsen, University of Wisconsin-Madison, 500 Lincoln Drive, Madison, WI 53706, USA.
References
- Alexander AL, Lee JE, Lazar M, Boudos R, DuBray MB, Oakes TR, Miller JN, Lu J, Jeong EK, McMahon WM, Bigler ED, Lainhart JE. Diffusion tensor imaging of the corpus callosum in autism. Neuroimage. 2007;34:61–73. doi: 10.1016/j.neuroimage.2006.08.032. [DOI] [PubMed] [Google Scholar]
- Allen-Brady K, Robison R, Cannon D, Varvil T, Villalobos M, Pingree C, Leppert MF, Miller J, McMahon WM, Coon H. Genome-wide linkage in Utah autism pedigrees. Molecular Psychiatry. 2010;15:1006–1015. doi: 10.1038/mp.2009.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aman MG, Kasper W, Manos G, Mathew S, Marcus R, Owen R, Mankoski R. Line-item analysis of the Aberrant Behavior Checklist: Results from two studies of aripiprazole in the treatment of irritability associated with autistic disorder. Journal of Child and Adolescent Psychopharmacology. 2010;20:415–422. doi: 10.1089/cap.2009.0120. [DOI] [PubMed] [Google Scholar]
- Aman MG, Mcdougle CJ, Scahill L, Handen B, Arnold LE, Johnson C, Stigler KA, Bearss K, Butter E, Swiezy NB. Medication and parent training in children with pervasive developmental disorders and serious behavior problems: Results from a randomized clinical trial. Journal of the American Academy of Child and Adolescent Psychiatry. 2009;48:1143–1154. doi: 10.1097/CHI.0b013e3181bfd669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aman MG, Singh NN, Stewart AW. The Aberrant Behavior Checklist: A behavior rating scale for the assessment of treatment effects. American Journal of Mental Deficiency. 1985;89:485–491. [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders (4th ed) 4th. Washington, DC: Author; 1994. [Google Scholar]
- Anckarsäter H. Central nervous changes in social dysfunction: Autism, aggression, and psychopathy. Brain Research Bulletin. 2006;69:259–265. doi: 10.1016/j.brainresbull.2006.01.008. [DOI] [PubMed] [Google Scholar]
- Bearss K, Johnson C, Smith T, Lecavalier L, Swiezy N, Aman M, McAdam DB, Butter E, Stillitano C, Minshawi N. Effect of parent training vs parent education on behavioral problems in children with autism spectrum disorder: A randomized clinical trial. Journal of the American Medical Association. 2015;313:1524–1533. doi: 10.1001/jama.2015.3150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosch OJ, Meddle SL, Beiderbeck DI, Douglas AJ, Neumann ID. Brain oxytocin correlates with maternal aggression: link to anxiety. The Journal of Neuroscience. 2005;25:6807–6815. doi: 10.1523/JNEUROSCI.1342-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bressler SL, Menon V. Large-scale brain networks in cognition: Emerging methods and principles. Trends in cognitive sciences. 2010;14:277–290. doi: 10.1016/j.tics.2010.04.004. [DOI] [PubMed] [Google Scholar]
- Brown EC, Aman MG, Havercamp SM. Factor analysis and norms for parent ratings on the Aberrant Behavior Checklist-Community for young people in special education. Research in Developmental Disabilities. 2002;23:45–60. doi: 10.1016/s0891-4222(01)00091-9. [DOI] [PubMed] [Google Scholar]
- Carroll D, Hallett V, McDougle CJ, Aman MG, McCracken JT, Tierney E, Arnold LE, Sukhodolsky DG, Lecavalier L, Handen BL. Examination of aggression and self-injury in children with autism spectrum disorders and serious behavioral problems. Child and Adolescent Psychiatric Clinics of North America. 2014;23:57–72. doi: 10.1016/j.chc.2013.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaste P, Klei L, Sanders SJ, Hus V, Murtha MT, Lowe JK, Willsey AJ, Moreno-De-Luca D, Yu TW, Fombonne E, Geschwind D, Grice DE, Ledbetter DH, Mane SM, Martin DM, Morrow EM, Walsh CA, Sutcliffe JS, Lese Martin C, Beaudet AL, Lord C, State MW, Cook EH, Jr, Devlin B. A genome-wide association study of autism using the simons simplex collection: does reducing phenotypic heterogeneity in autism increase genetic homogeneity? Biological Psychiatry. 2015;77:775–784. doi: 10.1016/j.biopsych.2014.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coccaro EF, McCloskey MS, Fitzgerald DA, Phan KL. Amygdala and orbitofrontal reactivity to social threat in individuals with impulsive aggression. Biological Psychiatry. 2007;62:168–178. doi: 10.1016/j.biopsych.2006.08.024. [DOI] [PubMed] [Google Scholar]
- Dennis M, Francis DJ, Cirino PT, Schachar R, Barnes MA, Fletcher JM. Why IQ is not a covariate in cognitive studies of neurodevelopmental disorders. Journal of the International Neuropsychological Society. 2009;15:331–343. doi: 10.1017/S1355617709090481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desikan RS, Segonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, Buckner RL, Dale AM, Maguire RP, Hyman BT, Albert MS, Killiany RJ. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage. 2006;31:968–980. doi: 10.1016/j.neuroimage.2006.01.021. [DOI] [PubMed] [Google Scholar]
- Dominick KC, Davis NO, Lainhart J, Tager-Flusberg H, Folstein S. Atypical behaviors in children with autism and children with a history of language impairment. Research in Developmental Disabilities. 2007;28:145–162. doi: 10.1016/j.ridd.2006.02.003. [DOI] [PubMed] [Google Scholar]
- Ducharme S, Hudziak JJ, Botteron KN, Ganjavi H, Lepage C, Collins DL, Albaugh MD, Evans AC, Karama S. Right anterior cingulate cortical thickness and bilateral striatal volume correlate with child behavior checklist aggressive behavior scores in healthy children. Biological Psychiatry. 2011;70:283–290. doi: 10.1016/j.biopsych.2011.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott CD. Differential Ability Scales-II (DAS-II) San Antonio: The Psychological Corporation; 1990. [Google Scholar]
- Farmer C, Butter E, Mazurek MO, Cowan C, Lainhart J, Cook EH, Dewitt MB, Aman M. Aggression in children with autism spectrum disorders and a clinic-referred comparison group. Autism. 2014;19:281–291. doi: 10.1177/1362361313518995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman J, Hastie T, Tibshirani R. Regularization paths for generalized linear models via coordinate descent. Journal of statistical software. 2010;33:1–22. [PMC free article] [PubMed] [Google Scholar]
- Hashimoto T, Tayama M, Miyazaki M, Sakurama N, Yoshimoto T, Murakawa K, Kuroda Y. Reduced brainstem size in children with autism. Brain and Development. 1992;14:94–97. doi: 10.1016/s0387-7604(12)80093-3. [DOI] [PubMed] [Google Scholar]
- Hendricks TJ, Fyodorov DV, Wegman LJ, Lelutiu NB, Pehek EA, Yamamoto B, Silver J, Weeber EJ, Sweatt JD, Deneris ES. Pet-1 ETS gene plays a critical role in 5-HT neuron development and is required for normal anxiety-like and aggressive behavior. Neuron. 2003;37:233–247. doi: 10.1016/s0896-6273(02)01167-4. [DOI] [PubMed] [Google Scholar]
- Horner RH, Carr EG, Strain PS, Todd AW, Reed HK. Problem behavior interventions for young children with autism: A research synthesis. Journal of Autism and Developmental Disorders. 2002;32:423–446. doi: 10.1023/a:1020593922901. [DOI] [PubMed] [Google Scholar]
- Jantz PB, Bigler ED, Froehlich AL, Prigge MB, Cariello AN, Travers BG, Anderson J, Zielinski BA, Alexander AL, Lange N, Lainhart JE. Wide Range Achievement Test in autism spectrum disorder: Test-retest stability. Psychological Reports. 2015;116:674–684. doi: 10.2466/03.15.PR0.116k24w8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanne SM, Mazurek MO. Aggression in children and adolescents with ASD: Prevalence and risk factors. Journal of Autism and Developmental Disorders. 2011;41:926–937. doi: 10.1007/s10803-010-1118-4. [DOI] [PubMed] [Google Scholar]
- Kirsch P, Esslinger C, Qiang C, Mier D, Lis S, Siddhanti S, Gruppe H, Mattay VS, Gallhofer B, Meyer-Lindenberg A. Oxytocin modulates neural circuitry for social cognition and fear in humans. Journal of Neuroscience. 2005;25:11489–11493. doi: 10.1523/JNEUROSCI.3984-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinhans NM, Pauley G, Richards T, Neuhaus E, Martin N, Corrigan NM, Shaw DW, Estes A, Dager SR. Age-related abnormalities in white matter microstructure in autism spectrum disorders. Brain Research. 2012;1479:1–16. doi: 10.1016/j.brainres.2012.07.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolla NJ, Matthews B, Wilson AA, Houle S, Bagby RM, Links P, Simpson AI, Hussain A, Meyer JH. Lower monoamine oxidase-a total distribution volume in impulsive and violent male offenders with antisocial personality disorder and high psychopathic traits: An [(11)C] harmine positron emission tomography study. Neuropsychopharmacology. 2015;40:2596–2603. doi: 10.1038/npp.2015.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamm C, Granic I, Zelazo PD, Lewis MD. Magnitude and chronometry of neural mechanisms of emotion regulation in subtypes of aggressive children. Brain and Cognition. 2011;77:159–169. doi: 10.1016/j.bandc.2011.06.008. [DOI] [PubMed] [Google Scholar]
- Lecavalier L, Leone S, Wiltz J. The impact of behaviour problems on caregiver stress in young people with autism spectrum disorders. Journal of Intellectual Disability Research. 2006;50:172–183. doi: 10.1111/j.1365-2788.2005.00732.x. [DOI] [PubMed] [Google Scholar]
- LeDoux JE. Emotion circuits in the brain. Annual Review of Neuroscience. 2000;23:155–184. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- Lockhart R, Taylor J, Tibshirani R, Tibshirani R. Package ‘covTest’: R package version 1.02. 2013 http://CRAN.R-project.org/package=covTest.
- Lockhart R, Taylor J, Tibshirani RJ, Tibshirani R. A significance test for the lasso. The Annals of Statistics. 2014;42:413–468. doi: 10.1214/13-AOS1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord C, Risi S, Lambrecht L, Cook EH, Jr, Leventhal BL, DiLavore PC, Pickles A, Rutter M. The Autism Diagnostic Observation Schedule—Generic: A standard measure of social and communication deficits associated with the spectrum of autism. Journal of Autism and Developmental Disorders. 2000;30:205–223. [PubMed] [Google Scholar]
- Lord C, Rutter M. Autism diagnostic interview-revised: A revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. Journal of Autism and Developmental Disorders. 1994;24:659–685. doi: 10.1007/BF02172145. [DOI] [PubMed] [Google Scholar]
- Lozier LM, Cardinale EM, VanMeter JW, Marsh AA. Mediation of the relationship between callous-unemotional traits and proactive aggression by amygdala response to fear among children with conduct problems. JAMA Psychiatry. 2014;71:627–636. doi: 10.1001/jamapsychiatry.2013.4540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcus RN, Owen R, Kamen L, Manos G, McQuade RD, Carson WH, Aman MG. A placebo-controlled, fixed-dose study of aripiprazole in children and adolescents with irritability associated with autistic disorder. Journal of the American Academy of Child and Adolescent Psychiatry. 2009;48:1110–1119. doi: 10.1097/CHI.0b013e3181b76658. [DOI] [PubMed] [Google Scholar]
- Marshburn EC, Aman MG. Factor validity and norms for the Aberrant Behavior Checklist in a community sample of children with mental retardation. Journal of Autism and Developmental Disorders. 1992;22:357–373. doi: 10.1007/BF01048240. [DOI] [PubMed] [Google Scholar]
- Maskey M, Warnell F, Parr JR, Le Couteur A, McConachie H. Emotional and behavioural problems in children with autism spectrum disorder. Journal of Autism and Developmental Disorders. 2013;43:851–859. doi: 10.1007/s10803-012-1622-9. [DOI] [PubMed] [Google Scholar]
- Mazurek MO, Kanne SM, Wodka EL. Physical aggression in children and adolescents with autism spectrum disorders. Research in Autism Spectrum Disorders. 2013;7:455–465. [Google Scholar]
- McCracken JT, McGough J, Shah B, Cronin P, Hong D, Aman MG, Arnold LE, Lindsay R, Nash P, Hollway J. Risperidone in children with autism and serious behavioral problems. New England Journal of Medicine. 2002;347:314–321. doi: 10.1056/NEJMoa013171. [DOI] [PubMed] [Google Scholar]
- Mehler MF, Purpura DP. Autism, fever, epigenetics and the locus coeruleus. Brain Res Rev. 2009;59:388–392. doi: 10.1016/j.brainresrev.2008.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paus T. Mapping brain development and aggression. Canadian Child and Adolescent Psychiatry Review. 2005;14:10–15. [PMC free article] [PubMed] [Google Scholar]
- Prigge MB, Lange N, Bigler ED, Merkley TL, Neeley ES, Abildskov TJ, Froehlich AL, Nielsen JA, Cooperrider JR, Cariello AN, Ravichandran C, Alexander AL, Lainhart JE. Corpus callosum area in children and adults with autism. Research in Autism Spectrum Disorders. 2013;7:221–234. doi: 10.1016/j.rasd.2012.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Core Team. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2015. [Google Scholar]
- Research Units on Pediatric Psychopharmacology (RUPP) Autism Network. Randomized, controlled, crossover trial of methylphenidate in pervasive developmental disorders with hyperactivity. Archives of General Psychiatry. 2005;62:1266–1274. doi: 10.1001/archpsyc.62.11.1266. [DOI] [PubMed] [Google Scholar]
- Rylands AJ, Hinz R, Jones M, Holmes SE, Feldmann M, Brown G, McMahon AW, Talbot PS. Pre- and postsynaptic serotonergic differences in males with extreme levels of impulsive aggression without callous unemotional traits: a positron emission tomography study using (11)c-dasb and (11)c-mdl100907. Biological Psychiatry. 2012;72:1004–1011. doi: 10.1016/j.biopsych.2012.06.024. [DOI] [PubMed] [Google Scholar]
- Saxbe D, Del Piero LB, Immordino-Yang MH, Kaplan JT, Margolin G. Neural mediators of the intergenerational transmission of family aggression. Developmental Psychopathology. 2016;28:595–606. doi: 10.1017/S0954579415000528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaefer TL, Vorhees CV, Williams MT. Mouse plasmacytoma-expressed transcript 1 knock out induced 5-HT disruption results in a lack of cognitive deficits and an anxiety phenotype complicated by hypoactivity and defensiveness. Neuroscience. 2009;164:1431–1443. doi: 10.1016/j.neuroscience.2009.09.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel A, Victoroff J. Understanding human aggression: New insights from neuroscience. International Journal of Law and Psychiatry. 2009;32:209–215. doi: 10.1016/j.ijlp.2009.06.001. [DOI] [PubMed] [Google Scholar]
- South M, Wolf JM, Herlihy LE. Future dimensions: Neuroscience applications to practice in child and adolescent psychology. Professional Psychology: Research and Practice. 2012;43:560–567. [Google Scholar]
- Sterzer P, Stadler C. Neuroimaging of aggressive and violent behaviour in children and adolescents. Frontiers in Behavioral Neuroscience. 2009;3:35. doi: 10.3389/neuro.08.035.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tibshirani R. Regression shrinkage and selection via the lasso. Journal of the Royal Statistical Society Series B (Methodological) 1996:267–288. [Google Scholar]
- Travers BG, Bigler ED, Tromp DPM, Adluru N, Froehlich AL, Ennis C, Lange N, Nielsen JA, Prigge MBD, Alexander AL, Lainhart JE. Longitudinal processing speed impairments in males with autism and the effects of white matter microstructure. Neuropsychologia. 2014;53:137–145. doi: 10.1016/j.neuropsychologia.2013.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Travers BG, Tromp DPM, Adluru N, Lange N, Destiche D, Ennis C, Nielsen JA, Froehlich AL, Prigge MBD, Fletcher PT, Anderson JS, Zielinski BA, Bigler ED, Lainhart JE, Alexander AL. Atypical development of white matter microstructure of the corpus callosum in males with autism: A longitudinal investigation. Molecular Autism. 2015;6:15. doi: 10.1186/s13229-015-0001-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visser TAW, Ohan JL, Whittle S, Yücel M, Simmons JG, Allen NB. Sex differences in structural brain asymmetry predict overt aggression in early adolescents. Social Cognitive and Affective Neuroscience. 2014;9:553–560. doi: 10.1093/scan/nst013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. WISC-III Wechsler Intelligence Scale for Children–third edition: Manual. San Antonio, TX: The Psychological Corporation; 1991. [Google Scholar]
- Weidenheim KM. Neurobiology of autism: An update. Salud Mental. 2001;24:3–9. [Google Scholar]
- World Health Organization. The ICD-10 classification of mental and behavioural disorders: Clinical descriptions and diagnostic guidelines. Geneva: World Health Organization; 1992. [Google Scholar]
- Zielinski BA, Prigge MB, Nielsen JA, Froehlich AL, Abildskov TJ, Anderson JS, Fletcher PT, Zygmunt KM, Travers BG, Lange N, Alexander AL, Bigler ED, Lainhart JE. Longitudinal changes in cortical thickness in autism and typical development. Brain. 2014;137:1799–1812. doi: 10.1093/brain/awu083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou H, Hastie T. Regularization and variable selection via the elastic net. Journal of the Royal Statistical Society: Series B (Statistical Methodology) 2005;67:301–320. [Google Scholar]


