Abstract
Background & aims
High-risk features of colonic polyps are based on size, number, and pathologic characteristics. Surveillance colonoscopy is often recommended according to these findings. This study aimed to determine whether the molecular characteristics of polyps might provide information about the risk of metachronous advanced neoplasia.
Methodology
We retrospectively included 308 patients with colonic polyps. A total of 995 polyps were collected and tested for somatic BRAF and KRAS mutations. Patients were classified into 3 subgroups, based on the polyp mutational profile at baseline, as follows: non-mutated polyps (Wild-type), at least one BRAF-mutated polyp, or at least one KRAS-mutated polyp. At surveillance, advanced adenomas were defined as adenomas ≥ 10 mm and/or with high grade dysplasia or a villous component. In contrast, advanced serrated polyps were defined as serrated polyps ≥ 10 mm in any location, located proximal to the splenic flexure with any size or with dysplasia.
Results
At baseline, 289 patients could be classified as wild-type (62.3%), BRAF mutated (14.9%), or KRAS mutated (22.8%). In the univariate analysis, KRAS mutations were associated with the development of metachronous advanced polyps (OR: 2.36, 95% CI: 1.22–4.58; P = 0.011), and specifically, advanced adenomas (OR: 2.42, 95% CI: 1.13–5.21; P = 0.023). The multivariate analysis, adjusted for age and sex, also showed associations with the development of metachronous advanced polyps (OR: 2.27, 95% CI: 1.15–4.46) and advanced adenomas (OR: 2.23, 95% CI: 1.02–4.85).
Conclusions
Our results suggested that somatic KRAS mutations in polyps represent a potential molecular marker for the risk of developing advanced neoplasia.
Introduction
Colorectal cancer (CRC) is a heterogeneous group of diseases that can develop through distinct pathways involving different genetic and epigenetic changes [1]. Conventional adenoma is the principal precursor of CRC [2] through the classical adenoma-carcinoma pathway, which represents around 75% of these tumours [3]. On the other hand, the serrated pathway has emerged as the second most significant pathway; it represents the progression of serrated lesions to CRC [4], and it is responsible for up to 20–30% of all CRCs [1, 5]. Colonoscopy is considered the main method for detecting and removing precursor lesions, through screening and surveillance for CRC [6]. Surveillance colonoscopy is often recommended according to the characteristics of polyps, mainly the size and number, determined at a baseline colonoscopy [7]. Although the number of strategies for screening could increase, due to emerging technologies in molecular marker applications [8–9], to date, no molecular information has been useful in predicting whether new lesions will be detected at follow-up.
KRAS and BRAF belong to the intracellular RAS/RAF/MEK/mitogen-activated protein kinase (MAPK) cascade, which mediates cellular responses to growth signals. Activating KRAS mutations occur in 30–50% of CRCs [10]. These mutations occur during the early to advanced stages of the polyp-to-carcinoma sequence. On the other hand, BRAF is mutated very early in the serrated pathway, and approximately 10% of all CRCs carry an activating mutation in this oncogene [11].
The present study aimed to determine whether molecular characteristics of polyps, specifically somatic BRAF and KRAS mutations, might provide information about the risk of developing metachronous advanced neoplasia during follow-up for patients diagnosed with polyps.
Materials and methods
Patients and subgroup classification
We retrospectively recruited patients diagnosed with polyps in a colonoscopic examination between the years 2007 and 2009 at the Hospital General Universitario of Alicante. All the included patients had at least one surveillance colonoscopy performed more than 6 months after the baseline examination. Data on surveillance colonoscopies were collected until December 2014. Colonoscopy was performed either on the basis of symptoms or as a follow-up surveillance after a CRC or adenoma excision. In these patients the first surveillance colonoscopy performed during the period of the study has been considered as the baseline colonoscopy in terms of subsequent follow-up. Clinicopathological information and patient personal history were also collected. Patients were excluded when they were diagnosed of CRC at the inclusion in the study or they were previously diagnosed with polyposis syndrome, Lynch syndrome, or inflammatory bowel disease.
This study was approved by the Ethics Committee of the Hospital General Universitario of Alicante, and all clinical data of patients were anonymized.
Samples
A total of 995 polyps from 308 patients were collected for histological and molecular analysis. These polyps were obtained from both, the baseline and subsequent surveillance colonoscopies during the period of the study. All polyps were removed endoscopically. Endoscopy and the corresponding histopathology reports were reviewed to collect information about the number, size, morphology, distribution, and pathology of polyps.
The polyps were categorised as conventional adenomas and serrated lesions. Conventional adenomas were differentiated as tubular, tubulovillous, or villous adenomas, according to standard criteria [12]. Serrated lesions were classified as hyperplastic polyps (HP), sessile serrated adenomas (SSA), traditional serrated adenomas (TSA) [13]. HPs were also classified as microvesicular type, globet type and mucine-poor hiperplastic polyps. A review of all polyps was performed by two experienced pathologists in our group (C.E. and C.A.), to avoid inter-observer errors.
Polyps were considered to be located in the right colon when they were in the ascending colon, transverse colon, or caecum. They were considered to be located in the left colon when they were in the descending colon (including the splenic flexure), sigmoid colon, or rectum.
Polyps were classified according to high risk features. Advanced adenomas were defined as adenomas ≥ 10 mm and/or with high grade dysplasia or a villous component. Advanced serrated lesions were defined as serrated lesions ≥ 10 mm in any location, located proximal to the splenic flexure with any size or with dysplasia [14–16].
For each polyp, samples from paraffin-embedded tissue were microdissected in ten, 5-μm-thick sections. Sample DNA was extracted with the QIAamp DNA Investigator kit (QIAGEN, Hilden Germany) and with the E.Z.N.A Forensic DNA kit (OMEGA Bio-tek, USA), according to manufacturer´s protocols.
Somatic BRAF and KRAS analysis
All polyps were tested for somatic BRAF and KRAS mutations
BRAF mutations at codon 600 (V600E) were identified with real time PCR (ABI PRISM 7500, Applied Biosystems, Foster City, CA, USA), based on the allelic discrimination method (Applied Biosystems, Foster City, CA, USA). We used specific TaqMan probes, as previously described by Benlloch et al. [17].
KRAS mutations at exon 2, which included codons 12 and 13, were identified with DNA Sanger sequencing (ABI3500 Genetic Analyzer, Applied Biosystems), as previously described [18].
Patients were classified into 3 subgroups, based on the mutational profile of their polyps at a baseline colonoscopy, as follows: 1) wild-type group (WT); patients with polyps at a baseline colonoscopy with no mutation in either the BRAF or KRAS gene; 2) BRAF group: patients with at least one BRAF-mutated polyp; and 3) KRAS group: patients with at least one KRAS-mutated polyp at a baseline colonoscopy. Patients with both BRAF and KRAS somatic mutation found at their polyps were excluded.
Statistical analysis
Data analyses were carried out to determine statistical significance with SPSS software (SPSS 19.0, Chicago, IL, USA). Parametric continuous variables are reported as the mean ± standard deviation (SD); nonparametric continuous variables are reported as the median and interquartile range (IQR). On the other hand, categorical variables are reported as frequencies or percentages. Differences between samples were determined with the Student t test or ANOVA analyses for parametric quantitative data. Statistical differences between the groups were analyzed using a chi-squared method for categorical data followed by Yates correction or Fisher’s exact test, where appropriate.
We included univariate and multivariate logistic regression models to determine the association between the detection of advanced lesions at surveillance and the clinical and the molecular characteristics of lesions at baseline. The multivariate analysis was performed after adjusting for the sex and age of patients. Also variables found to be significant in the univariate analyses were included in the multivariate analysis. Results are expressed as the odds ratios (OR) with 95% confidence intervals (95% CI).
Kaplan-Meier survival curves were compared with the log-rank test. P-values less than 0.05 were considered significant.
Results
Pathological and molecular characteristics of polyps
Nine hundred ninety-five polyps from 308 patients were reviewed for pathology and evaluated with molecular analyses. A total of 661 polyps (66.4%) were categorised as conventional adenomas (tubular adenoma, n = 593; tubulovillous, n = 63; villous adenoma, n = 5); and 334 polyps (33.6%) were categorised as serrated lesions (HP, n = 281; SSA, n = 45; TSA, n = 8). A total of 263 conventional adenomas (39.8%) were considered advanced, and a total of 87 serrated lesions were classified as advanced serrated lesions (26.0%).
The characteristics of polyps, based on their mutational profiles, are shown in Table 1. A total of 665 polyps were WT (72.0%), 124 had BRAF mutations (13.4%) and 135 had KRAS mutations (14.6%). As expected, BRAF mutations were extremely rare in adenomas; they were found in only 0.8% of all adenomas, and in 39.4% of serrated lesions. On the other hand, KRAS mutations were found in 11.6% of adenomas and 20.9% of serrated lesions. BRAF and KRAS mutated polyps were more frequently found in the left colon than in the right colon (P<0.0001; left colon 16.1% BRAF mutations and 17.2% KRAS mutations; right colon: 8.1% BRAF mutations and 9.4% KRAS mutations). Among advanced lesions, we observed that no advanced adenoma carried a BRAF mutation, but 45% of advanced serrated lesions harboured this mutation. Moreover, KRAS mutations were observed more frequently in advanced adenomas (22.0%) than in non-advanced adenomas (4.8%; P<0.0001). Polyps were more frequently larger than 10 mm in lesions with KRAS mutations (21.8% ≥10 mm and 11.3% <10 mm; P<0.0001). In addition, KRAS mutated polyps more frequently exhibited high grade dysplasia (low grade dysplasia: 11.1%; high grade dysplasia: 45.7%; P<0.0001).
Table 1. Molecular characteristics of polyps.
| WILD-TYPE 665 polyps |
BRAF mutation 124 polyps |
KRAS mutation 135 polyps |
P-value | |
|---|---|---|---|---|
| HISTOLOGY, n (%) | ||||
| Adenoma | 545 (87.6%) | 5 (0.8%) | 72 (11.6%) | <0.001* |
| Tubular | 509 (91.1%) | 5 (0.9%) | 45 (8.1%) | |
| Tubulovillous | 32 (55.2%) | 0 | 26 (44.8%) | |
| Villous | 4 (80.0%) | 0 | 1 (20.0%) | |
| Serrated lesions | 120 (39.7%) | 119 (39.4%) | 63 (20.9%) | |
| Hyperplastic polyps | 105 (41.8%) | 99 (39.4%) | 47 (18.7%) | <0.001† |
| -Microvesicular Type | 101 (43.2%) | 92 (39.3%) | 17.5%) | |
| -Goblet Type | 2 (25.0%) | 1 (12.5%) | (62.5%) | |
| -Mucine-Poor Type | 2 (22.2%) | 6 (66.7%) | 1 (11.1%) | |
| SSA | 14 (32.6%) | 19 (44.2%) | 10 (23.3%) | |
| TSA | 1 (12.5%) | 1 (12.5%) | 6 (75.0%) | |
| LOCATION, n (%) | ||||
| Right | 245 (82.5%) | 24 (8.1%) | 28 (9.4%) | <0.001 |
| Left | 415 (66.7%) | 100 (16.1%) | 107 (17.2%) | |
| SIZE, n (%) | ||||
| <10 mm | 444 (72.8%) | 97 (15.9%) | 69 (11.3%) | <0.001 |
| ≥10 mm | 198 (70.7%) | 21 (7.5%) | 61 (21.8%) | |
| GRADE OF DYSPLASIA, n (%) | ||||
| High | 19 (54.3%) | 0 | 16 (45.7%) | <0.001 |
| Low | 540 (87.2%) | 10 (1.6%) | 69 (11.1%) | |
| MORPHOLOGY, n (%) | ||||
| Pedunculated | 125 (75.3%) | 8 (4.8%) | 33 (19.9%) | 0.001 |
| Non-Pedunculated | 327 (72.2%) | 68 (15.0%) | 58 (12.8%) | |
| ADVANCED ADENOMAS, n (%) | ||||
| Yes | 191 (78.0%) | 0 | 54 (22.0%) | <0.001 |
| No | 354 (93.9%) | 5 (1.3%) | 18 (4.8%) | |
| ADVANCED SERRATED LESIONS, n (%) | ||||
| Yes | 31(38.8%) | 36 (45.0%) | 13 (16.3%) | 0.4 |
| No | 89 (40.1%) | 83 (37.4%) | 50 (22.5%) |
Abbreviations: SSA, sessile serrated adenoma; TSA, traditional serrated adenoma
Statistically significant results are represented in bold.
* P-value is referred to comparison between adenomas and serrated lesions.
† P-value is referred to comparison between the different types of serrated lesions.
Clinical and molecular characteristics of patients and the risk of developing metachronous advanced lesions
We retrospectively analysed the relationship between the clinical and molecular characteristics of polyps and the risk of developing metachronous neoplasia in a cohort of 308 patients. The mean age at diagnosis was 61 years (SD, 11.95; range 26–86) and the proportion of men was 62.3%.
A total of 289 cases were classified according to the mutational profiles of their polyps at a baseline colonoscopy. Nineteen cases were excluded, because they had equal proportions of KRAS and BRAF mutations (10 cases) or their polyps were not evaluable for BRAF and KRAS mutations (9 cases). The mean follow-up time was 36.6 months (SD: 15.6), with a median of 36 months (IQR: 25–49). The mean number of polyps diagnosed at baseline was 2.73 (SD: 2.43), with a median of 2 (IQR: 1–3). A subset of the patients included in this study has been previously diagnosed of CRC (n = 44, 15.2%) and/or previous polyps (n = 75, 26%). For these patients the first surveillance colonoscopy performed during the period of the study has been considered as the baseline colonoscopy.
According to their mutational profiles at baseline, 180 (62.3%) patients did not have any mutation in these two markers (WT group), 43 patients (14.9%) displayed BRAF mutations (BRAF group), and 66 patients (22.8%) displayed KRAS mutations (KRAS group). Table 2 shows the baseline characteristics of patients, according to the mutational status of their polyps. We observed that patients with KRAS mutations were older, frequently had more than 3 adenomas, and their polyps were ≥10 mm.
Table 2. Clinical characteristics of patients, according the polyp mutational profile assessed at a baseline colonoscopy.
| WILD-TYPE GROUP | BRAF GROUP | KRAS GROUP | P-value | |
|---|---|---|---|---|
| n = 180 | n = 43 | n = 66 | ||
| AGE, mean(SD) | 60.84 ±12.02 | 56.72±12.73 | 65.44±9.78 | 0.001 |
| SEX, n(%) | ||||
| Male | 110 (61.1%) | 25 (13.9%) | 45 (25.0%) | 0.5 |
| Female | 70 (64.2%) | 18 (16.5%) | 21 (19.3%) | |
| MONTHS OF FOLLOW-UP, mean(SD) | 35.77±15.17 | 37.19±16.93 | 38.36±16.06 | 0.5 |
| PERSONAL HISTORY OF CRC, n(%) | ||||
| No (n = 245) | 152 (62.0%) | 35 (14.3%) | 58 (23.7%) | 0.6 |
| Yes (n = 44) | 28 (63.6%) | 8 (18.2%) | 8 (18.2%) | |
| PREVIOUS POLYPS, n(%) | ||||
| No (n = 214) | 131 (61.2%) | 31 (14.5%) | 52 (24.3%) | 0.6 |
| Yes (n = 75) | 49 (65.3%) | 12 (16.0%) | 14 (18.7%) | |
| POLYP NUMBER, n(%) | ||||
| <3 | 126 (70%) | 26 (14.4%) | 28 (15.6%) | <0.001 |
| ≥ 3 | 54 (49.5%) | 17 (15.6%) | 38 (34.9%) | |
| ADENOMAS, n(%) | ||||
| <3 | 162 (65.3%) | 39 (15.7%) | 47 (19.0%) | 0.001 |
| ≥ 3 | 18 (43.9%) | 4 (9.8%) | 19 (46.3%) | |
| SERRATED LESIONS, n(%) | ||||
| <3 | 177 (63.2%) | 40 (14.3%) | 63 (22.5%) | 0.1 |
| ≥ 3 | 3 (33.3%) | 3 (33.3%) | 3 (33.3%) | |
| ≥10 mm POLYPS, n(%) | ||||
| No | 95 (68.3%) | 28 (20.1%) | 16 (11.5%) | <0.001 |
| Yes | 85 (56.7%) | 15 (10.0%) | 50 (33.3%) | |
| POLYPS IN THE RIGHT COLON, n(%) | ||||
| No | 107 (64.1%) | 28 (16.8%) | 32 (19.2%) | 0.2 |
| Yes | 73 (59.8%) | 15 (12.3%) | 34 (27.9%) | |
| POLYPS IN THE LEFT COLON, n(%) | ||||
| No | 35 (72.9%) | 3 (6.3%) | 10 (20.8%) | 0.1 |
| Yes | 145 (60.2%) | 40 (16.6%) | 56 (23.2%) |
Abbreviations: CRC, colorectal cancer; SD, standard deviation.
Statistically significant results are represented in bold.
During surveillance, a total of 401 lesions and 1 CRC were found. Pathologically, 237 were conventional adenomas and 164 serrated lesions. We classified 53 as advanced adenomas and 43 as advanced serrated lesions. The mean number of polyps at follow-up colonoscopies was 2.20 (SD: 2.95) polyps, with a median of 1 (IQR: 0–3).
Among the 289 patients with lesions, we investigated the risk of developing metachronous lesions for the different molecular subtypes. A total of 179 patients (61.9%) developed polyps at surveillance, 36 patients developed advanced adenomas (12.5%) and 26 patients advanced serrated lesions (9.0%).
In the univariate analysis, only the presence of a KRAS mutation in the polyp at baseline was associated with developing metachronous advanced polyps of any type (OR: 2.36, 95% CI: 1.22–4.58; P = 0.011 vs. non-mutated), and more specifically, advanced adenomas (OR: 2.42, 95% CI: 1.13–5.21; P = 0.023 vs. non-mutated) (Table 3). None of the other baseline characteristics (age, sex, previous CRC, high grade dysplasia, or size larger than 10 mm) were related to the development of advanced lesions or advanced adenomas at follow-up (Table 3). The baseline characteristics related to the development of advanced serrated lesions were male sex, previous CRC, and large lesions (Table 3).
Table 3. Univariate logistic regression analysis of risk of developing advanced lesions at surveillance, according to molecular and clinical characteristics of patients at the baseline colonoscopy.
| BASELINE CHARACTERISTICS | Risk of developing the indicated lesion at follow-up surveillance | ||||||||
| ADVANCED ADENOMAS | ADVANCED SERRATED LESIONS | ANY ADVANCED POLYP | |||||||
| n (%) | OR | P-value | n (%) | OR | P-value | n (%) | OR | P-value | |
| (95% CI) | (95% CI) | (95% CI) | |||||||
| CLASSIFICATION (n) | |||||||||
| Wild Type (180) | 18(10.0%) | 1 | 15(8.3%) | 1 | 28(15.6%) | 1 | |||
| BRAF (43) | 4(9.3%) | 0.92 | 0.9 | 3(7.0%) | 0.83 | 0.8 | 7(16.3%) | 1.06 | 0.9 |
| (0.29–2.88) | (0.23–2.99) | (0.43–2.61) | |||||||
| KRAS (66) | 14(21.2%) | 2.42 | 0.023 | 8(12.1%) | 1.52 | 0.4 | 20(30.3%) | 2.36 | 0.011 |
| (1.13–5.21) | (0.61–3.77) | (1.22–4.58) | |||||||
| AGE (mean±SD) | 63.81±10.34 | 0.2 | 59.31±10.76 | 0.4 | 62.25±10.55 | 0.5 | |||
| SEX (n) | |||||||||
| Male (180) | 25(13.9%) | 1 | 0.3 | 21(11.7%) | 1 | 0.049 | 40(22.2%) | 1 | 0.1 |
| Female (109) | 11(10.1%) | 0.69 | 5(4.6%) | 0.36 | 15(13.8%) | 0.56 | |||
| (0.33–1.48) | (0.13–0.99) | (0.29–1.07) | |||||||
| PREVIOUS CRC | |||||||||
| No | 27(11.0%) | 1 | 0.1 | 18(7.3%) | 1 | 0.025 | 42(17.1%) | 1 | 0.1 |
| Yes | 9(20.5%) | 2.08 | 8(18.2%) | 2.80 | 13(29.5%) | 2.03 | |||
| (0.90–4.78) | (1.14–6.92) | (0.98–4.19) | |||||||
| ≥3 ADENOMAS | |||||||||
| No | 27(10.9%) | 1 | 0.1 | 23(9.3%) | 1 | 0.7 | 45(18.1%) | 1 | 0.3 |
| Yes | 9(22.0%) | 2.30 | 3(7.3%) | 0.77 | 10(24.4%) | 1.46 | |||
| (0.99–5.34) | (0.22–2.70) | (0.66–3.18) | |||||||
| ADENOMAS HGD | |||||||||
| No | 32(12.1%) | 1 | 0.5 | 24(9.1%) | 1 | 0.9 | 50(18.9%) | 1 | 0.8 |
| Yes | 4(16.7%) | 1.46 | 2(8.3%) | 0.91 | 5(20.8%) | 1.13 | |||
| (0.47–4.53) | (0.20–4.12) | (0.40–3.18) | |||||||
| BASELINE CHARACTERISTICS | Risk of developing the indicated lesion at follow-up surveillance | ||||||||
| ADVANCED ADENOMAS | ADVANCED SERRATED LESIONS | ANY ADVANCED POLYP | |||||||
| n (%) | OR | P-value | n (%) | OR | P-value | n (%) | OR | P-value | |
| (95% CI) | (95% CI) | (95% CI) | |||||||
| VILLOUS COMPONENT | |||||||||
| No | 29(11.7%) | 1 | 0.3 | 24(9.7%) | 1 | 0.3 | 46(18.5%) | 1 | 0.6 |
| Yes | 7(17.1%) | 1.56 | 2(4.9%) | 0.48 | 9(22.0%) | 1.24 | |||
| (0.63–3.83) | (0.11–2.11) | (0.55–2.77) | |||||||
| SIZE ≥10 mm ADENOMAS | |||||||||
| No | 17(10.4%) | 1 | 0.2 | 20(12.3%) | 1 | 0.033 | 33(20.2%) | 1 | 0.6 |
| Yes | 19(15.1%) | 1.53 | 6(4.8%) | 0.36 | 22(17.5%) | 0.83 | |||
| (0.76–3.07) | (0.14–0.92) | (0.46–1.52) | |||||||
| ADVANCED SERRATED LESIONS AT BASELINE | |||||||||
| No | 30(11.8%) | 1 | 0.4 | 23(9.1%) | 1 | 0.9 | 47(18.5%) | 1 | 0.5 |
| Yes | 6(17.1%) | 1.55 | 3(8.6%) | 0.94 | 8(22.9%) | 1.31 | |||
| (0.59–4.03) | (0.27–3.32) | (0.56–3.05) | |||||||
| LOCATION | |||||||||
| Right | |||||||||
| No | 17(10.2%) | 1 | 0.2 | 14(8.4%) | 1 | 0.7 | 29(17.4%) | 1 | 0.4 |
| Yes | 19(15.6%) | 1.63 | 12(9.8%) | 1.19 | 26(21.3%) | 1.29 | |||
| (0.81–3.28) | (0.53–2.68) | (0.71–2.33) | |||||||
| Left | |||||||||
| No | 6(12.5%) | 1 | 1.0 | 1(2.1%) | 1 | 0.1 | 7(14.6%) | 1 | 0.4 |
| Yes | 30(12.4%) | 0.99 | 25(10.4%) | 5.44 | 48(19.9%) | 1.46 | |||
| (0.39–2.54) | (0.72–41.15) | (0.62–3.45) | |||||||
Abbreviations: CI, confidence interval; CRC, Colorectal cancer; HGD, high grade dysplasia; OR, odds ratio; SD, standard deviation
Statistically significant results are represented in bold.
This association between advanced lesions of any type at follow-up and a KRAS mutation was also independently observed in the multivariate analysis, after adjusting for age and sex (OR: 2.27, 95% CI: 1.15–4.46). Moreover, KRAS mutations were specifically associated with the development of metachronous advanced adenomas (OR: 2.23, 95% CI: 1.02–4.85). None of the clinical characteristics that were significantly associated with the development of advanced serrated lesions in the univariate analysis were identified as independent predictors in the multivariate analysis (Table 4).
Table 4. Multivariate analysis of clinical and molecular characteristics of patients, adjusted for age and sex.
| OUTCOME | OR | 95% CI | P-value | |
|---|---|---|---|---|
| Factors included in the analysis | Min. | Max. | ||
| ADVANCED ADENOMAS | ||||
| Molecular Classification | ||||
| -Wild-type Group | 1 | |||
| -BRAF Group | 0.99 | 0.31 | 3.12 | 1.0 |
| -KRAS Group | 2.23 | 1.02 | 4.85 | 0.044 |
| ADVANCED SERRATED LESIONS | ||||
| No Previous CRC | 1 | |||
| Previous CRC | 2.17 | 0.85 | 5.53 | 0.1 |
| Adenomas Size <10 mm or no adenomas | 1 | |||
| Adenomas Size ≥10 mm | 0.40 | 0.15 | 1.05 | 0.1 |
| ANY ADVANCED POLYP | ||||
| Molecular Classification | ||||
| -Wild-type Group | 1 | |||
| -BRAF Group | 1.08 | 0.43 | 2.71 | 0.9 |
| -KRAS Group | 2.27 | 1.15 | 4.46 | 0.018 |
Abbreviations: CI: confidence interval; CRC: Colorectal cancer; OR: odds ratio.
Statistically significant results are represented in bold.
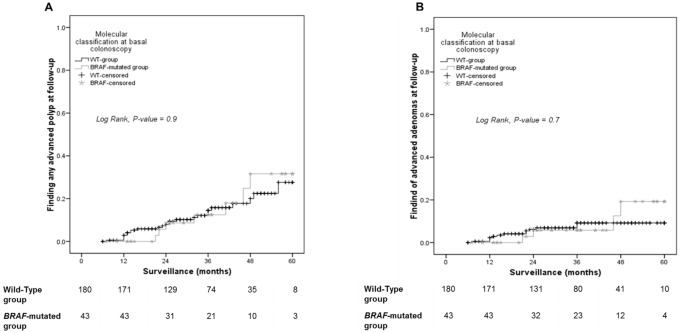
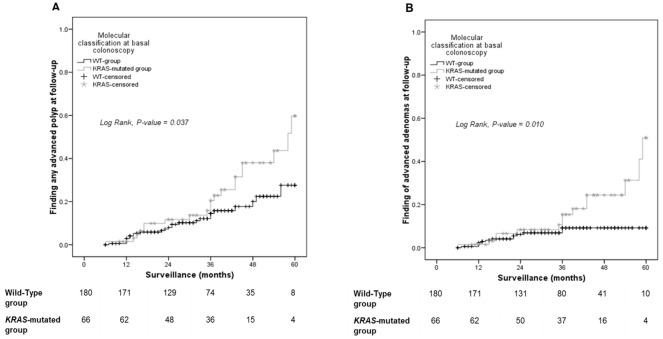
We performed Kaplan-Meier analyses to compare the risk of developing advanced polyps among patients with different baseline molecular characteristics. No differences were found between the BRAF and WT groups (log-rank for advanced polyps 0.9; log-rank for advanced adenomas 0.7) (Fig 1A and 1B). However, patients in the KRAS group developed advanced polyps (log-rank 0.037) and, more specifically, advanced adenomas (log-rank 0.010), at higher rates than patients in the WT group (Fig 2A and 2B).
Fig 1. Risk of developing advanced polyps based on BRAF mutational status at baseline colonoscopy.
Kaplan-Meier curves show the proportions of patients with WT or BRAF-mutated lesions that developed either (A) any advanced polyp or (B) advanced adenoma over time.
Fig 2. Risk of developing advanced polyps based on KRAS mutational status at baseline colonoscopy.
Kaplan-Meier curves show the proportions of patients with WT or KRAS-mutated lesions that developed either (A) any advanced polyp or (B) advanced adenomas over time.
Discussion
The main finding of this study was that patients with at least one polyp that harboured a KRAS mutation were at higher risk of developing advanced polyps, specifically, advanced adenomas, compared to patients with polyps that harboured BRAF mutations or no mutation. Moreover, the KRAS mutation was an independent predictor of the development of advanced polyps and advanced adenomas, and it was a stronger predictor than other characteristics, like the size or number of lesions at baseline. These results established the potential utility of molecular markers for stratifying risk among patients with colonic polyps. Our findings suggested that the KRAS somatic mutation would be a useful marker for predicting the development of metachronous advanced neoplasia.
In the first part of our study, we classified a series of 995 polyps into 3 groups, according to their molecular characteristics: WT, BRAF mutated, and KRAS mutated. This classification was consistent with previously proposed CRC classifications [19–20] that emphasised the molecular background characteristics of colonic neoplasms. In our study, we linked precursor CRC lesions to a molecular pathway with the aim of determining whether this molecular signature could predict the development of advanced lesions at follow-up. As expected and according with previous studies, BRAF mutations were rarely found in conventional adenomas [21–24]. However, we found BRAF mutations in less than 40% of serrated lesions, which was clearly less frequent than previously reported for this type of polyps [25–26]. Our population was selected, given that we only included patients with a follow-up surveillance colonoscopy. Thus, our results could not be directly compared to results found in the general population. However, the potential bias of our patient selection would be towards selecting individuals with more advanced lesions.
Previous studies aimed to correlate advanced histological features or size with somatic BRAF or KRAS mutations to predict the risk of potential malignancy of polyps. Those studies observed a strong association between KRAS mutations, villous component, high-grade dysplasia and polyp size [23, 27–29], which suggested that KRAS mutations might increase the risk of progression in sporadic colorectal adenomas [27, 30]. Moreover, other studies have reported a significant association between KRAS mutations and advanced adenomas [31]. Our results were consistent with those previous studies; however, we also observed, that the presence of KRAS mutations in polyps at baseline could be an independent risk factor for the development of metachronous advanced lesions. In a similar previous study, Nusko G et al. did not find that KRAS mutations were a reliable prognostic factor of metachronous neoplasia [32]. However the number of patients included in this study was very small and only one index adenoma of each patient at the first colonoscopy was analysed. In contrast, a higher number of patients were included in our study and moreover, all removed and available polyps from the baseline colonoscopy were analysed.
Surveillance colonoscopies are performed for polyps, due to the risk of developing advanced neoplasia. Recommendations for surveillance are based on different potential risk factors found in a baseline colonoscopy [33–34]. To date, the main known indicators of risk were polyp size, number, and a few pathological characteristics, such as the grade of dysplasia and the presence of a villous component [35–36]. In general, these recommendations are applicable to adenomas, but less evidence has supported follow-up recommendations for serrated lesions [37].
Molecular pathologic epidemiology is a relatively new field of epidemiology based on molecular classification of cancer that can help decipher interactions of environmental and lifestyle exposures with molecular pathology in cancer and premalignant tumors [38–39]. In the last few years, a classification system was developed for CRC molecular characteristics, based on BRAF, KRAS, and the CpG island methylator phenotype (CIMP) status, which could predict the prognosis and response to chemotherapy [19]. More recently, a comprehensive molecular classification of CRCs has also shown prognostic capability [40]. The present study was also designed along those lines, under the assumption that polyps, as precursor lesions for CRC, might also exhibit some early signatures of the pathway that could potentially lead to CRC. These pathway signatures could, at the same time, influence the risk of developing future lesions. Metachronous lesions that appear after polyp excision might develop under various conditions. On one hand, they might develop from missed or incompletely resected lesions, which might be related to the quality of the baseline colonoscopy. On the other hand, they may develop due to the biological characteristics of the lesions, which might promote rapid growth and progression to advanced states [41–42]. Both these possibilities could potentially explain the relationship between metachronous lesions and a carcinogenic pathway. For example, it is possible that KRAS mutated polyps might be more easily missed or incompletely resected than other types of polyps. Several reports have described the high risk of missing serrated lesions [43–46]; moreover, sessile serrated polyps were cited as a risk factor for incomplete endoscopic resection [47]. Although not all these lesions characteristically harboured KRAS mutations, a substantial proportion of KRAS mutated lesions were linked to the serrated pathway of carcinogenesis; thus, the difficulties in detecting and excising serrated polyps might, at least in part, apply to the association found here between KRAS mutations and the risk of developing advanced neoplasia. On the other hand, it is possible that KRAS mutated lesions might have a growth advantage. Moreover, it is also possible that a regional defect in the colon of patients that harboured KRAS mutated polyps might have exerted an effect that promoted the rapid development of these lesions after excision. Future studies should investigate all these potential explanations as well as potential relationships between molecular markers and lifestyle exposures in patients with colorectal polyps, following postulates of molecular pathological epidemiology [39].
Our study had several limitations. Importantly, it was a retrospective study, and our results must be confirmed with a prospective cohort, moreover it lacks a validation cohort that could confirm the results, avoiding potential selection bias. The study included only patients that received a second colonoscopy, and this population might not be completely representative of the general population. Another potential limitation was related to the definition of advanced serrated lesions. Currently, no standard definition has been established for advanced serrated lesions. This lack of definition hinders the formulation of a unified risk classification system for serrated polyps and adenomas. However, our findings provide information to support decisions about which polyps should be followed-up, due to the risk of developing advanced lesions and CRC, independent of polyp pathology. Very few studies have appropriately analysed risk factors for their ability to predict whether serrated polyps might develop into metachronous advanced lesions. Recommendations for the surveillance of these lesions varies among different guidelines. Some recent studies [15, 48] have shown that, among individuals with proximal, large serrated polyps, the risk of developing CRC is not lower than that of individuals with advanced adenomas. In this study, we adopted an arbitrary definition of advanced serrated polyps, based on previously recognised risk factors, including size, location, and the presence of dysplasia [15, 48–50]. Finally, we did not include analyses of the CIMP status or microsatellite instability (MSI) of polyps. CIMP, and particularly MSI, are late events in the serrated pathway of carcinogenesis; for the present study, we decided that, initially, we would study only early markers of the different carcinogenetic pathways. However, given our finding that BRAF would not be a useful marker for the risk of developing future advanced lesions, it is possible that adding CIMP status could improve our ability to characterise polyps in terms of risk.
In summary, this retrospective study was the first to find that KRAS mutations could play a potential role as a molecular marker for the risk of developing an advanced neoplasia during follow-up. These results should be confirmed in a prospective analysis, including a validation cohort. However, our findings could pave the way for going beyond size and number of lesions as main indicators for follow-up surveillance colonoscopies.
Data Availability
All relevant data are within the paper.
Funding Statement
This work was supported by the Instituto de Salud Carlos III (PI08/0726, INT-09/208, PI11/2630, INT-12-078, INT13-196, PI14/1386), FISABIO-ISABIAL foundation (UGP-13-221, UGP14-265), the Asociación Española contra el Cáncer (Fundación Científica GCB13131592CAST) and AIGPA, a private association that promotes research in gastrointestinal diseases in Alicante and also supported logistic aspects of the study, (this association declares no conflict of interest). Carla Guarinos and Mar Giner Calabuig received a predoctoral grant from Conselleria d’Educació de la Generalitat Valenciana (VALi+d. EXP ACIF/2010/018, ACIF/2016/002) and Eva Hernández-Illán received a grant from Instituto de Salud Carlos III (FI12/00233). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Jass JR. Classification of colorectal cancer based on correlation of clinical, morphological and molecular features. Histopathology. 2007; 50: 113–30. doi: 10.1111/j.1365-2559.2006.02549.x [DOI] [PubMed] [Google Scholar]
- 2.Fearon ER, Vogelstein B. A genetic model for colorectal tumorigenesis. Cell. 1990; 61: 759–67. [DOI] [PubMed] [Google Scholar]
- 3.Grady WM, Carethers JM. Genomic and epigenetic instability in colorectal cancer pathogenesis. Gastroenterology. 2008; 135: 1079–99. doi: 10.1053/j.gastro.2008.07.076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jass JR. Serrated adenoma and colorectal cancer. J Pathol. 1999; 187: 499–502. doi: 10.1002/(SICI)1096-9896(199904)187:5<499::AID-PATH309>3.0.CO;2-B [DOI] [PubMed] [Google Scholar]
- 5.Snover DC. Update on the serrated pathway to colorectal carcinoma. Hum Pathol. 2011; 42: 1–10. doi: 10.1016/j.humpath.2010.06.002 [DOI] [PubMed] [Google Scholar]
- 6.Nishihara R, Wu K, Lochhead P, Morikawa T, Liao X, Qian ZR, et al. Long-term colorectal-cancer incidence and mortality after lower endoscopy. N Engl J Med. 2013; 369: 1095–105. doi: 10.1056/NEJMoa1301969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bond JH. Colon polyps and cancer. Endoscopy. 2003; 35: 27–35. doi: 10.1055/s-2003-36410 [DOI] [PubMed] [Google Scholar]
- 8.Imperiale TF, Ransohoff DF. Understanding differences in the guidelines for colorectal cancer screening. Gastroenterology. 2010; 138: 1642–7 e1. doi: 10.1053/j.gastro.2010.03.027 [DOI] [PubMed] [Google Scholar]
- 9.Carethers JM. Biomarker-directed Targeted Therapy in Colorectal Cancer. J Dig Cancer Rep. 2015; 3: 5–10. [PMC free article] [PubMed] [Google Scholar]
- 10.Kressner U, Bjorheim J, Westring S, Wahlberg SS, Pahlman L, Glimelius B, et al. Ki-ras mutations and prognosis in colorectal cancer. Eur J Cancer. 1998; 34: 518–21. [DOI] [PubMed] [Google Scholar]
- 11.Davies H, Bignell GR, Cox C, Stephens P, Edkins S, Clegg S, et al. Mutations of the BRAF gene in human cancer. Nature. 2002; 417: 949–54. doi: 10.1038/nature00766 [DOI] [PubMed] [Google Scholar]
- 12.Hamilton SR, Bosman FT, Boffetta P, Ilyas M, Morreau H, Nakamura SI. Carcinoma of the colon and rectum In: Bosman FT, Carneiro F, Hruban RH, Theise ND, editors. WHO Classification of Tumours of the digestive system. Lyon: International Agency for Research on Cancer (IARC); 2010. pp. 134–46. [Google Scholar]
- 13.Snover D, Ahnen DJ, Burt RW, Odze RD. Serrated polyps of the colon and rectum and serrated polyposis In: Bosman FT, Carneiro F, Hruban RH, Theise ND, editors. WHO Classification of Tumours of the Digestive System. 4 ed Lyon, France: IARC Press; 2010. pp. 160–5. [Google Scholar]
- 14.Burt RW, Barthel JS, Dunn KB, David DS, Drelichman E, Ford JM, et al. NCCN clinical practice guidelines in oncology. Colorectal cancer screening. J Natl Compr Canc Netw. 2010; 8: 8–61. [DOI] [PubMed] [Google Scholar]
- 15.Erichsen R, Baron JA, Hamilton-Dutoit SJ, Snover DC, Torlakovic EE, Pedersen L, et al. Increased Risk of Colorectal Cancer Development Among Patients With Serrated Polyps. Gastroenterology. 2016; 150: 895–902 e5. doi: 10.1053/j.gastro.2015.11.046 [DOI] [PubMed] [Google Scholar]
- 16.Rex DK, Ahnen DJ, Baron JA, Batts KP, Burke CA, Burt RW, et al. Serrated lesions of the colorectum: review and recommendations from an expert panel. Am J Gastroenterol. 2012; 107: 1315–29; quiz 4, 30. doi: 10.1038/ajg.2012.161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Benlloch S, Paya A, Alenda C, Bessa X, Andreu M, Jover R, et al. Detection of BRAF V600E mutation in colorectal cancer: comparison of automatic sequencing and real-time chemistry methodology. J Mol Diagn. 2006; 8: 540–3. doi: 10.2353/jmoldx.2006.060070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guarinos C, Sanchez-Fortun C, Rodriguez-Soler M, Perez-Carbonell L, Egoavil C, Juarez M, et al. Clinical subtypes and molecular characteristics of serrated polyposis syndrome. Clin Gastroenterol Hepatol. 2013; 11: 705–11; quiz e46. doi: 10.1016/j.cgh.2012.12.045 [DOI] [PubMed] [Google Scholar]
- 19.Phipps AI, Limburg PJ, Baron JA, Burnett-Hartman AN, Weisenberger DJ, Laird PW, et al. Association between molecular subtypes of colorectal cancer and patient survival. Gastroenterology. 2015; 148: 77–87 e2. doi: 10.1053/j.gastro.2014.09.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sinicrope FA, Shi Q, Smyrk TC, Thibodeau SN, Dienstmann R, Guinney J, et al. Molecular markers identify subtypes of stage III colon cancer associated with patient outcomes. Gastroenterology. 2015; 148: 88–99. doi: 10.1053/j.gastro.2014.09.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yuen ST, Davies H, Chan TL, Ho JW, Bignell GR, Cox C, et al. Similarity of the phenotypic patterns associated with BRAF and KRAS mutations in colorectal neoplasia. Cancer Research. 2002; 62: 6451–5. [PubMed] [Google Scholar]
- 22.Spring KJ, Zhao ZZ, Karamatic R, Walsh MD, Whitehall VL, Pike T, et al. High prevalence of sessile serrated adenomas with BRAF mutations: a prospective study of patients undergoing colonoscopy. Gastroenterology. 2006; 131: 1400–7. doi: 10.1053/j.gastro.2006.08.038 [DOI] [PubMed] [Google Scholar]
- 23.Jass JR, Baker K, Zlobec I, Higuchi T, Barker M, Buchanan D, et al. Advanced colorectal polyps with the molecular and morphological features of serrated polyps and adenomas: concept of a 'fusion' pathway to colorectal cancer. Histopathology. 2006; 49: 121–31. doi: 10.1111/j.1365-2559.2006.02466.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lorentzen JA, Grzyb K, De Angelis PM, Hoff G, Eide TJ, Andresen PA. Oncogene Mutations in Colorectal Polyps Identified in the Norwegian Colorectal Cancer Prevention (NORCCAP) Screening Study. Clinical Medicine Insights Pathology. 2016; 9: 19–28. doi: 10.4137/CPath.s40143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kambara T, Simms LA, Whitehall VL, Spring KJ, Wynter CV, Walsh MD, et al. BRAF mutation is associated with DNA methylation in serrated polyps and cancers of the colorectum. Gut. 2004; 53: 1137–44. doi: 10.1136/gut.2003.037671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yang HM, Mitchell JM, Sepulveda JL, Sepulveda AR. Molecular and histologic considerations in the assessment of serrated polyps. Arch Pathol Lab Med. 2015; 139: 730–41. doi: 10.5858/arpa.2014-0424-RA [DOI] [PubMed] [Google Scholar]
- 27.Chen H, Lefferts JA, Schwab MC, Suriawinata AA, Tsongalis GJ. Correlation of polypoid colorectal adenocarcinoma with pre-existing adenomatous polyps and KRAS mutation. Cancer Genet. 2011; 204: 245–51. doi: 10.1016/j.cancergen.2011.04.002 [DOI] [PubMed] [Google Scholar]
- 28.Ishii T, Notohara K, Umapathy A, Mallitt KA, Chikuba H, Moritani Y, et al. Tubular adenomas with minor villous changes show molecular features characteristic of tubulovillous adenomas. Am J Surg Pathol. 2011; 35: 212–20. doi: 10.1097/PAS.0b013e318205df20 [DOI] [PubMed] [Google Scholar]
- 29.Maltzman T, Knoll K, Martinez ME, Byers T, Stevens BR, Marshall JR, et al. Ki-ras proto-oncogene mutations in sporadic colorectal adenomas: relationship to histologic and clinical characteristics. Gastroenterology. 2001; 121: 302–9. [DOI] [PubMed] [Google Scholar]
- 30.Yadamsuren EA, Nagy S, Pajor L, Lacza A, Bogner B. Characteristics of advanced- and non advanced sporadic polypoid colorectal adenomas: correlation to KRAS mutations. Pathol Oncol Res. 2012; 18: 1077–84. doi: 10.1007/s12253-012-9547-3 [DOI] [PubMed] [Google Scholar]
- 31.Yamane LS, Scapulatempo-Neto C, Alvarenga L, Oliveira CZ, Berardinelli GN, Almodova E, et al. KRAS and BRAF mutations and MSI status in precursor lesions of colorectal cancer detected by colonoscopy. Oncol Rep. 2014; 32: 1419–26. doi: 10.3892/or.2014.3338 [DOI] [PubMed] [Google Scholar]
- 32.Nusko G, Sachse R, Mansmann U, Wittekind C, Hahn E. K-RAS-2 gene mutations as predictors of metachronous colorectal adenomas. Scandinavian journal of gastroenterology. 1997; 32: 1035–41. [DOI] [PubMed] [Google Scholar]
- 33.Lieberman DA, Rex DK, Winawer SJ, Giardiello FM, Johnson DA, Levin TR. Guidelines for colonoscopy surveillance after screening and polypectomy: a consensus update by the US Multi-Society Task Force on Colorectal Cancer. Gastroenterology. 2012; 143: 844–57. doi: 10.1053/j.gastro.2012.06.001 [DOI] [PubMed] [Google Scholar]
- 34.Hassan C, Quintero E, Dumonceau J-M, Regula J, Brandão C, Chaussade S, et al. Post-polypectomy colonoscopy surveillance: European Society of Gastrointestinal Endoscopy (ESGE) guideline. Endoscopy. 2013; 45: 842–64. doi: 10.1055/s-0033-1344548 [DOI] [PubMed] [Google Scholar]
- 35.Saini SD, Kim HM, Schoenfeld P. Incidence of advanced adenomas at surveillance colonoscopy in patients with a personal history of colon adenomas: a meta-analysis and systematic review. Gastrointest Endosc. 2006; 64: 614–26. doi: 10.1016/j.gie.2006.06.057 [DOI] [PubMed] [Google Scholar]
- 36.Martinez ME, Thompson P, Messer K, Ashbeck EL, Lieberman DA, Baron JA, et al. One-year risk for advanced colorectal neoplasia: U.S. versus U.K. risk-stratification guidelines. Ann Intern Med. 2012; 157: 856–64. doi: 10.7326/0003-4819-157-12-201212180-00005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Janjua HG, Hogdall E, Linnemann D. Hyperplastic polyps of the colon and rectum—reclassification, BRAF and KRAS status in index polyps and subsequent colorectal carcinoma. APMIS. 2015; 123: 298–304. doi: 10.1111/apm.12355 [DOI] [PubMed] [Google Scholar]
- 38.Ogino S, Chan AT, Fuchs CS, Giovannucci E. Molecular pathological epidemiology of colorectal neoplasia: an emerging transdisciplinary and interdisciplinary field. Gut. 2011; 60: 397–411. doi: 10.1136/gut.2010.217182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ogino S, Nishihara R, VanderWeele TJ, Wang M, Nishi A, Lochhead P, et al. Review Article: The Role of Molecular Pathological Epidemiology in the Study of Neoplastic and Non-neoplastic Diseases in the Era of Precision Medicine. Epidemiology. 2016; 27: 602–11. doi: 10.1097/EDE.0000000000000471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Guinney J, Dienstmann R, Wang X, de Reynies A, Schlicker A, Soneson C, et al. The consensus molecular subtypes of colorectal cancer. Nat Med. 2015; 21: 1350–6. doi: 10.1038/nm.3967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Robertson DJ, Lieberman DA, Winawer SJ, Ahnen DJ, Baron JA, Schatzkin A, et al. Colorectal cancers soon after colonoscopy: a pooled multicohort analysis. Gut. 2014; 63: 949–56. doi: 10.1136/gutjnl-2012-303796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.le Clercq CM, Bouwens MW, Rondagh EJ, Bakker CM, Keulen ET, de Ridder RJ, et al. Postcolonoscopy colorectal cancers are preventable: a population-based study. Gut. 2014; 63: 957–63. doi: 10.1136/gutjnl-2013-304880 [DOI] [PubMed] [Google Scholar]
- 43.Rosty C, Hewett DG, Brown IS, Leggett BA, Whitehall VL. Serrated polyps of the large intestine: current understanding of diagnosis, pathogenesis, and clinical management. J Gastroenterol. 2013; 48: 287–302. doi: 10.1007/s00535-012-0720-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Oono Y, Fu K, Nakamura H, Iriguchi Y, Yamamura A, Tomino Y, et al. Progression of a sessile serrated adenoma to an early invasive cancer within 8 months. Dig Dis Sci. 2009; 54: 906–9. doi: 10.1007/s10620-008-0407-7 [DOI] [PubMed] [Google Scholar]
- 45.Anderson JC. Pathogenesis and management of serrated polyps: current status and future directions. Gut Liver. 2014; 8: 582–9. doi: 10.5009/gnl14248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.le Clercq CM, Winkens B, Bakker CM, Keulen ET, Beets GL, Masclee AA, et al. Metachronous colorectal cancers result from missed lesions and non-compliance with surveillance. Gastrointest Endosc. 2015; 82: 325–33 e2. doi: 10.1016/j.gie.2014.12.052 [DOI] [PubMed] [Google Scholar]
- 47.Pohl H, Srivastava A, Bensen SP, Anderson P, Rothstein RI, Gordon SR, et al. Incomplete polyp resection during colonoscopy-results of the complete adenoma resection (CARE) study. Gastroenterology. 2013; 144: 74–80 e1. doi: 10.1053/j.gastro.2012.09.043 [DOI] [PubMed] [Google Scholar]
- 48.Holme O, Bretthauer M, Eide TJ, Loberg EM, Grzyb K, Loberg M, et al. Long-term risk of colorectal cancer in individuals with serrated polyps. Gut. 2015; 64: 929–36. doi: 10.1136/gutjnl-2014-307793 [DOI] [PubMed] [Google Scholar]
- 49.Bouwens MW, Riedl RG, Bosman FT, Driessen A, Sanduleanu S. Large proximal serrated polyps: natural history and colorectal cancer risk in a retrospective series. J Clin Gastroenterol. 2013; 47: 734–5. doi: 10.1097/MCG.0b013e318293a656 [DOI] [PubMed] [Google Scholar]
- 50.Schreiner MA, Weiss DG, Lieberman DA. Proximal and large hyperplastic and nondysplastic serrated polyps detected by colonoscopy are associated with neoplasia. Gastroenterology. 2010; 139: 1497–502. doi: 10.1053/j.gastro.2010.06.074 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper.