Abstract
Currently used factors predicting disease recurrence in stage II colorectal cancer patients are not optimal for risk stratification. Thus, new biomarkers are needed. In this study the applicability of ezrin protein expression together with MSI status and BRAF mutation status were tested in predicting disease outcome in stage II colorectal cancer. The study population consisted of 173 stage II colorectal cancer patients. Paraffin-embedded cancer tissue material from surgical specimens was used to construct tissue microarrays (TMAs) with next-generation technique. The TMA-slides were subjected to following immunohistochemical stainings: MLH1, MSH2, MSH6, PMS2, ezrin and anti-BRAF V600E antibody. The staining results were correlated with clinicopathological variables and survival. In categorical analysis, high ezrin protein expression correlated with poor disease-specific survival (p = 0.038). In univariate analysis patients having microsatellite instabile / low ezrin expression tumors had a significantly longer disease-specific survival than patients having microsatellite stable / high ezrin expression tumors (p = 0.007). In multivariate survival analysis, the presence of BRAF mutation was associated to poor overall survival (p = 0.028, HR 3.29, 95% CI1.14–9.54). High ezrin protein expression in patients with microsatellite stable tumors was linked to poor disease-specific survival (p = 0.01, HR 5.68, 95% CI 1.53–21.12). Ezrin protein expression is a promising biomarker in estimating the outcome of stage II colorectal cancer patients. When combined with microsatellite status its ability in predicting disease outcome is further improved.
Introduction
Five-year survival in stage II colorectal cancer (CRC) is 70–80% [1,2]. Unfavorable prognostic factors for stage II CRC include lymphovascular invasion, less than 12 examined lymph nodes, poor differentiation grade, tumor spreading to the peritoneum or adjacent tissue structures as well as tumor obstruction or perforation. [3,4]. These risk factors have been utilized in the assessment of stage II colorectal cancer patients in need of postoperative adjuvant treatments. The benefit of chemotherapy in stage III colorectal cancer patients is apparent, but controversial in stage II colorectal cancer patients even with above-mentioned risk factors [5]. Consequently, there is a crucial need to discover new markers to better define those at highest danger of disease recurrence.
DNA mismatch repair competence is a feature associated with CRC outcome. Inactivation of genes responsible for mismatch repair competence cause microsatellite instability (MSI), which can be studied by expression of the gene products MLH1, MSH2, MSH6 and PMS2 or by PCR-based methods [6,7,8,9,10,11]. MSI is reported in about 15–20% of CRC [8,12]. MSI is linked to right-sided, poorly differentiated tumors with higher T stage and younger patient age [12]. Stage II CRCs with MSI, as demonstrated by immunohistochemistry of mismatch repair proteins, have a more favorable prognosis as compared to microsatellite stable (MSS) tumors [13,14,4]. Moreover, patients with defective mismatch repair (dMMR) stage II tumors do not seem to benefit from fluorouracil-based adjuvant chemotherapy [15,16]. MSI tumor can evolve in Lynch syndrome patients carrying a germ-line mutation in one MMR gene, or through sporadic events involving epigenetic silencing of the MLH1 gene [17].
BRAF gene encodes a protein kinase of the RAS/RAF/MEK-ERK signaling cascade, which is regulated by KRAS [18,19]. Previously, BRAF V600E mutation was shown to be an adverse prognostic factor for overall survival in stage II-III colon cancer [20]. MSS together with BRAF mutation is associated with poor prognosis in CRC [9,21]. On the contrary, MSI stage II tumors with BRAF V600E mutation are associated with a rather favorable prognosis [21]. Moreover, colorectal cancer with MSI phenotype and a concomitant BRAF mutation indicates a sporadic tumor, thus excluding Lynch syndrome [17,21].
Ezrin is a cytoskeleton-associated protein, which participates in cellular signaling, cell survival, proliferation and migration. Its association with malignant behavior has been suggested in several experimental models, and in several cancers strong ezrin expression correlates with inferior outcome. [22,23,24,25,26,27,28]. Our previous work has demonstrated the impact of ezrin expression on the outcome in metastatic CRC as well as in localized rectal cancer [27,28]. To our knowledge, the role of ezrin as a prognostic marker in stage II colorectal cancer has not been studied before.
In this work, we utilized tumor tissue collection form consecutive stage II CRC patients, together with extensive clinical, disease outcome and follow-up data to search for tissue-based prognostic markers. We report the association of MSI status, BRAF mutation status and ezrin protein expression with clinicopathological variables and patient outcome. Our results suggest that combined MSI and ezrin analysis can stratify tumors according to their clinical behavior.
Patients and methods
Study population
We collected archived paraffin-embedded tumor material from consecutive stage II CRC patients operated in Turku University Hospital in 2005–2012. This study was approved by Chief Executive Officer of TYKS-SAPA, Hospital District of Southwest Finland (T52/2014). The use of tissue material was approved by Scientific Steering Group of Auria Biobank (AB15-8108, 25.5.2012). The study was conducted in accordance with the Declaration of Helsinki. The clinical data were retrieved and histological samples collected and analyzed with the endorsement of the National Authority for Medico-Legal Affairs (VALVIRA). The patient records were accessed anonymously.
In 2005–2012 a total of 232 stage II CRC patients were radically operated in our hospital. Computed tomography (CT) of the abdomen and chest x-ray or CT had been performed preoperatively to rule out distant metastases. We carefully checked the patient files, including surgery and pathology reports and excluded patients with verified lymph node or distant metastases, those who had been operated with palliative-intent surgery, and also patients with other than adenocarcinoma histology (e.g. neuroendocrine tumors). Only patients with stage II CRC were included in the current study. For tumor staging, TNM7 classification of malignant tumors [29] was used. From the original cohort (n = 232), tumor material for MSI staining was available from 214 patients. For further BRAF and ezrin stainings, material was available from 173 patients. These patients (n = 173) were included in statistical analyses.
TMA construction
Tissue microarrays (TMA) were constructed and analyzed using the next-generation TMA technique [30]. Shortly, the appropriate formalin-fixed paraffin-embedded (FFPE) tissue specimens were chosen based on clinical data and retrieved from the pathology archives. A representative hematoxylin-eosin (H&E) section containing areas of invasive carcinoma was selected from each tumor. New H&E slides were produced, scanned (Pannoramic P250, 3DHistech) and uploaded into the university digital microscopy web portal (casecenter.utu.fi). Each slide was viewed using Pannoramic Viewer software (3DHistech). Using the 1.2 mm diameter annotation tool, annotations of different colors corresponding to various histological areas were placed onto each digital slide. Two annotations were placed in the center of the tumor, two in the tumor front and two in the normal colonic epithelium. The corresponding tissue cores were then transferred into the TMA blocks using an automated TMA instrument (TMA Grandmaster, 3DHistech) by overlaying each annotated digital slide with the corresponding tissue specimen. One tissue core containing benign tissue was selected from each tumor to act as a control. The constructed TMA blocks were sectioned, stained, scanned and uploaded into the web portal (casecenter.utu.fi) and each individual spot was scored by two pathologists (KS, JS). The resulting scores were combined with the clinical data for statistical analysis.
Immunohistochemistry
Immunohistochemical staining against MMR proteins is a useful screening method in research materials with paraffin-embedded TMA-samples. In contrast to PCR-based methods, it also readily provides information on the inactivated gene. Immunohistochemical stainings (IHC) were performed using standard procedures. Shortly, 3,5 μm sections were cut from the TMA blocks. They were stained with monoclonal antibodies against MLH1 (Clone G168-15BD Pharmingen, dilution: 1:5), MSH2 (Clone G219-1129, BD Pharmingen, dilution: 1:200) and MSH6 (Clone EP49, Epitomoc, dilution: 1:200). The signal was detected with UltraView Universal DAB Detection kit. For PMS2, Clone EPR3947 (Ventana/Roche, ready to use antibody) was used and the signal was detected with OptiView Universal DAB Detection Kit and amplification kit. To detect BRAF V600E mutation, BRAF RTU antibody (Clone VE1, Roche/Ventana) was used and the signal was detected with OptiView Universal DAB Detection kit. For ezrin staining, immunoglobulin G antibody to human ezrin (clone 3C12) [31] was used. All the stainings were performed with BenchMark XT (Ventana/Roche) using ultraVIEW Universal DAB Detection Kit (Ventana/Roche), except ezrin, which was done with LabVision immunoautomate (Thermo Fisher Scientific) using the Power Vision Plus poly HRP anti-mouse/rabbit/rat IgG detection kit.
Evaluation of immunohistochemical stainings
All IHC stainings were separately evaluated by two observers (KS and JS), blinded to clinical data. For MLH1, MSH2, MSH6, PMS2 and ezrin, inflammatory cells of the stroma were used as positive controls. For analyses of MSI (MLH1, MSH2, MSH6 and PMS2) also the cores from normal colonic epithelium were used as positive controls. As a positive control in evaluating the BRAF-stainings, we used BRAF V600E mutation-positive cancer tissue obtained from a CRC patient who did not belong to this study cohort. These IHC stainings were evaluated dichotomously as positive or negative. For ezrin protein expression, cytoplasmic staining was recorded [27, 28].Four staining categories were used: 0 for negative staining, 1 for weak staining (distinguishable from the background staining), 2 for moderate staining and 3 for strong staining (corresponding to immunoreactivity in lymphocytes) [27]. In addition, a category of non-evaluable was used for all stainings. For statistical purposes a dichotomous grading, ezrin low (negative or weak staining) and ezrin high (moderate or strong staining) was used.
Statistical analysis
Statistical analyses were performed with IBM SPSS version 23 with standard packages. Clinical data were analyzed in correlation with histological, immunohistochemical and mutational analysis data using χ2 or Fisher’s exact-test for discrete variables and one-way ANOVA for continuous variables. Overall survival (OS), disease free survival (DFS) and disease-specific survival (DSS) were calculated using Kaplan-Meier curves. Survival was analyzed with respect to (stratified to) different biomarkers using log-rank test. For multivariate analyses, the following variables were used: tumor grade, tumor-side, obstruction, perforation, vascular invasion, BRAF mutated/wild type, ezrin low/high and MSS/MSI combinations were included. Multivariate Cox proportional hazard regression model was used to adjust the survival curves for covariates and to obtain estimates on hazard ratios. All p-values were two-sided, and values less than 0.05 were considered statistically significant.
Results
General aspects of clinical patient characteristics
Altogether 173 patients were included in this study. The tumor was located in the proximal colon in 70 (40%), transverse colon in 19 (11%), descending colon in 8 (5%), sigmoid colon in 44 (25%) and rectum/rectosigmoideum in 32 (19%) patients. There were 30 (17%) T4-tumors included in the study. Vascular invasion was reported in 32 (18%) patients and preoperative bowel obstruction in 26 (15%) of patients. Adjuvant fluorouracil-based chemotherapy had been given to 51 (30%) patients. The median follow-up time was 57 months. At the latest follow-up data collection time point in September 2016, 116 patients (67%) were alive without CRC, 3 (2%) alive with CRC, 17 dead of CRC, 18 (10%) dead of other cancers and 19 (11%) dead of other causes than cancer. The clinical characteristics of the patients are shown in Table 1.
Table 1. The clinicopathological variables of the patient population included in the MSI, BRAF and Ezrin analyses (n = 173).
NA = not available, R0 = microscopically radical surgery, R1 macroscopically radical surgery, R2 macroscopically non-radical surgery.
| Variable | n (%) |
|---|---|
| Gender | |
| Female | 92 (53) |
| Male | 81 (47) |
| Age | |
| <70 years | 66 (38) |
| >70 years | 107 (62) |
| Postoperative stage | |
| T3N0 | 143 (83) |
| T4aN0 | 17 (10) |
| T4bN0 | 13 (7) |
| Tumor side | |
| Right | 89(51) |
| Left | 84 (48) |
| Tumor grade (analyzed from surgical specimens) | |
| G1 | 19 (11) |
| G2 | 114 (66)) |
| G3 | 40 (23) |
| Histology | |
| Conventional adenocarcinoma | 151 (87) |
| Mucinous adenocarcinoma | 22 (13) |
| Vascular invasion | |
| Yes | 32 (18) |
| No | 131 (76) |
| NA | 10 (6) |
| Lymph node count | |
| ≥12 lymph nodes examined | 138 (80) |
| <12 lymph nodes examined | 35 (20) |
| Radicality | |
| R0 | 162 (94) |
| R1 | 8 (5) |
| R2 | 3 (2) |
| Preoperative obstruction | |
| Yes | 26 (15) |
| No | 147 (85) |
| Tumor perforation | |
| Yes | 15 (9) |
| No | 157 (91) |
| NA | 1 (0) |
| Adjuvant chemotherapy | |
| Yes | 51 (30) |
| No | 121 (69) |
| NA | 1(0) |
General aspects of MSI staining
The results of the MSS/MSI analysis in relation to clinicopathological variables are shown in Table 2. Overall, 136 (79%) of the tumors were MSS and 37 (21%) were MSI high. MSI was significantly more common in the right-sided tumors (n = 30; 34%), as compared with the left-sided tumors (n = 7; 8%) (Pearson’s chi-square test, p = 0.0001). MSI was infrequent in well-differentiated tumors (1/39, 3%), but common in tumors with poor differentiation grade (15/39, 40%). Ten out of 22 (45%) mucinous cancers presented MSI. MSI status in relation to clinic-pathological variables is presented in Table 2.
Table 2. MSS/MSI status in relation to clinicopathological variables (n = 173).
NA = not available, R0 = microscopically radical surgery, R1 = macroscopically radical surgery, R2 = macroscopically non-radical surgery, CRC = colorectal cancer.
| Variable | MSS | MSI high | Significance (p) |
|---|---|---|---|
| n (%) | n (%) | ||
| Gender | 0.266 | ||
| Female | 69 (51) | 23 (62) | |
| Male | 67 (49) | 14 (38) | |
| Age | 0.707 | ||
| Under 70 years | 53 (39) | 13 (35) | |
| Over 70 years | 83 (61) | 24 (65) | |
| Postoperative stage | 0.253 | ||
| T3N0 | 115 (85) | 28 (76) | |
| T4aN0 | 13 (10) | 4 (11) | |
| T4bN0 | 8 (6) | 5 (13) | |
| Tumor side | 0.0001 | ||
| Right | 59 (43) | 30 (81) | |
| Left | 77 (57) | 7 (19) | |
| Tumor grade | 0.010 | ||
| G1 | 18 (13) | 1 (3) | |
| G2 | 93 (68) | 21 (57) | |
| G3 | 25 (18) | 15 (40) | |
| Histology | 0.009 | ||
| Conventional adenocarcinoma | 124 (91) | 27 (73) | |
| Mucinous adenocarcinoma | 12 (9) | 10 (27) | |
| Vascular invasion | 0.383 | ||
| Yes | 28 (21) | 4 (11) | |
| No | 101 (74) | 30 (81) | |
| NA | 7 (5) | 3 (8) | |
| Lymph node count | 0.646 | ||
| 12 or more examined | 107 (78) | 31 (84) | |
| Less than 12 examined | 29 (21) | 6 (16) | |
| Radicality of surgery | 0.446 | ||
| R0 | 128 (94) | 34 (92) | |
| R1 | 5 (4) | 3 (8) | |
| R2 | 3 (2) | 0 (0) | |
| Preoperative obstruction | 0.604 | ||
| Yes | 22 (16) | 4 (11) | |
| No | 114 (84) | 33 (89) | |
| Tumor perforation | 0.797 | ||
| Yes | 11 (8) | 4 (11) | |
| No | 124 (91) | 33 (89) | |
| NA* | 1 (1) | 0 (0) | |
| Adjuvant chemotherapy | 0.218 | ||
| Yes | 37 (27) | 14 (38) | |
| No | 99 (73) | 22 (59) | |
| NA* | 0 (0) | 1 (3) | |
| Disease-specific outcome | 0.660 | ||
| Alive without CRC | 93 (68) | 23 (62) | |
| Alive with CRC | 3 (2) | 0 (0) | |
| Dead of CRC | 13 (10) | 4 (13) | |
| Dead of other cancer | 13 (10) | 5 (13) | |
| Dead of other causes | 10 (7) | 5 (13) | |
| Dead cause unspecified | 4 (3) | 0 (0) |
General aspects of ezrin staining
The results of the ezrin stainings in relation to clinicopathological parameters are shown in Table 3. Generally, in 135 (78%) tumors, ezrin staining intensity was scored as low, and in 38 (28%) as high. High ezrin expression was more common in MSI tumors (19/37, 51%) than in MSS tumors (19/134, 14%) (Pearson’s chi-square test, p = 0.0001). There were no statistically significant differences in ezrin intensity according to clinicopathological variables, except for disease outcome (see below). Ezrin staining in relation to clinic-pathological variables is presented in Table 3.
Table 3. Ezrin expression in relation to clinicopathological variables (n = 173). CRC = colorectal cancer.
| Variable | Ezrin low | Ezrin high | Significance (p) |
|---|---|---|---|
| n (%) | n (%) | ||
| Gender | 0.272 | ||
| Female | 60 (44) | 21 (55) | |
| Male | 75 (56) | 17 (45) | |
| Age | 0.130 | ||
| Under 70 years | 56 (41) | 10 (26) | |
| Over 70 years | 79 (58) | 28 (74) | |
| Postoperative stage | 0.634 | ||
| T3N0 | 113 (84) | 30 (79) | |
| T4aN0 | 13 (10) | 4 10) | |
| T4bN0 | 9 (7) | 4 (10) | |
| Tumor side | 0.141 | ||
| Right | 65 (48) | 24 (63) | |
| Left | 70 (52) | 14 (37) | |
| Tumor grade | 0.119 | ||
| G1 | 14 (10) | 5 (13) | |
| G2 | 94 (70) | 20 (53) | |
| G3 | 27 (20) | 13 (34) | |
| Histology | 0.099 | ||
| Conventional adenocarcinoma | 121 (90) | 30 (79) | |
| Mucinous adenocarcinoma | 14 (10) | 8 (21) | |
| Vascular invasion | 0.677 | ||
| Yes | 26 (19) | 6 (16) | |
| No | 100 (74) | 31 (82) | |
| NA | 9 (7) | 1 (3) | |
| Lymph node count | 1.000 | ||
| 12 or more examined | 108 (80) | 30 (79) | |
| Less than 12 examined | 27 (20) | 8 (21) | |
| Radicality of surgery | 0.568 | ||
| R0 | 127 (94) | 35 (92) | |
| R1 | 6 (4) | 2 (5) | |
| R2 | 1 (1) | 1 (3) | |
| Preoperative obstruction | 0.607 | ||
| Yes | 19 (14) | 7 (18) | |
| No | 116 (86) | 31 (82) | |
| Tumor perforation | 0.476 | ||
| Yes | 10 (7) | 5 (13) | |
| No | 124 (92) | 33 (87) | |
| NA* | 1 (1) | 0 (0) | |
| Adjuvant chemotherapy | 1.000 | ||
| Yes | 40 (30) | 11 (29) | |
| No | 95 (70) | 26 (68) | |
| NA* | 0 (0) | 1 (3) | |
| MSI status | 0.001 | ||
| MSS | 117 (87) | 19 (50) | |
| MSI | 18 (13) | 19 (50) | |
| BRAF status | 0.001 | ||
| BRAF WT | 121 (91) | 25 (66) | |
| BRAF mutated | 12 (9) | 13 (34) | |
| Disease-specific outcome | 0.038 | ||
| Alive without CRC | 93 (69) | 23 (61) | |
| Alive with CRC | 3 (2) | 0 (0) | |
| Dead of CRC | 8 (6) | 9 (24) | |
| Dead of other cancer | 16 (12) | 2 (5) | |
| Dead of other causes | 11 (8) | 4 (11) | |
| Dead cause unspecified | 4 (3) | 0 (0) |
BRAF staining
BRAF staining was available from 171 patients. Of the tumors, 146 (85%) were BRAF wild type and 25 (15%) BRAF V600E mutated. The BRAF mutated tumors predominantly presented with MSI (21/25, 84%), whereas BRAF wild type tumors were mostly MSS (130/146, 89%). Of the BRAF wild type tumors only 25/146 (17%) showed high ezrin IHC, while 13/25 (52%) of BRAF mutant tumors were ezrin high (Pearson’s chi-square test, p = 0.0001). Combinatorial analysis of the three variables showed that BRAF wild type tumors were predominantly MSS / low ezrin (112/146, 77%), whereas 12/25 (48%) of the BRAF mutated tumors were MSI / high ezrin (Fisher’s exact test, p = 0.0001). Follow-up data according to BRAF status is presented in Table 4.
Table 4. BRAF status in relation to ezrin and MSS/MSI (n = 171).
| Variable | Ezrin MSS/Ezrin/MSI | Significance | |||
|---|---|---|---|---|---|
| (p) | |||||
| Ezrin low MSS | Ezrin low MSI | Ezrin high MSS | Ezrin high MSI | p = 0.0001 | |
| n (%) | n (%) | n (%) | n (%) | ||
| BRAF mutated | 3 (3) | 9 (50) | 1 (5) | 12 (63) | |
| n (%) | |||||
| BRAF wild type | 112 (97) | 9 (50) | 18 (95) | 7 (37) | |
| n (%) | |||||
Clinical correlations
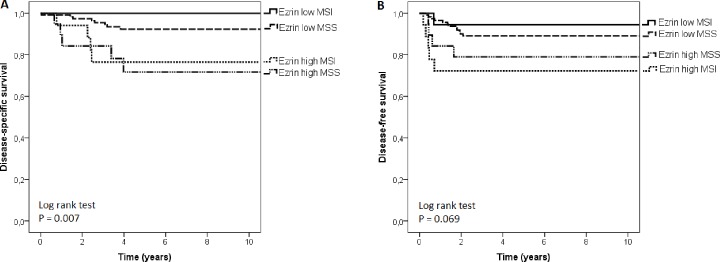
The clinical correlations of the MSI status and ezrin staining are shown in Tables 2 and 3. Altogether, high ezrin staining correlated with inverse DSS (Fisher’s exact test, p = 0.038). On the other, MSI status as a single variable did not correlate with survival. In categorical analysis of 5-year disease-specific survival time, 11 out of 18 (61%) patients with MSI / low ezrin were alive compared to only 4 out of 18 (21%) patients with MSS / high ezrin (Fisher’s exact test, p = 0.040). In univariate analysis, patients whose tumors were MSI / low ezrin tended to have the best OS probability, and those with MSI / high ezrin the worst, but the difference was not statistically significant (log-rank test, p = 0.235). Patients with MSI / low ezrin tumors had the longest DSS and those with MSS / high ezrin tumors had the shortest (log-rank test, p = 0.007). An example of staining patterns of patients belonging to groups of best and worst DSS are presented in Figs 1 and 2, respectively. Patients with MSI / low ezrin had the longest DFS and those with MSI / high ezrin had the shortest, but the difference was no statistically significant (log-rank test, p = 0.069). The survival curves are presented in Fig 3 and the results of univariate survival analysis in S1 Table.
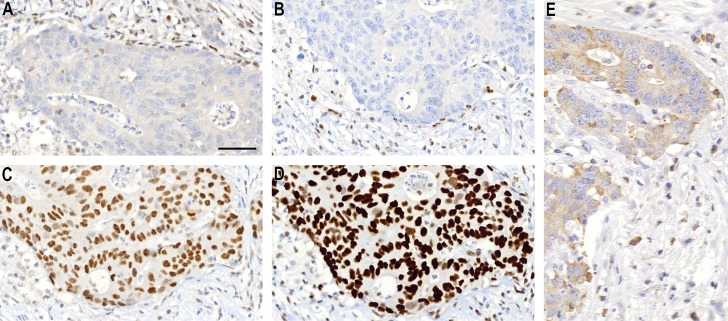
Fig 1. An example of a tumor with MSI-high features and weak ezrin expression.
MLH1 (A) and PMS2 (B) are negative in nuclei of cancer cells, while MSH2 (C) and MSH6 (D) show normal nuclear staining. Tumor cells show weak immunostaining for ezrin (E). This patient had a favorable prognosis.
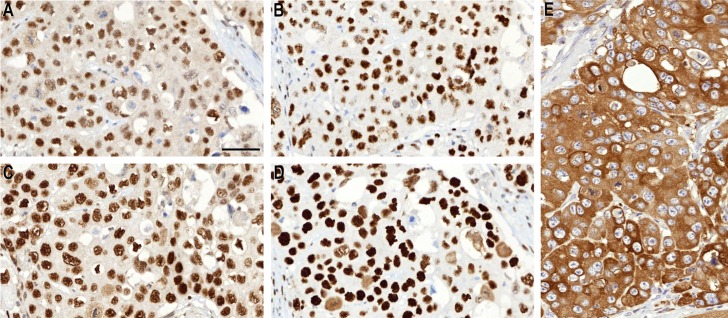
Fig 2. An example of a tumor with MSS features and strong ezrin expression.
MLH1 (A), PMS2 (B), MSH2 (C) and MSH6 (D) show normal nuclear staining in colorectal cancer cells. Tumor cells show strong immunostaining for ezrin (E). This patient had an unfavorable outcome.
Fig 3. Kaplan-Meyer survival analysis of stage II colorectal cancers based on MSI status and ezrin expression.
Disease-specific survival (A) and disease-free survival (B).
A summary of the multivariate analyses results is presented in Table 5. This table shows T4bN0 tumors to be associated with inferior OS (Cox model, HR 2.86, 95% CI [1.06–7.74], p = 0.038) and DFS (Cox model, HR 8.05, 95% CI [2.31–28.01], p = 0.001). Likewise, perforation was linked to inferior OS (Cox model, HR 3.8, 95% CI [1.57–9.17], p = 0.003), DSS (Cox model, HR 5.44, 95% CI [95% CI 1.3–22.75], p = 0.02), as well as DFS (Cox model, HR 4.87 95% CI [1.38–17.23]; p = 0.014). Moreover, the presence of BRAF mutation was associated to shortened OS (Cox model, HR 3.29, 95%CI [1.14–9.54], p = 0.028). High ezrin expression together with MSS were linked to shorter DSS (Cox model, HR 5.68, 95%CI [1.53–21.12], p = 0.01).
Table 5. Summary of the results in multivariate analysis.
| Variable (n) | Overall survival | Disease-specific survival | Disease-free survival | ||||||
|---|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | p value | HR | 95% CI | p value | HR | 95% CI | p value | |
| Stage T3N0 (143) | Reference category | Reference category | Reference category | ||||||
| Stage T4aN0 (17) | 1.76 | 0.64–4.83 | 0.275 | 3.40 | 0.72–15.98 | 0.121 | 3.04 | 0.82–11.33 | 0.097 |
| Stage T4bN0 (13) | 2.86 | 1.06–7.74 | 0.038 | 4.58 | 0.89–23.62 | 0.069 | 8.05 | 2.31–28.01 | 0.001 |
| Grade 1 (19) | Reference category | Reference category | Reference category | ||||||
| Grade 2 (114) | 0.50 | 0.20–1.29 | 0.153 | 0.93 | 0.13–6.56 | 0.946 | 0.82 | 0.13–5.21 | 0.838 |
| Grade 3 (40) | 0.53 | 0.18–1.53 | 0.241 | 0.68 | 0.08–6.12 | 0.732 | 1.27 | 0.18–8.87 | 0.809 |
| Right colon (89) | 1.35 | 0.69–2.65 | 0.378 | 1.27 | 0.37–4.36 | 0.702 | 1.04 | 0.39–2.83 | 0.933 |
| Vascular invasion (19) | 1.57 | 0.78–3.18 | 0.210 | 3.36 | 0.98–11.57 | 0.055 | 3.62 | 1.26–10.37 | 0.017 |
| Perforation (10) | 3.80 | 1.57–9.17 | 0.003 | 5.44 | 1.3–22.75 | 0.002 | 4.87 | 1.38–17.23 | 0.014 |
| Preop. obstruction (19) | 0.71 | 0.27–1.85 | 0.479 | 1.32 | 0.31–5.65 | 0.71 | 1.53 | 0.45–5.21 | 0.499 |
| BRAF mutation (12) | 3.29 | 1.14–9.54 | 0.028 | 1.41 | 0.20–9.90 | 0.728 | 1.00 | 0.20–5.07 | 0.997 |
| Ezrin low MSS (117) | Reference category | Reference category | Reference category | ||||||
| Ezrin low MSI (18) | 0.34 | 0.10–1.15 | 0.083 | 0.00 | 0.00-.000 | 0.986 | 0.78 | 0.09–6.66 | 0.824 |
| Ezrin high MSS (19) | 0.98 | 0.37–2.64 | 0.975 | 5.68 | 1.53–21.12 | 0.01 | 2.76 | 0.76–1.01 | 0.124 |
| Ezrin high MSI(19) | 0.76 | 0.26–2.21 | 0.619 | 3.19 | 0.61–16.74 | 0.17 | 3.01 | 0.78–11.66 | 0.110 |
Discussion
Stage II CRC patients possess a treatment challenge, because current diagnostic methods do not enable their accurate risk stratification. The purpose of this study was to test, whether analysis of ezrin, a promising prognostic marker, together with microsatellite instability and BRAF mutation status could be used for prognostication. Indeed, our results show ezrin as an independent prognostic marker for disease-specific survival in stage II CRC, and indicate this correlation to be further strengthened by concomitant microsatellite instability testing.
Previous studies by others and us have indicated an association between ezrin expression and CRC outcome. The earlier studies have been carried out with mixed cohorts, including various disease stages, which can lead to inaccurate conclusions. However, we are not aware of any studies that would have specifically focused on ezrin expression in stage II CRC. The current results indicate that tumors with high ezrin expression possess adverse biological features already at a stage, when the cancer has not yet disseminated. Importantly, as demonstrated by this study, these features are not associated with tumor location, histological grade, vascular invasion or other outcome-related clinicopathological features. Our results do not clarify the mechanism, by which ezrin may be linked with oncogenic properties. One explanation is that ezrin expression provides an advantage for the disseminating cells early on during metastatic seeding. Indeed, some previous studies have indicated a role for ezrin in this process [32,33]. Interestingly, ezrin turned out to be a stronger DSS predictor than any of the clinipathological factors, apart from tumor perforation.
Ezrin expression is linked to the activity of several oncogenic signaling cascades. Ezrin can act both as a regulator and/or a down-stream target in several signaling pathways, including Src, Akt-PI3K and PKA, and these associations have been suggested to be of importance in ezrin’s oncogenic properties [34,35,36]. Here, we found that ezrin expression correlated with BRAF mutation status; high ezrin immunoreactivity being significantly more common in BRAF V600E than BRAF wild-type tumors. This is a novel finding, there are no previous reports that would have linked ezrin with BRAF. Even if the specific mechanism of the connection between these two genes is unknown, the association of both high ezrin expression and BRAF mutation with the activity of several oncogenic signaling pathways might partly explain this interesting finding.
In this study, MSI status alone did not correlate with survival, although the superior prognosis of patients with MSI CRC over MSS tumors has been demonstrated earlier in many studies [37,38,13,39,21]. However, the combination of ezrin expression with MSI status stratified the patients to prognostic groups, in which patients with MSS and high ezrin expression had the shortest DSS and patients with MSI and low ezrin expression had the best DSS (log-rank test, p = 0.007). This correlation is of interest as high ezrin expression was significantly more infrequent in MSS tumors than MSI tumors. Why the prognostic role of ezrin is especially pronounced in MSS tumors awaits further studies.
With this university hospital area based cohort we could confirm earlier findings related to microsatellite instability and BRAF mutation status. Mucinous histology and poor differentiation grade were associated with MSI, which is in accordance with MSI high phenotype [40]. In the current study, about a fifth of the tumors were MSI high, and MSI high tumors were significantly more commonly right-sided, as reported previously for stage II tumors [37,13]. Sidedness in itself, however does not justify patient selection for possible adjuvant therapy in stage II CRC [41].
In the current study, 84% of BRAF mutated tumors were MSI, whereas most BRAF wild type tumors were MSS. BRAF mutation was also significantly linked to overall survival, the HR for mortality being 3.29 (95%CI [1.14–9.54], p = 0.028). Similar results have also been reported in previous studies, showing BRAF mutation to associate with increased mortality due to CRC [21,42]. There is evidence that MSI phenotype may compensate the poor prognostic effect of BRAF mutation [43], but this issue remains controversial [44]. BRAF mutation is also reported to rule out Lynch syndrome [17], which may be of help to the clinicians in counseling the patients and their families.
At the time-point the patients were treated, MSI-status was not routinely tested among stage II patients. Altogether, 37% of the patients had received adjuvant chemotherapy, according to possible high-risk factors including preoperative obstruction or perforation, vascular invasion, poor differentiation grade and T4-stage and depending on their overall health, general health and patient preference. Patients with MSI tumors are reported not to gain benefit from fluorouracil-based adjuvant chemotherapy [13,45]. This concerns especially stage II colorectal cancer patients, while there are conflicting results concerning stage III patients [46].
In conclusion, our study found a correlation between ezrin expression and DSS in stage II CRC, and this correlation was further strengthened by microsatellite instability analysis. Of the different tumor categories, DSS was longest in patients presenting with MSI / low ezrin tumors and shortest in MSS / high ezrin tumors. These results imply that ezrin staining can provide important prognostic information for estimating stage II patients’ individual risk of disease recurrence and progression.
Supporting information
(DOCX)
Acknowledgments
We are grateful to Mr Jaakko Liippo for his help with the digital pictures.
Data Availability
The sharing of even identified dataset of this study is restricted by Biobank Law of Finland (688/2012, Biobank Act: http://www.finlex.fi/fi/laki/kaannokset/2012/en20120688.pdf, see especially sections 26-27). To summarize, the dataset and samples encoded to defined projects can only be requested through permit authorization process from Auria Biobank for justifiable research projects (https://www.auriabiopankki.fi/?lang=en).
Funding Statement
The authors received salary for this project as follows: Khadija Slik: Finnish National Agency for Education CIMO, Cancer Society of South-West Finland; Eija Korkeila: The Special Government Funding (EVO) allocated to Turku University Hospital; and Jari Sundström: The Special Government Funding (EVO) allocated to Turku University Hospital. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Sato H, Maeda K, Sugihar K, Mochizuki H, Teramoto T, Kameoka S, et al. High-risk stage II colon cancer after curative resection. J Surg Oncol 2011;104:45–52. doi: 10.1002/jso.21914 [DOI] [PubMed] [Google Scholar]
- 2.Quasar Collaborative Group, Gray R, Barnwell J, McConkey C, Hills RK, et al. Adjuvant chemotherapy versus observation in patients with colorectal cancer: a randomized study. Lancet 2007;370:2020–9. doi: 10.1016/S0140-6736(07)61866-2 [DOI] [PubMed] [Google Scholar]
- 3.Berger AC, Sigurdson ER, LeVoyer T, Hanlon A, Mayer RJ, McDonald JS, et al. Colon cancer survival is associated with decreasing ratio of metastatic to examined lymph nodes. J Clin Oncol 2005;23(34):8706–12. doi: 10.1200/JCO.2005.02.8852 [DOI] [PubMed] [Google Scholar]
- 4.Dotan E and Cohen SJ. Challenges in the management of stage II colon cancer. Semin Oncol 2011;38(4): 511–520. doi: 10.1053/j.seminoncol.2011.05.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tsikitis VL, Larson DW, Huebner M, Lohse CM, Thompson PA. Predictors of recurrence free survival for patients with stage II and III colon cancer. BMC Cancer 2014;14:336 doi: 10.1186/1471-2407-14-336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kruhøffer M, Jensen JL, Laiho P, Dyrskjøt L, Salovaara R, D Arango D, et al. Gene expression signatures for colorectal cancer microsatellite status and HNPCC. Br J Cancer 2005; 92:2240–2248. doi: 10.1038/sj.bjc.6602621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang C, Rijnsoever M, Grieu F, Bydder S,Elsaleh H, Joseph D, et al. Prognostic significance of microsatellite instability and Ki-ras mutation type in stage II colorectal cancer. Oncology 2003;64(3):259–265. [DOI] [PubMed] [Google Scholar]
- 8.Lothe RA, Peltomaki P, Meling GI, Aaltonen LA, Nyström-Lahti M, Pylkkänen L, et al. Genomic instability in colorectal cancer: relationship to clinicopathological variables and family history. Cancer Res 1993;53:5849–5852. [PubMed] [Google Scholar]
- 9.Phipps AI, Buchanan DD, Makar KW, Burnett-Hartman AN, Coghill AE, Passarelli MN, et al. BRAF mutation status and survival after colorectal cancer diagnosis according to patient and tumor characteristics. Cancer Epidermiol Biomarkers Prev 2012;21:1792–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Phipps Ai, Lindor NM, Jenkins MA, Baron JA, Win AK, Gallinger S, et al. Colon and Rectal Cancer Survival by Tumor Location and Microsatellite Instability: The Colon Cancer Family Registry. Dis Colon Rectum 2013;56:937–44. doi: 10.1097/DCR.0b013e31828f9a57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shia J. Evolving approach and clinical significance of detecting DNA mismatch repair deficiency in colorectal carcinoma. Semin Diagn Pathol 2015;32(5):352–361. doi: 10.1053/j.semdp.2015.02.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Young J, Simms LA, Biden KG, Wynter C, Whitehall V, Karamatic R, et al. Features of colorectal cancers with high-level microsatellite instability occurring in familial and sporadic settings. Parallel pathways of tumorigenesis. Am J Pathol 2001;159(6):2107–2116. doi: 10.1016/S0002-9440(10)63062-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Popat S, Hubner R, Houlston RS. Systematic review of microsatellite instability and colorectal cancer prognosis. J Clin Oncol 2005;23:609–618. doi: 10.1200/JCO.2005.01.086 [DOI] [PubMed] [Google Scholar]
- 14.Merok MA, Ahlquist T, Røyrvik EC, Tufteland KF, Hektoen M, Sjo OH, et al. Microsatellite instability has a positive prognostic impact on stage II colorectal cancer after complete resection: results from a large, consecutive Norwegian series. Ann Oncol 2013;24(5):1274–82. doi: 10.1093/annonc/mds614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sargent DJ, Marsoni s, Monges G, Thibodeau SN, Labianca R, Hamilton SR, et al. Defective mismatch repair as a predictive marker for lack of efficacy of fluorouracil-based adjuvant therapy in colon cancer. J Clin Oncol 2010;10;28:3219–26. doi: 10.1200/JCO.2009.27.1825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mojarad E, Kashfi SM, Mirtalebi H, Taleghani MY, Azimzadeh P, Savabkar S, et al. Low Level of Microsatellite Instability Correlates with Poor Clinical Prognosis in Stage II Colorectal Cancer Patients. J Oncology 2016:2196703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Funkhouser WK, Lubin IM, Monzon FA, Zehnbauer BA, Evans JP, Ogino S, et al. Relevance, pathogenesis, and testing algorithm for mismatch repair–defective colorectal carcinomas: a report of the association for molecular pathology. J Mol Diagn 2012;14:91–103. doi: 10.1016/j.jmoldx.2011.11.001 [DOI] [PubMed] [Google Scholar]
- 18.Davies H, Bignell GR, Cox C, Stephens P, Edkins S, Clegg S, et al. Mutations of the BRAF gene in human cancer. Nature 2002;417:949–954. doi: 10.1038/nature00766 [DOI] [PubMed] [Google Scholar]
- 19.Rajagopalan H, Bardelli A, Lengauer C, Kinzler KW, Vogelstein B, Velcuescu VE. Tumorigenesis: RAF/RAS oncogenes and mismatch repair status. Nature 2002;418:934 doi: 10.1038/418934a [DOI] [PubMed] [Google Scholar]
- 20.Popovici V, Budinska E, Bosman FT, Tejpar S, Roth AD, Delorenzi M. Context-dependent interpretation of the prognostic value of BRAF and KRAS mutations in colorectal cancer. BMC Cancer 2013;13:439 doi: 10.1186/1471-2407-13-439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Seppälä TT, Böhm JP, Friman M, Lahtinen L, Väyrynen VM, Liipo TK, et al. Combination of microsatellite instability and BRAF mutation status for subtyping colorectal cancer. Br J Cancer 2015;112(12):1966–75. doi: 10.1038/bjc.2015.160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Heiska L, Carpen O. Src phosphorylates ezrin at tyrosine 477 and induces a phosphospecific association between ezrin and a kelch-repeat protein family member. J Biol Chem 2005;280:10244–52. doi: 10.1074/jbc.M411353200 [DOI] [PubMed] [Google Scholar]
- 23.Ilmonen S, Vaheri A, Asko-Seljävaara S, Carpen O. Ezrin in primary cutaneous melanoma. Mod Pathol 2005;18:503–10. doi: 10.1038/modpathol.3800300 [DOI] [PubMed] [Google Scholar]
- 24.Mäkitie T, Carpen O, Vaheri A, Kivelä T. Ezrin as a prognostic indicator and its relationship to tumor characteristics, in uveal malignant melanoma. Invest Ophthalmol Vis Sci 2001;42:2442–9. [PubMed] [Google Scholar]
- 25.Weng WH, Ahlén J, Aström K, Lui WO, Larsson C. Prognostic impact of immunohistochemical expression of ezrin in highly malignant soft tissue sarcomas. Clin Cancer Res 2005;11:198–204. [DOI] [PubMed] [Google Scholar]
- 26.Köbel M, Gradhand E, Zeng K, Schmitt WD, Kriese K, Lantzsch T, et al. Ezrin promotes ovarian carcinoma cell invasion and its retained expression predicts poor prognosis in ovarian carcinoma. Int J Gynecol Pathol 2006;25: 121–30. doi: 10.1097/01.pgp.0000185410.39050.ac [DOI] [PubMed] [Google Scholar]
- 27.Elzagheid A, Korkeila E, Bendardaf R, Buhmeida A, Heikkilä S, Vaheri A, et al. Intense cytoplasmic ezrin immunoreatctivity opredicts poor survival in colorectal cancer. Hum Pathol 2008;39:1737–1743. doi: 10.1016/j.humpath.2008.04.020 [DOI] [PubMed] [Google Scholar]
- 28.Korkeila E, Syrjänen K, Bendardaf R, Laulajainen M, Carpén O, Pyrhönen S, et al. Preoperative radiotherapy modulates ezrin expression and its value as a predictive marker in patients with rectal cancer. Hum Pathol 2011;42:384–392. doi: 10.1016/j.humpath.2010.08.004 [DOI] [PubMed] [Google Scholar]
- 29.Sobin LH, Gospodarowicz MK, Wittekind CH. TNM classification of malignant tumours, International Union Against Cancer: 7th ed Wiley-Blackwell, Singapore; 2010. [Google Scholar]
- 30.Zlobec I, Koelzer VH, Dawson H, Perren A, Lugli A. Next-generation tissue microarray (ngTMA)increases the quality of biomarker studies: an example using CD3, CD8, and CD45RO in the tumor microenvironment of six different solid tumor types. J Transl Med 2013;11:104:1–7. doi: 10.1186/1479-5876-11-104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Böhling TT, Turunen O, Jääskeläinen J, Carpén O, Sainio M, Wahlström T, et al. Ezrin expression in stromal cells of capillary hemangioblastoma. An immunohistochemical survey of brain tumours. Am J Pathol 1996;148:367–73. [PMC free article] [PubMed] [Google Scholar]
- 32.Horwitz V, Davidson B, Stern D, Tropé CG, Re’em TT, Reich R, et al. Ezrin is associated with disease progressionin ovarian carcinoma. PLOS ONE 2016;11(9):e1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kim EK, Park JM, Lim S, Choi JW, Kim HS, Seok H, et al. Activation of AMP-activated protein kinase is essential for lysophosphatidic acid-induced cell migration in ovarian cancer cells. J Biol Chem. 2011;286(27):24036–45. doi: 10.1074/jbc.M110.209908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shin HK, Ryu BJ, Choi S-W, Kim SH, Lee K. Inactivation of src-to-ezrin pathway: a possible mechanism in the ouabain-mediated Inhibition of A549 cell migration. Biomed Res Int 2015;537136 doi: 10.1155/2015/537136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Goler-Baron V, Sladkevich I, Assaraf YG. Inhibition of the PI3K-Akt signaling pathway disrupts ABCG2-rich extracellularvesicles and overcomes multidrug resistance in breast cancer cells. Biochem Pharmacol 2012;83(10):1340–1348. doi: 10.1016/j.bcp.2012.01.033 [DOI] [PubMed] [Google Scholar]
- 36.Kawaguchi K, Yoshida S, Hatano R, Asano S. Pathophysiological Roles of Ezrin/Radixin/Moesin Proteins. Biol Pharm Bull 2017;40, 381–390. doi: 10.1248/bpb.b16-01011 [DOI] [PubMed] [Google Scholar]
- 37.Ribic CM, Sargent DJ, Moore MJ, Thibodeau SN, French AJ, Goldberg RM, et al. Tumor-microsatellite instability status as a predictor of benefit from fluorouracil-based adjuvant chemotherapy for colon cancer N Engl J Med 2003;349:247–257. doi: 10.1056/NEJMoa022289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gryfe R, Kim H, Hsich ET, Aronson MD, Holowaty EJ, Bull SB, et al. Tumor microsatellite-instability and clinical outcome in young patients with colorectal cancer. N Engl J Med 2000;342:69–77. doi: 10.1056/NEJM200001133420201 [DOI] [PubMed] [Google Scholar]
- 39.de la Chapelle A and Hampel H. Clinical Relevance of Microsatellite Instability in Colorectal Cancer. J Clin Oncol 2010;28(20):3380–3387. doi: 10.1200/JCO.2009.27.0652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Boland CR and Goel A. Microsatellite instability in colorectal cancer. Gastroenterol 2010;138(6): ä2073–2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Weiss JM, Schumacher J, Allen GO, Neuman H, Lange EO, LoConte NK, et al. Adjuvant Chemotherapy for Stage II Right- and Left-Sided ColonCancer: Analysis of SEER-Medicare Data. Ann Surg Oncol 2014;21: 1781–1791. doi: 10.1245/s10434-014-3631-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gavin PG, Colangelo LH1,2,3, Fumagalli D, Tanaka N, Remillard MY, Yothers G, et al. Mutation profiling and microsatellite instability in stage II and III colon cancer: an assessment of their prognostic and oxaliplatin predictive value. Clin Cancer Res 2012;18:6531–41. doi: 10.1158/1078-0432.CCR-12-0605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lochhead P, Kuchiba A, Imamura Y, Liao X, Yamauchi M, Nishihara R, et al. Microsatellite instability and BRAF mutation testing in colorectal cancer prognostication. J Natl Cancer Inst 2013;105:1151–1156. doi: 10.1093/jnci/djt173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fariña-Sarasqueta A, van Lijnschoten G, Moerland E, Creemers GJ, Lemmens VE, Rutten HJ, et al. The BRAF V600E mutation is an independent prognostic factor for survival in stage II and stage III colon cancer patients Ann Oncol 2010;21:2396–2402. doi: 10.1093/annonc/mdq258 [DOI] [PubMed] [Google Scholar]
- 45.Jover R, Zapater P, Castells A, Llor X, Andreu M, Cubiella J, et al. The efficacy of adjuvant chemotherapy with 5-fluorouracil in colorectal cancer depends on the mismatch repair status. Eur J Cancer 2009;45:365–373, 2009. doi: 10.1016/j.ejca.2008.07.016 [DOI] [PubMed] [Google Scholar]
- 46.Labianca R, Nordlinger B, Beretta GD, Mosconi S, Mandalà M, Cervantes A, et al. Early colon cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2013;24 (Supplement 6): vi64–vi72. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
Data Availability Statement
The sharing of even identified dataset of this study is restricted by Biobank Law of Finland (688/2012, Biobank Act: http://www.finlex.fi/fi/laki/kaannokset/2012/en20120688.pdf, see especially sections 26-27). To summarize, the dataset and samples encoded to defined projects can only be requested through permit authorization process from Auria Biobank for justifiable research projects (https://www.auriabiopankki.fi/?lang=en).