Significance
The formation of the vascular network surrounding the central nervous system (CNS) is important for its subsequent vascularization. Previous work has suggested that the developing neural tube provides signals to recruit the endothelial cells that form the peri-neural vascular plexus (PNVP), a vascular network that surrounds the CNS. Here, we identify a critical role for CNS-resident progenitors in directing multistep angiogenic processes required for PNVP formation. Genetic ablation of CNS-resident progenitors in zebrafish affects the formation of the vascular network in tissues adjacent to the spinal cord. Genetic inactivation of Vegfab/Vegfr2 signaling phenocopies these vascular defects, and CNS-resident progenitors serve as a source of Vegfab. Mosaic overexpression of Vegfab in these progenitors induces ectopic blood vessels directed toward their endfeet.
Keywords: angiogenesis, spinal cord, zebrafish, radial glia, Vegf signaling
Abstract
Organ growth requires the coordinated invasion and expansion of blood vessel networks directed by tissue-resident cells and morphogenetic cues. A striking example of this intercellular communication is the vascularization of the central nervous system (CNS), which is driven by neuronal progenitors, including neuroepithelial cells and radial glia. Although the importance of neuronal progenitors in vascular development within the CNS is well recognized, how these progenitors regulate the vasculature outside the CNS remains largely unknown. Here we show that CNS-resident radial glia direct the vascularization of neighboring tissues during development. We find that genetic ablation of radial glia in zebrafish larvae leads to a complete loss of the bilateral vertebral arteries (VTAs) that extend along the ventrolateral sides of the spinal cord. Importantly, VTA formation is not affected by ablation of other CNS cell types, and radial glia ablation also compromises the subsequent formation of the peri-neural vascular plexus (PNVP), a vascular network that surrounds the CNS and is critical for CNS angiogenesis. Mechanistically, we find that radial glia control these processes via Vegfab/Vegfr2 signaling: vegfab is expressed by radial glia, and genetic or pharmacological inhibition of Vegfab/Vegfr2 signaling blocks the formation of the VTAs and subsequently of the PNVP. Moreover, mosaic overexpression of Vegfab in radial glia is sufficient to partially rescue the VTA formation defect in vegfab mutants. Thus, our findings identify a critical function for CNS-resident progenitors in the regulation of vascularization outside the CNS, serving as a paradigm for cross-tissue coordination of vascular morphogenesis and growth.
The vascular system functions in the delivery of oxygen, nutrients, and hormones, thereby providing fundamental support for tissue growth, homeostasis, and function. During development, the vascularization of the central nervous system (CNS) initiates by angiogenic sprouting from the peri-neural vascular plexus (PNVP) into neural tissues (1–4). This angiogenic sprouting event is directed primarily by morphogenetic cues that derive from CNS-resident cells. Neuronal progenitors, including neuroepithelial cells and radial glia, secrete factors, including Wnts and Vegfs, which promote the ingression of blood vessels into neural tissues (5–9). The metabolic state of neurons (10, 11) as well as signals from specialized CNS glia (12, 13) also play instructive roles in vascular patterning in the developing CNS. In contrast to these recent advances in our understanding of CNS vascularization, however, the cellular and molecular bases of vascular assembly around the CNS remain poorly understood.
We have recently begun to investigate the role of different CNS cell types in vascular development around the CNS by optimizing genetic ablation conditions for these cell types in zebrafish (14). This ablation method uses the bacterial enzyme Nitroreductase (NTR), which triggers cell apoptosis upon administration of a prodrug substrate, metronidazole (Mtz) (15, 16). Using cell type-specific promoters to drive NTR expression, different CNS cell types can be ablated in a spatially and temporally controlled manner. By using this ablation technology, we previously identified a requirement for radial glia during embryonic stages in limiting the oversprouting of the vasculature around the spinal cord, thereby ensuring the formation of a precisely patterned network (14). Prior work has suggested that the developing neural tube provides signals to recruit the endothelial cells (ECs) that form the PNVP (4, 17). However, the angiogenic events leading to PNVP formation as well as the cellular and molecular bases of this process remain largely unknown. By using unique attributes of the zebrafish model, we address these questions and identify a specific CNS cell type that is required to drive the formation of the PNVP around the spinal cord.
Results
Specific Cell-Type Ablation Using Genetic and Pharmacological Tools.
The developing zebrafish CNS is known to comprise different glial and neuronal cell types. The major cell types include radial glia neuronal progenitors, neurons, oligodendrocytes, and microglia. Unlike in the mammalian CNS, radial glia neuronal progenitors persist in many regions of the adult zebrafish CNS (18, 19), and to date, there is no clear evidence for bona fide astrocytes in the developing zebrafish spinal cord at the stages we analyzed in this study [3–30 days post fertilization (dpf)].
To investigate whether and how these CNS cell types control vascular development around the CNS, we ablated each of these cell types during development. To ablate radial glia, we used the TgBAC(gfap:gal4ff) line, in which Gal4ff is driven by the gfap regulatory elements contained in a BAC (14, 19). By crossing the TgBAC(gfap:gal4ff) line with the Tg(UAS-E1b:Eco.NfsB-mCherry) line, which contains an NTR-mCherry fusion cassette under the control of the upstream activator sequence (UAS), we generated TgBAC(gfap:gal4ff);Tg(UAS-E1b:Eco.NfsB-mCherry) fish, abbreviated Tg(gfap:NTR). To ablate neurons, we used Tg(elavl3:gal4-vp16);Tg(UAS-E1b:Eco.NfsB-mCherry) fish, abbreviated Tg(elavl3:NTR). To ablate oligodendrocytes, we used Tg(mbpa:gal4-vp16);Tg(UAS-E1b:Eco.NfsB-mCherry) fish, abbreviated Tg(mbpa:NTR). For microglia depletion, we used irf8 mutants, which were shown to lack microglia throughout development (20). Moreover, we treated embryos with a Sonic Hedgehog signaling inhibitor, cyclopamine, from 34 to 52 hours post fertilization (hpf), which was previously shown to result in a dramatic loss of spinal cord oligodendrocytes and their precursors (21).
We first confirmed that the Tg(gfap:NTR) line we used specifically labels GFAP-positive radial glia at the larval stage when we induced cell ablation (Fig. S1 A–B″), and we tested whether these transgenic (Tg) lines, as well as the cyclopamine treatment, could ablate the target cells effectively (Figs. S1 C–J″ and S2 A–D). We further observed that ablation of radial glia and neurons is specific for each of these cell types (Fig. S2 G–K″).
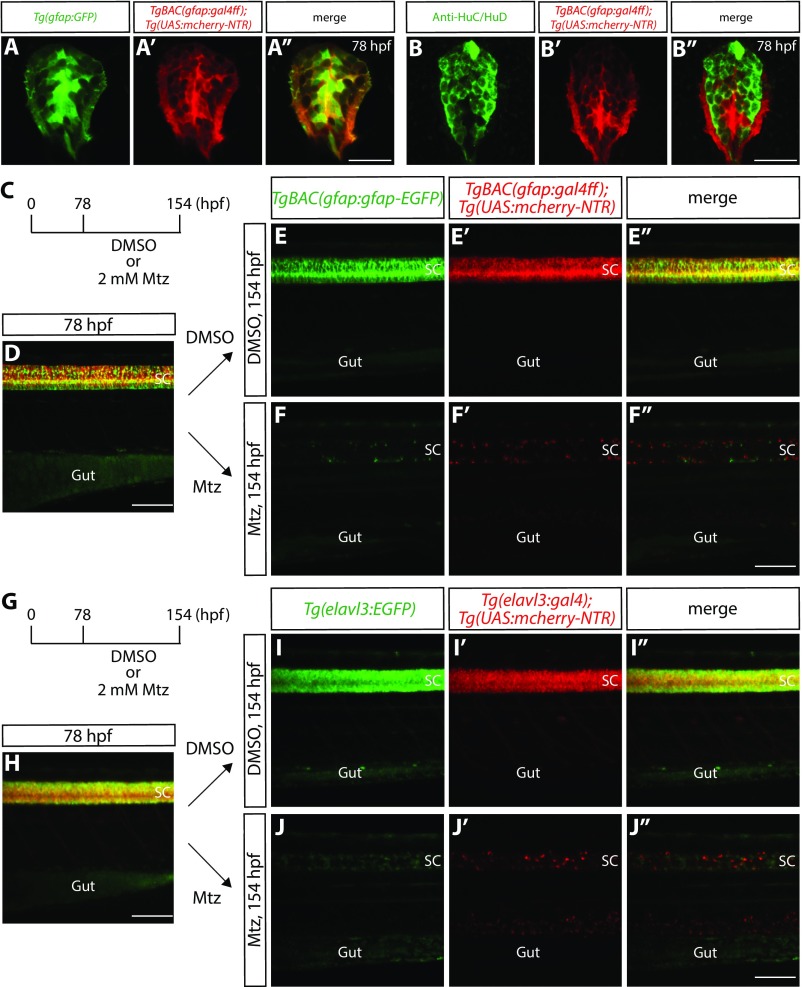
Fig. S1.
Radial glia and neuronal ablation by the NTR/Mtz-mediated cell ablation method. (A–A″) 78 hpf Tg(gfap:GFP);TgBAC(gfap:gal4ff);Tg(UAS:mcherry-NTR) trunk spinal cord section. GFP+ radial glia and mCherry+ cells are largely colocalized. (B–B″) 78 hpf TgBAC(gfap:gal4ff);Tg(UAS:mcherry-NTR) trunk spinal cord section immunostained for HuC/HuD (green). EGFP+ neurons and mCherry+ cells are mostly distinct. (C) Time course of NTR/Mtz-mediated cell ablation of radial glia for D–F″. (D) 78 hpf TgBAC(gfap:gfap-EGFP);TgBAC(gfap:gal4ff);Tg(UAS:mcherry-NTR) trunk. TgBAC(gfap:Gfap-EGFP) expression and TgBAC(gfap:gal4ff);Tg(UAS:mCherry-NTR) expression are observed in the spinal cord in an overlapping manner. SC, spinal cord. (E–E″ and F–F″) 154 hpf TgBAC(gfap:gfap-EGFP);TgBAC(gfap:gal4ff);Tg(UAS:mcherry-NTR) trunk after treatment with DMSO (E–E″) or Mtz (F–F″) starting at 78 hpf. Unlike DMSO-treated fish that show strong coexpression of TgBAC(gfap:Gfap-EGFP) and TgBAC(gfap:gal4ff);Tg(UAS:mCherry-NTR) in their spinal cord, Mtz-treated fish show a dramatic reduction of this coexpression. (G) Time course of NTR/Mtz-mediated cell ablation of neurons for H–J″. (H) 78 hpf Tg(elavl3:EGFP);Tg(elavl3:gal4);Tg(UAS:mcherry-NTR) trunk. Tg(elavl3:EGFP) expression and Tg(elavl3:gal4);Tg(UAS:mCherry-NTR) expression are observed in the spinal cord in an overlapping manner. (I–I″ and J–J″) 154 hpf Tg(elavl3:EGFP);Tg(elavl3:gal4);Tg(UAS:mcherry-NTR) trunk after treatment with DMSO (I–I″) or Mtz (J–J″) starting at 78 hpf. Unlike DMSO-treated fish that show strong coexpression of Tg(elavl3:EGFP) and Tg(elavl3:gal4);Tg(UAS:mCherry-NTR) in their spinal cord, Mtz-treated fish show a dramatic reduction of this coexpression. (Scale bars, 100 µm in D, F″, H, and J″ and 20 µm in A″ and B″.)
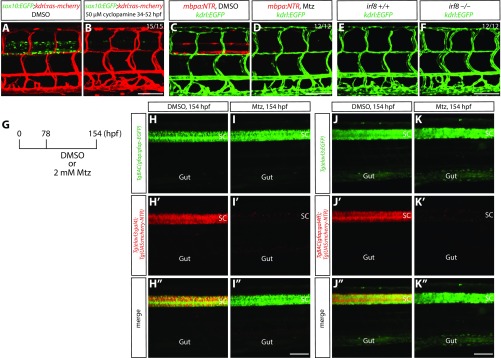
Fig. S2.
Ablation of oligodendrocytes, oligodendrocyte precursor cells, or microglia does not lead to a loss of VTAs; specificity of radial glia and neuronal ablation by the NTR/Mtz-mediated cell ablation method. (A and B) 178 hpf Tg(sox10:EGFP);Tg(kdrl:ras-mcherry) trunk after treatment with DMSO (A) or 50 µM cyclopamine (B) from 34 to 52 hpf. The loss of VTA phenotype observed after radial glia ablation is not seen in fish treated with cyclopamine, which show a dramatically reduced number of spinal cord oligodendrocytes and oligodendrocyte precursor cells (B). (C and D) 178 hpf Tg(mbpa:NTR);Tg(kdrl:EGFP) larval trunk after treatment with DMSO (C) or Mtz (D) from 78 to 178 hpf. Genetic ablation of oligodendrocytes does not lead to a loss of VTAs (D). (E and F) 178 hpf Tg(kdrl:EGFP) irf8+/+ (E) and irf8−/− (F) trunk vasculature. The loss of VTA phenotype observed after radial glia ablation is not seen in irf8−/− fish (F). (G) Experimental time course of NTR/Mtz-mediated cell ablation for H–K″. (H–H″ and I–I″) 154 hpf TgBAC(gfap:gfap-EGFP);Tg(elavl3:gal4);Tg(UAS:mcherry-NTR) trunk after treatment with DMSO (H–H″) or Mtz (I–I″) starting at 78 hpf. Mtz-treated fish show a dramatic reduction of Tg(elavl3:gal4);Tg(UAS:mCherry-NTR) expression in their spinal cord (I′), however TgBAC(gfap:Gfap-EGFP) expression appears unaffected (I) compared with DMSO-treated fish (H). (J–J″ and K–K″) 154 hpf Tg(elavl3:EGFP);TgBAC(gfap:gal4ff);Tg(UAS:mcherry-NTR) trunk after treatment with DMSO (J–J″) or Mtz (K–K″) starting at 78 hpf. Mtz-treated fish show a dramatic reduction of TgBAC(gfap:gal4ff);Tg(UAS:mCherry-NTR) expression in their spinal cord (K′). In contrast, Tg(elavl3:EGFP) expression is slightly, but not dramatically, reduced (K) compared with DMSO-treated fish that show strong expression of both TgBAC(gfap:gal4ff);Tg(UAS:mCherry-NTR) and Tg(elavl3:EGFP) expression in their spinal cord (J–J″). (Scale bar, 100 µm.)
Genetic Ablation of CNS Radial Glia, but Not of Other CNS Cell Types, Leads to a Complete Loss of the Vertebral Arteries.
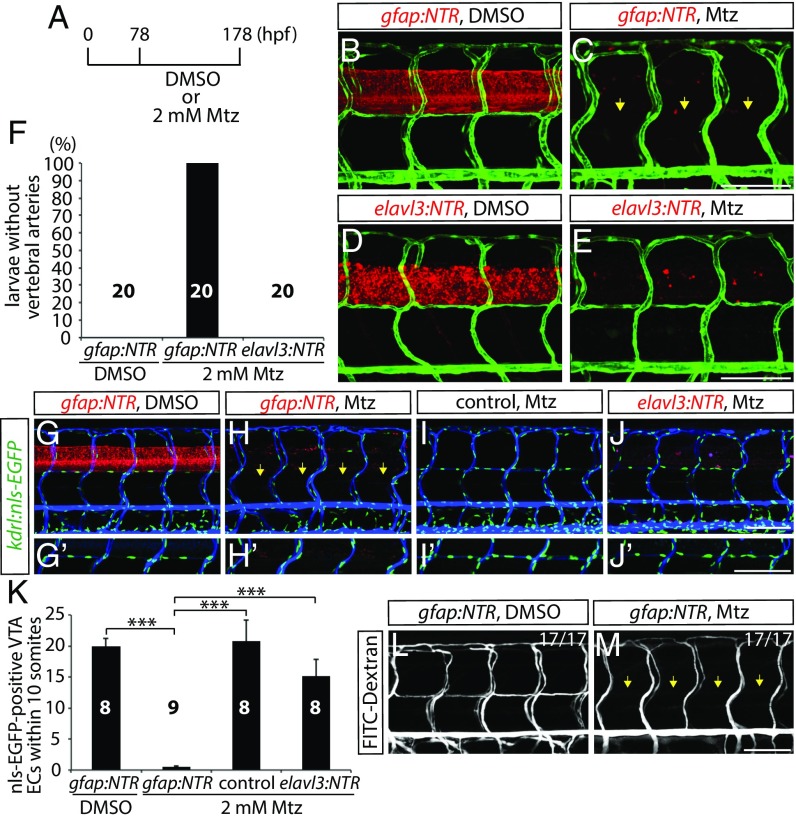
To determine whether and how these CNS cell types regulate vascular development, each Tg line was crossed with the EC reporter Tg line, Tg(kdrl:EGFP). We treated larvae with 2 mM Mtz starting from 78 hpf and assessed vascular development at 178 hpf (Fig. 1A). Interestingly, we observed that radial glia-ablated fish exhibited largely comparable patterns of the trunk vasculature to controls. However, we found that the vertebral arteries (VTAs), which develop along the ventrolateral sides of the spinal cord, were completely absent in 178 hpf radial glia-ablated fish (Fig. 1 B and C). This phenotype is fully penetrant and was observed in all of the radial glia-ablated animals analyzed (Fig. 1F and Table S1; n = 20). VTAs were present in all of the 178 hpf DMSO or 2 mM Mtz-treated control fish examined (Fig. 1 B and F; n = 20). Intriguingly, neuronal ablation did not lead to VTA formation defects (Fig. 1 D–F; n = 20). Importantly, fish depleted for oligodendrocytes, oligodendrocyte precursor cells, or microglia also did not exhibit this defect (Fig. S2 A–F), suggesting a specific role for radial glia in regulating VTA formation.
Fig. 1.
Genetic ablation of CNS radial glia in early larval stages leads to a complete loss of the VTAs. (A) Experimental time course for B–E, G–J, L, and M. (B–E) 178 hpf Tg(gfap:NTR) (B and C) and Tg(elavl3:NTR) (D and E) trunk vasculature visualized by Tg(kdrl:EGFP) expression after treatment with DMSO (B and D) or Mtz (C and E). Genetic ablation of radial glia, but not of neurons, leads to the loss of the VTAs (yellow arrows, C). (F) Percentage of larvae without VTAs, as judged by Tg(kdrl:EGFP) expression (10 somites examined per animal). (G–J) 178 hpf Tg(gfap:NTR) (G and H), control (I), and Tg(elavl3:NTR) (J) animals carrying the kdrl:nls-EGFP transgene, which were injected with Q-dot 655 nanocrystals after treatment with DMSO (G) or Mtz (H–J). Radial glia-ablated fish lack ECs in the position of the VTAs (yellow arrows, H). High-magnification images of ECs in the position of the VTAs are shown in G′–J′. (K) Quantification of nls-EGFP+ EC number in the position of the VTAs within 10 somites per animal. Values represent means ± SEM, ***P < 0.001. (L and M) 178 hpf Tg(gfap:NTR) trunk vasculature visualized by FITC-dextran microangiography after treatment with DMSO (L) or Mtz (M). Radial glia ablation leads to a loss of lumenized VTAs (yellow arrows, M). (Scale bar, 100 µm.)
Table S1.
Summary of the quantification data
| Sample name | Percentage, % | n | Mean ± SEM | SD |
| Fig. 1F | ||||
| gfap:NTR, DMSO | 0 | 20 | ||
| gfap:NTR, Mtz | 100 | 20 | ||
| elavl3:NTR, Mtz | 0 | 20 | ||
| Fig. 1K | ||||
| gfap:NTR, DMSO | 8 | 19.88 ± 1.36 | 3.833592124 | |
| gfap:NTR, Mtz | 9 | 0.44 ± 0.24 | 0.726483157 | |
| control, Mtz | 8 | 20.75 ± 3.42 | 9.67692395 | |
| elavl3:NTR, Mtz | 8 | 15.13 ± 2.66 | 7.510706644 | |
| Fig. 3I | ||||
| 1% DMSO | 16 | 15.69 ± 0.95 | 3.789788912 | |
| 2 μM Sunitinib | 16 | 0.5 ± 0.20 | 0.816496581 | |
| 2.5 μM SKLB1002 | 16 | 1.56 ± 0.80 | 3.182635176 | |
| 10 μM LY294002 | 16 | 3 ± 0.57 | 2.28035085 | |
| 5 μM DMH I | 16 | 13.38 ± 0.90 | 3.612478374 | |
| 3 μM PD158780 | 16 | 12.31 ± 1.33 | 5.338148868 | |
| Fig. 3J | ||||
| hsp:sflt1, no HS | 10 | 20.5 ± 1.44 | 4.552166761 | |
| hsp:sflt1, HS | 10 | 4.8 ± 2.25 | 7.11492953 | |
| control, HS | 10 | 13.5 ± 2.10 | 6.654154926 | |
| hsp:sflt4, HS | 10 | 13.5 ± 1.33 | 4.196559437 | |
| Fig. 3M | ||||
| vegfab+/+ | 0 | 20 | ||
| vegfab−/− | 100 | 20 | ||
| Fig. 4J | ||||
| gfap:NTR, DMSO | 14 | 7.57 ± 0.67 | 2.502745745 | |
| gfap:NTR, Mtz | 15 | 2.13 ± 0.42 | 1.641718032 | |
| vegfab−/− | 14 | 0.29 ± 0.13 | 0.468807231 | |
| hsp:sflt1, HS | 17 | 1.35 ± 0.45 | 1.835114999 | |
| hsp:sflt4, HS | 14 | 6.36 ± 0.75 | 2.790289284 | |
| Fig. S4I | ||||
| 1% DMSO | 12 | 13.92 ± 0.74 | 2.574643253 | |
| 50 μM SB203580 | 12 | 12.33 ± 0.77 | 2.674231694 | |
| 100 μM PF573228 | 12 | 12.33 ± 0.89 | 3.084663923 | |
| 500 nM U73122 | 12 | 13.17 ± 0.90 | 3.12855858 | |
| 10 μM SL327 | 12 | 13.17 ± 0.72 | 2.480224819 | |
| 100 μM SU6656 | 12 | 12.5 ± 0.67 | 2.315952582 | |
| 50 μM SH-6 | 12 | 5.83 ± 0.92 | 3.186144245 | |
| 50 nM Wortmannin | 12 | 5.92 ± 1.17 | 4.05548637 | |
| Fig. S6E | ||||
| WT | 10 | 0.2 ± 0.13 | 0.421637021 | |
| vegfab−/− | 10 | 0.1 ± 0.1 | 0.316227766 | |
| flt1−/− | 10 | 7.5 ± 0.37 | 1.178511302 | |
| flt1−/−; vegfab−/− | 10 | 6.8 ± 0.57 | 1.813529401 | |
| Fig. S6F | ||||
| WT | 0 | 10 | ||
| vegfab−/− | 100 | 10 | ||
| flt1−/− | 0 | 10 | ||
| flt1−/−; vegfab−/− | 100 | 10 | ||
| Fig. S8E | ||||
| control, Mtz | 20 | 6.95 ± 0.86 | 3.859029057 | |
| elavl3:NTR, Mtz | 16 | 5.5 ± 0.58 | 2.338090389 | |
| mbpa:NTR, Mtz | 20 | 6.5 ± 0.54 | 2.39517058 | |
| irf8−/− | 17 | 6.69 ± 0.97 | 4.011961283 |
Next, we quantified the number of ECs that form the VTAs using the Tg(kdrl:NLS-EGFP) reporter line after radial glia ablation. Consistent with the observations with Tg(kdrl:EGFP) expression, the number of ECs in the position of VTAs dramatically decreased in radial glia-ablated fish compared with control or neuron-ablated fish (Fig. 1 G–K and Table S1; n = 8–9 for each group). We also found the absence of lumenized VTAs by FITC-dextran microangiography (Fig. 1 L and M; n = 17). In control fish, FITC-dextran nanocrystals that were injected into the common cardinal vein circulated and labeled the trunk vasculature, including the VTAs (Fig. 1L). However, VTAs were not labeled by FITC-dextran nanocrystals in radial glia-ablated fish (Fig. 1M). These results indicate that radial glia ablation leads to a loss of the ECs that form the VTAs, resulting in the absence of these vessels. Thus, radial glia act as positive regulators of VTA formation.
VTAs Are Important for Subsequent Vascularization Around and Within the Spinal Cord.
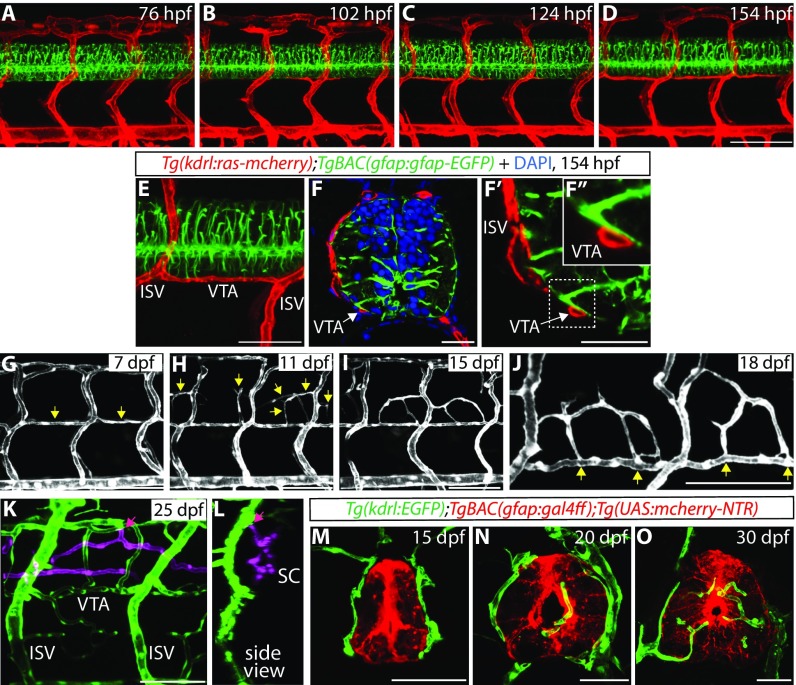
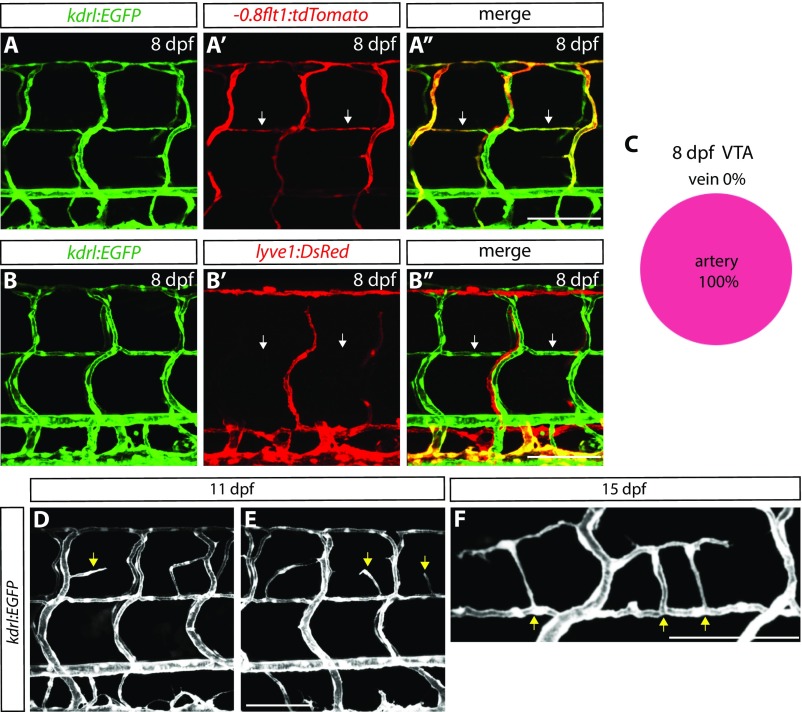
We next examined a developmental time course of VTA formation and observed that VTAs are formed later than other major trunk vessels such as the intersegmental vessels (ISVs) (Fig. 2 A–D). The cells that form the VTAs begin sprouting from the ISVs between 76 and 102 hpf, and VTA formation appears to be mostly complete by around 7–8 dpf (Fig. 2 A–D and Movie S1). We also observed that VTAs show strong expression of the Tg(-0.8flt1:tdTomato) arterial marker, but not the Tg(lyve1:DsRed) venous and lymphatic marker, at 8 dpf (Fig. S3 A–B″), suggesting that these vessels are of arterial identity (Fig. S3C; 132 VTAs quantified, n = 12 fish). Importantly, we found that cellular processes extending from radial glia, marked by TgBAC(gfap:Gfap-EGFP) expression, lie in close proximity to VTAs throughout development (Fig. 2 B–F″).
Fig. 2.
VTAs serve as critical scaffolds for subsequent vascularization around and within the spinal cord. (A–D) TgBAC(gfap:gfap-EGFP);Tg(kdrl:ras-mcherry) trunks at 76 (A), 102 (B), 124 (C), and 154 (D) hpf show a developmental time course of VTA formation. (E and F–F″) A 154 hpf TgBAC(gfap:gfap-EGFP);Tg(kdrl:ras-mcherry) trunk (E) and its sections counterstained with DAPI (F–F″). Radial glia endfeet and VTAs are physically adjacent (white arrows). High-magnification image of the region inside the dashed line (F′) is shown in F″. (G–I) Tg(kdrl:EGFP) trunk vasculature at different developmental stages. At 7 dpf, most VTAs are formed (G, yellow arrows). At 11 dpf, sprouting vessels emerge between dorsal ISVs (H, yellow arrows), and these vessels have established connections with neighboring VTAs or ISVs by 15 dpf (I). (J) High-magnification image of Tg(kdrl:EGFP) trunk vasculature at 18 dpf. Vessels around the spinal cord are connected to VTAs (yellow arrows). (K and L) z-stack projection images of 25 dpf Tg(kdrl:EGFP) trunk vasculature. Blood vessels inside the spinal cord are shown in magenta. Pink arrows point to the sites where angiogenic sprouting into the spinal cord takes place. It mostly occurs from the dorsolateral sides of the PNVP. (M–O) Tg(gfap:NTR);Tg(kdrl:EGFP) trunk sections at different developmental stages. At 15 dpf, blood vessels are not observed inside the spinal cord (M). Blood vessel invasion into the spinal cord is observed by 20 dpf (N). Increased number and density of spinal cord vessels are observed at 30 dpf (O). (Scale bars, 100 µm in D and G–K, 50 µm in E and M–O, and 20 µm in F and F′.)
Fig. S3.
Characterization of VTAs and the PNVP. (A–A″) 8 dpf Tg(kdrl:EGFP);Tg(-0.8flt1:tdTomato) trunk vasculature. Vessels that exhibit strong Tg(-0.8flt1:tdTomato) expression comprise those of arterial identity, and VTAs also show strong Tg(-0.8flt1:tdTomato) expression (white arrows). (B–B″) 8 dpf Tg(kdrl:EGFP);Tg(lyve1:DsRed) trunk vasculature. Vessels that show Tg(lyve1:DsRed) expression comprise those of venous and lymphatic identity, and VTAs are not positive for Tg(lyve1:DsRed) expression (white arrows). (C) Percentage of VTAs that show strong Tg(-0.8flt1:tdTomato) expression at 8 dpf (132 VTAs quantified in 12 fish). All of the VTAs examined at 8 dpf show strong Tg(-0.8flt1:tdTomato) expression. (D and E) 11 dpf Tg(kdrl:EGFP) larval trunk vasculature. Sprouting vessels in the dorsal trunk emerge from ISVs or VTAs (yellow arrows). (F) High-magnification image of Tg(kdrl:EGFP) trunk vasculature at 15 dpf. Vessels that emerge around the spinal cord are connected to the VTAs (yellow arrows). (Scale bar, 100 µm.)
At later developmental stages (11 dpf), we found that VTAs, as well as ISVs, initiate a secondary angiogenic program that vascularizes the peri-neural tissue between the dorsal ISVs and subsequently forms the PNVP, a vascular network that surrounds the spinal cord (Fig. 2 G and H, Fig. S3 D and E, and Movie S2). By 15 dpf, the sprouting vessels that derive from VTAs and ISVs further extend and connect with neighboring VTAs and ISVs (Fig. 2I). Importantly, we found that VTAs serve as critical scaffolds for PNVP formation (Fig. 2J and Fig. S3F). At around 17–18 dpf, when the PNVP becomes established, EC invasion into the spinal cord begins in its dorsolateral sides (Movie S3). These spinal cord-invading ECs originate from the PNVP (Fig. 2 K and L and Movie S3), and vessel growth is maintained through later stages (Fig. 2 M–O and Movies S4 and S5). These observations show that the VTAs serve as important vascular scaffolds for the subsequent vascularization in and around the spinal cord.
Vegfab/Vegfr2 Signaling Is Necessary for VTA Formation.
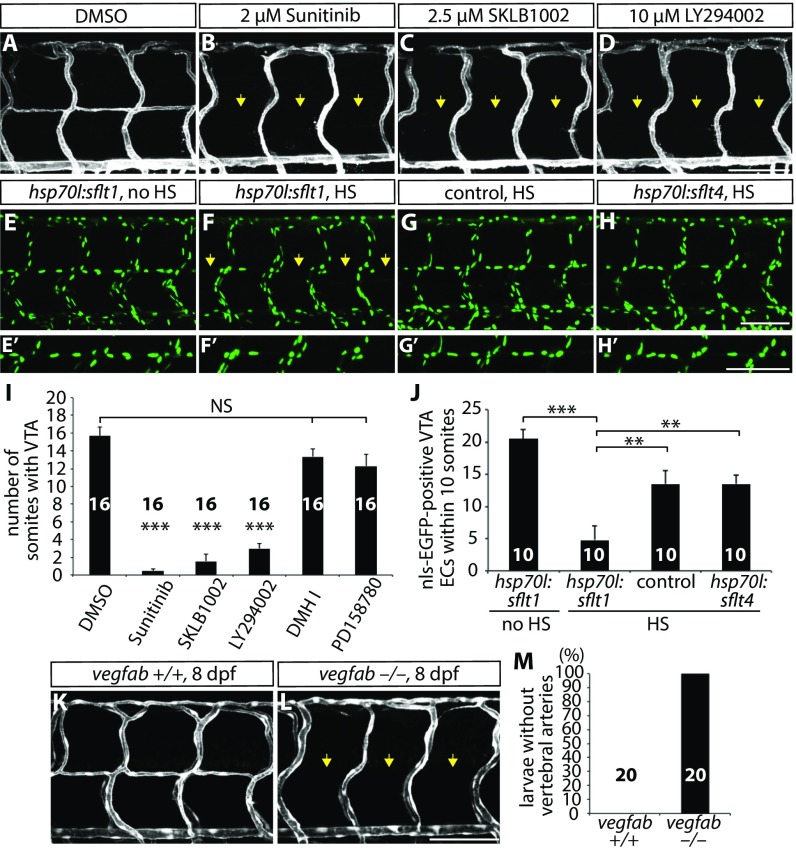
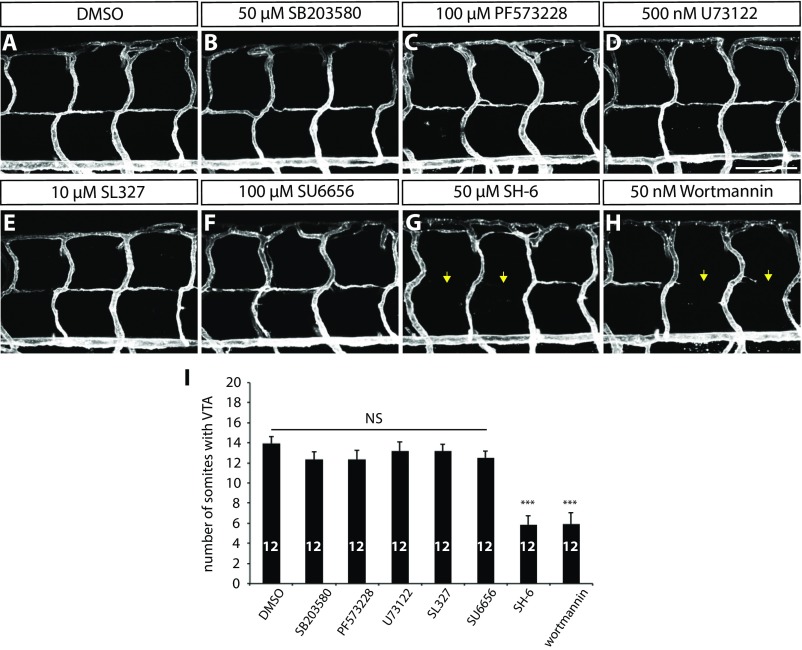
We next investigated how radial glia positively regulate VTA formation in vivo. To determine which angiogenic stimuli regulate VTA formation, we treated Tg(kdrl:ras-mcherry) fish with small molecules that inhibit distinct angiogenic signaling pathways. Specifically, we treated the larvae with the following compounds from 80 to 152 hpf: 2 µM sunitinib, a broad receptor tyrosine kinase inhibitor; 2.5 µM SKLB1002, a selective Vegfr2 inhibitor; 10 µM LY294002, a selective PI3 kinase inhibitor; 5 µM DMHI, a selective Bmp receptor inhibitor; and 3 µM PD158780, a selective ErbB receptor tyrosine kinase inhibitor. Among the chemicals tested, DMHI or PD158780 treatment did not affect VTA formation (Fig. 3I; n = 16 for each treatment group), suggesting that VTA formation is not regulated by BmpR or ErbB signaling. In contrast, VTA formation was dramatically inhibited in larvae treated with sunitinib, SKLB1002, or LY294002 (Fig. 3 A–D; quantification shown in Fig. 3I and Table S1; n = 16), suggesting that the Vegfr2 signaling pathway regulates this process. To investigate Vegfr2 effector pathways involved in VTA formation, we tested the following chemicals: 50 µM SB203580, a p38-MAPK inhibitor; 100 µM PF573228, a FAK inhibitor; 500 nM U73122, a PLC inhibitor; 10 µM SL327, a MEK1/2 inhibitor; 100 µM SU6656, a SRC kinase inhibitor; 50 µM SH-6, an AKT inhibitor; and 50 nM wortmannin, a PI3 kinase inhibitor. Interestingly, only when larvae were treated with SH-6 or wortmannin was VTA formation significantly inhibited (Fig. S4; n = 12). Similar results were obtained by treating the larvae with another PI3K inhibitor, LY294002 (Fig. 3D). Altogether, these data indicate that the PI3K-AKT pathway is a major Vegfr2 effector pathway required for VTA formation.
Fig. 3.
Vegfab/Vegfr2 signaling is required for VTA formation. (A–D) 152 hpf Tg(kdrl:ras-mcherry) trunk vasculature after treatment with DMSO (A), Sunitinib (B), SKLB1002 (C), or LY294002 (D) starting at 80 hpf. Sunitinib, SKLB1002, and LY294002 treatments significantly inhibit VTA formation (yellow arrows). (E–H) 178 hpf Tg(hsp70l:sflt1) (E and F), control (G), and Tg(hsp70l:sflt4) (H) trunk ECs visualized by Tg(kdrl:nls-EGFP) expression. Larvae were subjected to no heat shock (HS) (E) or multiple heat shocks (F–H). High-magnification images of ECs in the position of the VTAs are shown in E′–H′. Overexpression of sFlt-1 leads to a significantly reduced number of ECs in the position of the VTAs (yellow arrows, F). (I) Quantification of the average number of somites with VTAs for the experiments A–D (20 somites examined per animal). (J) Quantification of nls-EGFP+ EC number in the position of the VTAs within 10 somites per animal for the experiments E–H. (K and L) 8 dpf TgBAC(etv2:EGFP) vegfab+/+ (K) and vegfab−/− (L) trunk vasculature. All vegfab−/− larvae examined (n = 20) lacked VTAs (yellow arrows). (M) Percentage of larvae without VTAs, as judged by TgBAC(etv2:EGFP) expression. Values represent means ± SEM (I and J), **P < 0.01 and ***P < 0.001. (Scale bar, 100 µm.)
Fig. S4.
VTA formation in larvae treated with different small-molecule inhibitors. (A–H) 152 hpf Tg(kdrl:ras-mcherry) larval trunk vasculature after treatment with DMSO (A), SB203580 (B), PF573228 (C), U73122 (D), SL327 (E), SU6656 (F), SH-6 (G), and wortmannin (H) from 80 to 152 hpf. SH-6 and wortmannin treatments significantly inhibited VTA formation (yellow arrows). (Scale bar, 100 µm.) (I) Quantification of the average number of somites with VTAs for the experiments shown in A–H (20 somites examined per animal). Values represent means ± SEM (***P < 0.001 by ANOVA followed by Tukey’s HSD test).
We further tested these observations by using genetic tools that block Vegfr2 or Vegfr3 signaling in a temporally controlled manner. To this aim, we used two Tg lines: the Tg(hsp70l:sflt1) line, which upon heat shock overexpresses a soluble form of Vegfr1 (sFlt1), a decoy receptor for Vegfa, Vegfb, and PIGF (22), and the Tg(hsp70l:sflt4) line, which upon heat shock overexpresses a soluble form of Vegfr3 (sFlt4), a decoy receptor for Vegfc and Vegfd (22). We crossed the Tg(hsp70l:sflt1) and Tg(hsp70l:sflt4) lines with the Tg(kdrl:NLS-EGFP) line and heat shocked larvae every 12 h starting at 74 hpf until 7 dpf. We found that overexpression of sFlt1, but not sFlt4, significantly reduced the number of ECs that form VTAs (Fig. 3 E–H′; quantification shown in Fig. 3J and Table S1; n = 10). Altogether, these pharmacological and genetic data are consistent with a role for Vegfr2 signaling in VTA formation.
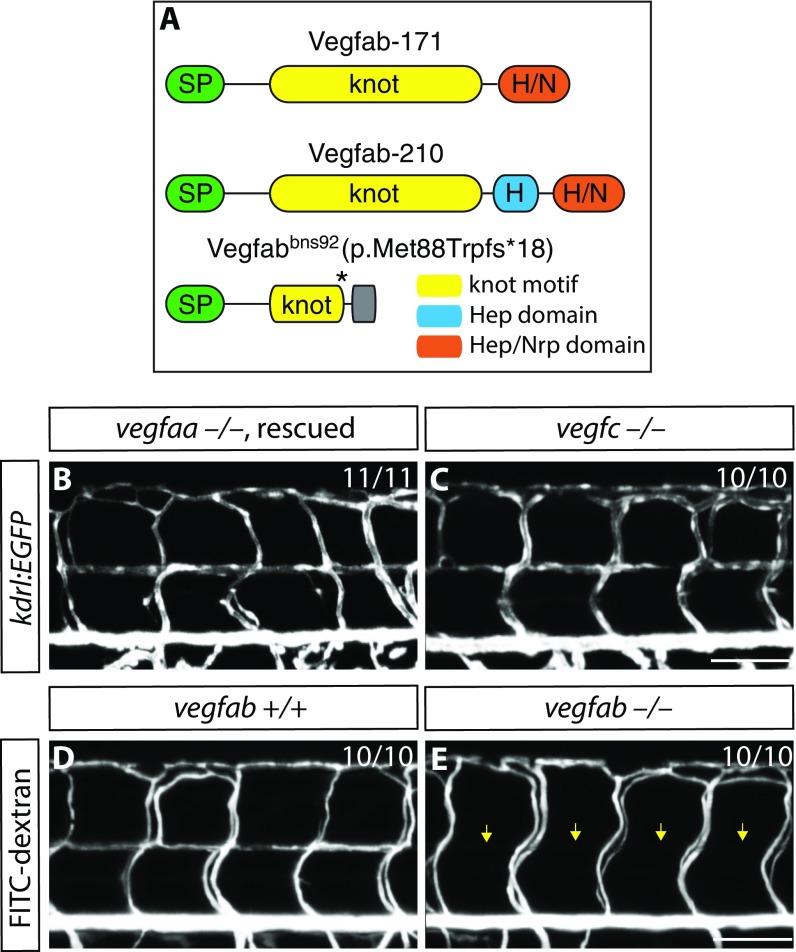
To identify the Vegfr2 ligand(s) regulating VTA formation, we analyzed different Vegfr2 ligand mutants, including vegfaabns1, vegfabbns92, and vegfchu6410 (23) mutant animals. We recently generated and characterized vegfaabns1 mutants that exhibit severe vasculogenesis and angiogenesis defects, leading to a lack of blood circulation, pericardial edema, and early larval death (24, 25). In addition, we generated a vegfabbns92 mutant allele by targeted genome editing using the CRISPR/Cas9 system (25). In this mutant, the cysteine residues critical for the knot motif are lost, thus affecting Vegf dimerization and function (25) (Fig. S5A). Therefore, vegfabbns92 is likely to be a severe or even a null allele (25). By carefully analyzing VTA formation in vegfaabns1, vegfabbns92, and vegfchu6410 mutant animals, we found that the vegfabbns92 mutants show a fully penetrant loss of VTA phenotype, as observed in radial glia-ablated fish (Fig. 3 K–M and Table S1; n = 20 for vegfab+/+ and vegfab−/−). We observed that VTAs never fully form in vegfabbns92 mutants examined up to 15 dpf (Fig. 3L). In contrast, vegfchu6410 or vegfaabns1 (a hsp70l:vegfaa165 transgene was used to rescue the early vasculogenesis and angiogenesis phenotypes of vegfaabns1 mutants by heat shock-induced overexpression of Vegfaa165 at 10 and 24 hpf) mutant animals do not exhibit a VTA formation defect [Fig. S5 B and C; n = 11 for Tg(hsp70l:vegfaa165) vegfaa−/− and n = 10 for vegfc−/− larvae]. We also documented the absence of lumenized VTAs in vegfabbns92 mutants by FITC-dextran microangiography (Fig. S5 D and E; n = 10). Thus, these analyses reveal that Vegfab signaling through Vegfr2 is essential for VTA formation.
Fig. S5.
VTA formation in vegfaabns1, vegfabbns92, and vegfchu6410 mutants. (A) Predicted domain structure of WT Vegfab isoforms -171, -210, and Vegfabbns92. Vegfab-171 and -210 consist of a signal peptide (SP), a knot motif, and heparin (Hep) and/or Hep/Nrp1 (Nrp) binding domains. vegfabbns92 encodes a truncated peptide (p.Met88Trpfs*18). (B and C) 8 dpf Tg(hsp70l:vegfaa165) vegfaa−/− (B) and vegfc−/− (C) larval trunk vasculature visualized by Tg(kdrl:EGFP) expression. Tg(hsp70l:vegfaa165) vegfaa−/− embryos were heat-shocked at 10 and 24 hpf to rescue their early vascular phenotype. VTAs are present in all of the vegfaa−/− (B; n = 11) and vegfc−/− (C; n = 10) larvae examined. (D and E) 8 dpf vegfab+/+ (D) and vegfab−/− (E) larval trunk vasculature visualized by FITC-dextran microangiography. vegfab−/− larvae lack lumenized VTAs (yellow arrows, E).
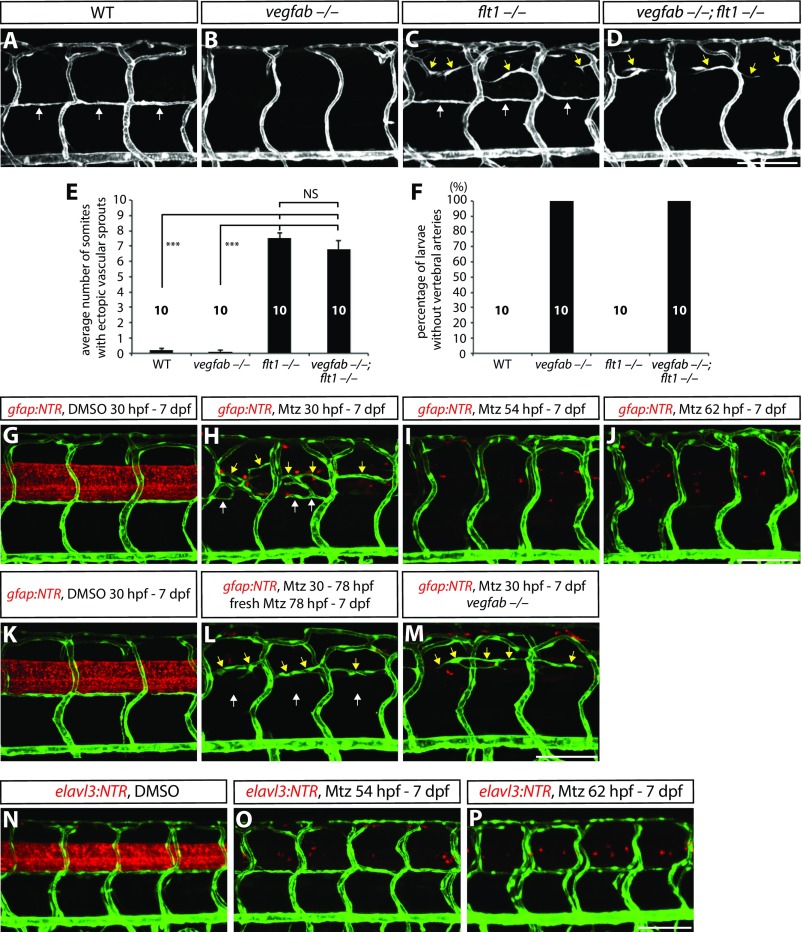
We next tested whether the depletion of sFlt1, a Vegf decoy receptor, rescues the lack of VTAs observed in vegfabbns92 mutants since a loss of sFlt1 causes ectopic vascular sprouting from dorsal ISVs (14, 26) and leads to increased Vegf bioavailability (22). To this aim, we analyzed flt1bns29;vegfabbns92 double mutants and observed that VTA formation was not rescued by increasing the bioavailability of other Vegfs (Fig. S6 A–D and F), providing further evidence that Vegfab is specifically required for VTA formation. We also observed that the ectopic ISV sprouting present in flt1bns29 mutants was also present in flt1bns29;vegfabbns92 double mutants (Fig. S6 A–E), suggesting that this ectopic sprouting process is regulated by Vegfs other than Vegfab. Furthermore, we found similar ISV oversprouting and/or a lack of VTA phenotypes when we performed radial glia ablation in Tg(gfap:NTR) animals by Mtz treatments starting at developmental stages earlier than 78 hpf (Figs. S6 G–M and S7), supporting a role for radial glia in the regulation of these two distinct processes. These phenotypes were not observed in larvae in which neurons were ablated by Mtz treatment starting at different developmental stages (14) (Fig. 1E and Fig. S6 N–P).
Fig. S6.
Ectopic ISV sprouting and VTA formation defects in larvae lacking flt1, vegfab, and/or radial glia. (A–D) 7 dpf WT (A), vegfabbns92/bns92 (B), flt1bns29/bns29 (C), and vegfabbns92/bns92;flt1bns29/bns29 (D) larval trunk vasculature visualized by TgBAC(etv2:EGFP) expression. VTAs are indicated by white arrows, ectopic vascular sprouts by yellow arrows. (E) Quantification of average number of somites that showed ectopic blood vessels in WT, vegfabbns92/bns92, flt1bns29/bns29, and vegfabbns92/bns92;flt1bns29/bns29 larvae (10 somites examined per animal; 10 animals examined per genotype). Values represent means ± SEM (*** indicates P < 0.001 by ANOVA followed by Tukey's HSD test). (F) Percentage of larvae without VTAs, as judged by TgBAC(etv2:EGFP) expression. All of the vegfab−/− and vegfab−/−;flt1−/− larvae lack VTAs. (G–J) 7 dpf Tg(gfap:NTR);Tg(kdrl:EGFP) animals that were treated with DMSO or Mtz starting at different developmental stages. Fish were treated with DMSO from 30 hpf to 7 dpf (G) or Mtz from 30 hpf to 7 dpf (H), 54 hpf to 7 dpf (I), or 62 hpf to 7 dpf (J). VTAs were completely absent in radial glia-ablated animals treated with Mtz from 54 hpf to 7 dpf (I) and 62 hpf to 7 dpf (J). VTAs were not correctly formed in radial glia-ablated animals treated with Mtz from 30 hpf to 7 dpf (H), yet vessels (white arrows) were occasionally observed near where VTAs usually form, in addition to ectopically sprouting vessels (yellow arrows) between the dorsal ISVs. (K and L) 7 dpf Tg(gfap:NTR);Tg(kdrl:EGFP) trunk vasculature after treatment with DMSO from 30 hpf to 7 dpf (K) or Mtz from 30 to 78 hpf followed by treatment with freshly prepared Mtz from 78 hpf to 7 dpf (L). Under these Mtz treatment conditions, a more complete ablation of radial glia was achieved (L) compared with the Mtz treatment shown in H. Although ectopic ISV sprouting was observed (yellow arrows, L), VTAs were absent under these Mtz treatment conditions (white arrows, L). (M) 7 dpf Tg(gfap:NTR);Tg(kdrl:EGFP) vegfab−/− trunk vasculature after treatment with Mtz from 30 hpf to 7 dpf. Although ectopic ISV sprouting was observed (yellow arrows), VTAs were absent in the radial glia-ablated vegfab−/− animal (M). This phenotype is similar to that observed in vegfabbns92/bns92;flt1bns29/bns29 larvae (D). (N–P) 7 dpf Tg(elavl3:NTR);Tg(kdrl:EGFP) animals that were treated with DMSO or Mtz starting at different developmental stages. Fish were treated with DMSO from 30 hpf to 7 dpf (N), or Mtz from 54 hpf to 7 dpf (O), or 62 hpf and 7 dpf (P). Unlike radial glia-ablated animals, Mtz-induced neuronal ablation starting at different developmental stages did not lead to a defect in trunk vascular patterning. (Scale bar, 100 µm.)
Fig. S7.
Trunk vascular patterning in 15 different radial glia-ablated animals after treatment with Mtz from 30 to 154 hpf. 154 hpf Tg(gfap:NTR);Tg(kdrl:EGFP) animals were treated with Mtz from 30 to 154 hpf. Confocal images were taken using a 20× (A–O) or 40× (A′–O′) objective lens. The region inside the dashed line in A is shown in A′, and the other 40× images (B′–O′) also correspond to the area that covers the four posterior ISVs shown in B–O. VTA formation was affected in all of the animals examined, although vessels were occasionally, and variably, observed in positions where VTAs usually form. Left to right in each image: anterior to posterior parts of the trunk. (Scale bar, 100 µm.)
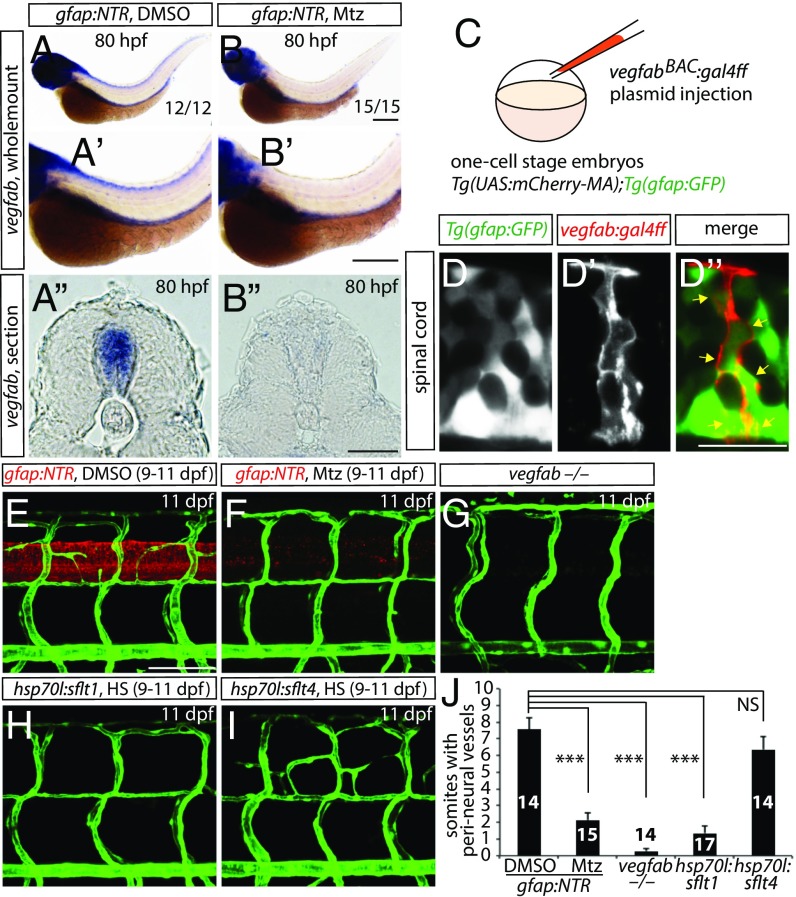
vegfab Is Expressed by Radial Glia, and Radial Glia Ablation Leads to Greatly Reduced vegfab Expression Within the Spinal Cord.
To examine the expression pattern of vegfab, we performed whole-mount in situ hybridization at 80 hpf when VTA formation takes place. We found that vegfab is strongly expressed in the head, spinal cord, and endoderm-derived organs at 80 hpf (Fig. 4 A–A″) and that vegfab expression in the brain and spinal cord, but not in endoderm-derived organs, is markedly reduced in radial glia-ablated fish (Fig. 4 A–B″). These data indicate that radial glia serve as a source of vegfab and/or regulate its expression within the spinal cord to promote VTA formation. To investigate whether vegfab is expressed in radial glia, we generated a BAC construct containing the vegfab regulatory elements and driving expression of Gal4ff and injected it into Tg(gfap:GFP);Tg(14XUAS:mCherry-MA) one-cell stage embryos (Fig. 4C). We found that mCherry-positive spinal cord cells were colabeled by Tg(gfap:GFP) expression at the stages when VTA formation occurs (3–7 dpf) as well as at later stages including 10 and 13 dpf (Fig. 4 D–D″). These data indicate that vegfab is expressed by spinal cord radial glia. Taken together, these findings suggest that radial glia serve as a source of Vegfab for VTA formation, which is dependent on Vegfab/Vegfr2 signaling.
Fig. 4.
vegfab is expressed by radial glia, and radial glia-ablated fish as well as vegfab mutants fail to develop a PNVP around the spinal cord. (A and B) Whole-mount in situ hybridization for vegfab expression in 80 hpf Tg(gfap:NTR) larvae treated with DMSO (A) or Mtz (B) starting at 30 hpf. High-magnification images of the trunk regions and their cryosections are shown in (A′ and B′) and (A″ and B″), respectively. Mtz-treated fish show decreased vegfab transcripts in the spinal cord compared with DMSO-treated fish. (C) Schematic diagram of the plasmid injection experiments (D–D″). (D–D″) 105 hpf Tg(UAS:mcherry-MA);Tg(gfap:GFP) trunk of fish injected with the vegfab BAC plasmid that drives Gal4ff. Injection of the plasmid drives mCherry expression in cells within the spinal cord (D′), which are also positive for Tg(gfap:GFP) expression (D). Most mCherry+ cells are also GFP+ (D″, yellow arrows). (E–I) 11 dpf Tg(gfap:NTR) trunk vasculature after treatment with DMSO (E) or Mtz (F) starting at 9 dpf; 11 dpf TgBAC(etv2:EGFP) vegfab−/− (G), Tg(kdrl:EGFP);Tg(hsp70l:sflt1) (H), and Tg(kdrl:EGFP);Tg(hsp70l:sflt4) (I) trunk vasculature after heat shocks (H and I). DMSO-treated control fish display vessels that emerge between dorsal ISVs around the spinal cord; however, vessel formation is severely impaired in radial glia-ablated larvae as well as in vegfab−/− animals. Overexpression of sFlt1 also blocked PNVP formation. (J) Quantification of the average number of somites with peri-neural blood vessels (10 somites examined per animal). Values represent means ± SEM, ***P < 0.001. (Scale bars, 200 µm in B–B″, 100 µm in E, and 20 µm in D″.)
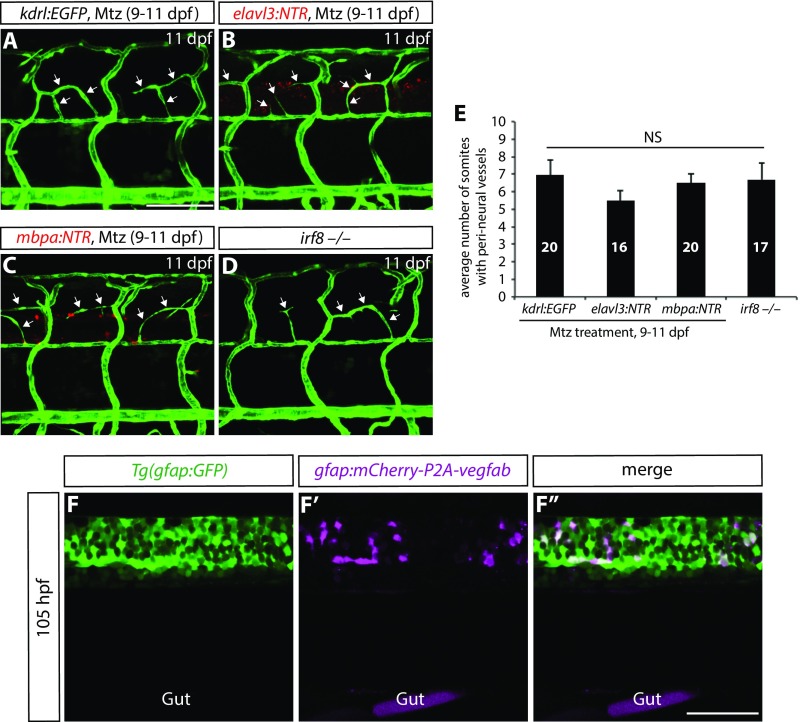
Radial Glia Ablation at Later Stages Leads to Compromised PNVP Formation.
We next asked whether PNVP formation was regulated by radial glia. To this aim, we treated Tg(gfap:NTR) larvae with DMSO or 2 mM Mtz from 9 to 11 dpf. Interestingly, we found that DMSO-treated larvae exhibited robust sprouting of vessels between the dorsal ISVs at 11 dpf (Fig. 4E; n = 14); however, radial glia-ablated fish showed a dramatically reduced number of these sprouting vessels (Fig. 4F; n = 15). In contrast, we did not observe a significant reduction in the number of these sprouting vessels in larvae in which neurons, oligodendrocytes, or microglia were ablated (Fig. S8 A–E; n = 16–20). These findings suggest that radial glia also direct PNVP formation. Mechanistically, we observed that vegfabbns92 mutants exhibited both VTA and PNVP formation defects (Fig. 4G; n = 14) and that genetic inhibition of Vegfr2, but not Vegfr3, signaling after VTA formation by overexpression of sFlt1 or sFlt4, respectively, blocked PNVP formation (Fig. 4 H–J and Table S1; n = 14–17). Taken together, these findings suggest that radial glia control the formation of both VTAs and PNVP in the mesodermal tissues around the spinal cord through Vegfab/Vegfr2 signaling and before the vascularization of the spinal cord.
Fig. S8.
Ablation of neurons, oligodendrocytes, or microglia does not lead to compromised PNVP formation; specific expression of gfap:mCherry-P2A-Vegfab in Tg(gfap:GFP)+ radial glia. (A–D) 11 dpf Tg(kdrl:EGFP) (A), Tg(elavl3:NTR);Tg(kdrl:EGFP) (B), and Tg(mbpa:NTR);Tg(kdrl:EGFP) (C) larval trunk vasculature after treatment with Mtz from 9 to 11 dpf. 11 dpf Tg(kdrl:EGFP) irf8−/− trunk vasculature is shown in D. Mtz-treated control fish (A) as well as larvae in which neurons (B), oligodendrocytes (C), or microglia (D) were depleted exhibit vessels (white arrows) that emerge between the dorsal ISVs around the spinal cord. (E) Quantification of the average number of somites with peri-neural blood vessels (10 somites examined per animal). Quantification was performed at 11 dpf. Values represent means ± SEM. (F–F″) 105 hpf trunk of Tg(gfap:GFP) fish injected with the gfap:mCherry-P2A-vegfab plasmid. Injection of the gfap:mCherry-P2A-vegfab plasmid drives mCherry expression in cells within the spinal cord (F′), which are also positive for Tg(gfap:GFP) expression (F). All mCherry+ cells are also GFP+ (F″). (Scale bars, 100 µm in A and 50 µm in F″.)
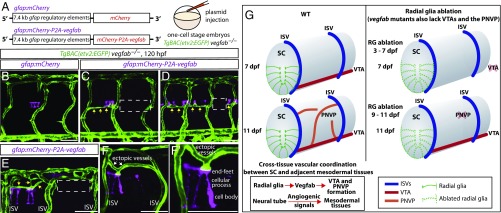
Mosaic Overexpression of Vegfab in Radial Glia Is Sufficient to Partially Rescue the Loss of VTAs in vegfab Mutants and Induce Ectopic Blood Vessels Directed Toward Their Endfeet.
To test whether forced Vegfab expression in spinal cord radial glia was sufficient to rescue the lack of VTAs observed in vegfabbns92 mutants, we generated plasmids that drive the expression of mCherry or mCherry-P2A-Vegfab under the control of the gfap promoter (27) (Fig. 5A). Injection of these plasmids into one-cell stage embryos led to mosaic overexpression of these transgenes in spinal cord radial glia as confirmed by mCherry expression in Tg(gfap:GFP)-positive radial glia (Fig. S8 F–F″). By injecting these plasmids into one-cell stage vegfabbns92 mutant embryos, we observed that mosaic overexpression of mCherry-P2A-Vegfab, but not mCherry, in radial glia was sufficient to rescue the absence of VTAs in vegfabbns92 mutants, leading to a partial recovery of VTAs (Fig. 5 B–D). Intriguingly, we also observed that Vegfab overexpression in radial glia led to the appearance of ectopic blood vessels in tissues adjacent to the spinal cord and that these ectopic vessels were directed preferentially toward their endfeet (Fig. 5 E–F′). Collectively, our data indicate that Vegfab derived from radial glia is presented to extraneural vessels through their endfeet.
Fig. 5.
Mosaic overexpression of Vegfab in radial glia is sufficient to partially rescue the loss of VTAs in vegfab mutants and induce ectopic blood vessels directed toward their endfeet. (A) Schematic diagram of the gfap:mCherry and gfap:mCherry-P2A-vegfab plasmid injection experiments (B–F′). (B–F′) 120 hpf TgBAC(etv2:EGFP) trunks of vegfab−/− fish injected with the gfap:mCherry (B) or gfap:mCherry-P2A-vegfab (C–F′) plasmid. Partial recovery of VTAs was observed in the vegfab−/− fish injected with the gfap:mCherry-P2A-vegfab plasmid (yellow arrows, C and D), while VTAs were absent in the vegfab−/− fish injected with the gfap:mCherry plasmid (B). As observed in the regions inside the dashed lines (C and D), VTAs were absent in the somites where mCherry+ radial glia were not present in close proximity and/or sufficient numbers. Ectopic blood vessel sprouting toward mCherry+ radial glia overexpressing Vegfab (yellow arrows, E) was not observed in somites lacking mCherry+ radial glia (e.g., the region inside the dashed white line in E). Ectopic vessels (white arrows) were directed toward the endfeet of mCherry+ radial glia overexpressing Vegfab (F and F′). (Scale bar, 50 µm.) (G) Schematic diagrams showing radial glia regulation of angiogenesis around the developing spinal cord. During development, radial glia endfeet lie in close proximity to the forming VTAs. Radial glia ablation in early larval stages leads to the loss of the VTAs. Radial glia ablation at later stages impairs PNVP formation. vegfab mutants phenocopy the vascular defects observed in radial glia-ablated larvae. Thus, radial glia act as positive angiogenic regulators in mesodermal tissues around the spinal cord, which in turn allows spinal cord vascularization. Vessels are shown only on one side of the spinal cord. RG, radial glia; SC, spinal cord.
Discussion
In this work, we identify a previously unappreciated role for CNS-resident progenitors in driving angiogenesis in tissues adjacent to the CNS (Fig. 5G). Specifically, we show that CNS radial glia ablation impairs the formation of the VTAs and PNVP, that Vegfab/Vegfr2 signaling mediates these angiogenic processes, that radial glia express vegfab at the stages when these vascular events occur, and that overexpression of Vegfab in radial glia is sufficient to rescue the loss of VTAs in vegfabbns92 mutants. Importantly, we find that these radial glia-directed angiogenic events are closely linked to spinal cord vascularization and provide high-resolution 3D reconstructions of the vasculature during these processes (Movies S1–S5). These findings will significantly advance our knowledge of the angiogenic steps leading to spinal cord vascularization.
How are the vascular networks around the CNS established during development? Previous work using neural tube and presomitic mesoderm cocultures has suggested that the developing neural tube provides VEGF signals to recruit the ECs that form the PNVP (4). Adding to this prior finding, we showed in vivo that ablation of radial glia, but not of other differentiated CNS cell types, leads to a defect in angiogenesis in tissues adjacent to the spinal cord, a process that is dependent on Vegfab/Vegfr2 signaling. These findings provide clear evidence that CNS-resident progenitors direct angiogenic processes not only within the CNS as previously described (5–9) but also in tissues outside the CNS, significantly adding to our understanding of the function of CNS progenitors during development. Also, these observations serve as an important example for a mechanism by which tissue vascularization is coordinated by interactions between neighboring tissues during development.
How are the angiogenesis events around the spinal cord spatiotemporally regulated? We found that angiogenesis in the mesodermal tissues around the spinal cord proceeds in a tightly controlled manner. After ISVs are formed, VTAs develop by angiogenic sprouting from ISVs. After VTA formation is complete, peri-neural vessels emerge from both ISVs and VTAs to form the PNVP around the spinal cord by connecting with these vessels. When these angiogenic steps are complete, spinal cord vascularization begins by angiogenic sprouting from the established PNVP into the spinal cord. Our data show that radial glia initiate this multistep angiogenic process at least in part via Vegfab. Given the observation that forced Vegfab expression in radial glia induced ectopic blood vessels directed toward their endfeet (Fig. 5 E–F′), we speculate that Vegfab secreted from radial glia may be presented to extraneural vessels through their endfeet to guide VTA and PNVP formation. VTA formation was not rescued by other Vegfs, such as Vegfaa, in the absence of Vegfab (Fig. S6 D and F), possibly because Vegfaa and Vegfab appear to have different properties (25). The Vegf decoy receptor sFlt1 was recently shown to limit the oversprouting of the dorsal ISVs around the spinal cord during early larval stages (3–6 dpf) (14, 26). In sflt1 mutants, sprouting vessels emerge from the ISVs around the spinal cord by 6 dpf (14, 26), in contrast to WT fish where this sprouting occurs at much later stages (10–15 dpf), indicating that sFlt1 is, at least in part, involved in the spatiotemporal regulation of these angiogenic processes.
We recently reported that radial glia ablation initiated at 30 hpf leads to selective oversprouting of venous ISVs around the spinal cord, showing a role for radial glia as negative angiogenic regulators (14). In contrast, here we show that inducing radial glia ablation at later stages leads to impaired formation of VTAs, indicating a role for radial glia as positive angiogenic regulators. These two contrasting observations could indicate that (i) spinal cord radial glia control angiogenesis differentially at distinct developmental stages, (ii) different ECs (i.e., venous vs. arterial) respond differently to the loss of radial glia, and/or (iii) distinct populations of radial glia extend their endfeet in close proximity to ISVs or VTAs and affect these vessels differently. When Tg(gfap:NTR) animals were treated with Mtz starting at 30 hpf, we occasionally observed vessels emerging in positions where VTAs form (Figs. S6H and S7); however, these vessels did not appear when we replaced the Mtz with freshly prepared Mtz at 78 hpf (Fig. S6L), suggesting that a more complete ablation of radial glia starting at 30 hpf abrogates VTA formation, an interpretation different from the one which might be drawn from looking at Fig. 2C in our previous report (14). Notably and consistent with our previous findings (14), radial glia ablation induced under this stronger Mtz treatment led to ectopic vISV sprouting (Fig. S6L).
Altogether, our identification of a critical role for CNS-resident progenitors in regulating angiogenesis in adjacent mesodermal tissues will advance our understanding of the mechanisms governing vascular network formation, in particular how tissue vascularization between adjacent organs is coordinated during development.
Materials and Methods
Detailed materials and methods are available in SI Materials and Methods.
Zebrafish.
Procedures involving animals were approved by the veterinary department of the Regional Board of Darmstadt.
Mtz Treatment.
Mtz treatment was performed as described previously (14).
Heat Shock Treatments.
Heat shock treatments were performed as previously described (14).
Statistical Analysis.
Statistical differences for mean values among multiple groups were determined using a one-way analysis of variance (ANOVA) followed by Tukey’s multiple comparison test.
SI Materials and Methods
Zebrafish Husbandry and Tg Lines.
All zebrafish husbandry was performed under standard conditions in accordance with institutional (University of California, San Francisco, and Max Planck Society) and national ethical and animal welfare guidelines. Tg(kdrl:EGFP)s843 (28), Tg(kdrl:NLS-EGFP)ubs1 (29), Tg(kdrl:Has.HRAS-mcherry)s896 (30), TgBAC(etv2:EGFP)ci1 (31), Tg(-0.8flt1:tdTomato)hu5333 (32), Tg(lyve1:DsRed)nz101 (33), TgBAC(gfap:gal4ff)s995 (14), TgBAC(gfap:gfap-gfp)zf167 (19), Tg(gfap:GFP)mi2001 (27), Tg(elavl3:gal4-vp16)psi1 (34), Tg(elavl3:EGFP)knu3 (35), Tg(mbpa:gal4-vp16)km5 (36), Tg(UAS-E1b:NfsB-mCherry)c264 (37), Tg(hsp70l:sflt1, cryaa-cerulean)bns80 (14), Tg(hsp70l:sflt4, cryaa-cerulean)bns82 (14), Tg(14XUAS:mCherry-MA)s1984t (38), Tg(-4.9sox10:EGFP)ba2 (39), irf8st96 (20), vegfaabns1 (25), vegfabbns92 (25), vegfchu6410 (23), and flt1bns29 (14) lines were used in this study. Fish embryos/larvae were raised at 28.5 °C. Fish larvae up to 8 dpf were analyzed without any feeding. Fish larvae analyzed beyond 8 dpf were transferred to a flowing water system and fed with SDS 100 (Special Diet Services) starting at 5 dpf. Fish were additionally fed twice daily with hatched brine shrimp (Claus GmbH) starting at 12 dpf.
Generation of the Tg(hsp70l:vegfaa165)s712 Line.
The Tg(hsp70l:vegfaa165)s712 line was generated by subcloning the coding sequence of vegfaa165 downstream of the zebrafish hsp70l promoter between AscI and PacI sites, in a pBluescript backbone containing I-Sce I meganuclease sites and the cryaa:cerulean cassette. The construct was injected into one-cell stage embryos with I-Sce I meganuclease (NEB) supplemented with 1× cut smart NEB buffer.
Mtz Treatment.
Mtz substrate (M3761; Sigma) was used at 2 mM dissolved in egg water containing 1% DMSO for all of the cell ablation experiments in this study. Larvae were incubated with freshly prepared 2 mM Mtz or 1% DMSO in egg water for all cell ablation experiments. For most experiments, larvae were treated with the Mtz solution without replacing it. For some experiments, the 2 mM Mtz solution was replaced with a freshly prepared solution at the time points indicated. Although a minority of the animals developed prominent pericardial edema and/or severe gross anatomical abnormalities after radial glia or neuronal ablation, a majority did not exhibit these abnormalities, except uninflated swim bladders and bent tails. We analyzed only the animals that did not exhibit pericardial edema or severe gross anatomical abnormalities.
Immunohistochemistry.
Immunohistochemistry was performed as previously described (40). The following primary antibody was used: mouse anti-HuC/HuD (Millipore at 1:200).
In Situ Hybridization.
Whole-mount in situ hybridization was performed as previously described (41). A digoxigenin (DIG)-labeled cRNA antiprobe for vegfab was transcribed in vitro using a DIG RNA labeling kit (Roche). Selected vegfab DNA sequences (687 bp) were amplified using the following primers: 5′-ATGTCTAACTTGCTTTCTGAGACC-3′ and 5′-TCACCTCCTTGGTTTGTCACATCTG-3′.
Confocal and Stereo Microscopy.
An LSM 700 confocal laser scanning microscope (Zeiss) was used for live and immunofluorescence imaging as previously described (14). Briefly, fish larvae were anesthetized with a low dose of tricaine, placed in a glass-bottom Petri dish (MatTek) with a layer of 1.2% low melt agarose, and imaged using W N-ACP 20×/0.5 and W Plan-Apochromat 40×/1.0 objective lenses. For FITC-Dextran microangiography, fluorescein isothiocyanate (FITC)-dextran, 2,000 kDa (Sigma), was injected into the common cardinal vein and imaged after 10 min.
Heat Shock Treatments.
Heat shock treatments were performed as previously described (14). Heat shock experiments for examining the number of ECs that form VTAs were performed as follows: Tg(hsp70l:sflt1, cryaa-cerulean)bns80;Tg(kdrl:NLS-EGFP)ubs1 and Tg(hsp70l:sflt4, cryaa-cerulean) bns82;Tg(kdrl:NLS-EGFP)ubs1 larvae, and their siblings were subject to one heat shock every 12 h starting at 74 until 178 hpf. Heat shock experiments for examining PNVP formation were performed as follows: Tg(hsp70l:sflt1, cryaa-cerulean)bns80;Tg(kdrl:EGFP)s843 and Tg(hsp70l:sflt4, cryaa-cerulean) bns82;Tg(kdrl:EGFP)s843 larvae were subject to one heat shock every 12 h from 9 until 11 dpf.
Pharmacological Inhibitor Treatments.
Pharmacological experiments that examined VTA formation were performed as follows: Tg(kdrl:Has.HRAS-mcherry)s896 larvae were treated with pharmacological inhibitors from 80 to 152 hpf by replacing the drug solution every 24 h. Final concentrations of the chemicals used were as follows: 2 µM sunitinib (Sigma), 2.5 µM SKLB1002 (Calbiochem), 10 µM LY294002 (Calbiochem), 5 µM DMHI (Calbiochem), 3 µM PD158780 (Tocris), 50 µM SB203580 (Sigma), 100 µM PF573228 (Sigma), 500 nM U73122 (Sigma), 10 µM SL327 (Sigma), 100 µM SU6656 (Sigma), 50 µM SH-6 (Enzo Life Sciences), and 50 nM wortmannin (Sigma). All listed chemicals were first prepared as 100× stock solutions dissolved in 100% DMSO and then diluted 100 times in egg water shortly before use. For the quantification of VTAs, the 10 somites directly anterior to the anal opening on both the left and right sides of the body were used for quantification (total 20 somites). Only the somites with a fully formed VTA were counted as positive.
Plasmid Injections.
For vegfab BAC plasmid injection, the DKEY-234H13 BAC clone (Source Bioscience) was engineered to replace the vegfab start codon with gal4ff by following the BAC recombineering protocol described previously (42). The engineered construct was injected into one-cell stage embryos from Tg(14XUAS:mCherry-MA)s1984t fish crossed to Tg(gfap:GFP)mi2001 fish, together with transposase mRNA transcribed in vitro. For gfap:mCherry and gfap:mCherry-P2A-vegfab plasmid injection, these plasmids were generated by cloning the sequence encoding mCherry or mCherry-P2A-Vegfab171 downstream of ∼7.4 kb gfap regulatory elements (27) flanked by tol2 sites. The 7.4 kb gfap regulatory elements were amplified by PCR using the gfap:GFP expression vector (27) as a template. The engineered constructs were injected into one-cell stage embryos from incrossed TgBAC(etv2:EGFP) vegfab−/− fish, together with transposase mRNA transcribed in vitro.
Quantification of Larvae Without VTAs.
Tg(kdrl:EGFP)s843 or TgBAC(etv2:EGFP)ci1 EC reporter fish were used to quantify larvae without VTAs. For the quantification after radial glia ablation or neuronal ablation, Tg(kdrl:EGFP)s843 fish were crossed to TgBAC(gfap:gal4ff)s995;Tg(UAS-E1b:NfsB-mCherry)c264 fish or Tg(elavl3:gal4-vp16)psi1;Tg(UAS-E1b:NfsB-mCherry)c264 fish, respectively, and treated with 2 mM Mtz from 78 to 178 hpf. The 10 somites directly anterior to the anal opening were examined on both sides of the body (defined as 10 somites), and the larvae without a fully formed VTA were quantified.
Quantification of EC Numbers.
Tg(kdrl:NLS-EGFP)ubs1 reporter fish were used to quantify the number of ECs. For the quantification of EC number after radial glia or neuronal ablation, Tg(kdrl:NLS-EGFP)ubs1 fish were crossed to TgBAC(gfap:gal4ff)s995;Tg(UAS-E1b:NfsB-mCherry)c264 or Tg(elavl3:gal4-vp16)psi1;Tg(UAS-E1b:NfsB-mCherry)c264 fish, respectively. For the quantification of EC number following the overexpression of sFlt1 or sFlt4, Tg(kdrl:NLS-EGFP)ubs1 fish were crossed to Tg(hsp70l:sflt1, cryaa-cerulean)bns80 or Tg(hsp70l:sflt4, cryaa-cerulean)bns82 fish, respectively. The 10 somites directly anterior to the anal opening were examined on both sides of the body (defined as 10 somites) to quantify the number of ECs in the position of the VTAs.
Quantification of Somites with Peri-Neural Blood Vessels.
Tg(kdrl:EGFP)s843 or TgBAC(etv2:EGFP)ci1 EC reporter fish were used to quantify the number of somites with peri-neural blood vessels at 11 dpf. For the quantification after radial glia ablation, Tg(kdrl:EGFP)s843 fish were crossed to TgBAC(gfap:gal4ff)s995;Tg(UAS-E1b:NfsB-mCherry)c264 fish and treated with 2 mM Mtz from 9 to 11 dpf. For the quantification after neuronal or oligodendrocyte ablation, Tg(kdrl:EGFP)s843 fish were crossed to Tg(elavl3:gal4-vp16)psi1;Tg(UAS-E1b:NfsB-mCherry)c264 or Tg(mbpa:gal4-vp16)km5;Tg(UAS-E1b:NfsB-mCherry)c264 fish, respectively, and treated with 2 mM Mtz from 9 to 11 dpf. The 10 somites directly anterior to the anal opening were examined at 11 dpf (defined as 10 somites), and the number of somites with peri-neural blood vessels in either side of the body was quantified and averaged.
Quantification of Ectopic Vessels.
TgBAC(etv2:EGFP)ci1 EC reporter fish were used to quantify ectopic ISV sprouts in WT, vegfabbns92, flt1bns29, and vegfabbns92;flt1bns29 mutant animals. The 11 ISVs directly anterior to the anal opening were used for quantification (defined as 10 somites), and the number of somites that showed ectopic blood vessels in either side of the body was quantified at 7 dpf and averaged.
3D Reconstruction of the Trunk Vasculature.
Tg(kdrl:EGFP)s843 EC reporter fish larvae were imaged using a LSM700 confocal microscope with a W Plan-Apochromat 40×/1.0 objective lens. Z-plane images were collected at every 1-µm interval and 3D reconstructed using the Imaris software (Bitplane). Blood vessels inside the spinal cord were colored in magenta using the masking function in Imaris.
Statistical Analysis.
Statistical differences for mean values among multiple groups were determined using a one-way analysis of variance (ANOVA) followed by Tukey’s multiple comparison test. The criterion for statistical significance was set at P < 0.05. Values represent means ± SEM. Error bars are SEM.
Supplementary Material
Acknowledgments
We thank Hae-Chul Park for fish lines, Petra Neeb and Rebecca Lee for technical assistance, and Michele Marass and Arica Beisaw for comments on the manuscript. This work was supported by funding from the Damon Runyon Cancer Research Foundation (DRG#2104-12), Human Frontier Science Program (LT001023/2012-L), Japan Society for the Promotion of Science, Excellence Cluster Cardio-Pulmonary System, and German Centre for Cardiovascular Research (to R.L.M.) and NIH Grant HL54737, the Packard Foundation, and the Max Planck Society (to D.Y.R.S.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1619300114/-/DCSupplemental.
References
- 1.Feeney JF, Jr, Watterson RL. The development of the vascular pattern within the walls of the central nervous system of the chick embryo. J Morphol. 1946;78:231–303. doi: 10.1002/jmor.1050780205. [DOI] [PubMed] [Google Scholar]
- 2.Strong LH. The early embryonic pattern of internal vascularization of the mammalian cerebral cortex. J Comp Neurol. 1964;123:121–138. doi: 10.1002/cne.901230111. [DOI] [PubMed] [Google Scholar]
- 3.Kurz H, Gärtner T, Eggli PS, Christ B. First blood vessels in the avian neural tube are formed by a combination of dorsal angioblast immigration and ventral sprouting of endothelial cells. Dev Biol. 1996;173:133–147. doi: 10.1006/dbio.1996.0012. [DOI] [PubMed] [Google Scholar]
- 4.Hogan KA, Ambler CA, Chapman DL, Bautch VL. The neural tube patterns vessels developmentally using the VEGF signaling pathway. Development. 2004;131:1503–1513. doi: 10.1242/dev.01039. [DOI] [PubMed] [Google Scholar]
- 5.Haigh JJ, et al. Cortical and retinal defects caused by dosage-dependent reductions in VEGF-A paracrine signaling. Dev Biol. 2003;262:225–241. doi: 10.1016/s0012-1606(03)00356-7. [DOI] [PubMed] [Google Scholar]
- 6.Raab S, et al. Impaired brain angiogenesis and neuronal apoptosis induced by conditional homozygous inactivation of vascular endothelial growth factor. Thromb Haemost. 2004;91:595–605. doi: 10.1160/TH03-09-0582. [DOI] [PubMed] [Google Scholar]
- 7.Stenman JM, et al. Canonical Wnt signaling regulates organ-specific assembly and differentiation of CNS vasculature. Science. 2008;322:1247–1250. doi: 10.1126/science.1164594. [DOI] [PubMed] [Google Scholar]
- 8.Daneman R, et al. Wnt/beta-catenin signaling is required for CNS, but not non-CNS, angiogenesis. Proc Natl Acad Sci USA. 2009;106:641–646. doi: 10.1073/pnas.0805165106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liebner S, et al. Wnt/beta-catenin signaling controls development of the blood-brain barrier. J Cell Biol. 2008;183:409–417. doi: 10.1083/jcb.200806024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lacoste B, et al. Sensory-related neural activity regulates the structure of vascular networks in the cerebral cortex. Neuron. 2014;83:1117–1130. doi: 10.1016/j.neuron.2014.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Whiteus C, Freitas C, Grutzendler J. Perturbed neural activity disrupts cerebral angiogenesis during a postnatal critical period. Nature. 2014;505:407–411. doi: 10.1038/nature12821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ye X, et al. Norrin, frizzled-4, and Lrp5 signaling in endothelial cells controls a genetic program for retinal vascularization. Cell. 2009;139:285–298. doi: 10.1016/j.cell.2009.07.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang Y, et al. Norrin/Frizzled4 signaling in retinal vascular development and blood brain barrier plasticity. Cell. 2012;151:1332–1344. doi: 10.1016/j.cell.2012.10.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Matsuoka RL, et al. Radial glia regulate vascular patterning around the developing spinal cord. eLife. 2016;5:e20253. doi: 10.7554/eLife.20253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Curado S, et al. Conditional targeted cell ablation in zebrafish: A new tool for regeneration studies. Dev Dyn. 2007;236:1025–1035. doi: 10.1002/dvdy.21100. [DOI] [PubMed] [Google Scholar]
- 16.Pisharath H, Rhee JM, Swanson MA, Leach SD, Parsons MJ. Targeted ablation of beta cells in the embryonic zebrafish pancreas using E. coli nitroreductase. Mech Dev. 2007;124:218–229. doi: 10.1016/j.mod.2006.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chapman DL, Papaioannou VE. Three neural tubes in mouse embryos with mutations in the T-box gene Tbx6. Nature. 1998;391:695–697. doi: 10.1038/35624. [DOI] [PubMed] [Google Scholar]
- 18.Than-Trong E, Bally-Cuif L. Radial glia and neural progenitors in the adult zebrafish central nervous system. Glia. 2015;63:1406–1428. doi: 10.1002/glia.22856. [DOI] [PubMed] [Google Scholar]
- 19.Lam CS, März M, Strähle U. gfap and nestin reporter lines reveal characteristics of neural progenitors in the adult zebrafish brain. Dev Dyn. 2009;238:475–486. doi: 10.1002/dvdy.21853. [DOI] [PubMed] [Google Scholar]
- 20.Shiau CE, Kaufman Z, Meireles AM, Talbot WS. Differential requirement for irf8 in formation of embryonic and adult macrophages in zebrafish. PLoS One. 2015;10:e0117513. doi: 10.1371/journal.pone.0117513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schebesta M, Serluca FC. olig1 expression identifies developing oligodendrocytes in zebrafish and requires hedgehog and notch signaling. Dev Dyn. 2009;238:887–898. doi: 10.1002/dvdy.21909. [DOI] [PubMed] [Google Scholar]
- 22.Ruiz de Almodovar C, Lambrechts D, Mazzone M, Carmeliet P. Role and therapeutic potential of VEGF in the nervous system. Physiol Rev. 2009;89:607–648. doi: 10.1152/physrev.00031.2008. [DOI] [PubMed] [Google Scholar]
- 23.Helker CSM, et al. The zebrafish common cardinal veins develop by a novel mechanism: Lumen ensheathment. Development. 2013;140:2776–2786. doi: 10.1242/dev.091876. [DOI] [PubMed] [Google Scholar]
- 24.Koenig AL, et al. Vegfa signaling promotes zebrafish intestinal vasculature development through endothelial cell migration from the posterior cardinal vein. Dev Biol. 2016;411:115–127. doi: 10.1016/j.ydbio.2016.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rossi A, et al. Regulation of Vegf signaling by natural and synthetic ligands. Blood. 2016;128:2359–2366. doi: 10.1182/blood-2016-04-711192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wild R, et al. Neuronal sFlt1 and Vegfaa determine venous sprouting and spinal cord vascularization. Nat Commun. 2017;8:13991. doi: 10.1038/ncomms13991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bernardos RL, Raymond PA. GFAP transgenic zebrafish. Gene Expr Patterns. 2006;6:1007–1013. doi: 10.1016/j.modgep.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 28.Jin SW, Beis D, Mitchell T, Chen JN, Stainier DY. Cellular and molecular analyses of vascular tube and lumen formation in zebrafish. Development. 2005;132:5199–5209. doi: 10.1242/dev.02087. [DOI] [PubMed] [Google Scholar]
- 29.Blum Y, et al. Complex cell rearrangements during intersegmental vessel sprouting and vessel fusion in the zebrafish embryo. Dev Biol. 2008;316:312–322. doi: 10.1016/j.ydbio.2008.01.038. [DOI] [PubMed] [Google Scholar]
- 30.Chi NC, et al. Foxn4 directly regulates tbx2b expression and atrioventricular canal formation. Genes Dev. 2008;22:734–739. doi: 10.1101/gad.1629408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Proulx K, Lu A, Sumanas S. Cranial vasculature in zebrafish forms by angioblast cluster-derived angiogenesis. Dev Biol. 2010;348:34–46. doi: 10.1016/j.ydbio.2010.08.036. [DOI] [PubMed] [Google Scholar]
- 32.Bussmann J, et al. Arteries provide essential guidance cues for lymphatic endothelial cells in the zebrafish trunk. Development. 2010;137:2653–2657. doi: 10.1242/dev.048207. [DOI] [PubMed] [Google Scholar]
- 33.Okuda KS, et al. lyve1 expression reveals novel lymphatic vessels and new mechanisms for lymphatic vessel development in zebrafish. Development. 2012;139:2381–2391. doi: 10.1242/dev.077701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stevenson TJ, et al. Hypoxia disruption of vertebrate CNS pathfinding through ephrinB2 is rescued by magnesium. PLoS Genet. 2012;8:e1002638. doi: 10.1371/journal.pgen.1002638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Park HC, et al. Analysis of upstream elements in the HuC promoter leads to the establishment of transgenic zebrafish with fluorescent neurons. Dev Biol. 2000;227:279–293. doi: 10.1006/dbio.2000.9898. [DOI] [PubMed] [Google Scholar]
- 36.Chung AY, et al. Generation of demyelination models by targeted ablation of oligodendrocytes in the zebrafish CNS. Mol Cells. 2013;36:82–87. doi: 10.1007/s10059-013-0087-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Davison JM, et al. Transactivation from Gal4-VP16 transgenic insertions for tissue-specific cell labeling and ablation in zebrafish. Dev Biol. 2007;304:811–824. doi: 10.1016/j.ydbio.2007.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Heap LA, Goh CC, Kassahn KS, Scott EK. Cerebellar output in zebrafish: An analysis of spatial patterns and topography in eurydendroid cell projections. Front Neural Circuits. 2013;7:53. doi: 10.3389/fncir.2013.00053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Carney TJ, et al. A direct role for Sox10 in specification of neural crest-derived sensory neurons. Development. 2006;133:4619–4630. doi: 10.1242/dev.02668. [DOI] [PubMed] [Google Scholar]
- 40.Matsuoka RL, et al. Transmembrane semaphorin signalling controls laminar stratification in the mammalian retina. Nature. 2011;470:259–263. doi: 10.1038/nature09675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Thisse C, Thisse B. High-resolution in situ hybridization to whole-mount zebrafish embryos. Nat Protoc. 2008;3:59–69. doi: 10.1038/nprot.2007.514. [DOI] [PubMed] [Google Scholar]
- 42.Bussmann J, Schulte-Merker S. Rapid BAC selection for tol2-mediated transgenesis in zebrafish. Development. 2011;138:4327–4332. doi: 10.1242/dev.068080. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.