Significance
Sleep-disordered breathing (SDB), characterized by intermittent hypoxia and sleep fragmentation during sleep, is common in the elderly and can contribute to cognitive decline, with significant impairment of executive function, including mental flexibility, problem solving, and working memory, which are under dorsolateral prefrontal cortex (DLPFC) control. The results of the present study suggest a potential mechanism of SDB in which a hypoxia-driven deficit of DLPFC γ-aminobutyric acid (GABA), the major inhibitory neurotransmitter, disrupts the neural excitation/inhibition balance which could lead to hyperexcitability, and potentially neuronal damage and cognitive decline. If validated, these findings, along with the proposed model, would represent a significant advance toward achieving a better understanding of the neurobiology of SDB and novel targets for interventions for the disorder.
Keywords: sleep-disordered breathing, hypoxia, GABA, inhibitory, hyperexcitability
Abstract
Sleep-disordered breathing (SDB) is a common disorder in aging that is associated with cognitive decline, including significant executive dysfunction, for which the neurobiological underpinnings remain poorly understood. Using proton magnetic resonance spectroscopy (1H MRS), this study assessed whether dysregulation of the homeostatic balance of the major inhibitory and excitatory amino acid neurotransmitter systems of γ-aminobutyric acid (GABA) and glutamate, respectively, play a role in SDB. Levels of GABA and those of the combined resonances of glutamate and glutamine (Glx), were measured by 1H MRS in the left dorsolateral prefrontal cortex (l-DLPFC) and bilateral hippocampal regions of 19 older adults (age ± SD: 66.1 ± 1.9 years) with moderate to severe SDB, defined as having an Apnea–Hypopnea Index (AHI) greater than 15 as assessed by polysomnography, and in 14 older adults (age ± SD: 62.3 ± 1.3 years) without SDB (AHI < 5). In subjects with SDB, levels of l-DLPFC GABA, but not Glx, were significantly lower than in control subjects (P < 0.0002). Additionally, there was a negative correlation between l-DLPFC GABA levels, but not Glx, and SDB severity by AHI (r = -0.68, P < 0.0001), and a positive correlation between l-DLPFC GABA levels, but not Glx, and minimal oxygen saturation during sleep (r = 0.62, P = 0.0005). By contrast, no group differences or oxygenation associations were found for levels of GABA or Glx in right or left hippocampal region. These findings are interpreted in terms of a pathophysiological model of SDB in which hypoxia-mediated inhibitory neurotransmission deficit in DLPFC could lead to hyperexcitability and, potentially neuronal dysfunction and cognitive decline.
Sleep-disordered breathing (SDB), prevalent in the elderly population (1, 2), is characterized by repeated episodes of hypopnea and apnea during sleep that lead to sleep fragmentation and intermittent hypoxia (3), which are believed to contribute to cognitive decline in affected individuals (4–6). However, the neural substrates of this association between hypoxia and cognitive decline and, in particular, impairment of executive function in SDB (7–12) are currently unknown. With mounting evidence suggesting that SDB-associated hypoxia can lead to hyperexcitability and neuronal damage (13–15), a likely neural substrate of cognitive decline in SDB might be an imbalance in neural excitation/inhibition homeostasis due to dysregulation of the primary inhibitory and excitatory amino acid neurotransmitter systems of γ-aminobutyric acid (GABA) and glutamate, respectively. However, in preclinical studies, the effects of hypoxia on GABA levels have been inconsistent, with some studies reporting increases (16, 17), while others have reported decreases of the inhibitory neurotransmitter (13, 15, 18). Likewise, the effects of hypoxia on glutamate levels have been inconsistent (19–21).
The aim of the present study was to investigate, in vivo in the human brain, whether more consistent and robust associations between hypoxia and GABA and/or glutamate can be obtained by targeting brain regions known to be involved in cognitive aging. Impairment of executive function in the dorsolateral prefrontal cortex (DLPFC) and memory decline in the hippocampus are the cognitive domains that are most affected with aging and are responsible for impairment of daily living in elderly individuals. Importantly, while SDB can affect multiple cognitive domains, there is significant evidence that executive function, which is under DLPFC control, is the most compromised cognitive domain in SDB (9–12).
Using proton magnetic resonance spectroscopy (1H MRS), a noninvasive neuroimaging technique that enables in vivo measurements of GABA levels and those of the combined glutamate and glutamine (commonly referred to as “Glx”), this study sought to test the hypothesis that GABA and/or Glx is altered in the left DLPFC (l-DLPFC) and bilateral hippocampal regions of older adults with SDB by determining both the magnitude and, due to the discrepancy in preclinical data, the direction of these alterations relative to healthy control subjects. In addition, secondary or exploratory analyses were conducted to assess whether there exist correlations between MRS measures of neurotransmitter levels and polysomnographic measures of oxygenation levels that might associate hypoxia with neural excitability and inflammatory markers in SBD.
Results
Sample Characteristics and Demographics.
The characteristics of the study participants are summarized in Table 1. Nineteen elderly subjects with an Apnea–Hypopnea Index (AHI) > 15 were classified as having moderate to severe SDB, whereas 14 with an AHI < 5 were classified as normal controls (Table 1). All participants had a clinical dementia rating (CDR) of zero, and the groups did not differ on age, gender, body mass index (BMI), or hypertension, or on the prevalence of diabetes or cardiovascular disease.
Table 1.
Patient demographics and clinical characteristics in control and SDB subjects
| Characteristic | Control (n = 14) | SDB (n = 19) | P value |
| Age, y | 62.3 ± 1.3 | 66.1 ± 1.9 | 0.14 |
| Female, % | 9 (64.3%) | 6 (31.6%) | 0.06 |
| BMI | 26.6 ± 1.2 | 29.6 ± 1.1 | 0.08 |
| Polysomnographic measures | |||
| AHI | 2.2 ± 0.5 | 31.7 ± 4.4 | <0.0001 |
| Minimum O2 saturation | 89.1 ± 0.7 | 80.5 ± 1.1 | <0.0001 |
| Average O2 saturation | 94.4 ± 0.4 | 92.9 ± 0.3 | 0.006 |
| O2 desaturation index | 1.8 ± 0.4 | 19.4 ± 3.7 | 0.0002 |
| Sleep efficiency | 77.3 ± 3.9 | 73.1 ± 3.1 | 0.40 |
| Total sleep time, min | 383.0 ± 19.4 | 375.1 ± 22.1 | 0.80 |
| Comorbid conditions | |||
| Hypertension | 1 (7.1%) | 4 (21.1%) | 0.37 |
| Diabetes | 0 (0%) | 1 (5.3%) | 1.0 |
| Hypothyroidism | 1 (7.1%) | 1 (5.3%) | 1.0 |
| Cardiovascular risk factors | 2 (14.3%) | 1 (5.3%) | 0.56 |
Mean ± SEM is presented for continuous variables and number (n) with percentage for categorical variables.
Group Metabolite Differences.
All 1H MRS scans yielded spectral data of sufficient quality to permit analysis. The levels of the unsuppressed voxel tissue water (W) did not differ between the SDB and healthy control groups (Table 2). Consequently, W-normalized GABA and Glx (i.e., GABA/W and Glx/W will henceforth be referred to simply as GABA or Glx). The proportions of gray matter in the left and right hippocampi were significantly higher in SDB compared with control subjects, and were therefore included as covariates in the analysis of covariance (ANCOVA) models for these brain regions.
Table 2.
Metabolite and W levels in control and SDB subjects for l-DLPFC and bilateral hippocampi
| Region | Measure | Control (n = 14) | SDB (n = 19) | P value (Cohen’s d effect size) |
| L-DLPFC | Internal W | 3.46 × 10−12 ± 0.12 × 10−−12 | 3.55 × 10−12 ± 0.07 × 10−12 | 0.50 (−0.27) |
| %GM | 0.40 ± 0.04 | 0.41 ± 0.04 | 0.69 (−0.16) | |
| Glx/W | 1.69 × 10−3 ± 0.15 × 10−3 | 1.39 × 10−3 ± 0.08 × 10−3 | 0.12 (0.77) | |
| GABA/W | 2.78 × 10−3 ± 0.15 × 10−3 | 2.12 × 10−3 ± 0.07 × 10−3 | 0.0002* (1.73) | |
| tCho/W | 1.09 × 10−2 ± 0.03 × 10−2 | 1.06 × 10−2 ± 0.04 × 10−2 | 0.26 (0.21) | |
| tCr/W | 1.33 × 10−2 ± 0.06 × 10−2 | 1.25 × 10−2 ± 0.04 × 10−2 | 0.11 (0.47) | |
| NAA/W | 2.23 × 10−2 ± 0.08 × 10−2 | 2.09 × 10−2 ± 0.07 × 10−2 | 0.68 (0.53) | |
| Left hippocampus | Internal W | 2.24 × 10−12 ± 0.09 × 10−12 | 2.19 × 10−12 ± 0.05 × 10−12 | 0.67 (0.17) |
| %GM | 0.59 ± 0.03 | 0.62 ± 0.03 | 0.03 (−0.91) | |
| Glx/W | 1.91 × 10−3 ± 0.12 × 10−3 | 1.81 × 10−3 ± 0.09 × 10−3 | 0.44 (0.28) | |
| GABA/W | 2.84 × 10−3 ± 0.19 × 10−3 | 3.08 × 10−3 ± 0.17 × 10−3 | 0.45 (−0.35) | |
| tCho/W | 1.24 × 10−2 ± 0.05 × 10−2 | 1.22 × 10−2 ± 0.05 × 10−2 | 0.75 (0.11) | |
| tCr/W | 1.17 × 10−2 ± 0.04 × 10−2 | 1.19 × 10−2 ± 0.04 × 10−2 | 0.34 (−0.16) | |
| NAA/W | 1.65 × 10−2 ± 0.09 × 10−2 | 1.65 × 10−2 ± 0.08 × 10−2 | 0.60 (0.01) | |
| Right hippocampus | Internal W | 2.08 × 10−12 ± 0.06 × 10−12 | 2.06 × 10−12 ± 0.06 × 10−12 | 0.80 (0.10) |
| %GM | 0.60 ± 0.03 | 0.62 ± 0.02 | 0.06 (−0.76) | |
| Glx/W | 1.77 × 10−3 ± 0.13 × 10−3 | 1.63 × 10−3 ± 0.10 × 10−3 | 0.49 (0.33) | |
| GABA/W | 2.40 × 10−3 ± 0.16 × 10−3 | 2.49 × 10−3 ± 0.14 × 10−3 | 0.76 (−0.15) | |
| tCho/W | 1.28 × 10−2 ± 0.09 × 10−2 | 1.32 × 10−2 ± 0.05 × 10−2 | 0.48 (−0.13) | |
| tCr/W | 1.14 × 10−2 ± 0.06 × 10−2 | 1.23 × 10−2 ± 0.05 × 10−2 | 0.34 (−0.48) | |
| NAA/W | 1.92 × 10−2 ± 0.10 × 10−2 | 1.90 × 10−2 ± 0.08 × 10−2 | 0.73 (0.05) |
Data are presented as mean ± SEM. Sample sizes for each region: l-DLPFC: 10 control and 18 SDB; left hippocampus: 11 control and 17 SDB; right hippocampus: 11 control and 16 SDB. Cohen’s d effect size is the standardized difference between the two means.
P value is significant after correction for multiple comparisons using the FDR (adjusted P = 0.001).
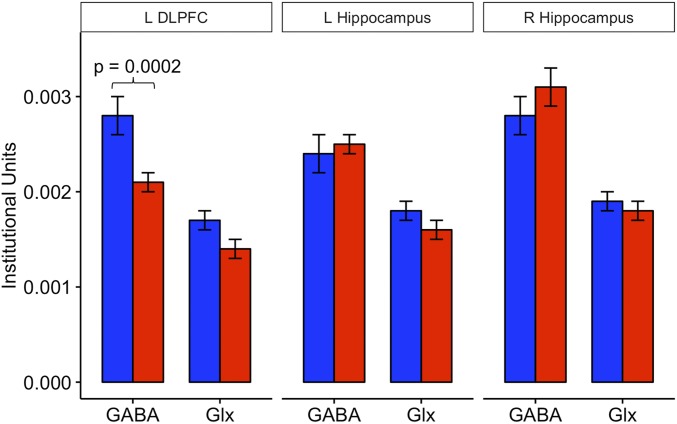
Table 2 provides the levels of GABA and Glx in the l-DLPFC and in the left and right hippocampi for the SDB and control groups. In the l-DLPFC (Fig. 1), GABA levels in elderly subjects with moderate to severe SDB were significantly lower than in control subjects [P = 0.0002, false discovery rate (FDR)-adjusted P = 0.001], with an effect size (Cohen’s d) of 1.73, while no significant differences in Glx (P = 0.12) were found. No group differences were found either in the left hippocampus for GABA (P = 0.45) or Glx (P = 0.44) or in the right hippocampus for GABA (P = 0.76) or Glx (P = 0.49). No other group differences were found in any other metabolite [N-acetyl-l-aspartate (NAA), total creatine (tCr), and total choline (tCho)] for either the l-DLPFC or hippocampi (Table 2).
Fig. 1.
Metabolite means for the l-DLPFC and left (L) and right (R) hippocampi. Blue bars represent older adult controls (AHI < 5), and red bars represent an older adult group with moderate to severe SDB (AHI > 15). The l-DLPFC GABA was lower in patients with moderate to severe SDB (red) compared with controls (blue) (P = 0.0002, FDR-adjusted P = 0.001). Bilateral hippocampi GABA and Glx were not different between the two groups.
Neurometabolite Associations with SDB Severity and Oxygenation.
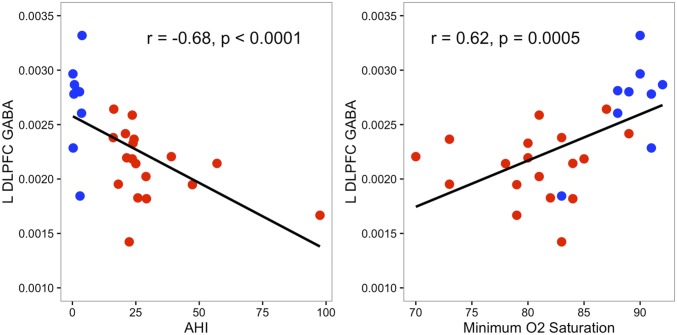
In the l-DLPFC, but not in the hippocampi, GABA levels negatively correlated with severity of SDB as measured by the AHI (r = −0.68, P < 0.0001; Fig. 2) and positively with minimum oxygen saturation (r = 0.62, P = 0.0005; Fig. 2). No other correlations among AHI, oxygen saturation, or any other neurometabolite were significant for either the l-DLPFC or the hippocampi.
Fig. 2.
Scatter plots characterizing associations of GABA level with AHI and minimal oxygen (O2) saturation in the l-DLPFC across all subjects. Subjects with SDB are shown in red, and controls are shown in blue.
Cytokine and Cortisol Levels.
Cortisol C (measured before bedtime) was lower at the trend level in SDB subjects compared with control subjects (P = 0.06; Table 3). None of the cytokines, including IL-6, IL-1β, and TNF-α, differed between SBD and control subjects.
Table 3.
Cortisol and cytokine measurements in control and SDB subjects
| Measure | Control (n = 14) | SDB (n = 19) | P value |
| Cortisol | |||
| Cortisol A | 0.42 ± 0.07 | 0.32 ± 0.05 | 0.36 |
| Cortisol B | 0.51 ± 0.07 | 0.43 ± 0.04 | 0.43 |
| Cortisol C | 0.18 ± 0.07 | 0.10 ± 0.02 | 0.06 |
| Average cortisol | 0.37 ± 0.05 | 0.30 ± 0.04 | 0.35 |
| Cytokines | |||
| IL-6, pg/mL | 2.72 ± 0.40 | 3.79 ± 0.82 | 0.44 |
| IL-1β, pg/mL | 0.36 ± 0.06 | 0.44 ± 0.09 | 0.50 |
| TNF-α, pg/mL | 4.76 ± 0.60 | 5.42 ± 0.47 | 0.52 |
Data are presented as mean ± SEM.
Discussion
Overview of the Main Results.
The present study has measured and reports MRS neurometabolic features in older adult subjects with SDB, finding robustly decreased levels of GABA in the l-DLPFC compared with healthy control subjects. In addition, GABA levels correlated negatively with the severity of sleep apnea as assessed with the AHI and positively with minimal oxygen saturation, both only in the l-DLPFC, indicating that high disease severity and low oxygenation, both of which are nonsalutary, are associated with decreased GABAergic tone. The state of hyperexcitability that has been reported in some preclinical models of hypoxia (13–15) may therefore result from decreased inhibitory neurotransmission tone. Significantly, the fact that the observed findings were limited only to GABA in the l-DLPFC, despite identical procedures having also been used to measure GABA and Glx in the hippocampus, suggests a regional specificity. This apparent regional specificity is unlikely to be due to differences in the proportions of gray matter, white matter, and cerebrospinal fluid (CSF) in each voxel because (i) the gray matter, white matter, and CSF fractions did not differ between the SDB and control groups in the l-DLPFC; (ii) the absence of findings in the hippocampal region was not altered when the group difference in gray matter fraction in this region was included as a covariate in the statistical models; and (iii) the effect size of the GABA deficit in the l-DLPFC was too large (Cohen’s d = 1.73) for the associated group difference to be fortuitous. Lastly, although the hippocampal voxel was relatively large, it should be noted that we had prescribed this voxel very tightly around the hippocampal structure to minimize the inclusion of nonhippocampal metabolite signals and brain tissues. Due to the irregular shape of the hippocampus, a degree of partial volume averaging was inevitable, and we had endeavored to account for it by performing tissue segmentation and then including the gray matter fraction, which differed between the groups, as a covariate in the statistical models. The absence of findings in the hippocampal region is therefore also quite likely genuine.
A precise balance between excitatory and inhibitory neurotransmitter systems is critical for normal brain function (22). The present finding of an l-DLPFC GABA deficit with no changes in Glx could be indicative of disrupted interneurons that have a key role in regulating neuroplasticity and neuronal function, as recently discussed (23–25). Thus, a disrupted inhibitory network could lead to neuronal dysfunction and cognitive decline (22).
Experimental Support and Pathophysiological and Mechanistic Implications.
The main findings of the present study of a robust and l-DLPFC–specific GABA deficit in SDB, which correlates with decreased oxygenation and increased disease severity, are consistent with most preclinical data, which have shown hypoxia to lead to a loss of GABA interneurons (15, 18, 26). A recent study in an adult rodent brain model of sleep apnea found a loss of the parvalbumin class of GABA interneurons in the prefrontal cortex (13), although other studies reported increased GABAergic activity (16, 17). These discrepant results, in fact, seem to confirm and stress the findings in the present study in suggesting that hypoxia may differentially affect GABA in different brain regions, as well as diverse classes of GABA interneurons. Furthermore, the duration of hypoxic exposure and potential differences between the human and rodent brain response to hypoxia may be other factors that can contribute to the discrepancies. Thus, while a robust GABA deficit was observed in the present study, this deficit was found only in the l-DLPFC and not in the hippocampi, suggesting a regional specificity for GABAergic alterations. In relation to the inflammatory markers, measurements were exploratory and did not reveal any differences.
In preclinical studies, hypoxia has been found not only to increase glutamate release but also to be associated with a glutamate transporter deficiency, which are conditions known to lead to glutamatergic excitotoxicity and neuronal loss (19, 20). However, rather than an increase in the glutamatergic component, the present study found no significant Glx difference between SDB and control subjects. Direct glutamatergic excitotoxicity therefore might not be the primary mechanism that underpins the reported likely hyperexcitability to hypoxia. Rather, it seems more plausible that hypoxia in human neural tissue, specifically in the l-DLPFC, predominantly affects GABAergic tone, which could, in turn, lead to hyperexcitability and neuronal damage. Since the inhibitory neural network is critical for neuroplasticity and function, its disruption can lead to neuronal dysfunction (22, 23, 25). GABAergic inhibition through cortical interneurons also regulates the degree of glutamatergic excitation (22). It is therefore conceivable that a GABA deficit could lead to excitotoxicity even without increased glutamate simply because the glutamatergic excitation threshold has been lowered as a result of the low inhibitory tone. Such a disruption of the excitatory/inhibitory homeostatic balance could be a mechanism that could trigger excitotoxicity and neuronal damage in SDB.
Empirical support for the postulated hypoxia-driven, GABAergic disinhibition-mediated hyperexcitability mechanism is provided by electrophysiological studies that have shown hyperexcitability in the setting of hypoxia (14, 15, 27). Alternatively, hyperexcitability may relate to a regionally specific imbalance in excitatory/inhibitory homeostasis, which seems to be supported by a recent study that found a GABA decrease and glutamate increase in the insular cortex of patients with obstructive sleep apnea (28). Such an excitatory/inhibitory neurotransmitter imbalance could synergistically lead to insular cortex hyperexcitability. While mechanisms of neurotransmission imbalance in different brain regions remain to be elucidated, evidence seems to support the interpretation that intermittent hypoxia in SDB is associated with a net increase in excitability, likely through GABAergic disinhibition, that could potentially lead to neural damage and cognitive deficits.
Recent evidence suggests that GABA levels decrease with aging (29, 30) and are related to cognitive performance, particularly working memory (31, 32). SDB may accelerate the aging process, especially in the prefrontal cortex, which is particularly vulnerable to cognitive deficits in aging (33, 34), with an imbalance in excitatory/inhibitory neural activity. A GABA deficit in DLPFC could potentially lead to hyperexcitability, neural damage, and impairment of executive function. Importantly, executive function has been suggested to be the predominantly affected cognitive domain in SDB (9–12), which could be consistent with our model of hyperexcitability in DLPFC leading to neural dysfunction. This model of hyperexcitability in SDB could also be an essential mechanism for increased risk for developing Alzheimer’s disease and should be further investigated, as previous studies have shown an association between SDB and Alzheimer’s disease (4, 6). Notably, release of both amyloid-β and tau, the neuropathological hallmarks of Alzheimer’s disease, has been suggested to be dependent on neuronal activity and to be increased in pathologically excessive excitable states (35–37). A more recent study has shown that propagation of tau is increased with hyperexcitability (37). Future work should investigate whether the present findings of increased excitability in SDB are implicated in increased accumulation, propagation, and toxicity of amyloid-β and tau, clarifying pathways and mechanisms of how SDB increases the risk of developing Alzheimer’s disease. Our findings implicating GABA in the pathophysiological mechanisms of SDB may also contribute to future studies that can ultimately help primarily prevent neural damage and cognitive decline in the elderly population and identify treatments, as maintaining executive function is critical for successful aging (38, 39). Future research should also examine if GABA changes in DLPFC are rescued with continuous positive airway pressure treatment in patients with SDB and if MRS could serve as a biomarker to regional neural metabolic state.
While the sample size is small and needs to be expanded and hippocampal region voxels are large, the strengths of this study include the use of polysomnography to assess sleep disorders and oxygenation levels, the gold standard technique for these purposes, and a specialized MRS “editing” technique that enables measurements of GABA and glutamatergic compounds noninvasively in the living brain. This has revealed a relationship of SDB to a possible path leading to neural damage and neurodegeneration that should be vigorously pursued. Limitations include, first, the relatively low detection sensitivity of GABA that necessitates sampling of large voxels. Second, the detected GABA resonance by J-editing is known to contain a proportion of coedited mobile macromolecules (40), and the Glx peak consists of the combined resonances of Glx, although we recently reported evidence suggesting that Glx measured by the J-editing technique either contains mainly glutamate or can be interpreted as such (41). Despite that, we observed a highly significant GABA deficit in the l-DLPFC (P = 0.0002), with a Cohen’s d effect size of 1.73 in older adults with SDB in comparison to controls that strongly correlates with oxygenation levels, minimal saturation (r = 0.62, P = 0.0005), and AHI (r = −0.68, P < 0.0001).
Conclusion.
This study has reported human brain data documenting a robust l-DLPFC GABA deficit in subjects with SDB. Along with the totality of available preclinical and clinical data, this GABAergic deficit has been interpreted as supporting a pathophysiological model of the disorder in which SDB, with intermittent hypoxia, leads to the observed GABA decrease and to the hyperexcitability that has been documented in preclinical studies. A disrupted inhibitory network in DLPFC could cause impairment in neuroplasticity and neural function, leading to executive dysfunction in SDB.
Methods
Subjects.
For this study, 19 older adult subjects with moderate to severe SDB, as assessed with the AHI, and 14 healthy older adult subjects without SDB were recruited at The Rockefeller University through referrals from the Center for Sleep Medicine at New York Presbyterian/Weill Cornell Medical Center. All subjects provided written informed consent to participate in this study, which was approved by the Institutional Review Boards of The Rockefeller University and Weill Cornell Medicine.
Inclusion criteria consisted of being between 55 and 95 y of age and having a normal ability to perform routine activities of daily living, including living independently in the community. Subjects were included based on the AHI, a measure of SDB severity based on polysomnographic measures of the average number of obstructive apneic and hypopneic events per hour of sleep. Moderate to severe SDB was defined as having an AHI > 15, and control subjects were required to have an AHI < 5. None of the subjects enrolled met the criteria for clinical dementia as assessed by the CDR. Subjects were excluded if they had a major neurological or psychiatric disorder or any medical condition impacting brain function. Exclusionary criteria also included a history of recent alcohol or illicit drug abuse, and other major medical conditions, including a recent myocardial infarction or failure, renal failure, liver disease, chronic obstructive pulmonary disease, or malignancy. Participants on medications that modulate glutamate or GABA levels (e.g., lamotrigine, lithium, opiates, psychostimulants, tricyclic antidepressants, benzodiazepines) were excluded. None of the participants had received treatment for SDB before or during the study. Lastly, a contraindication to undergoing MRI scans was exclusionary.
Magnetic Resonance Neuroimaging Procedures.
All neuroimaging studies, which included a limited structural brain MRI examination and three single-voxel MRS scans, were conducted on a research-dedicated, 3.0-T GE MR system with an eight-channel, phased-array head coil at the Citigroup Biomedical Imaging Center of Weill Cornell Medicine.
Structural MRI.
A three-plane, low-resolution, high-speed scout imaging series was conducted, followed by a series of high-resolution scans, consisting of standard axial, coronal, and sagittal T1-, T2-, and spin density-weighted scans that were appropriately obliqued for prescribing the 1H MRS voxels of interest. In addition, a T1-weighted spoiled gradient-recalled echo (SPGR) volumetric scan and an axial fast fluid-attenuated inversion recovery scan were performed for brain tissue segmentation and detection of exclusionary focal brain lesions, respectively.
1H MRS data acquisition.
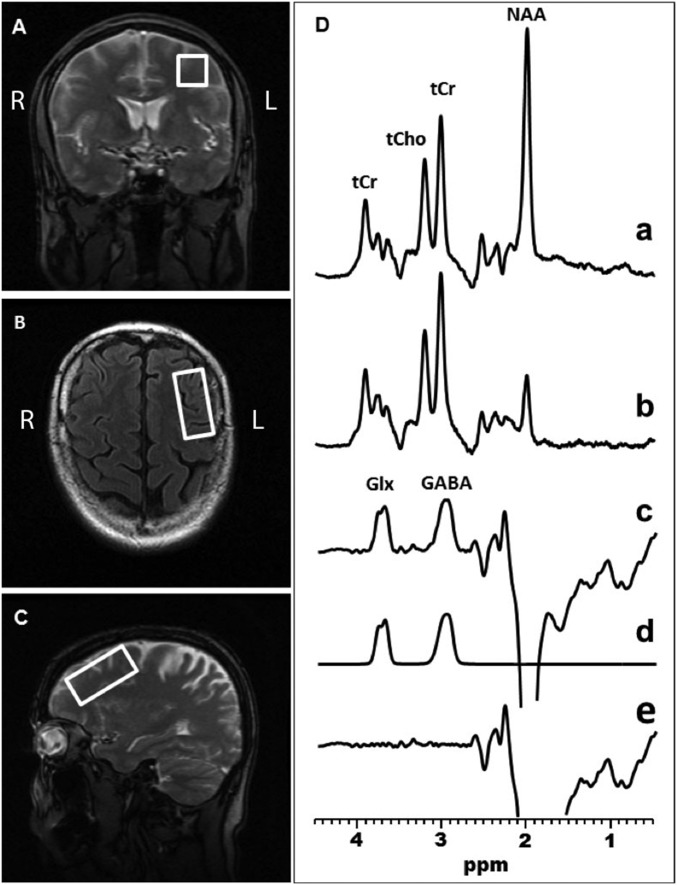
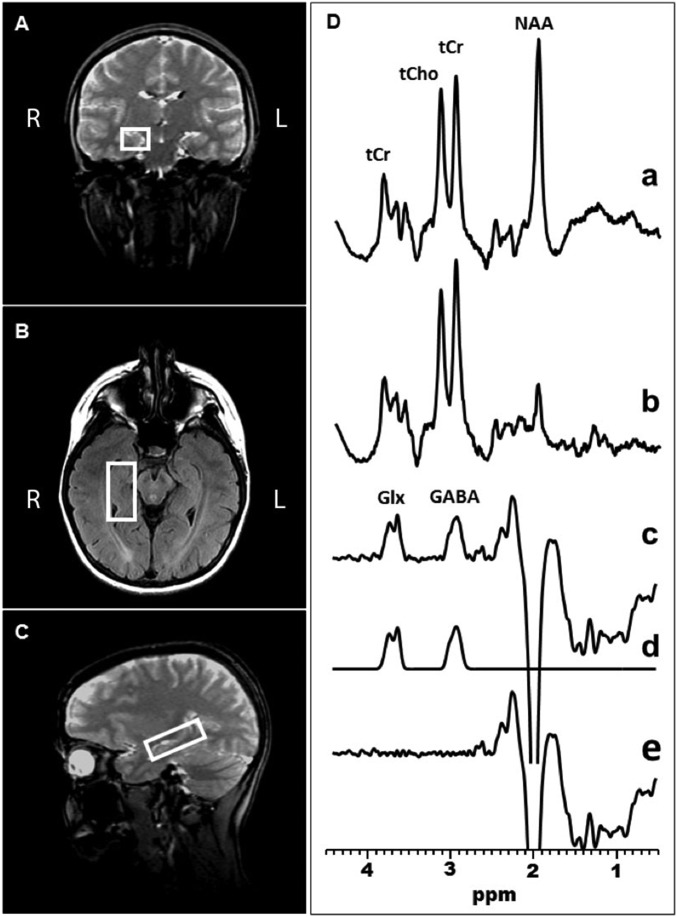
GABA-edited MRS data were acquired using the standard J-edited spin echo difference method (40, 42), and then processed as illustrated in Figs. 3 and 4 and as fully described recently (40). Spectra were recorded from a 2.0 × 2.0 × 4.5-cm3 voxel in the l-DLPFC (Fig. 3) and a 1.5 × 2.0 × 4.5-cm3 voxel in the right and left hippocampi (Fig. 4).
Fig. 3.
Coronal (A), transaxial (B), and sagittal (C) localizer images showing the size and location of the l-DLPFC voxel of interest. (D) Demonstration of in vivo human brain GABA and Glx detection with 1H MRS in the l-DLPFC: single-voxel subspectra acquired in 15 min with the editing pulse on and off and 290 interleaved averages (a and b, 580 total); spectrum (c), difference between spectra a and b showing the edited brain GABA and Glx resonances; spectrum (d), model fitting of the experimental spectrum (c) to obtain the GABA and Glx peak areas; spectrum (e), residual of the difference between spectra c and d.
Fig. 4.
Coronal (A), transaxial (B), and sagittal (C) localizer images showing the size and location of a voxel of interest in the right hippocampus. (D) Demonstration of in vivo human brain GABA and Glx detection with 1H MRS in the left hippocampus: single-voxel subspectra acquired in 15 min with the editing pulse on and off and 290 interleaved averages (a and b, 580 total); spectrum (c), difference between spectra a and b showing the edited brain GABA and Glx resonances; spectrum (d), model fitting of the experimental spectrum (c) to obtain the GABA and Glx peak areas; spectrum (e), residual of the difference between spectra c and d.
1H MRS data processing and quantitation.
Details of the MRS data quality assessment criteria and procedures used in this study to retain or reject spectra for inclusion in group analyses are provided in the supplementary online material of ref. 40. For all of the cases that fulfilled those quality assessment criteria, spectral peak areas, which are proportional to the concentrations of the associated metabolites, were obtained as illustrated in Figs. 3 and 4 (traces a–e) and as described recently (40). Briefly, the GABA and Glx resonances in the J-edited difference spectra were modeled as a linear combination of pseudo-Voigt lineshape functions and then fitted in the frequency domain using a robust and highly optimized public domain Levenberg–Marquardt nonlinear least-squares minimization routine (43). For normalization across subjects, the GABA and Glx levels were expressed semiquantitatively in “institutional units” as ratios of peak areas relative to the area of the synchronously acquired and similarly fitted unsuppressed voxel W signal. Levels of the other major brain metabolites, including NAA, tCr, and tCho, that are measured at the same time as GABA and Glx (Figs. 3 and 4) were similarly derived by spectral fitting and normalized to W.
Assessment of voxel tissue heterogeneity.
To estimate the proportions of gray matter, white matter, and CSF contained in each voxel of interest, MEDx software (Medical Numerics) was used to segment the brain tissue based on the signal-intensity histogram of each subject’s volumetric (SPGR) MRI. In-house software developed in MATLAB (MathWorks) was then implemented to generate a segmentation map for each voxel, from which the proportions of gray matter, white matter, and CSF were determined. These were then compared between the groups, and, in case of significant differences, percent gray matter was included in the statistical models as a covariate.
Overnight Polysomnography.
Standard nocturnal polysomnography (NPSG) was performed at the Center for Sleep Medicine of Weill Cornell Medicine using a Grass Comet XL system (Natus) with integrated Nonin oximetry and digital filter settings as recommended by the American Academy of Sleep Medicine. Respiratory effort was measured using Pro-Tech Crystal Trace piezo crystal belts placed around the rib cage and abdomen, and airflow was detected using a Pro-Tech PTAF lite pressure transducer. NPSG was performed at approximately each subject’s regular bedtime. Respiratory events were scored according to the 2007 American Academy of Sleep Medicine guidelines. Apnea was defined as a decrease in peak nasal pressure of >90% of baseline, lasting at least 10 s. Hypopnea was defined as a decrease of >30% of the baseline nasal pressure, lasting at least 10 s and associated with ≥4% drop in the oxyhemoglobin saturation (SaO2). The obstructive AHI values were calculated by computing the average number of obstructive apnea and hypopnea events per hour of sleep. Intermittent hypoxemia was determined by the number of periods lasting ≥6 s with SaO2 drops of ≥4%. These data were tabulated as the number of hypoxemic episodes per hour, generating the oxygen desaturation index. Also recorded were data on the percentage of time with SaO2 ≤ 90%, total time with SaO2 < 90%, and the lowest saturation levels.
Salivary Cortisol.
Diurnal cortisol activity was assessed based on a series of three saliva samples collected at home the day before the scheduled 1H MRS: sample A, upon waking (“when your eyes open and you are ready to get up”); sample B, 45 min later; and sample C, at bedtime (“right before getting into bed”). Samples were then assayed in duplicate aliquots of 25 μL, using the Salimetrics High Sensitivity Cortisol Assay Kit (Salimetrics SalivaLab), without modifications to the manufacturer’s protocol.
Cytokines.
Erenna immunoassays (EMD Millipore) were performed to measure IL-1β, IL-6, and TNF-α in blood samples obtained from subjects. This technology uses single-molecule technology, which can quantify fentogram levels of cytokines in different biological matrixes in a single-format assay. The assay consists of two sequential incubations with the target antibody (Ab) and the fluorophore-labeled detection Ab. The samples are transferred to two plates for washes to decrease background; finally, the plate is read in the Erenna instrument, which measures the number of Ab molecules bound to the target cytokine and fluorescence. By the algorithm of the Erenna software, the absolute concentration of each cytokine is calculated.
Statistical Analysis.
Demographic and clinical characteristic differences between control and SDB groups were assessed using two-tailed independent-sample t tests for continuous variables and χ2 tests for categorical variables. Comparisons of metabolite, cortisol, and cytokine levels were performed using ANCOVA with age. Gender and BMI were included as additional covariates in the model if the effects were significant. If there were significant group differences in voxel tissue composition, percent gray matter calculated as %GM/(%GM + %WM), where %WM is percent white matter, was included in the ANCOVA models. The Spearman correlation coefficient was used to test for associations among tissue W-normalized metabolite levels, AHI, and minimum O2 saturation. A P value ≤0.05 was considered statistically significant. The FDR was used to correct for multiple testing of the six primary outcomes: GABA and Glx in the DLPFC and bilateral hippocampi. The SAS Studio 3.4 statistical software package (SAS Institute, Inc.) was used to perform all of the analyses.
Acknowledgments
We thank Kathy Dowd for research coordination and Renee Zacharowicz for neuroimaging support. This work was supported by the Iris and Jumming Le Foundation, the Dana Foundation, the Rockefeller University Women & Science Initiative, NIH Grant 1 K76AG054772 (to A.C.P.), and NIH Grant 1 R01MH075895 (to D.C.S.). Partial support was provided by Grant UL1TR001866 from the National Center for Advancing Translational Sciences, NIH Clinical and Translational Science Award program.
Footnotes
The authors declare no conflict of interest.
References
- 1.Young T, et al. Sleep Heart Health Study Research Group Predictors of sleep-disordered breathing in community-dwelling adults: The Sleep Heart Health Study. Arch Intern Med. 2002;162:893–900. doi: 10.1001/archinte.162.8.893. [DOI] [PubMed] [Google Scholar]
- 2.Ancoli-Israel S, et al. Sleep-disordered breathing in community-dwelling elderly. Sleep. 1991;14:486–495. doi: 10.1093/sleep/14.6.486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Young T, et al. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med. 1993;328:1230–1235. doi: 10.1056/NEJM199304293281704. [DOI] [PubMed] [Google Scholar]
- 4.Spira AP, et al. Sleep-disordered breathing and cognition in older women. J Am Geriatr Soc. 2008;56:45–50. doi: 10.1111/j.1532-5415.2007.01506.x. [DOI] [PubMed] [Google Scholar]
- 5.Zimmerman ME, Aloia MS. Sleep-disordered breathing and cognition in older adults. Curr Neurol Neurosci Rep. 2012;12:537–546. doi: 10.1007/s11910-012-0298-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yaffe K, et al. Sleep-disordered breathing, hypoxia, and risk of mild cognitive impairment and dementia in older women. JAMA. 2011;306:613–619. doi: 10.1001/jama.2011.1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.El-Ad B, Lavie P. Effect of sleep apnea on cognition and mood. Int Rev Psychiatry. 2005;17:277–282. doi: 10.1080/09540260500104508. [DOI] [PubMed] [Google Scholar]
- 8.Spira AP, et al. Sleep-disordered breathing and functional decline in older women. J Am Geriatr Soc. 2014;62:2040–2046. doi: 10.1111/jgs.13108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Saunamäki T, Jehkonen M. A review of executive functions in obstructive sleep apnea syndrome. Acta Neurol Scand. 2007;115:1–11. doi: 10.1111/j.1600-0404.2006.00744.x. [DOI] [PubMed] [Google Scholar]
- 10.Beebe DW, Gozal D. Obstructive sleep apnea and the prefrontal cortex: Towards a comprehensive model linking nocturnal upper airway obstruction to daytime cognitive and behavioral deficits. J Sleep Res. 2002;11:1–16. doi: 10.1046/j.1365-2869.2002.00289.x. [DOI] [PubMed] [Google Scholar]
- 11.Beebe DW, Groesz L, Wells C, Nichols A, McGee K. The neuropsychological effects of obstructive sleep apnea: A meta-analysis of norm-referenced and case-controlled data. Sleep. 2003;26:298–307. doi: 10.1093/sleep/26.3.298. [DOI] [PubMed] [Google Scholar]
- 12.Saunamäki T, Himanen SL, Polo O, Jehkonen M. Executive dysfunction in patients with obstructive sleep apnea syndrome. Eur Neurol. 2009;62:237–242. doi: 10.1159/000232156. [DOI] [PubMed] [Google Scholar]
- 13.Yuan L, Wu J, Liu J, Li G, Liang D. Intermittent hypoxia-induced parvalbumin-immunoreactive interneurons loss and neurobehavioral impairment is mediated by NADPH-oxidase-2. Neurochem Res. 2015;40:1232–1242. doi: 10.1007/s11064-015-1586-1. [DOI] [PubMed] [Google Scholar]
- 14.Schiff SJ, Somjen GG. Hyperexcitability following moderate hypoxia in hippocampal tissue slices. Brain Res. 1985;337:337–340. doi: 10.1016/0006-8993(85)90071-x. [DOI] [PubMed] [Google Scholar]
- 15.Turovsky EA, Turovskaya MV, Kononov AV, Zinchenko VP. Short-term episodes of hypoxia induce posthypoxic hyperexcitability and selective death of GABAergic hippocampal neurons. Exp Neurol. 2013;250:1–7. doi: 10.1016/j.expneurol.2013.09.006. [DOI] [PubMed] [Google Scholar]
- 16.Wood JD, Watson WJ, Ducker AJ. The effect of hypoxia on brain gamma-aminobutyric acid levels. J Neurochem. 1968;15:603–608. doi: 10.1111/j.1471-4159.1968.tb08959.x. [DOI] [PubMed] [Google Scholar]
- 17.Madl JE, Royer SM. Glutamate dependence of GABA levels in neurons of hypoxic and hypoglycemic rat hippocampal slices. Neuroscience. 2000;96:657–664. doi: 10.1016/s0306-4522(99)00548-5. [DOI] [PubMed] [Google Scholar]
- 18.Romijn HJ, Ruijter JM, Wolters PS. Hypoxia preferentially destroys GABAergic neurons in developing rat neocortex explants in culture. Exp Neurol. 1988;100:332–340. doi: 10.1016/0014-4886(88)90112-4. [DOI] [PubMed] [Google Scholar]
- 19.Fung SJ, Xi M, Zhang J, Sampogna S, Chase MH. Apnea produces excitotoxic hippocampal synapses and neuronal apoptosis. Exp Neurol. 2012;238:107–113. doi: 10.1016/j.expneurol.2012.08.006. [DOI] [PubMed] [Google Scholar]
- 20.Dallas M, et al. Hypoxia suppresses glutamate transport in astrocytes. J Neurosci. 2007;27:3946–3955. doi: 10.1523/JNEUROSCI.5030-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kobayashi S, Millhorn DE. Hypoxia regulates glutamate metabolism and membrane transport in rat PC12 cells. J Neurochem. 2001;76:1935–1948. doi: 10.1046/j.1471-4159.2001.00214.x. [DOI] [PubMed] [Google Scholar]
- 22.Lehmann K, Steinecke A, Bolz J. GABA through the ages: Regulation of cortical function and plasticity by inhibitory interneurons. Neural Plast. 2012;2012:892784. doi: 10.1155/2012/892784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen JL, et al. Structural basis for the role of inhibition in facilitating adult brain plasticity. Nat Neurosci. 2011;14:587–594. doi: 10.1038/nn.2799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Castillo PE, Chiu CQ, Carroll RC. Long-term plasticity at inhibitory synapses. Curr Opin Neurobiol. 2011;21:328–338. doi: 10.1016/j.conb.2011.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kullmann DM, Moreau AW, Bakiri Y, Nicholson E. Plasticity of inhibition. Neuron. 2012;75:951–962. doi: 10.1016/j.neuron.2012.07.030. [DOI] [PubMed] [Google Scholar]
- 26.Romijn HJ. Preferential loss of GABAergic neurons in hypoxia-exposed neocortex slab cultures is attenuated by the NMDA receptor blocker D-2-amino-7-phosphonoheptanoate. Brain Res. 1989;501:100–104. doi: 10.1016/0006-8993(89)91031-7. [DOI] [PubMed] [Google Scholar]
- 27.Levin SG, Kalemenev SV, Godukhin OV. Hyperexcitability of neurons in field CAL1 evoked by transient episodes of hypoxia in hippocampal slices from rats of different ages. Neurosci Behav Physiol. 2005;35:585–588. doi: 10.1007/s11055-005-0097-y. [DOI] [PubMed] [Google Scholar]
- 28.Macey PM, et al. Obstructive sleep apnea is associated with low GABA and high glutamate in the insular cortex. J Sleep Res. 2016;25:390–394. doi: 10.1111/jsr.12392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gao F, et al. Edited magnetic resonance spectroscopy detects an age-related decline in brain GABA levels. Neuroimage. 2013;78:75–82. doi: 10.1016/j.neuroimage.2013.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Porges EC, et al. Frontal gamma-aminobutyric acid concentrations are associated with cognitive performance in older adults. Biol Psychiatry Cogn Neurosci Neuroimaging. 2017;2:38–44. doi: 10.1016/j.bpsc.2016.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yoon JH, Grandelis A, Maddock RJ. Dorsolateral prefrontal cortex GABA concentration in humans predicts working memory load processing capacity. J Neurosci. 2016;36:11788–11794. doi: 10.1523/JNEUROSCI.1970-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Michels L, et al. Frontal GABA levels change during working memory. PLoS One. 2012;7:e31933. doi: 10.1371/journal.pone.0031933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Raz N, et al. Selective aging of the human cerebral cortex observed in vivo: Differential vulnerability of the prefrontal gray matter. Cereb Cortex. 1997;7:268–282. doi: 10.1093/cercor/7.3.268. [DOI] [PubMed] [Google Scholar]
- 34.Dumitriu D, et al. Selective changes in thin spine density and morphology in monkey prefrontal cortex correlate with aging-related cognitive impairment. J Neurosci. 2010;30:7507–7515. doi: 10.1523/JNEUROSCI.6410-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bero AW, et al. Neuronal activity regulates the regional vulnerability to amyloid-β deposition. Nat Neurosci. 2011;14:750–756. doi: 10.1038/nn.2801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yamada K, et al. Neuronal activity regulates extracellular tau in vivo. J Exp Med. 2014;211:387–393. doi: 10.1084/jem.20131685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wu JW, et al. Neuronal activity enhances tau propagation and tau pathology in vivo. Nat Neurosci. 2016;19:1085–1092. doi: 10.1038/nn.4328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cabeza R, Anderson ND, Locantore JK, McIntosh AR. Aging gracefully: Compensatory brain activity in high-performing older adults. Neuroimage. 2002;17:1394–1402. doi: 10.1006/nimg.2002.1280. [DOI] [PubMed] [Google Scholar]
- 39.Kramer AF, et al. Ageing, fitness and neurocognitive function. Nature. 1999;400:418–419. doi: 10.1038/22682. [DOI] [PubMed] [Google Scholar]
- 40.Shungu DC, et al. Brain γ-aminobutyric acid (GABA) detection in vivo with the J-editing (1) H MRS technique: A comprehensive methodological evaluation of sensitivity enhancement, macromolecule contamination and test-retest reliability. NMR Biomed. 2016;29:932–942. doi: 10.1002/nbm.3539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shungu DC, et al. 2013. ‘Glx’ measured by J-editing/MEGA-PRESS is primarily ‘pure’ glutamate…or is it? Proceedings of the International Society for Magnetic Resonance Medicine (ISMRM.org, Concord, CA), p 21.
- 42.Rothman DL, Petroff OA, Behar KL, Mattson RH. Localized 1H NMR measurements of gamma-aminobutyric acid in human brain in vivo. Proc Natl Acad Sci USA. 1993;90:5662–5666. doi: 10.1073/pnas.90.12.5662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Markwardt CB. Non-linear least squares fitting in IDL with MPFIT. In: Bohlender D, Dowler P, Durand D, editors. Proceedings of Astronomical Data Analysis Software and Systems XVIII. Vol 411. Astronomical Society of the Pacific; San Francisco: 2009. pp. 251–254. [Google Scholar]