Significance
We report here the first measurements of molecular iodine (I2) in the Arctic atmosphere and iodide (I−) in the Arctic snowpack. Although iodine chemistry is expected to have significant impacts on Arctic atmospheric ozone destruction and new particle production, sparse measurements of atmospheric iodine have limited our ability to examine sources and impacts. We show, through sunlit and artificially irradiated snowpack experiments, that the coastal Arctic snowpack is capable of photochemical production and release of I2 to the boundary layer. This is supported by enrichment of the snowpack in I− compared with that expected from sea spray influence alone. Through photochemical modeling, we demonstrate that, at observed I2 levels, snowpack production can have a significant impact on Arctic atmospheric chemistry.
Keywords: atmosphere, iodine, cryosphere, snowpack, photochemistry
Abstract
During springtime, the Arctic atmospheric boundary layer undergoes frequent rapid depletions in ozone and gaseous elemental mercury due to reactions with halogen atoms, influencing atmospheric composition and pollutant fate. Although bromine chemistry has been shown to initiate ozone depletion events, and it has long been hypothesized that iodine chemistry may contribute, no previous measurements of molecular iodine (I2) have been reported in the Arctic. Iodine chemistry also contributes to atmospheric new particle formation and therefore cloud properties and radiative forcing. Here we present Arctic atmospheric I2 and snowpack iodide (I−) measurements, which were conducted near Utqiaġvik, AK, in February 2014. Using chemical ionization mass spectrometry, I2 was observed in the atmosphere at mole ratios of 0.3–1.0 ppt, and in the snowpack interstitial air at mole ratios up to 22 ppt under natural sunlit conditions and up to 35 ppt when the snowpack surface was artificially irradiated, suggesting a photochemical production mechanism. Further, snow meltwater I− measurements showed enrichments of up to ∼1,900 times above the seawater ratio of I−/Na+, consistent with iodine activation and recycling. Modeling shows that observed I2 levels are able to significantly increase ozone depletion rates, while also producing iodine monoxide (IO) at levels recently observed in the Arctic. These results emphasize the significance of iodine chemistry and the role of snowpack photochemistry in Arctic atmospheric composition, and imply that I2 is likely a dominant source of iodine atoms in the Arctic.
Atmospheric boundary layer ozone depletion events (ODEs), during which ozone (O3) in the lower troposphere rapidly drops from background levels of 30–40 ppb to below 10 ppb, have been observed during springtime in the polar regions for several decades (1, 2). Early measurements of filterable halogens (bromine, chlorine, and iodine) (3) showed a particularly strong correlation between filterable bromine and O3 concentrations, suggesting the catalytic destruction of O3 by bromine atoms (4). Subsequent observations of inorganic bromine (Br2, BrO, HOBr) in the polar regions (5–10) have elucidated the “bromine explosion” chemical mechanism (11, 12). Still, modeling studies suggest that this system is far from fully understood, and bromine chemistry alone cannot explain the full extent of ODEs that occur (13–16). The presence of iodine compounds, even at small mole ratios (moles of analyte/mole of air), may significantly increase the rate of O3 destruction during ODEs (13, 17, 18), due to the relatively large rate constant for the reaction of BrO with IO [k = 9.4 × 10−11 cm3⋅molecule−1⋅s−1 (19)] compared with the BrO self-reaction [k = 9.3 × 10−13 cm3⋅molecule−1⋅s−1 (20)] (R31, 33; Fig. 1). Recently, inorganic chlorine (Cl2, ClO) (21, 22) and iodine (IO, HIO3) (23, 24) have been observed in the Arctic, adding support to signs of the importance of iodine chemistry from early aerosol measurements (3). Although molecular iodine (I2) has not previously been observed in the Arctic, it has been observed at several midlatitude marine and coastal sites (25) and along the Antarctic coast (26), and IO has been observed in the Antarctic (16, 27, 28), and in the sub-Arctic (29). During recent measurements at Alert, Canada, IO was observed at levels up to 1.5 ppt (23). Iodine has recently been observed to contribute to atmospheric new particle formation (30) through the sequential addition of iodic acid (HIO3) at maximum Arctic mole ratios of ∼1 ppt (24), giving further evidence to the presence and importance of Arctic iodine chemistry.
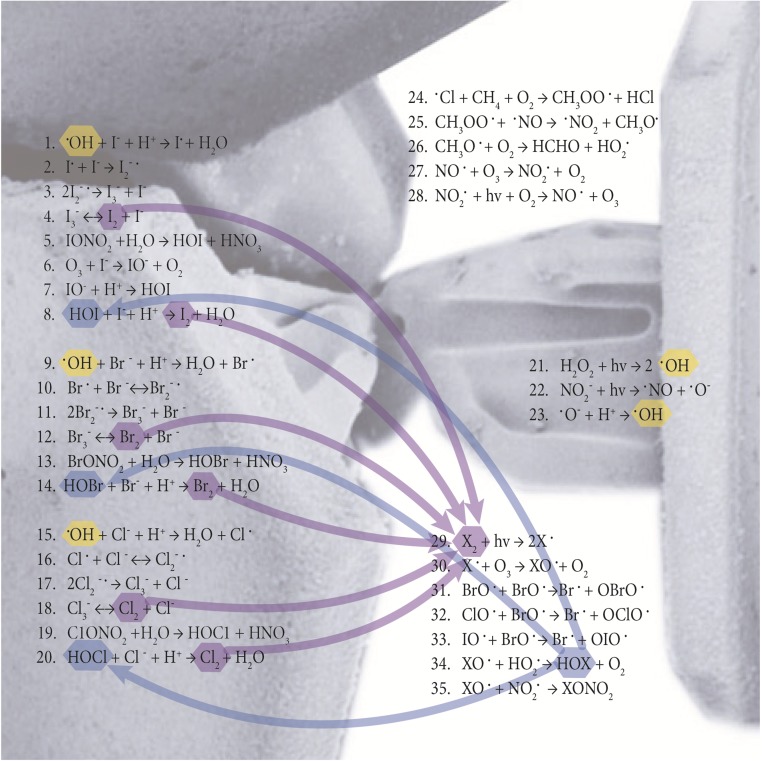
Fig. 1.
Snowpack halogen production and interstitial air halogen reactions. Major halogen reactions proposed to occur in the interstitial snowpack air and within the snow surface are shown. Oxidation of I− in the dark (R6–R8) is based on Carpenter et al. (47). Photochemical oxidation of Br− (R9–R12) is based on Abbatt et al. (38). Cl− and I− photochemical oxidation reactions (R15–R18 and R1–R4, respectively) are suggested to be analogous. Snow crystal SEM image is an open source image from the Electron and Confocal Microscopy Laboratory, Agricultural Research Service, US Department of Agriculture.
Although there is a clear indication of iodine chemistry in the Arctic, the source of the inorganic iodine has not been clear. In most midlatitude observations of I2 and IO, the source of inorganic iodine is believed to be macroalgae under oxidative stress, such as during low tide (31–33). In the Antarctic, observations have previously been ascribed to I2 production by sea ice diatoms, which are commonly found on the underside of both Arctic and Antarctic sea ice, followed by I2 diffusion through open brine channels to the sea ice surface (25, 34, 35). However, although the diffusion of I2 through brine channels has been modeled (34), it has not been directly observed. Whether iodine precursors in the Arctic are emitted from the open ocean (23, 29) or from sea ice-covered regions (24) has remained unclear. There are potential mechanistic pathways for both sources. Br2, Cl2, and BrCl production via photochemical reactions has been demonstrated in the Arctic saline snowpack (7, 9, 36) and from frozen substrates in laboratory experiments (37–44). I2 and triiodide (I3−) have recently been shown to be photochemically produced in Antarctic snow spiked with iodide (1–1,000 μM) (45), and iodate (IO3−) has also been shown to be photochemically active in frozen solutions (46). These studies show condensed phase iodine photochemistry, and although previous samples have lacked the physical and chemical characteristics of authentic snow, they suggest that photochemical production of I2, similar to that of Br2, Cl2, and BrCl production in the Arctic surface snowpack (7, 36), is probable. However, neither atmospheric I2 nor the production of I2 from snow samples with natural iodide (I−) levels has ever been reported.
Given the expected importance of iodine chemistry in the atmosphere (24, 25), snowpack iodine chemistry was investigated near Utqiaġvik, AK, in February 2014. Here, we report Arctic I2 measurements, in both the tropospheric boundary layer and snowpack interstitial air, coupled with measurements of I− in Arctic snow. The effect of radiation on halogen mole ratios in the snowpack interstitial air was examined through sunlit experiments, artificial irradiation experiments, and snowpack vertical profiles. In addition, the sensitivity of ozone depletion rates and IO mole ratios to tropospheric I2 was examined using a zero-dimensional photochemical model.
Results and Discussion
Snowpack Molecular Iodine Production.
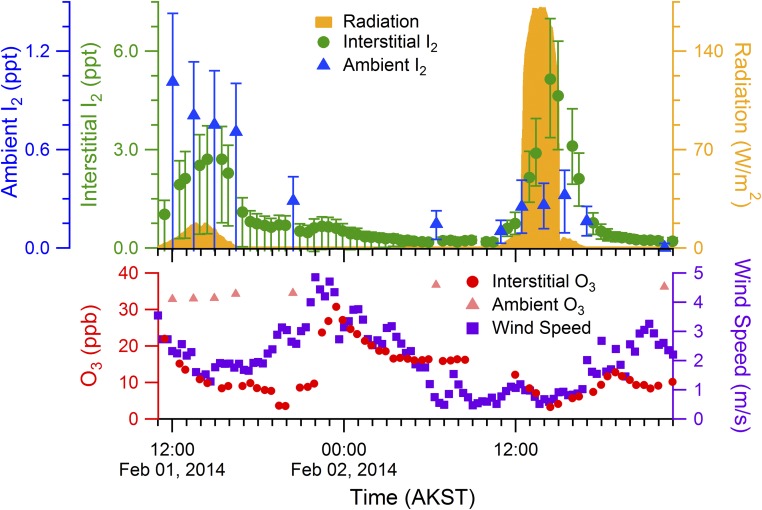
Here we report observations of I2 and snowpack I− in the Arctic. Gas-phase I2 was observed in the snowpack interstitial air at 10 cm below the sunlit snowpack surface on February 1 and 2, 2014, near Utqiaġvik, AK (Fig. 2). I2 mole ratios in the snowpack interstitial air peaked at 2.7–5.1 ppt in the early afternoon, just following the solar radiation maxima (Fig. 2). Coincident with these daytime maxima, I2 was observed in the boundary layer, 1 m above the snowpack surface, at mole ratios of ∼0.3–1.0 ppt (Fig. 2). Significantly more I2 was observed in the snowpack interstitial air on February 2 (maximum I2 5.1 ppt), which was sunny and clear (maximum radiation 172 W/m2), compared with February 1 (maximum I2 2.7 ppt), which was overcast (maximum radiation 18 W/m2), further supporting a photochemical production mechanism. Laboratory studies have shown that I2 can be produced from aqueous samples containing I− in the presence of O3 without light, via reactions 6–8 (Fig. 1) (47). During the night of February 1–2, average wind speeds rose from 2.0 m⋅s−1 to 5.9 m⋅s−1, leading to increased wind pumping, resulting in increasing O3 from 5 to 25 ppb in the snowpack interstitial air over the course of ∼30 min [22:00–22:30 Alaska Standard Time (AKST)] (Fig. 2). This presents an opportunity to examine the influence of O3 on dark oxidation and subsequent I2 formation. However, although an apparent small increase in I2 signal at a snowpack depth of 10 cm was observed during this time (Fig. 2), the I2 levels were never statistically significant different from zero. Therefore, these observations suggest that snowpack photochemical reactions were the predominant source of the observed I2 in the Arctic boundary layer.
Fig. 2.
I2, O3, radiation, and wind speeds during February 1–2, 2014. The diurnal profiles for I2 and O3 mole ratios, as well as the radiation and wind speeds, are shown as 20-min averages from February 1 to 2, 2014. Error bars are propagated uncertainties (SI Methods). Ambient measurements were conducted 1 m above the snowpack surface. Interstitial air measurements were conducted 10 cm below the snowpack surface. Fluctuations in interstitial air O3 mole ratios correlate with high wind speeds and are therefore likely due to wind pumping.
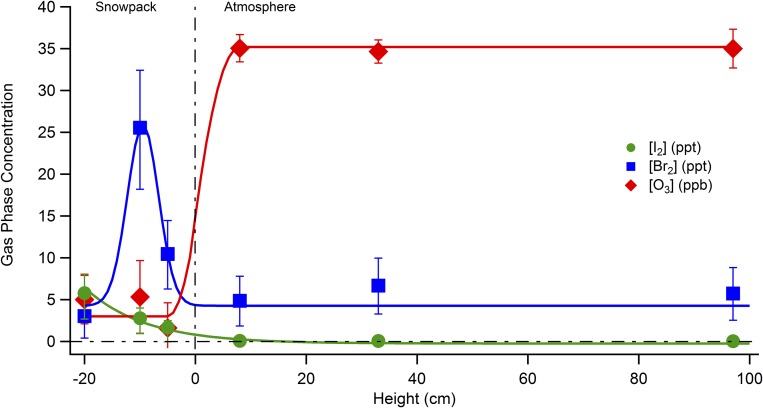
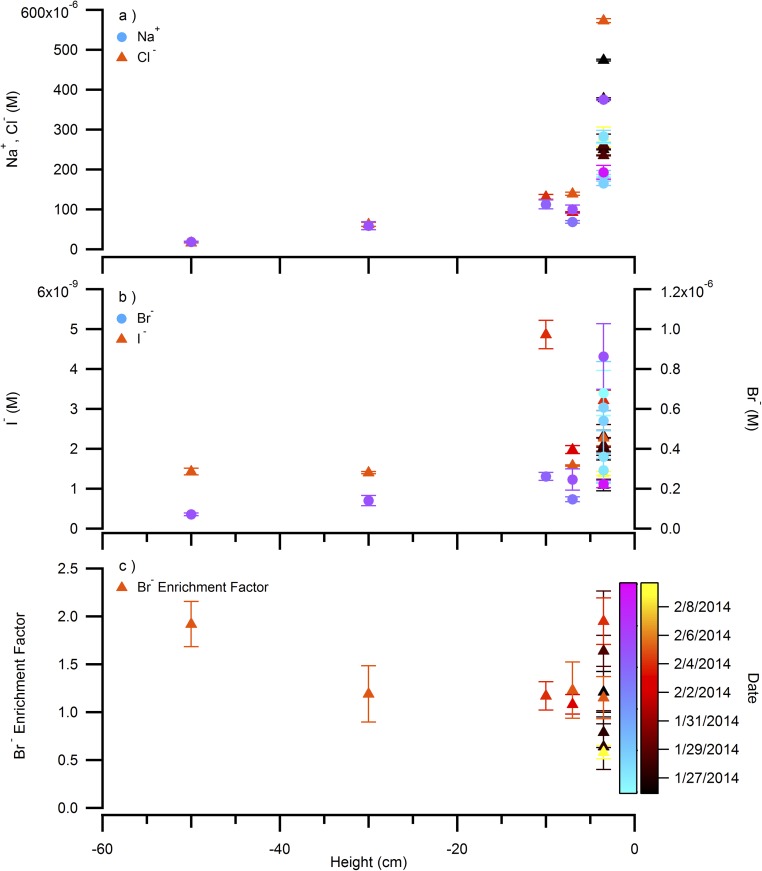
The photochemical nature of I2 production in the snowpack is further demonstrated by the differences in the vertical profiles of I2 and molecular bromine (Br2) within the snowpack interstitial air (Fig. 3). Gas-phase I2 and Br2 were simultaneously quantified at mole ratios up to 22 and 43 ppt, respectively, under sunlit conditions in the snowpack interstitial air, as shown in Fig. 3. Br2 showed peak mole ratios (43 ppt) just below the snowpack/atmosphere interface (within the top ∼10 cm) (Fig. 3 and Fig. S1). This is consistent with previous measurements, which showed a maximum in Br2 mole ratios within the top 7 cm of the snowpack air at Alert, Canada (9). In contrast, the I2 peak mole ratio (22 ppt) was observed at ∼40 cm below the snowpack surface, at least 30 cm deeper than the Br2 maximum (Fig. 3). At this snow depth, ambient light was attenuated (at 40 cm, ∼2% of 400 nm light remains) (48). The difference in behavior between I2 and Br2 with depth reflects two factors. First, I2 photolyzes nearly four times faster than Br2 (I2 Jmax = 2.9 × 10−3 s−1 vs. Br2 Jmax = 8.6 × 10−4 s−1 for above the snowpack on February 2, 2014). Second, whereas bromide (Br−) shows no consistent enrichment (relative to the seawater Br−/Na+ ratio) with depth (Fig. S2), I− was increasingly enriched with depth in January and February 2014 snow meltwater (Fig. 3). I− was observed at concentrations of 1.4–4.3 nM (Fig. S2) that are greatly enriched relative to sodium (Na+), at up to ∼1,900 times the seawater ratio (I−/Na+) (Fig. 3). The I− concentrations in the surface snowpack (top 7 cm) meltwater (2.0 ± 0.6 nM; Fig. S2) were sufficient to produce ∼1,600 ppt of I2, if I− were completely converted to I2 and contained in the snowpack interstitial air (SI Methods). In comparison, snow meltwater Br− ranged from partially depleted to double that in seawater (0.58–2.0 times the seawater Br−/Na+ ratio; Fig. S2). Previous measurements of Br−/Na+ ratios in coastal surface snow have shown bromide enrichments, relative to seawater, to increase from late winter (1.5–5 times the seawater Br−/Na+ ratio) through early spring (20–72 times the Br−/Na+ ratio in seawater), which is consistent with active heterogeneous recycling of bromine on the snowpack (49, 50). The much greater snowpack enrichment factor for I− suggests production and transport of iodine compounds from upwind snowpack; ocean or saline sea ice environments; or aerosols, and subsequent deposition on the downwind coastal snowpack. The exact source of I− to the snowpack remains undetermined, but the increasing enrichment of I− with depth indicates that iodine near the surface has migrated, either redepositing deeper in snowpack and/or being lost from the snowpack surface to the atmosphere, perhaps following polar sunrise. Although the source of I− enrichment in the Arctic snowpack requires further investigation, deposition of gas or particle phase iodine is consistent with earlier findings of enriched iodine in the aerosol phase (3). Future measurements of the spatial and temporal heterogeneity of snowpack I− enrichment are needed to elucidate the migration of iodine in the Arctic system.
Fig. 3.
Vertical profiles of near-surface atmospheric and snowpack interstitial air I2, Br2, and O3 mole ratios, as well as snow I− enrichment. Gas-phase measurements were made during daylight from 12:22 to 16:23 AKST on February 4, 2014, at heights above (positive) and below (negative) the snowpack surface. Error bars for species measured with CIMS (I2 and Br2) are propagated uncertainties (SI Methods). Error bars on the O3 measurements are the SDs of 9- to 22-min averages at each height. I− enrichment factors (the ratio of I− to Na+ in snow meltwater relative to the same ratio in seawater) are shown for snow samples collected from January 27 to February 5, 2014. I− enrichment factor error bars are the propagated error from three measurements of the I− concentration in a single sample. See Fig. S1 for an additional set of vertical profile measurements from February 3, 2014.
Fig. S1.
Vertical profiles of I2, Br2, and O3 mole ratios within and above the snowpack on February 3, 2014. Measurements were made alternating in and above the snowpack, starting closest to the snowpack surface. Height points are representative of the position of the snow probe inlet in the snowpack during measurement, and do not include possible errors due to air drawn from the other heights within the snowpack.
Fig. S2.
(A) Sodium (Na+), chloride (Cl−), (B) iodide (I−), and bromide (Br−) concentrations, and (C) Br− enrichment relative to sea water in snow as a function of depth in the snowpack and sampling date. Error bars are SDs from the average of three replicate measurements.
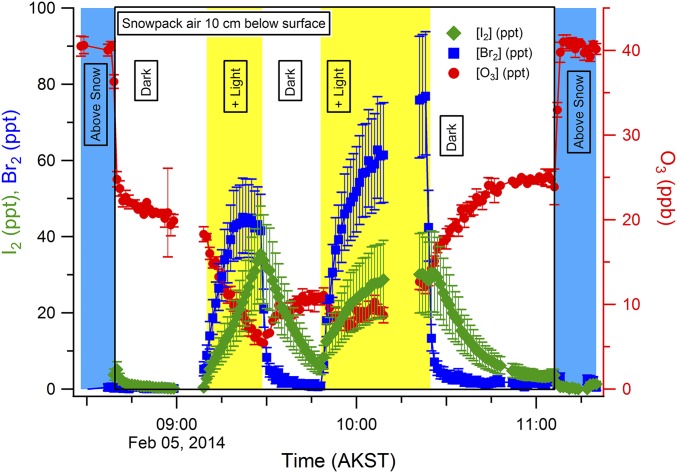
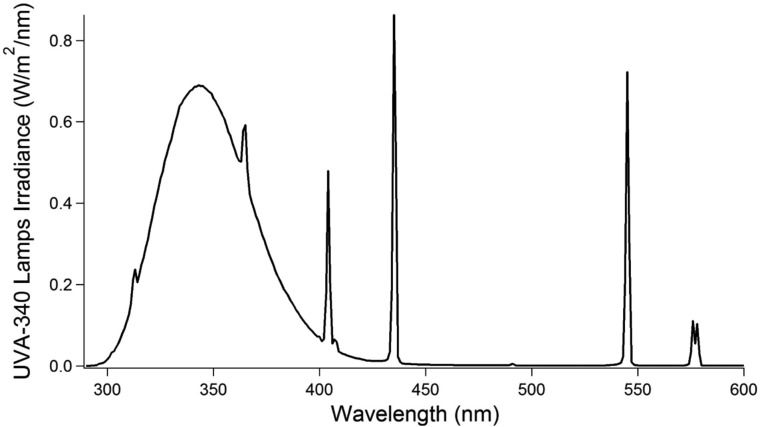
To further investigate photochemical I2 production, the snowpack was exposed to artificial UV light (Fig. 4). This experiment was conducted during the night and morning when solar radiation was low (<20 W/m2). When exposed to artificial light, the snowpack rapidly produced up to 35 ppt of gas-phase I2 at a depth of 10 cm (Fig. 4). The radiation spectrum of the lights used (Fig. S3) is adequate for the photolytic production of hydroxyl radicals from hydrogen peroxide and nitrite (Fig. 1, R21–R23), but not for significant I2 photolysis, which occurs most efficiently at wavelengths greater than 400 nm (20, 51). Upon snow illumination, Br2 was also quickly produced in the snowpack interstitial air, yielding mole ratios of 40–80 ppt (Fig. 4). With halogen production in the snowpack, O3 decayed rapidly, via the chemistry shown in Fig. 1. When the lights were turned off, both Br2 and I2 mole ratios decayed, and O3 partially recovered. This molecular halogen decline was likely controlled by dilution with ambient air (wind pumping), a lack of photochemical halogen production, and adsorption/desorption of halogen species onto the snow. Although snow grain chemical composition and exchange processes are complex (52), the rate of desorption from aqueous surfaces is often described as inversely proportional to the Henry's Law constant for that species (53). Because I2 is more soluble (kH = 41.9 M/atm at −20 °C) than Br2 (kH = 8.4 M/atm at −20 °C) (54), its rate of desorption from the disordered snow interface is expected to be slower, as shown in Fig. 4 by the slower decay in I2 mole ratios after illumination ceases, and from the slower initial rise in I2 mole ratios upon illumination. I2 and Br2 were again observed upon snowpack reillumination (Fig. 4). This demonstrates that I2 and Br2 are both characterized by condensed-phase photochemical production mechanisms.
Fig. 4.
Snowpack artificial irradiation experiment. Snowpack interstitial air Br2, I2, and O3 mole ratios are shown as 1-min averages for dark and artificial light measurement periods during an experiment on February 5, 2014. Error bars for I2 and Br2 are propagated uncertainties (SI Methods). The interstitial air measurements were bracketed by near-surface (5 cm above the snowpack surface) measurements of boundary-layer air. The duration of the experiment occurred before the sun rose, allowing for near-complete darkness when the artificial lights were off.
Fig. S3.
Radiation spectrum of the UVA 340 lamps used for artificial irradiation (67). The lamps have a peak irradiation wavelength at 340 nm. Aqueous nitrite absorbs very well in this region (20). Aqueous hydrogen peroxide photolyzes best at wavelengths less than 320 nm (20). Gas-phase I2 and Br2 have peak absorbance at 420 and 530 nm, respectively (20, 51).
The I2 multiphase photochemical production mechanism proposed here (Fig. 1) is analogous to that for Br2 production, which occurs first by condensed-phase photochemistry and then is greatly enhanced by gas-phase recycling of Br atoms in the presence of O3 (7, 37). The suggested mechanism for I2 production begins in the disordered interface of the aqueous phase on the snow grain surface (52) with oxidation of I− to an iodide radical by a photochemically produced oxidant (R1), likely the hydroxyl radical, produced by nitrite photolysis in Utqiaġvik snow (R22–R23) (55). The iodide radical then further reacts in solution to form I3− (R2–R3), which has been recently observed in snow samples spiked with I− (45). I3− then decomposes, forming I2 (R4). I2 can then be released from the condensed phase to the gas phase, where it rapidly photolyzes in the presence of sunlight. The resulting iodine atoms react with O3 to form IO (R30). IO can then react with HO2 to form HOI (R34) or NO2 to form IONO2 (R35), which can each then be redeposited onto the snow grain surface to reproduce I2 in a catalytic cycle similar to the bromine explosion (56). Interhalogen reactions may also participate in the production of molecular halogens. HOBr has also been shown to oxidize Cl− on frozen surfaces to form BrCl when the Br−/Cl− ratio is low (37, 39, 40). Similarly, HOI and IONO2 can react on frozen surfaces with Br− and Cl− to form IBr and ICl (57). The production of IBr via the reaction of HOI and Br− is up to ∼200 times faster (k = 3.3 × 1012 M−2⋅s−1) (58) than the production of Br2 via the reaction of HOBr and Br− (k = 1.6 × 1010 M−2⋅s−1) (59). Although the interactions between halogen species in the gas phase have received some study, the condensed-phase interactions of halogens have been significantly understudied (56).
Simulations of Arctic Ozone Destruction.
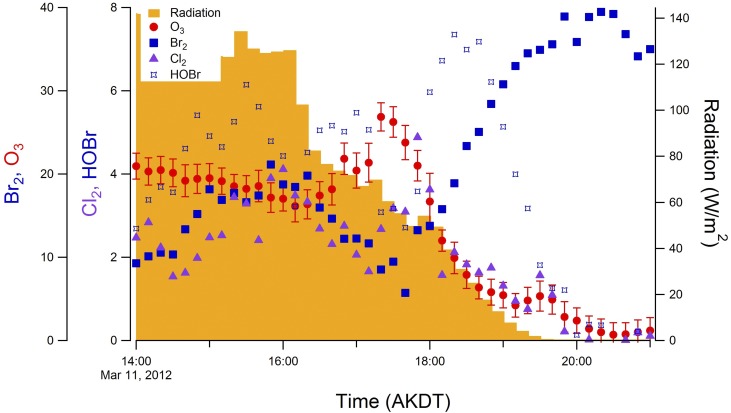
Because even small levels of I2 can significantly impact Arctic atmospheric chemistry, a zero-dimensional photochemical model was used to simulate O3 depletion and IO production. Because an ODE was not observed during the January to February 2014 study [ODEs typically begin in March in Utqiaġvik (2)], previous observations of atmospheric Br2, Cl2, and HOBr from the same location on March 11, 2012 were used to constrain the model (Fig. S4). On this day, atmospheric O3 decreased from ∼20 ppb to <1 ppb over the course of 7 h (Fig. 5A), with winds blowing from north to northeast over the consolidated snow-covered ice on the Beaufort Sea. The overall observed ozone depletion rate (3.0 ppb⋅h−1 over the 7-h period) is typical of a large number of ODEs observed over the snow-covered sea ice on the Arctic Ocean (average of 3.5 ppb⋅h−1) (60). The initial depletion (from 14:00–16:10 AKST) occurred at a rate of 2.2 ppb⋅h−1 and was interrupted by a local atmospheric mixing event (16:10–18:00 AKST), which is not possible to simulate with a zero-dimensional model.
Fig. S4.
Measurement data from March 11, 2012, used to constrain ambient photochemical modeling. Other starting mole ratios for the model can be found in Table S3.
Fig. 5.
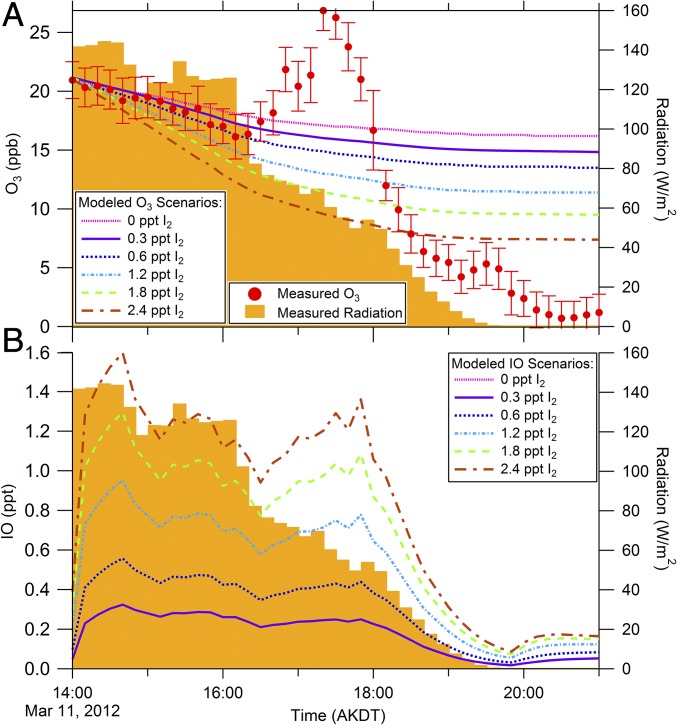
Model results show the influence of I2 on (A) tropospheric ozone depletion rates and (B) IO mole ratios. An ozone depletion event occurring on March 11, 2012 was simulated with I2 mole ratios between 0 and 2.4 ppt. Cl2, Br2, and HOBr were constrained to measurements as shown in Fig. S4. (A) Measured O3 with SDs of the 10-min average, and model results showing simulated O3 mole ratios. (B) Simulated IO mole ratios during the same period.
Given that I2 was only measured in early February in this study, it is plausible that higher mole ratios are present in March, when ODEs regularly occur. Therefore, the model was used to test the sensitivity of O3 depletion rates to I2 mole ratios from 0–2.4 ppt (Fig. 5A). Without the inclusion of iodine chemistry, O3 was simulated to deplete initially (14:00–16:10 AKST) at a rate of 1.3 ppb⋅h−1 (Fig. 5A), mostly from bromine atom chemistry. However, as shown in Fig. 5A, the addition of 0.3 ppt of I2 (as observed on February 2, 2014) increases the initial rate of ozone depletion by 31%, to 1.7 ppb⋅h−1. The best fit to the observed initial ozone depletion rate (2.2 ppb⋅h−1) corresponds to the inclusion of 0.6 ppt of I2 (within the range of our observations), which causes the model to simulate the initial depletion at a rate of at 2.1 ppb⋅h−1. The significant increase in the simulated ozone depletion rate with the inclusion of only 0.6 ppt I2 demonstrates the importance of even a small amount of iodine on the depletion of boundary layer O3. Higher, but still very modest and plausible, I2 mole ratios (compared with the ∼18 ppt of Br2 present) have a pronounced effect on the predicted O3. The addition of 2.4 ppt of I2 triples the rate of O3 depletion for the initial period (14:00–16:10 AKST) to 4.0 ppb⋅h−1.
The sensitivity of simulated IO concentrations to varying amounts of I2 on March 11, 2012 was also examined (Fig. 5B). The simulation containing 2.4 ppt of I2 produced a maximum of 1.6 ppt of IO, which is near the highest mole ratios observed (1.5 ppt) in Alert, Canada (23). The simulation with 0.3 ppt of I2 (February 2, 2014, maximum mole ratio) revealed IO mole ratios similar to those most commonly observed at Alert (∼0.3 ppt) (23). It should be noted, however, that there are significant uncertainties associated with modeling gas-phase iodine chemistry. Variations in the branching ratio for the products of the reaction of IO with itself can significantly change predicted IO mole ratios. The branching ratios used here (38% I + OIO, 16% I + I, 46% IOOI) may cause overestimation of IO by up to ∼10% (61). Additionally, because the photolysis of higher-order iodine oxides (I2O3 and larger) could cause the simulated O3 ratios shown here to be overestimated by up to 18% (17), sub-parts per trillion levels of I2 may result in even greater ozone depletion rates than predicted here. Even with these uncertainties in simulating iodine chemistry, our snowpack and ambient I2 observations along with our model results demonstrate that even a small amount of I2, at the observed levels, can significantly increase O3 depletion rates, while also producing realistic IO mole ratios.
SI Methods
The 2014 CIMS Measurements.
CIMS sampling in 2014 was conducted using a 25 °C heated 3.8-m-long, 1.3-cm ID FEP-Teflon line, which was attached directly to a custom-made three-way valve. The sampling end of this line was fitted with a 7-cm-long, 5-cm OD custom-machined PTFE snow probe (Fig. S5) intended to prevent snow from entering the sampling line. To minimize the recycling of HOBr into Br2 on the inlet line, as observed by Neuman et al. (65), as well as the recycling of HOI and/or IONO2 into I2, the line was rinsed with Milli-Q water and dried with ultra-high purity nitrogen (Air Liquide) between experiments. Holes in the snowpack for interstitial air measurements were drilled 2–3 cm deeper than the maximum depth to which the snow probe was inserted. Ambient measurements were conducted at 1 m above the snowpack surface for 30 min every 90 min during the day and two times overnight for the 2-d diurnal profile (Fig. 2).
Fig. S5.
The snow probe used to exclude snow from the sampling line during snowpack air measurement. Photograph courtesy of S. McNamara (University of Michigan, Ann Arbor, MI).
For vertical profile measurements, each height was sampled for 9–22 min, with sampling alternating between the overlying boundary layer and snowpack interstitial air. Interstitial measurements started closest to the snowpack surface and proceeded downward, whereas ambient measurements were done in no particular order. Fig. S1 shows an additional vertical profile conducted to a depth of 20 cm within the snowpack on February 3. The heights shown in Fig. 3 and Fig. S1 are the midline position of the snow probe, either in or above the snowpack. Due to the relatively high flow rate of air (7.5 lpm) being pulled from the snowpack, there was likely some vertical transport of species. The snowpack where sampling occurred was >70 cm deep for the duration of the campaign.
SF6− ion chemistry was used in February 2014 to measure I2 and Br2 using CIMS by monitoring at masses 254 amu (127I2−), 158 amu (79Br2−), and 160 amu (81Br79Br−). Br2 mole ratios were calculated using mass 160 amu; masses 158 and 160 showed the same trends (R2 = 0.977) and had an isotope ratio (158 amu/160 amu) of 0.5300 ± 0.0003 (natural isotope ratio = 0.5140). A constant flow rate of 7.5 lpm was continuously sampled into a custom-made three-way valve at the CIMS inlet, after which air was drawn through a 0.51-mm diameter orifice to the CIMS flow reactor (13 Torr), where electron transfer to the analyte using excess SF6− was achieved. SF6− was produced by passing 1.7 lpm of 5 ppm SF6 in N2 through a 210Po ionizer.
CIMS calibrations were performed using I2 and Br2 permeation devices (VICI) every 30 min to 2 h. The output gas of the I2 permeation device was flowed through an impinger into a NaHCO3 (30 mM)/NaHSO3 (5 mM) reducing solution. This solution quantitatively reduces I2 to I−, which was then analyzed using a Dionex DX500 ion chromatography system with an AG11 guard column and an AS11 analytical column with a sodium hydroxide gradient. Br2 and Cl2 permeation outputs were quantified using the method described by Liao et al. (5). The output gas of the Br2 and Cl2 permeation devices were flowed through impingers into a 2% (by weight) potassium iodide solution. The Br2/Cl2 then oxidizes I− to I3−, which is quantified using a UV-visible spectrometer (Spectronic 20 Genesys) at 352 nm. In 2014, the permeation rates were found to be 2.3(±0.8) × 10−11, 4.9(±0.6) × 10−10, and 8.1(±0.8) × 10−10 mol⋅min−1 of I2, Br2, and Cl2, respectively.
Background measurements were performed by passing the airflow through a glass wool scrubber, which quantitatively destroyed all three halogens, every 20 min to 1 h for 7–20 min each time. It has previously been shown that glass wool has >99% efficiency at removing halogen species (64, 65), and we found this to be true for I2, as well as Br2 and Cl2. There were no observable increases in the monitored masses when 62 ppt, 3.3 ppb, and 3.0 ppb of I2, Br2, and Cl2, respectively, were passed through the scrubber. There was some variation between mass 254 amu (127I2−) background measurements, both with ambient and ultra-zero grade air (Air Liquide), suggesting that there may be an interference created by the glass wool at mass 254 amu. This was particularly apparent during background measurements of the snowpack interstitial air, which were often higher than background measurements of ambient boundary layer Arctic air. It is unlikely that this variability in background measurements is due to an interference in the Arctic air, because scrubbed ambient air backgrounds and ultra-zero grade air backgrounds agreed well during individual tests, and no diurnal pattern was observed in background signals. It is also unlikely that this variability is due to inefficiency of the glass wool scrubber, which was found to be capable of removing >99% of 62 ppt of I2. Therefore, periodic background measurements in the ambient air were used for background subtraction for both the ambient air and snowpack interstitial air measurement data. The difference between these backgrounds and measured backgrounds in the snowpack interstitial air was accounted for in the measurement uncertainty expressed in figures.
For measurements conducted using SF6− ion chemistry in 2014, the duty cycle was 10 s, with a dwell time of 6 s for I2 (254 amu) and 300 ms for each of the Br2 masses (158 amu and 160 amu). Other masses, not discussed here, were monitored during the remaining time of the 10 s duty cycle. For SF6– ion chemistry, background measurements were conducted for a total of 7–20 min, corresponding to a 14.4- to 28.1-s integration period for each Br2 mass and a 252- to 720-s integration period for the I2 mass. The Br2 3σ LOD for these measurements ranged from 1.5 to 3.9 ppt. The I2 3σ LOD for these measurements ranged from 0.2 to 0.5 ppt. The propagated uncertainties, including line losses, in the 2014 I2 and Br2 mole ratios are calculated to be (−33%/+35% + LOD) and (−19%/+21% + LOD), respectively. The snowpack interstitial air I2 uncertainties shown in Figs. 2–4 and Fig. S1 reflect these uncertainties, as well as the difference between the snowpack interstitial air and above-snowpack background signals, applied to the negative uncertainty.
Tests for molecular halogen losses to the 2014 sampling line were completed both before the campaign in the laboratory and during the field campaign. In the laboratory, tests were done using several lengths of the 1.3-cm internal diameter fluorinated ethylene propylene line ranging from 2.44 to 7.32 m at room temperature. The greatest losses observed were 10% for I2 and 4% for Br2. In the field, line loss tests were performed at the end of the study with the same line as for sampling. Losses of 14% and 10% were observed for I2 and Br2, respectively. Because of the variability observed in line losses, the measurements presented in this paper have not been corrected for line losses. Instead, the maximum loss has been accounted for in the calculated measurement uncertainties.
2012 CIMS Measurements.
March 11, 2012 CIMS data, used for modeling, were obtained using hydrated I− [I(H2O)n−] chemistry. In 2012, masses 287 amu (I81Br79Br−), 289 amu (I81Br81Br−), 223 amu (IHO79Br−), 225 amu (IHO81Br−), 197 amu (I35Cl35Cl−), and 199 amu (I37Cl35Cl−) were monitored. As by Liao et al. (64), Peterson et al. (8), and Custard et al. (22), measurements were conducted by sampling from a 33-cm-long 4.6-cm ID aluminum pipe that extended ∼9 cm beyond the wall of the sampling building. A blower was used to pull a total flow rate of ∼300 lpm through the pipe with subsampling of 7.5 lpm from the centerline to the three-way valve. I(H2O)n− was produced in the flow reactor by passing 1.7 lpm of 5 ppm methyl iodide (CH3I) in N2 through a 210Po ionizer. To avoid any fluctuations due to ambient relative humidity, water was added in N2 (0.12 lpm) from a room temperature (∼20 °C) 1-L bubbler to the flow reactor, which was held at a constant pressure of 13 Torr. Background measurements were completed by flowing ambient air through a glass wool scrubber, as completed in 2014 and described above and by Liao et al. (5). Br2 and Cl2 permeation devices were used for calibration, with the permeation rate measured as described above and by Liao et al. (5). The sensitivity of HOBr (225 amu) relative to Br2 (287 amu) of 0.5 was used for HOBr quantification (5).
The 3σ limits of detection with I(H2O)n− chemistry for Br2 (287 amu), Cl2 (197 amu), and HOBr (225 amu) were calculated to be 3.9, 1.5, and 3.1 ppt, respectively, on average for 1 min. of CIMS measurements (corresponding to 2.8-s integration periods for each mass). Because the variation in the background is likely due to counting statistics (5), the limits of detection for the 10-min averaged data used for simulation are estimated at 1.2, 0.5, and 1.0 ppt for Br2, Cl2, and HOBr, respectively. The uncertainties in the 10-min averaged Br2, Cl2, and HOBr molar ratios used are calculated to be 14% + LOD, 25% + LOD, and (−28%/+34%) + LOD, respectively.
Auxiliary Measurements.
O3 measurements were obtained using a 2B Technologies model 205 dual-beam O3 monitor, which sampled from the same airflow as the CIMS instrument. After the three-way valve on the CIMS inlet, 1.3 lpm of air was diverted for O3 measurement. Radiation data were obtained from the NOAA Global Monitoring Division Earth Systems Research Laboratory (https://www.esrl.noaa.gov/gmd/). The NOAA measurement site was ∼5 km across flat tundra to the northeast (upwind) of the CIMS measurements.
Snow Meltwater Ion Concentrations.
Snow samples for ion chromatography analysis were collected in polyethylene bags using a polypropylene scoop. Before sampling, the scoop was rinsed with methanol (ACS grade) and air-dried. The sampler wore disposable gloves and remained downwind of the sampling site. The samples were maintained between −10 °C and −40 °C until they were melted, no more than 24 h before analysis. I− was measured in triplicate using an Agilent Technologies 1200 Series chromatography system equipped with a Thermo Scientific AS16 analytical column and an AG16 guard column was coupled with ESI Apex IR nebulizer and a Thermo Scientific Element XR inductively coupled plasma mass spectrometer. The 3σ limits of detection for I− were found to be 1.2–5.2 pM. The 100-μL injections of snowmelt samples, including water controls, were made using an Agilent 1260 series autosampler that was temperature controlled to 18 °C.
Snow meltwater Na+, Br−, and Cl− concentrations were measured in triplicate using a dual cation/anion ion chromatography system. Na+ (LOD 2 µM) was measured on a ICS-1100 system with an ultralow pressure trace cation concentrator column (TCC-ULP1; Dionex), a CG12A guard column (Dionex), and an CS12A analytical column (Dionex); methanesulfonic acid (20 mM) was used as an eluent. Br− (LOD 15 nM) and Cl− (LOD 0.8 µM) were measured on an ICS-2100 system with an ultralow pressure trace anion concentrator column (UTAC-ULP1; Dionex), a AG18 guard column (Dionex), and an AS18 analytical column (Dionex); a potassium hydroxide gradient generated by an EGC III KOH system was used as an eluent. Both the ICS-1100 and ICS-2100 systems used heated conductivity cells (DS6; Dionex) for detection.
Theoretical Limits on Snowpack Interstitial Air I2 Mole Ratios.
The theoretical maximum mole ratio of I2 in the snowpack interstitial air if I− were completely converted to I2, and completely contained in the snowpack interstitial air, is shown by Eq. S1:
| [S1] |
where [I−]avg is the average concentration of I− in the surface snowpack (2 nM), fs is the liquid water content of the snowpack as a unitless fraction (0.4), fa is the air fraction of the snowpack (0.6), and mvair is the mole volume of air at −20 °C (20.8 L/mol).
Time-Dependent Zero-Dimensional Model.
Boundary-layer photochemical modeling was conducted using a 7-h period of data (March 11, 2012) from the 2012 BROMEX campaign (68). The halogen species Cl2, Br2, and HOBr were constrained to CIMS measurements from 2012, and the sensitivity of the model to I2 mole ratios was tested. Measurements used to constrain the ambient model are shown in Fig. S4. Starting concentrations of other species can be found in Table S3. The model consisted of a series of photolytic and thermal chemical reactions. Photolytic reactions and maximum photolysis rates can be found in Table S1. Nonphotolytic chemical reactions can be found in Table S2, and include those found in Thompson et al. (13), as well as the formation of HIO3 through the reactions of OIO with OH and the equilibrium of I2O5 with water (61).
Table S3.
Constant (or starting) mole ratios used in modeling
| Species | Mole ratio |
| Hg | 122 ppq |
| HONO | 203 ppt |
| H2O | 907 ppm |
| H2 | 663 ppb |
| O2 | 252 ppth |
| O3* | 21.0 ppb |
| CO2 | 472 ppm |
| CO | 228 ppbv |
| CH4 | 2.28 ppm |
| CH3Br | 14.2 ppt |
| CHBr3 | 4.06 ppt |
| C2H2 | 317 ppt |
| C2H4 | 24.4 ppt |
| C2H6 | 2.11 ppb |
| C3H6 | 13.4 ppt |
| C3H8 | 610 ppt |
| PAN* | 200 ppt |
| MEK | 250 ppt |
| HCHO | 12.2 ppt |
| CH3CHO | 16.3 ppt |
| CH3COCH3 | 976 ppt |
| n-C4H10 | 244 ppt |
| i-C4H10 | 179 ppt |
Species not shown had a starting mole ratio of zero. Asterisks indicate starting mole ratios.
Table S1.
Maximum photolysis constants for the boundary layer air model
| Reaction | Jmax, s−1 | Ambient source |
| I2 → 2I | 3.76 × 10−2 | Scaled to Thompson et al. (13) |
| IO → I + O(3P) | 4.50 × 10−2 | Scaled to Thompson et al. (13) |
| OIO → IO + O(3P) | 3.84 × 10−4 | Scaled to Thompson et al. (13) |
| OIO → I +O2 | 8.09 × 10−3 | Scaled to Thompson et al. (13) |
| IOOI → 2IO | 3.71 × 10−3 | Scaled to Thompson et al. (13) |
| IOOI → 2I + O2 | 3.71 × 10−3 | Scaled to Thompson et al. (13) |
| HOI → I + OH | 1.26 × 10−3 | Scaled to Thompson et al. (13) |
| INO → I + NO | 2.07 × 10−2 | Scaled to Thompson et al. (13) |
| INO2 → I + NO2 | 5.53 × 10−4 | Scaled to Thompson et al. (13) |
| INO3 → IO + NO2 | 1.77 × 10−4 | Scaled to Thompson et al. (13) |
| INO3 → I + NO3 | 7.21 × 10−5 | Scaled to Thompson et al. (13) |
| IBr → I + Br | 1.69 × 10−2 | Scaled to Thompson et al. (13) |
| Br2 → 2Br | 1.09 × 10−2 | Scaled to Thompson et al. (13) |
| BrO → Br + O(3P) | 7.49 × 10−3 | Scaled to Thompson et al. (13) |
| HOBr → Br+ OH | 5.74 × 10−4 | Scaled to Thompson et al. (13) |
| BrNO2 → Br + NO2 | 3.75 × 10−5 | Scaled to Thompson et al. (13) |
| BrNO3 → BrO + NO2 | 2.90 × 10−4 | Calculated using TUV and radiation data |
| BrNO3 → Br + NO3 | 5.12 × 10−5 | Calculated using TUV and radiation data |
| ICl → I + Cl | 5.46 × 10−3 | Scaled to Thompson et al. (13) |
| BrCl → Br + Cl | 3.12 × 10−3 | Scaled to Thompson et al. (13) |
| Cl2 → 2Cl | 5.10 × 10−4 | Calculated using TUV and radiation data |
| ClO → Cl + O(3P) | 6.06 × 10−6 | Scaled to Thompson et al. (13) |
| OClO → ClO + O(3P) | 3.04 × 10−2 | Calculated using TUV and radiation data |
| HOCl → Cl + OH | 3.44 × 10−5 | Scaled to Thompson et al. (13) |
| ClNO2 → Cl + NO2 | 1.09 × 10−5 | Scaled to Thompson et al. (13) |
| ClNO3 → ClO + NO2 | 8.33 × 10−7 | Calculated using TUV and radiation data |
| ClNO3 → Cl + NO3 | 7.25 × 10−6 | Calculated using TUV and radiation data |
| O3 → O2 + O(1D) | 1.31 × 10−4 | Calculated using TUV and radiation data |
| H2O2 → 2OH | 8.45 × 10−7 | Calculated using TUV and radiation data |
| NO2 → NO + O(3P) | 2.13 × 10−3 | Calculated using TUV and radiation data |
| NO3 → NO + O2 | 1.11 × 10−2 | Calculated using TUV and radiation data |
| N2O5 → NO2 + NO3 | 3.78 × 10−6 | Calculated using TUV and radiation data |
| HNO2 → NO + OH | 4.47 × 10−4 | Calculated using TUV and radiation data |
| HNO3 → NO2 + OH | 3.62 × 10−8 | Calculated using TUV and radiation data |
| HNO4 → NO2 + HO2 | 1.81 × 10−7 | Calculated using TUV and radiation data |
| HCHO → CO + 2HO2 | 3.65 × 10−6 | Calculated using TUV and radiation data |
| HCHO → CO + H2 | 7.81 × 10−6 | Calculated using TUV and radiation data |
| CH3CHO→CH3O2+CO+HO2 | 2.65 × 10−7 | Calculated using TUV and radiation data |
| CH3OOH→HCHO+HO2+OH | 7.85 × 10−7 | Calculated using TUV and radiation data |
| C3H6O→C2H5OO+CO+HO2 | 3.46 × 10−7 | Calculated using TUV and radiation data |
| PAN→CH3COOO+NO2 | 4.33 × 10−8 | Calculated using TUV and radiation data |
As noted, most photolysis rate constants were scaled to Thompson et al. (13) by multiplying by the ratio of maximum NO2 photolysis rates.
Table S2.
Thermal chemical reactions used in the zero-dimensional modeling
| Reaction | Rate constant |
| O[1D] + M → O[3P] | 7.22 × 10−11 |
| O[3P] + O2 → O3 | 2.12 × 10−14 |
| O[1D] + H2O → 2OH | 2.2 × 10−10 |
| OH + O3 → HO2 | 3.84 × 10−14 |
| OH + HO2 → H2O | 1.34 × 10−10 |
| OH + H2O2 → HO2 + H2O | 1.52 × 10−12 |
| OH + O[3P] → O2 | 3.74 × 10−11 |
| OH + OH → H2O + O[3P] | 1.74 × 10−12 |
| OH + OH → H2O2 | 1.86 × 10−11 |
| OH + NO3 → HO2 + NO2 | 2.0 × 10−11 |
| HO2 + NO3 → HNO3 | 4.0 × 10−12 |
| HO2 + O3 → OH + 2O2 | 1.39 × 10−15 |
| HO2 + HO2 → H2O2 + O2 | 2.58 × 10−12 |
| NO + OH → HONO | 3.49 × 10−11 |
| NO + HO2 → NO2 + OH | 9.59 × 10−12 |
| NO + O3 → NO2 | 7.09 × 10−15 |
| NO + NO3 → NO2 + NO2 | 2.98 × 10−11 |
| NO2 + OH → HNO3 | 1.2 × 10−10 |
| NO2 + HO2 ←→ HNO4 | f: 8.6 × 10−12 |
| r: 1.32 × 10−4 | |
| NO2 + O3 → NO3 | 6.15 × 10−18 |
| NO2 + NO3 ←→ N2O5 | f: 1.83 × 10−12 |
| r: 3.76 × 10−5 | |
| NO2 + CH3COOO ←→ PAN | f: 1.4 × 10−11 |
| r: 3.1 × 10−8 | |
| NO3 + NO3 → NO2 + NO2 | 4.36 × 10−17 |
| N2O5 + H2O → HNO3 + HNO3 | 2.6 × 10−22 |
| HONO + OH → NO2 + H2O | 3.74 × 10−12 |
| HNO3 + OH → NO3 + H2O | 1.5 × 10−13 |
| HNO4 + OH → NO2 + H2O | 6.2 × 10−12 |
| CO + OH → HO2 + CO2 | 2.4 × 10−13 |
| CH4 + OH → CH3OO + H2O | 1.87 × 10−15 |
| C2H2 + OH → C2H2OH | 7.8 × 10−13 |
| C2H6 + OH → C2H5OO | 1.18 × 10−13 |
| C2H4 + OH → C2H4OH | 1.02 × 10−11 |
| C3H8 + OH → nC3H7O2 | 1.56 × 10−13 |
| C3H8 + OH → iC3H7O2 | 6.64 × 10−13 |
| C3H6 + OH → C3H6OH | 3.63 × 10−11 |
| C3H6O + OH → products | 2.51 × 10−11 |
| nC3H7O2 + NO → NO2 + C3H6O + HO2 | 5.4 × 10−11 |
| iC3H7O2 + NO → NO2 + CH3COCH3 + HO2 | 1.2 × 10−11 |
| nC4H10 + OH → nC4H9OO | 1.64 × 10−12 |
| iC4H10 + OH → CH3COCH3 + CH3OO | 1.65 × 10−12 |
| nC4H9OO + NO → n-Butanal + NO2 + HO2 | 5.4 × 10−11 |
| nC4H9OO + CH3OO → n-Butanal + HCHO + HO2 + HO2 | 6.7 × 10−13 |
| nC4H9OO + CH3OO → n-Butanal + CH3OH | 2.3 × 10−13 |
| nC4H9OO + CH3OO → nC4H9OH + HCHO | 2.3 × 10−13 |
| CH3OH + OH → CH3O | 7.09 × 10−13 |
| n-Butanal + OH → Products | 2.0 × 10−11 |
| CH3OO + HO2 → CH3OOH | 1.01 × 10−11 |
| C2H5OO + HO2 → C2H5OOH | 9.23 × 10−12 |
| CH3COOO + HO2 → CH3COOOH | 2.54 × 10−11 |
| C2H5OOH + OH → C2H5OO | 6.0 × 10−12 |
| CH3OO + CH3OO → HCHO + HO2 | 3.64 × 10−13 |
| CH3OOH + OH → HCHO + H2O + OH | 2.54 × 10−12 |
| CH3OOH + OH → CH3OO + H2O | 6.01 × 10−12 |
| CH3OO + HO2 → CH3OOH | 1.01 × 10−11 |
| CH3OO + NO → HCHO + HO2 + NO2 | 8.76 × 10−12 |
| CH3OO + nC3H7O2 → HCHO + C3H6O + HO2 + HO2 | 6.70 × 10−13 |
| CH3OO + nC3H7O2 → C3H6O + CH3OH | 2.3 × 10−13 |
| CH3OO + nC3H7O2 → HCHO + nC3H7OH | 2.3 × 10−13 |
| CH3OO + iC3H7O2 → HCHO + CH3COCH3 + HO2 + HO2 | 1.2 × 10−14 |
| CH3OO + iC3H7O2 → CH3COCH3 + CH3OH | 4.1 × 10−15 |
| CH3OO + iC3H7O2 → HCHO + iC3H7OH | 4.1 × 10−15 |
| CH3OO + C2H5OO → CH3CHO + HCHO + HO2 + HO2 | 2.0 × 10−13 |
| CH3OO + CH3COOO → HCHO + CH3OO + HO2 | 1.58 × 10−11 |
| C2H5OO + NO → CH3CHO + HO2 + NO2 | 8.68 × 10−12 |
| C2H5OO + HO2 → C2H5OOH | 9.23 × 10−12 |
| C2H5OO + CH3COOO → CH3CHO + CH3COO + HO2 | 4.0 × 10−12 |
| iC3H7O2 + HO2 → iPerox | 9.23 × 10−12 |
| nC3H7O2 + HO2 → nPerox | 9.23 × 10−12 |
| HCHO + OH → HO2 + CO | 9.3 × 10−12 |
| HCHO + HO2 → HOCH2O2 | 7.53 × 10−14 |
| HCHO + NO3 → HNO3 + HO2 + CO | 5.8 × 10−16 |
| CH3CHO + OH → CH3COOO + H2O | 1.98 × 10−11 |
| CH3CHO + NO3 → HNO3 + CH3COOO | 1.4 × 10−15 |
| CH3COCH3 + OH → H2O + CH3COCH2 | 1.37 × 10−13 |
| HOCH2O2 + NO → HCOOH + HO2 + NO2 | 8.68 × 10−12 |
| HOCH2O2 + HO2 → HCOOH + H2O | 2.0 × 10−12 |
| HOCH2O2 + HOCH2O2 → HCOOH + HCOOH + HO2 + HO2 | 1.0 × 10−13 |
| HCOOH + OH → HO2 + H2O + CO2 | 4.0 × 10−13 |
| CH3COOO + NO → CH3OO + NO2 + CO2 | 2.4 × 10−11 |
| CH3COOO + HO2 → CH3COOH + O3 | 1.87 × 10−11 |
| CH3COOO + CH3COOO → CH3COO + CH3COO | 2.5 × 10−11 |
| Cl2 + OH → HOCl + Cl | 2.85 × 10−14 |
| Cl + O3 → ClO | 1.02 × 10−11 |
| Cl + H2 → HCl | 3.5 × 10−15 |
| Cl + HO2 → HCl | 3.57 × 10−11 |
| Cl + HO2 → ClO + OH | 6.68 × 10−12 |
| Cl + H2O2 → HCl + HO2 | 2.11 × 10−13 |
| Cl + NO3 → ClO + NO2 | 2.4 × 10−11 |
| Cl + CH4 → HCl + CH3OO | 3.99 × 10−14 |
| Cl + C2H6 → HCl + C2H5OO | 5.36 × 10−11 |
| Cl + C2H4 → HCl + C2H5OO | 1.0 × 10−10 |
| Cl + MEK → HCl | 4.21 × 10−11 |
| Cl + C2H2 → ClC2CHO | 2.5 × 10−10 |
| Cl + C3H6 → HCl + C3H6Cl | 2.7 × 10−10 |
| Cl + C3H8 → HCl + iC3H7O2 | 1.65 × 10−10 |
| Cl + C3H8 → HCl + nC3H7O2 | 1.65 × 10−10 |
| Cl + C3H6O → HCl | 1.1 × 10−10 |
| Cl + iC4H10 → HCl + C4H9 | 1.3 × 10−10 |
| Cl + nC4H10 → HCl + C4H9 | 2.15 × 10−10 |
| Cl + n-Butanal → HCl + products | 1.1 × 10−10 |
| Cl + HCHO → HCl + HO2 + CO | 7.18 × 10−11 |
| Cl + CH3CHO → HCl + CH3COOO | 8.08 × 10−11 |
| Cl + CH3COCH3 → HCl + CH3COCH2 | 1.39 × 10−12 |
| Cl + CH3OOH → CH3OO + HCl | 2.36 × 10−11 |
| Cl + CH3OOH → CH2OOH + HCl | 3.54 × 10−11 |
| Cl + CHBr3 → HCl + Br + CBr2O | 2.9 × 10−13 [at 298 K] |
| Cl + OClO → ClO + ClO | 6.35 × 10−11 |
| Cl + ClNO3 → Cl2 + NO3 | 1.12 × 10−11 |
| Cl + PAN → HCl + HCHO + NO3 | 1.0 × 10−14 |
| Cl + HNO3 → HCl + NO3 | 1.0 × 10−16 |
| Cl + NO2 → ClNO2 | 1.43 × 10−12 [at 298 K] |
| Cl + HBr → HCl + Br | 4.48 × 10−12 |
| ClO + O[3P] → Cl + O2 | 1.6 × 10−11 |
| ClO + OH → Cl + HO2 | 2.45 × 10−11 |
| ClO + OH → HCl | 2.37 × 10−13 |
| ClO + HO2 → HOCl | 8.67 × 10−12 |
| ClO + CH3OO → Cl + HCHO + HO2 | 2.08 × 10−12 |
| ClO + CH3COOO → Cl + CH3OO + CO2 | 2.03 × 10−12 |
| ClO + NO → Cl + NO2 | 2.04 × 10−11 |
| ClO + NO2 → ClNO3 | 7.1 × 10−12 |
| ClO + ClO → Cl2 | 1.64 × 10−15 |
| ClO + ClO → Cl + Cl | 1.54 × 10−15 |
| ClO + ClO → Cl + OClO | 1.40 × 10−15 |
| OClO + OH → HOCl | 1.13 × 10−11 |
| OClO + NO → ClO + H2O | 1.51 × 10−13 |
| HOCl + OH → ClO + H2O | 4.0 × 10−13 |
| HCl + OH → Cl + H2O | 6.84 × 10−13 |
| ClNO3 + OH → HOCl + NO3 | 3.17 × 10−13 |
| HOCl + O[3P] → ClO + OH | 1.7 × 10−13 |
| Br + O3 → BrO | 6.75 × 10−13 |
| Br2 + OH → HOBr | 5.0 × 10−11 |
| Br + HO2 → HBr | 1.25 × 10−12 |
| Br + C2H2 → BrCH2CHO | 3.7 × 10−14 |
| Br + C2H4 → HBr + C2H5OO | 1.3 × 10−13 |
| Br + C3H6 → HBr + C3H5 | 1.60 × 10−12 |
| Br + HCHO → HBr + CO + HO2 | 6.75 × 10−13 |
| Br + CH3CHO → HBr + CH3COOO | 2.8 × 10−12 |
| Br + C3H6O → HBr | 9.7 × 10−12 |
| Br + n-Butanal → HBr | 9.7 × 10−12 |
| Br + CH3OOH → HBr + CH3OO | 4.03 × 10−15 |
| Br + NO2 → BrNO2 | 2.7 ×10−11 |
| Br + BrNO3 → Br2 + NO3 | 4.9 × 10−11 |
| Br + OClO → BrO + ClO | 1.43 × 10−13 |
| BrO + O[3P] → Br | 4.8 × 10−11 |
| BrO + OH → Br + HO2 | 4.93 × 10−11 |
| BrO + HO2 → HOBr | 3.38 × 10−11 |
| BrO + CH3OO → HOBr + CH2OO | 4.1 × 10−12 |
| BrO + CH3OO → Br + HCHO + HO2 | 1.6 × 10−12 |
| BrO + CH3COOO → Br + CH3COO | 1.7 × 10−12 |
| BrO + C3H6O → HOBr | 1.5 × 10−14 |
| BrO + NO → Br + NO2 | 2.48 × 10−11 |
| BrO + NO2 → BrNO3 | 1.53 × 10−11 |
| BrO + BrO → Br + Br + O2 | 2.82 × 10−12 |
| BrO + BrO → Br2 | 9.3 × 10−13 |
| BrO + HBr → HOBr + Br | 2.1 × 10−14 |
| HBr + OH → Br + H2O | 1.26 × 10−11 |
| CH3Br + OH → H2O + Br | 1.27 × 10−14 |
| CHBr3 + OH → H2O + Br | 1.2 × 10−13 |
| Cl + BrCl ←→ Br + Cl2 | f: 1.5 × 10−11 |
| r: 1.1 × 10−15 | |
| Cl + Br2 ←→ BrCl + Br | f: 1.2 × 10−10 |
| r: 3.3 × 10−1 | |
| BrO + ClO → Br + Cl | 7.04 × 10−12 |
| BrO + ClO → BrCl | 1.15 × 10−12 |
| BrO + ClO → Br + OClO | 9.06 × 10−12 |
| HOBr + OH → BrO + H2O | 5.0 × 10−13 |
| HOBr + Cl → BrCl + OH | 8.0 × 10−11 |
| HOBr + O[3P] → BrO + OH | 2.12 × 10−11 |
| I2 + O[3P] → IO + I | 1.25 × 10-10 [at 298 K] |
| IO + O[3P] → I | 1.4 × 10−10 [at 298 K] |
| I + HO2 → HI | 1.85 × 10−13 [at 298 K] |
| I + O3 → IO | 7.39 × 10−13 |
| I + NO → INO | 3.48 × 10−13 [at 298 K] |
| I + NO2 → INO2 | 5.76 × 10−12 [at 298 K] |
| I + NO3 → IO + NO3 | 1.0 × 10−12 [at 298 K] |
| I2 + NO3 → I + IONO2 | 1.5 × 10−12 [at 298 K] |
| HI + OH → I + H2O | 9.43 × 10−11 [at 298 K] |
| I2 + OH → HOI + I | 2.1 × 10−10 |
| IO + NO3 → OIO + NO2 | 9.0 × 10−12 |
| IO + HO2 → HOI | 8.40 × 10−11 [at 298 K] |
| IO + ClO → ICl +O2 | 3.16 × 10−12 [at 298 K] |
| IO + ClO → I + Cl + O2 | 3.95 × 10−12 |
| IO + ClO → I + OClO | 8.69 × 10−12 |
| IO + BrO → Br + OIO | 9.36 × 10−11 |
| IO + BrO → IBr + O2 | 4.32 × 10−11 |
| IO + BrO → Br + I + O2 | 7.2 × 10−12 |
| IO + IO → I + OIO | 4.41 × 10−11 |
| IO + IO → I + I | 1.84 × 10−11 |
| IO + IO ←→ IOOI | f: 5.34 × 10−11 |
| r: 1.3 × 10−4 | |
| IOOI → OIO + I | 2.1 × 10−1 |
| IO + NO → I + NO2 | 1.96 × 10−11 |
| IO + NO2 ←→ IONO2 | f: 4.61 × 10−11 |
| r: 8.36 × 10−7 | |
| OIO + NO → IO + NO2 | 9.78 × 10−12 |
| OIO + OH → HOI | 6.0 × 10−12 |
| OIO + OH → HIO3 | 5.8 × 10−10 |
| HOI + OH → IO | 2.0 × 10−13 |
| IO + OIO → I2O3 | 1.5 × 10−10 |
| OIO + OIO ←→ I2O4 | f: 1.0 × 10−10 |
| r: 4.4 × 10−4 | |
| IOOI + O3 → I2O3 | 1.0 ×10−12 |
| I2O3 + O3 → I2O4 | 1.0 ×10−12 |
| I2O4 + O3 → I2O5 | 1.0 × 10−12 |
| I2O5 + H2O ←→ HIO3 | f: 5.2 × 10−24 |
| r: 5.0 × 10−25 |
Conclusions
Here we report measurements of I2 in the Arctic. Low mole ratios (0.3–1.0 ppt) of I2 in the boundary layer air coupled with elevated I2 mole ratios in the snowpack interstitial air suggest that the snowpack is a source of I2 to the Arctic boundary layer. These results are supported by Arctic snowpack measurements of I−, which was greatly enriched relative to seawater, and more so with increasing depth. I2 is observed in the snowpack interstitial air under naturally sunlit conditions, and under artificial irradiation, but not in the dark, suggesting a photochemical production mechanism. The inclusion of observed molar ratios of I2 in a zero-dimensional model increases the ability of the model to simulate the initial rate of an observed ozone depletion event, and produces IO concentrations consistent with recent observations. Differences in the snowpack depth profiles of bromine and iodine species within both the snow phase (Br− and I−) and snowpack interstitial air (Br2 and I2) suggest that there are significant differences in bromine and iodine multiphase chemistry. The assumption that these species act similarly may be an oversimplification—one that can only be remedied through further measurements of production examining important chemical mechanisms and fundamental reaction rates and yields under both laboratory and field conditions.
The community’s challenge to properly simulate the chemical and physical processes that occur within and on the surface of snow grains (62) is especially daunting, because we do not currently understand the physical nature of the phase in which the chemistry is occurring (52, 62). New methodology is required to examine the chemical composition of ambient snow grain surfaces in situ. Although we lack comprehensive knowledge about the heterogeneous chemical processes of halogens on snow, we benefit greatly from real-world observations, such as those described herein. Multiphase interhalogen chemistry may also be important; however, there are no reported ambient measurements of the iodine molecular interhalogens (IBr and ICl). It is also unclear how the likely increasingly saline surface snowpack (from increasing first-year sea ice, sea spray production, and potentially decreasing snow depth), combined with increasing Arctic development (which may be changing acid deposition), are influencing springtime halogen chemistry. Iodine chemistry may have an especially large impact on atmospheric composition as the Arctic warms, given the prevalence of iodine chemistry in the marine midlatitudes (25).
Even at sub-parts per trillion levels of I2 in the Arctic atmosphere, iodine chemistry has significant impacts on atmospheric boundary layer oxidation capacity and composition, impacting pollutant fate and particle formation. Further simultaneous measurements of aerosol I−, snowpack I−, and I2 are needed to examine the movement of iodine between the aerosol, gas, and the snowpack phases. We now know that the coastal Arctic snowpack is a source of photochemically reactive inorganic iodine. This provides an abiotic source of iodine for new particle formation, expanding the potential importance of this chemical process to impacts on clouds (24, 25, 63). Given the dramatic impact of iodine on Arctic atmospheric composition, there is a need for further measurements of I2 in the ambient atmosphere to connect and elucidate the full cycling of iodine in the Arctic system.
Methods
Trace halogen gases were measured using chemical ionization mass spectrometry (CIMS), as described by Liao et al. (5, 64), Peterson et al. (8), and Custard et al. (22), on the Barrow Environmental Observatory (BEO), 5 km inland over tundra snowpack near Utqiaġvik, AK, on March 11, 2012, and February 1–5, 2014. A detailed description of the sampling, calibrations, background measurements, line loss tests, and uncertainties are described in SI Methods. Briefly, in 2012, CIMS, using IH2O− as the reagent ion, measured Br2, Cl2, and HOBr at ∼1 m above the snowpack surface [Peterson et al. (8), Custard et al. (22); SI Methods]. For 2014 measurements, the CIMS instrument was modified by the addition of an 18-cm-long PTFE-coated flow tube to the original 4.5-cm flow tube. CIMS measurements in 2014 were made using SF6− as the reagent ion; masses 254 amu (127I2−), 160 amu (81Br79Br −), and 158 amu (79Br2−) were monitored. Calibrations were performed using I2 and Br2 permeation devices (VICI) every 30 min to 2 h. Background measurements were performed every 20 min to 1 h, for 7–20 min, by passing the airflow through a glass wool scrubber, which quantitatively destroyed (>99%) the molecular halogens. For the I2 measurements, an apparent interference caused higher backgrounds when measuring in the snowpack interstitial air; therefore, only background measurements made above the snowpack were used. This uncertainty in the background is accounted for in the mole ratio uncertainties shown. In 2014, SDs of background signals resulted in 3σ limits of detection (LODs) for Br2 ranging from 1.5 to 3.9 ppt and for I2 ranging from 0.2 to 0.5 ppt. The method uncertainty in the I2 and Br2 mole ratios was (−33%/+35% + LOD) and (−19%/+21% + LOD), respectively. The 2014 and 2016 CIMS molecular halogen data are available through the NSF Arctic Data Center.
Interstitial snowpack air and depth profile sampling was conducted using a 380-cm-long, 1.3-cm ID FEP-Teflon line heated to 25 °C, which was attached directly to the CIMS sampling inlet. Estimated line losses, based on laboratory and field testing of the lines with permeation devices, were accounted for in the method uncertainties (SI Methods). To prevent heterogeneous recycling (65), the line was rinsed with Milli-Q water and dried with N2 before each experiment. A custom machined PTFE snow probe (Fig. S5) was used to prevent snow from entering the sampling line. A custom-built 61 × 61 cm Acrylite OP-4 cover (80% transmittance at 300 nm and ∼92% at 395 nm) with a 7.6-cm aluminum lip was pressed into the snow surface to prevent ambient air from being pulled directly into the snowpack and mixing with the interstitial air being sampled below (Fig. S6). Although dilution of the snowpack interstitial air by ambient air will occur to some extent, the O3 mole ratios measured during in-snowpack experiments were consistently much lower than those observed in the air above the snowpack and agreed with previous snowpack O3 observations (66, 67), suggesting that the mixing of ambient air into the snowpack was minimal. Any mixing of air within the snowpack due to high sampling rates would be expected to lessen the gradients shown in Fig. 3 and Fig. S1. O3 was measured using a 2B Technologies model 205 dual-beam O3 monitor. Artificial light was supplied by six Q-Lab UVA-340 halogen light bulbs (68) housed in a custom-built heated and insulated fixture. The light exited the fixture through a sheet of Acrylite OP-4, which insulated the light bulbs from the cold environment. The lamp housing was suspended ∼10 cm above the snow cover (Fig. S6).
Fig. S6.
Artificial light fixture suspended above the snowpack cover. As described in Methods, the artificial light fixture was suspended ∼10 cm above the Acrylite snow cover. The sampling line was inserted into the hole in the snowpack through a ∼6-cm-diameter hole, which was partially sealed using silicone sheets.
Snow samples were collected ∼50 m upwind (north to northeast) of the CIMS sampling site on the BEO using a polypropylene scoop, which was rinsed with ACS-grade methanol and air dried before sampling. Samples were stored frozen (−10 °C to −40 °C) in polyethylene bags until the day of analysis. An Agilent Technologies 1200 series ion chromatograph (IC) was paired with a Thermo Scientific Element XR inductively coupled plasma mass spectrometer (ICP-MS) for quantitation of I− in snowmelt samples. The 3σ limits of detection for iodide (I−) were 1.2–5.2 pM. Na+, Cl−, and Br− were determined using Dionex ICS-1100 and ICS-2100 chromatography systems, respectively, with conductivity detectors. Additional IC and IC-ICP-MS analysis details can be found in SI Methods. I− enrichment factors relative to seawater were calculated using the ratio of I− to Na+ in seawater off the coast of Iceland (I−/Na+ = 6.2 × 10−8) (69). Br− enrichment factors were calculated based on seawater off the coast of Utqiaġvik (Br−/Na+ = 2.0 × 10−3) (49).
Boundary layer modeling was constrained using Br2, HOBr, Cl2, and radiation data from a 7-h period on March 11, 2012, during the Bromine Ozone and Mercury Experiment (BROMEX) (Fig. S4) (7, 8, 22, 70). The zero-dimensional model is a series of explicit gas-phase reactions (Tables S1 and S2) and ref. 13. Initial gas-phase mole ratios (for species not constrained to observations) and photolysis rate constants are shown in Tables S1 and S3. Photolysis rate constants were obtained using the National Center for Atmospheric Research Tropospheric and UV (TUV) Radiation Model (https://www2.acom.ucar.edu/modeling/tropospheric-ultraviolet-and-visible-tuv-radiation-model) and scaled to radiation measurements from the NOAA Global Monitoring Division Earth Systems Research Laboratory (https://esrl.noaa.gov/gmd/).
Acknowledgments
We thank the staff of the Jonathan Amy Facility for Chemical Instrumentation at Purdue University for building the snow cover and light fixture. Solar radiation data were acquired by and obtained from the National Oceanic & Atmospheric Administration, Earth System Research Laboratory, Global Monitoring Division Solar Radiation group. Photolysis rates were obtained using the TUV model from the National Center for Atmospheric Research, Atmospheric Chemistry Division, Chemical Processes and Regional Modeling group. We also thank Ukpeaġvik Iñupiat Corporation Science and CH2MHILL Polar Services for field logistical support and T. Miller (Birck Nanotechnology Center) for nano-pure water for chromatography. P. Burroff-Murr (Purdue University) consulted on the graphic design of Fig. 1. Financial support was provided by NSF Division of Polar Programs ARC-1107695, PLR-1417906, and PLR-1417668. For the 2012 measurements, K.A.P. was supported by NSF Postdoctoral Fellowship in Polar Regions Research ARC-1103423. IC-ICPMS analyses were performed at the Environmental Molecular Sciences Laboratory, a national scientific user facility located at the Pacific Northwest National Laboratory (PNNL) and sponsored by the Office of Biological and Environmental Research of the US Department of Energy (DOE). PNNL is operated for the DOE by Battelle Memorial Institute under Contract DE-AC06-76RL0 1830.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1702803114/-/DCSupplemental.
References
- 1.Barrie LA, Bottenheim JW, Schnell RC, Crutzen PJ, Rasmussen RA. Ozone destruction and photochemical reactions at polar sunrise in the lower Arctic atmosphere. Nature. 1988;334:138–141. [Google Scholar]
- 2.Oltmans SJ. Surface ozone measurements in clean air. J Geophys Res. 1981;86:1174–1180. [Google Scholar]
- 3.Sturges WT, Barrie LA. Chlorine, bromine, and iodine in Arctic aerosols. Atmos Environ. 1988;22:1179–1194. [Google Scholar]
- 4.Barrie LA, den Hartog G, Bottenheim JW, Landsberger S. Anthroprogenic aerosols and gases in the lower troposphere at Alert Canada in April 1986. J Atmos Chem. 1989;9:101–127. [Google Scholar]
- 5.Liao J, et al. Observations of inorganic bromine (HOBr, BrO, and Br2) speciation at Barrow, Alaska, in spring 2009. J Geophys Res. 2012;117:D00R16. [Google Scholar]
- 6.Hausmann M, Platt U. Spectroscopic measurement of bromine oxide and ozone in the high Arctic during Polar Sunrise Experiment 1992. J Geophys Res. 1994;99:25399–25413. [Google Scholar]
- 7.Pratt KA, et al. Photochemical production of molecular bromine in Arctic surface snowpacks. Nat Geosci. 2013;6:351–356. [Google Scholar]
- 8.Peterson PK, et al. Dependence of the vertical distribution of bromine monoxide in the lower troposphere on meteorological factors such as wind speed and stability. Atmos Chem Phys. 2015;15:2119–2137. [Google Scholar]
- 9.Foster KL, et al. The role of Br2 and BrCl in surface ozone destruction at polar sunrise. Science. 2001;291:471–474. doi: 10.1126/science.291.5503.471. [DOI] [PubMed] [Google Scholar]
- 10.Hönninger G, Platt U. Observations of BrO and its vertical distribution during surface ozone depletion at Alert. Atmos Environ. 2002;36:2481–2489. [Google Scholar]
- 11.Simpson WR, et al. Halogens and their role in polar boundary-layer ozone depletion. Atmos Chem Phys. 2007;7:4375–4418. [Google Scholar]
- 12.Wennberg PO. Bromine explosion. Nature. 1999;397:299–301. [Google Scholar]
- 13.Thompson CR, et al. Interactions of bromine, chlorine, and iodine photochemistry during ozone depletions in Barrow, Alaska. Atmos Chem Phys. 2015;15:9651–9679. [Google Scholar]
- 14.Toyota K, McConnell JC, Staebler RM, Dastoor AP. Air–snowpack exchange of bromine, ozone and mercury in the springtime Arctic simulated by the 1-D model PHANTAS – Part 1: In-snow bromine activation and its impact on ozone. Atmos Chem Phys. 2014;14:4101–4133. [Google Scholar]
- 15.Thomas JL, et al. Modeling chemistry in and above snow at Summit, Greenland – Part 1: Model description and results. Atmos Chem Phys. 2011;11:4899–4914. [Google Scholar]
- 16.Saiz-Lopez A, et al. Boundary layer halogens in coastal Antarctica. Science. 2007;317:348–351. doi: 10.1126/science.1141408. [DOI] [PubMed] [Google Scholar]
- 17.Saiz-Lopez A, et al. Iodine chemistry in the troposphere and its effect on ozone. Atmos Chem Phys. 2014;14:13119–13143. [Google Scholar]
- 18.Calvert JG, Lindberg SE. Potential influence of iodine-containing compounds on the chemistry of the troposphere in the polar spring. I. Ozone depletion. Atmos Environ. 2004;38:5087–5104. [Google Scholar]
- 19.Rowley DM, Bloss WJ, Cox RA, Jones RL. Kinetics and products of the IO + BrO reaction. J Phys Chem A. 2001;105:7855–7864. [Google Scholar]
- 20.Sander SP, et al. 2006. Chemical kinetics and photochemical data for use in atmospheric studies (Jet Propulsion Lab, NASA, Pasadena, CA), Evaluation no. 15.
- 21.Liao J, et al. High levels of molecular chlorine in the Arctic atmosphere. Nat Geosci. 2014;7:91–94. [Google Scholar]
- 22.Custard KD, Pratt KA, Wang S, Shepson PB. Constraints on Arctic atmospheric chlorine production through measurements and simulations of Cl 2 and ClO. Environ Sci Technol. 2016;50:acs.est.6b03909. doi: 10.1021/acs.est.6b03909. [DOI] [PubMed] [Google Scholar]
- 23.Zielcke J. 2015. Observations of reactive bromine, iodine and chlorine species in the Arctic and Antarctic with differential optical absorption spectroscopy. PhD dissertation (Ruperto-Carola University, Heidelberg), 10.1017/CBO9781107415324.004.
- 24.Sipilä M, et al. Molecular-scale evidence of aerosol particle formation via sequential addition of HIO3. Nature. 2016;537:532–534. doi: 10.1038/nature19314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Saiz-Lopez A, et al. Atmospheric chemistry of iodine. Chem Rev. 2012;112:1773–1804. doi: 10.1021/cr200029u. [DOI] [PubMed] [Google Scholar]
- 26.Atkinson HM, et al. Iodine emissions from the sea ice of the Weddell Sea. Atmos Chem Phys. 2012;12:11229–11244. [Google Scholar]
- 27.Saiz-Lopez A, Chance K, Liu X, Kurosu TP, Sander SP. First observations of iodine oxide from space. Geophys Res Lett. 2007;34:L12812. [Google Scholar]
- 28.Frieß U, Deutschmann T, Gilfedder BS, Weller R, Platt U. Iodine monoxide in the Antarctic snowpack. Atmos Chem Phys. 2010;10:2439–2456. [Google Scholar]
- 29.Mahajan AS, et al. Evidence of reactive iodine chemistry in the Arctic boundary layer. J Geophys Res. 2010;115:D20303. [Google Scholar]
- 30.Allan JD, et al. Iodine observed in new particle formation events in the Arctic atmosphere during ACCACIA. Atmos Chem Phys. 2014;14:28949–28972. [Google Scholar]
- 31.Saiz-Lopez A, Plane JMC. Novel iodine chemistry in the marine boundary layer. Geophys Res Lett. 2004;31:L04112. [Google Scholar]
- 32.Mcfiggans G, et al. Direct evidence for coastal iodine particles from Laminaria macroalgae – linkage to emissions of molecular iodine. Atmos Chem Phys Atmos Chem Phys. 2004;4:701–713. [Google Scholar]
- 33.Saiz-Lopez A, et al. Modelling molecular iodine emissions in a coastal marine environment: The link to new particle formation. Atmos Chem Phys. 2006;6:883–895. [Google Scholar]
- 34.Saiz-Lopez A, Blaszczak-Boxe CS, Carpenter LJ. A mechanism for biologically-induced iodine emissions from sea-ice. Atmos Chem Phys. 2015;15:10257–10297. [Google Scholar]
- 35.Saiz-Lopez A, Blaszczak-Boxe CS. The polar iodine paradox. Atmos Environ. 2016;145:72–73. [Google Scholar]
- 36.Custard KD, Raso ARW, Shepson PB, Staebler RM, Pratt KA. 2017. Production and release of molecular bromine and chlorine from the Arctic coastal snowpack. ACS Earth Sp Chem 1: 10.1021/acsearthspacechem.7b00014.
- 37.Wren SN, Donaldson DJ, Abbatt JPD. Photochemical chlorine and bromine activation from artificial saline snow. Atmos Chem Phys. 2013;13:9789–9800. [Google Scholar]
- 38.Abbatt J, et al. Release of gas-phase halogens by photolytic generation of OH in frozen halide-nitrate solutions: An active halogen formation mechanism? J Phys Chem A. 2010;114:6527–6533. doi: 10.1021/jp102072t. [DOI] [PubMed] [Google Scholar]
- 39.Adams JW, Holmes NS, Crowley JN. Uptake and reaction of HOBr on frozen and dry NaCl/NaBr surfaces between 253 and 233 K. Atmos Chem Phys. 2002;2:79–91. [Google Scholar]
- 40.Huff AK, Abbatt JPD. Kinetics and product yields in the heterogeneous reactions of HOBr with ice surfaces containing NaBr and NaCl. J Phys Chem A. 2002;106:5279–5287. [Google Scholar]
- 41.Kirchner U, Benter T, Schindler RN. Experimental verification of gas phase bromine enrichment in reaction of HOBr with sea salt doped ice surfaces. Berichte der Bunsengesellschaft für Phys Chemie. 1997;977:975–977. [Google Scholar]
- 42.Oldridge NW, Abbatt JPD. Formation of gas-phase bromine from interaction of ozone with frozen and liquid NaCl/NaBr solutions: Quantitative separation of surficial chemistry from bulk-phase reaction. J Phys Chem A. 2011;115:2590–2598. doi: 10.1021/jp200074u. [DOI] [PubMed] [Google Scholar]
- 43.Oum KW, Lakin MJ, Finlayson-Pitts BJ. Bromine activation in the troposphere by the dark reaction of O3 with seawater ice. Geophys Res Lett. 1998;25:3923–3926. [Google Scholar]
- 44.Sjostedt SJ, Abbatt JPD. Release of gas-phase halogens from sodium halide substrates: Heterogeneous oxidation of frozen solutions and desiccated salts by hydroxyl radicals. Environ Res Lett. 2008 doi: 10.1088/1748-9326/3/4/045007. [DOI] [Google Scholar]
- 45.Kim K, et al. Production of molecular iodine and triiodide in the frozen solution of iodide: Implication for polar atmosphere. Environ Sci Technol. 2016;50:1280–1287. doi: 10.1021/acs.est.5b05148. [DOI] [PubMed] [Google Scholar]
- 46.Gálvez O, Baeza-romero MT, Sanz M, Saiz-Lopez A. Photolysis of frozen iodate salts as a source of active iodine in the polar environment. Atmos Chem Phys. 2016;16:12703–12713. [Google Scholar]
- 47.Carpenter LJ, et al. Atmospheric iodine levels influenced by sea surface emissions of inorganic iodine. Nat Geosci. 2013;6:108–111. [Google Scholar]
- 48.King MD, Simpson WR. Extinction of UV radiation in Arctic snow at Alert, Canada. J Geophys Res. 2001;106:12499–12507. [Google Scholar]
- 49.Simpson WR, Alvarez-aviles L, Douglas TA, Sturm M, Domine F. Halogens in the coastal snow pack near Barrow, Alaska: Evidence for active bromine air-snow chemistry during springtime. Geophys Res Lett. 2005;32:2–5. [Google Scholar]
- 50.Xu W, Tenuta M, Wang F. Bromide and chloride distribution across the snow-sea-ice-ocean interface: A comparative study between an Arctic coastal marine site and an experimental sea ice mesocosm. J Geophys Res Ocean. 2016;121:1063–1084. [Google Scholar]
- 51.Saiz-Lopez A, Saunders RW, Joseph DM, Ashworth SH, Plane JMC. Absolute absorption cross-section and photolysis rate of I2. Atmos Chem Phys. 2004;4:1443–1450. [Google Scholar]
- 52.Bartels-Rausch T, et al. A review of air-ice chemical and physical interactions (AICI): Liquids, quasi-liquids, and solids in snow. Atmos Chem Phys. 2014;14:1587–1633. [Google Scholar]
- 53.Jacob DJ. Heterogeneous chemistry and tropospheric ozone. Atmos Environ. 2000;34:2131–2159. [Google Scholar]
- 54.Sander R. Compilation of Henry’s law constants (version 4.0) for water as solvent. Atmos Chem Phys. 2015;15:4399–4981. [Google Scholar]
- 55.France JL, et al. Hydroxyl radical and NOx production rates, black carbon concentrations and light-absorbing impurities in snow from field measurements of light penetration and nadir reflectivity of onshore and offshore coastal Alaskan snow. J Geophys Res. 2012 doi: 10.1029/2011JD016639. [DOI] [Google Scholar]
- 56.Simpson WR, Brown SS, Saiz-Lopez A, Thornton JA, Glasow Rv. Tropospheric halogen chemistry: Sources, cycling, and impacts. Chem Rev. 2015;115:4035–4062. doi: 10.1021/cr5006638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Holmes NS, Adams JW, Crowley JN. Uptake and reaction of HOI and IONO on frozen and dry NaCl/NaBr surfaces and H2SO4. Phys Chem Chem Phys. 2001;9:1679–1687. [Google Scholar]
- 58.Troy RC, Kelley MD, Nagy JC, Margerum DW. Non-metal redox kinetics: Iodine monobromide reaction with iodide ion and the hydrolysis of IBr. Inorg Chem. 1991;30:4838–4845. [Google Scholar]
- 59.Beckwith RC, Wang TX, Margerum DW. Equilibrium and kinetics of bromine hydrolysis. Inorg Chem. 1996;35:995–1000. doi: 10.1021/ic950909w. [DOI] [PubMed] [Google Scholar]
- 60.Halfacre JW, et al. Temporal and spatial characteristics of ozone depletion events from measurements in the Arctic. Atmos Chem Phys. 2014;14:4875–4894. [Google Scholar]
- 61.Sommariva R, Bloss WJ, von Glasow R. Uncertainties in gas-phase atmospheric iodine chemistry. Atmos Environ. 2012;57:219–232. [Google Scholar]
- 62.Domine F, Bock J, Voisin D, Donaldson DJ. Can we model snow photochemistry? Problems with the current approaches. J Phys Chem A. 2013;117:4733–4749. doi: 10.1021/jp3123314. [DOI] [PubMed] [Google Scholar]
- 63.Saiz-Lopez A, et al. Estimating the climate significance of halogen-driven ozone loss in the tropical marine troposphere. Atmos Chem Phys. 2012;12:3939–3949. [Google Scholar]
- 64.Liao J, et al. A comparison of Arctic BrO measurements by chemical ionization mass spectrometry and long path-differential optical absorption spectroscopy. J Geophys Res. 2011;116:D00R02. [Google Scholar]
- 65.Neuman JA, et al. Bromine measurements in ozone depleted air over the Arctic Ocean. Atmos Chem Phys. 2010;10:6503–6514. [Google Scholar]
- 66.Peterson MC, Honrath RE. Observations of rapid photochemical destruction of ozone in snowpack interstitial air. Geophys Res Lett. 2001;28:511–514. [Google Scholar]
- 67.Van Dam B, et al. Dynamics of ozone and nitrogen oxides at Summit, Greenland: I. Multi-year observations in the snowpack. Atmos Environ. 2015;123:268–284. [Google Scholar]
- 68.Q-Lab Corporation 2011 Spectral power distribution for QUV with UVA-340 fluorescent lamps. Technical Bulletin LU-8052. Available at www.q-lab.com/products/lamps-optical-filters/lamps-and-optical-filters. Accessed December 3, 2014.
- 69.Waite TJ, Truesdale VW, Olafsson J. The distribution of dissolved inorganic iodine in the seas around Iceland. Mar Chem. 2006;101:54–67. [Google Scholar]
- 70.Nghiem SV, et al. Studying bromine, ozone, and mercury chemistry in the Arctic. Eos (Wash DC) 2013;94:2012–2013. [Google Scholar]