Significance
This paper investigates the organization of the active zone at ribbon synapses in the retina, using deletions of the active zone protein RIM-binding protein (RBP) as a tool. The results demonstrate that, at these synapses, which, different from other synapses, use presynaptic L-type calcium channels for triggering vesicle exocytosis, RBPs mediate the recruitment of L-type calcium channels adjacent to release sites, thereby allowing efficient stimulus-secretion coupling. Thus, this paper presents a demonstration of how calcium channels are organized at the presynaptic active zone of ribbon synapses.
Keywords: active zone, synaptic transmission, calcium current, nanodomain, RIM-BP
Abstract
Fast neurotransmitter release from ribbon synapses via Ca2+-triggered exocytosis requires tight coupling of L-type Ca2+ channels to release-ready synaptic vesicles at the presynaptic active zone, which is localized at the base of the ribbon. Here, we used genetic, electrophysiological, and ultrastructural analyses to probe the architecture of ribbon synapses by perturbing the function of RIM-binding proteins (RBPs) as central active-zone scaffolding molecules. We found that genetic deletion of RBP1 and RBP2 did not impair synapse ultrastructure of ribbon-type synapses formed between rod bipolar cells (RBCs) and amacrine type-2 (AII) cells in the mouse retina but dramatically reduced the density of presynaptic Ca2+ channels, decreased and desynchronized evoked neurotransmitter release, and rendered evoked and spontaneous neurotransmitter release sensitive to the slow Ca2+ buffer EGTA. These findings suggest that RBPs tether L-type Ca2+ channels to the active zones of ribbon synapses, thereby synchronizing vesicle exocytosis and promoting high-fidelity information transfer in retinal circuits.
At synapses, fast Ca2+-triggered neurotransmitter release occurs at specialized regions of the nerve terminal called active zones. Presynaptic active zones are composed of several large and evolutionarily conserved multidomain protein families, including Rab3-interacting molecules (RIMs), RIM-binding proteins (RBPs), MUNC13, ELKS, Bassoon, and Piccolo (1–3). Active zones perform at least four essential functions: They tether synaptic vesicles at release sites, they prime vesicles for fusion, they recruit Ca2+ channels into close proximity to release sites, and they align pre- and postsynaptic elements of synapses, possibly by organizing transsynaptic signaling complexes (3).
Recruitment of Ca2+ channels adjacent to release sites is essential for rapid and precise synaptic transmission. The underlying molecular mechanisms, however, are incompletely understood. Systematic analyses of RIM-deficient neurons have revealed that RIMs play a critical role in determining Ca2+-channel density and localization in conventional central synapses by two mechanisms (4, 5). First, RIMs’ central PDZ domains directly and selectively interact with C-terminal PDZ-domain-binding motifs of N- and P/Q-type Ca2+ channels (but not of L-type Ca2+ channels) to recruit them to the presynaptic active zone (5). Second, RIM proteins also indirectly interact with Ca2+ channels via RBPs. Specifically, RIMs contain central proline-rich sequences that tightly interact with SH3 domains of RBPs, which in turn directly bind to all presynaptic Ca2+-channel subtypes (see below).
RBPs are large multidomain proteins that are encoded by three genes in vertebrates: RBP1, RBP2, and RBP3 (6–8). RBP1 and RBP2 proteins are mainly produced in the brain while RBP3 is primarily synthesized in nonneural tissues (6). All RBPs are composed of one central and two C-terminal SH3 domains separated by three fibronectin-like-3 (FN3) domains. RBPs interact, via their SH3 domains, not only with RIMs but also with cytoplasmic proline-rich sequences of L-, P/Q-, and N-type Ca2+ channels (5, 8–10). The role of RBPs in presynaptic Ca2+-channel localization and function in conventional synapses has recently been addressed in flies and mice (11–13). In Drosophila, deletion of the single RBP gene reduced Ca2+-channel abundance and Ca2+ influx, decreased the number of docked vesicles, disrupted the integrity of the active zone cytomatrix, and dramatically impaired Ca2+-triggered neurotransmitter release (10, 13). In mice, however, deletion of RBP1 and RBP2 did not influence the properties or density of presynaptic P/Q- and N-type Ca2+ channels and produced only mild impairments in the extent of transmitter release in response to single spikes (11, 12). Remarkably, however, deletion of RBPs from murine synapses specifically interfered with the coupling of Ca2+ channels to synaptic vesicle exocytosis in response to spike trains and rendered action potential-mediated transmitter release highly unreliably under these conditions (11, 12).
Unlike RIMs, RBPs can bind not only P/Q- and N-type Ca2+ channels, but also L-type Ca2+ channels (9). In neurons, L-type Ca2+ channels mostly localize to dendritic compartments and are not required for neurotransmitter release. However, in ribbon-type synapses of the retina and inner ear, L-type Ca2+ channels are the source of Ca2+ influx for evoked neurotransmitter release. Ribbon synapses are specialized to support rapid and sustained transmitter release (refs. 14 and 15 and Fig. 1A, Left), and their eponymous synaptic ribbons are thought to provide a continuous supply of synaptic vesicles for release to support rapid and sustained release (16). In addition, L-type Ca2+ channels that mediate evoked release at ribbon synapses inactivate more slowly than N- and P/Q-type Ca2+ channels at central synapses (15, 17), allowing ribbon synapses to support more prolonged Ca2+ influx upon presynaptic depolarization. Lastly, fast neurotransmitter release from ribbon synapses is known to rely on Ca2+ nanodomains, which requires tight coupling of Ca2+ channels to release-ready synaptic vesicles at the presynaptic active zone (14, 18). Despite the obvious significance of the clustering of presynaptic Ca2+ channels in ribbon synapses, however, relatively little is known about the molecular mechanisms of such clustering.
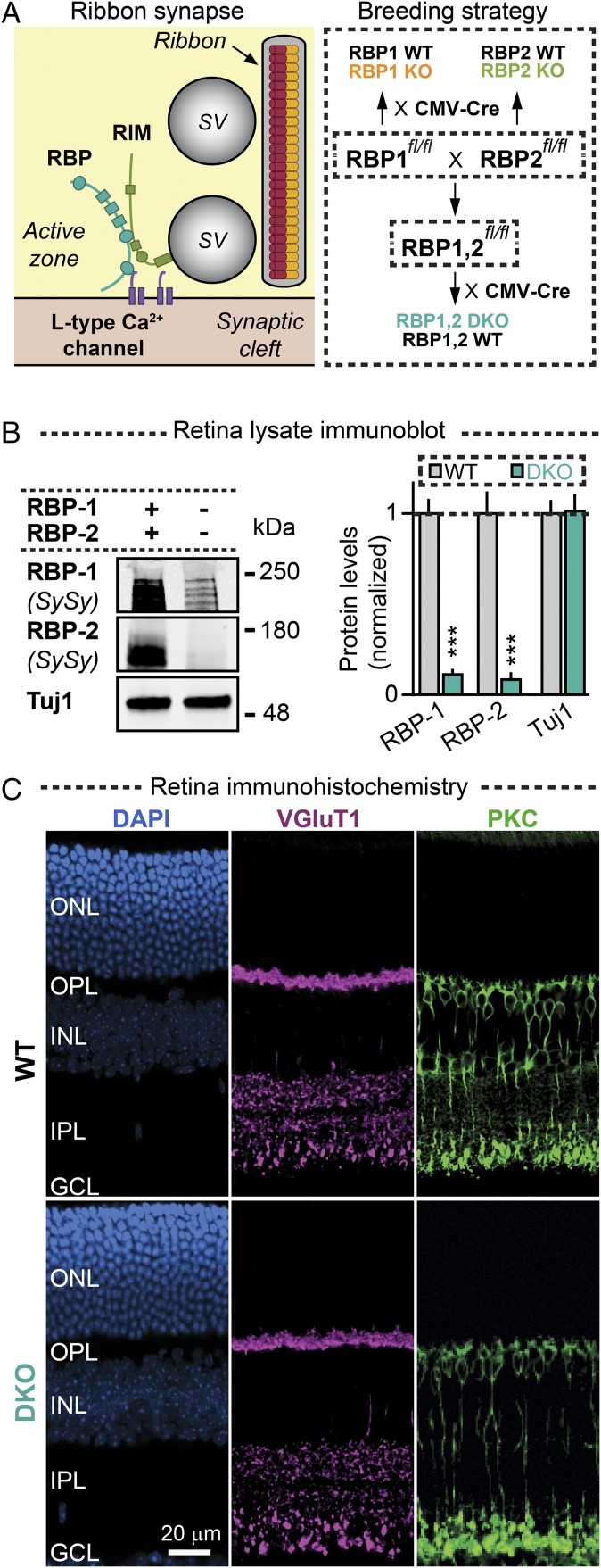
Fig. 1.
Genetic deletion of RBPs does not alter the overall architecture of the retina. (A, Left) Schematic of a ribbon synapse highlighting RBP interactions with RIM and L-type Ca2+ channels. (Right) Diagram of the breeding strategy used to generate constitutive RBP1 KO, RBP2 KO, and RBP1,2 DKO mice and their respective littermate controls (based on RBP1,2fl/fl mice described in ref. 11). (B) Immunoblot analysis of RBPs in retina lysates from WT and RBP DKO mice (Left, representative blots; Right, summary graphs) (means ± SD, n = 4 WT and RBP DKO mice; statistical analyses by Student’s t test; ***P < 0.001; n.s., nonsignificant). The remaining RBP immunoblotting signal in DKO mice is likely due to nonspecific antibody cross-reactivity. (C) Immunofluorescence images of thin retina sections obtained from a WT (Top) and an RBP DKO (Bottom) mouse. Retina cryosections were stained with DAPI (blue) and antibodies against vGlut1 (purple) and PKCα (green). GCL, ganglion cell layer; INL, inner nuclear layer; IPL, inner plexiform layer; ONL, outer nuclear layer; OPL, outer plexiform layer.
Here, we hypothesized that presynaptic RBPs recruit L-type Ca2+ channels to the active zone of ribbon synapses and mediate nanodomain coupling of L-type Ca2+ channels to release-ready vesicles. To test this hypothesis, we analyzed RBP-deficient mouse retinas using a combination of biochemistry, electrophysiology, and electron microscopy. Our results indicate that RBP1 and RBP2 determine the density and distribution of presynaptic L-type Ca2+ channels at ribbon synapses between rod bipolar and amacrine type-2 (AII) cells, thereby regulating the speed and synchrony of graded neurotransmitter release in retinal circuits.
Results
Deletion of RBPs Does Not Impact the Overall Architecture of the Retina.
To test the role of RBPs in ribbon synapses, we crossed RBP1, RBP2, or RBP1,2 conditional knockout (KO) mice (RBP1fl/fl, RBP2fl/fl, and RBP1,2fl/fl) (11) to a CMV-Cre mouse line that expressed cre-recombinase under control of the CMV minimal promoter in all tissues, including germ cells (19). In this manner, we generated constitutive RBP1 KO, RBP2 KO, or RBP1,2 double KO (DKO) mice (ref. 11 and Fig. 1 A and B).
We used light microscopy to study the structural organization of retinas obtained from WT and RBP-deficient DKO mice. Deletion of RBPs caused no major changes in the gross anatomy of the retina, including lamination of the two synaptic outer- and inner-plexiform layers, and did not alter the overall density of neurons of synapses (Fig. 1C). Thus, removal of RBPs does not cause major impairments in the architecture of the retina.
Deletion of RBPs Impairs Synaptic Transmission at Rod Bipolar Cells→AII Synapses.
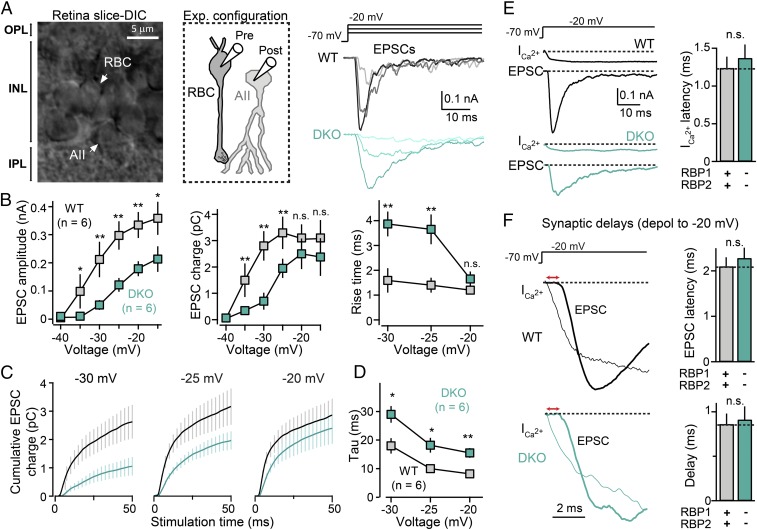
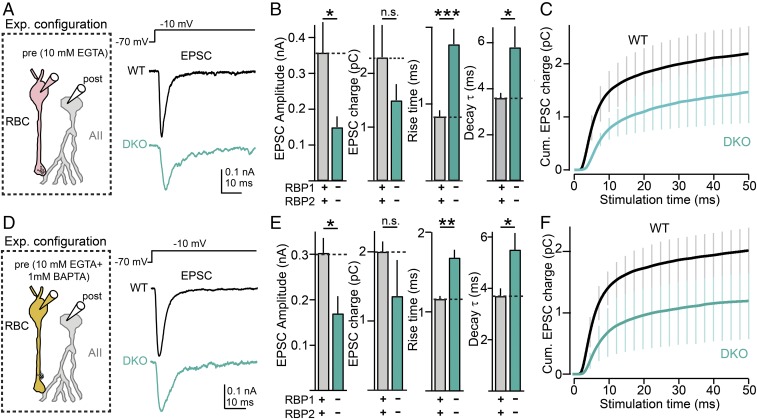
To study the role of RBPs in synaptic transmission at ribbon synapses, we performed high-resolution paired patch-clamp recordings from synaptically connected rod bipolar and AII amacrine cells (Fig. 2A). In these experiments, postsynaptic AII cells were maintained at a −70-mV holding potential, and presynaptic rod bipolar cells were sequentially depolarized from a holding potential of −70 mV to more positive potentials ranging from −50 mV to −20 mV; depolarizations were for 50 ms or 500 ms. These depolarization potentials are commonly observed in these synapses upon physiological activation with light (20, 21). As expected, in control retinas, presynaptic depolarizations above the L-type Ca2+-channel activation threshold (∼40 mV) induced robust Ca2+ currents and triggered pronounced fast synchronous neurotransmitter release, as measured via the peak amplitude of the corresponding excitatory postsynaptic currents (EPSCs) monitored in AII cells, as well as via the EPSC synaptic charge transfer (Fig. 2A). In contrast, deletion of RBPs strongly reduced presynaptic Ca2+ currents (Fig. 4 for full analysis of this phenotype) and led to a pronounced reduction in neurotransmitter release (Fig. 2 A, Right and B, Left and Center), consistent with the notion that genetic ablation of RBPs decreases the initial release probability in these synapses.
Fig. 2.
Paired recordings reveal that deletion of RBPs severely affects synaptic transmission at ribbon synapses. (A) Experimental design for paired recordings from rod bipolar cell→AII amacrine cell synapses (Left and Center) and representative traces (Right). (Left) Infrared-differential interference contrast image of a retina slice used for patch-clamp recordings. (Center) Schematic of the recording configuration. (Right Top) Diagram of presynaptic depolarizations of rod bipolar cells; (Right Bottom) Representative EPSCs recorded from postsynaptic AII amacrine cells in WT and RBP DKO mice in response to the presynaptic rod bipolar cell depolarizations. Note that presynaptic patch-pipette solutions routinely contain 1 mM BAPTA to mimic intracellular Ca2+ buffers (31, 41). (B) Summary graphs of peak amplitudes (Left), total charge transfer (Center), and 20 to 80% rise times (Right) of EPSCs induced by presynaptic depolarization to −40 →−15 mV) in WT (gray) and RBP DKO (blue) synapses. (C) RBP deletion impairs the total amount and kinetics of synaptic transmission induced by prolonged presynaptic depolarizations. Plots of the mean cumulative EPSC charge transfer as a function of the time of presynaptic depolarization from −70 mV to −30 (Left), −70 mV to −25 (Center), or −70 mV to −20 mV (Right) in control (black) and mutant (blue) synapses (n = 9 for both). (D) Summary plot of the kinetics of the EPSC charge transfer induced by presynaptic depolarizations as in C. (E and F) Measurements of Ca2+-current latency (E) and EPSC latency and synaptic delay (F). Presynaptic Ca2+ currents and postsynaptic EPSCs were simultaneously monitored using paired recordings from bipolar and AII amacrine cells from WT (black) and a RBP DKO (blue) synapse in these experiments. (E, Left) Representative dual recordings from rod bipolar cells and AII cells (Bottom). The protocol used to activate release from presynaptic bipolar cells is shown on Top and corresponds to depolarizations to −20 mV. (E, Right) Summary of Ca2+-current latencies in six RBP control and six RBP DKO synapses. (F, Left) Overlapping recordings of presynaptic ICa2+ (thin line) and postsynaptic EPSC (thick line) in a WT (Center) and a DKO (Bottom) synapse. The red horizontal arrows represent synaptic delays. Traces have been normalized in the y axes to allow more precise measurements of synaptic delays. (F, Right) Summary graphs of EPSC latency and synaptic delays for six control and six DKO synapses. All summary graphs are mean ± SD. Statistical analyses were performed by either Student’s t test (D–F) or by ANOVA followed by a Bonferroni post hoc test (B and C), comparing RBP DKO with WT (*P < 0.05, **P < 0.01; n.s., nonsignificant).
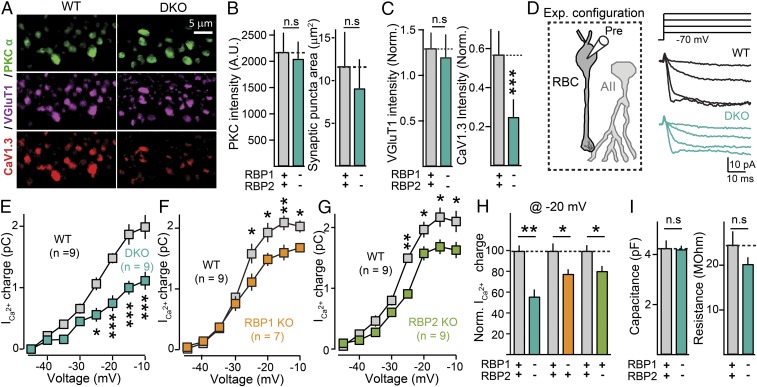
Fig. 4.
Deletion of RBPs reduces Ca2+ currents in presynaptic rod bipolar cells forming ribbon synapses on postsynaptic AII amacrine cells. (A) Selective loss of presynaptic L-type Ca2+ channels from ribbon synapses formed by rod bipolar cells. Representative confocal images of RBP WT (Left) and RBP DKO (Right) retina sections stained for PKCα (green, to identify rod bipolar neurons), VGlut1 (purple, to identify presynaptic terminals), and the L-type Ca2+-channel CaV1.3 (red). (B) Summary graphs of the intensity of PKCα fluorescent signals in WT(gray) and DKO (blue) rod bipolar cell terminals (Left), and terminal size (area, Right) defined by PKC staining. Number of experiments (images/mice): RBP WT, 79/3; RBP DKO, 70/3. (C) Relative vGluT1 (Left) and CaV1.3 (Right) staining intensity normalized by PKCα signals. Number of experiments (images/mice): RBP WT, 79/3; RBP DKO, 70/3. (D) Experimental configuration for Ca2+-current recordings (Left) and representative experiment (Right Top) schematic of depolarization protocol; (Right Bottom) Sample traces for an RBP control (black) and an RBP DKO (blue) bipolar cell. (E) Deletion of RBP1,2 severely impairs presynaptic Ca2+-current density. Summary plot of the Ca2+-current charge transfer over 50 ms as a function of the membrane voltage in RBP1,2 DKO (blue) rod bipolar cells and corresponding littermate controls (gray). (F and G) Incremental contribution of RBP1 (F) and RBP2 (G) to the presynaptic Ca2+-channel density of ribbon synapses. Same as E, but for littermate control (F and G, gray) and RBP1 KO (F, orange) or RBP2 KO (G, green) mice. (H) Summary graphs of the Ca2+-current charge transfer induced by a 50-ms depolarization to −20 mV in RBP1,2 DKO, RBP1 KO, or RBP2 KO mice, normalized to the controls analyzed in the same experiments. Number of experiments as in E–G. (I) Summary graphs of whole-cell capacitance (Left) and input resistance (Right) in the same rod bipolar cells used to measure whole-cell presynaptic Ca2+ currents. Number of experiments as in E. All summary graphs are mean ± SD. Statistical analyses were performed by either Student's t test (B, C, H, and I) or by ANOVA followed by a Bonferroni post hoc test (E–G), comparing RBP DKO with RBP WT (*P < 0.05, **P < 0.01, and ***P < 0.001; n.s., nonsignificant).
We next analyzed in detail the kinetics of synaptic transmission in the same experiments. We first measured rise times of postsynaptic responses and found that the kinetics of EPSCs was impaired by deletion of RBPs, as indicated by the increased 20 to 80% rise times (Fig. 2B, Right). To more precisely estimate the effect of the RBP deletion on transmission, we integrated synaptic responses recorded from AII cells for presynaptic depolarizations to −30, −25, and −20 mV (Fig. 2 C and D). We found that deletion of RBPs not only reduced overall synaptic transmission (Fig. 2C) but also made transmission significantly slower (Fig. 2D), consistent with our direct measurements of the EPSC parameters described above. We then estimated the Ca2+-current latency (Fig. 2E) (Δt between presynaptic depolarization and presynaptic Ca2+ current), EPSC latency (Fig. 2F, Left) (Δt between presynaptic depolarization and postsynaptic EPSC), and synaptic delay (Fig. 2F, Right) (difference between EPSC latency and Ca2+-current latency). These measurements were performed after depolarizing presynaptic bipolar cells to −20 mV. We failed to detect significant differences between control and RBP-deficient synapses in any of these parameters (Fig. 2 E and F). Together, our results thus indicate that RBPs control the extent and speed of evoked neurotransmitter release triggered by physiologically relevant patterns of presynaptic activation.
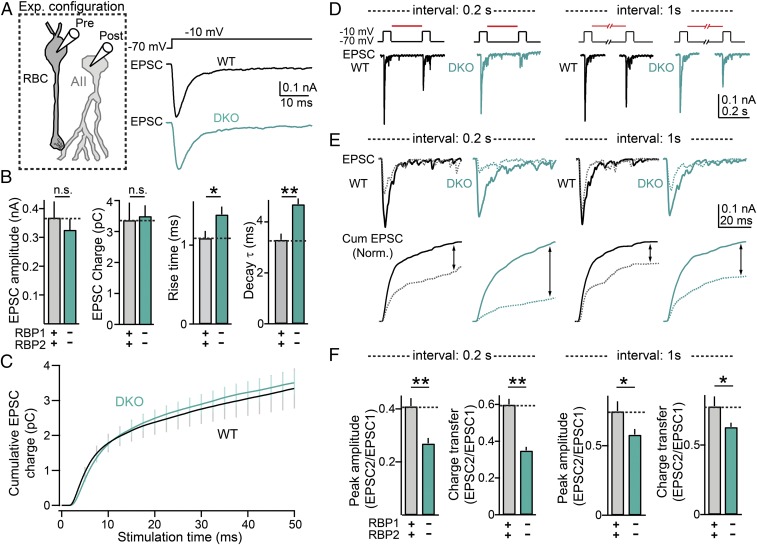
Deletion of RBPs Impairs the Kinetics of Emptying and Replenishing of the Readily Releasable Pool of Vesicles.
A recent study in Drosophila neuromuscular junctions suggested that RBPs contribute to the replenishment of synaptic vesicles into the readily releasable pool (RRP) (10), but no similar activity was detected in mammalian central synapses (11). To determine whether RBP performs an RRP replenishment function in mammalian ribbon synapses, we examined the size and kinetics of the RRP. We depolarized presynaptic rod bipolar cells from −70 mV to −10 mV for 50 ms and recorded postsynaptic responses from AII cells in voltage clamp mode (Fig. 3A). This protocol is known to induce maximal Ca2+ influx into the presynaptic terminals of bipolar cells, thereby triggering release of the entire RRP (22). Interestingly, we did not observe significant differences in the size of the RRP in DKO synapses compared with controls. Instead, we found that both the rise time and decay time of postsynaptic responses were significantly slower in RBP-deficient synapses (Fig. 3 B and C). This phenotype suggests a desynchronization of vesicle fusion upon removal of RBPs, which resembles the phenotype we recently identified in conventional central synapses in mice (11).
Fig. 3.
Deletion of RBPs impairs the speed of both RRP emptying and RRP replenishment. (A, Left) Schematic of recording configuration. (Right) Representative postsynaptic recordings in an RBP WT (black) and an RBP DKO (blue) synapse. Transmitter release was triggered by presynaptic rod bipolar cell depolarization from −70 to −10 mV for 50 ms. All these experiments were performed in the presence of 1 mM BAPTA in the presynaptic pipette. (B) Summary graphs of RRP properties. RRP size and properties were estimated by recording EPSCs in AII cells after maximal activation of presynaptic bipolar cells, as indicated in A. From Left to Right, the following parameters are displayed: EPSC amplitude, EPSC charge transfer, EPSC 20 to 80% rise time, and EPSC decay time constant. Number of experiments: RBP WT, seven pairs; RBP DKO, seven pairs. (C) Time course of RRP emptying. Summary graph of integrated EPSC charge transfer as a function of time. EPSCs were triggered by maximal presynaptic depolarization as indicated in A. (D) Measurements of RRP recovery kinetics in RBP WT (Left) and RBP DKO synapses (Right). (Top) Presynaptic protocols used to deplete the RRP and to measure RRP recovery. Note that RRP recovery was measured at two time intervals: 0.2 and 1 s. (Bottom) EPSCs used to estimate RRP size and recovery. (E) RRP replenishment in ribbon synapses. (Top) Overlapping EPSCs showing RRP recovery dynamics for 0.2-s (Left) and 1-s (Right) intervals in representative RBP WT and DKO synapses. (Bottom) Normalized integrated responses as a function of time for the same experiment presented above. (F) Summary graphs of RRP recovery for 0.2-s and for 1-s time intervals. From Left to Right, the following parameters are displayed: EPSC amplitude recovery at 0.2-s intervals, EPSC charge recovery at 0.2-s intervals, EPSC amplitude recovery at 1-s intervals, and EPSC charge recovery at 1-s intervals. Number of experiments: RBP WT, five pairs; RBP DKO, five pairs. All summary graphs are mean ± SD. Statistical analyses were performed by either Student’s t test (B and F) or by ANOVA followed by a Bonferroni post hoc test (C), comparing RBP DKO with RBP WT. (*P < 0.05, and **P < 0.01; n.s., nonsignificant).
We then measured the rate of RRP replenishment in control and RBP DKO synapses (Fig. 3 D–F). To measure replenishment rates, we first depleted the RRP with a 50-ms depolarizing pulse to −10 mV, as above, and then applied a second test pulse (50-ms depolarization to −10 mV) at intervals of 0.2 s or 1 s (Fig. 3D, Top). We observed that the degree of RRP recovery was significantly reduced in RBP DKO synapses compared with control synapses for both interstimulus intervals (Fig. 3 E and F). Altogether, these results indicate that RBPs do not control the total number of primed synaptic vesicles but regulate the speed at which primed synaptic vesicles can be released from ribbon terminals upon prolonged Ca2+ influx. Moreover, as observed in Drosophila neuromuscular synapses (10), our results indicate that RBPs are important for RRP refilling at ribbon synapses.
Deletion of RBPs Reduces the Density of Presynaptic L-Type Ca2+ Channels.
Our results above establish that RBPs strongly impair Ca2+-triggered release from ribbon synapses. Because RBPs directly interact with L-type Ca2+ channels that mediate release from these synapses (5, 9), we asked if removal of RBPs might disrupt Ca2+-channel density and/or function. We first studied how deletion of RBPs affects the level of presynaptic L-type Ca2+ channels by immunohistochemistry. We fixed retinas from control and RBP-deficient DKO mice with 4% paraformaldehyde, cut them into 50-µm-thick sections, and immunostained them with antibodies against CaV1.3. We observed a specific and significant reduction in the fluorescent signals for CaV1.3-containing Ca2+ channels in rod bipolar cell boutons, with no other obvious changes in bouton size or morphology (Fig. 4 A–C).
We then directly measured presynaptic Ca2+-channel density using patch-clamp electrophysiology. We prepared relatively thin (200-μm) retina slices from control and RBP DKO mice and patch-clamped presynaptic rod bipolar cells (Fig. 4D, Left). To determine if RBP deletion impacts presynaptic L-type Ca2+ channels, we sequentially depolarized bipolar cells for 50 ms from a holding membrane potential of −70 mV to more depolarized potentials, ranging from −40 mV to −10 mV with 5-mV increments, and measured the corresponding Ca2+ currents using somatic whole-cell voltage-clamp recordings (Fig. 4D, Right). Rod bipolar cells lack expression of Ca2+ channels in their somato-dendritic compartments, and thus Ca2+ currents recorded under these conditions arise exclusively from release-relevant channels localized at the nerve terminal (23). Remarkably, we found that removal of RBPs significantly reduced (∼50%) the peak amplitude and total charge of Ca2+ currents (Fig. 4 D, Right and E), without changing their kinetics and activation curve. The remaining Ca2+ currents in RBP DKO (∼50%) were completely blocked by nimodipine (10 μM).
We further studied if RBP1 and RBP2 independently or redundantly recruit Ca2+ channels at the active zone, comparing Ca2+ currents in rod bipolar cells of control mice or mice lacking only either RBP1 or RBP2. As shown in Fig. 4 F–H, deletion of either RBP1 or RBP2 reduced Ca2+ currents by ∼20 to 30% compared with control littermates, without changing the capacitance or membrane resistance of rod bipolar cells (Fig. 4I), suggesting that RBP1 and RBP2 are both required for presynaptic Ca2+-channel clustering and incrementally determine active zone L-type Ca2+-channel density in ribbon synapses. Taken together, these results indicate that, in contrast to conventional mammalian synapses (11, 12), RBPs at ribbon synapse determine the density of active zone L-type Ca2+ channels, which is similar to the role of RBP in invertebrate synapses (10, 13).
Deletion of RBPs Alters the Biochemical Composition but Not the Ultrastructure of Retinal Ribbon Synapses.
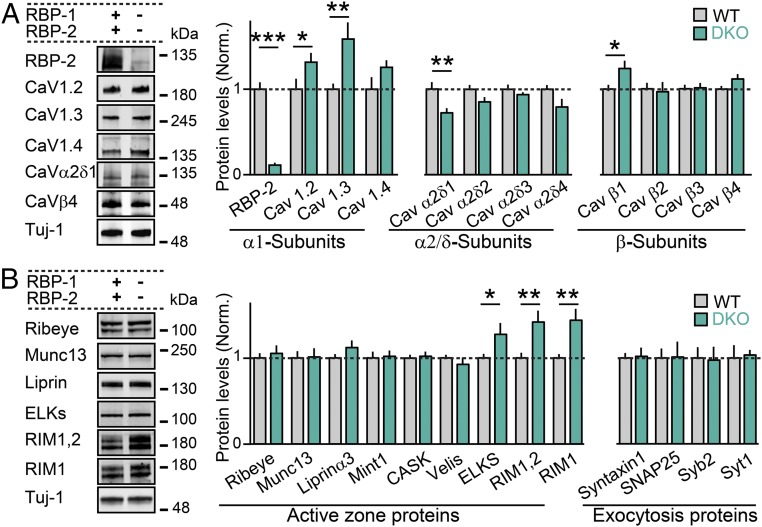
The strong reduction in presynaptic Ca2+-channel density in RBP-deficient presynaptic ribbon terminals prompted us to examine whether RBP deletion may also impair the overall biochemical composition of retinal synapses. To address this question, we compared the levels of different Ca2+-channel subtypes, active zone proteins, and proteins involved in late steps of synaptic vesicle exocytosis in extracts of retinas obtained from control or RBP DKO mice (Fig. 5). We first focused on the Cav1.2, Cav1.3, or Cav1.4 α-subunits of Ca2+ channels. Interestingly, we found that deletion of RBPs resulted in a significant increase in total expression of all α-subunits whereas the levels of β1–4 subunits remained unchanged (Fig. 5A). In contrast, the auxiliary α2σ subunits, which are thought to play a role in targeting functional Ca2+ channels to the synapse (24), were significantly reduced (Fig. 5A).
Fig. 5.
Retina protein levels upon removal of RBPs. (A) Quantitative Western blot analysis of primary and auxiliary L-type Ca2+-channel subunits in the mouse retina. (Left) Images of representative blots. (Right) Summary graphs for six RBP WT and seven RBP DKO experiments. (B) Same as in A, but for active zone and proteins involved in vesicle exocytosis. Number of experiments: six for WT retinas; seven for DKO retinas. All summary graphs are means ± SD. Statistical analyses were performed by Student’s t test comparing RBP DKO with RBP WT (*P < 0.05, **P < 0.01, and ***P < 0.001; n.s., nonsignificant).
We then measured the level of several active zone proteins (Fig. 5B). We found that Ribeye, Munc13, Liprinα3, Mint1, and CASK were not significantly changed. Surprisingly, RIMs and ELSKs, two important active zone molecules known to promote presynaptic clustering of Ca2+ channels (5, 25), were significantly increased in RBP-deficient retinas compared with WT controls. Finally, we assessed the levels of the SNARE proteins Syntaxin-1, SNAP-25, and Synaptobrevin-2, and of the fast Ca2+ sensor Synaptotagmin-1, but found no significant differences between control and RBP DKO retinas (Fig. 5B). Together, these results indicate that deletion of RBPs leads to selective but significant changes in retinal protein levels, which may directly or indirectly impair synaptic function.
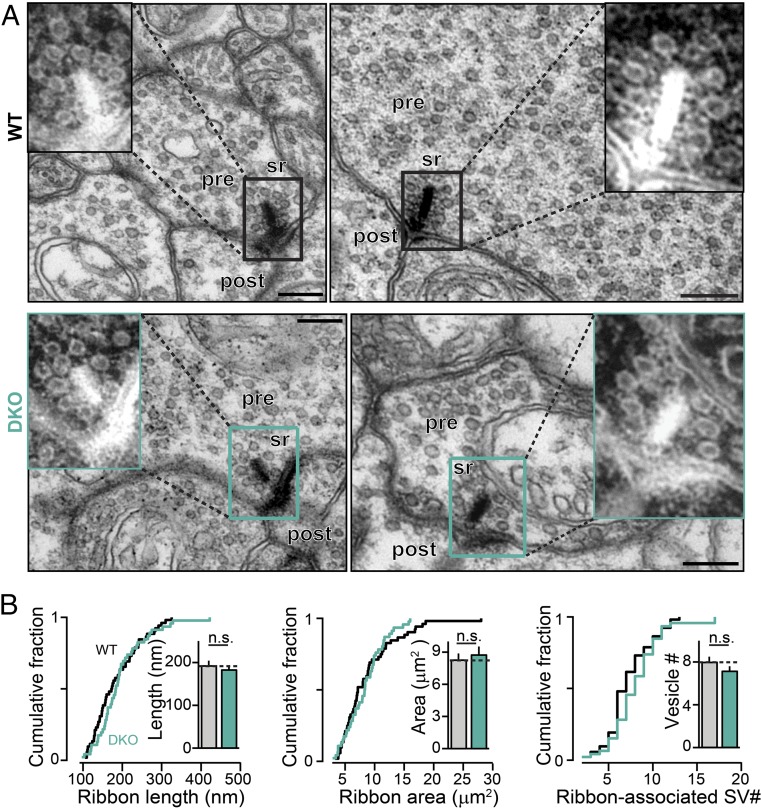
To determine if changes in protein composition of nerve terminals upon removal of RBPs might result in morphological alterations of synapses, we studied in detail the fine structure of retina ribbon synapses using transmission electron microscopy (TEM) (Fig. 6). We found that the overall ultrastructure of ribbon synapses appeared normal in RBP DKO mice, compared with control littermates (Fig. 6A). We quantitatively measured several parameters, including synaptic ribbon length and area, number of docked vesicles, and number of vesicles in close association to the synaptic ribbons. However, we did not detect any significant differences between control and RBP DKO groups (Fig. 6B). Viewed together, these findings establish that deletion of RBPs does not change the fine structure of ribbon-type synapses in mice.
Fig. 6.
Deletion of RBPs does not alter the ultrastructure of ribbon synapses. (A) High-resolution transmission electron microscopy images of retinal ribbon synapses in WT (Top) and RBP DKO (Bottom) retinas. (Insets) Enlarged images of the synaptic ribbons for each micrograph, with inverted contrast for better visualization of synaptic ribbons and associated vesicles. (Scale bar: 200 nm.) Post, postsynaptic; pre, presynaptic; sr, synaptic ribbon. (B) Cumulative distribution plots and bar graphs of the ribbon length (Left), ribbon area (Center), and total number of ribbon-associated vesicles (Right) in WT and RBP DKO retinas. n (synapses/mice) = 72/3 for WT; 97/3 for DKO sample. Summary graphs are means ± SD; statistical analyses were performed by the Kolmogorov–Smirnov (K-S) test (cumulative distributions) or Student’s t test (bar graphs; n.s., nonsignificant).
RBPs Couple Ca2+ Channels to Synaptic Vesicle Exocytosis at Bipolar Cells→AII Synapses.
Our results thus far indicate that ablation of RBPs decreases and desynchronizes Ca2+-triggered release (Fig. 2), impairs the kinetics of RRP emptying and replenishment (Fig. 3), and reduces presynaptic Ca2+-channel density in retina ribbon synapses (Fig. 4). These phenotypes can potentially be accounted for by increases in the physical distance between Ca2+ channels and primed synaptic vesicles at the presynaptic active zone in RBP-deficient synapses. To directly test this possibility, we loaded rod bipolar cell terminals with high concentrations (10 mM) of the slow Ca2+ chelator EGTA via the patch pipette, depolarized nerve terminals from −70 to 10 mV for 50 ms, and recorded the resulting evoked EPSCs in AII amacrine cells in the absence (Fig. 7 A–C) or presence (Fig. 7 D–F) of 1 mM BAPTA. The Ca2+ chelator EGTA, because of its intrinsically slow Ca2+-binding/unbinding rate, can selectively and efficiently suppress transmitter release mediated by Ca2+ channels loosely coupled to Ca2+ sensors attached to synaptic vesicles (26–30). Thus, changes in coupling distance between the Ca2+ channel and synaptic vesicles can be detected as changes in the relative EPSC sensitivity to defined presynaptic EGTA concentrations.
Fig. 7.
Deletion of RBPs impairs tight coupling of Ca2+ channels to synaptic vesicle exocytosis. (A–C) Impact of presynaptic 10 mM EGTA on evoked transmission at rod bipolar cell→AII amacrine cell synapses. (A, Left) Schematic of recording configuration. (Right) Representative postsynaptic recordings in an RBP WT (black, Center) and an RBP DKO (blue, Bottom) synapse. Transmitter release was triggered by depolarizing presynaptic rod bipolar cells from −70 to −10 mV for 50 ms (black, Top). (B) Impact of 10 mM EGTA on evoked synaptic transmission at rod bipolar cells→AII synapses, triggered as in A. Summary plots of multiple evoked EPSC parameters in RBP WT (gray) and RBP DKO (blue) are shown. From Left to Right, the following parameters are displayed: EPSC amplitude, EPSC charge transfer, EPSC 20 to 80% rise time, and EPSC decay time constant. Number of experiments: RBP WT, six pairs; RBP DKO, six pairs. (C) Time course of evoked EPSC in the presence of 10 mM EGTA in WT (black) and mutant (blue) synapses. EPSCs were triggered by maximal presynaptic depolarization as indicated in A. (D–F) Same as in A–C but in the presence of additional 1 mM BAPTA in presynaptic terminals. Number of experiments: RBP WT, six pairs; RBP DKO, five pairs. All summary graphs are means ± SD. Statistical analyses were performed by either Student’s t test (B and E) or by ANOVA followed by a Bonferroni post hoc test (C and F), comparing RBP DKO with RBP WT (*P < 0.05, **P < 0.01, and ***P < 0.001; n.s., nonsignificant).
We observed that high concentrations of EGTA (10 mM) did not change the magnitude of fast/synchronous transmitter release in WT rod bipolar cells→AII synapses, regardless of the presence of 1 mM BAPTA in presynaptic terminals (Fig. 7), indicating that fast/synchronous release in this synapse involves nanodomain coupling of Ca2+ channels to synaptic vesicle exocytosis (P > 0.05 for direct comparison of EPSC amplitude without and with EGTA) (Figs. 3 A and B and 7 D–F) (22, 31). In striking contrast, in RBP-deficient synapses, 10 mM EGTA reduced fast/synchronous EPSC peak amplitude by ∼50%; again, this change was observed in the presence or absence of 1 mM presynaptic BAPTA (Fig. 7) (for direct comparison of EGTA effects on EPSC amplitude in RBP DKO synapses, see Figs. 3 A and B and 7 D–F) (P < 0.05). Moreover, EPSC rise times and decay times were significantly increased by EGTA in RBP-deficient ribbon synapses, but the total synaptic charge transfer was not greatly changed (Fig. 7 B and E). These results suggest that RBPs control the physical distance between L-type Ca2+ channels and primed synaptic vesicles that mediate fast transmitter release from ribbon synapses.
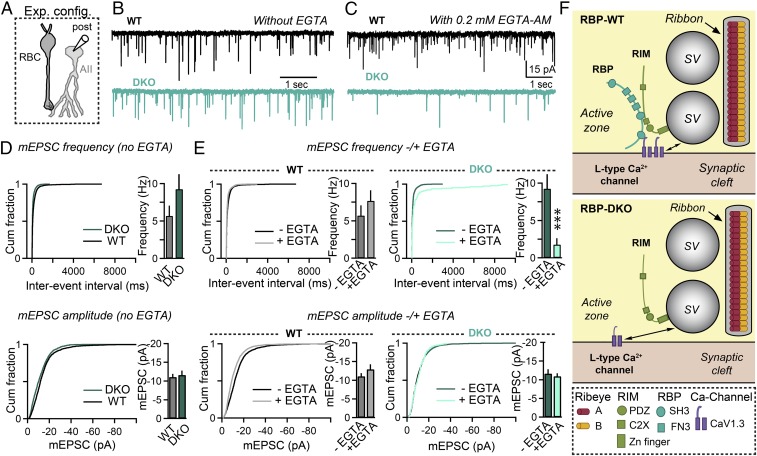
To further test this conclusion, we examined whether removal of RBPs also impairs coupling of Ca2+ channels to spontaneous synaptic vesicle exocytosis (Fig. 8). For this purpose, we measured the rate and properties of quantal miniature EPSCs (mEPSCs) in control and RBP-deficient AII cells in the absence and presence of EGTA (Fig. 8A). Ablation of RBPs did not significantly change the frequency, amplitude, or kinetic properties of AII mEPSCs (Fig. 8 A, B, and D). Moreover, incubation of the slices in 0.2 mM EGTA acetoxymethyl ester (EGTA-AM) for 45 min did not change the frequency, amplitude, or kinetic properties of mEPSCs in control slices (Fig. 8E). In RBP-DKO synapses, however, EGTA-AM treatment produced an almost complete ablation of mESPCs, with a reduction in mEPSC frequency of ∼90% but no major changes in mEPSC amplitude or kinetic properties (Fig. 8E). Since mEPSCs are thought to be triggered at least in part by stochastic Ca2+-channel opening (32), this result supports the notion that RBPs couple synaptic vesicles to Ca2+ channels.
Fig. 8.
Deletion of RBPs alters the sensitivity of quantal release to the slow Ca2+ buffer EGTA at rod bipolar cell to AII amacrine cell synapses. (A) Schematic of the recording configuration. (B and C) Spontaneous mEPSCs recorded from AII amacrine cells in RBP WT (black) and RBP mutant (blue) retinas, without external EGTA-AM (B) or in the presence of 0.2 mM EGTA-AM in the external solution (C). All these experiments were performed in the presence of GABAA receptor blockers. (D) Summary graphs of mEPSC frequency (Top) and mEPSC amplitude (Bottom) in RBP control (black) and RBP mutant (blue) retinas without EGTA-AM in the external solution. Number of experiments: RBP WT, 10 cells; RBP DKO, nine cells. (E) Summary graphs of mEPSC frequency (Top) and mEPSC amplitude (Bottom) in RBP control and RBP mutant retinas before (dark traces) and after (light traces) incubation with 0.2 mM EGTA-AM for 45 min. Number of experiments: RBP WT, nine cells; RBP DKO, nine cells. (F) Model summarizing the effects of deleting RBPs on the structure and function of retina ribbon synapses. All summary graphs are mean ± SD. Statistical analyses for data displayed in bar graphs were performed by Student’s t test, comparing RBP DKO with RBP WT, whereas statistical analyses for data displayed in cumulative distribution plots were performed by K-S test (***P < 0.001; n.s., nonsignificant).
Finally, we tested the effect of EGTA-AM on mEPSC frequency in RBP-deficient ribbon synapses via EGTA-AM “wash-in” experiments. We applied 0.2 μM EGTA-AM onto retina slices by perfusion and measured the effect of the EGTA-AM on mEPSC frequency in individual cells before and after EGTA-AM addition. Under these conditions, we found that EGTA-AM reduced mEPSC frequency by 62.7 ± 18.5% (n = 4, P < 0.05, estimated at 30 min after starting the perfusion of EGTA-AM). Again, these results are consistent with the notion that mEPSCs induced by stochastic opening of L-type Ca2+ channels are coupled to synaptic vesicles via RBPs in ribbon synapses.
Discussion
In the present study, we used a combination of morphological, biochemical, and electrophysiological analyses to assess the function of RBPs in ribbon synapses that are formed by rod bipolar cells on AII amacrine cells in the mouse retina. We found that, in these synapses, RBPs control the localization of presynaptic L-type Ca2+ channels at the active zone and determined the physical distance between these channels and release-ready synaptic vesicles for spontaneous and evoked transmitter release. We propose that, by tethering L-type Ca2+ channels to the active zone of ribbon synapses, RBPs promote efficient stimulus-secretion coupling and high-fidelity visual information transfer across retinal circuits.
We systematically dissected the functions of RBPs in the retinal ribbon synapses by constitutively deleting RBP1 and RBP2, the two major RBP isoforms expressed in neuronal tissues. In a series of detailed morphological studies using light and electron microscopy (Figs. 1 and 6), we showed that deletion of RBPs did not alter the overall organization of the retina or the ultrastructure of bipolar cell→AII amacrine cell ribbon synapses. These results differ from those reported in Drosophila, in which null mutations of RBP led to dramatic changes in the fine structure of neuromuscular synapses (13), but agree with analyses of RBP mutants in conventional central murine synapses that revealed no major morphological abnormalities in synapse ultrastructure (11, 12, 33). Thus, in mice, RBPs are likely not essential for the formation and/or maintenance of active zones as such.
Using high-resolution patch-clamp recordings from rod bipolar cells, we directly recorded presynaptic Ca2+ currents. We found that deletion of RBPs resulted in a 50% reduction in Ca2+-current density without changing Ca2+-current activation and deactivation kinetics (Fig. 4). Both RBP1 and RBP2 contributed to the Ca2+-channel density in an additive manner because separate deletions of either RBP isoform produced a 20 to 30% reduction in Ca2+ currents. Moreover, immunostaining of presynaptic bipolar cell terminals for Ca2+ channels showed that L-type Ca2+-channel levels were reduced in RBP mutants, indicating that RBPs are required for recruiting Ca2+ channels to the release sites. Interestingly, measurements of whole-retina Ca2+-channel levels by immunoblotting revealed an up-regulation of all major subtypes of pore-forming subunits of L-type Ca2+ channels, suggesting a compensatory increase in Ca2+-channel proteins (Fig. 5). The observation that L-type Ca2+-channel expression is increased in RBP-deficient retinas but that the presynaptic Ca2+-current density and Ca2+-channel levels are decreased suggests that the additional Ca2+-channel protein may be retained in intracellular compartments in the absence of targeting slots that are normally provided by RBPs.
Paired recordings from rod bipolar cells and AII amacrine cells revealed that removal of RBPs led to a dramatic reduction in the amount and speed of release triggered by presynaptic depolarization (Fig. 2). This is likely due to the fact that the remaining Ca2+ channels at active zones in the absence of RBPs (near 50%) are physically uncoupled from primed synaptic vesicles because evoked release in RBP DKO synapses was highly sensitive to the slow Ca2+-buffer EGTA (Fig. 7). Thus, RBPs appear to recruit L-type Ca2+ channels to ribbon synapse active zones and to place them in close proximity to the sites of exocytosis to enable tight “nanodomain” coupling of Ca2+ influx to Ca2+-triggered neurotransmitter release. It should be noted, however, that it is not clear whether the role of RBPs in coupling Ca2+ channels to release-ready vesicles as observed in retinal biopolar cell→AII cell synapses can be generalized to other ribbon synapses. For instance, in the hippocampus and cerebral cortex, the “tightness” of Ca2+-channel coupling to release-ready vesicles seems to be target- and cell type-specific (34, 35). Although different types of ribbon synapses appear to be more similar to each other than the different types of conventional synapses examined in studies on nanodomain coupling, it will be interesting to determine whether ribbon synapses other than bipolar cell→AII amacrine cell synapses are also controlled by RBPs.
In addition to their role in Ca2+-channel localization, we found that RBPs in ribbon synapses contribute to replenishment of synaptic vesicles into the RRP. This finding, different from the lack of ultrastructural changes in RBP-deficient synapses, is consistent with studies in Drosophila (10). Specifically, we observed that deletion of RBPs decelerated the kinetics of RRP emptying and replenishing. While the effect of the RBP deletion on RRP emptying can be readily explained by the impairments in the coupling of the Ca2+ channel to synaptic vesicle exocytosis, as discussed above, its effect on RRP replenishment suggests a specific role for RBPs in vesicle priming, as observed earlier (10, 33). The exact mechanism underlying RBP control of vesicle replenishment remains uncertain but is most likely related to the recruitment of Munc13, the most important priming factor at synapses, to release sites by RIMs, which in turn are bound to RBPs (3).
Deletion of RBPs did not have a major impact on spontaneous quantal minirelease in ribbon synapses. This was somewhat surprising because mEPSCs in AII amacrine cells are thought to depend, at least in part, on stochastic openings of presynaptic Ca2+ channels and extracellular Ca2+ influx (32). Thus, considering the strong reduction in the density of presynaptic Ca2+ channels, a reduction in the frequency of mEPSCs in RBP mutants would have been expected. The most parsimonious explanation for the lack of an effect of the RBP deletion on mEPSCs is that evoked and spontaneous transmitter release are mechanistically different and may depend on different pools of synaptic vesicles (36–38). Consistent with this hypothesis, previous studies—including our own—have established that, in conventional excitatory synapses, RBPs selectively control evoked but not spontaneous neurotransmitter release (11, 12, 33). Lastly, although the RBP deletion did not affect spontaneous minirelease under physiological conditions, it dramatically increased the EGTA sensitivity of spontaneous release (Fig. 8). This finding is consistent with the conclusion that the RBP deletion increases the distance between Ca2+ channels and the sites for spontaneous vesicle exocytosis, without controlling the total number of these sites at the active zone, but does not quite explain why the resting mEPSC frequency is unchanged, a question that will need further experimental studies.
Mechanistically, RBPs likely cooperate with RIMs to localize Ca2+ channels to the active zone of ribbon synapses (39) and to prime and tether vesicles at release sites, similar to their cooperative function at conventional central synapses (33). What is different between conventional central synapses and ribbon synapses, however, is that the latter use L-type Ca2+ channels for release whereas the former use N- and/or P/Q-type Ca2+ channels (40). This is important because RBPs directly bind to proline-rich sequences via their three SH3-type domains in all of these Ca2+ channels whereas RIMs bind only to N- and P/Q-type Ca2+ channels (5, 9). The observation that the RBP deletion leads to a depletion of presynaptic Ca2+ channels in rod bipolar cells forming ribbon synapses, but not in the standard calyx of Held synapse (refs. 11 and 33 and Fig. 4) supports the notion that L-type Ca2+ channels used by ribbon synapses mainly depend on RBPs for localization whereas N- and P/Q-type Ca2+ channels found in standard synapses, such as the calyx of Held, depend on both RBPs and RIMs. As a result, the molecular architecture of nano- and microdomains of Ca2+ influx in synapses differs dramatically between ribbon synapses and standard chemical synapses for reasons that remain to be explored but are likely related to the very different functional requirement imposed on these synapses.
Materials and Methods
Mice.
In this study, we used constitutive RBP-1 KO, RBP-2 KO, and RBP1,2 double KO (DKO) mice. These mouse lines were generated by crossing RBP-1fl, RBP-2fl, and RBP1,2fl mice to CMV-cre mice that deleted floxed exons in the germline (11) (Fig. 1A, Right). All experiments involving mice were performed in accordance with Stanford and Federal Guidelines and were approved by the Stanford Institutional Animal Care and Use Committee.
Electrophysiology of Ribbon Synapses.
Retinas from light-adapted, 4- to 8-week-old mice were harvested and cut in 200-μm slices using a vibratome. Patch-clamp recordings were performed in whole-cell voltage-clamp configuration either from presynaptic RBC cells (Fig. 4), postsynaptic AII cells (Fig. 8), or both cell types simultaneously (Figs. 2, 3, and 7). Recordings from identified presynaptic RBC cells were used to monitor L-type calcium current density and properties as previously described (31). Quantal release was monitored as postsynaptic mEPSCs using standard approaches (11, 31). Evoked release from ribbon synapses formed between RBC→AII cells was assessed using paired recordings as described previously (31). For details, see SI Materials and Methods.
Light Microscopy.
We studied the overall structure of the retina by immunohistochemistry, using retina cryosections and antibodies against vGlut1 and PKCα, and confocal microscopy. For details, see SI Materials and Methods.
Immunoblotting.
Immunoblotting experiments were performed as described elsewhere (33, 39). For details, see SI Materials and Methods. A list of antibodies used in the current study with corresponding dilutions and origin are presented in Table S1.
Table S1.
Antibodies used in the current study with corresponding dilutions and origin
| Antigen | Antibody | Dilution |
| Active zone proteins | ||
| RBP-1 (RIM-binding protein-1) | 316003 (SySy) | 1:1,000 |
| RBP-2 (RIM-binding protein-2) | 316103 (SySy) | 1:1,000 |
| RBP-2 (RIM-binding protein-2) | 4193 (custom) | 1:1,000 |
| RIM 1 (RIM1 central domains) | R809 (custom) | 1:2,000 |
| RIM 1/2 (1α/2α N terminus + rabphillin) | U1565 (custom) | 1:2,000 |
| Liprin α3 | 4396 (custom) | 1:5,000 |
| ELKS 1/2aB | 4790 (custom) | 1:500 |
| CASK | 75–000 (Neuromab) | 1:1,000 |
| Mint1 | P932 (custom) | 1;1000 |
| Veli1,2,3 | T813 (custom) | 1:1,000 |
| Munc13-1 | 126103 (SySy) | 1:1,000 |
| Ribeye | Maxeiner et al. (32) | 1:1,000 |
| Ca2+ channels | ||
| Ca2+v1.2-α1C voltage-gated channel | ACC-003 (Alomone) | 1:200 |
| Ca2+v1.3-α1D voltage-gated channel | ACC-005 (Alomone) | 1:200 |
| Ca2+v1.4-α1D voltage-gated channel | Gift from Frank Schmitz | 1:1,000 |
| Ca2+v-α2δ1 voltage-gated channel | ACC-015 (Alomone) | 1:500 |
| Ca2+v-α2δ2 voltage-gated channel | ACC-102 (Alomone) | 1:500 |
| Ca2+v-α2δ3 voltage-gated channel | ACC-103 (Alomone) | 1:500 |
| Ca2+v-α2δ4 voltage-gated channel | ACC-104 (Alomone) | 1:500 |
| Ca2+β1 voltage-gated channel | ACC-106 (Alomone) | 1:250 |
| Ca2+β2 voltage-gated channel | ACC-105 (Alomone) | 1:250 |
| Ca2+β3 voltage-gated channel | ACC-108 (Alomone) | 1:250 |
| Ca2+β4 voltage-gated channel | 75–054 (Neuromab) | 1:250 |
| Exocytosis proteins | ||
| Syntaxin 1 | HPC-1 (custom) | 1:500 |
| SNAP 25 | 71.1 (custom) | 1:2,000 |
| Synaptobrevin 2 | 69.1 (custom) | 1:2,500 |
| Synaptotagmin 1 | 41.1 (custom) | 1:1,000 |
| Loading control | ||
| TUJ1 | T2200 (Sigma) | 1:5,000 |
Electron Microscopy.
RBP1,2 WT or DKO mice were perfused through the heart with 2% glutaraldehyde/0.5% paraformaldehyde and 0.1 M Na-cacodylate (pH 7.4). The retinas were isolated and stained with 0.5% OsO4/0.8% K-ferricyanide and then embedded in resins, cut with an ultramicrotome, poststained with uranyl acetate and lead citrate, and analyzed under the electron microscope. For details, see SI Materials and Methods.
Data Analysis and Statistics.
All quantitative analysis was performed using custom-written macros in IgorPro. Data shown are means ± SEM; statistical analyses used are noted in all figure legends.
SI Materials and Methods
Animals.
All experiments were performed on 4- to 8-wk-old mice (C57BL/6J background) without identification of the genotype to the experimenter. To generate constitutive RBP1, RBP2, or RBP1,2 DKO, we crossed RBP1flox, RBP2flox, or RBP1,2flox mice (11) with a CMV-Cre mouse line (stock number 006054; The Jackson Laboratory) (Fig. 1B). Using this Cre line, removal of LoxP-flanked genes occurs in all tissues, including germ cells, before implantation (19). Thus, the CMV-Cre line allowed complete disruption of RBP1 and RBP2 genes to produce constitutive RBP1, RBP2, or RBP1,2 double KO mice (RBP DKO). Constitutive RBP1 KO, RBP2 KO, or RBP1,2 DKO were viable and fertile (see ref. 11 for more details) and did not display obvious vision phenotypes. The following oligonucleotide primers for genotyping RBP WT, RBP1 KO, RBP2 KO, or RBP1,2 DKO mice were used: TGGACATAGCAGAGGTCGTCC and CCCAGCTCTTCAGCATCTACC for RBP1 allele (WT, 585 bp; KO, 349 bp); and CAATGGCAGACTTCATGAGG and AAGGAGTCCCAGTGCATAGG for RBP2 allele (WT, 585 bp; KO, 351 bp). All experiments involving mice were performed in accordance with Stanford and Federal Guidelines and were approved by the Stanford Institutional Animal Care and Use Committee.
Immunohistochemistry and Light Microscopy.
To assess the impact of RBP1,2 removal on the gross structure of the retina, we used immunohistochemical techniques to label distinct retinal cell layers and laser-scanning microscopy to image and analyze them. Briefly, RBP WT or RBP DKO mice were anesthetized and decapitated. The eyes were enucleated and fixed in 4% paraformaldehyde in 0.9% NaCl solution for 30 min. Retinas were isolated and postfixed in 4% paraformaldehyde for 1 h, washed three to four times with PBS, and sectioned vertically (50 μm) using a Cryostat (CM3050 S; Leica) to preserve synaptic structures. Retina sections were then permeabilized with 0.4% Triton X-100, blocked with 3% goat serum, and subjected to conventional immunohistochemistry protocols using the following antibodies (overnight incubation at 4 °C): anti-PKCa (Sc-208, 1:200; Santa Cruz Biotechnology) to label bipolar cells, anti-VGluT1 to label bipolar cells terminals (Ab5905, 1:1,000; Millipore), and anti-Cav1.3 (ACC-005, 1:200; Alomone Labs) to label L-type Ca2+ channels. Samples were then washed three times in PBS (10 min each) and incubated for 2 h with secondary antibodies conjugated to different Alexa fluor dyes (Alexa 488 anti-rabbit, Alexa 586 anti-GP, Alexa 647 anti-mouse, 1:1,000; Invitrogen). Last, sections were mounted on coverslips using Vectashield with DAPI to label cell nuclei and imaged using a Nikon confocal microscope (A1RSi+, Confocal Microscope; Nikon) controlled by NIS-Elements AR software (Nikon). Samples were scanned in frame mode at 1,024 × 1,024 pixels/frame resolution. Then, 5 to 10 optical sections (1 µm each) along the z axis were taken for each sample and then compiled into a single maximal projection image for analysis. All of the acquisition parameters were kept constant between control and mutant samples. Quantification of puncta intensity was performed with NIS-Elements AR software (Nikon). Fluorescence signals derived from maximal projection images were segmented by intensity and size and binarized to isolate and quantify individual puncta-like structures, such as those shown in Fig. 4A. PKCα fluorescence was used to create a binary mask to define individual bipolar cell boutons, within which the fluorescence signals for VGluT1 and CaV1.3 were quantified and normalized, respectively, to PKCα signals from the same bipolar cell boutons. We used PKCα signal for signal normalization for two reasons: first, because PKCα is a relatively specific rod bipolar cell marker. Given that we analyzed synaptic transmission at RBC-AII ribbon synapses, we wanted to restrict our signal intensity analysis to RBC terminals only. Second, PKCα intensity itself did not change upon removal of RBPs, as shown in Fig. 4 B and C, and thus represented a good internal control for our experiments.
Transmission Electron Microscopy.
To study the ultrastructure of ribbon synapses, RBP WT or RBP DKO mice were anesthetized and perfused through the heart as described above, but using a solution that contained 2% paraformaldehyde/0.5% glutaraldehyde, 0.1 M Na-cacodylate buffer, pH 7.4. Then, their eyes were enucleated, and their retinas were isolated, postfixed in the same solution, and stained with 0.5% OsO4 (osmium tetroxide)/0.8% K-ferricyanide at room temperature for 30 min. This protocol is well suited to stain membranes (of synaptic vesicles, as well as of pre- and postsynaptic compartments) and synaptic ribbons and thus permitted accurate measurements of the nanoscale organization of synaptic vesicles within nerve endings.
All specimens were then stained en bloc with 2% aqueous uranyl acetate for 30 min, dehydrated in a graded series of ethanol to 100%, and embedded in Embed812 resin (Electron Microscopy Science) overnight. Thin sections (50 to 60 nm) were cut with a Leica ultramicrotome and poststained with uranyl acetate and lead citrate. Sample grids were examined in an FEI Tencai Biotwin transmission electron microscope at 80 kV. Images were taken using a Morada CCD camera controlled by iTEM software (Olympus). Quantitative measurements of synapse ultrastructure were performed using ImageJ software. Briefly, most elements of synaptic profiles were first manually traced (including the perimeter of presynaptic boutons, the x, y position of each synaptic vesicle, the entire area covered by synaptic ribbon, etc.), and then the bouton area, ribbon length, and other parameters were computed using built-in macros.
Western Blot and Protein Quantification.
For Western blot analysis, RBP WT or RBP DKO mice were killed by decapitation, and retinas were harvested as described above and incubated in 100 μL of a buffer containing 150 mM NaCl, 10 mM Tris, 0.1% SDS, 0.5% sodium cholate, 1% Nonidet P-40, 0.1% PMSF, and a mixture of proteinase inhibitors, pH 7.4. Samples were then triturated mechanically on ice, gently shook at 4 °C for 1 h, and spun down at 16,000 × g at 4 °C for 20 min. The supernatant was then mixed with 50 to 100 µL of 2× Laemmli sample buffer for cell lysis, and samples were gently shaken for 15 min at room temperature, and then boiled at 105 °C for 10 to 15 min. The 2× Laemmli sample buffer used for cell lysis contained 120 mM Tris⋅Cl, pH 6.8, 4% SDS, 20% glycerol, 0.02% bromophenol blue, and 4% β-mercaptoethanol. In all experiments, 25 to 50 μg of protein was loaded into 4 to 20% Tris/Glycine 1-mm-thick precast gradient gels (Bio-Rad), and blotted onto nitrocellulose transfer membranes using a semidry Trans-Blot Turbo Transfer System (Bio-Rad). Protein membranes were then incubated in a blocking solution containing 5% powder milk dissolved into 0.05% TBS-Tween for 2 h and incubated with primary antibodies overnight at 4 °C. Next morning, protein membranes were washed 3× in TBS-Tween, mixed with fluorescent secondary antibodies (donkey anti-rabbit IR Dye 680CW, 1:10,000; donkey anti-mouse IR Dye 800CW, 1:10,000; LI-COR Biosciences), and imaged with an Odyssey Infrared Imager and Odyssey software (LI-COR Biosciences). For protein quantitation, the total fluorescent intensity values were computed using Odyssey software, background-subtracted, and normalized to the loading control (Tuj1) and the control experiment (samples obtained from RBP WT mice). In Table S1, we show the antibodies used, with corresponding dilutions and origin.
Electrophysiology.
Slice preparation.
Light-adapted RBP WT or RBP1,2 DKO mice (4- to 8-wk-old) were deeply anesthetized with isofluorane (Henry Schein Animal Health) and decapitated. The eyes were enucleated, and the retinas were isolated in oxygenated artificial cerebrospinal fluid (ACSF) containing 119 mM NaCl, 26 mM NaHCO3, 10 mM glucose, 1.25 mM NaH2PO4, 2.5 mM KCl, 2 mM CaCl2, 1 mM MgCl2, 2 mM NaPyruvate, and 0.5 mM ascorbic acid. The midtemporal retinas were trimmed into rectangular pieces and then embedded in low-melting agar (2% in ACSF with Hepes substituted for NaHCO3). Then, 200-µm slices were cut using a Vibratome (Leica VT1200s). Slices were incubated for 1 h at 32 °C and then stored at 21 to 23 °C for experiments.
Data acquisition.
Data were acquired using a dual-channel HEKA EPC10 USB amplifier controlled by PatchMaster (Heka Instruments). To study synaptic transmission, paired whole-cell patch-clamp recordings were made from synaptically connected rod bipolar cells (RBCs) and AII amacrine cells, which were visually identified under infrared differential interference contrast (IR-DIC) video microscopy (Zeiss Axioskop 2) (22). In the mouse retina, both RBCs and AII amacrine cells are relatively small and thus difficult to patch with low-resistance glass pipettes. Therefore, we used relatively high-resistance glass pipettes (5 to 6 MΩ for presynaptic; 4 to 5 MΩ for postsynaptic) in our recordings.
Presynaptic recordings.
Whole-cell patch-clamp recordings of presynaptic Ca2+ current were made from visually identified RBCs. In some experiments, RBCs were filled with the fluorescence dye Alexa-594 (50 µM) through the recording pipette, to confirm their identity and synaptic connections with AII amacrine cells. RBCs were electrically compact and typically displayed membrane resistances >2 GΩ [WT, 7.7 ± 1.9 GΩ (n = 39); DKO, 6.7 ± 1.2 GΩ; P = 0.66]. RBC Ca2+ currents were evoked by step depolarizations from −70 mV holding potentials, to a series of more depolarized membrane potentials, ranging from −45 to −10 mV for 50 ms. Series resistance <30 MΩ was considered acceptable, and leak currents were normally small [WT, −18.4 ± 1.4 pA (n = 39); DKO, −17 ± 1.3 pA (n = 33); P = 0.64], suggesting good voltage-clamp control. RBC Ca2+ currents were pharmacologically isolated and recorded using the following internal solution: 120 mM Cs-methanesulfonate, 20 mM TEA-Cl, 20 mM Hepes, 1 mM BAPTA, 4 mM MgATP, 0.4 mM NaGTP, and 10 mM phosphocreatine. Current signals were leak-subtracted using P/4 protocols.
Postsynaptic recordings.
Postsynaptic recordings were performed from identified AII amacrine cells in retina slices. We used two criteria to unequivocally identify AII amacrine cells. First, we used IR-DIC microscopy to readily identify these cells based on their location in the inner nuclear layer of the retina and based on the characteristic inverted pear-like shape of their soma (22, 31). Second, we directly confirmed the nature of patched amacrine cells by loading them with a fluorescence dye (Alexa Fluo-594) through the recording pipette. AII cells displayed a characteristic morphology that could be readily distinguished from other cell types in the inner nuclear layer of the retina. Series resistance Rs < 20 MΩ were considered acceptable [WT, 15.9 ± 0.7 (n = 26); DKO, 15.2 ± 0.6 (n = 22); P = 0.43]. A2 amacrince cells have relatively large soma with extensive dendrites and relatively low membrane resistance [WT, 2 ± 0.2 GΩ (n = 26); DKO, 2.1 ± 0.4 GΩ (n = 22); P = 0.85]. Postsynaptic leak currents during voltage-clamp recording were as follows: WT, −52.1 ± 4.6 pA (n = 26); DKO, −49.3 ± 3.7 pA (n = 22); P = 0.29. EPSCs were recorded in the presence of picrotoxin (100 µM), strychnine (0.5 µM), and tetrodotoxin (TTX, 0.5 µM) in the bath solution, to block GABAA receptor, glycine receptors and voltage-gated Na+ channels, respectively. All reagents were purchased from Tocris unless otherwise specified.
Data Analysis.
Data analysis was performed in Igor Pro (Wavematrics) and MiniAnalysis (Synaptosoft). Statistical analyses were performed using the K-S test for cumulative distributions, Student’s t test for pairwise comparison, and ANOVA followed by a Tukey post hoc test for multiple comparisons.
Acknowledgments
We thank Prof. Frank Schmitz (University of Saarland) for the generous gift of Cav1.4 antibodies; and members of the T.C.S. laboratory for invaluable discussions and critical comments on earlier versions of this manuscript.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1702991114/-/DCSupplemental.
References
- 1.Rosenmund C, Rettig J, Brose N. Molecular mechanisms of active zone function. Curr Opin Neurobiol. 2003;13:509–519. doi: 10.1016/j.conb.2003.09.011. [DOI] [PubMed] [Google Scholar]
- 2.Schoch S, Gundelfinger ED. Molecular organization of the presynaptic active zone. Cell Tissue Res. 2006;326:379–391. doi: 10.1007/s00441-006-0244-y. [DOI] [PubMed] [Google Scholar]
- 3.Südhof TC. The presynaptic active zone. Neuron. 2012;75:11–25. doi: 10.1016/j.neuron.2012.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Han Y, Kaeser PS, Südhof TC, Schneggenburger R. RIM determines Ca2+ channel density and vesicle docking at the presynaptic active zone. Neuron. 2011;69:304–316. doi: 10.1016/j.neuron.2010.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kaeser PS, et al. RIM proteins tether Ca2+ channels to presynaptic active zones via a direct PDZ-domain interaction. Cell. 2011;144:282–295. doi: 10.1016/j.cell.2010.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mittelstaedt T, Schoch S. Structure and evolution of RIM-BP genes: Identification of a novel family member. Gene. 2007;403:70–79. doi: 10.1016/j.gene.2007.08.004. [DOI] [PubMed] [Google Scholar]
- 7.Wang Y, Liu X, Biederer T, Südhof TC. A family of RIM-binding proteins regulated by alternative splicing: Implications for the genesis of synaptic active zones. Proc Natl Acad Sci USA. 2002;99:14464–14469. doi: 10.1073/pnas.182532999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang Y, Sugita S, Sudhof TC. The RIM/NIM family of neuronal C2 domain proteins. Interactions with Rab3 and a new class of Src homology 3 domain proteins. J Biol Chem. 2000;275:20033–20044. doi: 10.1074/jbc.M909008199. [DOI] [PubMed] [Google Scholar]
- 9.Hibino H, et al. RIM binding proteins (RBPs) couple Rab3-interacting molecules (RIMs) to voltage-gated Ca(2+) channels. Neuron. 2002;34:411–423. doi: 10.1016/s0896-6273(02)00667-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Müller M, Genç Ö, Davis GW. RIM-binding protein links synaptic homeostasis to the stabilization and replenishment of high release probability vesicles. Neuron. 2015;85:1056–1069. doi: 10.1016/j.neuron.2015.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Acuna C, Liu X, Gonzalez A, Südhof TC. RIM-BPs mediate tight coupling of action potentials to Ca(2+)-triggered neurotransmitter release. Neuron. 2015;87:1234–1247. doi: 10.1016/j.neuron.2015.08.027. [DOI] [PubMed] [Google Scholar]
- 12.Grauel MK, et al. RIM-binding protein 2 regulates release probability by fine-tuning calcium channel localization at murine hippocampal synapses. Proc Natl Acad Sci USA. 2016;113:11615–11620. doi: 10.1073/pnas.1605256113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu KS, et al. RIM-binding protein, a central part of the active zone, is essential for neurotransmitter release. Science. 2011;334:1565–1569. doi: 10.1126/science.1212991. [DOI] [PubMed] [Google Scholar]
- 14.Jarsky T, Tian M, Singer JH. Nanodomain control of exocytosis is responsible for the signaling capability of a retinal ribbon synapse. J Neurosci. 2010;30:11885–11895. doi: 10.1523/JNEUROSCI.1415-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Matthews G, Fuchs P. The diverse roles of ribbon synapses in sensory neurotransmission. Nat Rev Neurosci. 2010;11:812–822. doi: 10.1038/nrn2924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schmitz F. Presynaptic [Ca(2+)] and GCAPs: Aspects on the structure and function of photoreceptor ribbon synapses. Front Mol Neurosci. 2014;7:3. doi: 10.3389/fnmol.2014.00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zanazzi G, Matthews G. The molecular architecture of ribbon presynaptic terminals. Mol Neurobiol. 2009;39:130–148. doi: 10.1007/s12035-009-8058-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mennerick S, Matthews G. Ultrafast exocytosis elicited by calcium current in synaptic terminals of retinal bipolar neurons. Neuron. 1996;17:1241–1249. doi: 10.1016/s0896-6273(00)80254-8. [DOI] [PubMed] [Google Scholar]
- 19.Schwenk F, Baron U, Rajewsky K. A cre-transgenic mouse strain for the ubiquitous deletion of loxP-flanked gene segments including deletion in germ cells. Nucleic Acids Res. 1995;23:5080–5081. doi: 10.1093/nar/23.24.5080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Euler T, Masland RH. Light-evoked responses of bipolar cells in a mammalian retina. J Neurophysiol. 2000;83:1817–1829. doi: 10.1152/jn.2000.83.4.1817. [DOI] [PubMed] [Google Scholar]
- 21.Oesch NW, Diamond JS. Ribbon synapses compute temporal contrast and encode luminance in retinal rod bipolar cells. Nat Neurosci. 2011;14:1555–1561. doi: 10.1038/nn.2945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Singer JH, Diamond JS. Sustained Ca2+ entry elicits transient postsynaptic currents at a retinal ribbon synapse. J Neurosci. 2003;23:10923–10933. doi: 10.1523/JNEUROSCI.23-34-10923.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Protti DA, Llano I. Calcium currents and calcium signaling in rod bipolar cells of rat retinal slices. J Neurosci. 1998;18:3715–3724. doi: 10.1523/JNEUROSCI.18-10-03715.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hoppa MB, Lana B, Margas W, Dolphin AC, Ryan TA. α2δ expression sets presynaptic calcium channel abundance and release probability. Nature. 2012;486:122–125. doi: 10.1038/nature11033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu C, et al. The active zone protein family ELKS supports Ca2+ influx at nerve terminals of inhibitory hippocampal neurons. J Neurosci. 2014;34:12289–12303. doi: 10.1523/JNEUROSCI.0999-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bucurenciu I, Kulik A, Schwaller B, Frotscher M, Jonas P. Nanodomain coupling between Ca2+ channels and Ca2+ sensors promotes fast and efficient transmitter release at a cortical GABAergic synapse. Neuron. 2008;57:536–545. doi: 10.1016/j.neuron.2007.12.026. [DOI] [PubMed] [Google Scholar]
- 27.Eggermann E, Bucurenciu I, Goswami SP, Jonas P. Nanodomain coupling between Ca2+ channels and sensors of exocytosis at fast mammalian synapses. Nat Rev Neurosci. 2011;13:7–21. doi: 10.1038/nrn3125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fedchyshyn MJ, Wang LY. Developmental transformation of the release modality at the calyx of Held synapse. J Neurosci. 2005;25:4131–4140. doi: 10.1523/JNEUROSCI.0350-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vyleta NP, Jonas P. Loose coupling between Ca2+ channels and release sensors at a plastic hippocampal synapse. Science. 2014;343:665–670. doi: 10.1126/science.1244811. [DOI] [PubMed] [Google Scholar]
- 30.Wang LY, Neher E, Taschenberger H. Synaptic vesicles in mature calyx of Held synapses sense higher nanodomain calcium concentrations during action potential-evoked glutamate release. J Neurosci. 2008;28:14450–14458. doi: 10.1523/JNEUROSCI.4245-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Luo F, Bacaj T, Südhof TC. Synaptotagmin-7 is essential for Ca2+-triggered delayed asynchronous release but not for Ca2+-dependent vesicle priming in retinal ribbon synapses. J Neurosci. 2015;35:11024–11033. doi: 10.1523/JNEUROSCI.0759-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Maxeiner S, Luo F, Tan A, Schmitz F, Südhof TC. How to make a synaptic ribbon: RIBEYE deletion abolishes ribbons in retinal synapses and disrupts neurotransmitter release. EMBO J. 2016;35:1098–1114. doi: 10.15252/embj.201592701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Acuna C, Liu X, Südhof TC. How to make an active zone: Unexpected universal functional redundancy between RIMs and RIM-BPs. Neuron. 2016;91:792–807. doi: 10.1016/j.neuron.2016.07.042. [DOI] [PubMed] [Google Scholar]
- 34.Hefft S, Jonas P. Asynchronous GABA release generates long-lasting inhibition at a hippocampal interneuron-principal neuron synapse. Nat Neurosci. 2005;8:1319–1328. doi: 10.1038/nn1542. [DOI] [PubMed] [Google Scholar]
- 35.Rozov A, Burnashev N, Sakmann B, Neher E. Transmitter release modulation by intracellular Ca2+ buffers in facilitating and depressing nerve terminals of pyramidal cells in layer 2/3 of the rat neocortex indicates a target cell-specific difference in presynaptic calcium dynamics. J Physiol. 2001;531:807–826. doi: 10.1111/j.1469-7793.2001.0807h.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ramirez DM, Kavalali ET. Differential regulation of spontaneous and evoked neurotransmitter release at central synapses. Curr Opin Neurobiol. 2011;21:275–282. doi: 10.1016/j.conb.2011.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ramirez DM, Khvotchev M, Trauterman B, Kavalali ET. Vti1a identifies a vesicle pool that preferentially recycles at rest and maintains spontaneous neurotransmission. Neuron. 2012;73:121–134. doi: 10.1016/j.neuron.2011.10.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sara Y, Virmani T, Deák F, Liu X, Kavalali ET. An isolated pool of vesicles recycles at rest and drives spontaneous neurotransmission. Neuron. 2005;45:563–573. doi: 10.1016/j.neuron.2004.12.056. [DOI] [PubMed] [Google Scholar]
- 39.Grabner CP, et al. RIM1/2-mediated facilitation of Cav1.4 channel opening is required for Ca2+-stimulated release in mouse rod photoreceptors. J Neurosci. 2015;35:13133–13147. doi: 10.1523/JNEUROSCI.0658-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Catterall WA. Voltage-gated calcium channels. Cold Spring Harb Perspect Biol. 2011;3:a003947. doi: 10.1101/cshperspect.a003947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Burrone J, Neves G, Gomis A, Cooke A, Lagnado L. Endogenous calcium buffers regulate fast exocytosis in the synaptic terminal of retinal bipolar cells. Neuron. 2002;33:101–112. doi: 10.1016/s0896-6273(01)00565-7. [DOI] [PubMed] [Google Scholar]