Significance
Interleukin-27 (IL-27) can promote or antagonize inflammatory responses. However, the cellular mechanisms underlying such highly diverse roles remain unclear. It was proposed that IL-27 exerts antiinflammatory functions by directly acting on conventional CD4 T cells to induce IL-10–producing cells that are implicated in controlling inflammatory responses. Here, we have developed a Treg-specific IL-27Rα–deficient animal and observed that it is highly susceptible to autoimmune disease induction despite the presence of IL-10–producing T cells. We also show that systemic administration of IL-27 fails to attenuate ongoing inflammation in the absence of Treg expression of IL-27Rα. Therefore, IL-27 acting on Tregs plays a nonreplaceable role in controlling autoimmune inflammation, providing a rationale to develop a therapy by targeting IL-27 on Tregs.
Keywords: autoimmunity, Foxp3+ regulatory T cells, IL-27, Tr1 cells
Abstract
Dysregulated Foxp3+ Treg functions result in uncontrolled immune activation and autoimmunity. Therefore, identifying cellular factors modulating Treg functions is an area of great importance. Here, using Treg-specific Il27ra−/− mice, we report that IL-27 signaling in Foxp3+ Tregs is essential for Tregs to control autoimmune inflammation in the central nervous system (CNS). Following experimental autoimmune encephalomyelitis (EAE) induction, Treg-specific Il27ra−/− mice develop more severe EAE. Consistent with the severe disease, the numbers of IFNγ- and IL-17–producing CD4 T cells infiltrating the CNS tissues are greater in these mice. Treg accumulation in the inflamed CNS tissues is not affected by the lack of IL-27 signaling in Tregs, suggesting a functional defect of Il27ra−/− Tregs. IL-10 production by conventional CD4 T cells and their CNS accumulation are rather elevated in Treg-specific Il27ra−/− mice. Analysis with Treg fate-mapping reporter mice further demonstrates that IL-27 signaling in Tregs may control stability of Foxp3 expression. Finally, systemic administration of recombinant IL-27 in Treg-specific Il27ra−/− mice fails to ameliorate the disease even in the presence of IL-27–responsive conventional CD4 T cells. These findings uncover a previously unknown role of IL-27 in regulating Treg function to control autoimmune inflammation.
Defective Foxp3+ regulatory CD4 T-cell (Treg) functions can result in uncontrolled autoimmune inflammation (1). However, the cellular mechanisms underlying the defects remain largely unclear. Inflammatory mediators may undermine the Treg function (2), or alternatively, inflammatory effector T cells may resist Treg-mediated suppression (3). Understanding pathways regulating the Treg function to control inflammatory responses is thus a subject of great importance.
IL-27 is a heterodimeric cytokine composed of the p28 and Ebi3 subunits, produced by activated Ag-presenting cells (4). It binds to IL-27 receptors (IL-27Rα:gp130) expressed on multiple cell types, including T lymphocytes (5). IL-27 controls T-cell turnover under steady-state conditions, and T-cell responses elicited by TLR agonist-based vaccine adjuvant induced immune responses (6, 7). In addition, IL-27 supports Th1 differentiation, inhibits Th17 differentiation, and induces IL-10–producing Foxp3− CD4 T cells that are implicated in autoimmune regulation (8–10). Indeed, Il27ra−/− mice are highly susceptible to experimental autoimmune encephalomyelitis (EAE), a myelin Ag-specific Th17-mediated autoimmunity in the central nervous system (CNS) (11, 12). We recently uncovered a key role of IL-27 in supporting Foxp3+ Treg function during T-cell–mediated intestinal inflammation (13), a model system in which Tregs are transferred into immunodeficient recipients. Thus, the exact role of IL-27 in Tregs under immune-competent settings remains unclear.
Here, we generated Treg-specific Il27ra−/− mice using the Cre-LoxP system and examined the role of IL-27 signaling in Tregs during autoimmune inflammation. Following EAE induction, the onset and initial progression of the disease were comparable in wild-type and Treg-specific Il27ra−/− mice. However, the latter mice were unable to recover from the acute disease and developed a progressive form of the disease. Equivalent numbers of Tregs were found in the inflamed CNS tissues in both groups of mice, suggesting a functional defect of Tregs. Interestingly, the generation of IL-10+ CD4 T cells under these conditions remained unaffected. Fate-mapping reporter mice with Treg-specific IL-27Rα deficiency further demonstrated that IL-27 may control Treg stability. Importantly, systemic administration of IL-27 into Treg-specific Il27ra−/− mice did not attenuate the disease despite the presence of conventional CD4 T cells capable of responding to IL-27. Taken together, our results demonstrate an indispensable role for IL-27 in regulating Treg function during autoimmune inflammation.
Results
Treg-Specific Deletion of IL-27Rα Does Not Alter Treg Development.
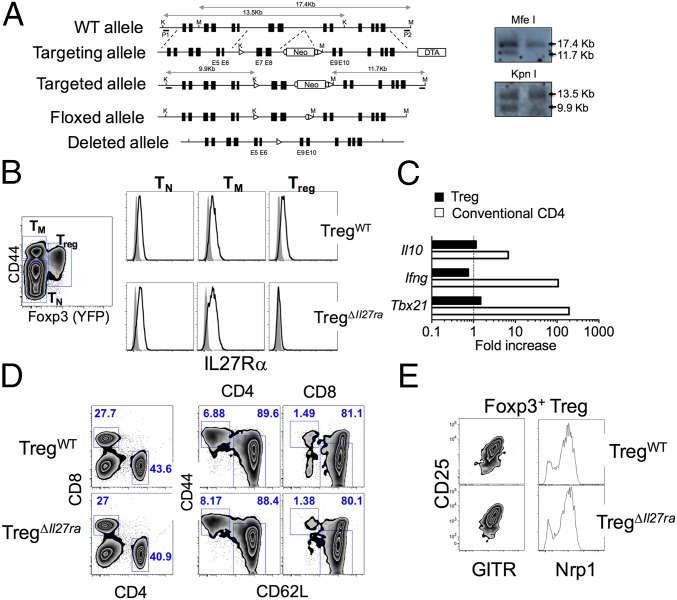
To test the role of IL-27 in Tregs, we generated Treg-specific Il27ra−/− mice using the Cre-loxP system. Analogous to the germline Il27ra−/− mice (14), exons 7 and 8, encoding a portion of the second fibronectin type III domain, were flanked by two loxP sites including the neomycin resistance cassette (Fig. 1A). Mice with floxed Il27ra allele were then bred to FLP-recombinase transgenic mice, and mice carrying a floxed allele without the neomycin cassette were obtained (Fig. 1A). Il27rafl/fl mice were then crossed to the Foxp3CreYFP mice to obtain Treg-specific Il27ra−/− (referred to as TregΔIl27ra) mice (Fig. 1A). It was recently noted that the Cre activity of Foxp3CreYFP mice could be promiscuous, and the Cre-mediated gene targeting can occur in non-Tregs (15). PCR analysis of genomic DNA amplifying the targeted exons of the Il27ra gene validated Treg-specific deletion of exons 7 and 8 only in Tregs (Fig. S1). Foxp3+ Tregs from TregΔIl27ra mice were the only population that lacked surface IL-27Rα expression (Fig. 1B), whereas naive and memory phenotype CD4 T-cell types from TregΔIl27ra and littermate control (Foxp3CreYFPIl27rafl/wt, referred to as TregWT) mice expressed comparable levels of IL-27Rα (Fig. 1B). In vitro stimulation with IL-27 induced Il10, Ifng, and Tbx21 expression in conventional CD4 but not in Tregs from TregΔIl27ra mice (Fig. 1C), while the expression was enhanced in WT Tregs by IL-27 stimulation (13). Moreover, naive CD4 T cells isolated from TregΔIl27ra and TregWT mice equally up-regulated T-bet and IL-10 and down-regulated IL-2 expression in response to IL-27 stimulation (16, 17) (Fig. S2). Neither CD8 T cells nor non-T cells expressed YFP, and only CD25+ CD4 T cells expressed YFP (Fig. S3). In vitro-activated YFP− T cells remained YFP− and IL-27Rα+. Therefore, IL-27Rα deletion in TregΔIl27ra mice is Foxp3+ Treg-specific.
Fig. 1.
Treg-specific Il27ra−/− mice. (A) Generation of neo-free Il27ra-floxed mice by the two-loxP and two-frt strategy. (B) IL-27Rα expression of naive (TN), memory phenotype (TM), and Foxp3+ Tregs (Treg) in Treg-specific Il27ra−/− (TregΔIl27ra) and littermate control (TregWT) mice. (C) Foxp3+ and conventional CD4 T cells were sorted from TregΔIl27ra mice and in vitro-stimulated with plate-coated anti-CD3/CD28 Abs in the presence of IL-27. Fold increases of the indicated genes were determined by qPCR analysis. (D and E) Lymph node cells were stained for CD4, CD8, CD44, and CD62L expression. Proportions of naive and effector/memory phenotype CD4 T cells (D) and surface phenotypes of Tregs (E) were examined. All of the experiments shown were repeated three times, and similar results were observed.
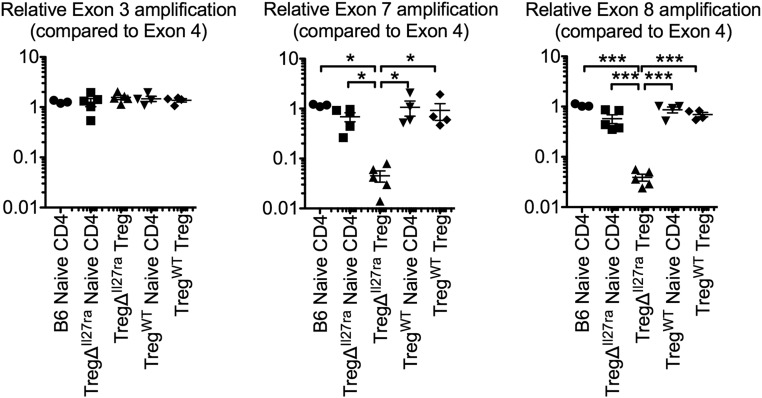
Fig. S1.
Treg-specific IL-27Rα deletion. Genomic DNA was isolated from FACS-sorted Foxp3− naive CD4, Foxp3+ Treg of TregΔIl27ra, and TregWT mice as well as naive CD4 T cells from C57BL/6 mice. PCR amplifying the Il27ra exons (3, 4, 7, 8) of the Il27ra gene was performed. Exons 7 and 8 represent genes targeted in TregΔIl27ra mice. Amplification of the indicated exon was normalized to the control exon 4. Each symbol represents an individually performed experiment.
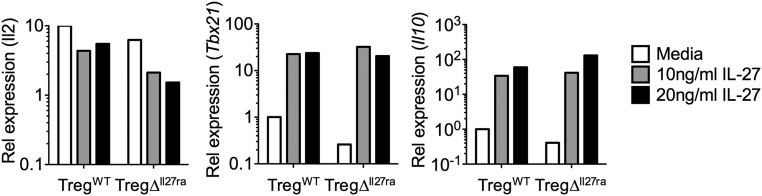
Fig. S2.
Naive CD4 T cells from TregWT mice and TregΔIl27ra mice respond equally to IL-27 stimulation. Foxp3− CD44low naive CD4 T cells were FACS-sorted from TregWT mice and TregΔIl27ra mice and in vitro-stimulated in the presence of IL-27 for 48 h. The expression of the indicated genes was determined by qPCR analysis.
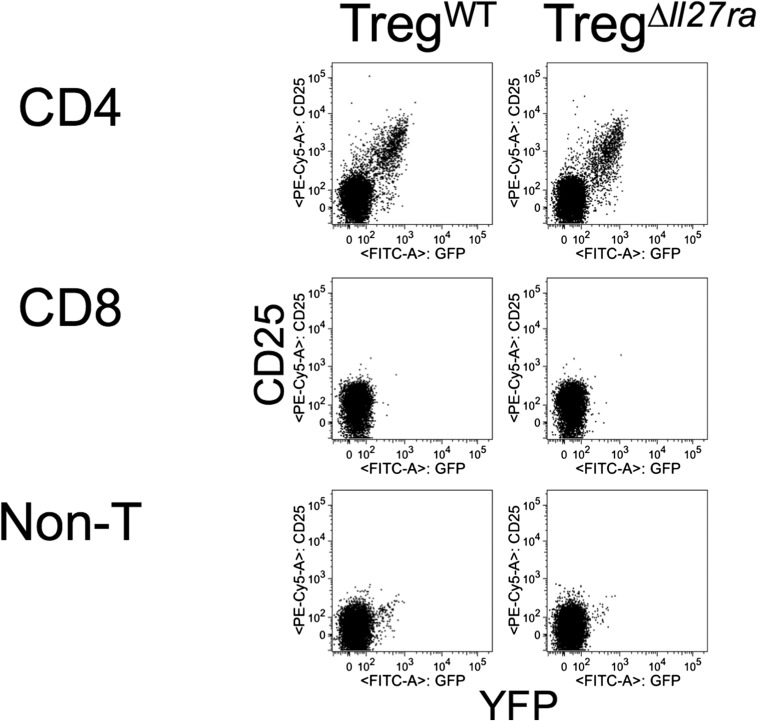
Fig. S3.
GFP expression of CD25+ CD4 T cells. Lymph node (LN) cells from TregWT mice and TregΔIl27ra mice were stained for CD25, CD4, and CD8 expression. CD25 and GFP expression of the indicated subsets was examined. The experiments were repeated three times, and similar results were observed.
TregΔIl27ra mice remained healthy, consistent with the germline Il27ra−/− mice (14). The total cellularity and the proportions of naive and effector/memory T cells in the thymus and periphery were comparable between the groups (Fig. 1D). Expression of the Treg-associated markers CD25, GITR, and Neuropilin-1 (Nrp1) was also comparable (Fig. 1E). Therefore, IL-27 signaling in Tregs seems dispensable for Treg development and homeostasis under steady-state conditions.
Treg-Specific Il27ra−/− Mice Develop Severe EAE.
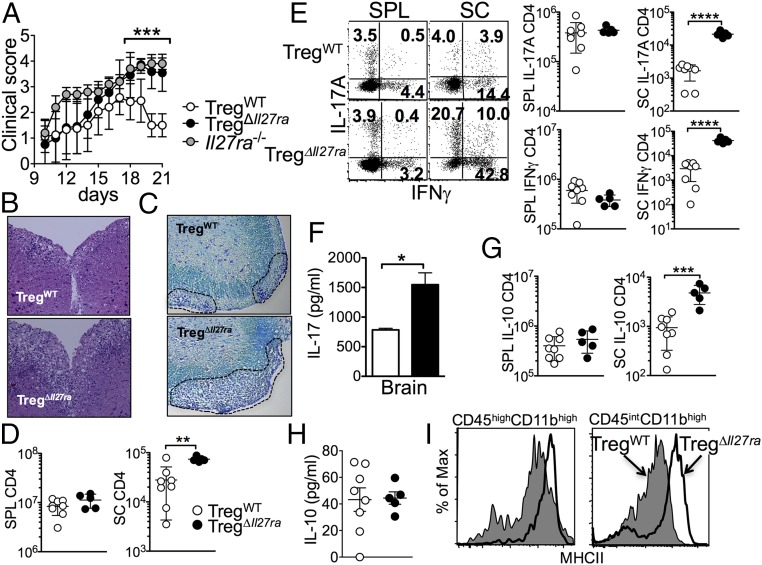
TregΔIl27ra and TregWT mice were induced for EAE by immunization with myelin oligodendrocyte glycoprotein (MOG) peptide 35–55 in complete Freund's adjuvant (CFA) followed by pertussis toxin injection. The onset and initial progression of the disease was similar between the groups (Fig. 2A). While the control TregWT mice reached peak disease around day 17 and slowly recovered from the disease, TregΔIl27ra mice continuously exhibited signs of severe EAE (Fig. 2A). Histopathologic examination demonstrated that the spinal cords of the TregΔIl27ra mice were heavily infiltrated with inflammatory cells compared with the control mice that showed less severe inflammation (Fig. 2B). The area of demyelination determined by Luxol fast blue (LFB) staining was substantially greater in TregΔIl27ra compared with that in control mice (Fig. 2C). Germline Il27ra−/− mice develop severe EAE (12). Interestingly, the progression of EAE in TregΔIl27ra mice after the peak of the disease was analogous to that in Il27ra−/− mice, although the clinical score of initial EAE induction (i.e., days 10–14) was markedly greater in Il27ra−/− mice (Fig. 2A). Consistently, the total numbers of CD4 T cells infiltrating the CNS were dramatically elevated in TregΔIl27ra, although CD4 T cells in the peripheral lymphoid tissues remained comparable (Fig. 2D). T cells expressing the proinflammatory cytokines IFNγ or IL-17 were more abundant in the CNS tissues of TregΔIl27ra mice (Fig. 2E). Brain cells stimulated with MOG peptide ex vivo secreted approximately twofold higher levels of IL-17 (Fig. 2F). We also noted that the level of IL-10–producing CNS infiltrating CD4 T cells was increased in TregΔIl27ra mice (Fig. 2G); however, IL-10 protein measured from the brain homogenates was comparable between the groups (Fig. 2H). Consistent with elevated inflammatory cytokine production in TregΔIl27ra mice, MHCII expression of the resident microglia (CD45int CD11bhigh) and infiltrating monocytes (CD45high CD11bhigh) was substantially higher in TregΔIl27ra mice (Fig. 2I).
Fig. 2.
Treg-specific Il27ra−/− mice develop severe EAE. (A) Groups of germline Il27ra−/− (n = 5), TregΔIl27ra (n = 10), and TregWT (n = 10) mice were induced for EAE. Clinical score was monitored daily. (B) Mice were killed 19 d post induction. H&E staining of the spinal cord. (Magnification: 10×.) (C) LFB staining of the spinal cord. (Magnification: 10×.) (D) Total CD4 T-cell numbers of the spleen and spinal cord at 19 d post induction. (E) Spleen and spinal cord cells were ex vivo-stimulated, and intracellular IFNγ/IL-17 expression was determined by FACS analysis. Absolute numbers of IFNγ/IL-17–producing CD4 T cells were enumerated. (F) CNS cells were isolated and ex vivo-stimulated with MOG peptide for 3 d. IL-17 secretion in the culture supernant was determined by ELISA. (G) Absolute numbers of IL-10–producing CD4 T cells. (H) IL-10 within inflamed brain tissues was determined by ELISA. (I) MHCII expression of microglia and infiltrating monocytes was measured. Each symbol represents an individually tested mouse. The results are representative of three independent experiments. *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001.
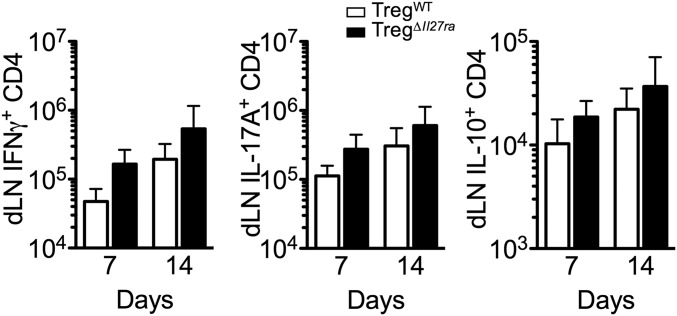
Severe EAE in TregΔIl27ra mice could be attributed to elevated generation of MOG-specific effector cells in the draining lymph nodes. IFNγ-, IL-17–, and IL-10–producing CD4 T cells in the draining lymphoid tissues were thus measured at different time points following EAE induction, when both groups display comparable clinical scores (i.e., days 7 and 14 post induction). The numbers of cytokine+ CD4 T cells in the draining inguinal lymph nodes were slightly elevated, although they did not reach statistical significance (Fig. S4). Thus, these results suggest that IL-27 signaling in Foxp3+ Tregs may play a more critical role in controlling effector T-cell responses at the target tissue sites.
Fig. S4.
CD4 T-cell cytokine production at different time points. Groups of TregΔIl27ra and TregWT mice were immunized with MOG peptide in CFA. Following 7 d and 14 d of immunization, draining inguinal LN cells were harvested, and IFNγ- and IL-17–producing CD4 T cells were enumerated by flow analysis. The data shown are representative of two independent experiments (n > 5).
The Role of IL-27 in Foxp3+ Tregs.
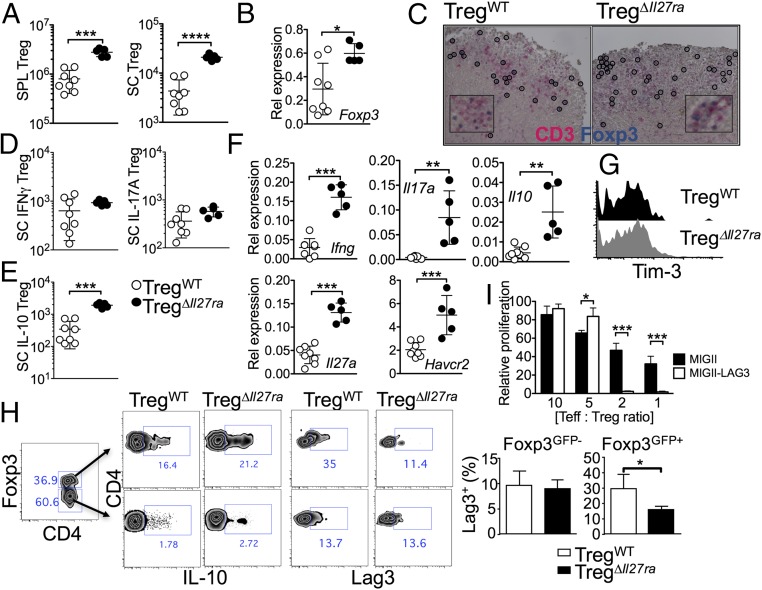
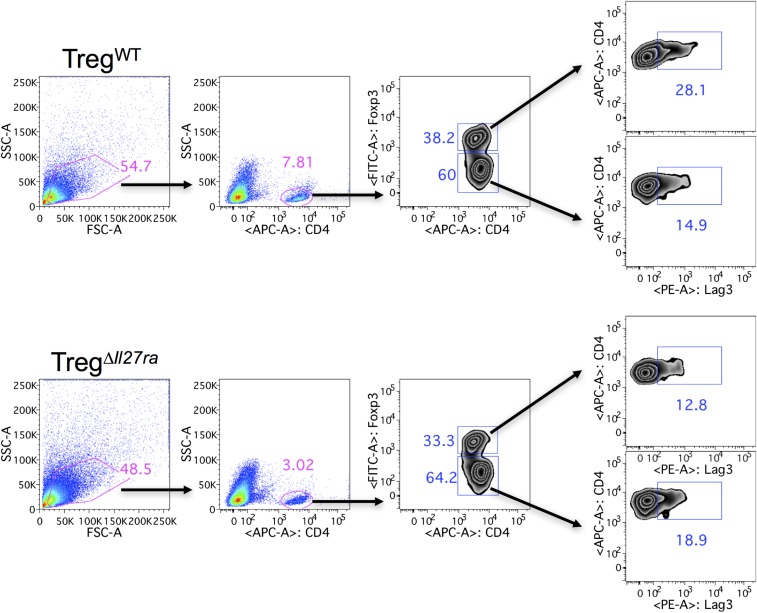
Tregs in the peripheral and CNS tissues of TregΔIl27ra mice with EAE were more abundant than those of TregWT mice, suggesting that the inability of Il27ra−/− Tregs to control the inflammation is likely due to defects in Treg functions rather than to Treg trafficking (Fig. 3A). Annexin-V binding of Tregs was similar between the groups, suggesting that Il27ra−/− Tregs do not exhibit defects in survival. Consistent with Treg numbers, Foxp3 mRNA expression was greater in the CNS tissues of TregΔIl27ra mice (Fig. 3B). Immunohistochemical analysis further demonstrated comparable infiltration of Foxp3+ Tregs in the CNS regardless of IL-27Rα expression (Fig. 3C). The expression of Treg markers, such as CTLA4, CD25, and CD39, was indistinguishable between TregWT and TregΔIl27ra mice. Under inflammatory conditions Tregs often express proinflammatory cytokines and acquire pathogenic function (18). However, Foxp3 (YFP)+ Treg expression of IFNγ and IL-17 was similar in both groups (Fig. 3D). Treg-derived IL-10 is considered a key mediator of Treg function (19). However, we found that the number of IL-10+ Tregs in TregΔIl27ra mice was even greater than that of TregWT mice (Fig. 3 E and H), suggesting that the IL-27–induced Treg suppressive function may be IL-10–independent (13). Consistent with the FACS data, Ifng, Il17a, Il10, and Il27a mRNA expression of the CNS tissues was significantly elevated in TregΔIl27ra mice (Fig. 3F). T-cell Ig and mucin domain-3 (TIM-3, Havcr2) is an inhibitory receptor expressed on IFNγ-producing CD4 T cells and implicated in suppressing effector T-cell function during autoimmune inflammation (20). IL-27 stimulation has previously been shown to induce Tim-3 in activated T cells (21). Havcr2 mRNA expression remained higher in the CNS tissues of TregΔIl27ra mice (Fig. 3F). However, surface Tim-3 expression on Tregs was comparable between the groups (Fig. 3G), suggesting that Tim-3 is not likely involved in IL-27–dependent Treg function. We previously reported that IL-27 induces the lymphocyte-activating gene 3 (Lag3) in Tregs and that Lag3 expression on Tregs is essential in mediating Treg function during intestinal inflammation in an IL-10–independent manner (13). Indeed, surface Lag3 expression on CNS infiltrating Foxp3+ Tregs was significantly lower in TregΔIl27ra mice, while CNS infiltrating effector CD4 T cells expressed comparable levels of Lag3 (Fig. 3H and Fig. S5). Overexpression of Lag3 in Tregs using a retroviral vector dramatically enhanced the Treg-suppressive function (Fig. 3I), suggesting that Lag3 enhances the Treg suppressive function. Taken together, IL-27 signaling in Foxp3+ Tregs, while essential for the Treg-suppressive function, has little impact on Treg survival and cytokine expression. IL-27 signaling up-regulates Treg expression of Lag3, which may regulate Treg functions to reduce autoimmune inflammation.
Fig. 3.
Foxp3+ Treg responses in Treg-specific Il27ra−/− mice. (A) GFP+ Tregs were enumerated in the spleen and spinal cords of TregΔIl27ra and TregWT mice at 19 d post induction. (B) Foxp3 mRNA expression in the brain tissue was measured by qPCR. (C) Immunohistochemistry analysis for CD3 and Foxp3 expression. (Magnification: C, 20×; Inset, 40×.) (D) Spinal cord cells from TregΔIl27ra and TregWT mice were ex vivo-stimulated, and IFNγ- and IL-17–expressing GFP+ Tregs were enumerated by FACS analysis. (E) IL-10–producing Tregs in the spinal cord. (F) mRNA expression of the indicated genes in the brain tissue was measured by qPCR analysis. (G) Treg expression of TIM-3. (H) Lag3 and IL-10 expression of conventional CD4 and Tregs was measured by FACS analysis. (I) Foxp3+-inducible Tregs were transduced with retroviral vectors expressing Lag3. Empty vector-transduced Tregs were used as controls. The transduced Tregs were subsequently used in a suppression assay as described in Methods. Each symbol represents an individually tested mouse. Mean ± SD from two to three independent experiments is shown. *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001.
Fig. S5.
Lag3 expression of brain-infiltrating T cells. TregΔIl27ra and TregWT mice were induced for EAE and killed at day 21 post induction. Mononuclear cells were isolated from the brain and stained for CD4 and Lag3. Zebra plots shown represent Lag3 expression of brain-infiltrating Foxp3+ Tregs and Foxp3− effector CD4 T cells. Similar results were observed in two independent experiments.
IL-27 Signaling in Foxp3+ Tregs Is Involved in Treg Stability.
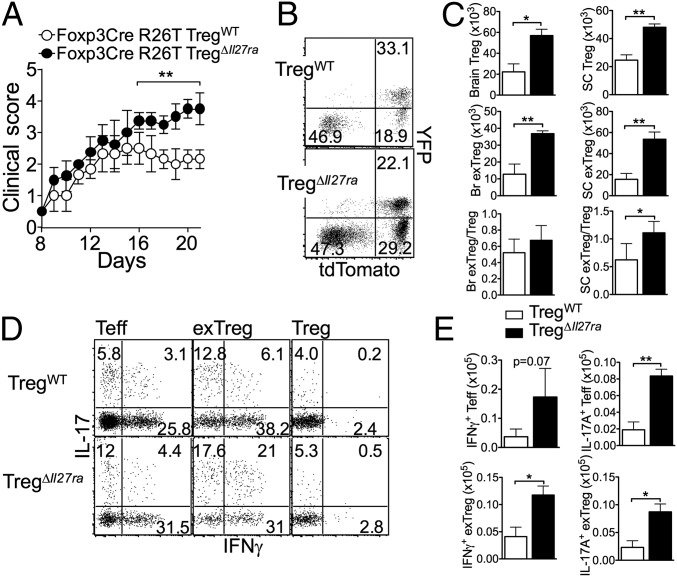
Tregs that lose Foxp3 expression (“exTregs”) are found in inflammatory sites, where cellular factors produced by inflammatory cells may trigger exTreg generation (22). In the preceding experiments, Tregs were defined based on Foxp3 (YFP) expression. However, if Foxp3− exTregs are generated, they are likely excluded from the analysis as they no longer express the YFP. To test whether the IL-27 signal alters Treg stability, we crossed fate-mapping Rosa26LSL(lox-stop-lox)-tdTomato mice to TregΔIl27ra mice. The resulting Rosa26LSL-tdTomato TregΔIl27ra mice have Tregs irreversibly marked with the tdTomato reporter. Groups of Rosa26LSL-tdTomato TregΔIl27ra and Rosa26LSL-tdTomato TregWT littermate control mice were induced for EAE. Analogous to TregΔIl27ra mice, Rosa26LSL-tdTomato TregΔIl27ra mice developed severe EAE, especially after the peak of the disease (Fig. 4A). Interestingly, we noted that a substantial proportion of tdTomato+ cells lost YFP expression, an indication of exTregs (Fig. 4B). Moreover, the level of exTregs generated was greater in the absence of IL-27Rα expression on Tregs (Fig. 4B). The absolute numbers of both Tregs and exTregs remained higher in Rosa26LSL-tdTomato TregΔIl27ra mice, and the ratio of exTregs to Tregs was greater in Rosa26LSL-tdTomato TregΔIl27ra mice (Fig. 4C). Regardless of IL-27Rα expression, exTregs expressed inflammatory cytokines, and the proportions of IFNγ and IL-17–expressing exTregs were even greater than those of effector CD4 T cells (Fig. 4D). Of note, the absolute numbers of IFNγ- and IL-17–producing effector T cells and exTregs were significantly higher in Rosa26LSL-tdTomato TregΔIl27ra than in TregWT mice (Fig. 4E). Thus, IL-27 signaling in Tregs may directly control Treg stability, or alternatively, exTreg generation may be elevated simply due to severe inflammatory conditions in TregΔIl27ra mice.
Fig. 4.
IL-27 signaling in Tregs controls Treg stability. (A) Groups of Rosa26CAG-tdTomato TregΔIl27ra and Rosa26CAG-tdTomato TregWT mice were induced for EAE. Clinical score was daily monitored. (B) Mice were killed 21 d post induction. YFP and tdTomato reporter expression of CD4 gated CNS cells is shown. (C) Absolute numbers of Tregs and exTregs in the CNS were calculated. (D) CNS cells were ex vivo-stimulated and intracellular IFNγ and IL-17 expression of Tconv, exTreg, and Tregs was determined by FACS analysis. (E) Absolute numbers of IFNγ- and IL-17–producing exTregs were calculated. The data shown are representative of two independent experiments (n = 6 ∼ 7). *P < 0.05; **P < 0.01.
Recombinant IL-27 Administration Has Little Therapeutic Impact in TregΔIl27ra Mice.
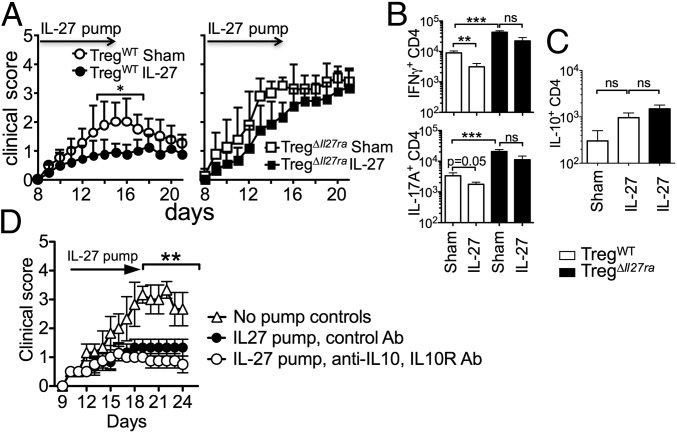
Unlike the previous findings that IL-27 reverses autoimmunity by inducing IL-10–producing Foxp3− CD4 cells (12, 21), our reports thus far demonstrate that IL-27 signaling in Foxp3+ Tregs is instrumental in controlling tissue inflammation. Recombinant rIL-27 administration prevents the development of encephalitogenic effector cell generation and suppresses active EAE (23). We thus tested whether rIL-27 has a therapeutic impact on ongoing EAE in TregΔIl27ra mice. If IL-27–induced IL-10–producing conventional CD4 T cells are critical for this protection, those mice will develop attenuated disease. However, if IL-27 signaling in Tregs is indispensable, those mice will not be protected from the disease. Osmotic pumps containing rIL-27 were implanted at the onset of the disease (on day 8 post immunization). As previously reported (23), rIL-27 administered attenuated ongoing EAE in TregWT mice compared with the sham group (Fig. 5A). CD4 T cells producing IFNγ or IL-17 were significantly reduced in IL-27–treated TregWT mice compared with the sham TregWT group (Fig. 5B). On the other hand, the therapeutic effect of IL-27 was not evident in TregΔIl27ra mice as rIL-27–treated animals still developed severe EAE (Fig. 5A). Furthermore, the numbers of effector CD4 T cells producing IFNγ or IL-17 accumulated in the CNS remained higher despite rIL-27 treatment in TregΔIl27ra mice (Fig. 5B). IL-27 can induce IL-10 production in T cells (10). IL-10+ CD4 T cells in the CNS tissues were elevated in rIL-27–treated mice, although the difference was not statistically different (Fig. 5C). Moreover, the numbers of IL-10+ CD4 T cells were comparable between TregWT and TregΔIl27ra mice treated with IL-27 (Fig. 5C). Finally, we tested whether the rIL-27 treatment effect is dependent on IL-10. B6 mice induced for EAE were treated with a rIL-27 pump as described above. Neutralizing anti–IL-10 mAb and blocking anti–IL-10R mAb were simultaneously injected into the treated groups. Antibody treatment did not abrogate the IL-27–mediated treatment effects (Fig. 5D), suggesting that the protection is IL-10–independent. Therefore, Treg-specific IL-27Rα deficiency appears to be responsible for the lack of treatment effect of IL-27.
Fig. 5.
IL-27 signaling in Tregs is essential to treat EAE. (A) TregWT and TregΔIl27ra mice were induced for EAE. Osmotic pump containing IL-27 was subcutaneously implanted (n = 9 ∼ 12) or sham surgery (n = 5 ∼ 6) was performed at 8 d post induction. The development of EAE was monitored. (B and C) Spinal cord cells from sham or IL-27–treated TregΔIl27ra and TregWT mice were ex vivo-stimulated, and intracellular IFNγ/IL-17 (B) and IL-10 (C) expression of CD4 T cells was determined by FACS analysis. The results shown are the mean ± SD of two to three independent experiments. (D) B6 mice (n = 5) were induced for EAE, and an osmotic pump containing IL-27 was implanted as described above. Anti–IL-10/anti–IL-10R mAbs (250 μg each) or control Ab was injected every 3 d. The development of EAE was monitored. *P < 0.05; **P < 0.01; ***P < 0.001.
Discussion
In earlier studies, susceptibility of Il27ra−/− mice to EAE was attributed to increased generation of IL-17+ CD4 T cells (12). Suppressive functions of Il27ra−/− Tregs measured in vitro remained intact. It was thus concluded that IL-27 acting on conventional effector T cells suppresses the generation of encephalitogenic Th17-type CD4 T cells (12). The loss of IL-27 also results in enhanced pathology characterized by altered T-cell responses during infections (17, 24). In the current study, we show that IL-27 signaling in Foxp3+ Tregs is indispensable for Treg function to control autoimmune inflammation in the CNS. Analogous to Il27ra−/− mice, Treg-specific Il27ra−/− mice developed severe EAE. CNS-infiltrating CD4 T cells displaying proinflammatory Th1/Th17 phenotypes were increased. Despite the severe disease in Treg-specific Il27ra−/− mice, CD4 T cells expressing IL-10 were abundant and the IL-10 level in the CNS homogenates was comparable. Systemic rIL-27 administration did not attenuate ongoing EAE when Tregs lacked IL-27Rα. Since conventional CD4 T cells express functional IL-27Rα under this condition, these results strongly suggest that IL-27 plays a nonredundant role in Treg functions to control autoimmune inflammation irrespective of IL-10+ conventional T cells.
The precise mechanisms by which IL-27 controls in vivo Treg function need further investigation. We recently found that Lag3 expression on Tregs is necessary for optimal Treg-suppressive function (13). Lag3 is one of the molecules highly up-regulated by IL-27 stimulation in Tregs (13, 17), and Lag3−/− Tregs are partially impaired in preventing T-cell–induced colitis (13), suggesting that Lag3 expression on Tregs induced in part via IL-27 stimulation may be critical for Tregs to control inflammation. In support of this possibility, Lag3 expression of Tregs but not of Foxp3− CD4 T cells in the inflamed CNS tissues was significantly lower in Treg-specific Il27ra−/− mice. Furthermore, Lag3 overexpression in Tregs was sufficient to improve their suppressive function, supporting a potential contribution of Lag3 to Treg functions. Unlike conventional CD4 T cells, in which Lag3 inhibits TCR signaling and T-cell activation (25), Lag3 may play a distinct role in Tregs by promoting Treg functions. It is also possible that Lag3 may inactivate APC function by engaging its ligand, MHCII, and inhibiting immune responses (26).
While IL-10 produced by conventional CD4 T cells has been implicated in the regulation of autoimmunity, IL-10 fails to control Th17-biased EAE (27). We similarly observed that the level of IL-10+ conventional CD4 T cells is comparable or even greater in TregΔIl27ra mice that develop severe inflammation even after systemic rIL-27 administration. It is thus possible that IL-27 signaling in Tregs may be critical especially in limiting encephalitogenic Th17 immune responses, after which IL-10+ conventional CD4 T cells may acquire regulatory functions to limit “Th1-type” responses. When Tregs are unable to receive IL-27 signals, “Th17-type” effector cells become resistant to IL-10, resulting in uncontrolled disease.
The finding that more Foxp3- exTregs accumulate in the inflamed tissues of TregΔIl27ra mice led us to conclude that IL-27 signaling in Tregs may control Treg stability. IL-27 signaling may induce factors that prevent Tregs from losing Foxp3 expression or that interfere with the action of inflammatory mediators that destabilize the Treg phenotypes (2). However, we found it interesting that the frequency of exTregs in the current study is rather high, considering that exTregs usually occur at a low frequency. Are these cells genuine exTregs? This becomes an important question of future investigation as exTregs may arise from activated conventional effector cells that “transiently” express Foxp3, during which they lose IL-27Rα (28). The precise origin of the exTreg phenotype cells during autoimmune inflammation remains to be carefully determined.
There is increasing evidence that IL-27 has broader effects in Treg homeostasis (5). IL-27 regulates Treg expansion by controlling IL-2 production (29). It promotes the emergence of a T-bet+, CXCR3+ Treg subset that suppresses parasite-specific effector responses at the sites of inflammation (17). IL-27 is also capable CTLA-4/CTLA-4 expression in collagen-induced arthritis (30), although the lack of IL-27R does not affect their expression under steady-state conditions (13). IL-27 induces multiple factors including chemokines (CCL3 and CCL4) in Tregs (13). It was recently shown that chemokines (especially CCL3 and CCL4) produced by Tregs play a key role in attracting pathogenic T cells for better suppression (31). Therefore, IL-27 may provide an upstream signal that attracts effector T cells to the proximity and subsequently potentiates Treg suppression via a contact-dependent mechanism, possibly through Lag3. IL-27 enhances IL-10 expression by Tregs; however, we previously reported that IL-10 secretion by Tregs is dispensable for Treg suppression of effector T-cell proliferation and cytokine production in a model of colitic inflammation (13). Indeed, IL-10 expression by Tregs is not altered by functionally defective Il27ra−/− Tregs, suggesting that an alternative mechanism rather than IL-10 production may be more crucial for IL-27–dependent Treg functions (27). IL-27 induces T-cell expression of TIM-3, an inhibitory receptor that interacts with its ligand, galectin-9, to suppress T-cell function (32). However, we found no evidence that IL-27 stimulation induces TIM-3 expression in Foxp3+ Tregs. A naturally occurring soluble form of IL-27Rα binds IL-27 and acts as a natural IL-27 antagonist (33). However, we think it unlikely as this should affect IL-27 signaling on all T-cell types in TregWT and TregΔIl27ra groups.
Treg functions can be compromised by inflammatory mediators, and such defective Treg functions can contribute to the manifestation of the diseases (34, 35). Our findings provide a rationale to use IL-27 as a tool to target Tregs to improve Treg-mediated regulation of chronic inflammation seen in autoimmune diseases including multiple sclerosis.
Methods
Mice.
B6 and B6LSL-tdtomato (B6-Gt(Rosa)26Sortm9(CAG-tdTomato)/J) mice were purchased from the Jackson Laboratory. Germline B6 Il27ra−/− mice were obtained from Amgen. Foxp3CreYFP mice were purchased from the Jackson Laboratory. Generation of mice carrying floxed Il27ra alleles was carried out as follows. A targeting vector was constructed by cloning fragments of the Il27ra gene amplified from a bacterial artificial chromosome library into a pNLF vector. A neomycin resistance gene cassette flanked by FRT sites was located 3′ of the targeted exons 7 and 8, which are also flanked by two LoxP sites. An expression cassette for the diphtheria toxin A chain was placed 3′ of the construct to negatively select homologous recombination. Murine B6 embryonic stem cells were electroporated with the construct and selected by using G418. DNA was extracted from G418-selected clones and tested for Southern blot analysis after MfeI and KpnI digestion. The presence of LoxP sites was genotyped using the following primers: 5′-TAGGTGCAGGATCAAAGCTGC-3′ and 5′-AGAAGCCTGGTGTGCTAGCC-3′. Il27ra-targeted mice were obtained and then crossed to C57BL/6-FLP transgenic mice (purchased from Jax). Mice carrying the Il27ra-floxed allele without the neomycin gene cassette were further crossed to Foxp3CreGFP mice to generate Treg-specific Il27ra−/− mice. The following primers were used for genotyping: 5′-TGTTGCATCTGCTGACTTTGG-3′ and 5′-AAGGAGTCAGGTATGGTGGC-3′. All animal experiments were performed in accordance with approved protocols for the Institutional Animal Care and Usage Committee of the Cleveland Clinic Foundation.
EAE Induction.
To induce EAE, mice were subcutaneously immunized with 300 μg MOG 35–55 peptide emulsified in incomplete Freund’s adjuvant (Sigma-Aldrich) supplemented with 5 mg/mL of Mycobacterium tuberculosis (H37Ra; Difco). Pertussis toxin (200 ng in 200 μL PBS; Sigma-Aldrich) was injected intraperitoneally on the day of immunization. Mice received a subcutaneous booster MOG 35–55 immunization on day 7. Animals were scored for clinical symptoms as follows: 0, no signs of disease; 1, flaccid tail or hind-limb weakness; 2, flaccid tail and hind-limb weakness and loss of righting reflex; 3, partial hind-limb paralysis; 4, complete hind-limb paralysis; 5, moribund or dead. In some experiments, an Alzet miniosmotic pump (Durect corporation) containing 400 ng carrier-free IL-27 (R&D Systems) was subcutaneously implanted 8 d post immunization. Control mice were anesthetized, and a sham surgery was made on the back instead. In some experiments, mice received anti–IL-10 (JES5-2A5) and anti–IL-10R (1B1.3A) mAbs (250 μg each) every 3 d.
Flow Cytometry.
For details of flow cytometry, see SI Methods.
ELISA.
For details of ELISA, see SI Methods.
Quantitative PCR.
CNS tissue was disrupted using a TissueLyser II (Qiagen). Total RNA was extracted from the tissue using a GeneJet RNA Purification Kit (Thermo Scientific). cDNA was subsequently synthesized using a SuperScript III reverse transcriptase (Invitrogen). Real-time quantitative PCR was performed using gene-specific primers and probe sets (Applied Biosystem) and an ABI 7500 PCR machine (Applied Biosystem). All values were normalized to the Gapdh expression.
Histology.
For details of the histology, SI Methods.
Retroviral Transduction and in Vitro Suppression Assay.
For details of the retroviral transduction and in vitro suppression assay, see SI Methods.
Statistics.
Statistical significance was determined by the Student’s t test or one-way ANOVA using the Prism 4 software (GraphPad). A P value of <0.05 was considered statistically significant.
SI Methods
Flow Cytometry.
Single cells were incubated with specific Ab together with rat anti-mouse FcγIII/II mAb (2.4G2) for 15 min on ice to minimize nonspecific binding. All of the antibodies used in this study were purchased from BD Biosciences or eBioscience. Antibodies specific for CD4 (RM4-5), CD8 (53–6.7), CD44 (IM7), CD62L (MEL-14), IL-27Rα (2918; BD), IL-10 (JES5-16E3), IL-17A (eBio17B7), IFNγ (XMG1.2), Foxp3 (FJK-16s), TIM3 (RMT3-23), and Lag-3 (C9B7W) mAbs were used. Cells were acquired using a FACS LSR II (BD Biosciences) and analyzed using FlowJo software (Treestar). For intracellular cytokine staining, isolated cells were ex vivo-stimulated with phorbol myristate acetate (10 ng/mL) and ionomycin (1 μM) for 4 h in the presence of 2 μM monensin (Calbiochem) during the last 2 h of stimulation. Cells were immediately fixed with 4% paraformaldehyde, permeabilized, and stained with fluorescence-conjugated antibodies.
ELISA.
Harvested brain tissues were homogenized in PBS. The homogenates were centrifuged at 10,000 × g for 10 min at 4 °C, and the supernatants were stored at −20 °C. IL-10 was measured by ELISA. In some experiments, CNS cells were incubated with MOG peptide for 3 d. MOG-specific IL-17 secretion in the culture supernatant was measured by ELISA. Abs used for the assays were purchased from eBioscience.
Histology.
Sections were fixed in acidic methanol (60% methanol, 10% acetic acid, 30% water), embedded in paraffin, and sectioned at 5 μm thickness. Foxp3 and CD3 T cells were demonstrated by a sequential double-staining method using rat monoclonal antibody to Foxp3 (clone: FJK-16s; eBioscience) and Rabbit polyclonal antibody to CD3 (Abcam) after heat-induced antigen retrieval. As secondary antibodies, Rat HRP-Polymer (Biocare Medical) was used for Foxp3 and Rabbit AP-Polymer (Biocare Medical) for CD3. Foxp3+ cells were stained with Vina Green chromogen (Biocare Medical) and CD3+ cells with Vulcan Fast Red (Biocare Medical). Hematoxylin was used for nuclear counterstain.
Genomic DNA qPCR.
Naive CD4 T cells (Foxp3− CD44low) and Tregs (Foxp3+) were FACS-sorted from B6, TregWT, and TregΔIl27ra mice. Genomic DNA was extracted using a genomic DNA isolation kit (Qiagen). The following primers were used to amplify exons 3 and 4 (control exons) and exons 7 and 8 (targeted exons): exon 3, sense 5′-ACAGAGTTGGGTGACCATTC-3′ and antisense 5′-AGGTTCACAGAG ACA GAG GA-3′; exon 4, sense 5′-CCAGATCTTCTCTCAAGTGGATATT-3′ and antisense 5′-CCGGAACTGACAGATGAGAAC-3′; exon 7, sense 5′-TGACTTACACAGTCTGGTTTGG-3′ and antisense 5′-GTGCTGTTGCCAGGAGAG-3′; exon 8, sense 5′-ATGGGAGCCCAGGGATAA-3′ and antisense 5′-CAGGCTGTCACCATCTTGAG-3′.
Retroviral Transduction and in Vitro Suppression Assay.
HEK 293T cells were transiently transfected using Fugene (Promega) with pMSCV-IRES-GFP (pMIG II) vector expressing LAG3. Control empty vector was used as a negative control. Naive CD4 T cells were activated in the presence of IL-2 and TGFβ to generate inducible Foxp3+ Tregs. Cells were subsequently transduced with retroviruses and polybrene (8 μg/mL) 24 h following activation. Transduced cells were cultured for an additional 2 d, and then FACS-sorted GFP+ cells were tested for in vitro suppression. Naive CD4 T cells labeled with carboxyfluorescein succinimidyl ester (Molecular Probes) were cocultured with T-cell–depleted splenocytes with anti-CD3 mAbs in the presence of a different number of transduced inducible Tregg cells for 3 d. Relative proliferation was calculated based on T-cell proliferation without Tregs.
Acknowledgments
We thank Jennifer Powers for cell sorting. This study was supported by National Multiple Sclerosis Society Grant RG-1411-02051 (to B.M.); the Crohn’s Colitis Foundation of America (B.M.); the American Asthma Foundation (B.M.); the Velosano Foundation (B.M.); and NIH Grants AI121524 and AI125247 (to B.M.) and CA203689 and AI108545 (to D.A.A.V.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. V.K.K. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1703100114/-/DCSupplemental.
References
- 1.Long SA, Buckner JH. CD4+FOXP3+ T regulatory cells in human autoimmunity: More than a numbers game. J Immunol. 2011;187:2061–2066. doi: 10.4049/jimmunol.1003224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pasare C, Medzhitov R. Toll pathway-dependent blockade of CD4+CD25+ T cell-mediated suppression by dendritic cells. Science. 2003;299:1033–1036. doi: 10.1126/science.1078231. [DOI] [PubMed] [Google Scholar]
- 3.Korn T, et al. Myelin-specific regulatory T cells accumulate in the CNS but fail to control autoimmune inflammation. Nat Med. 2007;13:423–431. doi: 10.1038/nm1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hunter CA, Kastelein R. Interleukin-27: Balancing protective and pathological immunity. Immunity. 2012;37:960–969. doi: 10.1016/j.immuni.2012.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yoshida H, Hunter CA. The immunobiology of interleukin-27. Annu Rev Immunol. 2015;33:417–443. doi: 10.1146/annurev-immunol-032414-112134. [DOI] [PubMed] [Google Scholar]
- 6.Do JS, Visperas A, Oh K, Stohlman SA, Min B. Memory CD4 T cells induce selective expression of IL-27 in CD8+ dendritic cells and regulate homeostatic naive T cell proliferation. J Immunol. 2012;188:230–237. doi: 10.4049/jimmunol.1101908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pennock ND, Gapin L, Kedl RM. IL-27 is required for shaping the magnitude, affinity distribution, and memory of T cells responding to subunit immunization. Proc Natl Acad Sci USA. 2014;111:16472–16477. doi: 10.1073/pnas.1407393111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Takeda A, et al. Cutting edge: Role of IL-27/WSX-1 signaling for induction of T-bet through activation of STAT1 during initial Th1 commitment. J Immunol. 2003;170:4886–4890. doi: 10.4049/jimmunol.170.10.4886. [DOI] [PubMed] [Google Scholar]
- 9.Diveu C, et al. IL-27 blocks RORc expression to inhibit lineage commitment of Th17 cells. J Immunol. 2009;182:5748–5756. doi: 10.4049/jimmunol.0801162. [DOI] [PubMed] [Google Scholar]
- 10.Awasthi A, et al. A dominant function for interleukin 27 in generating interleukin 10-producing anti-inflammatory T cells. Nat Immunol. 2007;8:1380–1389. doi: 10.1038/ni1541. [DOI] [PubMed] [Google Scholar]
- 11.Fitzgerald DC, et al. Suppression of autoimmune inflammation of the central nervous system by interleukin 10 secreted by interleukin 27-stimulated T cells. Nat Immunol. 2007;8:1372–1379. doi: 10.1038/ni1540. [DOI] [PubMed] [Google Scholar]
- 12.Batten M, et al. Interleukin 27 limits autoimmune encephalomyelitis by suppressing the development of interleukin 17-producing T cells. Nat Immunol. 2006;7:929–936. doi: 10.1038/ni1375. [DOI] [PubMed] [Google Scholar]
- 13.Do JS, et al. An IL-27/Lag3 axis enhances Foxp3+ regulatory T cell-suppressive function and therapeutic efficacy. Mucosal Immunol. 2016;9:137–145. doi: 10.1038/mi.2015.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yoshida H, et al. WSX-1 is required for the initiation of Th1 responses and resistance to L. major infection. Immunity. 2001;15:569–578. doi: 10.1016/s1074-7613(01)00206-0. [DOI] [PubMed] [Google Scholar]
- 15.Franckaert D, et al. Promiscuous Foxp3-cre activity reveals a differential requirement for CD28 in Foxp3+ and Foxp3− T cells. Immunol Cell Biol. 2015;93:417–423. doi: 10.1038/icb.2014.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Villarino AV, et al. IL-27 limits IL-2 production during Th1 differentiation. J Immunol. 2006;176:237–247. doi: 10.4049/jimmunol.176.1.237. [DOI] [PubMed] [Google Scholar]
- 17.Hall AO, et al. The cytokines interleukin 27 and interferon-γ promote distinct Treg cell populations required to limit infection-induced pathology. Immunity. 2012;37:511–523. doi: 10.1016/j.immuni.2012.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Komatsu N, et al. Pathogenic conversion of Foxp3+ T cells into TH17 cells in autoimmune arthritis. Nat Med. 2014;20:62–68. doi: 10.1038/nm.3432. [DOI] [PubMed] [Google Scholar]
- 19.Rubtsov YP, et al. Regulatory T cell-derived interleukin-10 limits inflammation at environmental interfaces. Immunity. 2008;28:546–558. doi: 10.1016/j.immuni.2008.02.017. [DOI] [PubMed] [Google Scholar]
- 20.Anderson AC, Anderson DE. TIM-3 in autoimmunity. Curr Opin Immunol. 2006;18:665–669. doi: 10.1016/j.coi.2006.09.009. [DOI] [PubMed] [Google Scholar]
- 21.Zhu C, et al. An IL-27/NFIL3 signalling axis drives Tim-3 and IL-10 expression and T-cell dysfunction. Nat Commun. 2015;6:6072. doi: 10.1038/ncomms7072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhou X, et al. Instability of the transcription factor Foxp3 leads to the generation of pathogenic memory T cells in vivo. Nat Immunol. 2009;10:1000–1007. doi: 10.1038/ni.1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fitzgerald DC, et al. Suppressive effect of IL-27 on encephalitogenic Th17 cells and the effector phase of experimental autoimmune encephalomyelitis. J Immunol. 2007;179:3268–3275. doi: 10.4049/jimmunol.179.5.3268. [DOI] [PubMed] [Google Scholar]
- 24.de Aquino MT, et al. IL-27 limits central nervous system viral clearance by promoting IL-10 and enhances demyelination. J Immunol. 2014;193:285–294. doi: 10.4049/jimmunol.1400058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hannier S, Tournier M, Bismuth G, Triebel F. CD3/TCR complex-associated lymphocyte activation gene-3 molecules inhibit CD3/TCR signaling. J Immunol. 1998;161:4058–4065. [PubMed] [Google Scholar]
- 26.Liang B, et al. Regulatory T cells inhibit dendritic cells by lymphocyte activation gene-3 engagement of MHC class II. J Immunol. 2008;180:5916–5926. doi: 10.4049/jimmunol.180.9.5916. [DOI] [PubMed] [Google Scholar]
- 27.Fitzgerald DC, et al. Independent and interdependent immunoregulatory effects of IL-27, IFN-β, and IL-10 in the suppression of human Th17 cells and murine experimental autoimmune encephalomyelitis. J Immunol. 2013;190:3225–3234. doi: 10.4049/jimmunol.1200141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Miyao T, et al. Plasticity of Foxp3(+) T cells reflects promiscuous Foxp3 expression in conventional T cells but not reprogramming of regulatory T cells. Immunity. 2012;36:262–275. doi: 10.1016/j.immuni.2011.12.012. [DOI] [PubMed] [Google Scholar]
- 29.Wojno ED, et al. A role for IL-27 in limiting T regulatory cell populations. J Immunol. 2011;187:266–273. doi: 10.4049/jimmunol.1004182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moon SJ, et al. In vivo action of IL-27: Reciprocal regulation of Th17 and Treg cells in collagen-induced arthritis. Exp Mol Med. 2013;45:e46. doi: 10.1038/emm.2013.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Patterson SJ, et al. T regulatory cell chemokine production mediates pathogenic T cell attraction and suppression. J Clin Invest. 2016;126:1039–1051. doi: 10.1172/JCI83987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhu C, et al. The Tim-3 ligand galectin-9 negatively regulates T helper type 1 immunity. Nat Immunol. 2005;6:1245–1252. doi: 10.1038/ni1271. [DOI] [PubMed] [Google Scholar]
- 33.Dietrich C, Candon S, Ruemmele FM, Devergne O. A soluble form of IL-27Rα is a natural IL-27 antagonist. J Immunol. 2014;192:5382–5389. doi: 10.4049/jimmunol.1303435. [DOI] [PubMed] [Google Scholar]
- 34.Viglietta V, Baecher-Allan C, Weiner HL, Hafler DA. Loss of functional suppression by CD4+CD25+ regulatory T cells in patients with multiple sclerosis. J Exp Med. 2004;199:971–979. doi: 10.1084/jem.20031579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Haas J, et al. Reduced suppressive effect of CD4+CD25high regulatory T cells on the T cell immune response against myelin oligodendrocyte glycoprotein in patients with multiple sclerosis. Eur J Immunol. 2005;35:3343–3352. doi: 10.1002/eji.200526065. [DOI] [PubMed] [Google Scholar]