Significance
The prevalent habitat of Escherichia coli is the predominantly anaerobic environment of the gastrointestinal tract of humans and other warm-blooded organisms. We found that, under anaerobic conditions, the presence of enolase in the RNA degradation machinery regulates cell morphology and induces E. coli filamentation by stabilizing a small RNA, DicF, that inhibits the cell division gene ftsZ. Cell filamentation has previously been linked to bacterial pathogenesis. In contrast to E. coli nonpathogenic strains, pathogenic E. coli strains possess multiple copies of sRNA DicF in their genomes. Our data provide a mechanism by which bacterial cells can adopt a filamentous form during infection under anaerobic conditions.
Keywords: RNase E, RNA decay, protein subcellular distribution, anaerobic conditions, cell shape
Abstract
Escherichia coli RNase E is an essential enzyme that forms multicomponent ribonucleolytic complexes known as “RNA degradosomes.” These complexes consist of four major components: RNase E, PNPase, RhlB RNA helicase, and enolase. However, the role of enolase in the RNase E/degradosome is not understood. Here, we report that presence of enolase in the RNase E/degradosome under anaerobic conditions regulates cell morphology, resulting in E. coli MG1655 cell filamentation. Under anaerobic conditions, enolase bound to the RNase E/degradosome stabilizes the small RNA (sRNA) DicF, i.e., the inhibitor of the cell division gene ftsZ, through chaperon protein Hfq-dependent regulation. RNase E/enolase distribution changes from membrane-associated patterns under aerobic to diffuse patterns under anaerobic conditions. When the enolase-RNase E/degradosome interaction is disrupted, the anaerobically induced characteristics disappear. We provide a mechanism by which E. coli uses enolase-bound degradosomes to switch from rod-shaped to filamentous form in response to anaerobiosis by regulating RNase E subcellular distribution, RNase E enzymatic activity, and the stability of the sRNA DicF required for the filamentous transition. In contrast to E. coli nonpathogenic strains, pathogenic E. coli strains predominantly have multiple copies of sRNA DicF in their genomes, with cell filamentation previously being linked to bacterial pathogenesis. Our data suggest a mechanism for bacterial cell filamentation during infection under anaerobic conditions.
Posttranscriptional regulation of RNAs is an important molecular mechanism for controlling gene expression, requiring various ribonucleases (RNases), including RNase E, which is an essential single-stranded endo-RNase involved in RNA processing and decay (1). RNase E has N-terminal catalytic and C-terminal scaffolding domains (2), with the latter responsible for assembling multicomponent ribonucleolytic complexes termed “RNA degradosomes.” Degradosomes consist of RNase E, PNPase 3′→5′ exoribonuclease, RhlB RNA helicase, and the glycolytic enzyme enolase (3, 4). Therefore, they can act on RNA internally (by RNase E) and/or externally (by PNPase) to catalyze the degradation of RNA into short fragments. Immunogold electron microscopy has shown that degradosomes exist in vivo and are tethered to the cytoplasmic membrane through the N-terminal region of RNase E (5). Binding of the N-terminal catalytic domain (amino acids 1–499) to the membrane stabilizes protein structure and increases both RNA cleavage activity and substrate affinity (6). Global analyses of aerobic Escherichia coli RNA degradosome functioning using DNA microarrays showed that decay of some mRNAs in vivo depends on the action of assembled degradosomes, whereas other mRNAs are impacted by degradosome proteins functioning independently of the complex (7–9). Some minor components of the degradosome, such as the inhibitors of RNase E, RraA and RraB (10), and ribosomal protein L4 (11), affect the stability of subsets of transcripts. Structural features or biochemical factors that target specific classes of mRNAs for degradosomal decay may exist.
E. coli is a metabolically versatile bacterium that is able to grow under aerobic and anaerobic conditions. Adaptation to environments with different O2 concentrations, which is vital for E. coli competitiveness and growth, requires reprogramming of gene expression and cell metabolism. E. coli uses one of three metabolic modes to support growth (12, 13), which depend on the availabilities of electron donors and acceptors. In the presence of O2, aerobic respiration allows complete oxidation of a growth substrate (such as glucose) and therefore is the most productive mode. Two alternative metabolic modes are available in the absence of O2, one of which is anaerobic respiration, which yields less energy than aerobic respiration because the substrate is only partially oxidized. The other O2-deficient mode is fermentation, which is the least productive mode since energy is generated only by substrate level phosphorylation. Thus, changes in E. coli physiology are provoked by changes in O2 availability.
The discovery of the multicomponent ribonucleolytic complexes associated with E. coli RNase E and their extensive characterization in vivo and in vitro have yielded a wealth of information regarding the structure and function of the complexes under aerobic growth conditions (see ref. 14 for a review). Enolase is a key enzyme of glycolysis, a process that generates ATP by converting glucose to pyruvate in either the presence (aerobic) or absence (anaerobic) of oxygen. Anaerobic glycolysis is thought to have been the primary means of energy production in ancient organisms before oxygen was at high atmospheric concentrations. This metabolic pathway is particularly essential under the anaerobic conditions faced by E. coli and other pathogenic bacteria in the intestine. In this paper we address the specific function of enolase in the bacterial degradosome under anaerobic growth (sometimes also referred to as “oxygen-limited growth”) conditions.
We found that under anaerobic conditions E. coli MG1655 cells are characterized by a predominantly (∼70%) filamentous morphology (>5 µm in length). Our study shows that in response to oxygen-limited conditions concentrations of RNase E protein are decreased, and its subcellular distribution is altered. We demonstrate that the anaerobically induced filamentous morphology is the result of a specific function of the enolase-bound RNA degradosome through small RNA DicF stabilization and FtsZ protein expression. Our results demonstrate the unique role of enolase for RNase E/degradosome-based regulation of bacterial morphology in response to oxygen-limited conditions and may provide a mechanistic explanation for some virulent E. coli strains whose morphological differentiation from rod to filamentous shape occurs under significantly low oxygen tension.
Results
RNase E Regulates Cell Filamentous Morphology and Is Oxygen-Level Dependent.
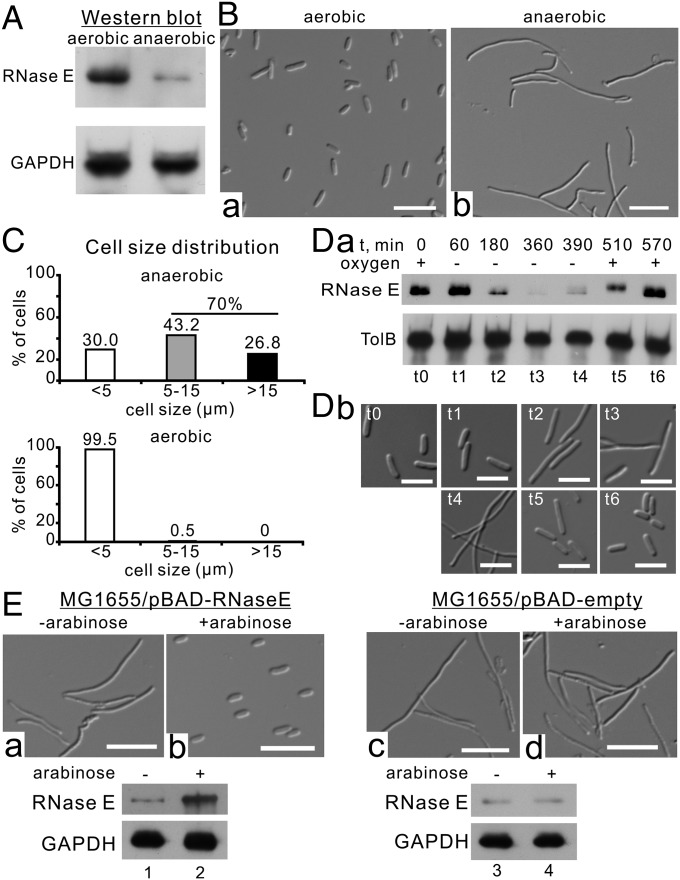
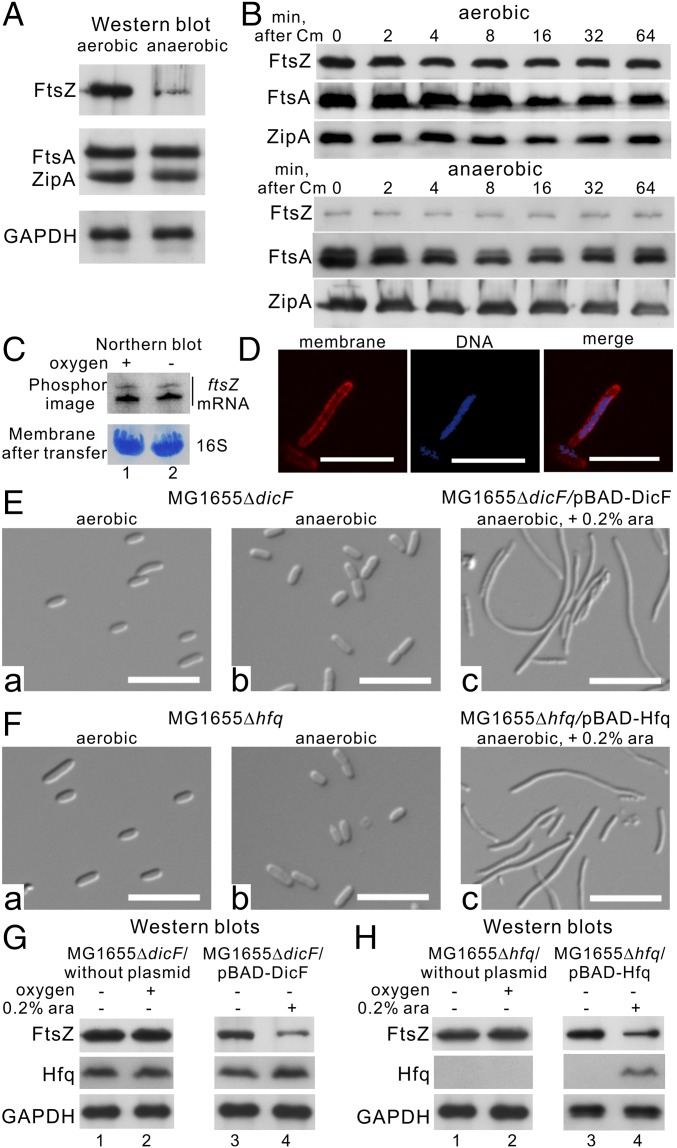
We determined RNase E protein levels in E. coli MG1655 under aerobic and anaerobic conditions by Western blotting and found that RNase E protein levels were significantly (∼4.0-fold) lower under anaerobic conditions (Fig. 1A and Fig. S1A). The decreased level of RNase E is due to active degradation of the protein (Fig. S1B). Microscopic observations showed that under anaerobic conditions E. coli MG1655 cells are characterized by a filamentous morphology (Fig. 1B), with ∼70% of the cells being >5 µm in length (Fig. 1C, Upper). In comparison, ∼99.5% of cells of the same strain grown under aerobic conditions are <5 µm long (Fig. 1C, Lower). The cells that are filamenting show OD increases for a long time, but the increase was slower than in cells grown under aerobic conditions (Fig. S2A).
Fig. 1.
RNase E is a key regulator of cell filamentation under anaerobic conditions. (A) Endogenous expression levels of RNase E under aerobic and anaerobic conditions. Whole-cell extracts were analyzed by SDS/PAGE and immunoblotted with the indicated antibodies. (B) E. coli MG1655 cell morphology under aerobic (B, a) and anaerobic (B, b) conditions. (Scale bars, 5 µm.) (C) Cell-size distribution of E. coli MG1655 cells under aerobic and anaerobic conditions. The lengths of cells were measured using the image analysis package MetaMorph (SI Materials and Methods). (D) RNase E protein levels (D, a) and cell morphology (D, b) under aerobic–anaerobic–aerobic alternating growth conditions. (Scale bars, 5 µm.) (E) Arabinose-induced expression of RNase E under anaerobic conditions. (Upper) Cell morphology. (Scale bars, 5 µm.) (Lower) RNase E protein levels.
Fig. S1.
RNase E protein levels and stabilities under aerobic and anaerobic conditions. (A) RNase E endogenous protein levels under aerobic (lines 1–4) and anaerobic (lane 5) conditions. Whole-cell extracts were analyzed by 10% SDS/PAGE and immunoblotted with the indicated antibodies. RNase E protein levels were significantly (∼4.0-fold) lower under anaerobic conditions. (B) RNase E protein stability under aerobic and anaerobic conditions. Cells were collected at the indicated times (0, 2, 4, 8, 16, and 32 min) after chloramphenicol (Cm) treatment, lysed, and assayed by Western blotting. The bands corresponding to endogenous RNase E and TolB (internal control) are indicated. RNase E is stable within 32 min under aerobic conditions, whereas the protein is completely degraded within 16 min under anaerobic conditions. (C) Flag-RNase E protein levels under aerobic–anaerobic–aerobic alternating growth conditions. Whole-cell extracts were analyzed by 10% SDS/PAGE and immunoblotted with the indicated antibodies. (D) Schematic representation of the aerobic–anaerobic–aerobic alternating experiment.
Fig. S2.
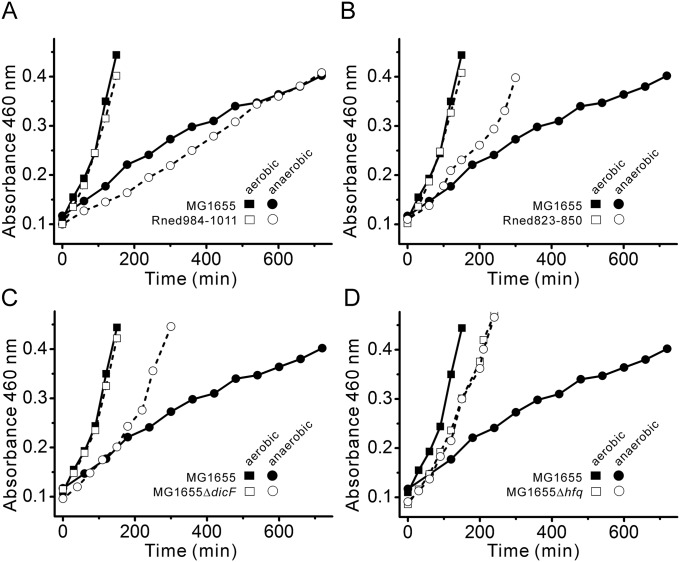
Growth curves of E. coli MG1655 and its isogenic mutant strains. Growth curves of E. coli MG1655 vs. Rned984–1011 (A), MG1655 vs. Rned823–850 (B), MG1655 vs. MG1655ΔdicF (C), and MG1655 vs. MG1655Δhfq (D) under aerobic and anaerobic conditions. For comparison, aerobic and anaerobic growth curves of MG1655 from A were replotted in B, C, and D. Under aerobic conditions, the doubling times were ∼86, ∼85, ∼84, ∼82, and ∼96 min for MG1655, Rned984–1011, Rned823–850, MG1655ΔdicF, and MG1655Δhfq, respectively. Under anaerobic conditions, the doubling times were ∼350, ∼333, ∼155, ∼141, and ∼100 min for MG1655, Rned984–1011, Rned823–850, MG1655ΔdicF, and MG1655Δhfq, respectively. For E. coli MG1655 grown under aerobic conditions, the number of cells was ∼3.3-fold greater than under anaerobic conditions (∼2.46 × 107 vs. ∼0.75 × 107 cells/mL of culture, respectively) (SI Materials and Methods).
Using aerobic–anaerobic–aerobic alternating growth conditions (Fig. S1D), we found that protein levels of chromosomal (untagged) or ectopically expressed RNase E using an arabinose-inducible promoter from the pBAD plasmid (Flag-tagged) decreased after shifting to anaerobic conditions but then were restored after reverting to aerobic conditions (Fig. 1 D, a, lanes t1–t6 and Fig. S1C, respectively), indicating that the amount of RNase E is correlated to the amount of oxygen. This additional experiment indicates that decreased RNase E protein levels are mainly due to active degradation of the protein. Cell morphology also cycled through rod–filamentous–rod shapes in accordance with aerobic–anaerobic–aerobic conditions (Fig. 1 D, b). Cell filamentation was completely resolved within ∼3 h after shifting back to aerobic conditions or on RNase E overexpression (Fig. 1 E, a and b and Movie S1). In contrast, cells transformed with the empty pBAD vector maintained a filamentous morphology under the same culture conditions (Fig. 1 E, c and d). Thus, our data demonstrate that acquisition of a filamentous morphology under anaerobic conditions is correlated with reduced RNase E protein levels.
Cell Filamentation Under Anaerobic Conditions Requires Enolase-Bound RNA Degradosomes.
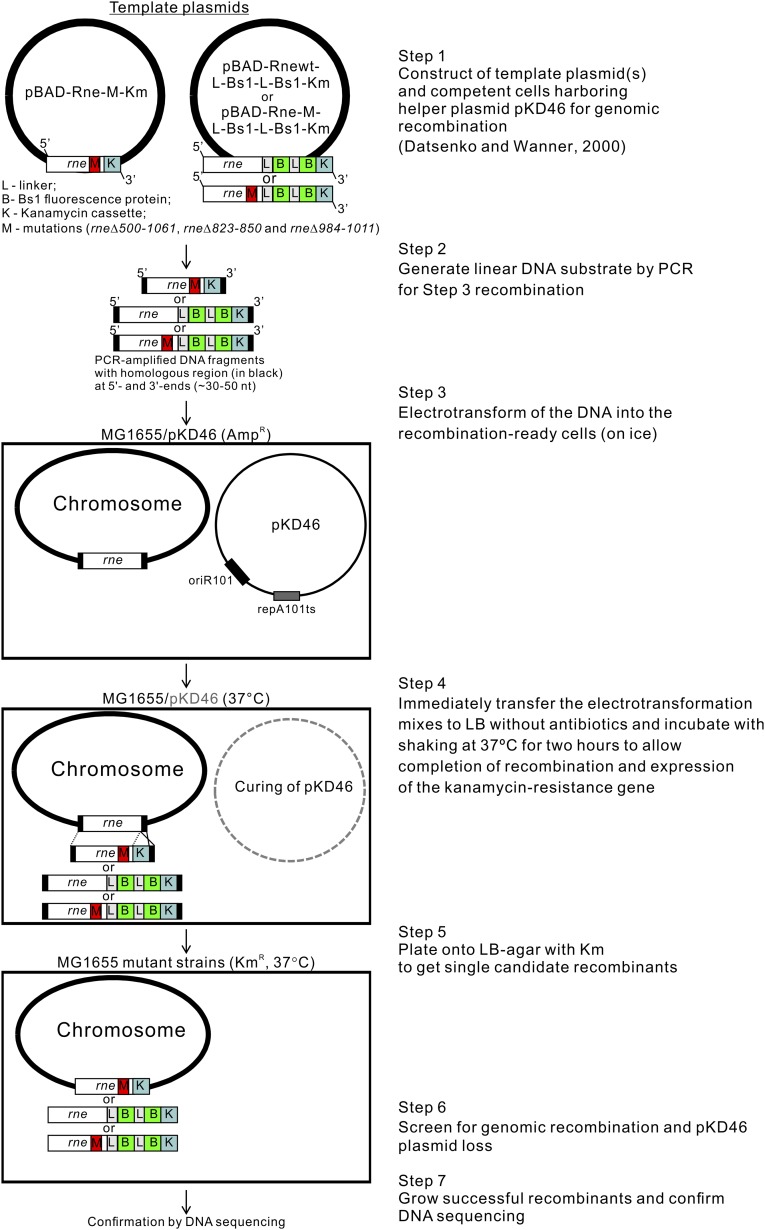
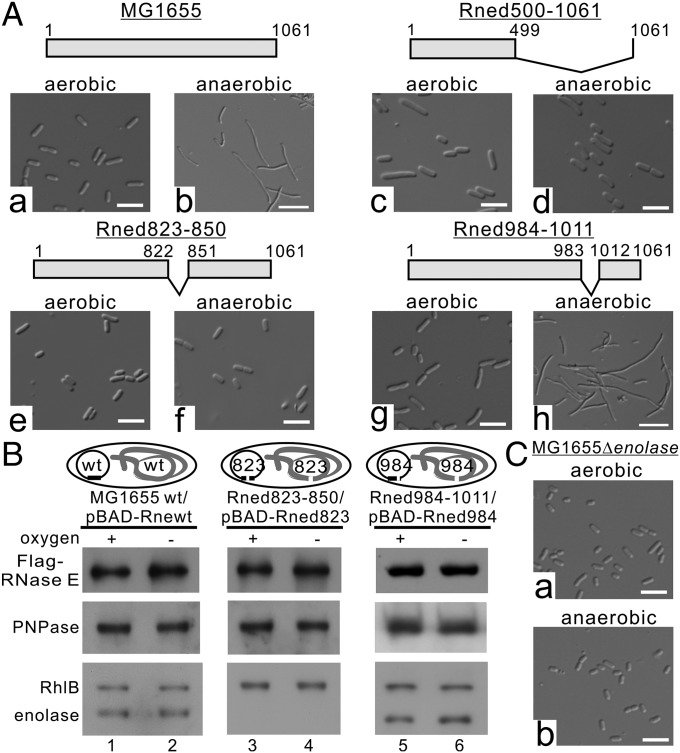
Since RNase E exists as multienzyme complexes (the RNA degradosomes) in cells (5), we investigated the possibility that RNA degradosomes are involved in E. coli acquiring the filamentous morphology under anaerobic conditions. We used the E. coli MG1655 strain to construct defined isogenic mutant strains (Fig. S3). We generated a MG1655 derivative that lacked degradosome formation by deleting amino acid residues 500–1,061 of the scaffolding domain of RNase E (hereafter Rned500–1061; see SI Materials and Methods). Rned500–1061 possesses only the N-terminal 499-aa residues of RNase E (Fig. 2 A, c and d). As shown in Fig. 2, under anaerobic conditions, Rned500–1061 had uniformly rod-shaped cells (∼2.0–2.5 µm) (Fig. 2 A, c and d), in contrast to MG1655 (Fig. 2 A, a and b), suggesting involvement of degradosome components in cell filamentation under anaerobic conditions. Since enolase is essential under anaerobic conditions, we wondered whether enolase bound to RNase E/degradosomes plays a role in anaerobically induced cell filamentation. We generated another strain (Rned823–850) in which the chromosome region encoding amino acid residues 823–850 of RNase E [constituting the RNase E microdomain for enolase recognition (15)] was deleted. The Rned823–850 strain could host PNPase and RhlB helicase, but not enolase, in degradosomes under both aerobic and anaerobic growth conditions (Fig. 2B, lanes 3 and 4). Like Rned500–1061, Rned823–850 exhibited a rod-shaped morphology under anaerobic conditions (Fig. 2 A, e and f). The growth rate of Rned823–850 was similar to that of MG1655 under aerobic conditions (Fig. S2B), but under anaerobic conditions Rned823–850 grew faster than MG1655 (Fig. S2B).
Fig. S3.
Schematic representation of the construction of mutants.
Fig. 2.
Cell filamentation under anaerobic conditions requires enolase binding to RNA degradosomes. (A) Cell morphology of E. coli MG1655 (a and b) and its derivative strains (c–h) under aerobic and anaerobic conditions. Schematic representations of RNase E and its endogenous (untagged) and plasmid-encoded (Flag-tagged) variants are presented above the respective images. (Scale bars, 5 µm.) (B) The degradosomes isolated from E. coli MG1655 and its derivatives, Rned823–500 and Rne984–1011. Protein aliquots containing equal amounts of Flag-RNase E and its variants were analyzed by SDS/PAGE and immunoblotted with the indicated antibodies. Schematic representations of RNase E and its endogenous (untagged) and plasmid-encoded (Flag-tagged) variants are presented above the respective images. (C) Cell morphology of the E. coli MG1655Δenolase mutant with endogenously deleted enolase under aerobic and anaerobic conditions. (Scale bars, 5 µm.)
We generated a control strain, Rned984–1011, in which the chromosome region encoding amino acid residues 984–1,011 of RNase E was deleted (SI Materials and Methods and Fig. S4). This region does not interfere with degradosome formation, and the protein ratios of RNase E, PNPase, enolase, and RhlB in degradosomes of this strain have a stoichiometry similar to that of the MG1655 strain (Fig. 2B, compare lanes 1 and 2 with lanes 5 and 6). Like the MG1655 strain, strain Rned984–1011 had a filamentous morphology under anaerobic conditions (Fig. 2 A, g and h). The growth rate of Rned984–1011 was similar to that of MG1655 under aerobic and anaerobic conditions (Fig. S2A), indicating that the deletion of residues 984–1,011 had no effect on cell growth.
Fig. S4.
De novo predicted structure of C-terminal RNase E. De novo predicted structure of C-terminal RNase E (A) and positions of regions (in red) that were deleted for mutant constructions (B–G). To minimize disruption of the predicted secondary structure of RNase E, we used unstructured regions or entire α- or β-structures for the construction of mutants. Based on growth characteristics and morphology under aerobic and anaerobic conditions, we used the Rned984–1011 mutant as a control strain in our experiments.
Since enolase is essential under anaerobic conditions to sustain bacterial growth using glucose as a carbon source but not using other carbon sources such as pyruvate, glycerate, or succinate, we performed a reciprocal experiment using a strain deleted for the eno gene (i.e., without enolase), grown with pyruvate-supplemented minimal medium, to address whether enolase is required for RNase E/degradosome anaerobically induced cell filamentation. This strain, MG1655Δeno, has intact full-length RNase E but lacks enolase and has a rod-shaped morphology, similar to the Rned500–1061 or Rned823–850 strains (Fig. 2C). Together, these data demonstrate that the filamentous morphology under anaerobic conditions requires enolase-bound RNA degradosomes.
Filamentous Cells Induced by Oxygen-Limited Conditions Have Limited Septum Formation and Decreased FtsZ Protein Expression Levels.
The filamentous morphology suggests limited septum formation under anaerobic conditions. Septum formation in E. coli begins by assembling three essential proteins—FtsZ, FtsA, and ZipA—to form a proto-ring attached to the midcell inner membrane (see ref. 16 for a review). As shown in Fig. 3A, FtsZ protein levels were significantly decreased in E. coli MG1655 under anaerobic conditions (in contrast, no difference in FtsA and ZipA protein levels was observed), suggesting that FtsZ total enzymatic activity is lower under anaerobic than in aerobic conditions. E. coli MG1655 also exhibited a limited capability for septum formation under anaerobic conditions (Fig. 3D), as is consistent with the reduced FtsZ levels. We confirmed that protein stability and abundance of ftsZ mRNA were equivalent under both aerobic and anaerobic conditions (Fig. 3 B and C). Therefore, anaerobic conditions result in the inhibition of FtsZ protein synthesis at the posttranscriptional level.
Fig. 3.
Both sRNA DicF and Hfq are necessary to reduce FtsZ protein levels, leading to cell filamentation under anaerobic conditions. (A) FtsZ, FtsA, and ZipA endogenous protein levels under aerobic and anaerobic conditions. Whole-cell extracts were analyzed by SDS/PAGE and immunoblotted with the indicated antibodies. (B) FtsZ, FtsA, and ZipA protein stability under both aerobic and anaerobic conditions. Cells were collected at the indicated times (0, 2, 4, 8, 16, 32, and 64 min) after chloramphenicol (Cm) treatment, lysed, and assayed by Western blotting. The bands corresponding to endogenous FtsZ, FtsA, and ZipA are indicated. (C) ftsZ mRNA steady-state levels under aerobic and anaerobic conditions. The bands corresponding to endogenous ftsZ mRNA (Northern blot) and 16S ribosomal RNA (membrane after transfer) are indicated. (D) Fluorescence images of anaerobically induced filamentous cells. Membrane (red) and DNA (blue) were visualized by FM 4-64 and Hoechst 33342 dyes, respectively. (E, a and b and F, a and b) Cell morphology of the MG1655ΔdicF and MG1655Δhfq mutants under aerobic and anaerobic conditions. (Scale bars, 5 µm.) (E, c and F, c) Cell morphology of the MG1655ΔdicF and MG1655Δhfq mutants after arabinose-induced expression of sRNA DicF (E, c) or Hfq (F, c), respectively, under anaerobic conditions. (Scale bars, 5 µm.) (G and H) Western blot analysis of the MG1655ΔdicF (G) and MG1655Δhfq (H) mutants under aerobic and anaerobic conditions and after arabinose-induced expression of sRNA DicF or Hfq under anaerobic conditions. The bands corresponding to endogenous FtsZ, Hfq, and GAPDH and ectopically expressed Hfq are indicated.
Both the sRNA DicF and Hfq Are Necessary to Reduce FtsZ Protein Levels and Induce Cell Filamentation Under Anaerobic Growth Conditions.
The sRNA DicF is responsible for decreased FtsZ protein levels through translational inhibition (17–19), so deletion of sRNA DicF should reveal a rod-shaped cell morphology in E. coli MG1655 under anaerobic conditions. Therefore, we analyzed the cell morphology of an endogenously deleted dicF strain, MG1655ΔdicF (20), under aerobic and anaerobic conditions. This strain exhibited a rod-shaped morphology (cell length ∼2.0–2.5 μm) (Fig. 3 E, a and b) under both aerobic and anaerobic conditions, and, tellingly, its FtsZ protein levels were unchanged under anaerobic conditions (Fig. 3G, lanes 1 and 2). The growth rate of MG1655ΔdicF was similar to that of MG1655 under aerobic conditions (Fig. S2C), but under anaerobic conditions MG1655ΔdicF grew faster than MG1655 (Fig. S2C), and its growth rate was similar to that of the Rned823–850 strain (compare Fig. S2 B and C).
Given that sRNA DicF is a known inhibitor of FtsZ (21), we first ectopically expressed sRNA DicF (53 nt) in MG1655ΔdicF using an arabinose-inducible promoter from the pBAD plasmid and observed that cells became filamentous aerobically (Fig. S5A). As in aerobically induced filamentation, cells regained their filamentous shape (cell length >5 μm) (Fig. 3 E, c), and FtsZ protein levels were reduced (Fig. 3G, lanes 3 and 4) following ectopic expression of sRNA DicF under anaerobic conditions. This aerobically or anaerobically induced filamentation by sRNA DicF overexpression was also observed in Rned823–850 cells (Fig. S5B), so it is not dependent upon the enolase-binding site in RNase E.
Fig. S5.
Cell morphologies of Rned823–850 and MG1655ΔdicF mutants upon arabinose-induced expression of sRNA DicF (53 nt) or its miniprecursors and DicF RNA amounts and stability in MG1655, Rned823–850, and N3431 strains. (A) Cell morphology of the MG1655ΔdicF mutants after arabinose-induced expression of sRNA DicF (53 nt) under aerobic conditions. (Scale bars, 5 µm.) (B) Cell morphology of the Rned823–850 mutants after arabinose-induced expression of sRNA DicF (53 nt) under aerobic (B, a) and anaerobic (B, b) conditions. (Scale bars, 5 µm.) (C, Right) Cell morphologies of E. coli MG1655ΔdicF before and after ectopic expression of the 63DicF1 DicF miniprecursor or a 63DicF2 variant under aerobic conditions. (Scale bars, 5 µm.) (Left) Northern blots upon arabinose ectopic expression under aerobic conditions. (Center) Schematic representations of the 63DicF1 DicF miniprecursor and the 63DicF2 variant and their RNase E cleavage sites. (D) Quantification of DicF RNA amounts under aerobic–anaerobic–aerobic alternating growth conditions. (E) 53-nt DicF RNA half-lives in MG1655 and Rned823–850 under anaerobic conditions. (F) Northern blot analyses of aerobic sRNA DicF levels in E. coli N3431 under permissive (30 °C) and nonpermissive (44 °C) temperature. Specific signals of probe 2 are indicated by arrowheads.
Many trans-encoded sRNAs, including DicF, require the chaperone protein Hfq for their stability and base-pairing activity under aerobic growth conditions (22–24). Hfq action on sRNA stability and function may also happen under anaerobic conditions. If so, deletion of the hfq gene should destabilize DicF and produce similar FtsZ protein levels under both aerobic and anaerobic conditions, and the cells should have a rod-shaped cell morphology under anaerobic conditions. Indeed, our MG1655Δhfq strain was rod-shaped (Fig. 3 F, a and b) under anaerobic conditions, and its FtsZ protein levels were similar under both aerobic and anaerobic conditions (Fig. 3H, lanes 1 and 2). MG1655Δhfq grew slightly more slowly than MG1655 (Fig. S2D) under aerobic conditions. However, under anaerobic conditions, MG1655Δhfq grew faster than MG1655 cells (Fig. S2D). In the MG1655Δhfq strain with ectopically expressed Hfq by the arabinose-inducible promoter, the cells again became filamentous under anaerobic conditions (cell length >5 μm) (Fig. 3 F, c), and FtsZ protein levels were reduced (Fig. 3H, lanes 3 and 4).
Thus, our results show that both sRNA DicF and the RNA chaperone Hfq are required for the filamentous transition of E. coli under anaerobic conditions. Since the level of FtsZ decreases under anaerobic conditions, it could be assumed that the level of its negative regulator, sRNA DicF, increases under the same conditions.
53-nt sRNA DicF Accumulates Under Anaerobic Conditions.
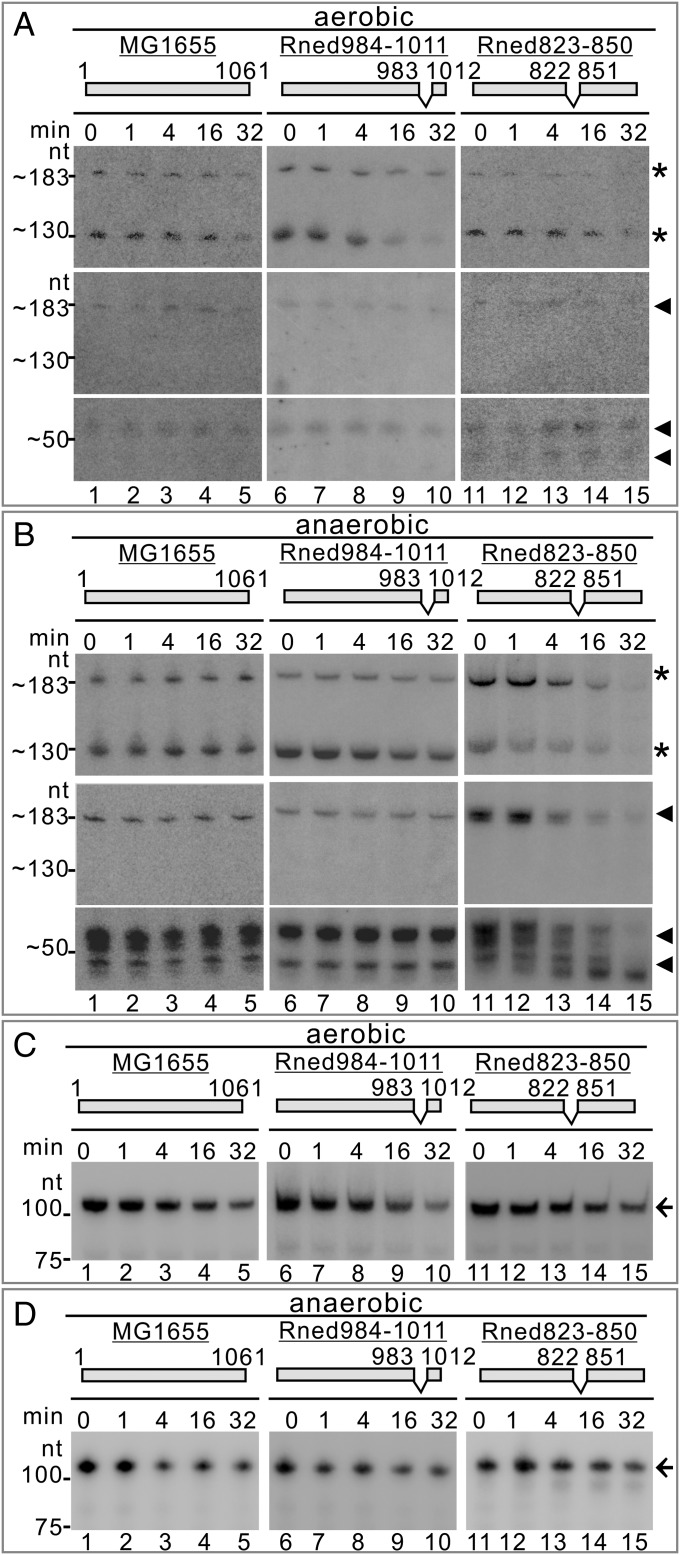
Pre-DicF RNA was transcribed from the dicA-ydfABC-dicF operon, and the transcript (∼850 nt long) was further processed by RNaseIII and RNase E via intermediate fragments (18) into mature 53-nt sRNA DicF. However, the processing steps to produce the mature sRNA are still unknown. Instead of using a strain deleted for dicF (as in Fig. 3)—namely, the MG1655ΔdicF mutant carrying the dicA-ydfABC-ΔdicF operon, which produces a truncated transcript with an unknown RNA secondary structure—we used a strain without the operon as a negative control to precisely identify pre-DicF–specific transcripts, intermediate RNA, and mature RNA products in the MG1655 strain by Northern blot analyses. To identify a strain lacking the entire dicA-ydfABC-dicF operon in its genome for use as a negative control for Northern blots, we conducted in silico analysis of E. coli genomes using the BioCyc Database Collection (https://biocyc.org). Interestingly, most commensal strains lack dicF in their genomes. In contrast, pathogenic E. coli strains have multiple copies of dicF (Fig. 4B and Table S1). In this study, we used E. coli Nissle 1917 as a negative control to identify the DicF-specific hybridization signal(s). We performed Northern blot analyses using two specific probes for the detection of sRNA DicF and its precursor RNA transcribed from the dicA-ydfABC-dicF operon (Fig. 4A) and compared the RNA steady-state levels under aerobic and anaerobic conditions in E. coli MG1655. Using internal radiolabeled antisense RNA probe 1 (Fig. 4A), we detected specific upper (∼183-nt) and lower (∼130-nt) bands (indicated by asterisks in Fig. 4 C, a, lanes 1 and 2) that were not present in the E. coli Nissle 1917 strain (Fig. 4 C, a, lanes 3 and 4). Using antisense RNA probe 2 (Fig. 4A), we detected several specific bands not present in the E. coli Nissle 1917 strain (indicated by arrowheads in Fig. 4 C, b; compare lanes 1 and 2 with lanes 3 and 4), one of which was the same upper band [∼183 nt; a product of RNase III processing (18)] detected by probe 1; the others were lower bands (∼50 nt) (Fig. 4 C, b, lanes 1 and 2). The upper band (∼183 nt) detected by both probes represented the 183-nt RNA precursor of sRNA DicF (which we refer to as “DicF precursor”), and the lower band (∼130 nt) detected only by probe 1 but not by probe 2 was the 130-nt 5′-upstream intermediate product arising from RNase E cleavage of the DicF precursor (which we refer to as the “130-nt product”) (Fig. 4A). The other RNase E cleavage products detected at the 3′ end of the DicF precursor, which were detected by probe 2 but not by probe 1, are variants of the 53-nt sRNA DicF (referred to as “53-nt DicF”) and/or its degradation products, represented by the multiple lower bands in Fig. 4 C, b.
Fig. 4.
DicF RNA levels are oxygen responsive, and DicF production (processing) and decay (degradation) depend on RNase E. (A) Schematic representation of the dicA-ydfABC-dicF operon with RNase III and RNase E cleavage sites (modified from ref. 18). Probes 1 (*) and 2 (◄) for Northern blots and the specific RNA fragments detected in this study are indicated. (B) Number of dicF genomic copies in nonpathogenic E. coli strains (Com, commensal; Env, environmental; Lab, laboratory) and pathogenic (AIEC, adherent-invasive; APEC, avian pathogenic; DAEC, diffusely adhering; EAEC, enteroaggregative; EHEC, enterohemorrhagic; EPEC, entheropathogenic; ETEC, enterotoxigenic; NMA, neonatal meningitis-associated; UPEC, uropathogenic). Each dot represents one E. coli strain. (C, a and b and D) Northern blot analyses of sRNA DicF in E. coli MG1655 (C) and in the MG1655Δhfq mutant (D) under aerobic and anaerobic conditions. Specific signals of probe 1 (*), probe 2 (◄), and nonspecific signals (black solid lines) are indicated at the right of each gel. (C, c) Northern blot analysis of sRNA Spot42 in E. coli MG1655 under aerobic and anaerobic conditions. Specific signal is indicated by a black arrow at the right of the gel. (E) DicF RNA levels under aerobic–anaerobic–aerobic alternating growth conditions. Specific signals of probe 1 (*) and probe 2 (◄) are indicated. (F) RNA stability of DicF, 130-nt product, or DicF precursor in the E. coli temperature-sensitive N3431 strain grown anaerobically at permissive (30 °C) and nonpermissive (44 °C) temperatures. Equal amounts of total RNA extracted from the cells before and after rifampicin treatment at the times indicated were analyzed by Northern blotting using probe 1 (*) and probe 2 (◄). (G, Right) Cell morphologies of E. coli MG1655ΔdicF before and after ectopic expression of the 63DicF1 DicF miniprecursor or a 63DicF2 variant under anaerobic conditions. (Scale bars, 5 µm.) (Left) Northern blots upon arabinose ectopic expression under anaerobic conditions are shown. (Center) Schematic representations of the 63DicF1 DicF miniprecursor and the 63DicF2 variant and their RNase E cleavage sites.
Table S1.
Number of dicF genomic copies in nonpathogenic and pathogenic E. coli
| Name | No. of sRNA DicF copies |
| Nonpathogenic strains | |
| Commensal | |
| E. coli Nissle 1917 | 0 |
| E. coli str. HS | 0 |
| E. coli str. SE11 | 0 |
| E. coli str. SE15 | 0 |
| E. coli str. IAI1 | 1 |
| E. coli str. ABU 82972 | 1 |
| E. coli str. KO11FL | 1 |
| Environmental | |
| E. coli str.SMS-3–5 | 1 |
| Laboratory: | |
| E. coli K-12 substr. MG1655 | 1 |
| E. coli K-12 substr. W3110 | 1 |
| E. coli K-12 substr. DH10B | 1 |
| E. coli ATCC 8739 | 1 |
| E. coli BL21(DE3) | 1 |
| E. coli BL21-Gold(DE3)pLysS AG | 1 |
| E. coli BW2952 | 1 |
| E. coli DH1 | 1 |
| E. coli W | 1 |
| E. coli P12b | 0 |
| Pathogenic strains | |
| Extraintestinal pathogenic | |
| Neonatal meningitis-associated | |
| E. coli IHE3034 | 2 |
| E. coli S88 | 1 |
| E. coli O7:K1 str. CE10 | 1 |
| Urinary tract infections | |
| Uropathogenic E. coli (UPEC) IHE3034 | 2 |
| Uropathogenic E. coli (UPEC) UMN026 | 2 |
| Uropathogenic E. coli (UPEC) UTI189 | 1 |
| Uropathogenic E. coli (UPEC) CFT073 | 1 |
| Intestinal pathogenic (diarrheagenic) | |
| Enterohemorrhagic E. coli (EHEC) | |
| Enterohemorrhagic E. coli (EHEC) O103:H2 str. 12009 | 6 |
| Enterohemorrhagic E. coli (EHEC) O26:H11 str. 11368 | 6 |
| Enterohemorrhagic E. coli (EHEC) O103:H2 str. EC4115 | 6 |
| Enterohemorrhagic E. coli (EHEC) O111:H str. 11128 | 5 |
| Enterohemorrhagic E. coli (EHEC) O157:H7 str. Xuzhou21 | 4 |
| Enterohemorrhagic E. coli (EHEC) O157:H7 str. EDL933 | 4 |
| Enterohemorrhagic E. coli (EHEC) O157:H7 str. Sakai | 4 |
| Enteroaggregative E. coli (EAEC) | |
| Enteroaggregative E. coli (EAEC) 55989 | 3 |
| Enteroaggregative E. coli (EAEC) 042 | 3 |
| Entheropathogenic E. coli (EPEC) | |
| Entheropathogenic E. coli (EPEC) O55:H7 str. RM12579 | 3 |
| Entheropathogenic E. coli (EPEC) O55:H7 str. CB9615 | 2 |
| Entheropathogenic E. coli (EPEC) E2348/69 | 2 |
| Entheropathogenic E. coli (EPEC) O55:H7 str. USDA 5905 | 2 |
| Entheropathogenic E. coli (EPEC) O55:H7 str. 3256–97 | 2 |
| Avian pathogenic E. coli (APEC) | |
| Avian pathogenic E. coli (APEC) str. APEC 01 | 2 |
| Diffusely adhering E. coli (DAEC) | |
| Diffusely adhering E. coli (DAEC) O127:H6 str. E2348/69 | 2 |
| Adherent-invasive E. coli (AIEC) | |
| Adherent-invasive E. coli (AIEC) LF82 | 1 |
| Adherent-invasive E. coli (AIEC) O83:H1 str. NRG 857C | 1 |
| Adherent-invasive E. coli (AIEC) UM146 | 1 |
| Enterotoxigenic E. coli (ETEC) | |
| Enterotoxigenic E. coli (porcine ETEC) UMNK88 | 3 |
| Enterotoxigenic E. coli (ETEC) H10407 | 1 |
| Enterotoxigenic E. coli (ETEC) E24377A | 1 |
To determine whether sRNA DicF is preferentially enriched under anaerobic conditions, we probed another Hfq-dependent Spot42 sRNA (25), which plays essential roles as a regulator of carbohydrate metabolism and uptake and expression of which is activated by glucose (26), and found that steady-state levels of the RNA decreased, rather than increased like DicF, under anaerobic conditions (Fig. 4 C, c). This result indicates that sRNA DicF is preferentially enriched under anaerobic conditions.
Although 53-nt DicF predominantly accumulated under oxygen-limited conditions (Fig. 4 C, b, lane 2), neither it nor its variants were detected in the MG1655Δhfq strain under either aerobic or anaerobic conditions (Fig. 4 D). This observation was expected, as Hfq specifically protects sRNA DicF from degradation under normal growth conditions (27); in the absence of Hfq, 53-nt DicF and its derivatives were rapidly cleaved and thus were not detected.
RNA Cleavage by RNase E Is Oxygen Responsive and Is Necessary for Cell Filamentation Under Oxygen-Limited Conditions.
Since 53-nt DicF accumulated under oxygen-limited conditions, we performed a Northern blot to determine the impact of oxygen levels on 53-nt DicF. Under aerobic–anaerobic–aerobic alternating growth conditions, 53-nt DicF and its precursor accumulated after shifting to anaerobiosis and then reverted to their original state after shifting back to aerobic conditions (Fig. 4E, compare lanes t0 and t1 with lanes t2 and t3 and with t4–t6, respectively, and Fig. S5D). We detected a similar pattern for the 130-nt product.
We then investigated whether RNase E is responsible for DicF precursor cleavage and production of the 130-nt and 53-nt DicF cleavage products. We used the RNase E temperature-sensitive strain N3431 (28), in which RNase E activity is inhibited under nonpermissive temperatures, since the DicF precursor should accumulate in the N3431 cells under nonpermissive temperatures. As shown in Fig. 4F, we found that after shifting to a nonpermissive temperature (44 °C) for 3 h, levels of the DicF precursor increased significantly compared with 30 °C (detected by both probes 1 and 2) (Fig. 4F, compare lane 1 under 44 °C vs. 30 °C). The 130-nt product was not produced at 44 °C but was detected at 30 °C (probe 1) (Fig. 4F, compare lane 1 under 44 °C vs. 30 °C). As expected, at 44 °C, levels of 53-nt DicF were comparable to those at 30 °C (probe 2) (Fig. 4F, Lower; compare lane 1 under 44 °C vs. 30 °C). In fact, 53-nt DicF was slowly degraded at both 30 °C and 44 °C (probe 2) (Fig. 4F, compare lanes 1–5 in the left and right panels). In contrast to 53-nt DicF, the 130-nt product was less stable (t1/2 ∼17 min at 30 °C) at permissive temperatures and was completely degraded after shifting to the 44 °C nonpermissive temperature for 3 h (probe 1) (Fig. 4F, compare lanes 1–5 in the left and right panels). Thus, our data indicate that RNase E is necessary for cleavage of the DicF precursor to produce the 130-nt product and 53-nt DicF and that degradation of the 53-nt DicF, but not the 130-nt product, is predominantly dependent on RNase E activity.
Since Hfq protects 53-nt DicF from degradation, and both Hfq and 53-nt DicF are necessary for cell filamentation under anaerobic conditions, we examined whether RNase E cleavage of the DicF precursor to generate 53-nt DicF is required for cell filamentation under anaerobic conditions. We introduced an arabinose-inducible pBAD-plasmid—expressed either as a 63DicF1 DicF miniprecursor containing the 53-nt sRNA DicF plus a 10-nt wild-type upstream region with an RNase E cleavage site (so it could be cleaved by RNase E) or a 63DicF2 variant in which the RNase E cleavage site had been replaced by 10 guanines in the 53-nt DicF upstream region (so it could not be cleaved by RNase E), as described in SI Materials and Methods. Unlike 63DicF1, 63DicF2 was not cleaved by RNase E under either anaerobic or aerobic conditions (left panels in Fig. 4G and Fig. S5C, respectively). As a result, in contrast to the 63DicF2 variant (Fig. 4 G, c and d and Fig. S5 C, c and d), a filamentous cell morphology was obtained for MG1655ΔdicF only upon expression of the 63DicF1 miniprecursor under anaerobic or aerobic conditions (Fig. 4 G, a and b, and Fig. S5 C, a and b, respectively). Our data demonstrate that RNase E cleavage (processing) is a necessary step for the production of functional 53-nt sRNA DicF that induces cell filamentation under anaerobic conditions.
Enolase in the Degradosome Stabilizes 53-nt sRNA DicF Under Anaerobic Conditions.
As shown in Fig. 2A, the MG1655 strain containing enolase-bound degradosomes is able to form filamentous cells under anaerobic conditions when functional 53-nt DicF accumulates. In contrast, the Rned823–850 strain containing enolase-free degradosomes lost the ability to form filamentous cells under the same conditions, suggesting that 53-nt DicF levels in this strain are not comparable to those in the MG1655 strain.
Northern blot analyses were performed to determine RNA abundances of 53-nt DicF, its precursor, and the 130-nt product in the MG1655, Rned984–1011, and Rned823–850 strains at different time points after rifampicin treatment under aerobic and anaerobic conditions. We found that the abundance and stability of all RNA species were relatively similar in all three strains under aerobic conditions (probes 1 and 2) (Fig. 5A, compare lanes 1–5, 6–10, and 11–15, respectively). In contrast, under anaerobic conditions, the abundance and stability of 53-nt DicF and the 183-nt precursor were lower for Rned823–850 than for the MG1655 and Rned984–1011 strains (probes 1 and 2) (Fig. 5B, compare lanes 11–15, 1–5, and 6–10, respectively). The RNA steady-state levels of the 130-nt product were similar under both aerobic and anaerobic conditions. However, its degradation rate was greater in Rned823–850 than in the MG1655 and Rned984–1011 strains under anaerobic conditions (Fig. 5B, compare lanes 11–15, 1–5, and 6–10, respectively). Our data show that disruption of the enolase and RNase E interaction results in faster degradation of the 130-nt product (t1/2 ∼12 min in Rned823–850 vs. t1/2 >32 min in MG1655) and 53-nt DicF under anaerobic conditions (t1/2 ∼17 min in Rned823–850 vs. t1/2 >32 min in MG1655) (Fig. S5E).
Fig. 5.
DicF processing and degradation are dependent on enolase being bound to RNase E. (A and B) sRNA DicF RNA half-lives in the E. coli MG1655, Rned984–1011, and Rned823–850 strains under aerobic (A) and anaerobic (B) conditions. RNA samples prepared from the cultures before and after rifampicin treatment at the times indicated were analyzed by Northern blotting using probe 1 (*) and probe 2 (◄). (C and D) RNA half-lives of sRNA Spot42 in the E. coli MG1655, Rned984–1011, and Rned823–850 strains under aerobic (C) and anaerobic (D) conditions. Specific signals are indicated by black arrows on the right side of each gel.
We used sRNA Spot42 again to monitor RNA half-life by Northern blot analysis. We found that Spot42 sRNA half-life is not changed under either aerobic or anaerobic conditions in all strains: MG1655, Rned823–850, and Rned984–1011 (Fig. 5 C and D). The data show that RNA turnover of Spot42 is not affected by deletion of the enolase-binding microdomain in RNase E. Thus, the anaerobically induced faster degradation of the sRNA DicF by the nonenolasic degradosomes in the Rned823–850 strain is specific. Collectively, our data show that the enolase association specifically helps stabilize DicF (and, possibly, its processing) but not Spot42 sRNA under anaerobic conditions.
Binding of Enolase to RNase E Does Affect Protein Levels and Impacts Subcellular Localization of RNase E Under Anaerobic Conditions.
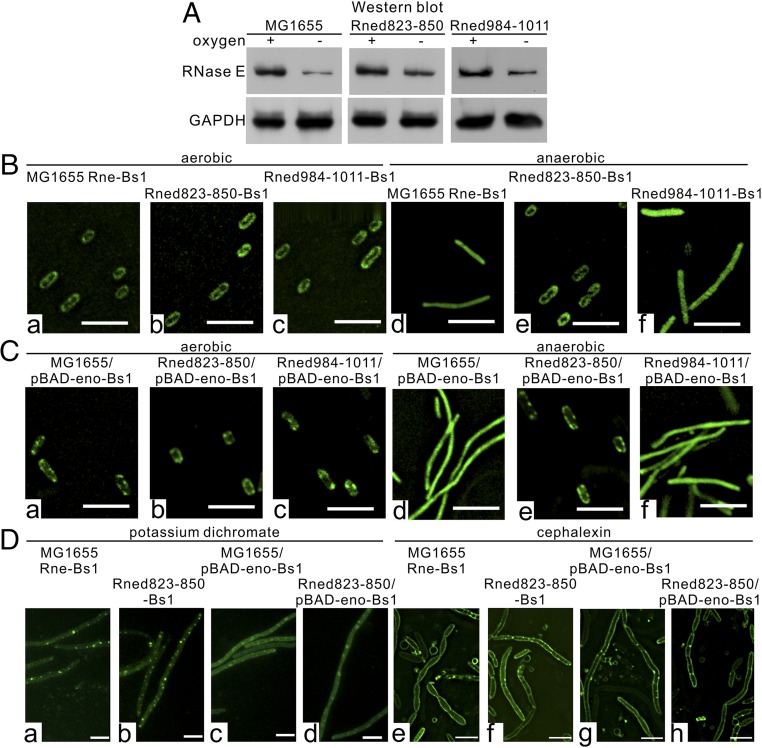
The fast turnover of the DicF precursor under anaerobic conditions in the Rned823–850 strain that lacks enolase in degradosomes could be caused by increased RNase E protein levels. Therefore, we determined the protein levels of RNase E/RNase E variants in the MG1655, Rned823–850, and Rned984–1011 strains under both aerobic and anaerobic conditions. We found that for all three strains the levels of RNase E or its derivative protein were reduced under anaerobic conditions (Fig. 6A). Quantitation indicated that RNase E protein levels decreased to a lesser extent under anaerobic conditions in the Rned823–850 mutant (∼2.5-fold) than in the MG1655 (∼4.0-fold) or Rned984–1011 (∼3.4-fold) strains.
Fig. 6.
The diffuse localization pattern of RNase E and enolase is specific to anaerobic conditions. (A) Endogenous expression levels of RNase E and its variants in the E. coli MG1655, Rned823–850, and Rned984–1011 strains under aerobic and anaerobic conditions. Whole-cell extracts were analyzed by SDS/PAGE and immunoblotted with the indicated antibodies. (B and C) Subcellular localization of RNase E and its variants (B) and enolase (C) under aerobic and anaerobic conditions in the E. coli MG1655, Rned823–850, and Rned984–1011 strains. (Scale bars, 5 µm.) (D) Subcellular localization of RNase E and its variant lacking the enolase-binding microdomain and enolase upon potassium dichromate (D, a–d) and cephalexin (D, e–h) treatments in the E. coli MG1655 and Rned823–850 strains under aerobic conditions. (Scale bars, 5 µm.)
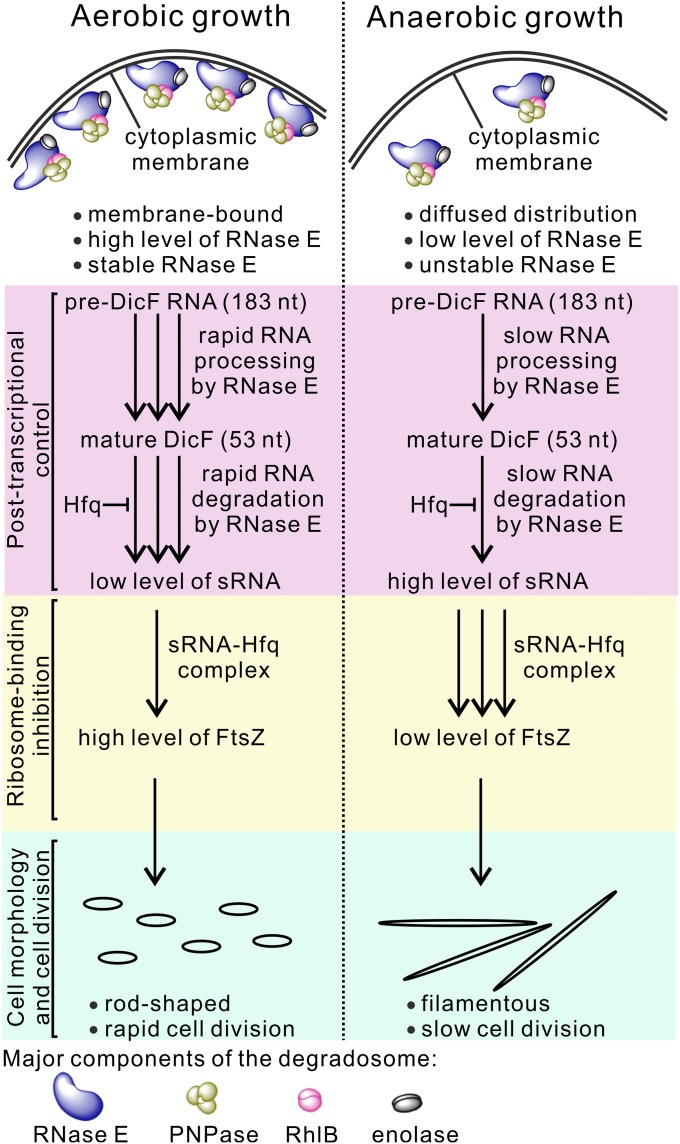
Previous studies have shown that RNase E–degradosome complexes are tethered to the cytoplasmic membrane in vivo (5) and that membrane-binding of the RNase E N terminus (which contains a catalytic domain) stabilizes protein structure and increases both RNA cleavage activity and substrate affinity (6). We investigated whether binding of enolase to RNase E may change the subcellular localization of RNase E under anaerobic conditions and thereby regulate protein stability and enzymatic activity and stabilize functional sRNA DicF in the MG1655 and Rned984–1011 strains. We used EvoGlow fluorescent reporter protein to study the subcellular localization of RNase E under aerobic and anaerobic conditions, as this fluorescent reporter can be detected under both conditions (29). We constructed MG1655 Rne-Bs1, Rned823–850-Bs1, and Rned984–1011-Bs1 strains in which the fluorescent reporter was fused to the chromosomal C terminus of RNase E through a polypeptide linker (SI Materials and Methods). Our results show that for the MG1655 or Rned984–1011 strains that host enolase in the degradosomes, the subcellular distribution of RNase E shifted from the cytoplasmic membrane under aerobic conditions to a diffuse pattern under anaerobic conditions (Fig. 6 B, a, d and c, and f, respectively). However, for the Rned823–850 strain, whose degradosomes lacked enolase, RNase E was cytoplasmic membrane-associated under both conditions (Fig. 6 B, b and e). Furthermore, our results show that whenever RNase E was tethered to the cytoplasmic membrane of MG1655 (Fig. 6 B, a), Rned823–850 (Fig. 6 B, b), or Rned984–1011 (Fig. 6 B, c) cells under aerobic conditions or of Rned823–850 (Fig. 6 B, e) cells under anaerobic conditions, 53-nt DicF turnover was rapid, whether or not enolase was hosted by the degradosomes. In agreement with this idea that association with the cytoplasmic membrane increases RNase E enzymatic activity, the Rned823–850 RNase E variant tethered to the cytoplasmic membrane under both aerobic and anaerobic conditions exhibited faster degradation of 53-nt DicF under both conditions (Figs. 5 A and B, lanes 11–15, and 6 B, b and e). When RNase E binds to enolase and is diffuse in the cytoplasm (under anaerobic conditions), and thus is not tethered to the cytoplasmic membrane (as it is under aerobic conditions), this may lock RNase E into a less active conformation for sRNA degradation, thereby stabilizing sRNA DicF.
To examine whether the diffuse localization pattern of RNase E and enolase is specific to anaerobically induced filamentous cells, we used other means of inducing cell filamentation under aerobic conditions (i.e., potassium dichromate and cephalexin). We found that the localization patterns of RNase E and enolase under these conditions (Fig. 6D) differed from those under anaerobiosis (Fig. 6 B and C). The pattern under potassium dichromate and cephalexin treatment is not affected by nonenolasic degradosomes (Fig. 6D, compare a and b with e and f). Thus, the diffused localization pattern of RNase E and enolase (Fig. 6 B, d and Fig. 6 C, d, respectively) is specific to anaerobically induced filamentous cells, and the localization pattern depends on how filamentation is induced (i.e., by low oxygen or potassium dichromate/cephalexin treatment).
To understand whether RNase E amounts affect the localization, we ectopically expressed RNase E fused with Bs1 protein using an arabinose-inducible promoter from the pBAD plasmid in the MG1655 Rne-Bs1 strain under anaerobic conditions and found that, upon low (namely, without ectopic expression) or increased (namely, after ectopic expression by arabinose) levels, RNase E has a similar diffused subcellular distribution (Fig. S6A). Thus, the decrease in RNase E amounts does not affect the localization pattern.
Fig. S6.
RNase E amounts do not affect the localization of the protein under anaerobic conditions, and the deletion of the enolase-binding site on RNase E does not affect the association of Hfq with RNase E. (A) The subcellular localizations (Upper) and protein levels (Lower) of RNase E upon arabinose-induced expression. RNase E was fused with Bs1 protein in the MG1655 Rne-Bs1 strain, and arabinose-induced expression was assessed according to indicated time under anaerobic conditions. (Scale bars, 5 µm.) (B) The degradosomes isolated from E. coli MG1655 and its derivatives, Rned823–500 and Rne984–1011. Protein aliquots containing equal amounts of Flag-RNase E and its variants were analyzed by 10% (for RNase E) or 15% (for Hfq) SDS/PAGE and immunoblotted with the indicated antibodies.
Collectively, our data demonstrate that under oxygen-limited conditions, enolase (in bound form to RNase E in the degradosome) is required for the filamentous transition of E. coli through its regulation of the subcellular localization, protein stability, and activity of RNase E.
SI Materials and Methods
Bacterial Strains and Growth Conditions.
The E. coli strains used in this study are listed in Table S2. The cells were grown in a 1-L Winpact Bench-Top Fermentor (Major Science, Inc.) with 0.75 L of M9 medium (50) supplemented with 0.4% glucose (or 0.4% pyruvate) under aerobic or anaerobic conditions, as previously described (51–53). Briefly, the cells from −80 °C glycerol stocks were first plated onto M9 agar plates containing 0.4% glucose (or 0.4% pyruvate) and supplemented with bacteriological agar (1.5 g/L). The resultant single colonies were subcultured in 50 mL of the M9 glucose or pyruvate medium in 250-mL Erlenmeyer flasks and were grown overnight in a shaking water bath at 37 °C and 200 rpm. To a 1-L Fermentor vessel containing 750 mL of the M9 glucose or pyruvate medium initially incubated for 6 h with a constant flow rate of 0.5 L/min of either sparged atmospheric air or pure nitrogen gas to maintain aerobic or anaerobic conditions, respectively, we added overnight cultures that had been diluted to an OD460 of 0.05. The pH, temperature, and agitation were maintained at 7.0, 37 °C, and 200 rpm, respectively. The cells were grown under aerobic or anaerobic conditions until the desired OD460 was reached and then were used for the experiments. For aerobic–anaerobic–aerobic alternating experiments (Fig. S1D), the cells were grown aerobically to OD460 ∼0.15 (t0) as described above. The culture was subsequently shifted to anaerobic conditions and then returned to aerobic conditions. Aliquots of the cells at 0 (t0), 60 (t1), 180 (t2), 360 (t3), 390 (t4), 510 (t5), and 570 (t6) min were drawn for Western and Northern blots and cell imaging. For experiments with the rne temperature-sensitive strain, the original N3431 (28) culture was subcultured in M9 glucose or pyruvate medium into two samples, and the cultures were simultaneously grown anaerobically at 30 °C. Then one culture was treated by rifampicin to inhibit new RNA synthesis, while the other culture was shifted to 44 °C for 3 h before treatment with rifampicin.
Table S2.
E. coli strains or plasmids used in this study
| Strain/plasmid name | Genotype/relevant markers | Description | Source |
| MG1655 | K-12, F−, λ−, ilvG−, rfb-50, rph-1 | Wild-type (wt) strain | (66) |
| N3431 | Hfr(PO1), lacZ43(Fs), λ−, rne-3071(ts), relA1, spoT1, thi-1 | RNase E temperature-sensitive (ts) strain | (28) |
| Nissle 1917 | — | In silico analysis showed that E. coli Nissle 1917 strain lacks the dicA-ydfABC-dicF operon, and therefore we used this strain as a negative control to identify specific hybridization signal(s) of the DicF transcripts. | (67) |
| MG1655ΔdicF | MG1655, ΔdicF::KanR | Mutant with endogenously deleted DicF sRNA | (20) |
| MG1655Δenolase | MG1655, Δeno::KanR | Mutant with endogenously deleted enolase | This study |
| MG1655Δhfq | MG1655, Δhfq::KanR | Mutant with endogenously deleted Hfq | This study |
| Rned500–1061 | MG1655, rneΔ500–1061::KanR | Mutant with endogenously deleted region encoding amino acids 500–1,061 of RNase E | This study |
| Rned823–850 | MG1655, rneΔ823–850::KanR | Mutant with endogenously deleted region encoding a 28-aa microdomain (amino acids 823–850) for enolase recognition in RNase E (15) | This study |
| Rned984-1011 | MG1655, rneΔ984–1011::KanR | Mutant with endogenously deleted region encoding a 28-aa region (amino acids 984–1011) within the C-terminal part of RNase E; alternative control for Rned823–850 | This study |
| MG1655 Rne-Bs1 | MG1655, rne-l-bs1-l-bs1::KanR | Mutant expressing RNase E polypeptide C-terminally fused with 2xBs1 fluorescence proteins in tandem through 20-aa linkers (L as a linker; LAEAAAKLAAAKEAAAKAAA). | This study |
| Rned823–850-Bs1 | MG1655, rneΔ823–850-l-bs1-l-bs1::KanR | Similar to MG1655 Rne-Bs1 but contains the rneΔ823–850 gene | This study |
| Rned984–1011-Bs1 | MG1655, rneΔ984–1011-l-bs1-l-bs1::KanR | Similar to MG1655 Rne-Bs1 but contains the rneΔ984–1011 gene | This study |
| pBAD-EBFP2 | AmpR | pBAD backbone vector was used for gene cloning. The expressed gene is under control of the araBAD promoter and is induced by arabinose. | Addgene |
| pKD46 | AmpR | Temperature-sensitive lambda red recombinase expression plasmid was used for strain constructions. | (55) |
| pKD4 | AmpR, KmR | Template plasmid was used for MG1655Δenolase and MG1655Δhfq mutant construction and amplification of the KmR cassette to prepare template plasmids (Fig. S3) for construction of pBAD-Rned500–1061-Km, pBAD-Rned823–850-Km, pBAD-Rned984–1011-Km, pBAD-Rnewt-l-Bs1-l-Bs1-Km, pBAD-Rned823–850-l-Bs1-l-Bs1-Km and pBAD-Rned984–1011-l-Bs1-l-Bs1-Km strains | (55) |
| pGLOW-Bs1 | AmpR | Template plasmid for PCR amplification of the bs1 gene encoding Bs1 fluorescence protein (29) | Jena Bioscience |
| pBAD-Rnewt | AmpR | The rne gene was cloned by rne_fw/rne_rev into pBAD-EBFP2 via NdeI/EcoRI. | This study |
| pBAD-empty | AmpR | Control vector for RNase E expression (without the rne gene). pBAD-Rnewt was reamplified with empty_fw/empty_rev and self-ligated. | This study |
| pBAD-Rnewt-Km | AmpR, KmR | The KmR cassette was cloned by Km_fw/Km_rev into pBAD-Rnewt via EcoRI. | This study |
| pBAD-Rned500–1061-Km | AmpR, KmR | Template for Rned500–1061 KmR mutant construction. pBAD-Rnewt-Km was reamplified with d1061_fw/d500_rev and self-ligated. See Fig. S3. | This study |
| pBAD-Rned823–850-Km | AmpR, KmR | Template plasmid for Rned823–850 KmR mutant construction. pBAD-Rnewt-Km was reamplified with d850_fw/d823_rev and self-ligated. See Fig. S3. | This study |
| pBAD-Rned984–1011-Km | AmpR, KmR | Template plasmid for Rned984–1011 KmR mutant construction. pBAD-Rnewt-Km was reamplified with d1011_fw/d984_rev and self-ligated. See Fig. S3. | This study |
| pBAD-FlagRnewt | AmpR | To clone 1xFlag at the 5′ end of the rne gene, the pBAD-Rnewt was reamplified with FLAG_fw/FLAG_rev and self-ligated. | This study |
| pBAD-FlagRned823–850 | AmpR | Similar to pBAD-FlagRnewt but contains the rneΔ823–850 gene. pBAD-FlagRnewt was reamplified with d850_fw/d823_rev and self-ligated. | This study |
| pBAD-FlagRned984–1011 | AmpR | Similar to pBAD-FlagRnewt but contains the rneΔ984–1011 gene. pBAD-FlagRnewt was reamplified with d1011_fw/d984_rev and self-ligated. | This study |
| pBAD-IR | AmpR | The dicA-dicB intergenic region (IR) containing dicF gene was cloned by IR_fw/IR_rev into pBAD-EBFP2 via NdeI/EcoRI. | This study |
| pBAD-63DicF1 | AmpR | Contains the dicF gene 53-nt wild type. pBAD-IR was reamplified with DicF53nt_fw/DicF_rev and self-ligated. | This study |
| pBAD-63DicF1 | AmpR | Contains the dicF gene plus the 10-nt wild type upstream region. pBAD-IR was reamplified with DicF10wt_fw/DicF_rev and self-ligated. | This study |
| pBAD-63DicF2 | AmpR | Similar to pBAD-63DicF1 but contains 10 guanines in the upstream region of the dicF gene. pBAD-IR was reamplified with DicF10G_fw/DicF_rev and self-ligated. | This study |
| pBAD-Hfq | AmpR | The hfq gene was cloned by hfq_fw/hfq_rev into pBAD-EBFP2 via NdeI/EcoRI. | This study |
| pBAD-Rnewt-l-Bs1-Km | AmpR, KmR | The intermediate plasmid for construction of pBAD-Rnewt-l-Bs1-l-Bs1-Km (see SI Materials and Methods for details) | This study |
| pBAD-Rnewt-l-Bs1-Bs1-Km | AmpR, KmR | Template plasmid for MG1655 Rne-Bs1 KmR mutant construction. See Fig. S3. | This study |
| pBAD-Rned823–850-l-Bs1-l-Bs1-Km | AmpR, KmR | Template plasmid for Rned823–850-Bs1 KmR mutant construction. pBAD-Rnewt-l-Bs1-l-Bs1-Km was reamplified with d850_fw/d823_rev and self-ligated. See Fig. S3. | This study |
| pBAD-Rned984–1011-l-Bs1-l-Bs1-Km | AmpR, KmR | Template plasmid for Rned984–1011-Bs1 KmR mutant construction. pBAD-Rnewt-l-Bs1-l-Bs1-Km was reamplified with d1011_fw/d984_rev and self-ligated. See Fig. S3. | This study |
| pBAD-eno-l-Bs1-l-Bs1 | AmpR, KmR | The eno gene was cloned into pBAD-Rnewt-l-Bs1-l-Bs1-Km (see SI Materials and Methods for details). | This study |
Plasmid Constructions and Protein and sRNA Expression.
The plasmids and oligonucleotides used in this study are listed in Tables S2 and S3, respectively. For ectopic expression of RNase E, we created pBAD-Rnewt plasmid by one-step in-frame insertion of the corresponding DNA fragment of the rne gene into the NdeI and EcoRI sites of pBAD-EBFP2 (ApR; Addgene). The cells were grown anaerobically as described above to OD460 ∼0.3 and then were induced with arabinose to a final concentration of 0.2% and incubated until all cells reverted to normal morphology (∼3 h). Aliquots of the cells before and after arabinose induction were withdrawn for cell imaging and Western blotting. To construct pBAD-FlagRnewt for ectopic expression of Flag-RNase E, the Flag sequence (5′-GACTACAAAGACGATGACGATAAA-3′) was inserted into pBAD-Rnewt by inverse PCR (54). pBAD-FlagdRned823–850 and pBAD-FlagdRned984–1011 plasmids were created by inverse PCR using pBAD-FlagRnewt as a template. Each culture of MG1655/pBAD-FlagRnewt, Rned823–850/pBAD-FlagdRned823–850, and Rned984–1011/pBAD-FlagdRned984–1011 was grown under either aerobic or anaerobic conditions until OD460 ∼0.3 and was induced further with arabinose (0.2%) for 30 min before Flag-RNase E protein isolation. Flag-RNase E and its derivatives were isolated using affinity chromatography as previously described (3, 11). After isolation equal protein aliquots from the fraction containing Flag-RNase E or its derivatives were analyzed by Western blotting. For ectopic expression of Hfq, we created the pBAD-Hfq plasmid by one-step in-frame insertion of the corresponding DNA fragment of the hfq gene into the NdeI and EcoRI sites of pBAD-EBFP2. For ectopic expression of sRNA DicF and its variants, we first constructed the pBAD-IR plasmid by in-frame insertion of the dicA–dicB intergenic region into the NdeI and EcoRI sites of plasmid pBAD-EBFP2. Then the pBAD-53DicF, pBAD-63DicF1, and pBAD-63DicF2 plasmids were created by inverse PCR. Each culture was grown under anaerobic conditions until OD460 ∼0.4 and then was induced with arabinose (0.2%) for 12 h before sampling for Western blotting and cell imaging. For ectopic expression of 2xBs1-tagged enolase, the pBAD-eno-l-Bs1-l-Bs1 plasmid was created in a single step by blunt-ended ligation of the eno gene DNA fragment into reamplified pBAD-Rnewt-l-Bs1-l-Bs1-Km plasmid (Bacterial Strain Constructions below). Each culture of MG1655/pBAD-eno-l-Bs1-Bs1, Rned823–850/pBAD-eno-l-Bs1-l-Bs1, and Rned984–1011/pBAD-eno-l-Bs1-l-Bs1 was grown under either aerobic or anaerobic conditions until OD460 ∼0.3 and was induced further with arabinose (0.2%) for 30 min before cell imaging. The individual DNA fragments corresponding to rne, eno, and hfq genes and the dicA–dicB intergenic region were prepared by PCR amplification from genomic DNA of E. coli K-12 strain MG1655. Each plasmid construct was confirmed by DNA sequencing.
Table S3.
Oligonucleotide primers used for construction of plasmids
| Name | Sequence (5′ to 3′) | Target |
| rne_fw | AGATATACATATGAAAAGAATGTTAATCAACGCAA | MG1655 rne gene |
| rne_rev | AGAATCGAATTCTTACTCAACAGGTTGCGGA | MG1655 rne gene |
| empty_fw | P-GAATTCGAAGCTTGGCTGTTTTGG | pBAD-EBFP2 |
| empty_rev | P-ATGTATATCTCCTTCTTAAAGTTAAACAAAATT | pBAD-EBFP2 |
| FLAG_fw | P-GACTACAAAGACGATGACGATAAAATGAAAAGAATGTTAATCAAC | pBAD-Rnewt |
| FLAG_rev | P-CATATGTATATCTCCTTCTTAAAGTTAAACAAAATTATTTCTAG | pBAD-Rnewt |
| d850_fw | P-CCGCAAGATGTACAGGTTGA | pBAD-FlagRnewt, pBAD-Rnewt-Km or pBAD-Rne-Bs1-Km |
| d823_rev | P-GGTTGGATAACGCTCGTCA | pBAD-FlagRnewt, pBAD-Rnewt-Km or pBAD-Rne-Bs1-Km |
| d1011_fw | P-CGCGCTCCAGCACCGGAATA | pBAD-FlagRnewt, pBAD-Rnewt-Km or pBAD-Rne-Bs1-Km |
| d984_rev | P-AGCGACTACTGGCGCGGCA | pBAD-FlagRnewt, pBAD-Rnewt-Km or pBAD-Rne-Bs1-Km |
| hfq_fw | AGATATACATATGGCTAAGGGGCAATCTTTACAAGA | pBAD-EBFP2 |
| hfq_rev | AGAATCGAATTCTTATTCGGTTTCTTCGCTGTCCT | pBAD-EBFP2 |
| IR_fw | AGATATACATATCATCAATGAGTTATCTTTTACCACAT | pBAD-EBFP2 |
| IR_rev | AGAATCGAATTCTATCGCGTCCTCAACAATGAATTTTG | pBAD-EBFP2 |
| DicF53nt_fw | P-TTTCTGGTGACGTTTGGCGG | pBAD-IR |
| DicF10wt_fw | P-TGTTATCAATTTTCTGGTGACGTTTGG | pBAD-IR |
| DicF10G_fw | P-GGGGGGGGGGTTTCTGGTGACGTTTGGCGGTAT | pBAD-IR |
| DicF_rev | P-GAGAAACAGTAGAGAGTTGCGATAAAAAGCGTC | pBAD-IR |
| GR_eno_fw | GTCTGGAGTTTCAGTTTAACTAGTGACTTGAGGAAAACCTAGAATTCGTGAACCTCTTCGA | pKD4 |
| GR_eno_rev | CAGCCCGGAGGCTGGCATTTTTAAATCAGATAAAGTCAGTCGAATTCACTAGTGATTTG | pKD4 |
| GR_hfq_fw | AAAGGTTCAAAGTACAAATAAGCATATAAGGAAAAGAGAGAGAATTCGTGAACCTCTTCGA | pKD4 |
| GR_hfq_rev | CAGGATCGCTGGCTCCCCGTGTAAAAAAACAGCCCGAAACCGAATTCACTAGTGATTTG | pKD4 |
| Km_fw | GAGTGAGAATTCGATTCTATTCCGAAGTTCCT | pKD4 |
| Km_rev | AGGAACTTCGGAATAGAATCGAATTC | pKD4 |
| d1061_fw | P-TAAGAATTCGATTCTATTCCGAAGTTCCTA | pBAD-Rnewt-Km |
| d500_rev | P-CATGCAGCTTCGGCAGCATGTA | pBAD-Rnewt-Km |
| GR_rne_fw | CGCAGCTTAGTCGTCAATGTAAGAATAATGAGTAAGTTACGATGAAAAGAATGTTAATC | pBAD-Rned500–1061-Km, pBAD-Rned823–850-Km, pBAD-Rned984–1011-Km, pBAD-Rnewt-l-Bs1-l-Bs1-Km, pBAD-Rned823–850-l-Bs1-l-Bs1-Km, pBAD-Rned984–1011-l-Bs1-l-Bs1-Km |
| GR_rne_rev | GCCCTGGCAGTTACCAGGGCTTGATTACTTTGAGCTAATTAGAATTCACTAGTGATTTG | pBAD-Rned500–1061-Km, pBAD-Rned823–850-Km, pBAD-Rned984–1011-Km, pBAD-Rnewt-l-Bs1-l-Bs1-Km, pBAD-Rned823–850-l-Bs1-l-Bs1-Km, pBAD-Rned984-1011-l-Bs1-l-Bs1-Km |
| rne_Bs1_rev | P-CTCAACAGGTTGCGGACGC | pBAD-Rnewt-Km, pBAD-Rnewt-l-Bs1-Km |
| Bs1_fw | P-CTCGCTGAGGCCGCCGCTAAAGAGGCCGCCGCTAAAGAGGCCGCCGCTAAAGCGGCCGCCATGGCTAGTTTTCAATCATTTGGGAT | pGLOW-Bs1 |
| Bs1_rev | P-TCACATAATCGGAAGCACTTTAAC | pGLOW-Bs1 |
| eno_fw | P-ATGTCCAAAATCGTAAAAATCATCGGTC | MG1655 rne gene |
| eno_rev | P-TTATGCCTGGCCTTTGATCTCTTT | MG1655 rne gene |
fw, forward; P-, 5′ phosphorylation; rev, reverse.
Bacterial Strain Constructions.
MG1655Δenolase and MG1655Δhfq mutant strains were constructed by replacement of the chromosomal eno and hfq gene, respectively, by a kanamycin (Km)-resistant cassette using the λRED system with pKD46 as described (55). MG1655 Rned500–1061, Rned823–850, Rned984–1011, MG1655 Rne-Bs1, Rned823–850-Bs1, and Rned984–1011-Bs1 mutant strains were constructed using a modified Datsenko and Wanner method (Fig. S3). Briefly, template pBAD-Rnewt-Km plasmid was generated in a single step by ligation of the pBAD-Rnewt plasmid digested by EcoRI with the Km-resistant cassette amplified from the pKD4 (55) plasmid (EcoRI restriction sites were added at the 3′ and 5′ ends of the cassette sequence). Thereafter, based on this plasmid, we performed inverse PCR to generate the pBAD-Rned500–1061-Km, pBAD-Rned823–850-Km, and pBAD-Rned984–1011-Km template plasmids used for Rned500–1061, Rned823–850, and Rned984–1011 mutant constructions, respectively. The template plasmid pBAD-Rne-l-Bs1-l-Bs1-Km was used for construction of mutants expressing RNase E polypeptide C-terminally fused with two fluorescence Bs1 proteins (to increase fluorescence signal) in tandem through linkers. The linker sequence contained 60 nucleotides (5′-CTC GCT GAG GCC GCC GCT AAA GAG GCC GCC GCT AAA GAG GCC GCC GCT AAA GCG GCC GCC-3′) and encoded 20 amino acids (LAEAAAKLAAAKEAAAKAAA), which formed an α-helix structure without any electronic charge (56). The plasmid was generated as follows: (i) the intermediate plasmid pBAD-Rnewt-l-Bs1-Km was created by blunt-ended ligation of reamplified pBAD-Rnewt-Km with amplified bs1 gene plus the linker sequence from the 5′ end of bs1 using pGLOW-Bs1 (ApR; Jena Bioscience) as a template. Within this step, we also added a SacI restriction site at the 5′ end and a NcoI restriction site at the 3′ end of the linker sequence. (ii) A second copy of the bs1 gene with the linker was inserted in tandem after the first copy of bs1 by blunt-ended ligation of reamplified pBAD-Rnewt-l-Bs1-Km with the amplified bs1 gene and linker (see above) to obtain pBAD-Rnewt-l-Bs1-l-Bs1-Km. Thereafter, based on this plasmid, we performed inverse PCR to generate the pBAD-Rned823–850-l-Bs1-l-Bs1-Km and pBAD-Rned984–1011-l-Bs1-l-Bs1-Km template plasmids used in this study for Rned823–850-Bs1 and Rned984–1011-Bs1 mutant constructions. Two hundred nanograms of each purified PCR product amplified by rne_fw/rne_rev primers (Table S3) using corresponding plasmids as templates was transformed into the cells by electroporation, which were then incubated in LB medium for 1 h at 37 °C. Mutants were directly selected as kanamycin-resistant colonies after incubation overnight at 37 °C on LB agar plates with kanamycin (25 µg/mL). After primary selection, individual colonies were screened for ampicillin sensitivity from the loss of the helper plasmid pKD46. Each plasmid construct was confirmed by DNA sequencing.
Western Blotting.
Western blot analysis was performed as previously described (57). Briefly, aliquots containing equal amounts of the whole-cell lysate (or equal protein aliquots from the fraction containing Flag-RNase E or its derivatives) were diluted with one volume of 2× sample buffer [100 mM Tris⋅HCl (pH 6.8), 4% SDS, 25% glycerol, 0.002% Bromophenol blue, 5% 2-mercaptoethanol] and heated at 95 °C for 5 min. The samples were ice-cooled and separated in 10% SDS polyacrylamide gels as described (58) and then transferred to PVDF Immobilon P membranes (Millipore) using a Mini Trans-Blot Cell (Bio-Rad) apparatus [100 V and constant 350 mA for 1 h, 4 °C; 1× transfer buffer: 25 mM Tris (pH 8.3), 192 mM glycine, 20% MeOH, and 0.1% SDS]. After blocking by SuperBlock T20 (TBS) Blocking Buffer (Thermo Scientific) for 30 min at RT, the membranes were washed three times by 1× Tris-buffered saline with Tween 20 buffer (TBST: 20 mM Tris, 150 mM NaCl and 0.1% Tween-20, pH 7.5) for 5 min at RT and were cut into smaller pieces and then incubated for 1 h at RT or overnight at 4 °C with individual primary polyclonal rabbit antibodies specific for degradosome components (anti-RNase E, anti-RhlB, anti-enolase, anti-PNPase) (3), RNA chaperone Hfq (anti-Hfq), bacterial cell division proteins (anti-FtsA, anti-FtsZ, anti-ZipA) (59), or membrane protein TolB used as a loading control (anti-TolB) (11), and monoclonal mouse anti-Flag (Sigma-Aldrich) or anti-GAPDH (SignalChem). The membranes were washed three times by 1× TBST buffer for 5 min at RT and then were incubated for 1 h at RT with secondary anti-IgG antibodies conjugated with HRP (GE Healthcare). The membranes were washed three times by 1× TBST buffer for 5 min at RT, and the protein bands recognized by each specific antibody were detected using an ECL Western blotting detection kit (GE Healthcare) and visualized by exposure of membranes to Classic Blue BX (MIDSCI) autoradiography films or captured by a BioSpectrum 600 Imaging System (UVP). The antibodies specific for the bacterial cell-division proteins (FtsA, FtsZ, and ZipA) and TolB were kindly provided by P. de Boer, Case Western Reserve University, Cleveland, OH, and K.-F. Chak, National Yang-Ming University, Taipei, Taiwan, respectively.
Measurement of Protein Stability.
The cells were grown aerobically or anaerobically as described above to OD460 ∼0.3. Measurement of RNase E, FtsZ, FtsA, and ZipA protein stability was performed as described (60). Briefly, a portion of cells was collected for the zero time point, and then chloramphenicol was added to the remaining culture (200 µg/mL) to completely inhibit new protein synthesis. Cells were collected at the indicated times (2, 4, 8, 16, 32, and 64 min), lysed, and assayed by a Western blot as described above to determine the amount of FtsZ, FtsA, and ZipA proteins.
Microscopy Imaging.
For cell imaging, 2 μL of the cell culture was added to the middle of a glass plate and then was covered with a cover slide and sealed. Differential interference contrast (DIC) images were captured using LSM510META-NLO (Carl Zeiss) or Axio Imager Z1 (Carl Zeiss) microscopes with 100× magnification. For anaerobically grown E. coli cells, the culture vessel was sealed and transferred for sampling into a 1025 Anaerobic System Glove Box (Forma Scientific). For aerobically grown E. coli cells, the sampling was archived in the laminar flow hood under normal conditions. The images were analyzed by ZEN 2.1 Blue Edition (Carl Zeiss).
For image-based cell-size calculation, transmitted light images were acquired at 60× magnification on an ImageXpress Micro XL Imaging System (Molecular Devices Inc.), and the lengths of cells were measured in micrometers using the image analysis package MetaMorph v7.6 (Molecular Devices Inc.).
For image-based cell counting, MG1655 cells were cultured aerobically or anaerobically in M9 medium supplemented with 0.4% glucose at 37 °C and 200 rpm until OD460 ∼0.4. Then, 1 mL of culture was withdrawn and added to stop solution (6:1, vol/vol); 1.8 µL of the culture was placed on a glass plate, covered, and sealed. Five randomly chosen tiles (0.5 × 0.5 mm) were imaged for each condition using an Axio Imager Z1 (Zeiss). Bacterial cell counts were obtained with MetaMorph v7.6 (Molecular Devices Inc.).
To check for the presence of septa or for subcellular localization of RNase E and enolase, we captured images of aerobically or anaerobically grown E. coli using a DeltaVision 3D deconvolution Olympus IX70 microscope (Olympus) and analyzed them with Imaris v8.4.1 software (Bitplane Inc.). For membrane and DNA visualization, 1 ng/μL FM 4-64 dye (Life Technologies) and 1 ng/μL Hoechst 33342 dye (Thermo Scientific), respectively, was added to cell cultures and incubated for 10 min at 37 °C before imaging.
RNA Isolation and Northern Blot Analysis.
Total RNA was isolated as described previously (61). Briefly, aliquots of cell cultures grown in M9/glucose medium as described above until OD460 ∼0.4 (or equivalent to OD460 ∼0.4) were mixed with cold stop solution (5% phenol in ethanol) at an 8:1 ratio. The cells were collected by centrifugation (15 min, 4 °C, 4,000 × g), suspended in 2 mL of lysis buffer [200 mM NaCl, 20 mM Tris–HCl (pH 7.5), 40 mM EDTA, 0.5% SDS], and incubated at 95 °C for 30 s. RNA was extracted from the cell lysates by acidic phenol (pH = 4.5), precipitated with isopropanol, washed with 80% ethanol, vacuum-dried for 3 min, and dissolved in RNase-free water (Ambion). Aliquots of total RNA (5 μg each or 15 μg for Rned823–850 mutant) were individually mixed with equal volumes of 2× RNA loading dye (0.03% Bromophenol Blue, 0.03% Xylene Cyanol FF, 0.5 mM EDTA in formamide), incubated at 65 °C for 10 min, chilled on ice, and separated on 3.5% (for ftsZ transcript) or 8% (for dicF transcript) polyacrylamide–urea gels. The fractionated RNAs were transferred to Zeta-Probe blotting membranes (Bio-Rad) by capillary method (overnight) and UV cross-linked (1,200 mJ) using Stratalinker UV Crosslinker 2400 (Stratagene). RNAs were colored by 0.3% methylene blue/0.3 M sodium acetate solution (pH = 5.2) for 1 min, further washed with milliQ water for 5 min on a shaker, and scanned by an Epson Perfection 4990 Photo scanner. The membranes were cut into smaller pieces and, after prehybridization with ULTRAhyb Hybridization Buffer (Ambion) at 65 °C (for internally labeled RNA probes) or ULTRAhyb-Oligo Hybridization Buffer (Ambion) at 42 °C (for DNA oligonucleotide probe) for 3 h, they were further hybridized with the specific internal [α-32P]ATP–labeled oligodeoxynucleotide (Table S4) or 5′-[γ-32P]ATP–labeled oligonucleotide probe (5′-ATTACTGACTGGGGCGGCTAAAATATTCAGCCAAATCCGATTACGTGAAGTAAAAGGTCT-3′) used to detect Spot42 RNAs. The internally labeled RNA probes were synthesized using an in vitro transcription kit (MAXIscript; Ambion) and DNA probe was 5′-labeled using T4 polynucleotide kinase (T4 PNK; Thermo Scientific) according to the manufacturer’s instructions. DNA templates for transcription of individual RNA probes were generated by PCR using gene-specific primers (Table S4). For size markers, we used RiboReady Color RNA Ladders (Amresco). After hybridization at 65 °C (internally labeled probes) or 42 °C (oligonucleotide probes) overnight, the membranes were washed twice with 20 mL of preheated wash buffer [5× SSC with 0.5% (wt/vol) SDS] at 65 °C (internally labeled probes) or 42 °C (oligonucleotide probes) for 30 min and exposed to Phosphor Imaging plates (FujiFilm) at −80 °C for 12–48 h. The RNA bands were detected using a Typhoon FLA 9000 Biomolecular Imager (GE Healthcare), and the relative amounts of individual RNA species in each band were calculated by normalizing its signal to either a 5S or a 16S rRNA signal.
Table S4.
Oligonucleotide primers used to amplify templates for in vitro transcription of RNA probes
| Name | Sequence (5′ to 3′) | Product length, bp | Target |
| FtsZ_fw | CCAATGACGCGGTGATTAAAG | 502 | ftsZ |
| FtsZ_rev | TAATACGACTCACTATAGGGCAGTTTGTCGTTCGGGATAGT | ||
| Probe1_fw | GTTATTCCTTCCCCGTTGAGGACACCGGGTTGTCAGGTTGACCATA | 66 | dicA-dicB intergenic region |
| Probe1_rev | TAATACGACTCACTATAGGGTATGGTCAACCTGACAACCCGGTGTCCTCAACGGGGAAGGAATAAC | ||
| Probe2_fw | TTTCTGGTGACGTTTGGCGGTATCAGTTTTACTCCGTGACTGCTCTGCCGCCCCCCTATAGTGAGTCGTATTA | 73 | dicF |
| Probe2_rev | TAATACGACTCACTATAGGGGGGCGGCAGAGCAGTCACGGAGTAAAACTGATACCGCCAAACGTCACCAGAAA |
Underlined nucleotides in the reverse primer correspond to T7 promoter; fw, forward; rev, reverse.
Induction of Cell Filamentation.
Cell filamentation was induced by potassium dichromate (K2Cr2O7) or cephalexin as previously described (62, 63). Briefly, the E. coli MG1655 or Rned823–850 strains were aerobically grown in M9 medium supplemented with 0.4% glucose to an optical density of 0.2, before being incubated with K2Cr2O7 (32 µM) or cephalexin (20 µM) for 180 min. Samples were then subjected to microscopic analysis.
Bioinformatics.
We used the Robetta prediction server (robetta.bakerlab.org) for de novo prediction of C-terminal RNase E (amino acids 511–1061). Within the predicted structure (Fig. S4A), we used 28-aa regions (i.e., the same number of amino acids as for the enolase-binding region) for construction of control strains. To minimize disruption of the predicted secondary structure of RNase E, we used unstructured regions or entire α- or β-structures for the construction of mutants. We chose six regions for deletion (Fig. S4 B–G) and constructed six mutants. We subsequently checked the cell phenotype of all six mutants under anaerobic conditions and found that the Rned984–1011 mutant had growth characteristics and a filamentous morphology under anaerobic conditions similar to the MG1655 strain (Fig. S2). Therefore, we used the Rned984–1011 mutant as a control strain in our experiments.
We used the BioCyc Database Collection (https://biocyc.org) to determine the number of dicF copies in the genomes of different E. coli strains.
Other Methods.
Plasmids were isolated using the HiYield Plasmid Kit Mini purification kit (Arrowtec) according to the manufacturer’s instructions. DNA samples for PCR were obtained by dispersing fresh bacterial colonies in 100 μL of sterile milliQ water, heating at 95 °C for 5 min, and then cooling on ice as described (64). PCR was performed using PfuUltra II Hotstart 2× PCR Master Mix (Agilent Technologies, Inc.). Amplifications were carried out in 50-μL volumes containing 0.4 μM reverse and forward primers and 1 μL of DNA sample (or 20 ng vector DNA) using a Veriti 96-well Thermal Cycler (Applied Biosystems). PCR was initiated by denaturation at 95 °C for 2 min, followed by 35 cycles of 95 °C for 20 s, 53 °C for 20 s, and 72 °C for 30 s (for eno and hfq genes and the dicA–dicB intergenic region) or 3 min (for rne gene or plasmids reamplification). Amplification products were visualized by 1% agarose gel electrophoresis and ethidium bromide (0.025%) staining to assess the sizes of the gene fragments. Products were purified using the HiYield Gel/PCR DNA Fragment Extraction Kit (Arrowtec) according to the manufacturer’s instructions. PCR product concentrations were quantified using a NanoDrop ND-1000 spectrophotometer (J&H Technology Co., Ltd). When necessary, PCR products were ligated by the Rapid DNA Ligation Kit (Thermo Scientific) according to the manufacturer’s instructions. The ligation mixture was used directly for bacterial transformation by the calcium chloride method (65). Plasmid constructs and purified PCR products were confirmed by DNA sequencing at the Institute of Biomedical Sciences DNA Sequencing Core, Academia Sinica.
Discussion
RNase E Enzymatic Activity and E. coli Cell Morphology.
A previous study (28) showed that strains carrying the rne-3071 mutation, which renders activity of RNase E thermolabile, fail to divide and grow as filaments at the nonpermissive temperature of 43 °C. Some filaments grew as big as 12 µm and contained up to four constriction sites. Over 75% of the filaments appeared to have at least one incomplete septum. Thus, it appears that the loss of RNase E activity is responsible for an early loss of the cell’s capacity to divide. In that study, the E. coli cells stopped cell growth/division nearly 3 h after being shifted to the nonpermissive temperature, but growth resumed when the culture was shifted back to 37 °C. In contrast, the anaerobically induced filamentous cells in our study are viable and continue to grow under anaerobic conditions. These cells have the ability to accumulate cell biomass under anaerobic conditions. Anaerobically induced cell filamentation was reversible, and the MG1655 strain reverted to a rod-shape morphology within ∼3 h after being returned to aerobic conditions (Fig. 1 D, b).
Although the absence of RNase E is lethal, the rne-deletion strain can be made viable by RNase G overexpression. Filamentous morphology was observed during culture in liquid medium under normal growth conditions in the KSL2000 strain, in which a chromosomal deletion in rne was complemented by a plasmid-borne rng gene under the control of an arabinose gene promoter (30). This filamentous morphology was completely reversed by complementation with full-length RNase E (30). We found that ectopic expression of RNase E also induced reversion to a rod-shaped morphology under anaerobic conditions (Fig. 1 E, a and b). Thus, RNase E enzymatic activity clearly has a role in determining E. coli cell morphology under both conditions. However, in the rne temperature-sensitive strain N3431 the levels of 53-nt DicF are very low (almost undetectable) under both permissive and nonpermissive temperatures (Fig. S5F), and the functional role of such a low level of sRNA under these conditions is therefore probably negligible.
Regulation of RNase E Activity.
Activity of RNase E can be regulated by inhibitors such as RraA, RraB (10), and ribosomal protein L4 (11). Previously, we found that RNase E associated with a membrane has increased enzymatic activity and substrate affinity (6), suggesting that compartmentation of RNase E plays a role in regulating its enzymatic activity in vivo. Moreover, here we have shown that RNase E has different subcellular distributions in the MG1655 and Rned984–1011 derivative strains. RNase E distribution depends on the presence or absence of oxygen, with the enzyme being tethered to the cytoplasmic membrane under aerobic conditions or being distributed in the cytoplasm under anaerobic conditions. We also found that RNA turnover and cleavage of the DicF precursor were faster under aerobic conditions than under anaerobic conditions in the MG1655 and Rned984–1011 strains. This result is consistent with the idea that RNase E substrate specificity/activity is differentially regulated by its subcellular distribution in the cell.
Enolasic RNA Degradosomes Are Necessary for Anaerobic-Responsive DicF RNA Degradation.
We have shown that enolase alters the subcellular localization of RNase E from the cytoplasmic membrane to diffuse patterns when it binds to the RNase E scaffolding region (Fig. 6B) and that cells became filamentous under anaerobic conditions (Fig. 1 B, a and b). Our findings also suggest that degradation of certain RNA species, such as sRNA DicF, takes place near the membrane and depends on high RNase E activity. Other RNAs found in the cytoplasm do not require high RNase E activity for their degradation. Although the deletion of the enolase-binding site on RNase E does not affect the association of Hfq with RNase E (Fig. S6B), we cannot exclude other possibilities, such as a mechanism by which Hfq protects sRNA from degradation that is differentially regulated by some unknown mechanism(s) related to RNase E or sRNA subcellular distributions.
RNase E degradosomes have been shown to be tethered to the cytoplasmic membrane by the N-terminal region of RNase E (amino acids 1–602) (5). Subsequently, an RNase E region (segment A; amino acids 562–587) that was proposed to have the propensity to form an amphipathic α-helix was found to be required for RNase E binding to cytoplasmic membranes (31). Recently, it was found that the N-terminal 499-aa residues of RNase E (NRne) interact with the cytoplasmic membrane through electrostatic interactions (6). NRne membrane-binding induces a structural change in NRne that may lead to a flexible protein conformation (6). In this study, we show that the subcellular membrane distribution of RNase E is the same in the MG1655 Rne-Bs1, Rned823–850-Bs1, and Rned984–1011-Bs1 variants under aerobic conditions, which indicates that the conformation of these RNase E derivatives is not altered. In contrast, the subcellular RNase E distribution is altered (to a diffuse pattern) under anaerobic conditions in the MG1655 Rne-Bs1 and Rned984–1011-Bs1 strains but not in Rned823–850, suggesting that the physical interaction of RNase E with enolase changes the protein conformation under those conditions, leading to an altered subcellular distribution of RNase E.
Enolase from the anaerobic Gram-negative bacterium Bacteroides fragilis, frequently found in intestines and known to cause intraabdominal infections, was found to be a heme-binding protein (32). It has been speculated that the original evolutionary function of hemoproteins was electron transfer in ancestral cyanobacteria-like organisms before the appearance of molecular oxygen (33). Upon binding of a diatomic gas, such as O2, CO, or NO, heme undergoes conformational changes (34) that can induce conformational changes in its interacting partner protein(s). We hypothesize that enolase in the E. coli enolasic degradosome is a heme-binding protein and that the binding of the diatomic gas to the heme protein induces conformational changes in RNase E/degradosomes. As a result, RNase E/degradosome attachment or detachment from the membrane can be regulated in response to oxygen levels. Whether the RNase E inhibitors RraA, RraB, or L4 can change the subcellular localization of RNase E is unknown.
Enolase in the RNA degradosome has been reported to be involved in the rapid decay of glucose transporter ptsG mRNA under aerobic growth conditions (35). Bacteria should have different strategies to regulate RNA levels to adapt their metabolism and thereby survive in different conditions. We suggest that alteration of RNase E cleavage by enolase in the degradosome may depend on factors such as environmental growth conditions, substrate specificity, and substrate availability, which allow the degradosome to quickly target specific RNA species for degradation.
Functions of Enolase Outside Glycolytic Metabolism and Its Role in Anaerobically Induced Cell Filamentation.
Enolase is primarily known for its role in glycolysis, a metabolic pathway involved in ATP and NADH production. It also plays a central role in metabolism by providing its intermediates to several other pathway processes (36). This metabolic pathway is particularly essential under anaerobic conditions. Enolase is essential for sustaining bacterial growth, with E. coli mutants with a disrupted eno gene being unable to grow without additional medium supplements such as pyruvate, glycerate, or succinate. It is known that other glycolytic enzymes, such as phosphoglucose isomerase and glyceraldehyde-3-phosphate dehydrogenase, exhibit other functions in addition to their major enzymatic activities in various organisms (37). We have shown that enolase has a degradosome-specific function and plays a role in altering RNase E cellular distribution/activity, resulting in cells having a filamentous shape under oxygen-limited conditions. The role of enolase in regulating sRNA degradation is another example of one of its multiple, so-called “moonlighting,” functions. In addition, our data suggest that binding of enolase to the cytoplasmic membrane is independent of RNase E, and thus it probably has multiprotein partners on the membrane, such as the glycolytic complex (38).
Oxygen sensing is a fundamental biological process necessary for the adaptation of living organisms to variable habitats and physiological situations. One current model of oxygen sensing is based on a heme protein capable of reversibly binding oxygen (39). Since enolase from another anaerobic Gram-negative bacterium, B. fragilis, is a heme-binding protein (32), we hypothesize that E. coli enolase may be an oxygen-level sensor (see above).
Furthermore, structural analysis of enolase from the anaerobic protozoan Entamoeba histolytica indicates that it contains 2-PGA in the active site and exists in the open conformation; the Mg2+(II) ion is absent from the active site (40). These studies also suggest that anaerobic conditions might change the conformation and oligomerization of E. coli enolase, as well as its ion-binding affinity, thereby altering the activity and function of the enzyme. Anaerobic conditions may also change the conformation of RNase E and major components of the degradosome such as RhlB helicase and PNPase and their enzymatic activities as well as the composition of the degradosome. These topics would be interesting avenues for future research.
Role of Cell Filamentation.
Bacteria are the oldest organisms known, and, interestingly, the first bacteria had a filamentous morphology (41). At that time the Earth’s atmosphere was almost without oxygen (42). Thus, a filamentous morphology was likely the first organismal form.
For many years, filamentous bacteria have been considered to be the overstressed, sick, and dying members of the population (43). More in-depth analyses have revealed that filamentous members of some communities have vital roles in the bacterial population’s continued existence. Recently, filamentation has been implicated in bacterial survival during exposure to environmental stresses, which include host effectors, protist predators, and antimicrobial therapies (43). Differentiation into the filamentous form is also important for the survival of pathogenic bacteria (44–46). It remains unclear whether the molecular mechanisms that lead to filamentation are shared by these scenarios. In fact, there is evidence to suggest that multiple mechanisms are involved in the inhibition of septation that leads to filamentation. Our data provide a mechanism (Fig. 7) by which E. coli and perhaps other pathogenic bacteria can switch from a rod-shaped to the filamentous morphology that might be necessary for survival within host tissues and for virulence. The working model shows how RNase E, enolasic degradosomes, and sRNA regulate cell shape and division (limited septum formation) (Fig. 7). Authentic homologs of RNase E are found in many γ-proteobacteria such as Yersinia pseudotuberculosis (47) and Vibrio angustum (48), and their RNase E interacts with RhlB, enolase, and PNPase to form an RNA degradosome homologous (49) to the multienzyme complex in E. coli. We postulate that other facultative anaerobic and anaerobic bacteria possessing enolasic RNA degradosomes exhibit similar regulation of cell filamentation.
Fig. 7.
RNase E, enolasic degradosomes, and sRNA mediate the regulation of cell shape and division. Growth conditions, the components of degradosomes, and what happens when the bacteria are under aerobic or anaerobic conditions are shown.
Materials and Methods
Bacterial Strains, Plasmids, and Growth Conditions.
Strains, plasmids, and oligonucleotide sequences are in Tables S2–S4. Construction details and growth conditions are provided in SI Materials and Methods.
Additional materials and procedures are provided in SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank Dr. J. O’Brien and G. Cohen for help in editing the manuscript; Dr. K.-F. Chak (National Yang-Ming University) for the specific antibody for TolB; Prof. P. de Boer (Case Western Reserve University) for the specific antibodies for FtsA, FtsZ, and ZipA; W.-S. Wang (S.L.-C. laboratory) for the specific antibodies for Hfq; S.-P. Lee [Institute of Molecular Biology (IMB) Imaging Core Facility, Academia Sinica] for excellent technical support; Dr. J.-D. Huang (University of Hong Kong) for the MG1655ΔdicF strain; Dr. C.-H. Teng (National Cheng Kung University) for the E. coli Nissle1917 strain; and Dr. S.-M. Yu (IMB, Academia Sinica) for providing access to the Anaerobic System Glove Box. This work was supported by Grants 104-2311-B-001-011-MY3 from the Taiwan Ministry of Science and Technology (MOST) and by Grants AS 034006 and 022323 from Academia Sinica (to S.L.-C.). O.N.M. received MOST Postdoctoral Fellowships 104-2811-B-001-094 and 105-2811-B-001-094.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1703731114/-/DCSupplemental.
References
- 1.Mackie GA. RNase E: At the interface of bacterial RNA processing and decay. Nat Rev Microbiol. 2013;11:45–57. doi: 10.1038/nrmicro2930. [DOI] [PubMed] [Google Scholar]
- 2.Kaberdin VR, et al. The endoribonucleolytic N-terminal half of Escherichia coli RNase E is evolutionarily conserved in Synechocystis sp. and other bacteria but not the C-terminal half, which is sufficient for degradosome assembly. Proc Natl Acad Sci USA. 1998;95:11637–11642. doi: 10.1073/pnas.95.20.11637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Miczak A, Kaberdin VR, Wei CL, Lin-Chao S. Proteins associated with RNase E in a multicomponent ribonucleolytic complex. Proc Natl Acad Sci USA. 1996;93:3865–3869. doi: 10.1073/pnas.93.9.3865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Py B, Higgins CF, Krisch HM, Carpousis AJ. A DEAD-box RNA helicase in the Escherichia coli RNA degradosome. Nature. 1996;381:169–172. doi: 10.1038/381169a0. [DOI] [PubMed] [Google Scholar]
- 5.Liou GG, Jane WN, Cohen SN, Lin NS, Lin-Chao S. RNA degradosomes exist in vivo in Escherichia coli as multicomponent complexes associated with the cytoplasmic membrane via the N-terminal region of ribonuclease E. Proc Natl Acad Sci USA. 2001;98:63–68. doi: 10.1073/pnas.011535498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Murashko ON, Kaberdin VR, Lin-Chao S. Membrane binding of Escherichia coli RNase E catalytic domain stabilizes protein structure and increases RNA substrate affinity. Proc Natl Acad Sci USA. 2012;109:7019–7024. doi: 10.1073/pnas.1120181109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bernstein JA, Khodursky AB, Lin PH, Lin-Chao S, Cohen SN. Global analysis of mRNA decay and abundance in Escherichia coli at single-gene resolution using two-color fluorescent DNA microarrays. Proc Natl Acad Sci USA. 2002;99:9697–9702. doi: 10.1073/pnas.112318199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bernstein JA, Lin PH, Cohen SN, Lin-Chao S. Global analysis of Escherichia coli RNA degradosome function using DNA microarrays. Proc Natl Acad Sci USA. 2004;101:2758–2763. doi: 10.1073/pnas.0308747101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tseng YT, Chiou NT, Gogiraju R, Lin-Chao S. The protein interaction of RNA helicase B (RhlB) and polynucleotide phosphorylase (PNPase) Contributes to the homeostatic control of cysteine in Escherichia coli. J Biol Chem. 2015;290:29953–29963. doi: 10.1074/jbc.M115.691881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gao J, et al. Differential modulation of E. coli mRNA abundance by inhibitory proteins that alter the composition of the degradosome. Mol Microbiol. 2006;61:394–406. doi: 10.1111/j.1365-2958.2006.05246.x. [DOI] [PubMed] [Google Scholar]
- 11.Singh D, et al. Regulation of ribonuclease E activity by the L4 ribosomal protein of Escherichia coli. Proc Natl Acad Sci USA. 2009;106:864–869. doi: 10.1073/pnas.0810205106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sawers G. The aerobic/anaerobic interface. Curr Opin Microbiol. 1999;2:181–187. doi: 10.1016/S1369-5274(99)80032-0. [DOI] [PubMed] [Google Scholar]
- 13.Unden G, Bongaerts J. Alternative respiratory pathways of Escherichia coli: Energetics and transcriptional regulation in response to electron acceptors. Biochim Biophys Acta. 1997;1320:217–234. doi: 10.1016/s0005-2728(97)00034-0. [DOI] [PubMed] [Google Scholar]
- 14.Górna MW, Carpousis AJ, Luisi BF. From conformational chaos to robust regulation: The structure and function of the multi-enzyme RNA degradosome. Q Rev Biophys. 2012;45:105–145. doi: 10.1017/S003358351100014X. [DOI] [PubMed] [Google Scholar]
- 15.Nurmohamed S, McKay AR, Robinson CV, Luisi BF. Molecular recognition between Escherichia coli enolase and ribonuclease E. Acta Crystallogr D Biol Crystallogr. 2010;66:1036–1040. doi: 10.1107/S0907444910030015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rico AI, Krupka M, Vicente M. In the beginning, Escherichia coli assembled the proto-ring: An initial phase of division. J Biol Chem. 2013;288:20830–20836. doi: 10.1074/jbc.R113.479519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Faubladier M, Bouché JP. Division inhibition gene dicF of Escherichia coli reveals a widespread group of prophage sequences in bacterial genomes. J Bacteriol. 1994;176:1150–1156. doi: 10.1128/jb.176.4.1150-1156.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Faubladier M, Cam K, Bouché JP. Escherichia coli cell division inhibitor DicF-RNA of the dicB operon. Evidence for its generation in vivo by transcription termination and by RNase III and RNase E-dependent processing. J Mol Biol. 1990;212:461–471. doi: 10.1016/0022-2836(90)90325-G. [DOI] [PubMed] [Google Scholar]
- 19.Balasubramanian D, Ragunathan PT, Fei J, Vanderpool CK. A prophage-encoded small RNA controls metabolism and cell division in Escherichia coli. mSystems. 2016;1:e00021-15. doi: 10.1128/mSystems.00021-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jin Y, Watt RM, Danchin A, Huang JD. Small noncoding RNA GcvB is a novel regulator of acid resistance in Escherichia coli. BMC Genomics. 2009;10:165. doi: 10.1186/1471-2164-10-165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tétart F, Bouché JP. Regulation of the expression of the cell-cycle gene ftsZ by DicF antisense RNA. Division does not require a fixed number of FtsZ molecules. Mol Microbiol. 1992;6:615–620. doi: 10.1111/j.1365-2958.1992.tb01508.x. [DOI] [PubMed] [Google Scholar]
- 22.Aiba H. Mechanism of RNA silencing by Hfq-binding small RNAs. Curr Opin Microbiol. 2007;10:134–139. doi: 10.1016/j.mib.2007.03.010. [DOI] [PubMed] [Google Scholar]
- 23.Vogel J, Luisi BF. Hfq and its constellation of RNA. Nat Rev Microbiol. 2011;9:578–589. doi: 10.1038/nrmicro2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moll I, Afonyushkin T, Vytvytska O, Kaberdin VR, Bläsi U. Coincident Hfq binding and RNase E cleavage sites on mRNA and small regulatory RNAs. RNA. 2003;9:1308–1314. doi: 10.1261/rna.5850703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Møller T, et al. Hfq: A bacterial Sm-like protein that mediates RNA-RNA interaction. Mol Cell. 2002;9:23–30. doi: 10.1016/s1097-2765(01)00436-1. [DOI] [PubMed] [Google Scholar]
- 26.Sahagan BG, Dahlberg JE. A small, unstable RNA molecule of Escherichia coli: Spot 42 RNA. I. Nucleotide sequence analysis. J Mol Biol. 1979;131:573–592. doi: 10.1016/0022-2836(79)90008-1. [DOI] [PubMed] [Google Scholar]
- 27.Zhang A, et al. Global analysis of small RNA and mRNA targets of Hfq. Mol Microbiol. 2003;50:1111–1124. doi: 10.1046/j.1365-2958.2003.03734.x. [DOI] [PubMed] [Google Scholar]
- 28.Goldblum K, Apririon D. Inactivation of the ribonucleic acid-processing enzyme ribonuclease E blocks cell division. J Bacteriol. 1981;146:128–132. doi: 10.1128/jb.146.1.128-132.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Drepper T, et al. Reporter proteins for in vivo fluorescence without oxygen. Nat Biotechnol. 2007;25:443–445. doi: 10.1038/nbt1293. [DOI] [PubMed] [Google Scholar]
- 30.Tamura M, et al. RNase E maintenance of proper FtsZ/FtsA ratio required for nonfilamentous growth of Escherichia coli cells but not for colony-forming ability. J Bacteriol. 2006;188:5145–5152. doi: 10.1128/JB.00367-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Khemici V, Poljak L, Luisi BF, Carpousis AJ. The RNase E of Escherichia coli is a membrane-binding protein. Mol Microbiol. 2008;70:799–813. doi: 10.1111/j.1365-2958.2008.06454.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Izumi A, Otto B, Heddle J, Park SY, Tame JRH. 2P005 crystal structure of heme binding protein P46 from Bacteroides fragilis. Seibutsu Butsuri. 2004;44:S111. [Google Scholar]
- 33.Dewilde S, Van Hauwaert ML, Peeters K, Vanfleteren J, Moens L. Daphnia pulex didomain hemoglobin: Structure and evolution of polymeric hemoglobins and their coding genes. Mol Biol Evol. 1999;16:1208–1218. doi: 10.1093/oxfordjournals.molbev.a026211. [DOI] [PubMed] [Google Scholar]
- 34.Milani M, et al. Structural bases for heme binding and diatomic ligand recognition in truncated hemoglobins. J Inorg Biochem. 2005;99:97–109. doi: 10.1016/j.jinorgbio.2004.10.035. [DOI] [PubMed] [Google Scholar]
- 35.Morita T, Kawamoto H, Mizota T, Inada T, Aiba H. Enolase in the RNA degradosome plays a crucial role in the rapid decay of glucose transporter mRNA in the response to phosphosugar stress in Escherichia coli. Mol Microbiol. 2004;54:1063–1075. doi: 10.1111/j.1365-2958.2004.04329.x. [DOI] [PubMed] [Google Scholar]
- 36.Díaz-Ramos A, Roig-Borrellas A, García-Melero A, López-Alemany R. α-Enolase, a multifunctional protein: Its role on pathophysiological situations. J Biomed Biotechnol. 2012;2012:156795. doi: 10.1155/2012/156795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kim JW, Dang CV. Multifaceted roles of glycolytic enzymes. Trends Biochem Sci. 2005;30:142–150. doi: 10.1016/j.tibs.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 38.Campanella ME, Chu H, Low PS. Assembly and regulation of a glycolytic enzyme complex on the human erythrocyte membrane. Proc Natl Acad Sci USA. 2005;102:2402–2407. doi: 10.1073/pnas.0409741102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lopez-Barneo J, Pardal R, Ortega-Sáenz P. Cellular mechanism of oxygen sensing. Annu Rev Physiol. 2001;63:259–287. doi: 10.1146/annurev.physiol.63.1.259. [DOI] [PubMed] [Google Scholar]
- 40.Schulz EC, Tietzel M, Tovy A, Ankri S, Ficner R. Structure analysis of Entamoeba histolytica enolase. Acta Crystallogr D Biol Crystallogr. 2011;67:619–627. doi: 10.1107/S0907444911016544. [DOI] [PubMed] [Google Scholar]
- 41.Taylor EL, Taylor TN, Krings M. Precambrian life. In: Thomas NT, editor. Paleobotany: The Biology and Evolution of Fossil Plants. Academic; San Diego: 2009. pp. 43–70. [Google Scholar]
- 42.Lyons TW, Reinhard CT, Planavsky NJ. The rise of oxygen in Earth’s early ocean and atmosphere. Nature. 2014;506:307–315. doi: 10.1038/nature13068. [DOI] [PubMed] [Google Scholar]
- 43.Justice SS, Hunstad DA, Cegelski L, Hultgren SJ. Morphological plasticity as a bacterial survival strategy. Nat Rev Microbiol. 2008;6:162–168. doi: 10.1038/nrmicro1820. [DOI] [PubMed] [Google Scholar]
- 44.Chauhan A, et al. Mycobacterium tuberculosis cells growing in macrophages are filamentous and deficient in FtsZ rings. J Bacteriol. 2006;188:1856–1865. doi: 10.1128/JB.188.5.1856-1865.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Piao Z, Sze CC, Barysheva O, Iida K, Yoshida S. Temperature-regulated formation of mycelial mat-like biofilms by Legionella pneumophila. Appl Environ Microbiol. 2006;72:1613–1622. doi: 10.1128/AEM.72.2.1613-1622.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Stackhouse RR, Faith NG, Kaspar CW, Czuprynski CJ, Wong AC. Survival and virulence of Salmonella enterica serovar enteritidis filaments induced by reduced water activity. Appl Environ Microbiol. 2012;78:2213–2220. doi: 10.1128/AEM.06774-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yang J, Jain C, Schesser K. RNase E regulates the Yersinia type 3 secretion system. J Bacteriol. 2008;190:3774–3778. doi: 10.1128/JB.00147-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Erce MA, Low JK, Wilkins MR. Analysis of the RNA degradosome complex in Vibrio angustum S14. FEBS J. 2010;277:5161–5173. doi: 10.1111/j.1742-4658.2010.07934.x. [DOI] [PubMed] [Google Scholar]
- 49.Aït-Bara S, Carpousis AJ. RNA degradosomes in bacteria and chloroplasts: Classification, distribution and evolution of RNase E homologs. Mol Microbiol. 2015;97:1021–1135. doi: 10.1111/mmi.13095. [DOI] [PubMed] [Google Scholar]
- 50.Sambrook J, Fritsch EF, Maniatis T. Molecular Cloning: A Laboratory Manual. 2nd Ed Cold Spring Harbor Lab Press; Cold Spring Harbor, NY: 1989. [Google Scholar]
- 51.Omilusik K. The role of metabolic by-product secretion by Escherichia coli in relation to intracellular NADH concentration and re-dox potential. J Exp Microbiol Immunol. 2001;1:1–8. [Google Scholar]
- 52.Covert MW, Knight EM, Reed JL, Herrgard MJ, Palsson BO. Integrating high-throughput and computational data elucidates bacterial networks. Nature. 2004;429:92–96. doi: 10.1038/nature02456. [DOI] [PubMed] [Google Scholar]
- 53.Wlaschin AP, Trinh CT, Carlson R, Srienc F. The fractional contributions of elementary modes to the metabolism of Escherichia coli and their estimation from reaction entropies. Metab Eng. 2006;8:338–352. doi: 10.1016/j.ymben.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 54.Hemsley A, Arnheim N, Toney MD, Cortopassi G, Galas DJ. A simple method for site-directed mutagenesis using the polymerase chain reaction. Nucleic Acids Res. 1989;17:6545–6551. doi: 10.1093/nar/17.16.6545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Datsenko KA, Wanner BL. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc Natl Acad Sci USA. 2000;97:6640–6645. doi: 10.1073/pnas.120163297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Arai R, Ueda H, Kitayama A, Kamiya N, Nagamune T. Design of the linkers which effectively separate domains of a bifunctional fusion protein. Protein Eng. 2001;14:529–532. doi: 10.1093/protein/14.8.529. [DOI] [PubMed] [Google Scholar]
- 57.Towbin H, Staehelin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: Procedure and some applications. Proc Natl Acad Sci USA. 1979;76:4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 59.Hale CA, de Boer PA. Recruitment of ZipA to the septal ring of Escherichia coli is dependent on FtsZ and independent of FtsA. J Bacteriol. 1999;181:167–176. doi: 10.1128/jb.181.1.167-176.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chen C, Deutscher MP. RNase R is a highly unstable protein regulated by growth phase and stress. RNA. 2010;16:667–672. doi: 10.1261/rna.1981010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lin-Chao S, Cohen SN. The rate of processing and degradation of antisense RNAI regulates the replication of ColE1-type plasmids in vivo. Cell. 1991;65:1233–1242. doi: 10.1016/0092-8674(91)90018-t. [DOI] [PubMed] [Google Scholar]
- 62.Brandi G, Schiavano GF, Albano A, Cattabeni F, Cantoni O. The effect of K2Cr2O7 on the growth and morphology of Escherichia coli. Biol Trace Elem Res. 1989;21:271–275. doi: 10.1007/BF02917263. [DOI] [PubMed] [Google Scholar]
- 63.Scheu PD, Steinmetz PA, Dempwolff F, Graumann PL, Unden G. Polar localization of a tripartite complex of the two-component system DcuS/DcuR and the transporter DctA in Escherichia coli depends on the sensor kinase DcuS. PLoS One. 2014;9:e115534. doi: 10.1371/journal.pone.0115534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hammarlöf DL, Liljas L, Hughes D. Temperature-sensitive mutants of RNase E in Salmonella enterica. J Bacteriol. 2011;193:6639–6650. doi: 10.1128/JB.05868-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Dagert M, Ehrlich SD. Prolonged incubation in calcium chloride improves the competence of Escherichia coli cells. Gene. 1979;6:23–28. doi: 10.1016/0378-1119(79)90082-9. [DOI] [PubMed] [Google Scholar]
- 66.Blattner FR, et al. The complete genome sequence of Escherichia coli K-12. Science. 1997;277:1453–1462. doi: 10.1126/science.277.5331.1453. [DOI] [PubMed] [Google Scholar]
- 67.Reister M, et al. Complete genome sequence of the gram-negative probiotic Escherichia coli strain Nissle 1917. J Biotechnol. 2014;187:106–107. doi: 10.1016/j.jbiotec.2014.07.442. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.