Significance
Research of the past decades has shown that biodiversity promotes ecosystem functions including primary productivity. However, most studies focused on experimental communities at small spatial scales, and little is known about how these findings scale to nonexperimental, real-world ecosystems at large spatial scales, despite these systems providing essential ecosystem services to humans. Here, we show that primary productivity, its temporal stability, and the decadal trend of a prolonged growing season strongly increase with biodiversity across heterogeneous landscapes, which is consistent over vast environmental, climatic, and altitudinal gradients. Our findings thus underline the critical role biodiversity plays for ecosystem functioning and responses to environmental change in heterogeneous, real-world ecosystems at the landscape scale.
Keywords: ecosystem function and services; EVI and NDVI land surface phenology; large spatial scale; nonexperimental, real-world ecosystems; plant, bird, and butterfly species richness
Abstract
Experiments have shown positive biodiversity-ecosystem functioning (BEF) relationships in small plots with model communities established from species pools typically comprising few dozen species. Whether patterns found can be extrapolated to complex, nonexperimental, real-world landscapes that provide ecosystem services to humans remains unclear. Here, we combine species inventories from a large-scale network of 447 1-km2 plots with remotely sensed indices of primary productivity (years 2000–2015). We show that landscape-scale productivity and its temporal stability increase with the diversity of plants and other taxa. Effects of biodiversity indicators on productivity were comparable in size to effects of other important drivers related to climate, topography, and land cover. These effects occurred in plots that integrated different ecosystem types (i.e., metaecosystems) and were consistent over vast environmental and altitudinal gradients. The BEF relations we report are as strong or even exceed the ones found in small-scale experiments, despite different community assembly processes and a species pool comprising nearly 2,000 vascular plant species. Growing season length increased progressively over the observation period, and this shift was accelerated in more diverse plots, suggesting that a large species pool is important for adaption to climate change. Our study further implies that abiotic global-change drivers may mediate ecosystem functioning through biodiversity changes.
Field and laboratory studies in which the diversity of plant species was experimentally manipulated have demonstrated that species loss can decrease many ecosystem functions including primary productivity (1) and its temporal stability (2). These biodiversity-ecosystem functioning (BEF) studies have revealed generally positive effects of species richness on primary productivity and also shed light on the mechanisms that promote productivity under these conditions (3). Niche differentiation among species can enhance community-level productivity through complementary resource use, decreased competition (3), and reduced density-dependent herbivore and pathogen pressure (4). Facilitation can increase community-level productivity via positive effects of one species on another. For example, legumes often symbiotically fix atmospheric dinitrogen which subsequently becomes available also to nonlegume species (3). Finally, positive sampling effects occur if more diverse communities include species with high productivity and these species reach dominance (3). Although there is considerable variability among the ecosystems investigated, biodiversity effects on primary productivity generally are substantial with metaanalyses showing that they can be as large as effects of other drivers of environmental change such as drought, fire, or eutrophication (5, 6).
Field experiments in which the diversity of communities is manipulated can only address effects that occur at the scale of small plots (typically <100 m2), and in newly created ecosystems over relatively short periods of time (7). Also, the communities used in these studies typically are comprised of a random selection of species from relatively small species pools of one trophic group of organisms, in most cases plants (7). These settings markedly contrast conditions in nonexperimental, natural, or seminatural, “real-world” ecosystems (8). In such systems, the diversity of multiple trophic groups often varies in concert (9) and the composition of communities is determined by nonrandom community assembly processes (8). Nonexperimental ecosystems typically are more complex and closer to steady state than experimental plots in which plant species assemblages often need to be maintained by regular weeding (10). Finally, experimental studies, even if they are repeated across large geographic scales (11), lack the landscape-scale environmental context (e.g., heterogeneity, environmental adversity, species pool) that may influence BEF relationships (12).
The dramatic loss of diversity both globally (13) and in many places also locally (14) is one of the most pressing environmental problems of our time (15). Real-world ecosystems provide critical ecosystem services to humans (16), and it therefore is crucial to evaluate whether the consequences of species loss identified in BEF experiments also hold under complex natural and seminatural conditions. Here, we used 447 plots 1 km2 in size and spread regularly across six biogeographic regions (BGR) and an altitude range of 249–2,819 m above sea level (a.s.l.) in Switzerland (Central Europe) to evaluate whether plant productivity is related to the biodiversity found in these plots (Fig. 1 and Materials and Methods). The species diversity of vascular plants, breeding birds, and butterflies was obtained from surveys carried out twice in 2001–2013 in the frame of a national biodiversity monitoring program (BDM; ref. 17; biodiversitymonitoring.ch). Proxies of primary productivity were derived from satellite-sensed vegetation indices [Moderate Resolution Imaging Spectroradiometer enhanced vegetation index (MODIS EVI); ref. 18]. Specifically, we tested whether landscape-scale biodiversity measures of plant, bird, and butterfly communities promoted our proxy of primary productivity, and its temporal stability. Capitalizing on the high spatial and temporal resolution of the productivity data, we analyzed whether these effects were caused by higher momentary vegetation activity, or by an extended growing season. We further tested whether growing season length (GSL) increased throughout our observation period, and whether this change depended on biodiversity. Finally, we evaluated the magnitude of all biodiversity effects in relation to the magnitude of effects of other drivers related to climate, topography, and land cover.
Fig. 1.
Swiss BDM sampling design. Dots represent a systematic grid of 509 plots, each 1 km2 in size, spanning six BGRs (names and altitude ranges shown in map). A denser sampling grid is used in the Jura Mountains and Southern Alps because of their smaller area. For our analysis, we used a subset of 447 plots (gray).
Results
Primary Productivity.
We derived two proxies of primary productivity from a remotely sensed vegetation activity index (MODIS EVI; see Materials and Methods for details). characterizes average growing season productivity, whereas EVIGS integrates EVI over the growing season, i.e., also factors in changes in GSL (Fig. 2). We ran all analyses for both and EVIGS but only report results for EVIGS because effects were very similar for both dependent variables. EVIGS increased strongly with the diversity of vascular plants (Splants; Fig. 3B; F1,378 = 172, P < 0.001). A similar effect was found when using an index (S) that combined the diversity of all taxa using an ordination technique (Fig. 3A; F1,379 = 240, P < 0.001). The study area was composed of distinct BGRs (Fig. 1). Biodiversity differed among BGR and was negatively correlated with altitude (Pearson’s product moment correlation r = −0.48 for S and r = −0.50 for Splants), leading to a partial confounding of effects. Estimated effects of biodiversity became smaller when adjusted for altitude (Fig. 3 A and B; solid lines) or BGR but remained highly significant for both S and Splants (Table S1). Biodiversity effects were independent of altitude and BGR [interaction of S and Splants with BGR and with altitude: not significant (n.s.)]. We also included covariates related to topography, climate, and land cover into our models (Table S2), but similar to BGR and altitude, effects of biodiversity remained highly significant even when fitted after these terms (effect sizes: Table S1).
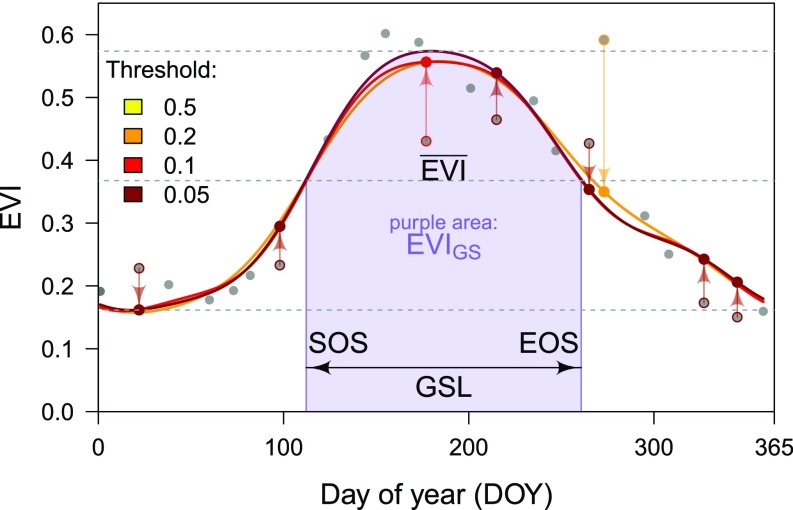
Fig. 2.
Example of a 1-y EVI time series and the metrics derived from this data. Original EVI data (gray dots) were approximated as sum of three harmonics (dark-red line). In a step-wise process, data exceeding given thresholds were replaced with model predictions (arrows). See Materials and Methods for details. From the final fit, we derived GSL, start and end of growing season (SOS and EOS), and the proxies of primary productivity growing-season EVI () and the integral of EVI over the growing season (EVIGS). Over all years and plots, averaged 0.42 and EVIGS averaged 0.23, corresponding to a gross (net) primary productivity of 970 (896) g of C·m−2·y−1 for the same time and area (MODIS MOD17A2H and MOD17A3H products; ref. 54).
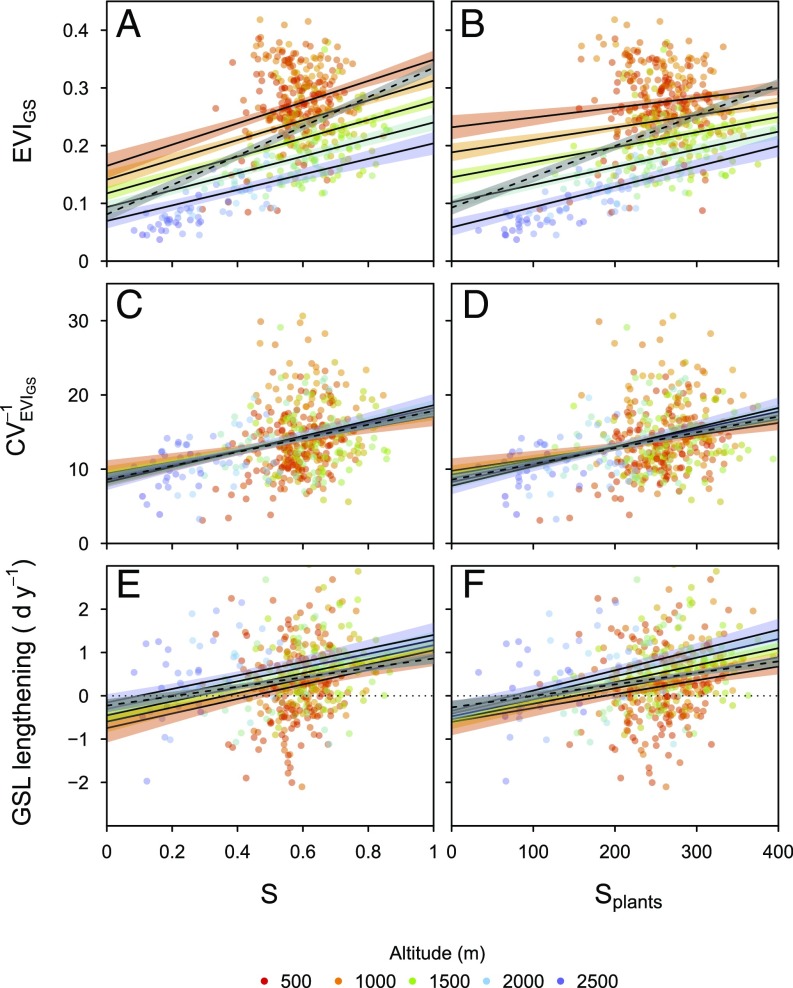
Fig. 3.
Biodiversity effects on primary productivity (proxy: EVIGS; A and B), its temporal stability (; C and D), and on the trend in growing season lengthening in 2000–2015 (GSL-lengthening; E and F). Effects were tested using an indicator combining the species richness of several taxa (S: plants, birds, butterflies), or of vascular plants alone (Splants). Dashed lines, overall effects; solid lines, model predictions for given altitudes; shaded areas, SEs of model predictions; P < 0.001 for all effects of biodiversity; n = 447 plots; see Table S1 for models and F statistics.
Table S1.
Effects of biodiversity (S and Splants) on proxies of productivity (EVIGS), the stability of productivity (), and GSL lengthening (d·y−1) when fitted after covariates related to climate, land cover, and topography
| Model terms | S | Splants | |||||||||||
| EVIGS | GSL lengthening, d·y−1 | EVIGS | GSL lengthening, d·y−1 | ||||||||||
| BGR | Covariate | Effect size | Significance | Effect size | Significance | Effect size | Significance | Effect size* | Significance | Effect size* | Significance | Effect size* | Significance |
| — | — | 0.25 ± 0.02 | F1,379 = 240*** | 9.2 ± 1.4 | F1,445 = 45*** | 1.1 ± 0.3 | F1,444 = 18*** | 0.053 ± 0.004 | F1,378 = 172*** | 2.1 ± 0.3 | F1,444 = 43*** | 0.27 ± 0.06 | F1,442 = 19*** |
| Alt. | 0.15 ± 0.02 | F1,437 = 64*** | 9.3 ± 1.6 | F1,438 = 35*** | 1.6 ± 0.3 | F1,437 = 31*** | 0.027 ± 0.005 | F1,437 = 32*** | 2.2 ± 0.4 | F1,443 = 33*** | 0.40 ± 0.07 | F1,441 = 34*** | |
| Slope | 0.27 ± 0.02 | F1,402 = 278*** | 9.1 ± 1.4 | F1,443 = 45*** | 1.1 ± 0.3 | F1,443 = 17*** | 0.059 ± 0.004 | F1,407 = 206*** | 2.1 ± 0.3 | F1,443 = 43*** | 0.26 ± 0.06 | F1,443 = 19*** | |
| N-Aspect | 0.24 ± 0.02 | F1,375 = 220*** | 11.0 ± 1.3 | F1,443 = 70*** | 0.9 ± 0.3 | F1,443 = 13*** | 0.050 ± 0.004 | F1,374 = 157*** | 2.5 ± 0.3 | F1,442 = 65*** | 0.24 ± 0.06 | F1,441 = 15*** | |
| Irrad. | 0.23 ± 0.02 | F1,415 = 158*** | 9.6 ± 1.5 | F1,443 = 41*** | 1.2 ± 0.3 | F1,444 = 18*** | 0.046 ± 0.005 | F1,410 = 103*** | 2.2 ± 0.4 | F1,444 = 39*** | 0.30 ± 0.07 | F1,442 = 20*** | |
| Precip. | 0.25 ± 0.02 | F1,399 = 212*** | 9.0 ± 1.4 | F1,443 = 42*** | 1.2 ± 0.3 | F1,443 = 22*** | 0.051 ± 0.004 | F1,395 = 151*** | 2.0 ± 0.3 | F1,444 = 40*** | 0.29 ± 0.06 | F1,443 = 23*** | |
| Temp. | 0.18 ± 0.02 | F1,410 = 95*** | 10.4 ± 1.5 | F1,442 = 45*** | 1.4 ± 0.3 | F1,444 = 24*** | 0.034 ± 0.005 | F1,409 = 55*** | 2.4 ± 0.4 | F1,442 = 43*** | 0.35 ± 0.7 | F1,444 = 26*** | |
| Grass | 0.23 ± 0.02 | F1,376 = 237*** | 8.7 ± 1.4 | F1,443 = 39*** | 1.1 ± 0.3 | F1,442 = 17*** | 0.050 ± 0.004 | F1,376 = 185*** | 2.0 ± 0.3 | F1,443 = 39*** | 0.26 ± 0.06 | F1,441 = 18*** | |
| Forest | 0.29 ± 0.02 | F1,379 = 232*** | 5.0 ± 1.5 | F1,441 = 11** | 0.8 ± 0.3 | F1,444 = 7** | 0.058 ± 0.005 | F1,381 = 157*** | 1.2 ± 0.4 | F1,440 = 10** | 0.20 ± 0.07 | F1,443 = 9** | |
| LCrichness | 0.24 ± 0.02 | F1,380 = 178*** | 10.4 ± 1.5 | F1,444 = 50*** | 1.3 ± 0.3 | F1,442 = 22*** | 0.048 ± 0.004 | F1,376 = 124*** | 2.4 ± 0.3 | F1,443 = 47*** | 0.31 ± 0.06 | F1,440 = 23*** | |
| Arable | 0.26 ± 0.02 | F1,382 = 268*** | 9.1 ± 1.4 | F1,443 = 45*** | 1.1 ± 0.3 | F1,443 = 17*** | 0.055 ± 0.004 | F1,383 = 195*** | 2.1 ± 0.3 | F1,443 = 43*** | 0.26 ± 0.06 | F1,442 = 19*** | |
| Urban | 0.25 ± 0.02 | F1,376 = 241*** | 9.5 ± 1.4 | F1,443 = 47*** | 1.2 ± 0.3 | F1,443 = 21*** | 0.054 ± 0.004 | F1,374 = 174*** | 2.2 ± 0.3 | F1,441 = 47*** | 0.30 ± 0.06 | F1,441 = 24*** | |
| Yes | — | 0.25 ± 0.02 | F1,387 = 236*** | 9.5 ± 1.4 | F1,438 = 46*** | 0.9 ± 0.3 | F1,438 = 13*** | 0.053 ± 0.004 | F1,382 = 164*** | 2.2 ± 0.3 | F1,434 = 45*** | 0.24 ± 0.06 | F1,435 = 15*** |
| Alt. | 0.18 ± 0.02 | F1,416 = 78*** | 7.2 ± 1.7 | F1,439 = 17*** | 1.3 ± 0.3 | F1,438 = 18*** | 0.031 ± 0.005 | F1,415 = 39*** | 1.7 ± 0.4 | F1,438 = 17*** | 0.33 ± 0.07 | F1,436 = 20*** | |
| Slope | 0.27 ± 0.02 | F1,402 = 261*** | 9.2 ± 1.4 | F1,437 = 44*** | 0.9 ± 0.3 | F1,438 = 12*** | 0.056 ± 0.004 | F1,400 = 184*** | 2.2 ± 0.3 | F1,434 = 42*** | 0.22 ± 0.06 | F1,436 = 14*** | |
| N-Aspect | 0.24 ± 0.02 | F1,381 = 218*** | 11.3 ± 1.3 | F1,437 = 71*** | 0.8 ± 0.3 | F1,437 = 10** | 0.050 ± 0.004 | F1,376 = 152*** | 2.6 ± 0.3 | F1,434 = 67*** | 0.21 ± 0.06 | F1,434 = 12*** | |
| Irrad. | 0.25 ± 0.02 | F1,422 = 185*** | 8.6 ± 1.5 | F1,436 = 33*** | 1.1 ± 0.3 | F1,434 = 16*** | 0.050 ± 0.005 | F1,417 = 120*** | 2.0 ± 0.4 | F1,439 = 32*** | 0.28 ± 0.07 | F1,437 = 18*** | |
| Precip. | 0.25 ± 0.02 | F1,398 = 212*** | 8.8 ± 1.5 | F1,439 = 37*** | 1.1 ± 0.3 | F1,438 = 15*** | 0.052 ± 0.004 | F1,389 = 144*** | 2.1 ± 0.3 | F1,436 = 36*** | 0.26 ± 0.06 | F1,435 = 17*** | |
| Temp. | 0.19 ± 0.02 | F1,394 = 108*** | 9.8 ± 1.7 | F1,433 = 35*** | 1.0 ± 0.3 | F1,437 = 11*** | 0.037 ± 0.005 | F1,392 = 62*** | 2.3 ± 0.4 | F1,428 = 34*** | 0.26 ± 0.07 | F1,434 = 13*** | |
| Grass | 0.23 ± 0.02 | F1,392 = 226*** | 9.0 ± 1.4 | F1,437 = 40*** | 1.0 ± 0.3 | F1,437 = 14*** | 0.049 ± 0.004 | F1,390 = 171*** | 2.2 ± 0.3 | F1,435 = 41*** | 0.24 ± 0.06 | F1,434 = 15*** | |
| Forest | 0.28 ± 0.02 | F1,383 = 224*** | 5.3 ± 1.6 | F1,431 = 11*** | 0.6 ± 0.3 | F1,438 = 5* | 0.057 ± 0.005 | F1,381 = 144*** | 1.2 ± 0.4 | F1,426 = 11*** | 0.17 ± 0.07 | F1,434 = 6* | |
| LCrichness | 0.24 ± 0.02 | F1,387 = 176*** | 10.9 ± 1.5 | F1,435 = 51*** | 1.1 ± 0.3 | F1,434 = 16*** | 0.048 ± 0.005 | F1,377 = 117*** | 2.5 ± 0.4 | F1,428 = 49*** | 0.27 ± 0.07 | F1,428 = 17*** | |
| Arable | 0.26 ± 0.02 | F1,386 = 262*** | 9.3 ± 1.4 | F1,435 = 45*** | 0.9 ± 0.3 | F1,437 = 13*** | 0.054 ± 0.004 | F1,381 = 184*** | 2.2 ± 0.3 | F1,432 = 44*** | 0.23 ± 0.06 | F1,434 = 14*** | |
| Urban | 0.26 ± 0.02 | F1,383 = 240*** | 9.7 ± 1.4 | F1,435 = 47*** | 1.0 ± 0.3 | F1,436 = 15*** | 0.054 ± 0.004 | F1,377 = 170*** | 2.3 ± 0.3 | F1,429 = 47*** | 0.26 ± 0.06 | F1,431 = 18*** | |
Alt., mean plot altitude; BGR, the model does (yes) or does not (-) first account for effects of biogeographic region before the covariate; covariate, the specific covariate, which was included in the fixed-effects term before the biodiversity variables; effect size, coefficients (mean ± SE); Irrad., annual shortwave irradiation; LCrichness, number of land cover types in plot; N-aspect, North-south component of aspect; Precip., annual precipitation; S, the biodiversity index for all taxa combined; significance, F values with associated degrees of freedom and P values; Slope, average slope in degrees; Splants, the biodiversity of plants; Temp., mean annual temperature; Forest, Grass, Urban, and Arable, fraction of plot covered by forest, grassland, urban, or arable land; for details see SI Materials and Methods and Table S2. (n = 447; *P < 0.05; **P < 0.01; ***P < 0.001; n.s. not significant).
Effect size is given for an increase of 100 species.
Table S2.
Covariates related to land cover, topography, and climate that were selected for the analysis presented in the main text
| Category | Covariate | Time of collection | Time of retreival | Spatial resolution | Product name/source |
| Land cover | Grass (Fractional meadow/Pasture cover) | 2004–2009 | January 10, 2016 | 100 m | NOAS04/Swiss Federal Statistical Office (FSO), GEOSTAT |
| Forest (Fractional forest cover) | |||||
| LCrichness (Number of different land-cover types) | |||||
| Climate | Irradiation (SIS; Wm−2; yearly mean) | 2004–2012 | November 26, 2016 | 0.0208 decimal degree (2.3 km West-East and 1.6 km South-North) | msg.SIS.M/Swiss Federal Office of Meteorology and Climatology (MeteoSwiss) |
| Temperature (T; °C; yearly mean) | 2000–2014 | TabsM/Swiss Federal Office of Meteorology and Climatology (MeteoSwiss) | |||
| Precipitation (P; mm; yearly sum) | 2000–2014 | RhiresM /Swiss Federal Office of Meteorology and Climatology (MeteoSwiss) | |||
| Topography | Altitude (m.a.s.l) | 1978–2001 | May 22, 2016 | 25 m | DHM25/Swiss Federal Office of Topography (swisstopo) |
| Slope (°) | |||||
| N-Aspect: North-south component of the aspect (-1 if south-exposed; 1 if north-exposed) |
Temporal Stability of Primary Productivity.
We quantified the temporal stability of productivity as the inverse coefficient of variation of EVIGS in the years 2000–2015 (). This metric increased strongly with biodiversity (S: F1,445 = 45, P < 0.001; Splants: F1,444 = 43, P < 0.001; Fig. 3 C and D and Table S1). Biodiversity remained statistically significant when fitted after altitude (S: F1,438 = 35, P < 0.001; Splants: F1,443 = 33, P < 0.001; Fig. 3 C and D and Table S1), but altitude explained no additional variation when fitted after biodiversity. Effects of biodiversity also were independent of altitude (S × altitude and Splants × altitude: n.s.).
Long-Term Changes in Growing Season Length.
The 16-y average GSL (Materials and Methods and Fig. 2) decreased with altitude, but showed no effect of biodiversity after accounting for altitude. However, GSL increased by 0.39 ± 0.07 d·y−1 over the observation period (F1,996 = 34; P < 0.001), and this rate of change was significantly accelerated with biodiversity (Fig. 3 E and F; S: F1,444 = 18, P < 0.001; Splants: F1,442 = 19; P < 0.001; Table S1). This diversity effect on growing season prolongation was mediated by effects on start of season (SOS; Fig. 2; S: F1,428 = 60, P < 0.001; Splants: F1,424 = 58; P < 0.001), with no parallel effect on end of season (EOS; Fig. 2; S and Splants: n.s.). The biodiversity effects on the rate of change of SOS and GSL were independent of altitude (S × altitude and Splants × altitude: n.s.), and, notably, the rates of change of SOS and GSL were similar across all altitude ranges.
Magnitude of Biodiversity Effects.
While climatic, topographic, and land cover-related covariates explained some variance in our data, the metaanalytic effect sizes (Zr values derived from F statistics in linear models; ref. 19) of both overall and altitude-corrected biodiversity effects were among the largest of all tested explanatory variables (Fig. 4), for all biodiversity metrics.
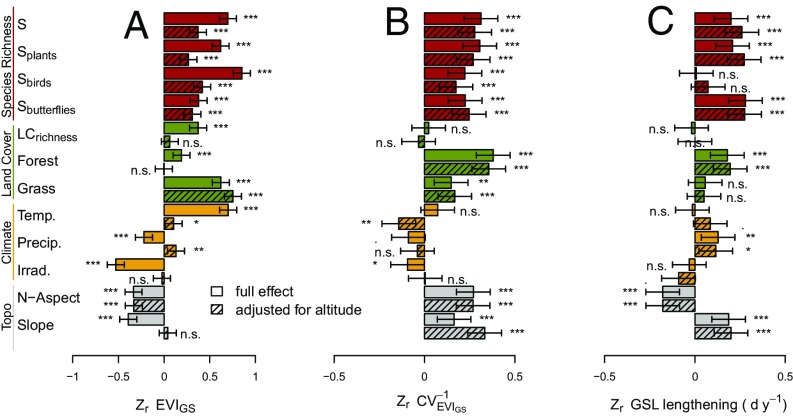
Fig. 4.
Magnitude of effects of biodiversity, land cover, and climatic and topographic drivers of productivity (A; EVIGS), the temporal stability of productivity (B; ), and growing season lengthening over the 2000–2015 observation period (C). Data show normalized effect sizes (z-transformed, based on F ratios) as used in metaanalysis, with (hashed bars) or without (white bars) prior correction for effects of altitude. Biodiversity ranged among the variables with the largest effect sizes, irrespective of adjustment for altitude. Forest and Grass, fraction of plot covered by forest or grassland; LCrichness, number of land cover types in plot; for details see SI Materials and Methods and Table S2. Error bars show 95% confidence intervals; n = 447; *P < 0.05; **P < 0.01; ***P < 0.001; see Table S1 for models and F statistics. Irrad., annual shortwave irradiation; N-aspect, North-south component of aspect; Precip., annual precipitation; Slope, average slope; Temp., mean annual temperature.
Effects of climatic covariates on EVIGS (Fig. 4A) were largely altitude-mediated, i.e., their effects vanished when adjusted for altitude. This was to some extent also the case for topographic and land cover-related covariates. Our biodiversity variables were much less confounded with altitude. Very similar patterns were found for the stability of productivity (Fig. 4B) and growing season lengthening (Fig. 4C) except that altitude had much less of an effect, i.e., overall and altitude-corrected effects were similar in size for most of the explanatory variables.
Interrelation of Drivers.
Structural equation models (SEM) showed that biodiversity (S) was positively related to primary productivity (EVIGS) and GSL [Fig. 5A; P() = 0.5]. Effects of S on EVIGS followed two paths: First, EVIGS increased because of positive effects mediated by a higher growing season vegetation activity (; standardized path coefficient of 0.14, P < 0.001); second, EVIGS also increased because of indirect, positive effects through an increase in GSL (standardized path coefficient of 0.21, P < 0.001). This SEM explained 82% and 62% of the variation in EVIGS and GSL, respectively. Additional SEM (Fig. S1) without indirect path from S through GSL showed significant positive links from biodiversity (S) to EVIGS, the stability of productivity (), and GSL lengthening, and support the notion that primary productivity was in part promoted by S through enhanced temporal stability but not through growing season lengthening. The exogenous variables in our SEM were correlated, some highly (Fig. 5B). However, path coefficients for biodiversity were in the same range as coefficients of other exogenous variables, indicating that substantial amounts of the overall effects were simultaneously driven by multiple drivers, of which S was a very important one.
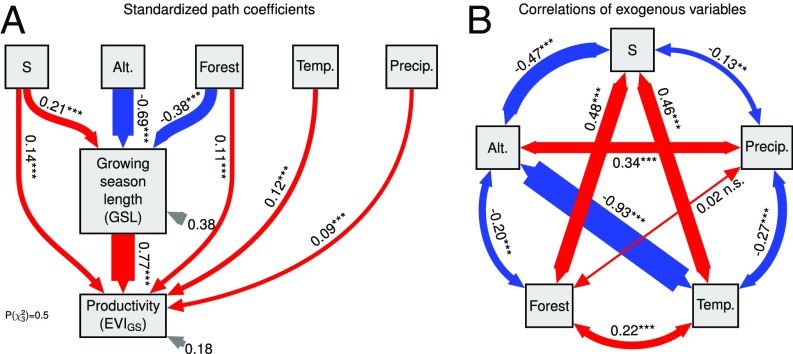
Fig. 5.
Path diagram of effects of biodiversity on productivity (EVIGS) that are mediated by or independent of changes in GSL. The structural equation model accounts for altitude (Alt.), and variables related to land cover and climate. Standardized path coefficients (A) and correlation of exogenous variables (B) are shown separately. Other drivers were tested but removed from the model because they had no statistically significant path coefficients. Gray arrows, residual variances; n = 447; *P < 0.05; **P < 0.01; ***P < 0.001. See Fig. S1 for additional structural equation models including and GSL lengthening. Forest, fraction of plot covered by forest; Precip., annual precipitation; Temp., mean annual temperature.
Fig. S1.
Path diagrams showing effects of biodiversity (S) on 16-y average productivity (proxy: EVIGS; A, D, and E), its temporal stability (; B and D) and the temporal trend of growing season lengthening (GSL lengthening; C and E). In addition to biodiversity, the structural equation models account for influences of altitude (Alt.) and variables related to land cover and climate. Only the standardized path coefficients are shown because the correlation of exogenous variables is described in Fig. 5B. Residual variances of the response variables are depicted with gray arrows (n = 447; *P < 0.05; **P < 0.01; ***P < 0.001; n.s., not significant). Forest, fraction of plot covered by forest; Precip., annual precipitation; Temp., mean annual temperature.
Biodiversity and land-cover richness (LCrichness), a measure of landscape diversity, were positively correlated (Pearson’s product moment correlation; S: r = 0.36; Splants: r = 0.35). We fitted SEMs with the additional exogenous variable LCrichness. However, including LCrichness did not decrease path coefficients for S. We also tested whether effects of diversity depended on LCrichness but this was not the case (S × LCrichness: n.s. in linear mixed models).
Discussion
Our analysis indicates that biodiversity is tightly linked to primary productivity and its temporal stability in large field plots spanning extensive environmental, climatic, and topographic gradients in Switzerland (Central Europe). Our plot network contrasts markedly with biodiversity experiments with respect to size, structural complexity, and age of the communities investigated. The effects we report here nevertheless are comparable to the ones observed in experimental studies in small plots, suggesting that the positive effects of biodiversity on ecosystem functioning, in particular on productivity, also exist in real-world landscape-scale ecosystems that integrate different land cover types (i.e., metaecosystems; ref. 20).
Drivers of primary productivity generally are correlated in nonexperimental studies of BEF relationships across natural landscapes (21–23). The individual contributions of these drivers thus are difficult to disentangle. The amount of explained variance shared by potential explanatory variables can be explored with methods that include multiple regression models, path analysis, or variance partitioning schemes. However, even if applied in an educated way these methods can only suggest likely boundaries for effect sizes and do not allow an unequivocal attribution of effects to particular drivers or mechanisms. In our study, altitude was the primary factor that explained variation in productivity and was negatively correlated with biodiversity. Comparing the biodiversity effects on productivity that we found to findings from experiments therefore remains difficult. Metaanalytic normalized effect sizes (Zr) were in the range of ∼0.4–0.8 for overall effects of biodiversity and shrank to 0.3–0.4 when first adjusted for altitude. These Zr values place the biodiversity effects in our study above the median response reported for primary producers in the metaanalysis of ref. 1.
BEF relationships in experiments have been attributed to mechanisms that include so-called complementarity and sampling effects (3). We cannot disentangle these mechanisms in our data because we cannot break down community-level or even metaecosystem-level vegetation indices into contributions from individual species. However, the pattern we found integrates effects over several hundred communities with nearly 2,000 vascular plant species from different habitats. The communities we investigated (on average 250 plant species per study plot) therefore did not share a common species set, and we did not find evidence that responses were driven by a few particular species.
Biodiversity was linked to an increased temporal stability of primary productivity, which again suggests that BEF relationships observed in experiments with small plots (2) also apply in natural systems at the landscape scale. Effects on stability have traditionally been expressed as resistance to or resilience from disturbance. Our study included extreme events such as the extreme heat wave and drought in summer 2003 (24). However, this driver did not affect our study network homogeneously, with more severe impacts at low altitude and positive effects in alpine areas (25). Given that biodiversity was generally higher at low altitude, i.e., in areas that were particularly badly affected by this heat wave, we would have expected such altitude-dependent disturbance effects to mask positive effects of biodiversity rather than to promote them.
It has been argued that biodiversity effects in experiments originate in part from poorly performing low-diversity communities that typically result from random species selection (26) and regular weeding (10). In our study, agricultural land typically was species-poor and often periodically bare or low in ground cover, similar to the situation in experiments. To test whether our findings were biased by the presence of agricultural land, we repeated all analyses excluding plots that contained agricultural land (SI Materials and Methods); however, the pattern of positive BEF relationships remained (Figs. S2 and S3), supporting the idea that poorly performing low diversity plots were less important in the real-world landscapes we investigated. The strong biodiversity effects we found thus are all the more remarkable.
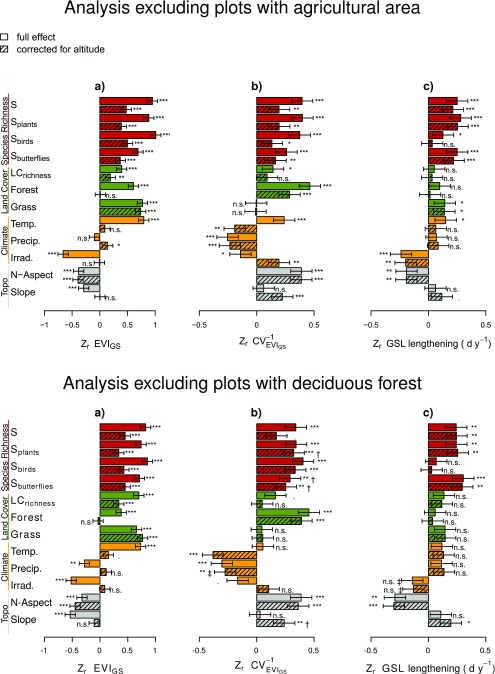
Fig. S2.
Reanalysis of data subsets excluding agricultural land (Upper; n = 254) and deciduous forest (Lower; n = 128): Biodiversity effects on primary productivity (proxy: EVIGS; A), its temporal stability (., B), and the temporal trend of growing season lengthening (GSL-lengthening, C) in the years 2000–2015. These results are very similar to the analysis of the full dataset (cf. Fig. 4). Effect sizes marked with † are derived from models with modeled isotropic (instead of anisotropic) spatial autocorrelation, whereas the ‡ symbol depicts effect sizes from models without considering the spatial autocorrelation. In these cases, models with anisotropic spatial autocorrelation did not converge. See Fig. 4 for further explanations.
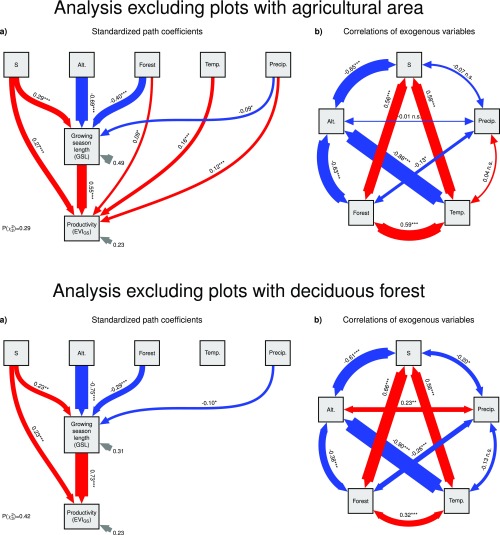
Fig. S3.
Reanalysis of data subsets excluding agricultural land (Upper; n = 254) and deciduous forest (Lower; n = 128): Path diagrams showing effects of biodiversity on productivity (proxy: EVIGS) that are mediated by or independent of changes in GSL. Besides biodiversity, the structural equation models account for influences of altitude (Alt.) and variables related to land cover and climate. The standardized path coefficients (A) and the correlation of exogenous variables (B) are shown separately. Gray arrows, residual variances of the response variables; *P < 0.05; **P < 0.01; ***P < 0.001; Forest, fraction of plot covered by forest; n.s., not significant; Precip., annual precipitation; Temp., mean annual temperature. These results are very similar to the analysis of the full dataset (cf. Fig. 5).
The biodiversity effects on productivity and its temporal stability were statistically robust. They remained highly significant when we repeated our analyses accounting for the presence or abundance of any particular land-cover type, indicating that they did not originate from a confounding of biodiversity with any particular land-cover type (Table S1). These effects also were consistent across the different biogeographic regions and altitudes of Switzerland.
Positive biodiversity effects on primary productivity were in part driven by changes in GSL, i.e., overall effects resulted not only from increased but also from prolonged vegetation activity. Our findings show that biodiversity also was related to an enhanced longer-term (decadal) trend toward a prolonged growing season. Global observations of trends in vegetation phenology indicate an earlier onset of vegetation activity in spring and a delayed senescence in fall for many locations (27). These phenological shifts are generally attributed to warming, which is typically more pronounced at higher altitudes (28). The capacity of species to adapt their phenology to climate change is important for their survival, in particular when species are unable to migrate to other habitats with suitable climate (29). Studies have shown that variation in phenology allows for the coexistence of species through temporal complementarity (30). Conversely, our results promote the idea that high biodiversity provides a greater capacity for plant communities to respond to emerging opportunities for activity and growth in the shoulder season. In other words, climate warming seems to create additional environmental niche space that can be filled, given a sufficiently large source species pool (31). Hence, our study provides evidence that biodiversity is a critical determinant of the phenological sensitivity (29) of communities and, thus, also the long-term performance of ecosystems in real-world landscapes. Biodiversity mediated growing season lengthening by shifting the start but not the end of the season. One possible interpretation is that the start of season phenology in vascular plants is more strongly linked to temperature, and to processes regulated by species-specific, variable genetic pathways (e.g., genes related to responses to winter-chilling; ref. 32). In contrast, the regulation of end of season is related to a more complex interplay of multiple environmental cues and conserved ontogenetic factors common to most plant species, which will limit the potential for community-level changes with species composition and diversity (32). Irrespective of the mechanisms involved, the effects we report here are large, with biodiversity substantially modulating decadal trends in season lengthening. We therefore argue that considering biodiversity may help to understand and predict community-level trends in phenology, irrespective of the underlying variable, idiosyncratic, or context-dependent responses of individual species, which are challenging to predict (33).
Evergreen and deciduous vegetation exhibit different seasonal amplitudes in EVI. In our study, the fraction of evergreen forest increases with altitude. To rule out the possibility of related biases on our estimates of GSL, we reanalyzed a subset of the original data, where study plots with 10% or more forested area classified as “deciduous” or “mixed-deciduous” were excluded (SI Materials and Methods). However, phenology estimates did not change and BEF patterns stayed similar (Figs. S2 and S3).
An advantage of observational studies is that they involve more realistic conditions than experiments in which community composition is directly manipulated. However, a caveat of the observational approach is that the directionality of effects cannot be inferred with certainty. Early studies relating biodiversity and primary productivity across habitats have coined the concept of a hump-shaped relationship with high biodiversity at intermediate productivity (34). This has been explained by resource limitation in low-productivity environments and competitive exclusion (34), or reduced heterogeneity of limiting resources in high-productivity environments (35). Empirical evidence for this hump-shaped relationship is mixed, in part possibly because it is confounded with other important drivers of productivity and diversity (36). Such drivers include biogeographic constraints on the species pool, spatial heterogeneity, and disturbance (37). We have deliberately analyzed effects of biodiversity on primary productivity, which is the perspective adopted in BEF experiments. Our study nevertheless remains correlational, and a reverse causality or a common third cause are also conceivable. For example, positive effects of resource availability on both diversity and productivity may have occurred in marginal environments such as alpine regions. However, agricultural activities in regions such as the Swiss central midlands have led to large-scale nitrogen deposition from airborne transport of volatilized fertilizer nitrogen (38). Similar effects are found from rainout of air pollution from traffic and fossil fuel-based heating along the pre-Alps (39). In such areas, species loss may be found as a consequence of eutrophication, which would go along with a negative rather than positive biodiversity–productivity relationship due to competitive exclusion. Given that biodiversity may have simultaneously acted as driver of and response to productivity, the overall net relation we observed here might ultimately underestimate the importance of biodiversity in promoting productivity. Assuming that effects of productivity on biodiversity would dominate and result in a positive correlation of the two would be inconsistent with the hump-back model. The positive effects of biodiversity on the temporal stability of productivity and the lengthening of the growing season provide additional support for at least a strong partial cause–effect directionality from biodiversity to productivity at the landscape scale.
As is typical in observational studies, effects of the different drivers we inspected were not fully independent. Hence, effects of environmental drivers of productivity were partly mediated via biodiversity (and vice versa), in line with earlier studies (40, 41). Despite dominant influences of altitude-related climatic effects, residual effects of biodiversity on productivity and season lengthening were substantial. These large landscape-scale effects of biodiversity extrapolate similar evidence from experimental studies that show that biodiversity can be as important as other drivers of global change (5, 6).
The combined diversity of the different taxa (S) often explained more variation in the analyzed variables than plant species richness alone (Splants). This supports the idea that the diversity of taxa other than plants reflects elements of plant species richness that were not captured in the vegetation surveys, or that these metrics are indicative of independent properties of the investigated ecosystems that are relevant for their functioning (e.g., structural complexity; ref. 42).
In conclusion, we demonstrate that biodiversity effects can be found at large spatial scales in real-world ecosystems. These effects are at least as large as the ones reported from small-scale experimental systems, despite different community assembly processes at play. Ecosystem services are provided in real-world landscapes and are of enormous economic value (16), which has raised concerns about whether they will be maintained at current levels, given the ongoing, unprecedented rates of biodiversity loss (13). Our results indicate that biodiversity indeed is critical for the provision of these ecosystem services. We show that, in real landscapes, biodiversity is as important as other environmental drivers, including climate, land cover, and topography. For example, we provide evidence that climate change translates more effectively into a longer growing season and, therefore, productivity when a sufficiently large species pool is available. Ultimately, this implies that, if global environmental change affects the composition of biological communities, a significant part of the overall effect of these changes may be a biodiversity effect in disguise.
SI Materials and Methods
Primary Productivity and Growing Season.
EVI time series were downloaded for the years 2000–2015 and georeferenced to the Swiss CH1903+ grid. Data were then quality-filtered. We kept all MODIS EVI pixels that conformed to the following criteria:
-
i)
Quality of EVI data product: We downloaded the MOD13Q1 product (18) in HDF format and filtered out low quality data by relying on the “VI usefulness information” coded in bits 2–5 of the MOD13Q1 “VI Quality Layer”: We discarded all measurements with quality 8 (binary 10002) or worse.
-
ii)
Minimum number of measurements per year: We filtered out pixels in years with less than 15 EVI measurements available after step i).
-
iii)
Maximum noise in raw EVI data: We filtered out pixels where more than half of the original data points were replaced during the HANTS fitting process (47).
-
iv)
Thresholds for LSP metrics: A minimum SOS was set to day 20 of the year, a maximum EOS was set to day 362. Pixels with SOS and EOS beyond these boundaries were discarded. Pixels with a mean EVI of 0.12 or lower were flagged as not vegetated and also discarded. Pixels with a growing-season EVI amplitude of 0.1 or less above the yearly mean were discarded as well because they did not allow a reliable determination of the growing season.
We then processed all data on a per pixel and year basis (years 2000–2015). Data were smoothed using a modified implementation of the HANTS algorithm (47). Our procedure was based on Fourier synthesis to estimate amplitudes and phase of three dominant frequencies for each MODIS pixel and year. Unlike standard fast Fourier transformation techniques, this algorithm also is applicable to unequally spaced data. Seasonal courses of EVI were fitted using a robust nonlinear procedure, adopting a Huber M-estimator that was used to iteratively reweigh data passed to a Levenberg–Marquard algorithm. As usual in the HANTS approach, the fit was repeated applying sequentially decreasing thresholds of 0.5, 0.2, 0.1, and 0.05 raw EVI values. Data with residuals exceeding these thresholds were replaced by model predictions. See Fig. 2 and Methods for the further processing and the derivation of GSL and productivity proxies.
Land Cover, Topography, and Climate: Description of Covariates and Sources.
For each study plot, covariates related to land cover, topography, and climate were determined (selected covariates and respective times of collection and retrieval, spatial resolution, and data source are listed in Table S2). These specific covariates were selected based on a prior RDA (https://cran.r-project.org/web/packages/vegan/index.html), which helped to reduce colinearity. However, we also wanted keep important, interpretable, well-known drivers of ecosystem functioning in the dataset, despite their strong correlation (e.g., in the case of mean annual temperature and altitude). All data were averaged over the years 2000–2015.
Land-cover information was derived from point data with 100-m spatial resolution (product name: NOAS04) available from the Swiss Federal Statistical Office, GEOSTAT (https://www.bfs.admin.ch/bfs/de/home/dienstleistungen/geostat/geodaten-bundesstatistik/boden-nutzung-bedeckung-eignung/arealstatistik-schweiz/standardnomenklatur.html). We aggregated the original classification of 17 land-cover types into eight classes (forest, grassland, agricultural, urban, urban green, water, unproductive, bare land). From these data, we derived the fractional covers of each of the eight land-cover types. Land-cover richness was determined as the number of different land-cover types in each 1-km2 study plot.
Topographic data (i.e., altitude, slope, and north-south component of the aspect; values averaged for the 1-km2 plots) were derived from a digital elevation model (product name: DHM25) provided by the Swiss Federal Office of Topography (swisstopo; https://shop.swisstopo.admin.ch/en/products/height_models/dhm25).
Climate data (i.e., mean annual precipitation, temperature, and surface incoming shortwave radiation) were obtained using interpolated gridded monthly temperature, precipitation and radiation data; (product names: TabsM; RhiresM; msg.SIS.M) provided by the Swiss Federal Office of Meteorology and Climatology (MeteoSwiss; a description of these data can be accessed under www.meteoswiss.admin.ch/content/dam/meteoswiss/de/service-und-publikationen/produkt/raeumliche-daten-temperatur/doc/ProdDoc_TabsM.pdf for temperature or www.meteoswiss.admin.ch/content/dam/meteoswiss/de/service-und-publikationen/produkt/raeumliche-daten-niederschlag/doc/ProdDoc_RhiresM.pdf for precipitation or www.meteoswiss.admin.ch/content/dam/meteoswiss/de/service-und-publikationen/produkt/raeumliche-daten-globalstrahlung/doc/ProdDoc_MSG.SIS.pdf for irradiation).
Data Subsets Excluding 1-Km2 Plots with Agricultural Land or Deciduous Forest.
To test whether results depended on the presence of agricultural land or deciduous vegetation in the 1-km2 study plots, we created two data subsets where these land-cover types where excluded. Using these datasets, we repeated all analyses that we had performed with the full dataset (n = 447). Results are summarized in Figs. S2 and S3.
The first dataset (n = 254) contained only plots without any agricultural land. The second dataset (n = 128) contained only plots with at least 90% of the forested area classified as “evergreen” or “evergreen-mixed.” We could not increase this threshold to 100% because only 14 plots containing “evergreen” or “evergreen-mixed” forest would have remained. The forest type classification was obtained from the Swiss Federal Statistical Office, GEOSTAT (https://www.bfs.admin.ch/bfs/de/home/dienstleistungen/geostat/geodaten-bundesstatistik/boden-nutzung-bedeckung-eignung/abgeleitete-und-andere-daten/waldmischungsgrad-schweiz.html; product name: Waldmischungsgrad der Schweiz; time of collection: 1990/92; spatial resolution: 25 m).
Materials and Methods
Study Design.
We used a systematic network of 447 plots of 1 × 1 km in size that are part of the Swiss Biodiversity Monitoring Program (BDM; ref. 17). These plots are systematically spread across the entire 41,248 km2 of Switzerland and cover six biogeographic regions that form distinct units with respect to climate, edaphic conditions, and distribution patterns of fauna and flora (ref. 43 and Fig. 1). After excluding plots without vegetation (e.g., lakes, snow fields, scree slopes) or insufficient remote-sensing data, of the original 509 BDM plots, a total of 447 plots with complete data and spanning an altitudinal range of 249–2,819 m a.s.l. remained for our analysis.
Biodiversity.
In each 1-km2 plot, vascular plant and butterfly species were monitored along 2,500 × 5 m transects following standardized field protocols (44). Breeding bird species were monitored along a plot-specific route with an average length of 5 km following the standardized method of the Common Breeding Bird Survey (45). Monitoring events took place in 5-y intervals. We derived average species richness from the first two monitoring events in 2001–2013. This procedure revealed the presence of 1,931 vascular plant, 152 breeding bird, and 188 butterfly species in the 447 1-km2 study plots.
Since primary productivity is largely driven by plants, we focused on vascular plant species richness (Splants) as a measure of biodiversity. We expected the species richness of breeding birds (Sbirds) and butterflies (Sbutterflies) to reflect additional aspects of the overall biodiversity and the structural complexity of biotic communities (42) in the plots, which may also be relevant for ecosystem functioning. We therefore calculated an aggregated indicator of the biodiversity of all taxa (S), which we obtained from the first ordination axis of a principal component analysis combining the species richness of all three taxa. This axis explained 63% of the variation in the species richness data, with loadings of 0.69, 0.55, and 0.47 for vascular plant, breeding bird, and butterfly species richness, respectively. To simplify the interpretation of this biodiversity metric, we rescaled the ordination axis so that values of zero and one corresponded to the complete absence of species and to the simultaneous presence of the maximum number of plant (n = 394), bird (n = 57), and butterfly (n = 78) species that were found in any plot.
Primary Productivity and Growing Season.
We derived growing season vegetation activity and GSL from satellite-borne data (MODIS; ref. 18) with a spatial resolution of ≈250 m and a temporal resolution of 16 d. We used the EVI, which, similarly to the normalized difference vegetation index (NDVI), quantifies photosynthetically active vegetation from the ratio of red and near-infrared reflected light but uses blue band data to correct for scattering by aerosols (46). EVI time series were smoothed using a modified implementation of the harmonic analysis of time series algorithm (HANTS; ref. 47) (Fig. 2, and SI Materials and Methods). Many methods exist to determine growing season start (SOS), end (EOS), and length (GSL) from remote sensing data (48), with no universally accepted best approach (49). We used the NDVIratio method (50), which defines SOS as day of year at which EVI first exceeded the mean of its annual minimum and maximum value. Similarly, EOS indicates the first day of the year at which EVI fell below this threshold. This method is widely applied (48), yields results that are consistent with ground-measured plant phenology (49), and is robust with regard to different annual shapes of vegetation activity (51). Average growing season vegetation activity (), a first proxy of primary productivity, was estimated as average EVI in the SOS to EOS time span. A second proxy, EVIGS, integrates EVI values over the growing season:
| [1] |
We quantified the temporal stability of primary productivity as reciprocal coefficient of variation of yearly EVIGS for the years 2000–2015 (). We also derived the temporal trend in seasonality (SOS, EOS, and GSL) over the 16-y period by linearly regressing these data against time. EVI-derived data were mapped to the 1-km2 study plots by computing area-weighted means for potentially vegetated land surfaces (i.e., excluding water, rock, glaciers) in each 1-km2 plot and year.
Land Cover, Topography, and Climate.
For each 1-km2 plot, covariates related to land cover, topography, and climate were determined (SI Materials and Methods for details). In brief, land-cover information was derived from point data with 100-m spatial resolution. We classified each point into eight classes (forest, grassland, agricultural, urban, urban green, water, unproductive, bare land) and calculated their fractional cover in each 1-km2 plot. Land-cover richness (LCrichness) was determined as number of land cover types present. Topographic data (mean plot value of altitude, slope, and north-south component of the aspect) were derived from a digital elevation model with 25-m spatial resolution. Climate data (mean annual precipitation, temperature, and surface incoming shortwave radiation) were obtained using interpolated gridded monthly temperature, precipitation, and radiation data. All data were averaged over the time period 2000–2015.
Statistical Analysis.
We tested effects of biodiversity on EVIGS,, SOS, EOS, and GSL and the temporal trend and stability of these parameters using analysis of variance based on general linear mixed models, using R 3.3 (r-project.org) and ASReml (VSN International). Biodiversity and plot covariates were included as fixed effects and fitted sequentially. The spatial correlation among plots was fitted as anisotropic exponential distance-decay of residual correlation. As plot covariates, we evaluated a total of 41 topographic and climatic indicators potentially related to vegetation activity. Variable selection was guided by redundancy analysis (RDA; https://cran.r-project.org/web/packages/vegan/index.html), allowing to pick representative variables with strong explanatory power from highly collinear sets. We finally settled on biogeographic region (BGR; factor with six levels) and altitude (continuous variable) because these terms integrate important climatic, topographic, and edaphic drivers and explained most variance. We determined their degree of confounding with biodiversity by fitting the biodiversity term before and after these covariates, i.e., we tested for (i) overall effects of biodiversity across regions and altitude and (ii) effects of biodiversity within biogeographic regions and constant altitude. We further determined 57 covariates characterizing land cover; these included the area of specific land cover types, land cover richness, and patch structure (e.g., largest patch, edge lengths, patch cohesion indices; ref. 52); we selected a subset of these using RDA, as described above, and included these in further models, together with a few climatic and topographic covariates we considered conceptually so important that we kept them, despite correlations with altitude and BGR (Tables S1 and S2). We quantified the relative importance of these covariates by calculating normalized effect sizes (Fisher’s z transformation based on correlation coefficients derived from F ratios; ref. 19), with and without prior adjustments for the effect of altitude. Finally, we integrated the likely causal relationships among variables in a structural equation model (53). Starting from a saturated model, we removed nonsignificant paths with small path coefficients, until a model remained in which all path coefficients were significantly different from zero and for which there was no significant deviation between observed and model-implied covariance among the variables (χ2 test; P > 0.05). These models were fitted by maximum likelihood, using the lavaan software (lavaan.ugent.be). Here, we focus on the most parsimonious of a wide array of models that we systematically explored. Additional models are provided as SI Materials and Methods (Table S1).
Acknowledgments
We thank the BDM, in particular Tobias Roth and Hintermann & Weber AG, and Irene Garonna and Rogier de Jong who provided advice on remote sensing and vegetation indices. NASA hosted the online Data Pool from which we downloaded the MODIS data. This study was funded by the University of Zurich Research Priority Program Global Change and Biodiversity (URPP GCB).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. S.N. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1703928114/-/DCSupplemental.
References
- 1.Balvanera P, et al. Quantifying the evidence for biodiversity effects on ecosystem functioning and services. Ecol Lett. 2006;9:1146–1156. doi: 10.1111/j.1461-0248.2006.00963.x. [DOI] [PubMed] [Google Scholar]
- 2.Gross K, et al. Species richness and the temporal stability of biomass production: A new analysis of recent biodiversity experiments. Am Nat. 2014;183:1–12. doi: 10.1086/673915. [DOI] [PubMed] [Google Scholar]
- 3.Hooper DU, et al. Effects of biodiversity on ecosystem functioning: A consensus of current knowledge. Ecol Monogr. 2005;75:3–35. [Google Scholar]
- 4.Civitello DJ, et al. Biodiversity inhibits parasites: Broad evidence for the dilution effect. Proc Natl Acad Sci USA. 2015;112:8667–8671. doi: 10.1073/pnas.1506279112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hooper DU, et al. A global synthesis reveals biodiversity loss as a major driver of ecosystem change. Nature. 2012;486:105–108. doi: 10.1038/nature11118. [DOI] [PubMed] [Google Scholar]
- 6.Tilman D, Reich PB, Isbell F. Biodiversity impacts ecosystem productivity as much as resources, disturbance, or herbivory. Proc Natl Acad Sci USA. 2012;109:10394–10397. doi: 10.1073/pnas.1208240109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wardle DA. Do experiments exploring plant diversity-ecosystem functioning relationships inform how biodiversity loss impacts natural ecosystems? J Veg Sci. 2016;27:646–653. [Google Scholar]
- 8.Duffy JE. Why biodiversity is important to the functioning of real-world ecosystems. Front Ecol Environ. 2009;7:437–444. [Google Scholar]
- 9.Haddad NM, et al. Plant species loss decreases arthropod diversity and shifts trophic structure. Ecol Lett. 2009;12:1029–1039. doi: 10.1111/j.1461-0248.2009.01356.x. [DOI] [PubMed] [Google Scholar]
- 10.Schmid B, et al. Biodiversity and Ecosystem Functioning: Synthesis and Perspectives. Oxford Univ Press; Oxford: 2002. The design and analysis of biodiversity experiments; pp. 61–75. [Google Scholar]
- 11.Hector A, et al. Plant diversity and productivity experiments in european grasslands. Science. 1999;286:1123–1127. doi: 10.1126/science.286.5442.1123. [DOI] [PubMed] [Google Scholar]
- 12.Lepš J. What do the biodiversity experiments tell us about consequences of plant species loss in the real world? Basic Appl Ecol. 2004;5:529–534. [Google Scholar]
- 13.Pimm SL, et al. The biodiversity of species and their rates of extinction, distribution, and protection. Science. 2014;344:1246752. doi: 10.1126/science.1246752. [DOI] [PubMed] [Google Scholar]
- 14.Brooks TM, et al. Habitat loss and extinction in the hotspots of biodiversity. Conserv Biol. 2002;16:909–923. [Google Scholar]
- 15.Naeem S, Duffy JE, Zavaleta E. The functions of biological diversity in an age of extinction. Science. 2012;336:1401–1406. doi: 10.1126/science.1215855. [DOI] [PubMed] [Google Scholar]
- 16.Costanza R, et al. The value of the world’s ecosystem services and natural capital. Nature. 1997;387:253–260. [Google Scholar]
- 17.Weber D, Hintermann U, Zangger A. Scale and trends in species richness: Considerations for monitoring biological diversity for political purposes. Glob Ecol Biogeogr. 2004;13:97–104. [Google Scholar]
- 18.Didan K. 2015 MOD13Q1 MODIS/Terra Vegetation Indices 16-Day L3 Global 250m SIN GridV006. NASA EOSDIS Land Processes DAAC. Available at https://doi.org/10.5067/modis/mod13q1.006. Accessed January 16, 2016.
- 19.Rosenthal R. Parametric measures of effect size. In: Cooper H, Hedges LV, editors. The Handbook of Research Synthesis. Russell Sage Foundation; New York: 1994. pp. 231–244. [Google Scholar]
- 20.Loreau M, Mouquet N, Holt RD. Meta-ecosystems: A theoretical framework for a spatial ecosystem ecology. Ecol Lett. 2003;6:673–679. [Google Scholar]
- 21.Barrufol M, et al. Biodiversity promotes tree growth during succession in subtropical forest. PLoS One. 2013;8:e81246. doi: 10.1371/journal.pone.0081246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Paquette A, Messier C. The effect of biodiversity on tree productivity: From temperate to boreal forests. Glob Ecol Biogeogr. 2011;20:170–180. [Google Scholar]
- 23.Vilà M, et al. Disentangling biodiversity and climatic determinants of wood production. PLoS One. 2013;8:e53530. doi: 10.1371/journal.pone.0053530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ciais P, et al. Europe-wide reduction in primary productivity caused by the heat and drought in 2003. Nature. 2005;437:529–533. doi: 10.1038/nature03972. [DOI] [PubMed] [Google Scholar]
- 25.Jolly WM, Dobbertin M, Zimmermann NE, Reichstein M. Divergent vegetation growth responses to the 2003 heat wave in the Swiss Alps. Geophys Res Lett. 2005;32:L18409. [Google Scholar]
- 26.Huston MA. Hidden treatments in ecological experiments: Re-evaluating the ecosystem function of biodiversity. Oecologia. 1997;110:449–460. doi: 10.1007/s004420050180. [DOI] [PubMed] [Google Scholar]
- 27.Menzel A. Plant phenological “fingerprints”. In: Schwartz MD, editor. Phenology: An Integrative Environmental Science. Springer, Dordrecht; The Netherlands: 2013. pp. 335–350. [Google Scholar]
- 28.Pepin N, et al. Elevation-dependent warming in mountain regions of the world. Nat Clim Chang. 2015;5:424–430. [Google Scholar]
- 29.Cleland EE, et al. Phenological tracking enables positive species responses to climate change. Ecology. 2012;93:1765–1771. doi: 10.1890/11-1912.1. [DOI] [PubMed] [Google Scholar]
- 30.Rathcke B, Lacey EP. Phenological patterns of terrestrial plants. Annu Rev Ecol Syst. 1985;16:179–214. [Google Scholar]
- 31.Hutchinson GE. An Introduction to Population Ecology. Yale Univ Press; New Haven, CT: 1978. [Google Scholar]
- 32.Wilczek AM, et al. Genetic and physiological bases for phenological responses to current and predicted climates. Philos Trans R Soc Lond B Biol Sci. 2010;365:3129–3147. doi: 10.1098/rstb.2010.0128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ibáñez I, et al. Forecasting phenology under global warming. Philos Trans R Soc Lond B Biol Sci. 2010;365:3247–3260. doi: 10.1098/rstb.2010.0120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Grime JP. Competitive exclusion in herbaceous vegetation. Nature. 1973;242:344–347. [Google Scholar]
- 35.Tilman D. Resource Competition and Community Structure. Princeton Univ Press; Princeton: 1982. [PubMed] [Google Scholar]
- 36.Grace JB. The factors controlling species density in herbaceous plant communities: An assessment. Perspect Plant Ecol Evol Syst. 1999;2:1–28. [Google Scholar]
- 37.Gillman LN, Wright SD. The influence of productivity on the species richness of plants: A critical assessment. Ecology. 2006;87:1234–1243. doi: 10.1890/0012-9658(2006)87[1234:tiopot]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 38.Rihm B. 1996. Critical loads of nitrogen and their exceedances: Eutrophing atmospheric depositions (FOEFL, Bern, Switzerland), Environmental Series No. 275.
- 39.Rogora M, et al. An overview of atmospheric deposition chemistry over the Alps: Present status and long-term trends. Hydrobiologia. 2006;562:17–40. [Google Scholar]
- 40.Hautier Y, et al. Plant ecology. Anthropogenic environmental changes affect ecosystem stability via biodiversity. Science. 2015;348:336–340. doi: 10.1126/science.aaa1788. [DOI] [PubMed] [Google Scholar]
- 41.Isbell F, et al. Nutrient enrichment, biodiversity loss, and consequent declines in ecosystem productivity. Proc Natl Acad Sci USA. 2013;110:11911–11916. doi: 10.1073/pnas.1310880110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.MacArthur R, MacArthur JW. On bird species-diversity. Ecology. 1961;42:594–598. [Google Scholar]
- 43.Wohlgemuth T. Biogeographical regionalization of Switzerland based on floristic data: How many species are needed? Biodivers Lett. 1996;3:180–191. [Google Scholar]
- 44.BDM Coordination Office . Swiss Biodiversity Monitoring BDM. Description of Methods and Indicators. Federal Office for the Environment; Bern, Switzerland: 2014. pp. 1–104. [Google Scholar]
- 45.Kéry M, Royle JA, Schmid H. Modeling avian abundance from replicated counts using binomial mixture models. Ecol Appl. 2005;15:1450–1461. [Google Scholar]
- 46.Huete A, et al. Overview of the radiometric and biophysical performance of the MODIS vegetation indices. Remote Sens Environ. 2002;83:195–213. [Google Scholar]
- 47.Roerink GJ, Menenti M, Verhoef W. Reconstructing cloudfree NDVI composites using Fourier analysis of time series. Int J Remote Sens. 2000;21:1911–1917. [Google Scholar]
- 48.de Beurs KM, Henebry GM. Spatio-temporal statistical methods for modelling Land Surface Phenology. In: Hudson IL, Keatley MR, editors. Phenological Research: Methods for Environmental and Climate Change Analysis. Springer; New York: 2010. pp. 177–208. [Google Scholar]
- 49.White MA, et al. Intercomparison, interpretation, and assessment of spring phenology in North America estimated from remote sensing for 1982-2006. Glob Change Biol. 2009;15:2335–2359. [Google Scholar]
- 50.White MA, Thornton PE, Running SW. A continental phenology model for monitoring vegetation responses to interannual climatic variability. Global Biogeochem Cycles. 1997;11:217–234. [Google Scholar]
- 51.Garonna I, et al. Strong contribution of autumn phenology to changes in satellite-derived growing season length estimates across Europe (1982-2011) Glob Change Biol. 2014;20:3457–3470. doi: 10.1111/gcb.12625. [DOI] [PubMed] [Google Scholar]
- 52.McGarigal K. 2015 Fragstats Help. (University of Massachusetts, Amherst, MA), Version 4.2. Available at www.umass.edu/landeco/research/fragstats/documents/fragstats.help.4.2.pdf. Accessed August 17, 2017.
- 53.Grace JB. Structural Equation Modeling and Natural Systems. Cambridge Univ Press; Cambridge, UK: 2006. [Google Scholar]
- 54.Running SW, Zhao M. 2015 MOD17A2(3)H MODIS/Terra Gross (Net) Primary Productivity 8-Day (Yearly) L4 Global 500m SIN Grid V006. NASA EOSDIS Land Processes DAAC. Available at https://doi.org/10.5067/modis/mod17a2(3)h.006. Accessed February 2, 2017.