Significance
Since Down syndrome (DS) is caused by trisomy of chromosome 21, prenatal diagnosis of DS is now possible. Nonetheless, parents of a fetus diagnosed with DS have only two choices: terminate the pregnancy or prepare to raise a child with a serious disability. We developed a new compound, ALGERNON (altered generation of neurons), to provide a third option to these parents. Treatment of pregnant dams with ALGERNON prevented morphological brain abnormalities including a thinned cortical plate. Remarkably, these offspring exhibited normal cognitive behavior compared with untreated offspring with DS. ALGERNON has therapeutic potential for treating not only DS, but also numerous neurodevelopmental disorders.
Keywords: developmental disorder, Down syndrome, neurogenesis
Abstract
Down syndrome (DS) caused by trisomy of chromosome 21 is the most common genetic cause of intellectual disability. Although the prenatal diagnosis of DS has become feasible, there are no therapies available for the rescue of DS-related neurocognitive impairment. A growth inducer newly identified in our screen of neural stem cells (NSCs) has potent inhibitory activity against dual-specificity tyrosine phosphorylation-regulated kinase 1A (DYRK1A) and was found to rescue proliferative deficits in Ts65Dn-derived neurospheres and human NSCs derived from individuals with DS. The oral administration of this compound, named ALGERNON (altered generation of neurons), restored NSC proliferation in murine models of DS and increased the number of newborn neurons. Moreover, administration of ALGERNON to pregnant dams rescued aberrant cortical formation in DS mouse embryos and prevented the development of abnormal behaviors in DS offspring. These data suggest that the neurogenic phenotype of DS can be prevented by ALGERNON prenatal therapy.
Down syndrome (DS) is the most common congenital cause of intellectual disability, occurring in 1 in every 757 live-born infants in the United States (1). Although the level of DS-related disability ranges from mild to severe, DS nonetheless has a substantial impact on the life of affected individuals and parents and is associated with significant socioeconomic burden. The major cause of intellectual disability in DS is thought to be defects in neurogenesis that are already present during prenatal life (2, 3). As DS is caused by trisomy of human chromosome 21, DS can be diagnosed through a prenatal chromosome analysis. Accordingly, prenatal therapy to normalize aberrant neurogenesis in the fetal DS brain is an awaited therapeutic option.
The largest region of human chromosome 21 is conserved in the orthologous region of mouse chromosome 16. Accordingly, several mouse models of DS such as the Ts65Dn and Ts1Cje models have been generated to study the etiology of DS (4). Studies of these mice have reported prenatal brain abnormalities similar to those observed in human DS. Both Ts65Dn and Ts1Cje mice exhibit growth retardation in the neocortical wall due to impaired cell cycle arrest, resulting in compromised neuronal differentiation during embryogenesis (5, 6). Aberrant neurogenesis has also been observed in adult DS model mice (7, 8). Behaviorally, DS model mice display features relevant to human DS, including deficits in learning and memory (4).
A number of educational approaches have been implemented for the therapeutic rehabilitation of cognitive disability in individuals with DS; however, an improved learning environment in the absence of any molecular intervention has limited utility for restoring DS-related intellectual disability (9). Since neuronal alterations manifest prenatally, it can be hypothesized that pharmacological treatment during the prenatal period of brain development has the potential to fully rescue DS-related brain abnormalities and intellectual disability. To realize the possibility of a prenatal therapy for DS, we performed a screen of our kinase inhibitor-focused library for compounds promoting the growth of neural stem cells (NSCs) and identified compounds that enhance NSC proliferation through the inhibition of dual-specificity tyrosine phosphorylation-regulated kinase 1A (DYRK1A), which is encoded in the DS critical region. One of these compounds, dubbed ALGERNON (altered generation of neurons), restored impaired NSC proliferation both in vitro and in vivo and prevented the development of learning deficits in DS model mice.
Results
Compound Screening.
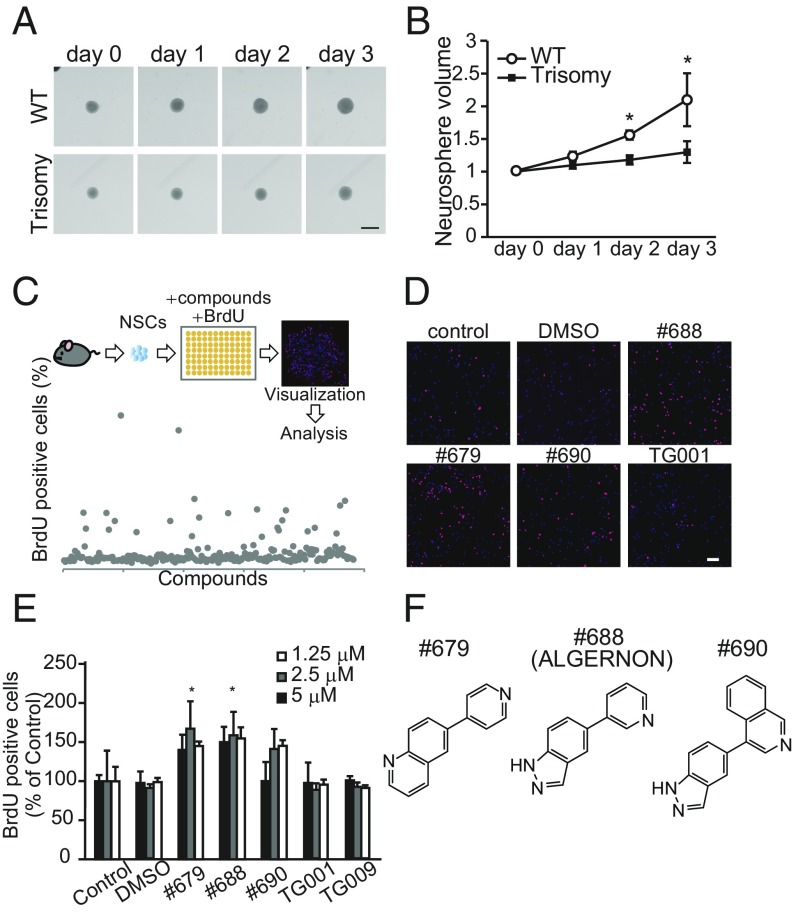
To perform an in vitro assessment of aberrant neurogenesis occurring in DS mice (7, 8), we isolated and cultured NSCs from Ts65Dn DS model mice. Consistent with previous observations in vivo, neurosphere cultures prepared from DS mice showed delayed growth (Fig. 1 A and B) relative to cultures prepared from WT mice. To identify compounds capable of reversing deficits in NSC proliferation, we screened our chemical library for compounds promoting NSC growth, assessed by measuring BrdU incorporation (Fig. 1C). Compounds #679, #688, and #690 (Fig. 1F) significantly increased the ratio of BrdU-positive cells (Fig. 1 D and E). As our chemical library specifically targets kinases, we performed a panel assay of 309 kinases to evaluate the specificity of #688 and found that #688 was a selective inhibitor of the DYRK and CLK families (SI Appendix, Fig. S1 and Table S1). The effective bis (heteroaryl) compounds (Fig. 1F) showed ATP-competitive IC50 values of 0.128 μM (#679), 76.9 nM (#688), and 0.364 μM (#690) for DYRK1A (SI Appendix, Fig. S2). Of note, control compounds TG001 and TG009 (CLK inhibitors without inhibitory activity against DYRK1A) (10) did not increase BrdU incorporation (Fig. 1 D and E). In DYRK1A-overexpressing cells, candidate compounds suppressed the phosphorylation of tau, a well-characterized substrate of DYRK1A (11), in a dose-dependent manner (SI Appendix, Fig. S3A). The #688 also suppressed the phosphorylation of endogenous tau in primary hippocampal neurons (SI Appendix, Fig. S3B), indicating that these compounds suppress endogenous DYRK1A.
Fig. 1.
Screening for NSC growth-promoting compounds. (A and B) Assessment of proliferation of DS model NSCs. (A) Representative images of neurosphere cultures derived from WT and Ts65Dn DS model mice are shown. (Scale bar, 400 μm.) (B) Trisomy neurospheres showed reduced growth volumes. Data are normalized to day 0 and represent the average of at least three independent experiments. (C) Schematic of experiments for screening NSC growth-promoting compounds. Representative images (D) and quantitative analyses (E) of NSCs treated with the indicated compounds are shown. Proliferating NSCs were pulse-labeled with BrdU and visualized in red. (Scale bar, 100 μm.) Data were normalized to nontreated control wells and averaged from three independent experiments [F(6,16) = 2.84, P = 7.68E-06]. (F) The structures of compounds #679, #688 (ALGERNON), and #690. *P < 0.05.
DYRK1A Promotes NSC Proliferation.
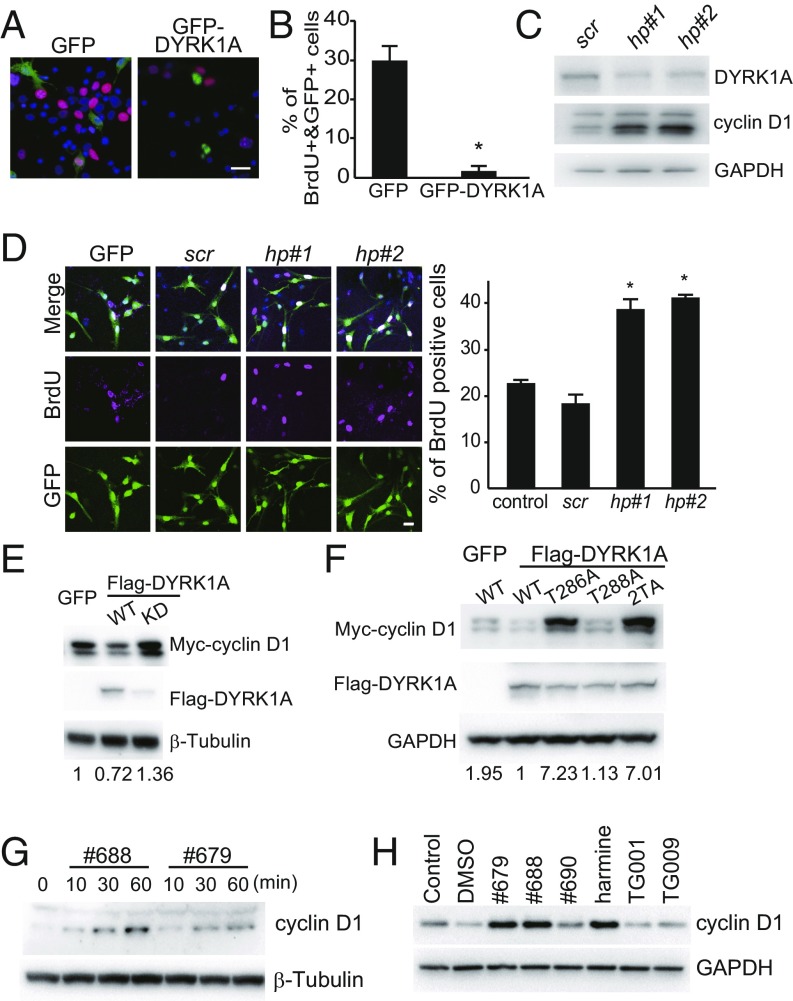
Since compounds promoting NSC proliferation showed potent inhibitory activity against DYRK1A, we examined the involvement of DYRK1A in NSC proliferation. We overexpressed GFP-DYRK1A in NSCs and labeled proliferating cells with BrdU (Fig. 2A). While ∼30% of GFP-expressing NSCs showed BrdU incorporation, minimal BrdU incorporation was observed in GFP-DYRK1A–expressing NSCs (Fig. 2B), indicating that DYRK1A negatively regulated NSC proliferation. To confirm this finding, we delivered short-hairpin (sh) RNA against DYRK1A to murine-cultured neurospheres using lentiviral constructs. GFP coexpression was used to confirm construct delivery, and those shRNAs targeting either the coding sequence (hp#1) or the 3′ untranslated region (3′ UTR; hp#2) of mouse DYRK1A reduced DYRK1A expression (Fig. 2C). Measurement of BrdU incorporation revealed that the depletion of DYRK1A enhanced NSC proliferation (Fig. 2D). Together, these results clearly indicated that DYRK1A is a negative regulator of NSC proliferation.
Fig. 2.
Effects of DYRK1A overexpression or knockdown on NSC proliferation (A–D) and cyclin D1 expression (E–J). (A) Representative images and (B) quantitative analyses of NSCs expressing GFP or DYRK1A are shown. BrdU incorporation is visualized in red. (Scale bar, 10 μm.) (C) NSCs were transfected with lentiviral vectors expressing scramble shRNA (scr) and EGFP, DYRK1A #1 shRNA (hp#1) and EGFP, or Dyrk1A #2 shRNA (hp#2) and EGFP. Effective DYRK1A knockdown was observed 3 d posttransfection. (D, Left) Representative images of NSCs transfected with the indicated lentiviral vectors and labeled for BrdU incorporation (magenta). (Scale bar, 20 μm.) (Right graph) Ratios of BrdU double-positive cells to GFP-positive cells (% BrdU+&GFP+/GFP+) in DYRK1A-knockdown NSCs [F(4,12) = 4.06, P = 0.000149]. (E) Cyclin D1 expression in DYRK1A-expressing and DYRK1A kinase dead mutant-expressing cells. (F) DYRK1A coexpressed with Myc-cyclin D1 WT, T286A, T288A, or T286AT288A (2TA) mutants. The values at the bottom of the panels indicate protein levels normalized to control values. (E–H) Effects of NSC growth-promoting compounds #679, #688 (ALGERNON), and #690 on cyclin D1 expression. HEK293 cells (G) and NSCs (H) were treated with NSC growth-promoting compounds (#679, #688, and #690), a DYRK1A inhibitor (harmine), and structurally related negative control compounds (TG001 and TG009) (5 μM each) for the indicated durations or 24 h, respectively. *P < 0.05.
DYRK1A Phosphorylates Cyclin D1 and Induces Its Degradation.
To determine the mechanism by which DYRK1A negatively regulates NSC proliferation, we next investigated its effect on cyclins, given the observation that DYRK1A knockdown up-regulated the expression of cyclin D1 in NSCs (Fig. 2C). The effect of DYRK1A inhibition on cyclin D1 expression is the most prominent among other cell cycle-regulating cyclins (SI Appendix, Fig. S4A). We introduced expression vectors for GFP or Flag-DYRK1A with Myc-cyclin D1 into HEK293 cells and observed reduced Myc-cyclin D1 expression in Flag-DYRK1A–expressing cells relative to GFP controls (Fig. 2E). Alternatively, overexpression of the kinase-dead DYRK1A K188R mutant increased Myc-cyclin D1 expression (Fig. 2E). Cytosolic cyclin D1 is constantly degraded via proteasome activity (12) (SI Appendix, Fig. S4B), so we examined the effect of DYRK1A on cyclin D1 expression and phosphorylation at Thr286, an important phosphorylation site regulating its nuclear export. We observed that the DYRK1A-mediated reduction in Myc-cyclin D1 expression was abolished by a T286A mutation at Thr286, but not by the same mutation at Thr288 (Fig. 2F). Thus, DYRK1A must phosphorylate cyclin D1 at Thr286 to induce its degradation. Next, we examined the effect of our newly identified DYRK1A inhibitors on cyclin D1 expression in vitro. Treatment of HEK293 cells with #679 or #688 dramatically increased cyclin D1 expression within 60 min (Fig. 2G). Treatment with #679 or #688 also increased cyclin D1 expression in NSCs (Fig. 2H), whereas control compounds TG001 and TG009 had no effects on cyclin D1 expression. These data indicate that DYRK1A is involved in proliferation through the regulation of cyclin D1 expression.
Suppression of DYRK1A Rescues Impaired NSC Proliferation in Murine Models of DS and Human DS Cells.
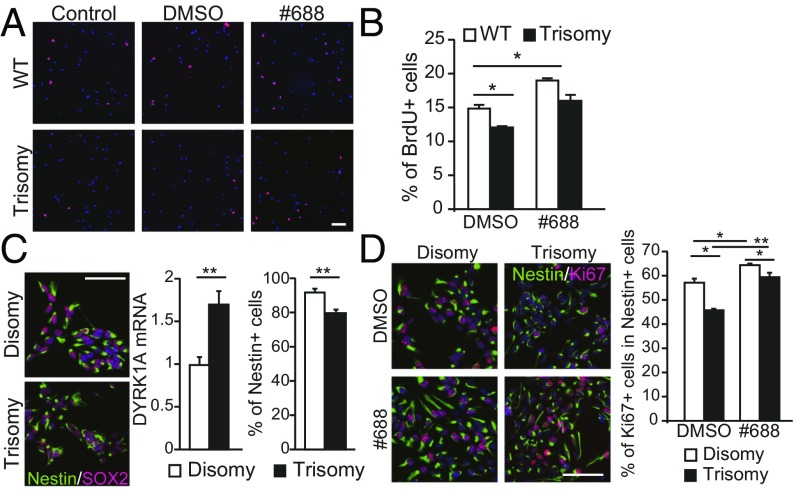
Next, we confirmed the ability of compound #688 to promote the growth of Ts65Dn-derived neurospheres. We observed an increased number of BrdU-positive cells in Ts65Dn-derived neurospheres that were treated with compound #688; moreover, the number of BrdU-positive cells was similar to that observed in WT neurospheres (Fig. 3 A and B).
Fig. 3.
Recovery of slowed cell cycle progression in trisomy NCSs (A and B), and NSCs (C and D) isolated from individuals with DS after #688 treatment. (A) Representative images of BrdU-positive NSCs (red) prepared from WT and Ts65Dn trisomy mice treated with DMSO vehicle or ALGERNON are shown. (Scale bar, 100 μm.) (B) Effect of ALGERNON treatment on the ratio of BrdU-positive cells in trisomy NSCs. Data were averaged from three independent experiments [F(3,8) = 4.06, P = 0.0064]. (C) Generation of NSCs from DS-iPSCs. A disomy control was naturally acquired in the process of generating iPSCs from fibroblasts. DS-derived NSCs were confirmed by immunolabeling for Nestin and SOX2 (Left) (Scale bar, 100 μm.). DS-NSCs showed a higher level of DYRK1A mRNA and fewer Nestin-positive cells (Right graphs). (D) Representative images (Left) and quantification (Right graph) of proliferating Ki67-positive cells in the Nestin-positive population [F(3,9) = 3.86, P = 1.23E-05]. *P < 0.05; **P < 0.01.
We next investigated the proliferation rate of human fibroblasts derived from euploid (control) individuals and individuals with DS. DS-derived fibroblasts showed reduced proliferation (SI Appendix, Fig. S4C), consistent with our observations in the murine DS model (Fig. 1 A and B). We examined the role of DYRK1A in human fibroblast proliferation by specific knockdown of DYRK1A and found that the repression of DYRK1A restored the proliferative capacity of DS-derived fibroblasts to a level comparable to that of euploid-derived fibroblasts (SI Appendix, Fig. S4C).
Next, we examined whether treatment with #688 rescues impaired proliferation in DS-derived human fibroblasts. While DS-derived fibroblast cultures (Tri#1 and Tri#2) showed significantly delayed growth, cultures treated with #688 showed growth values that approached euploid control values (SI Appendix, Fig. S4D). Additionally, a majority of DS-derived fibroblasts were in the G1 phase, whereas fewer cells were in the S and G2/M phases (SI Appendix, Fig. S4 E and F). This is in agreement with our finding that DYRK1A phosphorylated cyclin D1 to regulate its expression level/degradation. Knockdown of DYRK1A in DS-derived fibroblasts normalized the population of cells in the G1 phase to euploid control levels (SI Appendix, Fig. S4 E and F). Treatment of DS-derived fibroblasts with #688 produced similar results (SI Appendix, Fig. S4 G and H). Taken together, these data indicated that #688 restores the proliferative capacity of NSCs derived from DS model mice, as well as human fibroblasts derived from individuals with DS. Furthermore, these results were confirmed in patient-derived NSCs/neural progenitor cells (NPCs) (Fig. 3 C and D). We generated DS-NSCs/NPCs from induced pluripotent stem cells (iPSCs) of individuals with DS (Fig. 3C). Trisomic NSCs/NPCs showed higher expression of DYRK1A mRNA and fewer Nestin-positive cells (Fig. 3C), which is consistent with a previous observation (13). Importantly, #688 treatment increased the population of Ki67-positive cells (Fig. 3D) to disomic control levels and normalized the increased population of G1-phase DS-NSCs/NPCs as well (SI Appendix, Fig. S4I). Thus, it is indicated that #688 rescued impaired proliferation in DS-derived human cells.
Compound #688 (ALGERNON) Enhances Neurogenesis in the Dentate Gyrus of the Hippocampus.
To evaluate the bioavailability of #688 in mice, we measured drug concentrations in plasma and brain tissue after different modes of administration (SI Appendix, Fig. S5). Compound #688 was maintained at a concentration above the effective dose in plasma for at least 4 h after oral administration (SI Appendix, Fig. S5B); moreover, concentrations of #688 were detected in the brain after both oral and s.c. administration at a dose of 10 mg/kg (SI Appendix, Fig. S5 A and C).
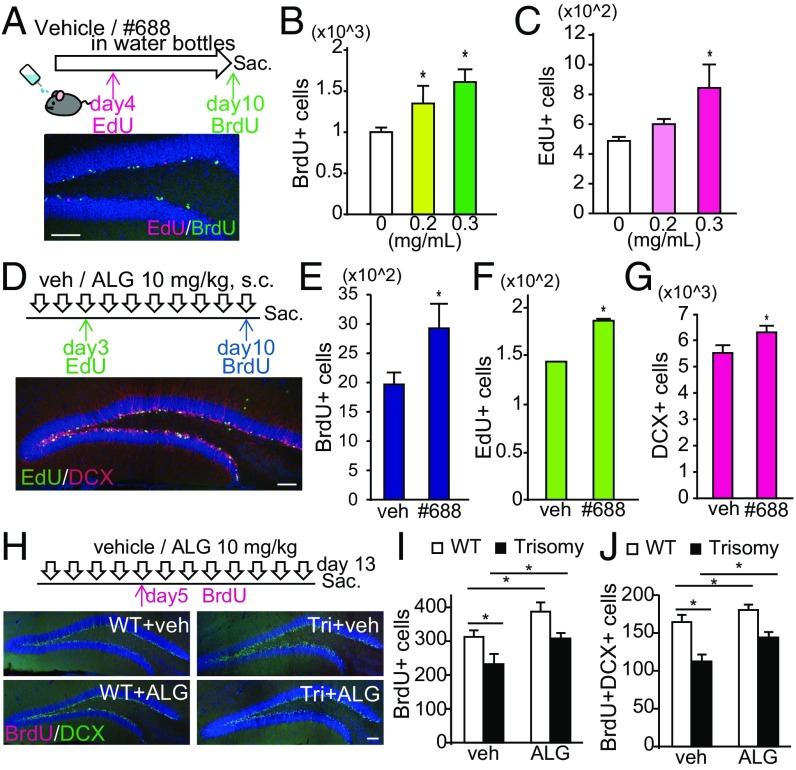
Compound #688 was water-soluble up to 4 mg/mL in 5% Tween 80 (data not shown), and this solution was orally administered to mice for 10 d in drinking water. Mice were then injected with 5-ethynyl-2′-deoxyuridine (EdU) (50 mg/kg) on day 4 and BrdU (50 mg/kg) on day 10 (Fig. 4A, Upper) to label proliferating cells and subsequently killed 24 h after BrdU injection. EdU and BrdU incorporation were evaluated in the dentate gyrus of the hippocampus (Fig. 4A, Lower). BrdU-positive cells were increased in the dentate gyrus of #688-treated mice (Fig. 4B), suggesting that #688 promoted neurogenesis within 10 d after treatment initiation. Similar results were obtained in the analysis of EdU-positive cells, which were proliferating by day 4 and survived for an additional week (Fig. 4C). Thus, the compound #688 was renamed ALGERNON (altered generation of neurons).
Fig. 4.
ALGERNON promotes neurogenesis in the dentate gyrus of the adult mouse hippocampus (A–C) and rescues impaired neurogenesis in Ts1Cje mice (D–F). (A, Upper) Experimental timeline for assessing NSC proliferation after EdU and BrdU injections following ALGERNON treatment via drinking water. (A, Lower) Representative image of proliferating cells at day 4, the survival of these cells 1 wk later (EdU, red), and proliferating cells at day 10 (BrdU, green). (Scale bar, 100 μm.) (B and C) Quantified BrdU-positive cells and EdU-positive cells in the dentate gyrus of the hippocampus in each group, respectively (n = 3). (D, Upper) Experimental timeline for assessing NSC proliferation and differentiation after EdU and BrdU injections following s.c. ALGERNON administration. (D, Lower) Representative image of newly generated immature neurons positive for DCX (red) and a cell proliferation marker (EdU, green) in the dentate gyrus of the hippocampus. (Scale bar, 100 μm.) (E–G) Quantification of BrdU-positive cells (E), EdU-positive cells (F), and DCX-positive cells (G). n = 10 per group. (H, Upper) Experimental timeline for assessing NSC proliferation and differentiation after BrdU injections following ALGERNON treatment. (H, Lower) Representative images of newly generated immature neurons positive for DCX (green) and a proliferation marker (BrdU, red) in the dentate gyrus of the hippocampus from WT and trisomy mice. (Scale bar, 100 μm.) (I and J) Quantified BrdU-positive cells and BrdU/DCX double-positive cells in each group, respectively (n = 8). F(3,28) = 6.99, P = 0.0015 (I); F(3,28) = 6.88, P = 0.0014 (J). *P < 0.05.
We next investigated whether newly generated cells could differentiate into neurons following ALGERNON treatment. ALGERNON (10 mg/kg, s.c.) was administered to mice followed by EdU injection on day 3 and BrdU injection on day 10, and mice were killed 24 h after BrdU injection (Fig. 4D, Upper). EdU incorporation and the expression of doublecortin (DCX), which is transiently expressed in newborn cells during early neuronal differentiation, were analyzed. As expected, ALGERNON treatment significantly increased EdU-positive and BrdU-positive cells (Fig. 4 E and F). Numbers of DCX-positive (Fig. 4G) and EdU/DCX-double-positive cells (SI Appendix, Fig. S6A) were also increased by ALGERNON treatment, indicating that new NSCs generated on day 3 differentiated into young neurons by day 10. Of note, the ratio of EdU/DCX double-positive cells was similar to that between ALGERNON-treated and vehicle-treated mice (SI Appendix, Fig. S6B), indicating that ALGERNON did not suppress neuronal differentiation, but did enhance precursor proliferation.
We analyzed EdU incorporation alongside the expression of NeuN, a marker of mature neurons, in the dentate gyrus of the hippocampus following ALGERNON treatment. Mice were killed 4 wk after EdU injection (SI Appendix, Fig. S6C, Upper). The number of EdU/NeuN double-positive cells was increased (SI Appendix, Fig. S6D), while the ratio of EdU/NeuN double-positive cells was comparable between ALGERNON-treated and vehicle-treated mice (SI Appendix, Fig. S6E), indicating that newborn neurons successfully differentiated into mature neurons after ALGERNON treatment. We further examined which type of NSCs in the subgranular zone of the dentate gyrus of the hippocampus responded to ALGERNON. We labeled radial glia-like NSCs with anti-GFAP immunolabeling and found that ALGERNON treatment more potently increased the BrdU/GFAP double-positive radial glial cells, which represent quiescent NSCs (SI Appendix, Fig. S6 F–H).
We next asked whether ALGERNON could rescue impaired neurogenesis in the Ts1Cje mouse model of DS. We administered ALGERNON or vehicle to WT and Ts1Cje mice at 9 wk old for 12 d, injected BrdU on day 5, and killed them on day 13 (Fig. 4H, Upper). The analysis revealed that ALGERNON significantly increased the number of BrdU-positive (Fig. 4I) and BrdU/DCX double-positive cells (Fig. 4J) in the dentate gyrus of the hippocampus, indicating that ALGERNON treatment effectively enhanced neurogenesis in DS mice.
ALGERNON Rescues Cortical Formation in Ts1Cje Embryos.
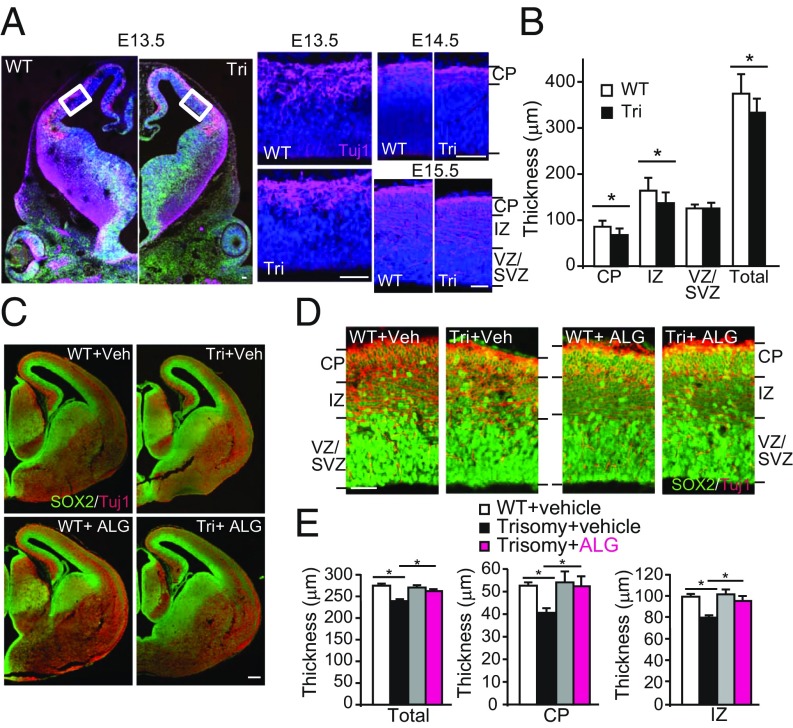
Given that early defects in embryonic brain development are a critical component of dysfunction in DS, we analyzed embryonic cortical development in the Ts1Cje mouse model (Fig. 5A). The neocortical layers consist of the ventricular zone/subventricular zone (VZ/SVZ), where the NSC/neural precursor cells reside (14); the intermediate zone (IZ), which contains migrating neurons and axon tracts; and the cortical plate (CP), which includes migrating and differentiating neurons. Analysis of Tuj1 staining in E13.3–E15.5 embryos demonstrated thinning of the neocortical wall in DS model embryos compared with WT embryos (Fig. 5B). While there was no difference in the thickness of the VZ/SVZ between Ts1Cje and WT embryos, the IZ and CP were thinner in model embryos compared with WT embryos (Fig. 5B).
Fig. 5.
Prenatal administration of ALGERNON normalizes the thickness of the cortical layer in Ts1Cje trisomy embryos. (A and B) Analysis of cortical development in Ts1Cje DS model embryos. (A) Representative images of coronal sections from WT and trisomy (Tri) embryos at E13.5 with enlarged images of the cortical walls denoted by white boxes. Matched sections at E14.5 and E15.5 are also shown. (Scale bar, 50 μm.) The cortical plate was identified with the neuronal marker Tuj1 (magenta). (B) Quantification of cortical layer thickness. The neocortical wall, intermediate zone (IZ), and CP were evaluated at E15.5. (C and D) Representative images of coronal sections of WT and trisomy of E15 embryos from dams treated with vehicle or ALGERNON. [Scale bars, 200 μm (C) and 50 μm (D).] (E) Quantification of cortical thicknesses in C–E (n = 5). F(3,16) = 3.59, P = 0.00045 (total); F(3,16) = 6.12, P = 0.00029 (CP); and F(3,16) = 8.76, P = 0.0068 (IZ). *P < 0.05.
To examine the utility of ALGERNON as a prenatal therapy for DS, we administered ALGERNON to pregnant DS model dams from E10 to E15 and examined the ability of ALGERNON to normalize impaired proliferation during development (SI Appendix, Fig. S7A, Upper). Oral administration of ALGERNON to pregnant dams successfully distributed ALGERNON to the developing brains of embryos (SI Appendix, Fig. S7A, Lower). In an analysis of corticogenesis, we found that ALGERNON administration to dams rescued CP and IZ thinning observed in untreated DS model embryos to a level approximating that in WT embryos (Fig. 5 C–E).
Prenatal ALGERNON Prevents the Development of Impaired Cognitive Behaviors in Ts1Cje DS Model Mice.
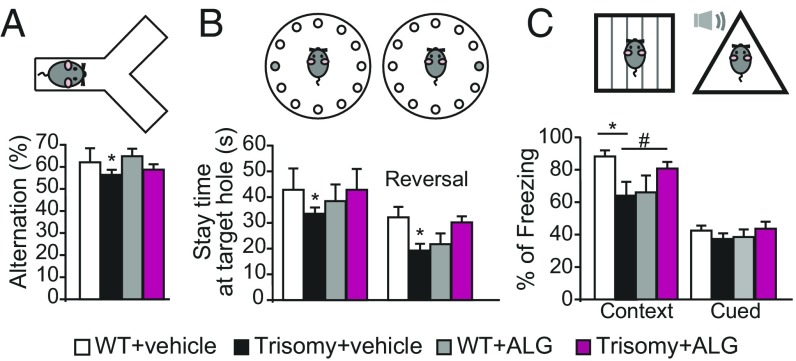
We next assessed whether behavioral abnormalities observed in DS model mice were normalized by prenatal ALGERNON therapy. Offspring treated with ALGERNON prenatally showed no difference in general neurologically regulated conditions such as body weight, rectal temperature, wire-hang ability, and grip strength (SI Appendix, Fig. S7 B–E). Trisomy mice showed decreased percentages of alternation in the Y-maze task, indicating an impairment in spontaneous exploration behavior; in contrast, ALGERNON-treated DS offspring showed improved percentage alternation scores compared with vehicle-treated trisomy mice (Fig. 6A). We further examined a hippocampus-dependent spatial memory task, the Barnes maze. In the acquisition phase, all groups learned the location of an escape box based on spatial cues (SI Appendix, Fig. S7F). In probe tests, Ts1Cje trisomy mice showed impaired spatial memory compared with WT mice (Fig. 6B); moreover, ALGERNON-treated DS offspring performed better than vehicle-treated DS mice (Fig. 6B, Left graph), and improved learning was also confirmed in the reversal learning test (Fig. 6B, Right graph). Associative learning was tested with fear conditioning. In Ts1Cje DS mice, cued fear memory was not altered, but hippocampus-dependent contextual memory was impaired with respect to WT mice (Fig. 6C); this is consistent with a previous report in another DS model, Ts65Dn (15). ALGERNON-treated DS offspring did not exhibit impairments in contextual fear learning compared with WT mice (Fig. 6C). We analyzed whether prenatal treatment was capable of rescuing adult neurogenesis in the brains of offspring after the behavior tests. Prenatal treatment rescued the malformation of corticogenesis during brain development (Fig. 5 C–E), but not the altered neurogenesis in trisomic offspring (SI Appendix, Fig. S7G). These data strongly suggest that ALGERNON therapy in the embryonic developmental stage prevented deficits in cognitive brain function related to the DS model.
Fig. 6.
Prenatal administration of ALGERNON prevents the development of impaired cognitive behaviors in trisomy mice. Behavior analysis of (A) Y-maze [F(3,35) = 2.72, P = 0.047]. (B) Barnes maze spatial memory task [F(3,35) = 3.46, P = 0.09 (Left graph); F(3,35) = 2.93, P = 0.016 (Right graph, reversal learning)]. (C) Fear conditioning [F(3,35) = 2.82, P = 0.039) (context); F(3,35) = 2.87, P = 0.77 (cued)]. *P < 0.05; #P = 0.116. WT, n = 9; trisomy, n = 11; vehicle-treated WT, n = 7 and ALGERNON-treated trisomy, n = 12.
Discussion
In the present study, we sought to develop compounds that can restore impaired neurogenesis in DS/DS models and identified a DYRK1A inhibitor, ALGERNON. By using ALGERON, we demonstrated that balancing DYRK1A activity can restore aberrant brain development and prevent cognitive deficits in DS model mice. Epigallocatechin gallate (EGCG), a catechin found in green tea, has also been reported to inhibit Dyrk1A (16); however, EGCG has profound influences on many other signaling pathways as an antioxidant/metal chelator and known inhibitor of proteasomes, matrix metalloproteinase, dihydrofolate reductase, DNA methyltransferase, topoisomerase II, and telomerase (17, 18). De la Torre et al. (19) reported that EGCG treatment improved learning deficits in Ts65Dn mice, but two recent studies refuted the former study (20, 21). Harmine is also known to inhibit DYRK1A, but produces serious adverse effects including hallucinations due to its monoamine oxidase (MAO)-A inhibitory activity, which occurs at a much lower dose than that required for DYRK inhibition (IC50 < 1 nM). In the present study, we tested the ability of ALGERNON to inhibit MAO-A activity and confirmed that the IC50 value of ALGERNON for MAO-A is much higher than the effective dose for DYRK1A (SI Appendix, Fig. S8). Thus, ALGERNON appears to provide effective DYRK1A inhibition without the promiscuity of EGCG or side effects of harmine.
DYRK1A phosphorylates various proteins including tau, cyclin D1, caspase-9, Notch, Gli1, Sprouty2, and the CTD of Poll II (22). The diversity of DYRK1A substrate proteins and the overt pathology caused by gene dosage imbalance suggest that DYRK1A is a key signaling integrator. The fact that DYRK1A has many substrates, including cyclin D1, raises the concern that the inhibition of DYRK1A with ALGERNON treatment, especially during a critical period of brain development, may produce side effects affecting growth and proliferation of many tissues. Importantly, offspring administered ALGERNON in utero showed no differences in body weight, rectal temperature, righting reflex, whisker twitch, ear twitch, reaching behavior, wire-hang performance, grip strength, and reaction to noise (key jangling) (SI Appendix, Fig. S7 B–E). Considering that the main role of DYRK1A is regulating brain development, the effects of ALGERNON treatment on other tissues are likely to be considerably smaller than those on neural development. In addition, we confirmed that repetitive administration of ALGERNON to adult mice for at least 7 wk did not alter the increase in body weight and amount of food consumption significantly from those values in vehicle control animals (SI Appendix, Fig. S9 A and B). We saw no abnormal neurological behaviors after 4 wk of ALGERNON administration (SI Appendix, Fig. S9 C–E). We also quantified the level of cleaved caspase-3 in the dentate gyrus of the hippocampus, since it could be activated through the inhibition of DYRK1A, given that caspase-9 is a reported substrate of the protein (23). However, no significant difference was observed (SI Appendix, Fig. S6I).
Moreover, DYRK1A has been reported to regulate neural differentiation (24), raising the possibility that inhibition of DYRK1A suppresses the generation of mature neurons. Indeed, NSC cultures treated with #688/ALGERNON for 4 d showed a reduced ratio of Tuj1-positive cells, a neuronal differentiation marker (SI Appendix, Fig. S6J). This seems reasonable if #688 keeps NSCs from proliferating, and thus represses differentiation, especially in vitro where the compounds are not metabolized. In our hands, s.c. ALGERNON at the described dose increased the number of proliferating cells without affecting the rate of differentiation or number of differentiated cells compared with vehicle-treated animals (Fig. 4 G–J and SI Appendix, Fig. S6 A–E) in contrast to in vitro experiments. One possible explanation for this observation is that the concentrations of ALGERNON used in this study were sufficient to stabilize cyclin D1 and enhance NSC proliferation, but did not suppress neuronal differentiation because of a short pharmacokinetic duration. We confirmed this by washing out #688 from NSC cultures in vitro after 2 d of initial differentiation. This resulted in normal differentiation of the NSCs treated with #688 initially (SI Appendix, Fig. S6J). This suggests that #688 promotes proliferation but does not affect differentiation. Thus, ALGERNON may have utility for the treatment aberrant brain development caused by imbalanced DYRK1A expression. Importantly, this is a successful case of prenatal therapy promoting neurogenesis during brain development. Notably, Zika virus infection is associated with an increased rate of microcephaly, and DYRK1A is reported to be up-regulated in Zika-infected human NPCs that exhibit dysregulated cell cycle progression and attenuated growth (25). Although the direct pathogenic link between Zika virus-induced microcephaly and up-regulated DYRK1A has yet to be elucidated, ALGERNON may also be applicable for the prevention or rescue of Zika virus-associated microcephaly.
In addition, adult neurogenesis persists throughout life (26) as a dynamic and highly regulated process that is modulated by various physiological and pathological factors. Neurogenesis facilitates the formation of new neural circuits in the adult brain by supplying new neurons to existing circuits in response to learning, pharmacological treatment, traumatic brain injury, and other stimuli (27, 28). Based on our finding that ALGERNON enhances adult neurogenesis in vivo, ALGERNON treatment may offer a new opportunity for the direct modification of basic brain tissue structure and function. Thus, ALGERNON has therapeutic potential not only for the treatment of DS, but also for a wide range of disorders involving progressive or permanent neuronal loss such as neurodegenerative diseases and traumatic brain injury.
Methods
Screening for Growth Inducers.
Screening was performed against our kinase-focused library of 717 compounds (29–31). Detailed information is available in SI Methods.
Study Approval.
All animal protocols were reviewed and approved by the Animal Research Committee, Graduate School of Medicine, Kyoto University.
Immunohistochemistry.
Male adult mouse brains were fixed in 4% paraformaldehyde, and 40-μm sections were cut with a vibratome (Leica). To collect embryonic brains, embryos were perfused with PBS, and brains were removed and immersed in 4% paraformaldehyde for 1 h. After equilibrating in 30% sucrose/PBS, 20-μm frozen sections were cut with a cryostat (Leica). After antigen retrieval using HistoOne (Nacalai Tesque), tissues were stained with anti-BrdU (Santa Cruz), anti-DCX (CST), anti-NeuN (Millipore), anti-Tuj1 (Covance), and anti-SOX2 (Millipore). Nuclei were stained using Hoechst reagent (Nacalai Tesque). EdU staining was performed using the Click-iT EdU Alexa Fluor 555 Imaging Kit (Life Technologies) according to the manufacturer’s instructions. Images were captured using a confocal microscope (SP8; Zeiss). Quantification was performed on every sixth section by an operator blinded to the conditions. The layers adjacent to both the upper (closest to the glass slide) and lower surfaces (closest to the coverslip) were excluded from quantification to avoid double-counting errors. The number of counted cells was multiplied by six to provide an estimate of the number of cells in the dentate gyrus.
Statistics.
Results obtained from more than three experiments are expressed as the mean ± SEM. Statistically significant differences were determined using a two-tailed, unpaired Student’s test or one-way analysis of variance (ANOVA) followed by a Tukey–Kramer comparison test. A P value of less than 0.05 was considered to be significant and marked with a single asterisk (*); a P value less than 0.01 was marked with a double asterisk (**).
Supplementary Material
Acknowledgments
We thank Mr. Kohei Araki and Ms. Keiko Wanezaki for technical assistance; the M.H. laboratory members for helpful discussion; and the Medical Research Support Center, the Radioisotope Research Center, and the Animal Facility of Kyoto University for assistance and the use of their equipment. This work was supported by Grants-in-Aid from the Japan Agency for Medical Research and Development (AMED) (to M.H. and T.H.); AMED-CREST (Create Revolutionary Technological Seeds for Science and Technology Innovation) (to M.H. and T.H.); the Ministry of Education, Culture, Sports, Science, and Technology (MEXT) of Japan (to M.H., T.H., and A.N.-K.); the Ministry of Health, Labour and Welfare of Japan (to M.H.); the Platform for Drug Discovery, Informatics, and Structural Life Science of MEXT, Japan (to M.H. and T.H.); and the Mochida Memorial Foundation (to A.N.-K.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1704143114/-/DCSupplemental.
References
- 1.Parker SE, et al. National Birth Defects Prevention Network Updated national birth prevalence estimates for selected birth defects in the United States, 2004-2006. Birth Defects Res A Clin Mol Teratol. 2010;88:1008–1016. doi: 10.1002/bdra.20735. [DOI] [PubMed] [Google Scholar]
- 2.Bartesaghi R, Guidi S, Ciani E. Is it possible to improve neurodevelopmental abnormalities in Down syndrome? Rev Neurosci. 2011;22:419–455. doi: 10.1515/RNS.2011.037. [DOI] [PubMed] [Google Scholar]
- 3.Dierssen M. Down syndrome: The brain in trisomic mode. Nat Rev Neurosci. 2012;13:844–858. doi: 10.1038/nrn3314. [DOI] [PubMed] [Google Scholar]
- 4.Rueda N, Flórez J, Martínez-Cué C. Mouse models of Down syndrome as a tool to unravel the causes of mental disabilities. Neural Plast. 2012;2012:584071. doi: 10.1155/2012/584071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chakrabarti L, Galdzicki Z, Haydar TF. Defects in embryonic neurogenesis and initial synapse formation in the forebrain of the Ts65Dn mouse model of Down syndrome. J Neurosci. 2007;27:11483–11495. doi: 10.1523/JNEUROSCI.3406-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Reeves RH, et al. A mouse model for Down syndrome exhibits learning and behaviour deficits. Nat Genet. 1995;11:177–184. doi: 10.1038/ng1095-177. [DOI] [PubMed] [Google Scholar]
- 7.Bianchi P, et al. Early pharmacotherapy restores neurogenesis and cognitive performance in the Ts65Dn mouse model for Down syndrome. J Neurosci. 2010;30:8769–8779. doi: 10.1523/JNEUROSCI.0534-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Contestabile A, et al. Lithium rescues synaptic plasticity and memory in Down syndrome mice. J Clin Invest. 2013;123:348–361. doi: 10.1172/JCI64650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Potier MC, Reeves RH. Editorial: Intellectual disabilities in Down syndrome from birth and throughout life: Assessment and treatment. Front Behav Neurosci. 2016;10:120. doi: 10.3389/fnbeh.2016.00120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Muraki M, et al. Manipulation of alternative splicing by a newly developed inhibitor of Clks. J Biol Chem. 2004;279:24246–24254. doi: 10.1074/jbc.M314298200. [DOI] [PubMed] [Google Scholar]
- 11.Liu F, et al. Overexpression of Dyrk1A contributes to neurofibrillary degeneration in Down syndrome. FASEB J. 2008;22:3224–3233. doi: 10.1096/fj.07-104539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Diehl JA, Zindy F, Sherr CJ. Inhibition of cyclin D1 phosphorylation on threonine-286 prevents its rapid degradation via the ubiquitin-proteasome pathway. Genes Dev. 1997;11:957–972. doi: 10.1101/gad.11.8.957. [DOI] [PubMed] [Google Scholar]
- 13.Hibaoui Y, et al. Modelling and rescuing neurodevelopmental defect of Down syndrome using induced pluripotent stem cells from monozygotic twins discordant for trisomy 21. EMBO Mol Med. 2014;6:259–277. doi: 10.1002/emmm.201302848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dehay C, Kennedy H. Cell-cycle control and cortical development. Nat Rev Neurosci. 2007;8:438–450. doi: 10.1038/nrn2097. [DOI] [PubMed] [Google Scholar]
- 15.Faizi M, et al. Comprehensive behavioral phenotyping of Ts65Dn mouse model of Down syndrome: Activation of β1-adrenergic receptor by xamoterol as a potential cognitive enhancer. Neurobiol Dis. 2011;43:397–413. doi: 10.1016/j.nbd.2011.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guedj F, et al. Green tea polyphenols rescue of brain defects induced by overexpression of DYRK1A. PLoS One. 2009;4:e4606. doi: 10.1371/journal.pone.0004606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mandel SA, et al. Cell signaling pathways and iron chelation in the neurorestorative activity of green tea polyphenols: Special reference to epigallocatechin gallate (EGCG) J Alzheimers Dis. 2008;15:211–222. doi: 10.3233/jad-2008-15207. [DOI] [PubMed] [Google Scholar]
- 18.Patra SK, Rizzi F, Silva A, Rugina DO, Bettuzzi S. Molecular targets of (-)-epigallocatechin-3-gallate (EGCG): Specificity and interaction with membrane lipid rafts. J Physiol Pharmacol. 2008;59(Suppl 9):217–235. [PubMed] [Google Scholar]
- 19.De la Torre R, et al. Epigallocatechin-3-gallate, a DYRK1A inhibitor, rescues cognitive deficits in Down syndrome mouse models and in humans. Mol Nutr Food Res. 2014;58:278–288. doi: 10.1002/mnfr.201300325. [DOI] [PubMed] [Google Scholar]
- 20.Stringer M, Abeysekera I, Dria KJ, Roper RJ, Goodlett CR. Low dose EGCG treatment beginning in adolescence does not improve cognitive impairment in a Down syndrome mouse model. Pharmacol Biochem Behav. 2015;138:70–79. doi: 10.1016/j.pbb.2015.09.002. [DOI] [PubMed] [Google Scholar]
- 21.Stagni F, et al. Short- and long-term effects of neonatal pharmacotherapy with epigallocatechin-3-gallate on hippocampal development in the Ts65Dn mouse model of Down syndrome. Neuroscience. 2016;333:277–301. doi: 10.1016/j.neuroscience.2016.07.031. [DOI] [PubMed] [Google Scholar]
- 22.Fernández-Martínez P, Zahonero C, Sánchez-Gómez P. DYRK1A: The double-edged kinase as a protagonist in cell growth and tumorigenesis. Mol Cell Oncol. 2015;2:e970048. doi: 10.4161/23723548.2014.970048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Laguna A, et al. The protein kinase DYRK1A regulates caspase-9-mediated apoptosis during retina development. Dev Cell. 2008;15:841–853. doi: 10.1016/j.devcel.2008.10.014. [DOI] [PubMed] [Google Scholar]
- 24.Canzonetta C, et al. DYRK1A-dosage imbalance perturbs NRSF/REST levels, deregulating pluripotency and embryonic stem cell fate in Down syndrome. Am J Hum Genet. 2008;83:388–400. doi: 10.1016/j.ajhg.2008.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tang H, et al. Zika virus infects human cortical neural progenitors and attenuates their growth. Cell Stem Cell. 2016;18:587–590. doi: 10.1016/j.stem.2016.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Altman J. Are new neurons formed in the brains of adult mammals? Science. 1962;135:1127–1128. doi: 10.1126/science.135.3509.1127. [DOI] [PubMed] [Google Scholar]
- 27.Cameron HA, Glover LR. Adult neurogenesis: Beyond learning and memory. Annu Rev Psychol. 2015;66:53–81. doi: 10.1146/annurev-psych-010814-015006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Danzer SC. Depression, stress, epilepsy and adult neurogenesis. Exp Neurol. 2012;233:22–32. doi: 10.1016/j.expneurol.2011.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kii I, et al. Selective inhibition of the kinase DYRK1A by targeting its folding process. Nat Commun. 2016;7:11391. doi: 10.1038/ncomms11391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sako Y, et al. Development of an orally available inhibitor of CLK1 for skipping a mutated dystrophin exon in Duchenne muscular dystrophy. Sci Rep. 2017;7:46126. doi: 10.1038/srep46126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yamamoto M, et al. CDK9 inhibitor FIT-039 prevents replication of multiple DNA viruses. J Clin Invest. 2014;124:3479–3488. doi: 10.1172/JCI73805. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.