Significance
Few patients with pancreatic cancer survive longer than 5 y, in part because most patients are identified only after their disease has progressed to an advanced stage. In this study, we show how combining mutations in circulating tumor DNA (ctDNA) with protein markers can result in a screening test with improved sensitivity while retaining specificity. The combination of the ctDNA and protein markers was superior to any single marker. Moreover, the combination detected nearly two-thirds of pancreatic cancers that had no evidence of distant metastasis at the time of surgical resection. The strategy may represent an approach to detect cancers of many types at an earlier stage.
Keywords: early cancer detection, liquid biopsy, circulating tumor DNA, protein biomarkers, pancreatic cancer
Abstract
The earlier diagnosis of cancer is one of the keys to reducing cancer deaths in the future. Here we describe our efforts to develop a noninvasive blood test for the detection of pancreatic ductal adenocarcinoma. We combined blood tests for KRAS gene mutations with carefully thresholded protein biomarkers to determine whether the combination of these markers was superior to any single marker. The cohort tested included 221 patients with resectable pancreatic ductal adenocarcinomas and 182 control patients without known cancer. KRAS mutations were detected in the plasma of 66 patients (30%), and every mutation found in the plasma was identical to that subsequently found in the patient’s primary tumor (100% concordance). The use of KRAS in conjunction with four thresholded protein biomarkers increased the sensitivity to 64%. Only one of the 182 plasma samples from the control cohort was positive for any of the DNA or protein biomarkers (99.5% specificity). This combinatorial approach may prove useful for the earlier detection of many cancer types.
Pancreatic ductal adenocarcinoma (PDAC, hereafter “pancreatic cancer”) is the third leading cause of cancer death and is predicted to become the second most common cause in the United States by 2030 (1). Pancreatic cancer is notoriously lethal, with fewer than 9% of patients surviving 5 y after diagnosis (2). The poor prognosis of patients with pancreatic cancer is in part due to the fact that 80–85% of patients are diagnosed at advanced stages, when either tumor invasion into the surrounding major vessels or distant metastases are evident upon radiologic studies (3). At this late point in the disease, pancreatic cancer is not amenable to surgical resection, and the 3-y survival rate is <5%. In contrast, a 5-y survival of almost 60% is reported for very small, localized tumors; among resectable cancers, the smaller the tumor, the better the prognosis (4–8).
Pancreatic cancer is not different from other cancers with respect to its strong correlation between tumor stage and prognosis (4). Very few patients with cancers of the lung, colon, esophagus, or stomach who have distant metastasis at the time of diagnosis survive for more than 5 y (9). The size of cancers is also important in a general sense, in that smaller tumors have less often metastasized than larger tumors at the time of diagnosis and are therefore more likely to be curable by surgery alone. Even when cancers have metastasized to distant sites, a smaller burden of disease is much more easily managed than bulky lesions (10). Thus, adjuvant chemotherapeutic agents administered to patients with micrometastases stemming from a colorectal cancer can be curative in nearly 50% of cases (11–13). The same chemotherapeutic agents delivered to patients with metastatic lesions that are radiologically visible produce virtually no cures (14).
It is therefore evident that the earlier detection of cancers is one key to reducing deaths from these diseases, including pancreatic cancer. In addition to offering the possibility of surgical resection, newly developed adjuvant chemotherapeutic and emerging immunotherapy regimens will undoubtedly prove more efficacious in patients with minimal disease beyond that which is curable surgically (15). Biomarkers in the circulation provide one of the best ways, in principle, to detect cancers at an earlier stage. Historically, the type of biomarkers used to monitor cancers were proteins (16) and included carcinoembryonic antigen (CEA), carbohydrate antigen 19-9 (CA19-9), and cancer antigen 125 (CA125). These biomarkers have proven useful for following patients with known disease, but none has been approved for screening purposes, in part because of their low sensitivity or specificity (17–19). More recently, mutant DNA has been explored as a biomarker. The concept underlying this approach, often called “liquid biopsies,” is that cancer cells, like normal self-renewing cells, turn over frequently. DNA released from the dying cells can escape into bodily fluids such as urine, stool, and plasma (20–30). An advantage of using mutant DNA in the circulation as a biomarker is its exquisite specificity. Every cell within a cancer has a core set of somatic mutations in driver genes that are responsible for their clonal growth (31). In contrast, normal cells do not clonally expand during adulthood, and the fraction of normal cells that have any specific somatic mutation is extremely low.
Most studies of circulating tumor DNA (ctDNA) have focused on following patients with cancer rather than on evaluating its use in screening settings. Available data indicate that ctDNA is elevated in >85% of patients with advanced forms of many cancer types (22, 24). However, it has been shown that a considerably smaller fraction of patients with earlier stages of cancer have detectable levels of ctDNA in their plasma (22, 24). In the current study, we determined whether ctDNA and protein biomarkers can be combined to increase the sensitivity of detection of resectable pancreatic cancer under conditions that preserve high specificity.
Results
Characteristics of Patients with PDAC and Presumed Healthy Controls.
Two hundred and twenty-one patients with surgically resectable pancreatic cancer were evaluated in this study. The histopathological and clinical characteristics of these patients are summarized in Table S1. A total of 182 individuals of similar age with no known history of cancer, autoimmune disease, or chronic kidney disease acted as the healthy control cohort.
Table S1.
General demographics and histopathology of patients with pancreatic cancer
| Pancreatic cancer, n = 221 | |
| Age, y, mean ± SD | 66.9 ± 10.5 |
| Gender, n (%) | |
| Male | 121 (54.8) |
| Female | 100 (45.2) |
| Race, n (%) | |
| White | 188 (85.1) |
| African-American | 4 (1.8) |
| Hispanic | 1 (0.5) |
| Asian | 12 (5.4) |
| Other or unknown | 16 (7.2) |
| Classic symptoms of PDAC, n (%) | |
| Absent | 45 (20.4) |
| Present | 176 (79.6) |
| Abdominal pain, n (%) | |
| Absent | 127 (57.5) |
| Present | 94 (42.5) |
| Jaundice, n (%) | |
| Absent | 110 (49.8) |
| Present | 111 (50.2) |
| Tumor size, cm, median (IQR) | 3.0 (2.4–3.8) |
| Tumor size, n (%) | |
| ≤1.00 cm | 2 (0.9) |
| 1.01–1.50 cm | 22 (10.0) |
| 1.51–2.00 cm | 12 (5.4) |
| 2.01–2.50 cm | 47 (21.3) |
| 2.51–3.00 cm | 38 (17.2) |
| 3.01–3.50 cm | 36 (16.3) |
| 3.51–4.00 cm | 22 (10.0) |
| >4.00 cm | 42 (19.0) |
| AJCC T stage, n (%) | |
| T1 | 22 (10.0) |
| T2 | 46 (20.8) |
| T3 | 153 (69.2) |
| AJCC N stage, n (%) | |
| N0 | 51 (23.1) |
| N1 | 170 (76.9) |
| AJCC M stage, n (%) | |
| M0 | 221 (100.0) |
| M1 | — |
| AJCC stage, n (%) | |
| IA | 12 (5.4) |
| IB | 17 (7.7) |
| IIA | 22 (10.0) |
| IIB, | 170 (76.9) |
| Grade of tumor differentiation, n (%) | |
| Well/moderately differentiated | 141 (63.8) |
| Poorly differentiated | 80 (36.2) |
| Perineural invasion, n (%) | |
| Absent | 26 (11.8) |
| Present | 195 (88.2) |
| Lymphovascular invasion, n (%) | |
| Absent | 76 (34.4) |
| Present | 145 (65.6) |
| Number of harvested nodes, median (IQR) | 19 (15–27) |
| Number of harvested nodes, n (%) | |
| ≤20 nodes | 111 (50.2) |
| >20 nodes | 110 (49.8) |
| Number of positive nodes, median (IQR) | 2 (1–5) |
| Positive nodes, n (%) | |
| Absent | 51 (23.1) |
| Present | 170 (76.9) |
IQR, interquartile range.
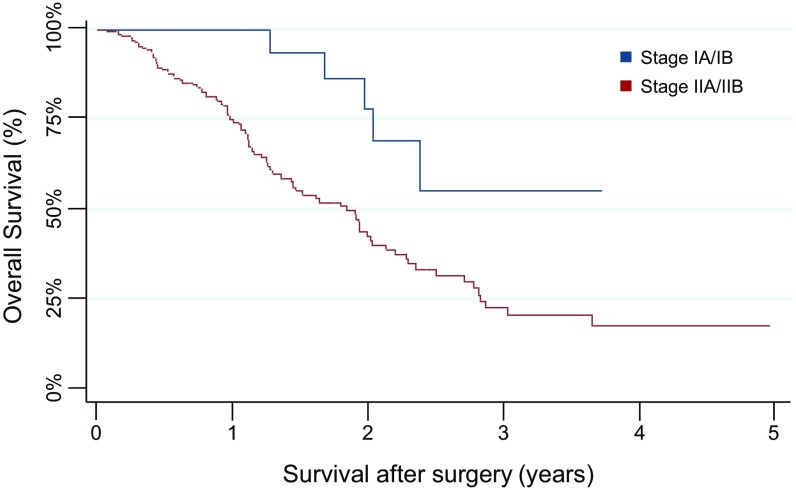
Twenty percent of the patients had no symptoms typically associated with pancreatic cancer. The size of the primary tumors at presentation ranged from 0.6 cm to 13 cm, with a median size of 3.0 cm. The most common stage at presentation was American Joint Commission on Cancer (AJCC) stage IIB, accounting for 77% of patients, with the remaining patients harboring stage IA (5%), stage IB (8%), or stage IIA (10%) disease (Table S1). Patient survival correlated with stage, as graphically depicted in Fig. S1 and as expected from prior clinical studies (32).
Fig. S1.
Kaplan–Meier survival plot of the 221 PDAC patients included in this study stratified by AJCC stage (stage IA or IB: blue curve; stage IIA or IIB: red curve).
A PCR-Based Assay to Identify Tumor-Specific KRAS Mutations in Plasma Samples.
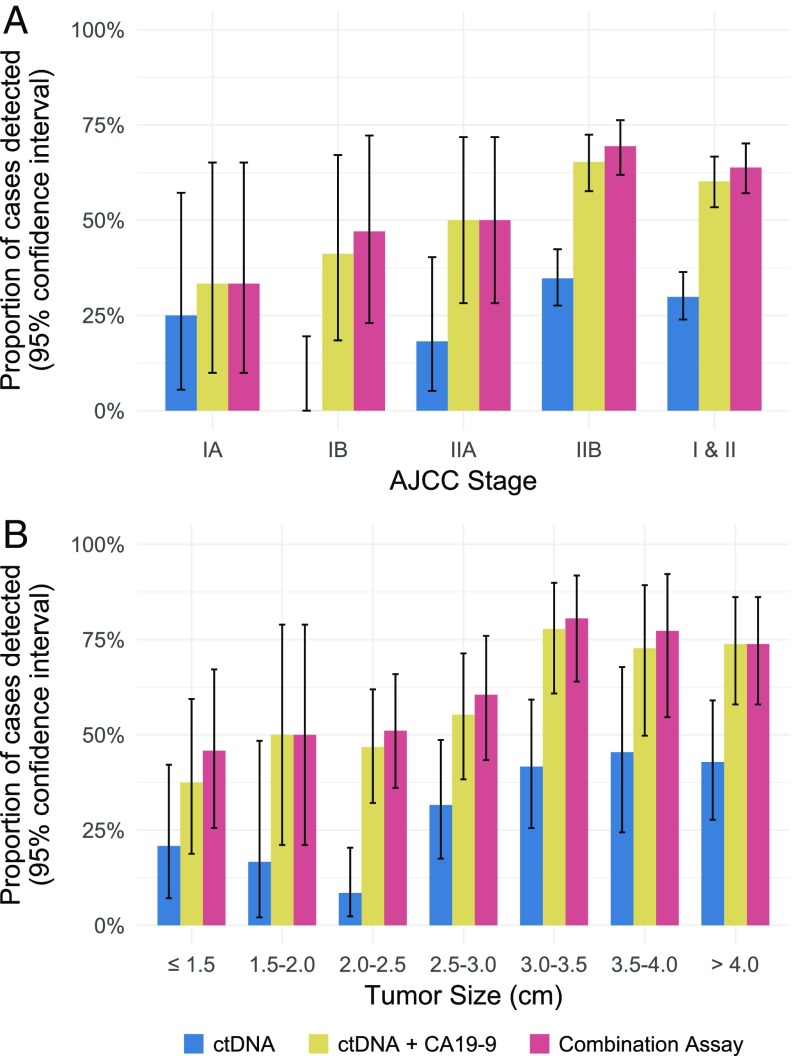
We designed a PCR-based assay that could simultaneously assess the two codons (codons 12 and 61) of the KRAS gene that are most frequently mutated in PDAC as well as surrounding codons. The assay used a sensitive technology called the “Safe-Sequencing System” (Safe-SeqS) (33). Safe-SeqS incorporates molecular barcodes that uniquely label each template molecule, thereby drastically minimizing the errors that routinely occur in massively parallel sequencing. This approach can identify one mutant template among as many as 10,000 normal templates. Using this technology, we identified KRAS mutations in the plasma of 66 of the 221 (30%: 95% CI 24–36%) pancreatic cancer cases (Table 1, Table S2, and Dataset S1). Sixty-two (94%) and four (6%) of the mutations were at codons 12 and 61, respectively, with G > T transversions most commonly observed (Dataset S1). Mutations were found more frequently in stage II patients than in stage I patients (Fig. 1A, Table 1, Table S2, and Dataset S1). Additionally, while the mutant allele frequency did not correlate with tumor size (Fig. S2A and Dataset S1), mutations were found more frequently in larger tumors than in smaller tumors (Fig. 1B, Table 2, Table S2, and Dataset S1).
Table 1.
Proportion of samples stratified by AJCC stage detected with each individual assay and all combinations thereof
| % samples detected (95% confidence interval) | |||||
| Assay type | Stage IA, 12 cases | Stage IB, 17 cases | Stage IIA, 22 cases | Stage IIB, 170 cases | Stage I and II, 221 cases |
| ctDNA | 25 (5–57) | 0 (0–20) | 18 (5–40) | 35 (28–42) | 30 (24–36) |
| CA19-9 | 17 (2–48) | 41 (18–67) | 36 (17–59) | 54 (46–62) | 49 (43–56) |
| CEA + HGF + OPN | 25 (5–57) | 6 (0–29) | 14 (3–35) | 19 (14–26) | 18 (13–24) |
| ctDNA + CA19-9 | 33 (10–65) | 41 (18–67) | 50 (28–72) | 65 (58–72) | 60 (53–67) |
| ctDNA + CEA + HGF + OPN | 33 (10–65) | 6 (0–29) | 32 (14–55) | 47 (39–55) | 42 (35–48) |
| CA19-9 + CEA + HGF + OPN | 25 (5–57) | 47 (23–72) | 36 (17–59) | 59 (52–67) | 54 (47–61) |
| Combination assay | 33 (10–65) | 47 (23–72) | 50 (28–72) | 69 (62–76) | 64 (57–70) |
Table S2.
General demographics and histopathology of patients with pancreatic cancer by KRAS status
| Pancreatic cancer with detectable KRAS, n = 66 | Pancreatic cancer with undetectable KRAS, n = 155 | P value | |
| Age, y, mean ± SD | 66.2 ± 10.8 | 67.3 ± 10.3 | 0.473 |
| Gender, n (%) | 0.070 | ||
| Male | 30 (24.8) | 91 (75.2) | |
| Female | 36 (36.0) | 64 (64.0) | |
| Race, n (%) | 0.889 | ||
| White | 55 (29.3) | 133 (70.7) | |
| African-American | 1 (25.0) | 3 (75.0) | |
| Hispanic | — | 1 (100.0) | |
| Asian | 5 (41.7) | 7 (58.3) | |
| Other or unknown | 2 (40.0) | 3 (60.0) | |
| Classic symptoms of PDAC, n (%) | 0.194 | ||
| Absent | 49 (27.8) | 127 (72.2) | |
| Present | 17 (37.8) | 28 (62.2) | |
| Abdominal pain, n (%) | 0.361 | ||
| Absent | 41 (32.3) | 86 (67.7) | |
| Present | 25 (26.6) | 69 (73.4) | |
| Jaundice, n (%) | 0.528 | ||
| Absent | 35 (31.8) | 75 (68.2) | |
| Present | 31 (27.9) | 80 (72.1) | |
| Tumor size, cm, median (IQR) | 3.5 (3.0–4.5) | 2.7 (2.2–3.5) | 0.002 |
| Tumor size, n (%) | 0.004 | ||
| ≤ 1.00 cm | — | 2 (100.0) | |
| 1.01–1.50 cm | 5 (22.7) | 17 (77.3) | |
| 1.51–2.00 cm | 2 (16.7) | 10 (83.3) | |
| 2.01–2.50 cm | 4 (8.5) | 43 (91.5) | |
| 2.51–3.00 cm | 12 (31.6) | 26 (68.4) | |
| 3.01–3.50 cm | 15 (41.7) | 21 (58.3) | |
| 3.51–4.00 cm | 10 (45.5) | 12 (54.6) | |
| >4.01 cm | 18 (42.9) | 24 (57.1) | |
| AJCC T stage, n (%) | 0.003 | ||
| T1 | 5 (22.7) | 17 (77.3) | |
| T2 | 5 (10.9) | 41 (89.1) | |
| T3 | 56 (36.6) | 97 (63.4) | |
| AJCC N stage, n (%) | 0.004 | ||
| N0 | 7 (13.7) | 44 (86.3) | |
| N1 | 59 (34.7) | 111 (65.3) | |
| AJCC M stage, n (%) | |||
| M0 | 66 (29.9) | 155 (70.2) | — |
| M1 | — | ||
| AJCC stage, n (%) | 0.013 | ||
| IA | 3 (25.0) | 9 (75.0) | |
| IB | — | 17 (100.0) | |
| IIA | 4 (18.2) | 18 (81.8) | |
| IIB | 59 (34.7) | 111 (65.3) | |
| Grade of tumor differentiation, n (%) | 0.118 | ||
| Well/moderately differentiated | 37 (26.2) | 104 (79.8) | |
| Poorly differentiated | 29 (36.3) | 51 (63.7) | |
| Perineural invasion, n (%) | 0.086 | ||
| Absent | 4 (15.4) | 22 (84.6) | |
| Present | 62 (31.8) | 133 (68.2) | |
| Lymphovascular invasion, n (%) | 0.078 | ||
| Absent | 17 (22.4) | 59 (77.6) | |
| Present | 49 (33.8) | 96 (66.2) | |
| Number of harvested nodes, median (IQR) | 20 (17–28) | 19 (14–27) | 0.047 |
| Number of harvested nodes, n (%) | 0.355 | ||
| ≤20 nodes | 30 (27.0) | 81 (73.0) | |
| >20 nodes | 36 (32.7) | 74 (67.3) | |
| Number of positive nodes, median (IQR) | 4.0 (2.0–7.0) | 1 (0.0–4.0) | <0.001 |
| Positive nodes, n (%) | 0.004 | ||
| Absent | 7 (13.7) | 44 (86.3) | |
| Present | 59 (34.7) | 111 (65.3) |
Fig. 1.
Combining ctDNA KRAS mutations with protein biomarkers increases sensitivity for early detection of PDAC. (A) Sensitivities of ctDNA KRAS mutations alone, ctDNA KRAS mutations plus CA19-9, and ctDNA KRAS mutations with CA19-9 and other proteins (combination assay) with respect to AJCC stage. (B) Sensitivities of ctDNA KRAS mutations alone, ctDNA KRAS mutations plus CA19-9, and ctDNA KRAS mutations with CA19-9 and other proteins (combination assay) with respect to tumor size. Error bars represent 95% CIs.
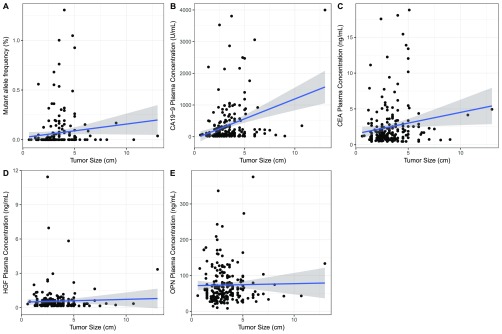
Fig. S2.
Correlation between triplex assay markers and tumor size. (A) KRAS mutations were found more frequently in larger tumors than smaller tumors, but the MAF did not correlate with tumor size (Pearson’s r = 0.039). (B) In patients with elevated CA19-9, the CA19-9 plasma concentration weakly correlates with tumor size (Pearson’s r = 0.287). (C–E) Plasma levels of CEA (C), HGF (D), and OPN (E) were less dependent on tumor size than KRAS mutations or CA19-9 (CEA: Pearson’s r = 0.153; HGF: Pearson’s r = 0.037; OPN: Pearson’s r = 0.018). Shaded regions represent 95% CIs.
Table 2.
Proportion of samples stratified by tumor size detected with each individual assay and all combinations thereof
| Assay type | % samples detected (95% confidence interval) | ||||||
| ≤ 1.5 cm, 24 cases | 1.5–2.0 cm, 12 cases | 2.0–2.5 cm, 47 cases | 2.5–3.0 cm, 38 cases | 3.0–3.5 cm, 36 cases | 3.5–4.0 cm, 22 cases | >4.0 cm, 42 cases | |
| ctDNA | 21 (7–42) | 17 (2–48) | 9 (2–20) | 32 (18–49) | 42 (26–59) | 45 (24–68) | 43 (28–59) |
| CA19-9 | 25 (10–47) | 33 (10–65) | 43 (28–58) | 45 (29–62) | 58 (41–74) | 59 (36–79) | 67 (50–80) |
| CEA + HGF + OPN | 25 (10–47) | 8 (0–38) | 17 (8–31) | 21 (10–37) | 8 (2–22) | 18 (5–40) | 24 (12–39) |
| ctDNA + CA19-9 | 38 (19–59) | 50 (21–79) | 47 (32–62) | 55 (38–71) | 78 (61–90) | 73 (50–89) | 74 (58–86) |
| ctDNA + CEA + HGF + OPN | 38 (19–59) | 25 (5–57) | 26 (14–40) | 47 (31–64) | 47 (30–65) | 55 (32–76) | 50 (34–66) |
| CA19-9 + CEA + HGF + OPN | 38 (19–59) | 33 (10–65) | 47 (32–62) | 53 (36–69) | 64 (46–79) | 64 (41–83) | 67 (50–80) |
| Combination assay | 46 (26–67) | 50 (21–79) | 51 (36–66) | 61 (43–76) | 81 (64–92) | 77 (55–92) | 74 (58–86) |
The number of mutant templates in the plasma could be calculated from the mutant allele fraction and the concentration of DNA in each plasma sample (Dataset S1). This number was often very low, with 25 (38%) of the patients with detectable KRAS mutations having fewer than two mutant templates per milliliter of plasma. The average number of mutant templates per milliliter of plasma was 5.3 (Dataset S1). These results emphasize that extremely sensitive techniques are required to effectively detect the mutations in early-stage pancreatic cancer patients. KRAS mutations were observed in only one of the 182 individuals in the presumed healthy cohort, a 69-y-old male with no known cancer.
The basis for the liquid biopsy concept is that the mutant DNA templates identified in the circulation are derived from cancers. It was therefore important to determine whether the KRAS mutations identified in these patients’ plasma samples were also present in their primary carcinomas. We were able to obtain primary carcinomas from 50 of the 66 patients with detectable KRAS mutations in their plasma. In all 50 cases, the mutation found in the plasma was identical to that found in the primary carcinoma, providing another, orthogonal measure of specificity.
Simultaneous Assessment of CA19-9 and KRAS Mutations in Plasma.
We sought to determine whether a combination of the KRAS ctDNA test with CA19-9, the best-known PDAC biomarker (17, 34), would result in improved sensitivity compared with the KRAS ctDNA test alone. Recent studies have shown that CA19-9 can be elevated in patients with pancreatic cancer 2 y before diagnosis (35). However, CA19-9 elevations have also been observed in nonmalignant conditions, and 5% of the population cannot produce the CA19-9 antigen due to germline genetic variation, limiting its use for screening purposes (17). However, we reasoned that CA19-9 might prove useful as a screening biomarker if the threshold for scoring a result as positive was sufficiently high. We chose a threshold of 100 U/mL based on prior data that this level is not found among healthy individuals who do not have a clinical history of pancreaticobiliary disease (36).
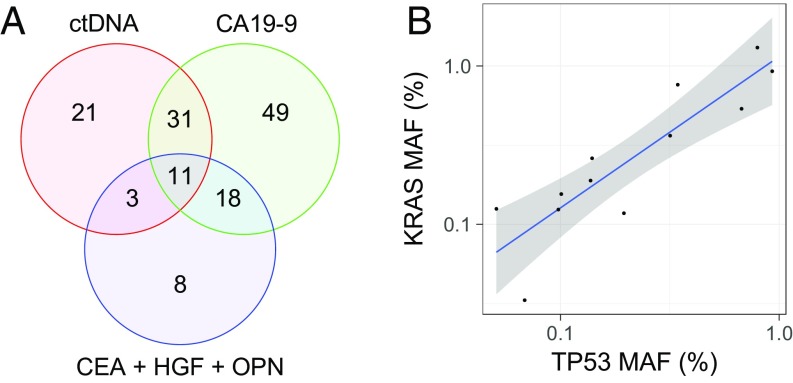
Using this predefined high threshold, CA19-9 was detected in 109 of the 221 (49%: 95% CI 43–56%) patients with pancreatic cancer and in none of the 182 healthy controls, confirming its specificity when used in this way (Table 1, Table S3, and Datasets S1 and S2). As expected, the number of patients with detectable CA19-9 levels increased with stage and tumor size (Fig. 1, Tables 1 and 2, Table S3, and Dataset S1). The most important question addressed in the current study was whether these two biomarkers—KRAS mutations and a positive CA19-9 score—were independent indicators of the presence of disease. We found that the overlap was only partial, as indicated in the Venn diagram in Fig. 2A. Although 42 patients (19%) had elevated CA19-9 levels as well as detectable KRAS mutations in their plasma, 91 additional patients had either mutations in KRAS or elevated CA19-9 but not both (Fig. 2A). Thus, the combined sensitivity of these analyses was 60% (95% CI 53–67%), higher than the sensitivity of either alone (Fig. 1 and Table 1). Importantly, the two assays could be combined without substantially increasing the false-positive rate because each was extremely specific at the thresholds used.
Table S3.
General demographics and histopathology of patients with pancreatic cancer by CA19-9 status
| Pancreatic cancer with detectable CA19-9, n = 109 | Pancreatic cancer with undetectable CA19-9, n = 112 | P value | |
| Age, y, mean ± SD | 65.9 ± 10.24 | 67.9 ± 10.6 | 0.136 |
| Gender, n (%) | 0.721 | ||
| Male | 61 (50.4) | 60 (49.6) | |
| Female | 48 (48.0) | 52 (52.0) | |
| Race, n (%) | 0.598 | ||
| White | 95 (50.5) | 93 (49.5) | |
| African-American | 2 (50.0) | 2 (50.0) | |
| Hispanic | 1 (100.0) | — | |
| Asian | 7 (58.3) | 5 (41.7) | |
| Other or unknown | 4 (25.0) | 12 (75.0) | |
| Classic symptoms of PDAC, n (%) | 0.690 | ||
| Absent | 21 (46.7) | 24 (53.3) | |
| Present | 88 (50.0) | 88 (50.0) | |
| Abdominal pain, n (%) | 0.207 | ||
| Absent | 58 (45.7) | 69 (54.3) | |
| Present | 51 (54.3) | 43 (45.7) | |
| Jaundice, n (%) | 0.841 | ||
| Absent | 55 (50.0) | 55 (50.0) | |
| Present | 54 (48.7) | 57 (51.3) | |
| Tumor size, cm, median (IQR) | 3.4 (2.5–4.5) | 2.8 (2.1–3.5) | <0.001 |
| Tumor size, n (%) | 0.028 | ||
| ≤ 1.00 cm | — | 2 (100.0) | |
| 1.01–1.50 cm | 6 (27.3) | 16 (72.7) | |
| 1.51–2.00 cm | 4 (33.3) | 8 (66.7) | |
| 2.01–2.50 cm | 20 (42.5) | 27 (57.5) | |
| 2.51–3.00 cm | 17 (44.7) | 21 (55.3) | |
| 3.01–3.50 cm | 21 (58.3) | 15 (41.7) | |
| 3.51–4.00 cm | 13 (59.1) | 9 (40.9) | |
| >4.01 cm | 28 (66.7) | 14 (33.3) | |
| AJCC T stage, n (%) | 0.001 | ||
| T1 | 4 (18.2) | 18 (81.8) | |
| T2 | 18 (39.1) | 28 (60.9) | |
| T3 | 87 (56.9) | 66 (43.1) | |
| AJCC N stage, n (%) | 0.009 | ||
| N0 | 17 (33.3) | 34 (66.7) | |
| N1 | 92 (54.1) | 78 (45.9) | |
| AJCC M stage, n (%) | — | ||
| M0 | 109 (49.3) | 112 (50.7) | |
| M1 | — | — | |
| AJCC stage, n (%) | 0.035 | ||
| IA | 2 (16.7) | 10 (83.3) | |
| IB | 7 (41.2) | 10 (58.8) | |
| IIA | 8 (36.4) | 14 (63.6) | |
| IIB | 92 (54.1) | 78 (45.9) | |
| Grade of tumor differentiation, n (%) | 0.666 | ||
| Well/moderately differentiated | 68 (48.2) | 73 (51.8) | |
| Poorly differentiated | 41 (51.3) | 39 (48.7) | |
| Perineural invasion, n (%) | 0.044 | ||
| Absent | 8 (30.8) | 18 (69.3) | |
| Present | 101 (51.8) | 94 (48.2) | |
| Lymphovascular invasion, n (%) | <0.001 | ||
| Absent | 25 (32.9) | 51 (67.1) | |
| Present | 84 (57.9) | 61 (42.1) | |
| Number of harvested nodes, median (IQR) | 19 (16–26) | 20 (14–29) | 0.973 |
| Number of harvested nodes, n (%) | 0.544 | ||
| ≤20 nodes | 57 (51.3) | 54 (48.7) | |
| >20 nodes | 52 (47.3) | 58 (52.7) | |
| Number of positive nodes, median (IQR) | 3 (1–6) | 2 (0–4) | 0.038 |
| Positive nodes, n (%) | 0.009 | ||
| Absent | 17 (33.3) | 34 (66.7) | |
| Present | 92 (54.1) | 78 (45.9) |
Fig. 2.
(A) Combining ctDNA and protein markers increases sensitivity because a large proportion of patients are detected by only one marker. The Venn diagram shows the number of patients detected by ctDNA KRAS mutations (red circle), CA19-9 (green circle), the three other protein biomarkers (blue circle), and by combinations thereof (overlapping regions). Eighty patients (36% of the total) were not detectable by any of the three makers. (B) MAF of KRAS and TP53 mutations are strongly correlated (Pearson’s r = 0.885) in the plasma of the 12 patients whose plasma contained detectable amounts of both mutations, providing validation of the reliability of the ctDNA assay and its quantitative nature. The shaded region represents the 95% CI.
Further Increasing Sensitivity by Inclusion of Other Protein Biomarkers.
Encouraged by the results described above, we sought to further increase sensitivity by combining ctDNA KRAS mutations and CA19-9 with other protein biomarkers. In a pilot study on a small number of pancreatic cancer samples independent from those studied here, we evaluated the potential utility of other proteins that had been found to be elevated in cancer, including alpha-fetoprotein (AFP), CA15-3, leptin, IL-6, CEA, CA-125, IL-8, sFas, prolactin, osteopontin (OPN), basic FGF2, hepatocyte growth factor (HGF), cytokeratin-19 fragment (CYFRA 21-1), human epididymis protein 4 (HE4), TGF-α, growth/differentiation factor 15 (GDF15), dickkopf-related protein 1 (DKK1), neuron-specific enolase (NSE), osteoprotegerin (OPG), TIMP metallopeptidase inhibitor 1 (TIMP-1), TIMP metallopeptidase inhibitor 2 (TIMP-2), mesothelin, midkine, kallikrein-6, CD44, AXL receptor tyrosine kinase, soluble human epidermal growth factor receptor 2 (sHER2), soluble epidermal growth factor receptor (sEGFR), soluble urokinase-type plasminogen activator receptor (suPAR), and soluble platelet endothelial cell adhesion molecule 1 (sPECAM-1) (37). Of these 29 protein biomarkers, five—CEA (38), HGF (39), midkine (40), OPN (41), and prolactin (42)—appeared to be most promising.
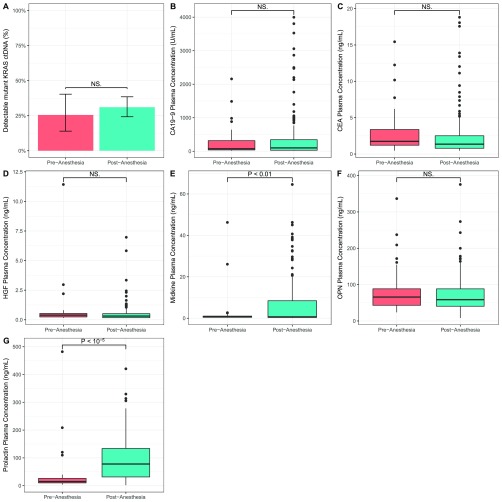
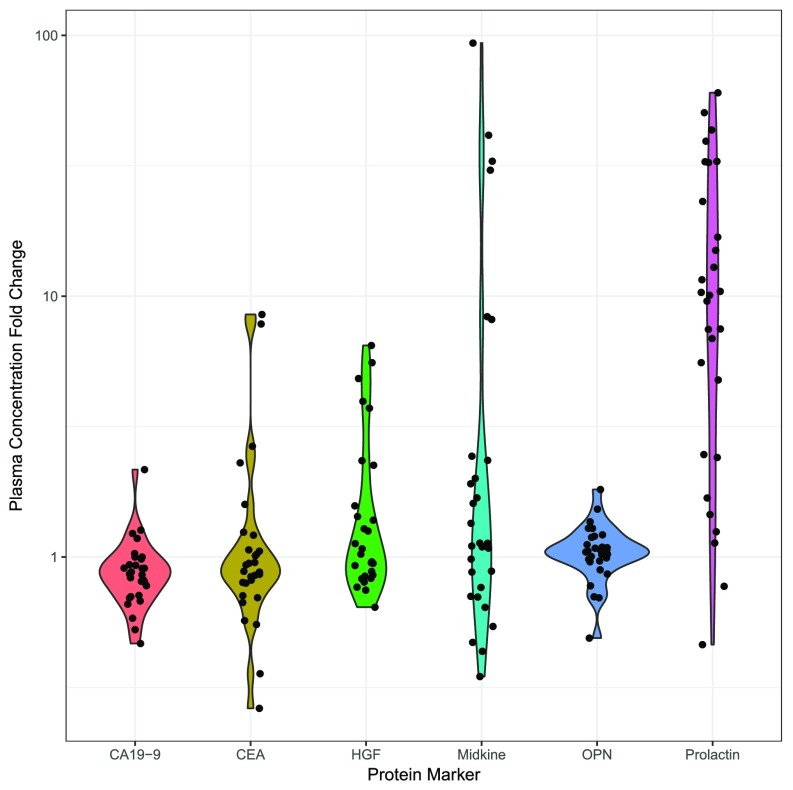
When the levels of these five markers were evaluated in plasmas from our 221-patient pancreatic cancer cohort, we observed an association between the plasma concentrations of prolactin and midkine and sample collection site (P < 0.01, χ2 test, df = 5), suggesting that blood collection conditions might have elevated the levels of these two markers. There was no significant correlation between CA19-9, CEA, HGF, or OPN levels and collection sites, nor was there any correlation between the presence of KRAS ctDNA mutations and collection site. Upon further investigation, we noted that the levels of prolactin and midkine were significantly elevated in samples that were collected after the administration of anesthesia but before surgical excision (Fig. S3). The results on prolactin were consistent with previous studies showing that anesthetics elevate the levels of this protein (43). To ensure that anesthesia did not affect the levels of the other protein biomarkers described above, we collected paired plasma samples before and immediately after the administration of anesthesia in 29 new patients. The only proteins found to be elevated by anesthesia were prolactin and midkine (P < 0.05, Wilcoxon signed-rank test) (Fig. S4), in perfect accord with the correlation between collection site and protein levels noted above. We therefore excluded prolactin and midkine from further analysis.
Fig. S3.
Levels of prolactin (G) and midkine (E) were significantly elevated in samples that were collected after the administration of anesthesia but before surgical excision. In contrast, no difference in the proportion of samples with mutant KRAS ctDNA (A), CA19-9 plasma concentration (B), CEA plasma concentration (C), HGF plasma concentration (D), and OPN plasma concentration (F) was observed between samples collected before or after the administration of anesthesia. P > 0.05 (exact permutation t test), NS., not significant.
Fig. S4.
Fold change in protein biomarker levels from 29 pairs plasma samples collected before and immediately after the administration of anesthesia. Of the six markers evaluated, only prolactin and midkine were found to be elevated by anesthesia, in perfect agreement with the correlation between collection site and protein levels.
Unlike CA19-9, no predefined threshold exists for the use of CEA, HGF, or OPN as markers for pancreatic cancer. As a result, we determined appropriate thresholds in an independent set of 273 plasma samples from healthy controls. To be conservative, we chose thresholds for each protein that were 10% higher than the maximum values observed in any of the 273 normal plasma samples. Notably, when these thresholds were applied to the independent test set of 182 plasma samples, all three protein markers maintained 100% specificity (Dataset S2). The sensitivity of each of these three markers was less than that obtained with KRAS mutations or CA19-9 when each marker was used alone, but their levels were less dependent on stage and size than KRAS mutations or CA19-9 (Table 1, Fig. S2, Tables S4–S6, and Dataset S1). In combination with KRAS mutations and CA19-9 assays, this five-member biomarker panel (“combination assay”) detected 141 (64%: 95% CI 57–70%) of the 221 resectable cancers (Figs. 1A and 2A, Table 1, Fig. S5, Table S7, and Dataset S1).
Table S4.
General demographics and histopathology of patients with pancreatic cancer by HGF status
| Pancreatic cancer with detectable HGF, n = 23 | Pancreatic cancer with undetectable HGF, n = 198 | P value | |
| Age, y, mean ± SD | 68.1 ± 9.4 | 66.8 ± 10.6 | 0.579 |
| Gender, n (%) | 0.251 | ||
| Male | 10 (8.3) | 111 (91.7) | |
| Female | 13 (13.0) | 87 (87.0) | |
| Race, n (%) | 0.011 | ||
| White | 16 (8.5) | 172 (91.5) | |
| African-American | — | 4 (100.0) | |
| Hispanic | 1 (100.0) | — | |
| Asian | 3 (25.0) | 9 (75.0) | |
| Other or unknown | 3 (18.7) | 13 (81.3) | |
| Classic symptoms of PDAC, n (%) | 0.044 | ||
| Absent | 1 (2.2) | 44 (97.8) | |
| Present | 22 (12.5) | 154 (87.5) | |
| Abdominal pain, n (%) | 0.588 | ||
| Absent | 12 (9.5) | 115 (90.5) | |
| Present | 11 (11.7) | 83 (88.3) | |
| Jaundice, n (%) | 0.524 | ||
| Absent | 10 (9.1) | 100 (90.9) | |
| Present | 13 (11.7) | 98 (88.3) | |
| Tumor size, cm, median (IQR) | 2.7 (2.0–4.0) | 3.0 (2.4–3.8) | 0.619 |
| Tumor size, n (%) | 0.648 | ||
| ≤ 1.00 cm | — | 2 (100.0) | |
| 1.01–1.50 cm | 5 (22.7) | 17 (77.3) | |
| 1.51–2.00 cm | 1 (8.3) | 11 (91.7) | |
| 2.01–2.50 cm | 4 (8.5) | 43 (91.5) | |
| 2.51–3.00 cm | 4 (10.5) | 34 (89.5) | |
| 3.01–3.50 cm | 2 (5.6) | 34 (94.4) | |
| 3.51–4.00 cm | 2 (9.1) | 20 (90.9) | |
| >4.01 cm | 5 (11.9) | 37 (88.1) | |
| AJCC T stage, n (%) | 0.822 | ||
| T1 | 3 (13.6) | 19 (86.4) | |
| T2 | 4 (8.7) | 42 (91.3) | |
| T3 | 16 (10.5) | 137 (89.5) | |
| AJCC N stage, n (%) | 0.717 | ||
| N0 | 6 (11.8) | 45 (88.2) | |
| N1 | 17 (10.0) | 153 (90.0) | |
| AJCC M stage, n (%) | — | ||
| M0 | 23 (10.4) | 198 (89.6) | |
| M1 | — | ||
| AJCC stage, n (%) | 0.364 | ||
| IA | 3 (25.0) | 9 (75.0) | |
| IB | 1 (5.9) | 16 (94.1) | |
| IIA | 2 (9.1) | 20 (90.9) | |
| IIB | 17 (10.0) | 153 (90.0) | |
| Grade of tumor differentiation, n (%) | 0.757 | ||
| Well/moderately differentiated | 14 (9.9) | 127 (90.1) | |
| Poorly differentiated | 9 (11.3) | 71 (88.7) | |
| Perineural invasion, n (%) | 0.841 | ||
| Absent | 3 (11.5) | 23 (88.5) | |
| Present | 20 (10.3) | 175 (89.7) | |
| Lymphovascular invasion, n (%) | 0.967 | ||
| Absent | 8 (10.5) | 68 (89.5) | |
| Present | 15 (10.3) | 130 (89.7) | |
| Number of harvested nodes, median (IQR) | 20 (17–28) | 19 (15–27) | 0.173 |
| Number of harvested nodes, n (%) | 0.261 | ||
| ≤20 nodes | 9 (8.1) | 102 (91.9) | |
| >20 nodes | 14 (12.7) | 96 (87.3) | |
| Number of positive nodes, median (IQR) | 2 (0–5) | 2 (1–5) | 0.965 |
| Positive nodes, n (%) | 0.717 | ||
| Absent | 6 (11.8) | 45 (88.2) | |
| Present | 17 (10.0) | 153 (90.0) |
Table S6.
General demographics and histopathology of patients with pancreatic cancer by OPN status
| Pancreatic cancer with detectable OPN, n = 15 | Pancreatic cancer with undetectable OPN, n = 206 | P value | |
| Age, y, mean ± SD | 70.3 ± 11.4 | 66.7 ± 10.4 | 0.204 |
| Gender, n (%) | 0.515 | ||
| Male | 7 (5.8) | 114 (94.2) | |
| Female | 8 (8.0) | 92 (92.0) | |
| Race, n (%) | 0.440 | ||
| White | 13 (6.9) | 175 (93.1) | |
| African-American | — | 4 (100.0) | |
| Hispanic | — | 1 (100.0) | |
| Asian | — | 12 (100.0) | |
| Other or unknown | 2 (12.5) | 14 (87.5) | |
| Classic symptoms of PDAC, n (%) | 0.971 | ||
| Absent | 12 (6.8) | 164 (93.2) | |
| Present | 3 (6.7) | 42 (93.3) | |
| Abdominal pain, n (%) | 0.837 | ||
| Absent | 9 (7.1) | 118 (92.9) | |
| Present | 6 (6.4) | 88 (93.6) | |
| Jaundice, n (%) | 0.803 | ||
| Absent | 7 (6.4) | 103 (93.6) | |
| Present | 8 (7.2) | 103 (92.8) | |
| Tumor size, cm, median (IQR) | 2.5 (2.4–3.0) | 3.0 (2.4–3.8) | 0.312 |
| Tumor size, n (%) | 0.266 | ||
| ≤ 1.00 cm | — | 2 (100.0) | |
| 1.01–1.50 cm | 3 (13.6) | 19 (86.4) | |
| 1.51–2.00 cm | — | 12 (100.0) | |
| 2.01–2.50 cm | 5 (10.6) | 42 (89.4) | |
| 2.51–3.00 cm | 4 (10.5) | 34 (89.5) | |
| 3.01–3.50 cm | — | 36 (100.0) | |
| 3.51–4.00 cm | — | 22 (100.0) | |
| >4.01 cm | 3 (7.1) | 39 (92.9) | |
| AJCC T stage, n (%) | 0.200 | ||
| T1 | 3 (13.6) | 19 (86.4) | |
| T2 | 1 (2.2) | 45 (79.8) | |
| T3 | 11 (7.2) | 142 (92.8) | |
| AJCC N stage, n (%) | 0.733 | ||
| N0 | 4 (7.8) | 47 (92.2) | |
| N1 | 11 (6.5) | 159 (93.5) | |
| AJCC M stage, n (%) | — | ||
| M0 | 15 (6.8) | 206 (93.2) | |
| M1 | — | ||
| AJCC stage, n (%) | 0.052 | ||
| IA | 3 (25.0) | 9 (75.0) | |
| IB | — | 17 (100.0) | |
| IIA | 1 (4.6) | 21 (95.4) | |
| IIB | 11 (6.5) | 159 (93.5) | |
| Grade of tumor differentiation, n (%) | 0.811 | ||
| Well/moderately differentiated | 10 (7.1) | 131 (92.9) | |
| Poorly differentiated | 5 (6.3) | 75 (93.8) | |
| Perineural invasion, n (%) | 0.845 | ||
| Absent | 2 (7.7) | 24 (92.3) | |
| Present | 13 (6.7) | 182 (93.3) | |
| Lymphovascular invasion, n (%) | 0.929 | ||
| Absent | 5 (6.6) | 71 (93.4) | |
| Present | 10 (6.9) | 135 (93.1) | |
| Number of harvested nodes, median (IQR) | 26 (19–28) | 19 (15–27) | 0.197 |
| Number of harvested nodes, n (%) | 0.059 | ||
| ≤20 nodes | 4 (3.6) | 107 (96.4) | |
| >20 nodes | 11 (10.0) | 99 (99.0) | |
| Number of positive nodes, median (IQR) | 4 (0–6) | 2 (1–5) | 0.547 |
| Positive nodes, n (%) | 0.733 | ||
| Absent | 4 (7.8) | 47 (92.2) | |
| Present | 11 (6.5) | 159 (93.5) |
Fig. S5.
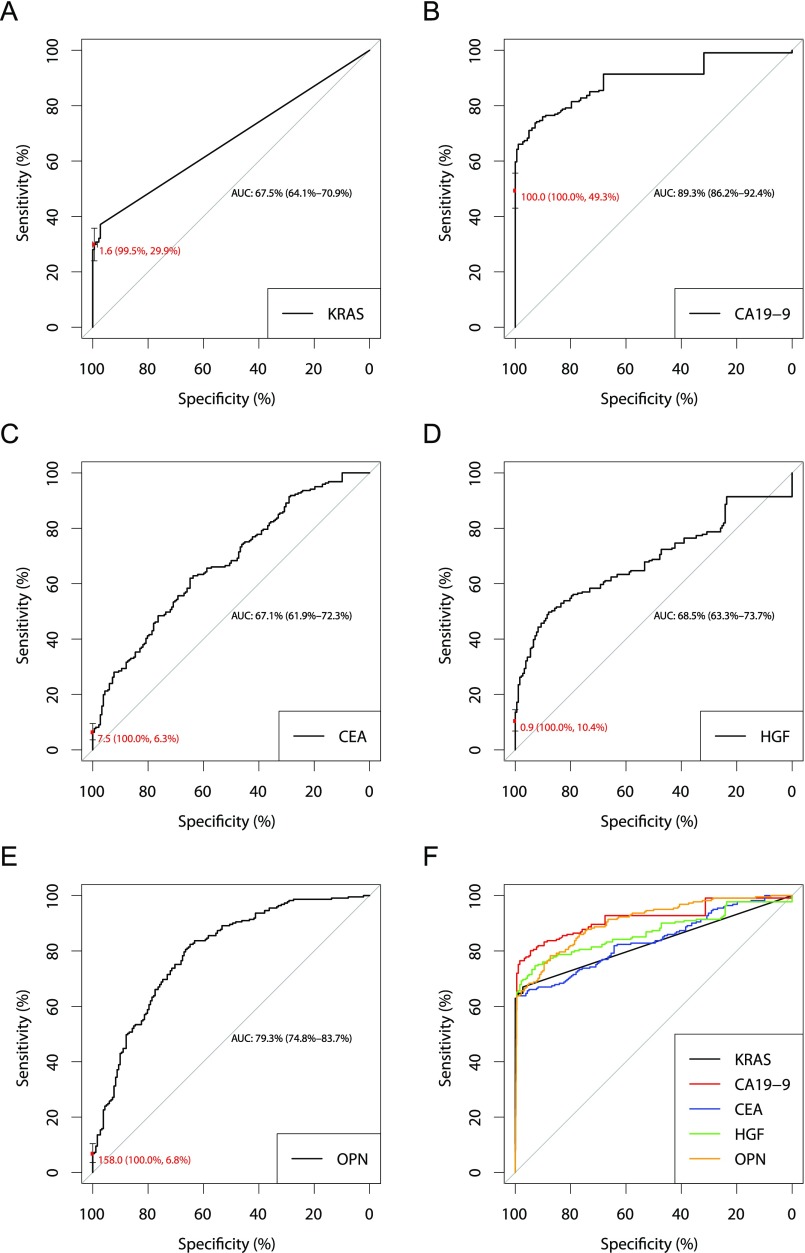
Receiver operator characteristic (ROC) curves for KRAS mutations (A), CA19-9 (B), CEA (C), HGF (D), OPN (E), and the combination assay (F). (A–E) ROC curves demonstrate the performance of each combination assay biomarker individually. The red points on the curves indicate the marker performance at the thresholds used in the combination assay. Error bars represent 95% CIs for sensitivity and specificity at the particular threshold (red font). (F) ROC curves demonstrating the performance of the combination assay when the KRAS threshold was varied and CA19-9, CEA, HGF, and OPN thresholds were fixed at the levels used in the combination assay (black curve), the CA19-9 threshold was varied and KRAS, CEA, HGF, and OPN thresholds were fixed at the levels used in the combination assay (red curve), the CEA threshold was varied and KRAS, CA19-9, HGF, and OPN thresholds were fixed at the levels used in the combination assay (blue curve), the HGF threshold was varied and KRAS, CA19-9, CEA, and OPN thresholds were fixed at the levels used in the combination assay (green curve), and the OPN threshold was varied and KRAS, CA19-9, CEA, and HGF thresholds were fixed at the levels used in the combination assay (orange curve). The intersection of these three curves designates the overall performance of the triplex assay (64% sensitivity, 99.5% sensitivity).
Table S7.
General demographics and histopathology of patients with pancreatic cancer by combination assay status
| Pancreatic cancer with detectable combination assay, n = 141 | Pancreatic cancer with undetectable combination assay, n = 80 | P value | |
| Age, y, mean ± SD | 66.5 ± 10.1 | 67.7 ± 11.09 | 0.430 |
| Gender, n (%) | 0.238 | ||
| Male | 73 (60.3) | 48 (39.7) | |
| Female | 68 (68.0) | 32 (32.0) | |
| Race, n (%) | 0.240 | ||
| White | 119 (63.3) | 69 (36.7) | |
| African-American | 2 (50.0) | 2 (50.0) | |
| Hispanic | 1 (100.0) | — | |
| Asian | 11 (91.7) | 1 (8.3) | |
| Other or unknown | 8 (50.0) | 8 (50.0) | |
| Classic symptoms of PDAC, n (%) | 0.354 | ||
| Absent | 114 (64.8) | 62 (35.2) | |
| Present | 27 (60.0) | 18 (40.0) | |
| Abdominal pain, n (%) | 0.771 | ||
| Absent | 80 (62.9) | 47 (37.0) | |
| Present | 61 (64.9) | 33 (35.1) | |
| Jaundice, n (%) | 0.741 | ||
| Absent | 69 (62.7) | 41 (37.3) | |
| Present | 72 (64.9) | 39 (35.1) | |
| Tumor size, cm, median (IQR) | 3.2 (2.5–4.0) | 2.5 (2.1–3.4) | 0.002 |
| Tumor size, n (%) | 0.013 | ||
| ≤ 1.00 cm | — | 2 (100.0) | |
| 1.01–1.50 cm | 11 (50.0) | 11 (50.0) | |
| 1.51–2.00 cm | 6 (50.0) | 6 (50.0) | |
| 2.01–2.50 cm | 24 (51.1) | 23 (48.9) | |
| 2.51–3.00 cm | 23 (60.5) | 15 (39.5) | |
| 3.01–3.50 cm | 29 (80.6) | 7 (19.4) | |
| 3.51–4.00 cm | 17 (77.3) | 5 (22.7) | |
| >4.01 cm | 31 (73.8) | 11 (26.2) | |
| AJCC stage, n (%) | 0.002 | ||
| T1 | 8 (36.4) | 14 (63.6) | |
| T2 | 25 (54.4) | 21 (45.6) | |
| T3 | 108 (70.6) | 45 (29.4) | |
| AJCC N stage, n (%) | 0.002 | ||
| N0 | 23 (45.1) | 28 (54.9) | |
| N1 | 118 (69.4) | 52 (30.6) | |
| AJCC M stage, n (%) | — | ||
| M0 | 141 (63.8) | 80 (36.2) | |
| M1 | — | — | |
| AJCC stage, n (%) | 0.012 | ||
| IA | 4 (33.3) | 8 (66.7) | |
| IB | 8 (47.1) | 9 (52.9) | |
| IIA, | 11 (50.0) | 11 (50.0) | |
| IIB | 118 (69.4) | 52 (30.6) | |
| Grade of tumor differentiation, n (%) | 0.389 | ||
| Well/moderately differentiated | 87 (61.7) | 54 (38.3) | |
| Poorly differentiated | 54 (67.5) | 26 (32.5) | |
| Perineural invasion, n (%) | 0.119 | ||
| Absent | 13 (50.0) | 13 (50.0) | |
| Present | 128 (65.6) | 67 (34.4) | |
| Lymphovascular invasion, n (%) | 0.005 | ||
| Absent | 39 (51.3) | 37 (48.7) | |
| Present | 102 (70.3) | 43 (29.7) | |
| Number of harvested nodes, median (IQR) | 20 (16–27) | 18 (14–27) | 0.044 |
| Number of harvested nodes, n (%) | 0.285 | ||
| ≤20 nodes | 67 (60.4) | 44 (39.6) | |
| >20 nodes | 74 (67.3) | 35 (32.7) | |
| Number of positive nodes, median (IQR) | 3 (1–6) | 1 (0–3) | <0.001 |
| Positive nodes, n (%) | 0.002 | ||
| Absent | 23 (45.1) | 28 (54.9) | |
| Present | 118 (69.4) | 52 (30.6) |
Table S5.
General demographics and histopathology of patients with pancreatic cancer by CEA status
| Pancreatic cancer with detectable CEA, n = 14 | Pancreatic cancer with undetectable CEA, n = 207 | P value | |
| Age, y, mean ± SD | 68.6 ± 12.0 | 66.8 ± 10.4 | 0.539 |
| Gender, n (%) | 0.139 | ||
| Male | 5 (4.1) | 116 (95.9) | |
| Female | 9 (9.0) | 91 (91.0) | |
| Race, n (%) | 0.083 | ||
| White | 12 (6.4) | 176 (93.6) | |
| African-American | — | 4 (100.0) | |
| Hispanic | — | 1 (100.0) | |
| Asian | — | 12 (100.0) | |
| Other or unknown | 2 (12.5) | 14 (87.5) | |
| Classic symptoms of PDAC, n (%) | 0.560 | ||
| Absent | 2 (4.4) | 43 (95.6) | |
| Present | 12 (6.8) | 164 (93.2) | |
| Abdominal pain, n (%) | 0.594 | ||
| Absent | 9 (7.1) | 118 (92.9) | |
| Present | 5 (5.3) | 89 (94.7) | |
| Jaundice, n (%) | 0.593 | ||
| Absent | 6 (5.5) | 104 (94.5) | |
| Present | 8 (7.2) | 103 (92.8) | |
| Tumor size, cm, median (IQR) | 4.0 (2.7–5.0) | 3.0 (2.4–3.7) | 0.343 |
| Tumor size, n (%) | 0.313 | ||
| ≤ 1.00 cm | — | 2 (100.0) | |
| 1.01–1.50 cm | 2 (9.1) | 20 (90.9) | |
| 1.51–2.00 cm | — | 12 (100.0) | |
| 2.01–2.50 cm | 1 (2.1) | 46 (97.9) | |
| 2.51–3.00 cm | 2 (5.3) | 36 (94.7) | |
| 3.01–3.50 cm | 1 (2.8) | 35 (97.2) | |
| 3.51–4.00 cm | 2 (9.1) | 20 (90.9) | |
| >4.01 cm | 6 (14.3) | 36 (85.7) | |
| AJCC T stage, n (%) | 0.936 | ||
| T1 | 1 (4.6) | 21 (95.5) | |
| T2 | 3 (6.5) | 43 (93.5) | |
| T3 | 10 (6.5) | 143 (93.5) | |
| AJCC N stage, n (%) | 0.144 | ||
| N0 | 1 (1.9) | 50 (98.1) | |
| N1 | 13 (7.7) | 157 (92.3) | |
| AJCC M stage, n (%) | — | ||
| M0 | 14 (6.3) | 207 (93.7) | |
| M1 | — | ||
| AJCC stage, n (%) | 0.360 | ||
| IA | 1 (8.3) | 11 (91.7) | |
| IB | — | 17 (100.0) | |
| IIA | — | 22 (100.0) | |
| IIB | 13 (7.7) | 157 (92.4) | |
| Grade of tumor differentiation, n (%) | 0.969 | ||
| Well/moderately differentiated | 9 (6.4) | 132 (93.6) | |
| Poorly differentiated | 5 (6.3) | 75 (93.8) | |
| Perineural invasion, n (%) | 0.579 | ||
| Absent | 1 (3.9) | 25 (96.2) | |
| Present | 13 (6.7) | 182 (93.3) | |
| Lymphovascular invasion, n (%) | 0.291 | ||
| Absent | 3 (3.9) | 73 (96.1) | |
| Present | 11 (7.6) | 134 (92.4) | |
| Number of harvested nodes, median (IQR) | 22 (18–26) | 19 (15–27) | 0.345 |
| Number of harvested nodes, n (%) | 0.262 | ||
| ≤20 nodes | 5 (4.5) | 106 (95.5) | |
| >20 nodes | 9 (8.2) | 101 (91.8) | |
| Number of positive nodes, median (IQR) | 4 (2–7) | 2 (1–5) | 0.185 |
| Positive nodes, n (%) | 0.144 | ||
| Absent | 1 (1.9) | 50 (98.1) | |
| Present | 13 (7.7) | 157 (92.3) |
Some of the patients detectable by the combination assay were of particular note. Forty-five (20%) of the patients in this study had no symptoms classically associated with pancreatic cancer (Table S1 and Dataset S1). The combination assay identified 27 (60%) of these individuals, of whom 19 (70%) had no evidence of recurrence with a median follow-up of 12 mo (range 3–16 mo) (Table S7 and Dataset S1). Of the 29 patients with the earliest stages of disease recognized by the AJCC (stages IA and IB), 12 (41%) were detectable using the combination assay (Fig. 1A), seven of whom (58%) had no evidence of recurrence at the study termination with a median follow-up of 19 mo (range 2–25 mo).
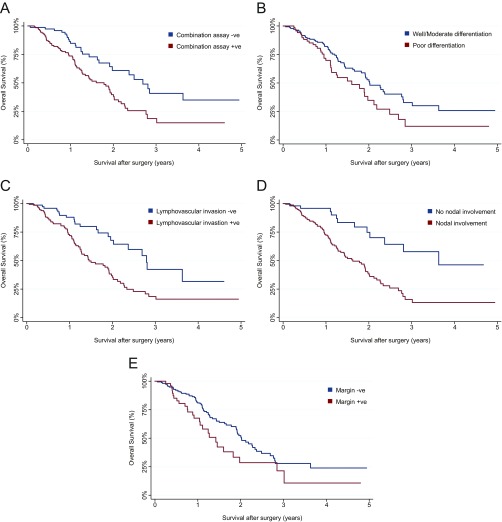
Another notable but sobering result from our study was that patients with poorer survival were more likely to have a positive test. Of the entire 221 pancreatic cancer patients studied, 122 (56%) patients were alive at the termination of the study, with a median follow-up of 13 mo (7–21 mo). We found that the combination assay provided prognostic value that was independent of conventional clinical and histopathologic features. In particular, multivariate analyses showed that the independent predictors of overall survival were combination assay status [hazards ratio (HR) = 1.76, 95% CI 1.10–2.84, P = 0.018], increasing age (HR = 1.04, 95% CI 1.02–1.06, P = 0.001), grade of differentiation (poorly differentiated, HR = 1.72, 95% CI 1.11–2.66, P = 0.015), lymphovascular invasion (present, HR = 1.81, 95% CI 1.06–3.09, P = 0.028), nodal disease (present, HR = 2.35, 95% CI 1.20–4.61, P = 0.013), and margin status (HR = 1.59, 95% CI 1.01–2.55, P = 0.050) (Fig. S6).
Fig. S6.
Kaplan–Meier survival plots stratified by independent predictors of overall survival identified by multivariate analysis. (A) Combination assay status (HR = 1.76, 95% CI 1.10–2.84, P = 0.018). (B) Grade of differentiation (poorly differentiated, HR = 1.72, 95% CI 1.11–2.66, P = 0.015). (C) Lymphovascular invasion (present, HR = 1.81, 95% CI 1.06–3.09, P = 0.028). (D) Nodal disease (present, HR = 2.35, 95% CI 1.20–4.61, P = 0.013). (E) Margin status (HR = 1.59, 95% CI 1.01–2.55, P = 0.050).
Inclusion of TP53 in a Multiplex Assay.
While nearly all pancreatic cancers harbor mutations within KRAS, a large fraction (∼75%) also contains mutations in TP53 (44–46). Furthermore, TP53 is the mostly commonly mutated gene in cancer (31), making it an attractive target for ctDNA detection in future studies involving other tumor types. We wished to determine whether the mutant allele frequencies (MAF) of TP53 in the plasma correlated with those of KRAS and also whether a mutant TP53 assay in plasma might add to the sensitivity of the mutant KRAS assay. For this purpose, we evaluated the 152 carcinomas for which matched tumor and plasma samples were available. We first searched for mutations at one of the “hotspots” identified in previous genome-wide studies of PDAC (44). A total of 64 (42%) carcinomas contained a TP53 mutation at one of these positions. We then determined whether these same mutations could be identified in the plasma of these 64 patients, using Safe-SeqS–based assays similar to that described above for KRAS but using primers specific for particular TP53 mutations.
We identified TP53 mutations in 13 (20%) of the 64 plasma samples (Table S8). Two observations were of interest. First, 12 of the 13 plasma samples containing a detectable TP53 mutation also contained a detectable KRAS mutation. Thus, TP53 mutation assays did not substantially increase sensitivity for pancreatic cancer detection, as expected from the high prevalence of KRAS mutations noted above. Second, there was a strong correlation between the MAF of TP53 and KRAS mutations in the plasma of the 12 patients whose plasma contained detectable amounts of both mutations (Pearson’s r = 0.885) (Fig. 2B). This provides yet another validation of the reliability of the ctDNA assay and its quantitative nature.
Table S8.
TP53 plasma mutations identified in 13 PDAC patients with TP53-mutant tumors
| Sample ID | Plasma DNA concentration, ng/mL | Mutation identified in plasma | Mutant mean allele frequency, % | Mutant fragments/mL plasma |
| PANC 335 | 7.44 | TP53 p.R196*, c.586C > T | 0.795 | 18.2 |
| PANC 336 | 7.66 | TP53 p.G266E, c.797G > A | 0.929 | 21.9 |
| PANC 352 | 10.88 | TP53 p.R175H, c.524G > A | 0.673 | 22.6 |
| PANC 387 | 6.22 | TP53 p.R175H, c.524G > A | 0.195 | 3.7 |
| PANC 467 | 3.79 | TP53 p.R248Q, c.743G > A | 0.344 | 4.0 |
| PANC 468 | 5.76 | TP53 p.S241F, c.722C > T | 0.139 | 2.5 |
| PANC 469 | 3.35 | TP53 p.C238F, c.713G > T | 0.051 | 0.5 |
| PANC 545 | 6.85 | TP53 p.Y236C, c.707A > G | 0.137 | 2.9 |
| PANC 547 | 6.01 | TP53 p.Y234C, c.701A > G | 0.069 | 1.3 |
| PANC 552 | 5.60 | TP53 p.C238Y, c.713G > A | 0.317 | 5.5 |
| PANC 692 | 5.50 | TP53 p.V272M, c.814G > A | 0.101 | 1.7 |
| PANCA 1105 | 6.43 | TP53 p.H193Y, c.577C > T | 0.098 | 1.9 |
| PANCA 1109 | 15.44 | TP53 p.Y234C, c.701A > G | 0.351 | 16.7 |
Discussion
The major conclusion of this study is that assays for genetic alterations can be combined with assays for elevated proteins to increase the sensitivity of a blood test for low-stage pancreatic cancers. We were able to detect 64% of these cancers through this combination test, including some patients with a favorable prognosis. One of the critical design features of our study was that we included only patients with resectable pancreatic cancers and excluded all patients with advanced disease (i.e., stage III or IV). Although this exclusion reduces the sensitivity that could be achieved by evaluating all pancreatic cancer patients, regardless of stage, the resectable cases are the most important group with respect to evaluating a screening technology.
Whether combining ctDNA and protein markers could increase sensitivity over either alone was not known. In fact, it was conceivable that the patients with detectable circulating protein markers would largely overlap those releasing DNA into the circulation. This was of particular concern for early-stage cancer patients, because both ctDNA and protein-based markers are known to be considerably higher in patients with advanced cancers than in those with earlier-stage cancers (17, 19, 22).
Another important aspect of our study is that we achieved very high specificity (99.5%: 95% CI 97–100%), observing only one false positive among 182 healthy individuals of average age 64 y. Given the relative infrequency of cancer in the general population, the specificity of any potentially useful blood-based screening test for pancreatic cancer has to be high, preferably >99%. Otherwise, the number of false positives will greatly exceed the number of true positives (i.e., have suboptimal positive predictive value) (47). This stringency for screening tests is not required for tests to monitor disease in patients with known cancer. For monitoring, specificity can be relaxed somewhat in the interest of obtaining higher sensitivity. We achieved high specificity in two ways. First, we used ctDNA as one of the components of the test. KRAS mutations are exquisitely specific for neoplasia, and their specificity is limited by technical rather than biological factors. The incorporation of molecular barcoding into our assays (Safe-SeqS) minimizes the false-positive results from sequencing that are the major technical issues confronting any ctDNA-based assays. KRAS mutations are particularly suitable for early-detection strategies because they are rarely found in clones arising during age-associated clonal hematopoiesis. Such clones, which may represent early forms of myelodysplasia, are a potential source of false-positive ctDNA assays. The vast majority of such mutations occur within nine genes (DNMT3A, TET2, JAK2, ASXL1, TP53, GNAS, PPM1D, BCORL1, and SF3B1) (48–50), posing challenges for the use of these genes as biomarkers in ctDNA-based assays. Second, we used high thresholds for scoring the protein markers as positive. These thresholds were based on prior studies in the literature or on an independent set of controls, allowing us to avoid positive scores in the vast majority of healthy patients (36). We could afford to use these high thresholds without an overall reduction in sensitivity because the ctDNA assay added sensitivity on its own and the ctDNA-positive cases only partially overlapped the protein-biomarker–positive cases (Figs. 1 and 2A and Table 1).
Protein biomarkers have been combined in the past to achieve higher sensitivity (51). For example, nearly 20 y ago it was shown that combining CA19-9 and TIMP-1 was more sensitive for the detection of PDAC than either biomarker alone (34). More recently, it was shown that the combination of CA19-9, TIMP-1, and LRG-1 was more sensitive for the detection of early PDAC than CA19-9 alone (52). The combination of protein biomarkers with ultrasensitive ctDNA is by comparison novel. For example, a recent study evaluated a combination of ctDNA and CA19-9 for pancreatic cancer but found no benefit to combining the biomarkers over CA19-9 alone, perhaps due to inadequate sensitivity of the test used in detecting KRAS mutations (53). Furthermore, the specificity for ctDNA achieved in that study was relatively low, reducing its suitability for screening.
Our study demonstrates that the majority of patients with resectable pancreatic cancers can be detected through a noninvasive blood test. However, there are several limitations of the study that should be acknowledged. Patients with pancreaticobiliary disease, such as ascending cholangitis, cholecystitis, or chronic pancreatitis, can have high levels of CA19-9 (17). Our goal is to develop a test that will be used in healthy, asymptomatic individuals. Individuals with ascending cholangitis, cholecystitis, or acute pancreatitis are almost always symptomatic. Incidentally detected chronic pancreatitis has been reported; however, the risk of this is extremely low, with an estimated prevalence of 0.49 per 100,000 (54–56), so this should not reduce specificity in practice. Another limitation is that, even though the majority of patients with resectable pancreatic cancer in our cohort could be detected, most patients still died from disease recurrence following surgery. These results make it clear that it will be necessary to detect cancers at earlier stages than those in our cohort to realize the maximum potential of earlier diagnosis, i.e., detection of individuals that can be cured by surgery alone.
On the other hand, the results we obtained may underestimate the survival benefits of early detection. The majority of the patients we studied, even though they had resectable cancers, were symptomatic, and their cancers were discovered only by virtue of their symptoms. Accordingly, 77% of patients in our cohort were stage IIB, and the median size of tumors in these patients was 3 cm. In a screening study of asymptomatic individuals, it is expected that a greater proportion of earlier-stage patients with smaller tumors will be discovered. This optimistic expectation is somewhat tempered by the fact that our combination assay was more sensitive for the detection of patients with larger tumors and patients with a poorer prognosis than for patients with smaller tumors, even though all tumors were surgically resectable (Fig. 1B, Table 2, Fig. S6, and Table S7). Another caveat to the use of this test for screening is that KRAS mutations are found in the circulation of patients with cancer types other than those of the pancreas, primarily cancers of the lung (57), and CA19-9, CEA, HGF, and OPN expression is elevated in several other cancer types (36, 58–60). Thus, patients testing positive would have to undergo appropriate imaging studies for tumor localization. Despite these limitations, our study establishes proof of principle for a large, prospective study in which these and other related issues can be evaluated.
The current study also lays a foundation for evaluation of patients at high risk for PDAC, which is a key strategy in implementation of early-detection technologies (61). As an example, new-onset diabetes is associated with an increased risk for pancreatic cancer. Approximately 1% of diabetic patients aged 50 y and older are diagnosed with pancreatic cancer within 3 y of first meeting criteria for diabetes (62). With an incidence of 1%, the positive predictive value/negative predictive value of the combination assay are expected to be 54% and 99.6%, respectively, in this population, which is well within the range of currently approved screening tests for cancers.
The applicability of our combination strategy to other cancers remains to be determined. Its success will largely depend on the extent of release of ctDNA from other cancer types coupled with the availability of protein and other biomarkers for those types. Available evidence indicates that many cancers have detectable ctDNA in their earliest stages, often more commonly than observed in pancreatic cancer (22). Similarly, a large number of protein biomarkers have already been described for the detection of numerous cancer types (16). In theory, these protein biomarkers could be thresholded in the way described here, offering cautious optimism for the use ctDNA–protein combinations to detect a variety of cancer types (22).
Materials and Methods
Detailed materials and methods are available in SI Materials and Methods. Briefly, DNA was purified from plasma using a QIAsymphony circulating DNA kit (catalog no. 1091063; Qiagen). Custom primers containing a unique identifier (UID) and amplicon-specific sequence (Table S9) were used to amplify plasma DNA, and the resulting products were sequenced on an Illumina MiSeq or HiSeq instrument. Protein biomarker plasma concentrations were determined using Luminex bead-based immunoassays on the Bio-Plex 200 platform (Bio-Rad). Plasma samples were scored as positive if the sample contained a KRAS mutation or if the concentration of CA19-9, CEA, HGF, or OPN was greater than 100 U/mL, 7.5 ng/mL, 0.92 ng/mL, or 158 ng/mL, respectively. All samples were obtained following approval by the Institutional Review Boards for Human Research at The Johns Hopkins Medical Institutions, Walter and Eliza Hall Institute of Medical Research, Indiana University, Memorial Sloan-Kettering Cancer Center, University of Pittsburgh, Mayo Clinic, Asan Medical Center, University of Ulsan College of Medicine, San Raffaele Scientific Institute Research Hospital, and The University of Texas MD Anderson Cancer Center, and informed consent was obtained.
Table S9.
Primer sequences for multiplex PCR assays
| Amplicon name | Amplicon-specific forward primer sequence | Amplicon-specific reverse primer sequence | Gene | hg19 coordinates | Region of interest length (bp) | Amplicon length (bp) |
| KRAS_0057_0064 | GAGAAACCTGTCTCTTGGATATTCTC | TGTACTGGTCCCTCATTGCAC | KRAS | chr12:25,380,244–25,380,315 | 25 | 72 |
| KRAS_012_+13_-4 | CGTCAAGGCACTCTTGCC | TGCTGAAAATGACTGAATATAAACTTGT | KRAS | chr12:25,398,259–25,398,326 | 22 | 68 |
| TP53_342 | TCCTCTGTTGCTGCAGATCC | TGAGTTCCAAGGCCTCATTC | TP53 | chr17:7,573,976–7,574,049 | 34 | 74 |
| TP53_298_306 | AAAGGGGAGCCTCACCAC | ACCGCTTCTTGTCCTGCTT | TP53 | chr17:7,576,994–7,577,064 | 34 | 71 |
| TP53_0280_0289 | GTGAGGCTCCCCTTTCTTG | CGTGTTTGTGCCTGTCCTG | TP53 | chr17:7,577,050–7,577,121 | 34 | 72 |
| TP53_0272_0283 | GGGACGGAACAGCTTTGAG | GCGGAGATTCTCTTCCTCTGT | TP53 | chr17:7,577,068–7,577,143 | 36 | 76 |
| TP53_262_267 | AACACGCACCTCAAAGCTG | TGCCTCTTGCTTCTCTTTTCCT | TP53 | chr17:7,577,116–7,577,188 | 32 | 73 |
| TP53_0248_0261 | GTGGCAAGTGGCTCCTGA | CATGGGCGGCATGAAC | TP53 | chr17:7,577,480–7,577,555 | 42 | 76 |
| TP53_233_245 | TGGCTCTGACTGTACCACCA | ATGGGCCTCCGGTTCAT | TP53 | chr17:7,577,529–7,577,606 | 41 | 78 |
| TP53_0227_0236 | CCCATGCAGGAACTGTTACAC | GGCCTGTGTTATCTCCTAGGTTG | TP53 | chr17:7,577,550–7,577,627 | 34 | 78 |
| TP53_219_224 | TAACCCCTCCTCCCAGAGAC | TTTTCGACATAGTGTGGTGGTG | TP53 | chr17:7,578,139–7,578,216 | 36 | 78 |
| TP53_208_218 | TTGCGTGTGGAGTATTTGGA | AGACCTCAGGCGGCTCATAG | TP53 | chr17:7,578,173–7,578,248 | 36 | 76 |
| TP53_0196_0205 | CGAAAAGTGTTTCTGTCATCCA | GCCCCTCCTCAGCATCTTAT | TP53 | chr17:7,578,211–7,578,284 | 32 | 74 |
| TP53_188_195 | CGCAAATTTCCTTCCACTCG | CTGATTCCTCACTGATTGCTCTT | TP53 | chr17:7,578,244–7,578,314 | 28 | 71 |
| TP53_172A | AGCCCCAGCTGCTCACC | ACATGACGGAGGTTGTGAGG | TP53 | chr17:7,578,355–7,578,427 | 36 | 73 |
| TP53_167_177 | TGGCCATCTACAAGCAGTCA | GAGCAGCGCTCATGGTG | TP53 | chr17:7,578,382–7,578,451 | 33 | 70 |
| TP53_151_163 | CGTCATGTGCTGTGACTGCTT | CAGCTGTGGGTTGATTCCA | TP53 | chr17:7,578,420–7,578,500 | 41 | 81 |
| TP53_126_131 | GGCCAGTTGGCAAAACATCT | GCCCTGACTTTCAACTCTGTCT | TP53 | chr17:7,578,516–7,578,593 | 36 | 78 |
SI Materials and Methods
Plasma, White Blood Cell, and Tumor DNA Samples.
Samples were obtained following approval by the IRBs for Human Research at each institution and informed consent. Patients with stage IA, IB, IIA, or IIB (considered resectable) PDAC who had had peripheral blood collected before surgery, had not received neoadjuvant therapy, and had undergone surgical resection at the participating institutions between April 2011 and May 2016 were included in the study. General demographics, surgical pathology, and AJCC stage (seventh edition) were documented. The healthy cohort consisted of peripheral blood samples obtained from 182 individuals, average age 64 y, with no history of cancer. The pancreatic cancer and healthy control samples were collected and processed in an identical manner.
DNA was purified from 7.5 mL plasma using a QIAsymphony circulating DNA kit (catalog no. 1091063; Qiagen), as specified by the manufacturer. Tumor tissues were formalin-fixed and paraffin-embedded according to standard histopathologic procedures and were macrodissected under a microscope to ensure a neoplastic cellularity of >30%. DNA was purified with a QIAsymphony DP DNA Midi Kit (catalog no. 937255; Qiagen) as specified by the manufacturer. DNA concentrations were assessed by fluorescence using SYBR Green I (catalog no. S7585; Thermo Fisher).
Mutation Detection and Analysis.
For amplification of DNA from plasma, primer pairs were designed to amplify 66- to 80-bp segments containing regions of interest from the KRAS and TP53 genes (Table S9). These primers were used to amplify DNA in six independent 25-μL reactions as previously described (25). Reactions were purified with AMPure XP beds (Beckman Coulter) and eluted in 50 μL of Buffer EB (Qiagen). A fraction (5 μL) of purified PCR products was then amplified in a second round of PCR, as previously described (25). The PCR products were purified with AMPure and sequenced on an Illumina MiSeq or HiSeq 4000 instrument.
The template-specific portion of the reads was matched to reference sequences using custom scripts written in SQL and C#. Reads from a common template molecule were then grouped based on the UIDs that were incorporated as molecular barcodes (32). Artifactual mutations introduced during the sample preparation or sequencing steps were reduced by requiring a mutation to be present in >90% of reads in each UID family.
Evaluation of Plasma Proteins.
The Bio-Plex 200 platform (Bio-Rad) was used to determine the concentration of multiple target proteins in the plasma samples. Luminex bead-based immunoassays were performed following the manufacturer’s protocols, and concentrations were determined using five-parameter log curve fits (using Bio-Plex Manager 6.0) with vendor-provided standards and quality controls. Plasma samples were diluted sixfold for assay of CA19-9, CEA, HGF, OPN, and prolactin and fivefold for assay of midkine. Plasma samples were scored as positive if the concentration of CA19-9, CEA, HGF, or OPN was greater than 100 U/mL, 7.5 ng/mL, 0.92 ng/mL, or 158 ng/mL, respectively. The dynamic ranges of these immunoassays for CA19-9, CEA, HGF, OPN, prolactin, and midkine were 2.74–2,000 U/mL, 78.19–57,000 pg/mL, 27.43–20,000 pg/mL, 548.7–400,000 pg/mL, 137.17–100,000 pg/mL, and 13.72–10,000 pg/mL, respectively.
Algorithm for Classifying ctDNA Status.
The classification of a sample’s ctDNA status was obtained from a statistical test comparing the normalized mutation frequencies of the sample of interest to a distribution of normal controls. Specifically, the MAF, defined as the ratio between the number of supermutants and the number of UIDs, was first normalized based on the observed MAFs in a set of normal controls for each mutation. Following this mutation-specific normalization, the MAF of each mutation in each well was compared with a reference distribution of MAFs built from normal controls with all mutations included, and a P value was calculated from this distribution. The lowest P value among all mutations detected in a given sample was deemed the “top mutation.” The classification of a sample’s ctDNA status was based on whether the P value of this top mutation was below or above a given threshold. The threshold was selected based on a desired specificity observed among an independent set of normal controls. Thus, no training was performed on any other sample except these controls; in particular, neither the 182 healthy controls nor the 221 pancreatic cancer patients described in the main text were included in the controls used for training the algorithm.
Statistical Analysis.
Continuous variables were reported as means and SDs or medians and range as deemed necessary, while categorical variables were reported as whole numbers and percentages. CIs for sensitivities were calculated using a binomial distribution. Survival curves were estimated using Kaplan–Meier method, and differences between curves were investigated with the log-rank test. Statistically significant variables in the univariate analyses were subjected to multivariable Cox proportional hazard regression model. HR and 95% CIs for variables included in the multivariable model were reported. A P value <0.05 was considered statistically significant.
Supplementary Material
Acknowledgments
We thank C. Blair and K. Judge from The Ludwig Center at Johns Hopkins for expert technical and administrative assistance and Dr. Dae Wook Hwang, Department of Hepatobiliary and Pancreas Surgery, Asan Medical Center, University of Ulsan College of Medicine; J. Brooks, K. Chaffee, and W. Bamlet of the Mayo Clinic; and Dr. Herb Zeh of the University of Pittsburgh for their assistance with enrollment for this study. This work was supported by the Lustgarten Foundation for Pancreatic Cancer Research, The Virginia and D.K. Ludwig Fund for Cancer Research, The Conrad N. Hilton Foundation, The Sol Goldman Center for Pancreatic Cancer Research, The Michael Rolfe Pancreatic Cancer Research Foundation, The Joseph L. Rabinowitz Fund for Pancreatic Cancer Research, Susan Wojcicki and Dennis Troper, The Benjamin Baker Scholarship, The Commonwealth Foundation, National Health and Medical Research Council Grant APP1060804, The John Templeton Foundation, and NIH Grants P30-CA006973, P50-CA062924, P50-CA102701, CA06973, CA152753, CA196403, CA200468, GM-07309, and U01CA152753.
Footnotes
Conflict of interest statement: C.M.S. and M.T.Y.-S. are founders and coowners of B9, Inc. and have no conflicts of interest with respect to the new technology described in this article. N.P., K.W.K., and B.V. are founders of Personal Genome Diagnostics, Inc. and PapGene, Inc. K.W.K. and B.V. are members of the Scientific Advisory Board of Sysmex-Inostics and Morphotek. B.V. is also a member of the Scientific Advisory Board of Exelixis GP. These companies and others have licensed technologies from Johns Hopkins, including those related to early diagnostics. N.P., K.W.K., and B.V. are the inventors of some of these technologies and receive equity or royalties from their licenses. The terms of these arrangements are being managed by the university in accordance with its conflict of interest policies. N.P., K.W.K., and B.V. have no conflicts of interest with respect to the new technology described in this article, as defined by the Johns Hopkins University policy on conflict of interest.
Data deposition: The sequence reported in this paper has been deposited in the GenBank database (accession no. EGAS00001002444).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1704961114/-/DCSupplemental.
References
- 1.Rahib L, et al. Projecting cancer incidence and deaths to 2030: The unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res. 2014;74:2913–2921. doi: 10.1158/0008-5472.CAN-14-0155. [DOI] [PubMed] [Google Scholar]
- 2.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 3.Ryan DP, Hong TS, Bardeesy N. Pancreatic adenocarcinoma. N Engl J Med. 2014;371:2140–2141. doi: 10.1056/NEJMc1412266. [DOI] [PubMed] [Google Scholar]
- 4.Ansari D, et al. Relationship between tumour size and outcome in pancreatic ductal adenocarcinoma. Br J Surg. 2017;104:600–607. doi: 10.1002/bjs.10471. [DOI] [PubMed] [Google Scholar]
- 5.Jung KW, et al. Clinicopathological aspects of 542 cases of pancreatic cancer: A special emphasis on small pancreatic cancer. J Korean Med Sci. 2007;22(Suppl):S79–S85. doi: 10.3346/jkms.2007.22.S.S79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Egawa S, et al. Clinicopathological aspects of small pancreatic cancer. Pancreas. 2004;28:235–240. doi: 10.1097/00006676-200404000-00004. [DOI] [PubMed] [Google Scholar]
- 7.Ishikawa O, et al. Minute carcinoma of the pancreas measuring 1 cm or less in diameter–collective review of Japanese case reports. Hepatogastroenterology. 1999;46:8–15. [PubMed] [Google Scholar]
- 8.Tsuchiya R, et al. Collective review of small carcinomas of the pancreas. Ann Surg. 1986;203:77–81. doi: 10.1097/00000658-198601000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Howlader N, et al. 2016 SEER Cancer Statistics Review, 1975-2013 (National Cancer Institute, Bethesda, MD) Available at https://seer.cancer.gov/csr/1975_2013/. Accessed April 25, 2017.
- 10.Bozic I, et al. Evolutionary dynamics of cancer in response to targeted combination therapy. Elife. 2013;2:e00747. doi: 10.7554/eLife.00747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Semrad TJ, Fahrni AR, Gong IY, Khatri VP. Integrating chemotherapy into the management of oligometastatic colorectal cancer: Evidence-based approach using clinical trial findings. Ann Surg Oncol. 2015;22(Suppl 3):S855–S862. doi: 10.1245/s10434-015-4610-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moertel CG, et al. Fluorouracil plus levamisole as effective adjuvant therapy after resection of stage III colon carcinoma: A final report. Ann Intern Med. 1995;122:321–326. doi: 10.7326/0003-4819-122-5-199503010-00001. [DOI] [PubMed] [Google Scholar]
- 13.André T, et al. Improved overall survival with oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment in stage II or III colon cancer in the MOSAIC trial. J Clin Oncol. 2009;27:3109–3116. doi: 10.1200/JCO.2008.20.6771. [DOI] [PubMed] [Google Scholar]
- 14.Dy GK, et al. Long-term survivors of metastatic colorectal cancer treated with systemic chemotherapy alone: A North Central Cancer Treatment Group review of 3811 patients, N0144. Clin Colorectal Cancer. 2009;8:88–93. doi: 10.3816/CCC.2009.n.014. [DOI] [PubMed] [Google Scholar]
- 15.Huang AC, et al. T-cell invigoration to tumour burden ratio associated with anti-PD-1 response. Nature. 2017;545:60–65. doi: 10.1038/nature22079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liotta LA, Petricoin EF., 3rd The promise of proteomics. Clin Adv Hematol Oncol. 2003;1:460–462. [PubMed] [Google Scholar]
- 17.Lennon AM, Goggins M. Pancreatic Cancer. Springer; New York: 2010. Diagnostic and therapeutic response markers; pp. 675–701. [Google Scholar]
- 18.Clarke-Pearson DL. Clinical practice. Screening for ovarian cancer. N Engl J Med. 2009;361:170–177. doi: 10.1056/NEJMcp0901926. [DOI] [PubMed] [Google Scholar]
- 19.Locker GY, et al. ASCO ASCO 2006 update of recommendations for the use of tumor markers in gastrointestinal cancer. J Clin Oncol. 2006;24:5313–5327. doi: 10.1200/JCO.2006.08.2644. [DOI] [PubMed] [Google Scholar]
- 20.Haber DA, Velculescu VE. Blood-based analyses of cancer: Circulating tumor cells and circulating tumor DNA. Cancer Discov. 2014;4:650–661. doi: 10.1158/2159-8290.CD-13-1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dawson SJ, et al. Analysis of circulating tumor DNA to monitor metastatic breast cancer. N Engl J Med. 2013;368:1199–1209. doi: 10.1056/NEJMoa1213261. [DOI] [PubMed] [Google Scholar]
- 22.Bettegowda C, et al. Detection of circulating tumor DNA in early- and late-stage human malignancies. Sci Transl Med. 2014;6:224ra24. doi: 10.1126/scitranslmed.3007094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kinde I, et al. Evaluation of DNA from the Papanicolaou test to detect ovarian and endometrial cancers. Sci Transl Med. 2013;5:167ra4. doi: 10.1126/scitranslmed.3004952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang Y, et al. Detection of somatic mutations and HPV in the saliva and plasma of patients with head and neck squamous cell carcinomas. Sci Transl Med. 2015;7:293ra104. doi: 10.1126/scitranslmed.aaa8507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang Y, et al. Detection of tumor-derived DNA in cerebrospinal fluid of patients with primary tumors of the brain and spinal cord. Proc Natl Acad Sci USA. 2015;112:9704–9709. doi: 10.1073/pnas.1511694112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang Y, et al. Diagnostic potential of tumor DNA from ovarian cyst fluid. ElLife. 2016;5:e15175. doi: 10.7554/eLife.15175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Springer S, et al. A combination of molecular markers and clinical features improve the classification of pancreatic cysts. Gastroenterology. 2015;149:1501–1510. doi: 10.1053/j.gastro.2015.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Forshew T, et al. Noninvasive identification and monitoring of cancer mutations by targeted deep sequencing of plasma DNA. Sci Transl Med. 2012;4:136ra68. doi: 10.1126/scitranslmed.3003726. [DOI] [PubMed] [Google Scholar]
- 29.Vogelstein B, Kinzler KW. Digital PCR. Proc Natl Acad Sci USA. 1999;96:9236–9241. doi: 10.1073/pnas.96.16.9236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dressman D, Yan H, Traverso G, Kinzler KW, Vogelstein B. Transforming single DNA molecules into fluorescent magnetic particles for detection and enumeration of genetic variations. Proc Natl Acad Sci USA. 2003;100:8817–8822. doi: 10.1073/pnas.1133470100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vogelstein B, et al. Cancer genome landscapes. Science. 2013;339:1546–1558. doi: 10.1126/science.1235122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Allen PJ, et al. Multi-institutional validation study of the American Joint Commission on Cancer (8th edition) changes for T and N staging in patients with pancreatic adenocarcinoma. Ann Surg. 2017;265:185–191. doi: 10.1097/SLA.0000000000001763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kinde I, Wu J, Papadopoulos N, Kinzler KW, Vogelstein B. Detection and quantification of rare mutations with massively parallel sequencing. Proc Natl Acad Sci USA. 2011;108:9530–9535. doi: 10.1073/pnas.1105422108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhou W, et al. Identifying markers for pancreatic cancer by gene expression analysis. Cancer Epidemiol Biomarkers Prev. 1998;7:109–112. [PubMed] [Google Scholar]
- 35.O’Brien DP, et al. Serum CA19-9 is significantly upregulated up to 2 years before diagnosis with pancreatic cancer: Implications for early disease detection. Clin Cancer Res. 2015;21:622–631. doi: 10.1158/1078-0432.CCR-14-0365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kim JE, et al. Clinical usefulness of carbohydrate antigen 19-9 as a screening test for pancreatic cancer in an asymptomatic population. J Gastroenterol Hepatol. 2004;19:182–186. doi: 10.1111/j.1440-1746.2004.03219.x. [DOI] [PubMed] [Google Scholar]
- 37.Uhlén M, et al. Proteomics. Tissue-based map of the human proteome. Science. 2015;347:1260419. doi: 10.1126/science.1260419. [DOI] [PubMed] [Google Scholar]
- 38.Nazli O, Bozdag AD, Tansug T, Kir R, Kaymak E. The diagnostic importance of CEA and CA 19-9 for the early diagnosis of pancreatic carcinoma. Hepatogastroenterology. 2000;47:1750–1752. [PubMed] [Google Scholar]
- 39.Di Renzo MF, Poulsom R, Olivero M, Comoglio PM, Lemoine NR. Expression of the Met/hepatocyte growth factor receptor in human pancreatic cancer. Cancer Res. 1995;55:1129–1138. [PubMed] [Google Scholar]
- 40.Ikematsu S, et al. Serum midkine levels are increased in patients with various types of carcinomas. Br J Cancer. 2000;83:701–706. doi: 10.1054/bjoc.2000.1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Koopmann J, et al. Evaluation of osteopontin as biomarker for pancreatic adenocarcinoma. Cancer Epidemiol Biomarkers Prev. 2004;13:487–491. [PubMed] [Google Scholar]
- 42.Levina VV, et al. Biological significance of prolactin in gynecologic cancers. Cancer Res. 2009;69:5226–5233. doi: 10.1158/0008-5472.CAN-08-4652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Thorpe JD, et al. Effects of blood collection conditions on ovarian cancer serum markers. PLoS One. 2007;2:e1281. doi: 10.1371/journal.pone.0001281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jones S, et al. Core signaling pathways in human pancreatic cancers revealed by global genomic analyses. Science. 2008;321:1801–1806. doi: 10.1126/science.1164368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Biankin AV, et al. Australian Pancreatic Cancer Genome Initiative Pancreatic cancer genomes reveal aberrations in axon guidance pathway genes. Nature. 2012;491:399–405. doi: 10.1038/nature11547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Waddell N, et al. Australian Pancreatic Cancer Genome Initiative Whole genomes redefine the mutational landscape of pancreatic cancer. Nature. 2015;518:495–501. doi: 10.1038/nature14169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lennon AM, et al. The early detection of pancreatic cancer: What will it take to diagnose and treat curable pancreatic neoplasia? Cancer Res. 2014;74:3381–3389. doi: 10.1158/0008-5472.CAN-14-0734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Xie M, et al. Age-related mutations associated with clonal hematopoietic expansion and malignancies. Nat Med. 2014;20:1472–1478. doi: 10.1038/nm.3733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jaiswal S, et al. Age-related clonal hematopoiesis associated with adverse outcomes. N Engl J Med. 2014;371:2488–2498. doi: 10.1056/NEJMoa1408617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Genovese G, et al. Clonal hematopoiesis and blood-cancer risk inferred from blood DNA sequence. N Engl J Med. 2014;371:2477–2487. doi: 10.1056/NEJMoa1409405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dong T, Liu CC, Petricoin EF, Tang LL. Combining markers with and without the limit of detection. Stat Med. 2014;33:1307–1320. doi: 10.1002/sim.6027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Capello M, et al. Sequential validation of blood-based protein biomarker candidates for early-stage pancreatic cancer. J Natl Cancer Inst. 2017;109:djx004. doi: 10.1093/jnci/djw266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Le Calvez-Kelm F, et al. KRAS mutations in blood circulating cell-free DNA: A pancreatic cancer case-control. Oncotarget. 2016;7:78827–78840. doi: 10.18632/oncotarget.12386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wilcox CM, et al. Chronic pancreatitis pain pattern and severity are independent of abdominal imaging findings. Clin Gastroenterol Hepatol. 2015;13:552–560, quiz e528–e559. doi: 10.1016/j.cgh.2014.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Frøkjær JB, Olesen SS, Drewes AM. Fibrosis, atrophy, and ductal pathology in chronic pancreatitis are associated with pancreatic function but independent of symptoms. Pancreas. 2013;42:1182–1187. doi: 10.1097/MPA.0b013e31829628f4. [DOI] [PubMed] [Google Scholar]
- 56.Bahuva R, Walsh RM, Kapural L, Stevens T. Morphologic abnormalities are poorly predictive of visceral pain in chronic pancreatitis. Pancreas. 2013;42:6–10. doi: 10.1097/MPA.0b013e318258cd9c. [DOI] [PubMed] [Google Scholar]
- 57.Herbst RS, Heymach JV, Lippman SM. Lung cancer. N Engl J Med. 2008;359:1367–1380. doi: 10.1056/NEJMra0802714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Thomas DS, et al. Evaluation of serum CEA, CYFRA21-1 and CA125 for the early detection of colorectal cancer using longitudinal preclinical samples. Br J Cancer. 2015;113:268–274. doi: 10.1038/bjc.2015.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Di Renzo MF, et al. Overexpression and amplification of the met/HGF receptor gene during the progression of colorectal cancer. Clin Cancer Res. 1995;1:147–154. [PubMed] [Google Scholar]
- 60.El-Tanani MK, et al. The regulation and role of osteopontin in malignant transformation and cancer. Cytokine Growth Factor Rev. 2006;17:463–474. doi: 10.1016/j.cytogfr.2006.09.010. [DOI] [PubMed] [Google Scholar]
- 61.Kalinich M, et al. An RNA-based signature enables high specificity detection of circulating tumor cells in hepatocellular carcinoma. Proc Natl Acad Sci USA. 2017;114:1123–1128. doi: 10.1073/pnas.1617032114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chari ST, et al. Probability of pancreatic cancer following diabetes: A population-based study. Gastroenterology. 2005;129:504–511. doi: 10.1053/j.gastro.2005.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.