Significance
Myelin is a potent inhibitor of axon regeneration. In the central nervous system, failure to clear myelin debris after injury presents a major roadblock to recovery. In contrast, rapid myelin clearance in the peripheral nervous system (PNS) contributes to this system’s remarkable regenerative capacity, but the mechanisms involved have remained incompletely understood. In this work, we set out to identify novel mechanisms of PNS myelin clearance to generate new ideas about activating myelin clearance in the injured CNS. We provide evidence that Schwann cells, myelinating glia of the PNS, engulf myelin debris using two receptors, Axl and Mertk. We hypothesize that astrocytes have the potential to use this same mechanism to engulf myelin debris after CNS injury.
Keywords: myelin, phagocytosis, Wallerian degeneration, Schwann cell, regeneration
Abstract
Ineffective myelin debris clearance is a major factor contributing to the poor regenerative ability of the central nervous system. In stark contrast, rapid clearance of myelin debris from the injured peripheral nervous system (PNS) is one of the keys to this system’s remarkable regenerative capacity, but the molecular mechanisms driving PNS myelin clearance are incompletely understood. We set out to discover new pathways of PNS myelin clearance to identify novel strategies for activating myelin clearance in the injured central nervous system, where myelin debris is not cleared efficiently. Here we show that Schwann cells, the myelinating glia of the PNS, collaborate with hematogenous macrophages to clear myelin debris using TAM (Tyro3, Axl, Mer) receptor-mediated phagocytosis as well as autophagy. In a mouse model of PNS nerve crush injury, Schwann cells up-regulate TAM phagocytic receptors Axl and Mertk following PNS injury, and Schwann cells lacking both of these phagocytic receptors exhibit significantly impaired myelin phagocytosis both in vitro and in vivo. Autophagy-deficient Schwann cells also display reductions in myelin clearance after mouse nerve crush injury, as has been recently shown following nerve transection. These findings add a mechanism, Axl/Mertk-mediated myelin clearance, to the repertoire of cellular machinery used to clear myelin in the injured PNS. Given recent evidence that astrocytes express Axl and Mertk and have previously unrecognized phagocytic potential, this pathway may be a promising avenue for activating myelin clearance after CNS injury.
Tight regulation of debris clearance is an essential and highly conserved process required for successful development as well as maintenance of homeostasis and immune tolerance in mature tissues (1). Defects in clearance of debris are associated with diverse pathologies including retinitis pigmentosa, chronic obstructive pulmonary disease and asthma, atherosclerosis, and Alzheimer’s disease (2–4). In addition to being required in developing and healthy tissues, efficient debris clearance is essential for successful tissue repair following injury, when large quantities of cellular debris accumulate over a short time span. The cell types responsible for debris clearance include professional phagocytes recruited from the circulation, such as macrophages, as well as less renowned tissue-resident phagocytes. Elucidating the molecular mechanisms used by cells to clear debris during health and after injury is a critical step toward understanding how debris clearance goes awry in disease.
A remarkable example of efficient debris clearance following injury is removal of myelin debris from the lesioned peripheral nerve. Following peripheral nerve injury, the nerve distal to injury degenerates through the process of Wallerian degeneration, producing a large quantity of myelin debris. Within only 2 to 3 wk after injury, the majority of this debris is cleared from the distal nerve (5). This rapid clearance of peripheral myelin debris is essential for successful axon regeneration and functional recovery, and yet the molecular mechanisms that underlie it remain incompletely understood (6–8). The postinjury scenario in the peripheral nerve is in stark contrast to that of the injured CNS, where myelin debris persists for months to even years after injury and is a major molecular roadblock to brain and spinal cord repair (9, 10). Given this contrast, we reasoned that furthering mechanistic understanding of peripheral myelin clearance would not only answer important outstanding questions in peripheral nerve biology, but may also provide insight into ways to promote myelin debris clearance in the injured CNS.
Myelin debris clearance in the injured peripheral nerve is a collaborative effort accomplished by multiple cell types. Our laboratory and others have shown that macrophages recruited into the nerve from peripheral circulation use complement- and Fc receptor-mediated mechanisms to help accomplish peripheral nervous system (PNS) myelin clearance. Accordingly, the abrogation of either of these pathways in macrophages leads to decreases in macrophage phagocytosis and delays in myelin clearance as well as impaired regeneration and functional recovery (7, 10). Two types of tissue-resident glial cells have also been recognized to contribute to myelin debris removal from the injured peripheral nerve: Schwann cells and perineurial cells (11–13). Despite recent progress toward a molecular understanding of the Schwann cell response to injury, our understanding of the Schwann cell-mediated mechanism of myelin removal has remained incomplete (14, 15).
Here we report efforts to enhance our mechanistic understanding of myelin removal by Schwann cells. To begin, we examine the time course of Schwann cell and macrophage contributions to this process to establish the time window during which Schwann cell-mediated myelin clearance is at its peak. We then investigate two clearance mechanisms, autophagy and phagocytosis, that might underlie Schwann cell clearance of myelin. Next, using data gleaned from RNA sequencing (RNAseq) analysis of Schwann cells acutely purified from the intact and injured peripheral nerve, we identify candidate phagocytic pathways that might be necessary for this process. Finally, we test our candidates using transgenic mice and in vitro and in vivo assays of Schwann cell myelin clearance.
Results
Time Course of Schwann Cell-Mediated Myelin Clearance.
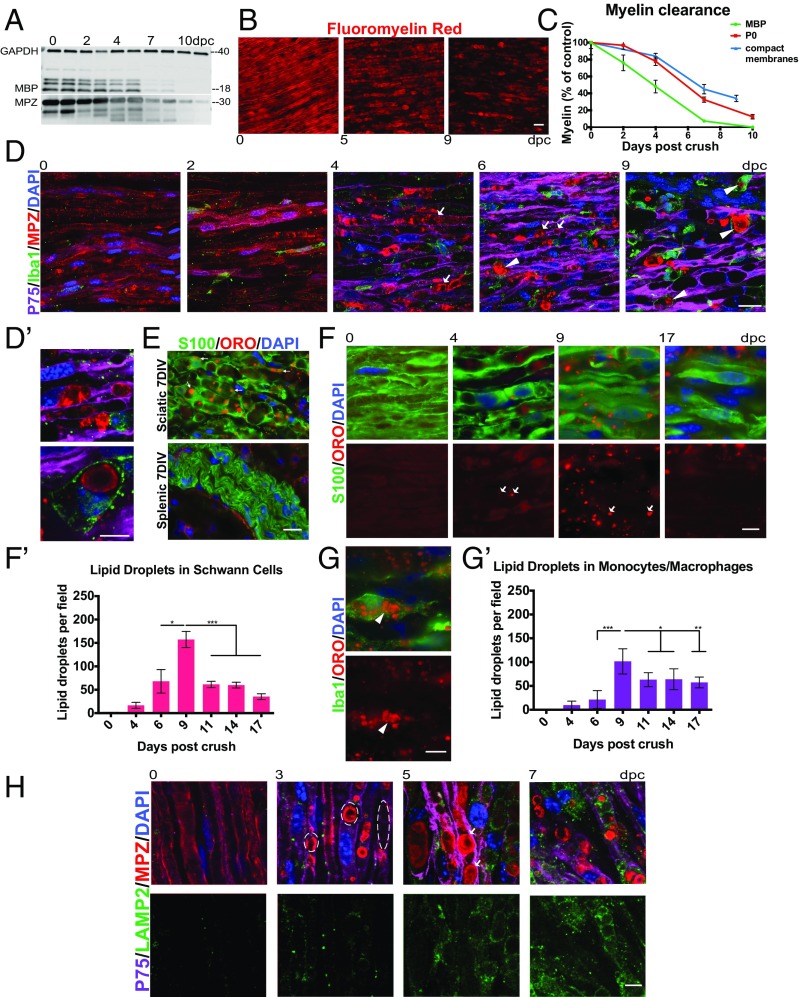
We first established the time frame of myelin clearance after PNS injury by measuring the quantity of residual myelin protein and compact membranes in the mouse sciatic nerve distal to the site of nerve crush injury at multiple time points. We measured the myelin proteins myelin protein zero (MPZ or P0) and myelin basic protein (MBP) using Western blotting of distal nerve lysates (Fig. 1A). Compact myelin membranes were measured by staining cryosections of the distal nerve with the lipophilic dye FluoroMyelin red (Fig. 1B). In agreement with previously published findings from our laboratory and others, we found that myelin clearance is already under way in the mouse peripheral nerve by 2 d after injury and is ∼70 to 80% complete by 8 to 10 d after injury (Fig. 1C).
Fig. 1.
Schwann cells and macrophages contribute to myelin clearance after nerve crush. (A) Western blot depicting myelin protein clearance from the crushed peripheral nerve. Each lane represents a separate sciatic nerve, and each well was loaded with the same amount of total nerve protein. (B) Representative images depicting myelin compact membrane clearance from the sciatic nerve visualized using FluoroMyelin dye (red). (Scale bar, 20 μm.) (C) Quantification of myelin protein and compact membrane clearance. n = 3 nerves for each FluoroMyelin time point. n = 5 nerves for each MBP and MPZ time point. All data are presented as mean ± SEM. (D) Confocal single-z-plane images of intact and degenerating whole-mount sciatic nerves at 0, 2, 4, 6, and 9 dpc stained with p75 (Schwann cells; purple), Iba1 (monocytes/macrophages; green), MPZ (myelin; red), and DAPI (nuclei; blue). Arrows indicate myelin that appears to be “inside” Schwann cells. Arrowheads indicate macrophages that have engulfed myelin debris. (Scale bar, 20 μm.) (D′) Higher-magnification image of a phagocytic macrophage at 9 dpc and Schwann cell association with myelin at 6 dpc. (Scale bar, 10 μm.) (E) Oil red O accumulation in sciatic and splenic nerves degenerated in vitro for 7 d. DIV, days in vitro. (Scale bar, 20 μm.) (F and G) Time course of oil red O lipid droplet accumulation in Schwann cells at 0, 4, 9, and 17 d after injury (F) and macrophages at 9 d after injury (G). Cryosections were stained with S100 (Schwann cells; green) and Iba1 (macrophages; green). Lipid droplets are red (oil red O). Nuclei are blue (DAPI). Arrows indicate lipid droplets in Schwann cells. Arrowheads indicate lipid droplets in macrophages. (Scale bars, 10 μm.) (F′ and G′) Quantification of lipid droplet accumulation in Schwann cells and macrophages from 0 to 17 dpc. n = 3 nerves and 6 fields of view for each time point. All data are presented as mean ± SEM. (H) Time course of lysosome accumulation in the sciatic nerve at 0, 3, 5, and 7 dpc. Whole-mount sciatic nerves are stained with p75 (Schwann cells; purple), MBP (myelin; red), LAMP2 (lysosomes; green), and DAPI (nuclei; blue). Dashed lines outline myelin ovoids. Arrows indicate myelin that appears to be inside Schwann cells. (Scale bar, 10 μm.) *P < 0.05, **P < 0.01, ***P < 0.001.
To elucidate the timing of individual cell-type contributions to peripheral myelin clearance, we assessed the myelin clearance activity of monocytes/macrophages and Schwann cells at 2, 4, 6, and 9 d after sciatic nerve crush. We first used immunohistochemistry (IHC) for MPZ in combination with macrophage/monocyte marker Iba1 and Schwann cell marker p75 to visualize clearance of myelin debris by both cell types. Throughout our immunohistochemical studies, Schwann cells were differentiated from perineurial cells, which are also p75- and S100-immunoreactive, by their location within the nerve, elongated cellular morphology, and characteristic association with axons and myelin (16). We interpreted the presence of myelin proteins inside of cells stained for Iba1 as evidence for myelin degradation by monocytes/macrophages. Monocyte/macrophage degradation of myelin debris was apparent beginning at 6 d after injury and was even more pronounced at 9 d after injury (Fig. 1D, arrowheads and Fig. 1D′, Top). In contrast, we found that immunostaining for p75 and myelin protein was insufficient to deduce Schwann cell-mediated degradation of myelin. This difficulty arose from the fact that the intact myelin sheath is already intimately associated with Schwann cell cytoplasm in the uninjured nerve, and from the fact that physical fragmentation of the myelin sheath by Schwann cells ∼2 d after nerve injury to form myelin ovoids results in large amounts of segmented myelin that appears within the Schwann cell but has not necessarily begun to be cleared or degraded by the cell (Fig. 1D, arrows and Fig. 1D′, Bottom).
To specifically detect Schwann cell degradation of myelin, we turned to the lipid dye oil red O (ORO), which stains droplets of neutral lipids that arise following degradation of the polar phospholipids of cell membranes (17). As expected, ORO brightly stains lipid droplets and only dimly stains intact myelin and myelin ovoids, allowing us to specifically identify and quantify lipid degradation (Fig. 1F). In our system, the ORO signal is specific to myelin degradation, since it was nearly absent from mostly nonmyelinated degenerating splenic nerves (Fig. 1E). A time course of ORO-positive droplet formation revealed that myelin lipid degradation products accumulate in Schwann cells beginning at 4 d after injury, peak around 9 d after injury, and subside significantly by 2 wk after injury (Fig. 1F′), allowing us to conclude that Schwann cells contribute most heavily to myelin clearance during this time window. ORO accumulation in macrophages followed a very similar time course, also peaking at 9 days post crush (dpc), consistent with our IHC observations (Fig. 1 G and G′). As further evidence for degradative activity, Schwann cell lysosome abundance, measured by immunohistochemistry using antibodies to LAMP2, also increased over the first week after nerve injury (Fig. 1H).
Lysosomes are interlinked with two main intracellular processes: endocytosis/phagocytosis and autophagy. We designed experiments to determine whether these mechanisms mediate Schwann cell clearance of myelin. Our first set of experiments aimed to assess whether Schwann cells require autophagy to degrade myelin after peripheral nerve crush injury.
Assessment of the Role of Autophagy in Myelin Debris Clearance.
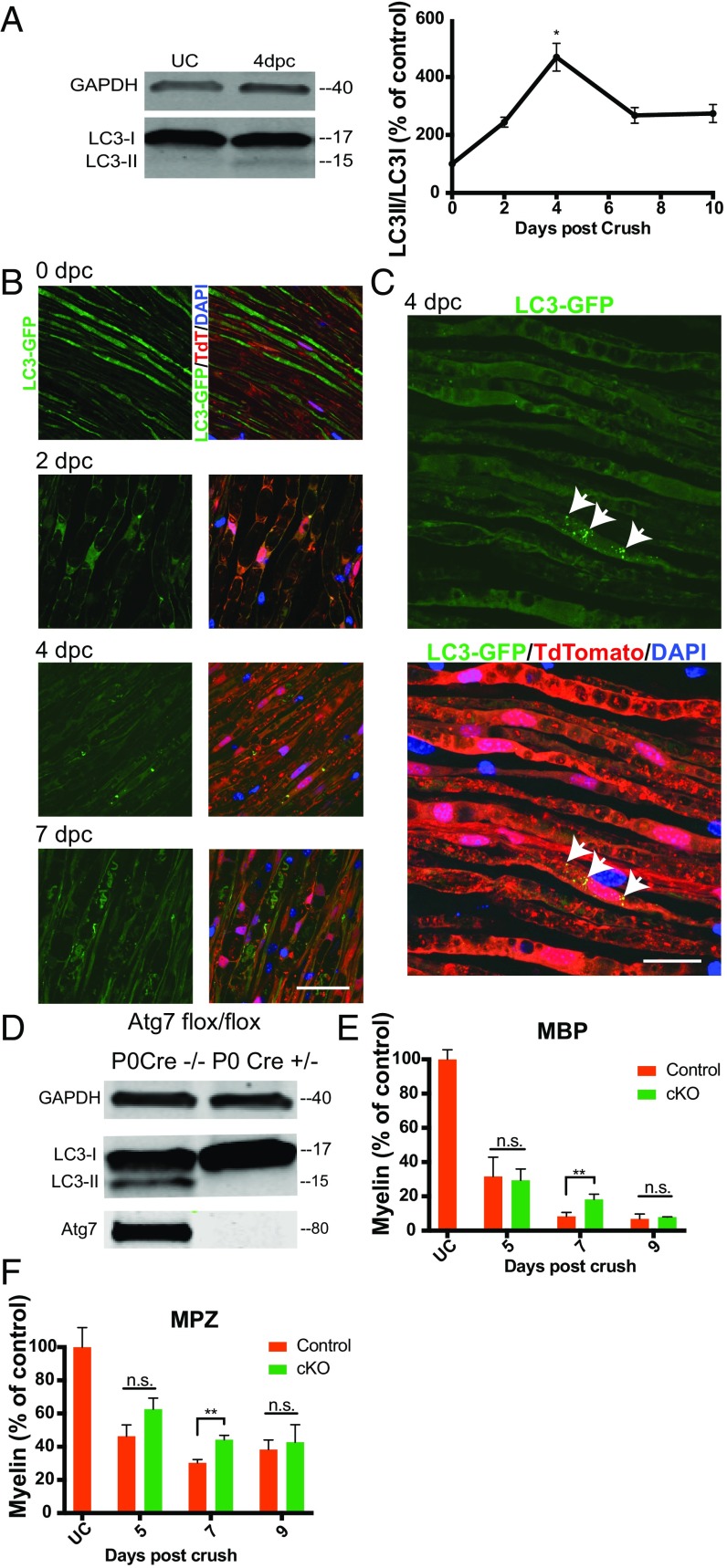
Lipidation of cytosolic mammalian myosin light chain 3 (LC3) to become LC3-phosphatidylethanolamine (LC3-PE) results in transient association of this protein with nascent autophagosomes and is critical for autophagosome formation (18). We established a time course of autophagosome formation after peripheral nerve crush by examining the ratio of unlipidated LC3 to LC3-PE at multiple time points using Western blotting, and found that this measure increased modestly after nerve crush, peaking at 4 d after injury (Fig. 2A).
Fig. 2.
Schwann cell autophagy contributes to myelin clearance after nerve crush. (A) Western blot of whole-sciatic nerve protein lysate at 0 (UC) and 4 dpc and graph of LC3II/LC3I values for the first 10 d post injury. Each Western blot lane represents a separate sciatic nerve, and each well was loaded with the same amount of total nerve protein. All data are presented as mean ± SEM. n = 3 nerves for each time point. (B) Representative confocal single-z-plane images of whole-mount sciatic nerves from LC3-GFP+/−;P0 Cre+/−;loxSTOPlox tdtomato flox/+ mice at 0, 2, 4, and 7 dpc. Autophagosomes are green (LC3-GFP), Schwann cells are red (tdtomato), and nuclei are blue (DAPI). (Scale bar, 50 μm.) (C) Maximum-intensity projection from a stack of confocal images of tissue at 4 dpc. Arrows denote autophagosomes in a Schwann cell. (Scale bar, 20 μm.) (D) Western blot of immunopanned Schwann cells purified 5 d after crush from Atg7 flox/flox;P0 Cre+/− and Atg7 flox/flox;P0 Cre−/− mice. Each lysate was prepared from all of the Schwann cells purified from two sciatic nerves. (E and F) Quantification of Western blots of residual myelin proteins MBP and MPZ in sciatic nerves from Atg7 flox/flox P0 Cre+/− mice. Protein lysates were generated from single nerves, and each Western blot well was loaded with the same amount of total nerve protein. n = 3 or 4 nerves per time point per genotype. cKO, Atg7 flox/flox,P0 Cre+/−; control, Atg7 flox/flox;P0 Cre−/−. Data are presented as mean ± SEM. n.s., not significant; *P < 0.05, **P < 0.01.
We next used mice expressing the GFP fusion protein LC3-GFP to determine what cell type was up-regulating autophagy and the frequency of autophagosome formation in the injured nerve. The LC3-GFP fusion protein allows the visualization of autophagosomes when LC3-PE associates with the autophagosome membrane, but is diffusely distributed throughout the cytoplasm in the absence of autophagy (19). We crossed these LC3-GFP mice to a line of mice expressing cytoplasmic tdtomato in Schwann cells (loxSTOPlox tdtomato × P0 Cre) to obtain mice with green autophagosomes and red Schwann cells. Examination of whole-mount sciatic nerves from these mice at 2, 4, and 7 d after injury revealed that autophagosome formation occurs in Schwann cells after sciatic nerve crush and reaches a maximum at 4 dpc, in agreement with the results of our Western blotting experiment (Fig. 2 B and C). Abundance of autophagosomes was highly variable, however, with most cells exhibiting only very sparse or no autophagosomes.
To test whether autophagy is necessary for Schwann cell degradation of myelin after nerve crush injury, as has recently been shown after nerve transection, we generated mice in which Schwann cells are unable to perform autophagy due to deletion of essential autophagy protein atg7 (floxed Atg7 × P0 Cre) (20–22). These mice did not display any obvious behavioral abnormalities before their use for experiments at 8 to 12 wk of age. Western blotting of sciatic nerve Schwann cells purified from these animals by immunopanning at 5 dpc indicated that conversion of LC3 to LC3-PE had been inhibited as expected (Fig. 2D). Western blot analysis of residual peripheral myelin proteins MPZ and MBP from these autophagy-deficient Atg7flox/flox;P0 Cre+/− and littermate control Atg7flox/flox;P0 Cre−/− nerves indicated a significant reduction in myelin clearance in sciatic nerves with autophagy-deficient Schwann cells at 7 d after crush injury. By 9 d after injury, this difference was no longer significant. The results of these experiments led us to conclude that autophagy contributes to Schwann cell-mediated clearance of myelin debris in mice after sciatic nerve crush. Autophagy appears insufficient to fully account for Schwann cell-mediated myelin degradation, however, given the paucity of autophagosomes observed in the postcrush nerve, in contrast with the abundance of ORO-positive lipid droplets within Schwann cells at 6 and 9 dpc.
Assessment of the Role of Schwann Cell Phagocytosis in Clearing Myelin Debris.
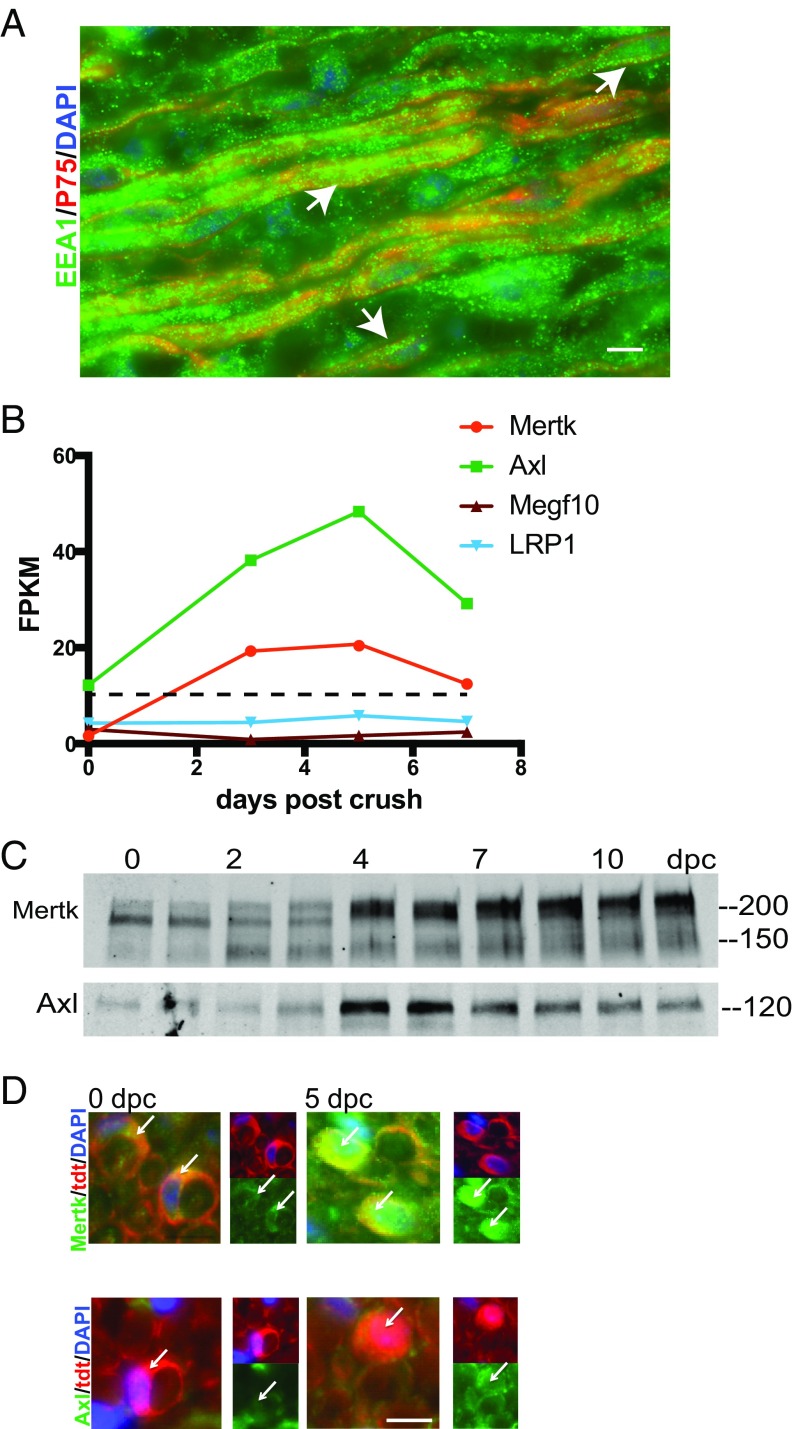
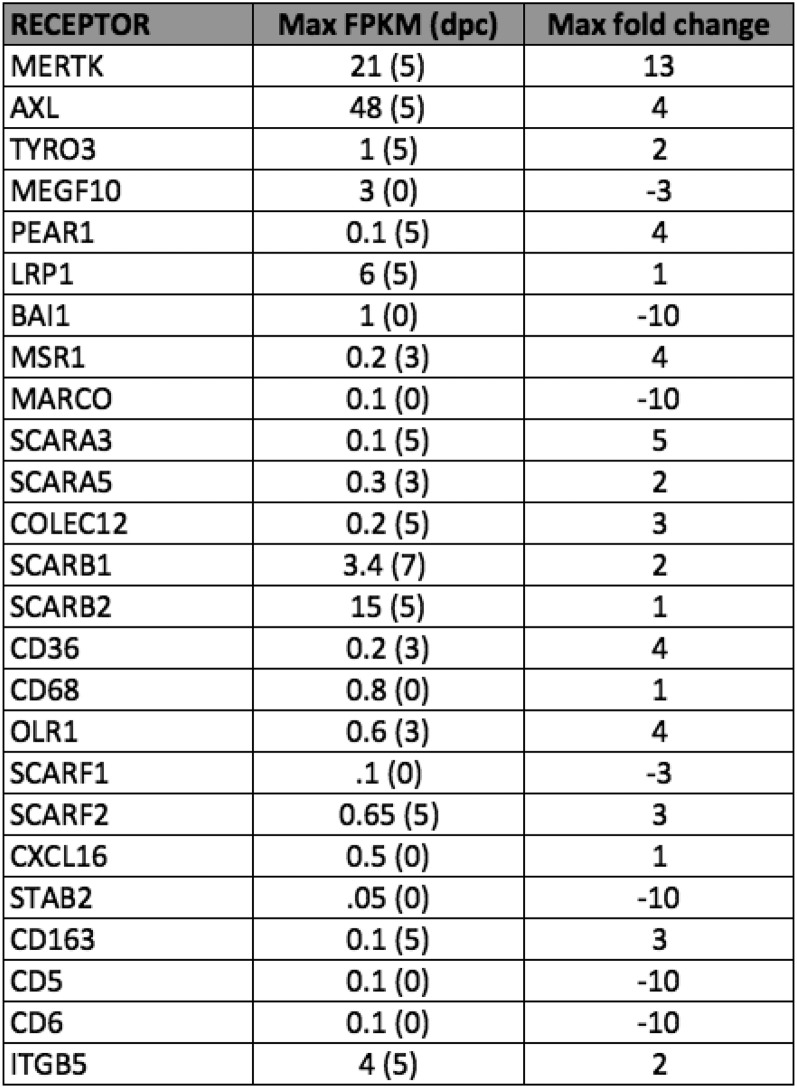
We wanted to take an unbiased look at alternate mechanisms Schwann cells might use to clear myelin. To begin this search, we used an RNAseq database recently generated by our laboratory that compares the transcriptomes of Schwann cells acutely purified from the intact and crushed rat sciatic nerve at 0, 3, 5, and 7 dpc. DAVID software analysis of our gene lists enabled us to take a first look at pathways enriched in Schwann cells after nerve injury (23, 24). Notably, the Gene Ontology term “endosome” was significantly enriched in our gene lists in comparison with the rat reference genome and enriched at increasing levels of significance over time after injury, with P values of 0.08 in the uncrushed nerve, 0.003 at 3 dpc, 0.007 at 5 dpc, and 0.001 at 7 dpc, suggesting that Schwann cells might use phagocytosis in addition to autophagy to clear myelin debris. Indeed, we found by immunohistochemistry using antibodies to endosome-specific protein EEA1 that endosomes are very abundant in Schwann cells after nerve crush injury (Fig. 3A). To our knowledge, there is no existing transgenic mouse that is globally deficient in phagocytosis. Therefore, to assess the necessity of phagocytosis for Schwann cell-mediated myelin clearance, we sought to identify candidate molecular pathways that we could then manipulate. We mined our RNAseq datasets for transcripts of known phagocytic receptors to identify specific pathways that might underlie Schwann cell phagocytosis of myelin debris. We selected candidate phagocytic receptors based on two criteria: up-regulation by twofold or more after nerve injury, and maximum fragments per kilobase million (FPKM) of at least 10. Two phagocytic receptors met both of these criteria: Axl and Mertk, both members of the TAM family of receptor tyrosine kinases with known roles in debris clearance in other systems (Fig. S1 and Fig. 3B) (3, 25). Interestingly, Megf10 and LRP1, both phagocytic receptors with known phagocytic functions in other types of glia, were expressed at FPKM <6 (25, 26). We confirmed up-regulation of these receptors in the injured mouse sciatic nerve at the protein level by Western blotting and specifically in Schwann cells by using immunostaining (Fig. 3 C and D).
Fig. 3.
Schwann cells up-regulate the phagocytic receptors Axl and Mertk after nerve crush. (A) Representative IHC image of a sciatic nerve 9 dpc stained for endosomes (EEA1; green), Schwann cells (p75; red), and nuclei (DAPI; blue). (Scale bar, 10 μm.) Arrows indicate regions of colocalization of EEA1 and p75 immunoreactivity. (B) Graphical illustration of the time course of expression of select phagocytic genes in acutely purified rat Schwann cells at 0, 3, 5, and 7 dpc. The dotted line represents an average FPKM of 11 for the dataset. FPKM values are averaged across two samples for each time point. (C) Western blot showing up-regulation of Mertk and Axl protein after mouse sciatic nerve crush. Protein lysates were generated from single nerves, and each Western blot well was loaded with the same amount of total nerve protein. (D) Cross-sections of loxSTOPlox tdtomato flox/+;P0 Cre+/− mouse sciatic nerve at 0 and 5 dpc stained by IHC with antibodies to Mertk and Axl (green) and DAPI. Arrows highlight Schwann cells in each field. (Scale bar, 10 μm.)
Fig. S1.
Table depicting rat Schwann cell expression of phagocytic receptors before and after sciatic nerve crush. Data obtained from RNAseq analysis of acutely purified P18 rat Schwann cells at 0, 3, 5, and 7 dpc. Schwann cells were purified according to ref. 39. Table indicates maximum FPKM across the time points sampled and timing of peak RNA abundance (in dpc) as well as fold change of FPKM relative to the uncrushed state. FPKM values represent average of two replicates for each time point.
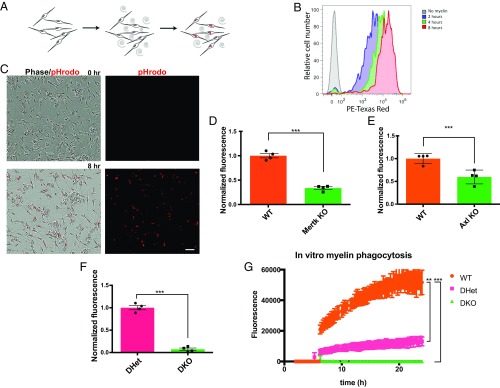
Are Axl and Mertk required for Schwann cell phagocytosis of myelin? We first assessed this question in primary culture. We began by purifying myelin from peripheral nerves and labeling it with the pH-sensitive dye pHRODO, which fluoresces brightly in acidic cellular compartments such as lysosomes but exhibits only dim fluorescence in the cytosol. When purified wild-type Schwann cells isolated from injured sciatic nerves were fed pHRODO-labeled myelin, the cells readily engulfed the labeled debris and exhibited pHRODO fluorescence. Although outside the realm of this study, Schwann cells were also observed to cluster over several hours of incubation with myelin debris, suggesting migration toward debris over time. We could quantify the level of pHRODO fluorescence per cell using flow cytometry (FACS) analysis following trypsinization of the Schwann cells or per field using live-cell microscopy (Fig. 4 A–C). We performed our FACS-based in vitro phagocytosis assay using Schwann cells purified from the sciatic nerves of WT mice and mice lacking Axl or Mertk. The results of these experiments revealed that in comparison with Schwann cells purified from wild-type littermate controls, Schwann cells lacking both copies of Axl or Mertk exhibited significant 40–50% defects in myelin phagocytosis (Fig. 4 D and E). Based on these findings, we were very interested in knowing if Schwann cells lacking both Axl and Mertk would exhibit an even greater defect in phagocytic ability. Indeed, double-mutant Schwann cells were almost completely unable to phagocytose myelin debris (Fig. 4F). We confirmed these data using live-cell microscopy of Axl/Mertk WT, double-heterozygous (DHet), and double-mutant (DKO) Schwann cells over a 24-h period of coincubation with pHRODO-labeled myelin debris (Fig. 4G). Interestingly, this experiment revealed a significant defect in the phagocytic ability of Axl/Mertk DHet Schwann cells relative to WT controls, suggesting haploinsufficiency of these receptors, at least in the in vitro setting.
Fig. 4.
Schwann cells use Axl and Mertk to clear myelin debris in vitro. (A) Schematic illustration of a Schwann cell in vitro myelin phagocytosis assay. (B) Representative FACS tracing of Schwann cells analyzed by flow cytometry after no exposure and 2-, 4-, and 8-h exposure to pHRODO-labeled myelin. (C) Representative live-cell images of Schwann cells before and 8 h following addition of pHRODO-labeled myelin to culture media. (Scale bar, 50 μm.) (D–F) Results of an in vitro phagocytosis assay performed on Schwann cells purified from Axl−/−, Mertk−/−, and Axl/Mertk double-mutant Schwann cells and their littermate controls. Cells were purified from sciatic nerves 6 d after crush. Myelin phagocytosis was quantified using flow cytometry 2 to 3 h after addition of pHRODO-labeled PNS myelin debris. n = 4 for each genotype. Data are presented as mean ± SEM. (G) Quantification of integrated fluorescence per live-cell imaging field of Schwann cells coincubated with pHRODO-labeled myelin at 2 to 24 h after addition of myelin. n = 3 for each genotype: wild type, double heterozygote, and double knockout. Data are presented as mean ± SEM. **P < 0.01, ***P < 0.001.
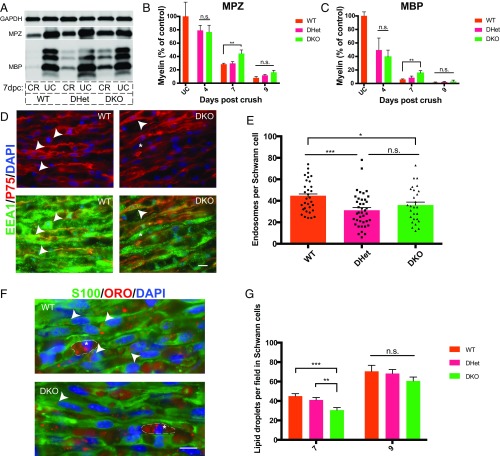
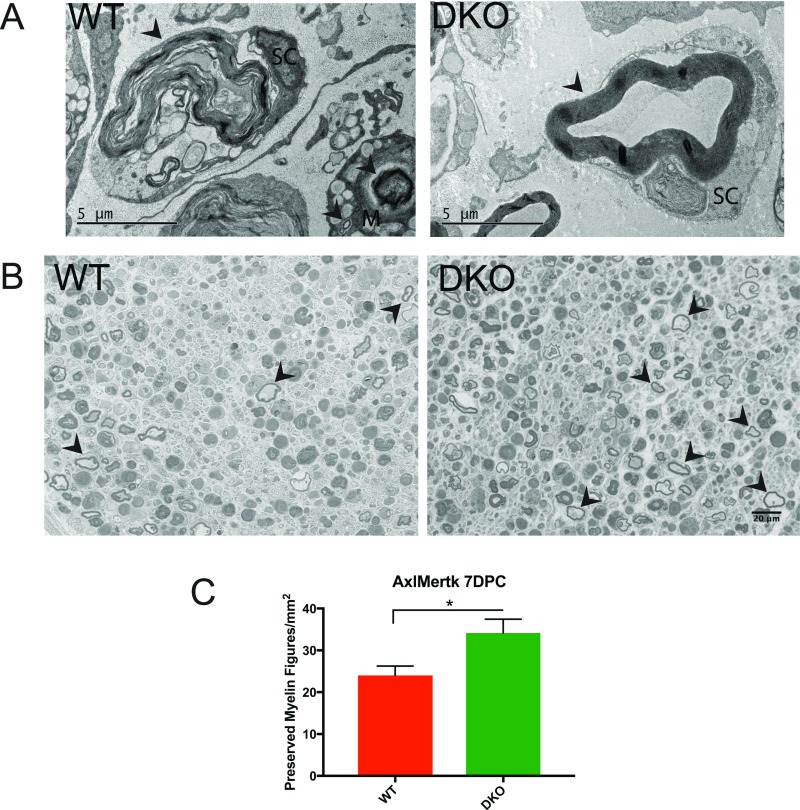
We next tested the necessity of the Axl and Mertk pathways for myelin clearance after peripheral nerve injury in vivo. We quantified residual myelin proteins MPZ and MBP 7 and 9 d after crush in sciatic nerves from Axl−/−;Mertk−/− (DKO) and Axl+/−;Mertk+/− (DHet) littermates as well as wild-type controls. As observed in our autophagy experiments, we found a significant decrease in myelin protein clearance in nerves lacking both Axl and Mertk at 7 dpc in comparison with Axl/Mertk WT nerves (Fig. 5 A–C). To further characterize this phenotype, we examined myelin morphology in WT and DKO nerves at 7 dpc using electron microscopy (EM), reasoning that if Axl/Mertk mutant Schwann cells were unable to engulf myelin debris, myelin in these mutant nerves would persist in a more preserved state than in WT injured nerves. By EM, myelin in both WT and DKO nerves existed at various stages of degradation at 7 dpc, with highly degraded myelin figures localized within vacuoles of Schwann cells and macrophages, consistent with previous ultrastructural studies of Wallerian degeneration (27). Upon close examination, we noted that preserved, nonvacuolar myelin figures, each defined as a single ring of closely opposed myelin membranes (no onion bulb appearance) not located within an intracellular vacuole, appeared more abundant in DKO nerves in comparison with WT nerves (Fig. S2A). This phenotype was statistically significant when quantified using toluidine blue-stained thick sections by a blinded observer (Fig. S2 B and C). Without 3D reconstruction EM, we were unable to determine with certainty whether these preserved myelin figures were extracellular or associated with a Schwann cell nucleus out of the plane of section. These data corroborated our Western blot findings and provided additional evidence for a delay in degradation of myelin in Axl/Mertk double-mutant nerves.
Fig. 5.
Schwann cells use Axl and Mertk to clear myelin debris in vivo. (A) Representative Western blot showing myelin protein (MBP and MPZ) levels in wild-type, Axl/Mertk double-heterozygous, and Axl/Mertk double-knockout mouse sciatic nerves at 0 and 7 dpc. Protein lysates were generated from single nerves, and each Western blot well was loaded with the same amount of total nerve protein. CR, crushed; UC, uncrushed. (B and C) Quantification of MPZ- and MBP-stained Western blots of protein lysates from WT, Axl/Mertk double-heterozygous, and Axl/Mertk double-knockout mouse sciatic nerves at 0, 4, 7, and 9 dpc. n = 3 to 10 per genotype per time point. Data are presented as mean ± SEM. (D) Representative IHC images of cryosections of 9-dpc WT and Axl/Mertk DKO mouse sciatic nerves stained with antibodies to EEA1 (endosomes) and p75 (Schwann cells). Arrowheads indicate colocalization of EEA1 and p75, while asterisks indicate EEA1 immunoreactivity in a p75-negative cell, presumably a macrophage. (Scale bar, 10 μm.) (E) Quantification of IHC images of EEA1- and p75-labeled Schwann cells. Graph of average endosome abundance (EEA1 puncta) per Schwann cell at 9 dpc in Schwann cells from WT, Axl/Mertk double-heterozygous, and Axl/Mertk double-knockout mice. Eight cells were blindly selected and analyzed per animal. n = 4 or 5 animals per genotype. Data are presented as mean ± SEM. (F) Representative IHC images of 7-dpc cryosections of WT and Axl/Mertk DKO mouse sciatic nerves stained with ORO (lipid droplets) and antibodies to S100 (Schwann cells). Arrowheads indicate ORO within an S100-positive cell, while asterisks indicate ORO-positive droplets in S100-negative cells, presumably macrophages. (Scale bar, 10 μm.) (G) Quantification of ORO-positive lipid droplet abundance per field at 7 and 9 dpc in blindly selected and analyzed fields of IHC-stained tissue from WT, Axl/Mertk double-heterozygous, and Axl/Mertk double-knockout mice. n = 4 to 10 animals per genotype per time point. Four images were analyzed per animal. Data are presented as mean ± SEM. *P < 0.05, **P < 0.01, ***P < 0.001.
Fig. S2.
Loss of Axl/Mertk leads to retention of preserved myelin figures. (A) Electron microscopy images taken of wild-type (WT) and Axl/Mertk double-knockout (DKO) mouse sciatic nerves at 7 dpc. Arrowheads indicate myelin at various stages of degradation within or in close association with Schwann cells (SC) and macrophages (M). Note the basal lamina surrounding the Schwann cells. The DKO image features a preserved myelin figure, found to be significantly more abundant in DKO nerves than in WT nerves. (Scale bar, 5 μm.) (B) Representative images of toluidine blue-stained 1-μm sections of WT and DKO nerves at 7 dpc. Arrowheads indicate examples of preserved myelin figures (nonexhaustive labeling). (Scale bar, 20 μm.) (C) Quantification of preserved myelin figures in WT and DKO nerves at 7 dpc. n = 3 animals for each genotype. Two images were analyzed per animal. Data are presented as mean ± SEM. *P < 0.05.
To confirm that reduced myelin clearance in Axl/Mertk DKO nerves was Schwann cell-mediated, we compared the number of oil red O droplets as well as the number of endosomes in Axl/Mertk WT, double-heterozygous, and double-knockout Schwann cells post injury (Fig. 5 E–G). We measured endosome abundance using an antibody to EEA1, an endosome-specific protein. Schwann cells in Axl/Mertk double-knockout nerves exhibited reduced numbers of both oil red O droplets as well as endosomes in comparison with the Schwann cells of WT controls. In our endosome experiment, we again saw evidence for potential haploinsufficiency of Axl and Mertk, this time in vivo, as endosome number was also significantly reduced in DHet Schwann cells relative to WT controls. These experiments demonstrated that Schwann cells use Axl and Mertk to clear myelin debris in vivo.
Discussion
The Peak Periods of Schwann Cell- and Macrophage-Mediated Myelin Clearance Are More Coincident than Previously Believed.
Previously, myelin clearance in the injured peripheral nerve had been thought to occur in two stages: an initial Schwann cell-mediated period of clearance 0 to 6 d after injury, followed by a second, macrophage-mediated period of clearance. This picture of myelin clearance is based on studies by our laboratory and others demonstrating that knockdown of specific pathways involved in macrophage-mediated clearance results in a delay in myelin clearance, but only from day 5–6 post injury onward (6, 7). Here we show that although Schwann cells partition their myelin sheath to form myelin ovoids during the first 2–3 d after injury, intracellular generation of lipid droplets in Schwann cell (SC) cytoplasm follows a very similar time course as the appearance of lipid droplets in macrophages, beginning at ∼4 d after injury and peaking at 9 d post injury (Fig. 1).
Schwann Cells Use Autophagy to Clear Myelin Debris.
Descriptive studies have long postulated the involvement of autophagy and/or phagocytosis in myelin clearance by Schwann cells (28, 29). Indeed, the intimate association of myelin with Schwann cell cytoplasm both before and after injury makes autophagy an attractive mechanism for myelin clearance. Alternatively, the involvement of macrophages in myelin phagocytosis implies accessibility of myelin debris to phagocytic cell types, making phagocytosis by Schwann cells a plausible mechanism for clearance as well. Here we have provided evidence that both of these processes contribute to Schwann cell-mediated myelin clearance. Gomez-Sanchez et al. recently demonstrated that Schwann cells use autophagy to degrade myelin after nerve transection, a process they coined “myelinophagy” (22). These findings were supported in a subsequent publication by Jang et al. (20). Given the known association between nutrient restriction and autophagy and the more drastic disruption in distal nerve blood supply caused by transection injury in comparison with crush injury, we wondered if these findings would apply in a nerve crush scenario as well (30). Our data corroborate these studies showing the involvement of autophagy in Schwann cell-mediated myelin clearance and validate this mechanism in a crush model of nerve injury (Fig. 2). However, our findings also indicate that autophagy alone does not account for all of the myelin clearance activity exhibited by Schwann cells after nerve crush.
Schwann Cells Help to Clear Myelin Debris by the Axl/Mertk Phagocytic Pathways.
Using a Schwann cell RNAseq transcriptome to identify candidate phagocytic pathways involved in myelin clearance, we have shown that two phagocytic receptors, Axl and Mertk, are up-regulated by Schwann cells after injury and contribute to Schwann cell clearance of myelin in vitro and in vivo (Figs. 3–5). The TAM family of receptors is well-known for its involvement in phagocytosis by tissue-resident phagocytes: by retinal pigment epithelial cells in the retina, Sertoli cells in the testis, and astrocytes in the brain (25, 31). Our study adds a cell type, debris type, and tissue setting to this list and raises several issues for consideration.
First, we show that both Schwann cell autophagy and Schwann cell phagocytosis contribute to myelin clearance after nerve injury. In vivo, however, the impact of each of these mechanisms on the amount of myelin debris remaining in the injured nerve is constrained to a relatively short time window which begins around a week after injury and ends by 9 d post injury (Fig. 5). It is very likely that the role of these pathways in our mutant mice is masked by compensatory increased phagocytosis by macrophages recruited into the nerve after injury. We conducted preliminary studies to test this hypothesis using clodronate liposomes to reduce macrophage number (32). These studies were unable to test our prediction due to an inability to reduce macrophage number more than 50% using this method and an observed significant reduction in Schwann cell phagocytosis in wild-type clodronate-treated animals versus untreated animals. The fact that clodronate treatment, itself, led to reduced myelin clearance by Schwann cells raises the possibility that macrophages are required for Schwann cells to attain their full phagocytic potential. Indeed, our RNAseq transcriptomes indicate that Gas6, one of the bridging molecules required for TAM receptor function, is highly expressed by macrophages isolated from the injured peripheral nerve. Other possible compensatory mechanisms include activation of alternate Schwann cell pathways and increased clearance by yet other cell types such as perineurial cells (11). The redundancy of multiple cellular and molecular mechanisms to mediate myelin clearance in the injured peripheral nerve highlights the complexity of this process and its importance for successful nerve repair. In future experiments, it would be interesting to observe the effect on myelin clearance in a system where Schwann cells are unable to perform both phagocytosis and autophagy. Additionally, it may be the case that autophagy and/or TAM-mediated phagocytosis contribute to regulating myelin turnover and maintenance in the healthy peripheral nerve, which has only a very sparse population of resident macrophages.
A fascinating area of future study is understanding how myelin, which is protected from attack by phagocytic cells in the healthy nerve, becomes a phagocytic target after injury. At a structural level, one possibility is that following myelin ovoid formation, myelinating Schwann cells shed their myelin into the extracellular space to be engulfed by debris-scavenging phagocytes. Another possibility is that phagocytes play an active role in promoting release of myelin from intact myelin sheaths by inducing local out-foldings in the myelin sheath akin to the myelinosome formation recently reported by Romanelli et al. in a mouse model of multiple sclerosis (33). In this latter scenario, myelin may never be apparent in the extracellular milieu, since it would be directly pinched off from the intact myelin sheath by phagocytic processes. At a molecular level, “eat me” signals on the surface of myelin may be exposed or up-regulated after injury, leading to interaction with phagocytic receptors and promoting engulfment, as is observed in clearance of apoptotic cells (2).
Finally, the specificity of Axl and Mertk receptors for myelin debris among the multiple kinds of cellular debris present in the postinjury nerve has yet to be thoroughly investigated. Schwann cells may use these receptors to clear axonal as well as myelin debris. Alternatively, macrophages rather than Schwann cells may clear axon debris after injury. Using our growing knowledge of the molecular interactions that enable phagocytes to engage with targets, it will be very interesting to understand how specific cell types and molecular pathways are coordinated to accomplish debris clearance in the peripheral nerve (1, 2).
Myelin Phagocytosis Pathways May Be Dysfunctional After CNS Injury.
Why CNS glia fail to clear myelin debris after CNS injury is a critically important question. Persistent myelin debris in the injured CNS milieu is one of the major obstacles to successful CNS repair (34). Unlike Schwann cells, oligodendrocytes, the myelinating glia of the CNS, do not express phagocytic machinery and do not contribute to myelin debris clearance (22, 34, 35). Macrophages do not enter the distal segment of axotomized CNS axon pathways, as the blood–brain barrier does not break down and microglia appear capable of only a limited degree of myelin debris clearance (10, 36). Recent work from our laboratory, however, has shown that mouse astrocytes express phagocytic pathways and that they phagocytose synaptic debris in the developing and adult brain using Mertk, Megf10, and LRP1 and robustly phagocytose myelin debris in vitro (25, 37). Why then, are astrocytes unable to phagocytose myelin debris after CNS injury? Gene profiling of reactive astrocytes following CNS injury demonstrates that many components of these phagocytic pathways are down-regulated and that after CNS axotomy a type of reactive astrocyte is generated that has little ability to phagocytose (37, 38). As we continue to uncover the Schwann cell-mediated mechanism of myelin debris clearance, it will be interesting to use our findings to diagnose specific deficiencies in myelin clearance pathways after CNS injury and to devise therapeutic strategies for activating this process.
Materials and Methods
Immunohistochemistry and Fluorescent Dye Labeling.
Nerves were paraformaldehyde (PFA)-fixed for 4 h on ice, cryopreserved with 30% sucrose in PBS overnight (4 °C), embedded in 2:1 OCT:30% sucrose, and sliced (10 to 12 μm) as longitudinal or cross-sections. Slides were dried at 60 °C, placed in PBS, blocked for 1 h [room temperature (RT), in 10% serum/0.2% Triton X-100 in PBS], and incubated with primary antibodies overnight (4 °C, in 10% serum/0.2% Triton X-100 in PBS). They were then washed with PBS, given secondary antibodies for 1 h (RT, in 10% serum/0.2% Triton X-100 in PBS), washed in PBS, and coverslipped in Vectashield with DAPI (Vector Laboratories).
For sciatic nerve whole mounts, nerves were PFA-fixed for 4 h on ice. Each nerve’s perineurium was carefully removed with forceps, permeabilized with methanol on dry ice, and then blocked with 10% serum/1% Triton X-100/PBS overnight at 4 °C. Nerves were incubated with primary antibodies in 10% serum/1% Triton X-100/PBS for 48 h (4 °C), washed with PBS/1% Triton X-100, and then incubated with secondary antibodies in 10% serum/1% Triton X-100/PBS for 48 h (4 °C). After incubation with secondary antibodies, nerves were washed with 1% Triton X-100/PBS, put through 25%, 50%, and 75% glycerol in PBS (>6 h each, 4 °C), and then mounted. Oil red O staining (Abcam; ab150678) was performed according to the manufacturer’s instructions. FluoroMyelin staining was performed with FluoroMyelin Red Fluorescent Myelin Solution (Life Technologies) according to the manufacturer’s instructions. The following antibodies were used for immunostaining: rabbit anti-S100 (Dako; 1:100), goat anti-P75NTR (Neuromics; 1:500), rabbit anti-Iba1 (Wako; 1:300), rabbit anti-EEA1 (Abcam; 1:200), chicken anti-MPZ (Millipore; 1:100), rat anti-LAMP2 (Abcam; 1:100), goat anti-Axl (R&D Systems; 1:100), and goat anti-Mertk (R&D Systems; 1:100). Secondaries used were donkey or goat Alexa Fluor 488/594/568/405 (highly cross-absorbed; Invitrogen)-conjugated and used at 1:1,000 on cryosections and at 1:500 for whole mounts.
For staining of splenic and sciatic nerves degenerated in vitro, following removal of epineurium and superfluous connective tissue, nerves were cut into 5-mm segments, transferred to DMEM supplemented with 5% FBS, and incubated at 37 °C/5% CO2 until fixation and staining as described above.
Western Blotting.
On dry ice, nerves were ground using ReadyPrep Mini Grinders (Bio-Rad) for 15 s. RIPA (200–400 μL) with cOmplete Ultra tablets (Roche) was added, and samples were ground for another 30 s and then centrifuged (16,000 × g, 4 °C). For Western blots of whole-nerve lysates, protein concentration was determined using a BCA Kit (Pierce), and equal amounts of total protein were loaded per well. For Western blots of immunopanned Schwann cell lysate, cells were lysed by addition of RIPA with cOmplete Ultra tablets (Roche) and the entire lysate was loaded onto the gel. Samples were run through an SDS/polyacrylamide gel and transferred onto PVDF membranes (Immobilon-FL 0.45 μm; Millipore). Blots were washed, dried (RT, overnight), rehydrated, blocked (Odyssey buffer; LI-COR), and incubated with relevant primary and secondary (IRDye 680LT/800CW; LI-COR) antibodies. The following primary antibodies were used for Western blotting experiments: rabbit anti-LC3 (Novus Biologicals; 1:1,000), rabbit anti-ATG7 (Cell Signaling Technology; 1:750), chicken anti-GAPDH (ProSci; 1:1,000), mouse anti-MPZ (obtained as a gift from Juan J. Archelos, University of Graz, Graz, Austria; 1:10,000), rat anti-MBP (Abcam; 1:1,000), goat anti-Axl (R&D Systems; 1:1,000), and goat anti-Mertk (R&D Systems; 1:1,000). Western blots were labeled with IRDye 680LT/800CW secondary antibodies (LI-COR; 1:20,000).
In Vitro Schwann Cell Phagocytosis Assay.
Schwann cells were purified 6 d after sciatic nerve crush from 8- to 13-wk-old mice according to the methods described in ref. 39. Cells were plated at equal density into wells of a 96-well plate. The following day, cells in each well were rinsed once with Dulbecco's phosphate-buffered saline (DPBS) and fed media supplemented with 5% FCS and 1 μL pH-sensitive dye (pHRODO)–labeled crude PNS myelin purified according to ref. 37. Myelin was quantified using the BCA assay and used at a protein concentration of 800 mg/mL. For flow cytometry analysis, Schwann cells were trypsinized after incubation at 37 °C/5% CO2 for 2–3 h and resuspended in 30% FCS on ice. Data were collected at the Stanford Shared FACS Facility obtained using the facility’s LSR II.UV. SCs were identified by forward scatter, side scatter, and viability (DAPI) gating. Mean PE Texas red fluorescence (pHRODO) was calculated for each cell sample. For live-cell imaging, cells were incubated at 37 °C/5% CO2 and each well was imaged at 15-min intervals using an IncuCyte Zoom Live-Cell Analysis System (Essen BioScience). Integrated fluorescence intensity was quantified for each well. Images displaying no fluorescence due to evident error in automated focal plane were excluded from analysis.
Mice.
C57BL/6 mice were obtained from Charles River. Mertk and Axl mutant mice were obtained from Greg Lemke at The Salk Institute, San Diego, CA. Atg7f/f mice were obtained from David Sulzer at Columbia University, New York, NY, with consent from Masaaki Komatsu at the Tokyo Metropolitan Institute of Medical Science, Tokyo, Japan. LC3-GFP mice were obtained from Thomas Rando at Stanford University with consent from Noboru Mizushima, RIKEN BRC, Ibaraki, Japan. P0 Cre and loxSTOPlox tdtomato mice were obtained from JAX (stock nos. 017927 and 007914). Animals were housed and handled in accordance with the guidelines of the Administrative Panel on Laboratory Animal Care of Stanford University. Unless noted otherwise, all mice were 8–13 wk of age at the time of the experiment, and comparisons between genotypes were made between matched littermate controls. For experiments involving Axl/Mertk mutant mice, double-knockout animals were matched with littermate double-heterozygous controls. WT controls for these experiments were age-matched only (to the closest week). For electron microscopy experiments, double-knockout animals were age-matched only (to the closest week) with WT controls.
Statistical Analysis.
All measurements in this study are presented as means ± SEM. Significance was determined with two-tailed unpaired Student’s t test, and P < 0.05 was considered significant. *P < 0.05, **P < 0.01, ***P < 0.001. For analysis of Axl/Mertk live-cell imaging data, a two-way ANOVA with Tukey test was performed.
Sciatic Nerve Crush.
All surgical experiments were performed under 2.5% isoflurane. Sciatic nerve crush injury was performed as previously described (40). Briefly, the sciatic nerve was exposed at midthigh level on the left side of the animal and crushed with smooth forceps for 30 s. The upper thigh was shaved and sterilized using isopropanol. A 1-cm incision was made using a scalpel, and the nerve was visualized via blunt dissection using forceps. The left sciatic nerve was crushed at midthigh for 10 s using forceps marked with sterile graphite to mark the crush site. Carprofen (5 mg/kg s.c.) was administered for analgesia.
Electron Microscopy Image Acquisition and Analysis.
Transmission electron microscopy of sciatic nerves was performed in conjunction with the Stanford Cell Sciences Imaging Facility supported by NIH 1S10RR02678001. Sciatic nerves were processed essentially as described for optic nerves (41). Briefly, mice were killed by CO2 inhalation. Sciatic nerves were dissected out and postfixed in cold Karlsson–Schultz fixative (2.5% glutaraldehyde, 4% PFA in phosphate buffer, pH 7.3) at 4 °C, treated with 2% osmium tetroxide in cold Karlsson–Schultz fixative, serially dehydrated, and embedded in EMbed 812 (EMS; 14120); 85-nm sections were transferred onto formvar/carbon-coated 50-mesh copper grids and stained for 30 s in 3% uranyl acetate in 50% acetone followed by staining for 3 min in 0.2% lead citrate. Images were acquired with a JEOL 1400 transmission electron microscope; 1-μm sections were stained with toluidine blue and imaged with a Zeiss Axio Imager M1. For quantification of preserved myelin figures in toluidine blue-stained sections, preserved myelin figures were counted for two 40× images of each nerve. Analysis of toluidine blue sections was blinded to genotype.
Epifluorescent Image Acquisition and Analysis.
Epifluorescence images were taken with a Zeiss Axio Imager M1. Confocal images were taken with an LSM 710 confocal microscope at the Stanford Neuroscience Microscopy Service, supported by NIH NS069375. Live-cell images were taken using the IncuCyte Zoom Live-Cell Analysis System (Essen BioScience). Image analysis was carried out using Fiji ImageJ and Image Studio. Western blots were imaged with the Odyssey CLx Imaging System (LI-COR) in 700- and 800-nm channels.
Acknowledgments
We thank members of the B.A.B. laboratory, notably Mariko Bennett and Anja Scholze, for discussions and support. We also thank Greg Lemke for helpful comments on experimental design, and David Parkinson and Xin-Peng Dun for advice on the whole-mount staining protocol. This work was supported by the Dr. Miriam and Sheldon G. Adelson Medical Research Foundation (B.A.B.), Christopher and Dana Reeve Foundation (B.A.B.), NIH Grants EY11310, NS069375, 1S10RR02678001, and NIH S10 Shared Instrument Grant S10RR027431-01 (to B.A.B.), NIH Grant T32 HD007249 Developmental and Neonatal Biology Training Program (to A.B.L.), National Institute of Mental Health Grants T32GM007365 and F30MH106261, and Bio-X Predoctoral Fellowship (to S.A.S.), and the Stanford Medical Scientist Training Program and School of Medicine (A.B.L.). W.-S.C. is supported by National Research Foundation of Korea (NRF) Grants NRF-2016M3C7A1905391 and NRF-2016R1C1B3006969 funded by the Korean government (MSIP). J.B.Z. is a Career Transition Award Fellow of the National Multiple Sclerosis Society.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1710566114/-/DCSupplemental.
References
- 1.Arandjelovic S, Ravichandran KS. Phagocytosis of apoptotic cells in homeostasis. Nat Immunol. 2015;16:907–917. doi: 10.1038/ni.3253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Elliott MR, Ravichandran KS. Clearance of apoptotic cells: Implications in health and disease. J Cell Biol. 2010;189:1059–1070. doi: 10.1083/jcb.201004096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Burstyn-Cohen T, et al. Genetic dissection of TAM receptor-ligand interaction in retinal pigment epithelial cell phagocytosis. Neuron. 2012;76:1123–1132. doi: 10.1016/j.neuron.2012.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hong S, et al. Complement and microglia mediate early synapse loss in Alzheimer mouse models. Science. 2016;352:712–716. doi: 10.1126/science.aad8373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gaudet AD, Popovich PG, Ramer MS. Wallerian degeneration: Gaining perspective on inflammatory events after peripheral nerve injury. J Neuroinflammation. 2011;8:110. doi: 10.1186/1742-2094-8-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vargas ME, Watanabe J, Singh SJ, Robinson WH, Barres BA. Endogenous antibodies promote rapid myelin clearance and effective axon regeneration after nerve injury. Proc Natl Acad Sci USA. 2010;107:11993–11998. doi: 10.1073/pnas.1001948107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dailey AT, Avellino AM, Benthem L, Silver J, Kliot M. Complement depletion reduces macrophage infiltration and activation during Wallerian degeneration and axonal regeneration. J Neurosci. 1998;18:6713–6722. doi: 10.1523/JNEUROSCI.18-17-06713.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brown MC, Perry VH, Hunt SP, Lapper SR. Further studies on motor and sensory nerve regeneration in mice with delayed Wallerian degeneration. Eur J Neurosci. 1994;6:420–428. doi: 10.1111/j.1460-9568.1994.tb00285.x. [DOI] [PubMed] [Google Scholar]
- 9.Schwab ME. Nogo and axon regeneration. Curr Opin Neurobiol. 2004;14:118–124. doi: 10.1016/j.conb.2004.01.004. [DOI] [PubMed] [Google Scholar]
- 10.Vargas ME, Barres BA. Why is Wallerian degeneration in the CNS so slow? Annu Rev Neurosci. 2007;30:153–179. doi: 10.1146/annurev.neuro.30.051606.094354. [DOI] [PubMed] [Google Scholar]
- 11.Lewis GM, Kucenas S. Perineurial glia are essential for motor axon regrowth following nerve injury. J Neurosci. 2014;34:12762–12777. doi: 10.1523/JNEUROSCI.1906-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu HM, Yang LH, Yang YJ. Schwann cell properties: 3. C-fos expression, bFGF production, phagocytosis and proliferation during Wallerian degeneration. J Neuropathol Exp Neurol. 1995;54:487–496. [PubMed] [Google Scholar]
- 13.Perry VH, Tsao JW, Fearn S, Brown MC. Radiation-induced reductions in macrophage recruitment have only slight effects on myelin degeneration in sectioned peripheral nerves of mice. Eur J Neurosci. 1995;7:271–280. doi: 10.1111/j.1460-9568.1995.tb01063.x. [DOI] [PubMed] [Google Scholar]
- 14.Arthur-Farraj PJ, et al. c-Jun reprograms Schwann cells of injured nerves to generate a repair cell essential for regeneration. Neuron. 2012;75:633–647. doi: 10.1016/j.neuron.2012.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Napoli I, et al. A central role for the ERK-signaling pathway in controlling Schwann cell plasticity and peripheral nerve regeneration in vivo. Neuron. 2012;73:729–742. doi: 10.1016/j.neuron.2011.11.031. [DOI] [PubMed] [Google Scholar]
- 16.Peleshok JC, Ribeiro-da-Silva A. Neurotrophic factor changes in the rat thick skin following chronic constriction injury of the sciatic nerve. Mol Pain. 2012;8:1. doi: 10.1186/1744-8069-8-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mehlem A, Hagberg CE, Muhl L, Eriksson U, Falkevall A. Imaging of neutral lipids by oil red O for analyzing the metabolic status in health and disease. Nat Protoc. 2013;8:1149–1154. doi: 10.1038/nprot.2013.055. [DOI] [PubMed] [Google Scholar]
- 18.Klionsky DJ, et al. Guidelines for the use and interpretation of assays for monitoring autophagy. Autophagy. 2012;8:445–544. doi: 10.4161/auto.19496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mizushima N, Kuma A. Autophagosomes in GFP-LC3 transgenic mice. Methods Mol Biol. 2008;445:119–124. doi: 10.1007/978-1-59745-157-4_7. [DOI] [PubMed] [Google Scholar]
- 20.Jang SY, et al. Autophagic myelin destruction by Schwann cells during Wallerian degeneration and segmental demyelination. Glia. 2016;64:730–742. doi: 10.1002/glia.22957. [DOI] [PubMed] [Google Scholar]
- 21.Komatsu M, et al. Impairment of starvation-induced and constitutive autophagy in Atg7-deficient mice. J Cell Biol. 2005;169:425–434. doi: 10.1083/jcb.200412022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gomez-Sanchez JA, et al. Schwann cell autophagy, myelinophagy, initiates myelin clearance from injured nerves. J Cell Biol. 2015;210:153–168. doi: 10.1083/jcb.201503019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huang DW, Sherman BT, Lempicki RA. Systematic and integrative analysis of large gene lists using DAVID Bioinformatics Resources. Nature Protoc. 2009;4:44–57. doi: 10.1038/nprot.2008.211. [DOI] [PubMed] [Google Scholar]
- 24.Huang DW, Sherman BT, Lempicki RA. Bioinformatics enrichment tools: Paths toward the comprehensive functional analysis of large gene lists. Nucleic Acids Res. 2009;37:1–13. doi: 10.1093/nar/gkn923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chung WS, et al. Astrocytes mediate synapse elimination through MEGF10 and MERTK pathways. Nature. 2013;504:394–400. doi: 10.1038/nature12776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ziegenfuss JS, et al. Draper-dependent glial phagocytic activity is mediated by Src and Syk family kinase signalling. Nature. 2008;453:935–939. doi: 10.1038/nature06901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Reichert F, Saada A, Rotshenker S. Peripheral nerve injury induces Schwann cells to express two macrophage phenotypes: Phagocytosis and the galactose-specific lectin MAC-2. J Neurosci. 1994;14:3231–3245. doi: 10.1523/JNEUROSCI.14-05-03231.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Satinsky D, Pepe FA, Liu CN. The neurolemma cells in peripheral nerve degeneration and regeneration. Exp Neurol. 1964;9:441–451. doi: 10.1016/0014-4886(64)90052-4. [DOI] [PubMed] [Google Scholar]
- 29.Holtzman E, Novikoff AB. Lysosomes in the rat sciatic nerve following crush. J Cell Biol. 1965;27:651–669. doi: 10.1083/jcb.27.3.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ravikumar B, et al. Regulation of mammalian autophagy in physiology and pathophysiology. Physiol Rev. 2010;90:1383–1435. doi: 10.1152/physrev.00030.2009. [DOI] [PubMed] [Google Scholar]
- 31.Lemke G. Biology of the TAM receptors. Cold Spring Harb Perspect Biol. 2013;5:a009076. doi: 10.1101/cshperspect.a009076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Van Rooijen N, Sanders A. Liposome mediated depletion of macrophages: Mechanism of action, preparation of liposomes and applications. J Immunol Methods. 1994;174:83–93. doi: 10.1016/0022-1759(94)90012-4. [DOI] [PubMed] [Google Scholar]
- 33.Romanelli E, et al. Myelinosome formation represents an early stage of oligodendrocyte damage in multiple sclerosis and its animal model. Nat Commun. 2016;7:13275. doi: 10.1038/ncomms13275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brosius Lutz A, Barres BA. Contrasting the glial response to axon injury in the central and peripheral nervous systems. Dev Cell. 2014;28:7–17. doi: 10.1016/j.devcel.2013.12.002. [DOI] [PubMed] [Google Scholar]
- 35.Cahoy JD, et al. A transcriptome database for astrocytes, neurons, and oligodendrocytes: A new resource for understanding brain development and function. J Neurosci. 2008;28:264–278. doi: 10.1523/JNEUROSCI.4178-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Safaiyan S, et al. Age-related myelin degradation burdens the clearance function of microglia during aging. Nat Neurosci. 2016;19:995–998. doi: 10.1038/nn.4325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liddelow SA, et al. Neurotoxic reactive astrocytes are induced by activated microglia. Nature. 2017;541:481–487. doi: 10.1038/nature21029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zamanian JL, et al. Genomic analysis of reactive astrogliosis. J Neurosci. 2012;32:6391–6410. doi: 10.1523/JNEUROSCI.6221-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lutz AB. Purification of Schwann cells from the neonatal and injured adult mouse peripheral nerve. Cold Spring Harb Protoc. 2014;2014:1312–1319. doi: 10.1101/pdb.prot074989. [DOI] [PubMed] [Google Scholar]
- 40.Ma CH, et al. Accelerating axonal growth promotes motor recovery after peripheral nerve injury in mice. J Clin Invest. 2011;121:4332–4347. doi: 10.1172/JCI58675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Möbius W, et al. Electron microscopy of the mouse central nervous system. Methods Cell Biol. 2010;96:475–512. doi: 10.1016/S0091-679X(10)96020-2. [DOI] [PubMed] [Google Scholar]