Significance
Autoantibody directed against the pancreatic zinc transport 8 (ZnT8) is used as a major biomarker for identifying and staging presymptomatic type 1 diabetes (T1D). Owing to difficulties in maintaining the tertiary structure outside a membrane environment, full-length ZnT8 has been somewhat limited for ZnT8A detection. In this work, we realized efficient ZnT8A detection using a proteoliposome-based full-length ZnT8 (PLR-ZnT8) self-antigen immobilized on a plasmonic gold platform (pGOLD). Proteoliposomes immobilized on pGOLD improved the proper folding and structural stability of full-length ZnT8 and thus enabled autoreactive sites accessible to ZnT8A, leading to the superior microarray-based assay performance of ZnT8A detection. This proteoliposome-based strategy opens up a new way to develop effective self-antigens to capture autoantibodies for disease diagnosis.
Keywords: full-length ZnT8, proteoliposome, autoantibody, type 1 diabetes, plasmonic
Abstract
Identified as a major biomarker for type 1 diabetes (T1D) diagnosis, zinc transporter 8 autoantibody (ZnT8A) has shown promise for staging disease risk and disease diagnosis. However, existing assays for ZnT8 autoantibody (ZnT8A) are limited to detection by soluble domains of ZnT8, owing to difficulties in maintaining proper folding of a full-length ZnT8 protein outside its native membrane environment. Through a combined bioengineering and nanotechnology approach, we have developed a proteoliposome-based full-length ZnT8 self-antigen (full-length ZnT8 proteoliposomes; PLR-ZnT8) for efficient detection of ZnT8A on a plasmonic gold chip (pGOLD). The protective lipid matrix of proteoliposomes improved the proper folding and structural stability of full-length ZnT8, helping PLR-ZnT8 immobilized on pGOLD (PLR-ZnT8/pGOLD) achieve high-affinity capture of ZnT8A from T1D sera. Our PLR-ZnT8/pGOLD exhibited efficient ZnT8A detection for T1D diagnosis with ∼76% sensitivity and ∼97% specificity (n = 307), superior to assays based on detergent-solubilized full-length ZnT8 and the C-terminal domain of ZnT8. Multiplexed assays using pGOLD were also developed for simultaneous detection of ZnT8A, islet antigen 2 autoantibody, and glutamic acid decarboxylase autoantibody for diagnosing T1D.
The incidence of type 1 diabetes (T1D) in children has been increasing by 3–5% annually worldwide since the 1960s (1), which makes the sensitive and specific diagnosis of T1D imperative. Detection of T1D-related autoantibodies can quantify the extent of risk for onset of symptomatic T1D, and also afford enrollment into trials seeking to prevent overt disease development. Unlike the earlier established autoantibodies against insulin, islet antigen 2 (IA2), and glutamic acid decarboxylase (GAD) as three major biomarkers for T1D diagnosis, zinc transporter 8 autoantibody (ZnT8A) was more recently identified as another major biomarker for T1D diagnosis through bioinformatics (2), expanding the panel of T1D diagnostic autoantibodies. In prediabetic individuals, ZnT8A appears in the prodromal phase months to years before the clinical onset of disease, and its detection can improve the accuracy of T1D prediction (3), making ZnT8A one of the most important biomarkers for evaluating the risk of T1D development.
ZnT8 is a specialized zinc transporter found predominantly in insulin secretory granules of pancreatic beta cells (4). It is a multispanning transmembrane protein consisting of two functional modules, a transmembrane domain responsible for zinc transport and a cytosolic zinc-sensing unit of the N-terminal domain (NTD) and the C-terminal domain (CTD) (5). The transport activity of ZnT8 yields a highly enriched granular zinc content for crystalline packaging of insulin molecules in complex with zinc ions (6). During insulin secretion, granule exocytosis exposes ZnT8 to the surface membrane, subjecting its transmembrane domain (TMD) to extracellular ZnT8A surveillance (7). The cytosolic domains of ZnT8 become accessible to ZnT8A only after the destruction of beta cells (2). Approximately one-half of the ZnT8 structure lies within six membrane-spanning regions, presenting a challenge in developing assays for ZnT8A detection due to the difficulty of maintaining its tertiary structure outside a membrane environment.
Thus far, most assays for ZnT8A detection are based on antigens of cytosolic domains of ZnT8 (i.e., CTD and NTD) or construction of single-chain molecules fusing the two domains. Because the CTD encompassing amino acids 275–369 can produce robust detection performance relative to the less efficient detection performance based on the NTD, ZnT8A detection has focused on two CTD variants that differ by a single amino acid at position 325: arginine (325R) and tryptophan (325W). Although the combination of two CTD variants has been used for effective ZnT8A detection, the best candidate for ZnT8A detection may be full-length ZnT8 that contains complete autoreactive sites accessible to ZnT8A. However, due to the instability of purified full-length ZnT8 protein in its detergent-solubilized form, thus far full-length ZnT8 has not been used successfully for ZnT8A detection.
Here we show that a single variant of full-length ZnT8, 325R, can be used for highly sensitive and specific ZnT8A detection on a plasmonic gold chip (pGOLD). Through expression in human embryonic kidney 293 (HEK293) cells, purification of the monodispersed full-length ZnT8 protein, and subsequent reconstitution within liposomes, a complex (∼146 nm) of full-length ZnT8 proteoliposomes (PLR-ZnT8) with high bio-activity was obtained at a high purity (>95%). When immobilized on pGOLD, proteoliposomes can maintain the proper folding of full-length ZnT8, making autoreactive sites accessible to ZnT8A. With PLR-ZnT8 and a near-infrared (NIR) fluorescence-enhanced pGOLD platform (8–15), we achieved excellent assay performance of ZnT8A detection with ∼76% sensitivity and ∼97% specificity for T1D diagnosis using 307 human serum samples from 138 patients with T1D and 169 healthy individuals. The results were superior to those of assays based on other ZnT8-related antigens (∼2% sensitivity and ∼94% specificity based on DS-ZnT8 and ∼59% sensitivity/∼94% specificity based on CTD). Furthermore, the pGOLD-based microarray also demonstrated the potential of multiplexed detection of ZnT8A, IA2A, and GADA for future T1D diagnosis.
Results and Discussion
Preparation of High-Quality Proteoliposomes PLR-ZnT8.
Human ZnT8 is encoded by the SLC30A8 gene expressed in pancreatic beta cells to produce two protein isoforms. Isoform-1 is a full-length 369-aa protein, and isoform-2 is a product of alternative splicing with a 49-aa deletion from the N terminus (16). The NTD of ZnT8 is largely missing in isoform-2. A major arginine variation at position 325 (325R) of human ZnT8 is an immunoreactivity determinant for ZnT8A (17). We overexpressed the human 325R variant of isoform-2 in HEK293 cells, and purified the recombinant full-length ZnT8 in detergent micelles (DS-ZnT8) or proteoliposomes (PLR-ZnT8) (Fig. 1A). (Details of the experimental procedures are provided in SI Appendix, Materials and Methods.)
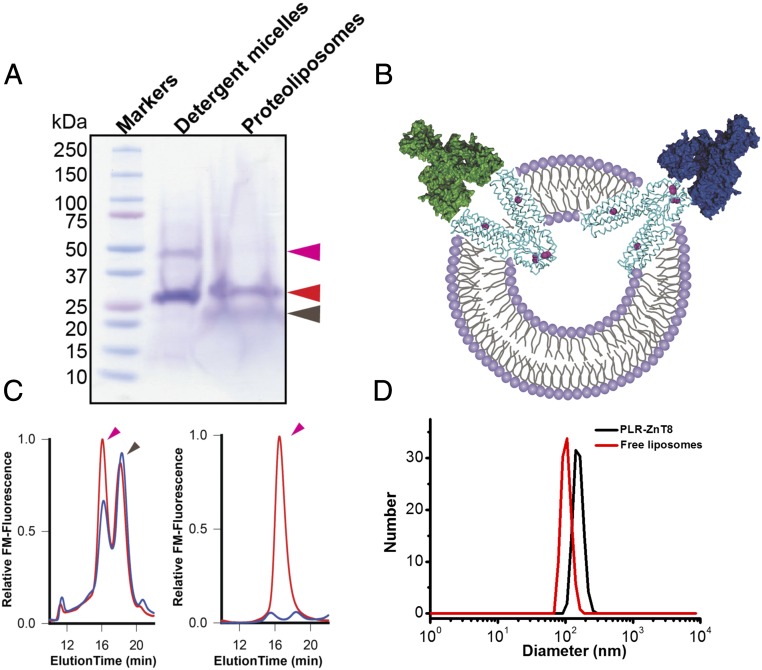
Fig. 1.
Preparation of high-quality full-length ZnT8 antigens. (A) Purified full-length ZnT8 in detergent micelles (DS-ZnT8) or proteoliposomes (PLR-ZnT8) shown by Coomassie blue staining of a SDS/PAGE gel. Magenta, red, and dark-brown arrows indicate full-length ZnT8 dimer, monomer, and lipids, respectively. (B) Schematic of transmembrane orientations of full-length ZnT8 in reconstituted proteoliposomes as marked. Double-layer circles, cyan ribbons, magenta spheres, and green or blue autoantibodies represent liposomes, full-length ZnT8, zinc ions, and ZnT8A binding to the extracellular or intracellular surface of full-length ZnT8, respectively. (C) Size-exclusion HPLC profiles of PLR-ZnT8 before (red) and after (blue) vacuum drying (Left) and of DS-ZnT8 before (red) and after (blue) vacuum drying (Right). Magenta and black arrows indicate full-length ZnT8 and lipids, respectively. (D) DLS analysis of ZnT8-free liposomes and PLR-ZnT8. The size of liposomes increased from ∼104 to ∼146 nm after reconstitution of full-length ZnT8.
DS-ZnT8 was stable in the aqueous solution for only a few hours before forming insoluble protein aggregates, accompanied by an irreversible loss of zinc transport activity. In contrast, PLR-ZnT8, which mediates rapid zinc uptake into proteoliposomes (18), remained functionally active for days on ice, and could be stored indefinitely in liquid nitrogen. Resolubilization of PLR-ZnT8 with detergents yielded well-folded DS-ZnT8 that was subjected to rapid denaturation, as indicated by a progressive loss of the initial monodispersed protein population on size-exclusion high-performance liquid chromatography (HPLC) (SI Appendix, Fig. S1). Multiple copies of ZnT8 proteins were incorporated in a single proteoliposome in mixed transmembrane orientations, exposing both the CTD and TMD to ZnT8A binding (Fig. 1B). Different from DS-ZnT8 in the aqueous solution, PLR-ZnT8 is a solid-phase antigen that can be separated from the aqueous phase by ultracentrifugation and stored as a lipid-like pellet. Analytical size-exclusion HPLC analysis of detergent extracts of solid PLR-ZnT8 pellets revealed a major monodispersed full-length ZnT8 specie followed by a lipid peak, indicating well-preserved structural integrity of full-length ZnT8 in a wet lipid matrix (Fig. 1C).
To further examine the structural integrity of full-length ZnT8 in a completely dried lipid matrix, we vacuum-dried PLR-ZnT8 and then rehydrated the proteoliposomes to mimic the experimental conditions of the subsequent pGOLD microarray-based assay. HPLC analysis showed that a large majority of full-length ZnT8 remained intact after being dried and rehydration (Fig. 1C). In sharp contrast, DS-ZnT8 was short-lived, losing the monodispersed HPLC profile (Fig. 1C). These results indicate that the lipid matrix of proteoliposomes enhanced the biostability of the reconstituted full-length ZnT8. According to dynamic light scattering analysis (DLS), the size of ZnT8-free liposomes centered on ∼104 nm with a narrow distribution and increased to ∼146 nm after reconstitution of full-length ZnT8 (Fig. 1D).
PLR-ZnT8 Immobilized on pGOLD for Efficient ZnT8A Detection.
We previously developed a pGOLD substrate for NIR fluorescence-enhanced biological detections, and performed a pilot study of T1D autoantibody assay using a small number of human serum and whole blood samples, such as finger-prick samples (10). Here we adopted this pGOLD (Nirmidas Biotech) platform to evaluate the ability of PLR-ZnT8 for ZnT8A detection in hundreds of human serum samples. For simply printed PLR-ZnT8 on a pGOLD slide, proteoliposomes presented a large number of full-length ZnT8 free of direct contact or interactions with the gold surface, efficiently maintaining the proper folding of full-length ZnT8 and thereby enabling autoreactive sites accessible to ZnT8A. The gold nano-islands on pGOLD were arranged in such a way that the edges of adjacent islands conformed to each other for the generation of abundant nano-gaps that support strong electric field enhancement. This and surface plasmonic resonance coupling with NIR fluorophore-excited states afforded increased radiative emission and thus fluorescence enhancement by ∼100-fold (10), boosting the sensitivity of the NIR-based bioassay. PLR-ZnT8 immobilized on pGOLD (PLR-ZnT8/pGOLD) was used to capture ZnT8A in human serum samples diluted by 20-fold with FBS, and the captured autoantibody was subsequently labeled with NIR fluorophore-conjugated secondary antibody (i.e., anti-human IgG-IRDye800). The amount of ZnT8A was then analyzed through the fluorescence intensity of the fluorophore (Fig. 2A).
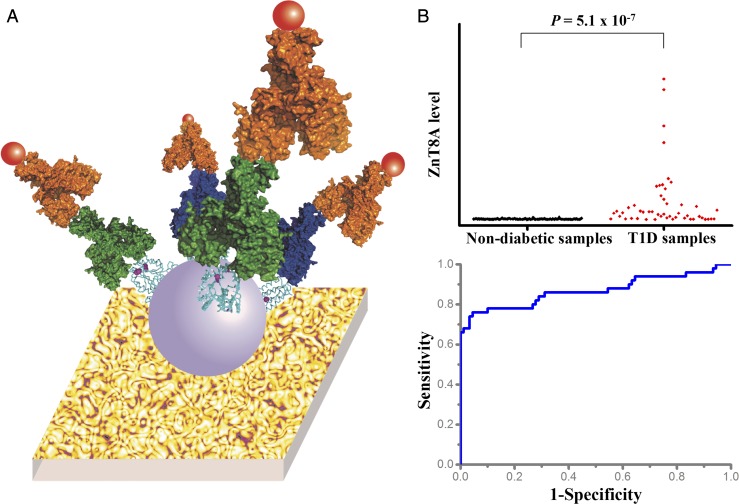
Fig. 2.
PLR-ZnT8 immobilized on pGOLD for ZnT8A detection. (A) Schematic of PLR-ZnT8 immobilized on pGOLD for ZnT8A detection. The substrate, purple spheres, cyan ribbons, red spheres, yellow spheres, and green or blue autoantibodies represent pGOLD, liposomes, full-length ZnT8, IRDye800 fluorophores, secondary antibody anti-human IgG, and ZnT8A binding to the extracellular or intracellular surface of full-length ZnT8, respectively. (B) Scatterplot and ROC analysis of ZnT8A levels detected by PLR-ZnT8 immobilized on pGOLD in 140 human serum samples from 50 T1D patients and 90 healthy individuals provided by the IASP, showing ∼76% sensitivity and ∼95% specificity.
We participated in the 2016 Islet Autoantibody Standardization Program (IASP) workshop to evaluate the ability of PLR-ZnT8/pGOLD for ZnT8A detection. In 140 human serum samples from 50 T1D patients and 90 healthy individuals, PLR-ZnT8/pGOLD achieved ∼66% sensitivity and ∼100% specificity by blind tests (sensitivity adjusted to 99% specificity, ∼68%). The report from the IASP, which summarized all participating assays of ZnT8A detection for T1D diagnosis in the 2016 workshop, adopted sensitivity adjusted to 95% specificity as an important index for assessing assay performance. To better compare performance, our assay performance could be rationally adjusted to ∼76% sensitivity and ∼95% specificity by lowering the cutoff according to a receiver operating characteristic (ROC) curve analysis (P = 5.0 × 10−7) (Fig. 2B). These results outperformed ZnT8A detection using a single variant of CTD, and matched the best ZnT8A detection using two variants of CTD based on other methods (i.e., radioimmunoassay, luciferase immunoprecipitation systems, and ELISA) among all participating assays in the 2016 IASP workshop (SI Appendix, Table S1).
Subsequently, another set of 167 human serum samples from 88 T1D patients and 79 healthy individuals provided by University of Florida were combined with serum samples obtained from the IASP to form a large set of samples (i.e., 307 in total, including 138 samples from T1D patients and 169 from nondiabetic individuals) for further evaluation of ZnT8A detection by PLR-ZnT8/pGOLD. With this combined serum set, our PLR-ZnT8/pGOLD assay still achieved ∼76% sensitivity and ∼97% specificity with a positive predictive value (PPV) of ∼95% and a negative predictive value (NPV) of ∼83%, effectively discriminating T1D samples from nondiabetic samples (P = 4.2 × 10−22), making this one of the best-performing ZnT8A assays yet reported (2, 19) (Fig. 3). To confirm the reproducibility of our PLR-ZnT8/pGOLD assay, we repeated the serum screening twice and found good correlation between two sets of measurements of ZnT8A (R2 = 0.87) (SI Appendix, Fig. S2).
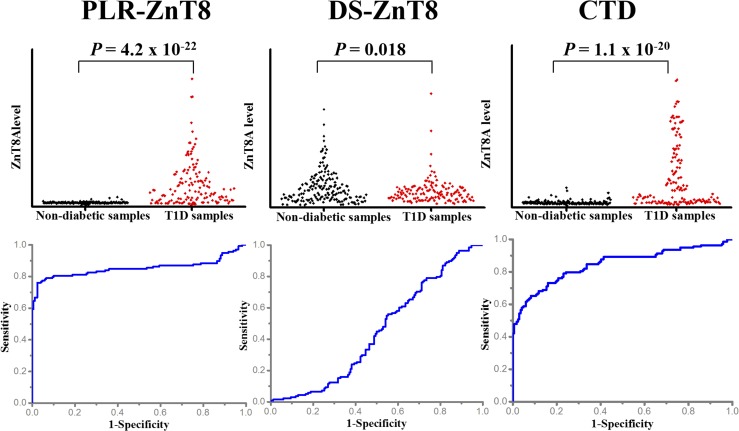
Fig. 3.
The superiority of PLR-ZnT8 immobilized on pGOLD for ZnT8A detection. Scatterplot and ROC analysis of ZnT8A levels detected by different ZnT8-related antigens immobilized on pGOLD in 307 human serum samples from 138 T1D patients and 169 healthy individuals provided by the IASP and University of Florida. The assay performance was ∼76% sensitivity and ∼97% specificity for PLR-ZnT8, ∼2% sensitivity and ∼94% specificity for DS-ZnT8, and ∼59% sensitivity and ∼94% specificity for CTD. All antigens used were of the 325R variant.
For comparison, DS-ZnT8 and CTD were also immobilized on pGOLD in parallel with PLR-ZnT8. (Unless mentioned otherwise, all ZnT8-related antigens we used were the 325R variant.) Under the same experimental conditions, DS-ZnT8 did not afford useful ZnT8A detection, presumably due to the loss of structural integrity during the assay, while CTD provided ∼17% lower sensitivity than PLR-ZnT8 (Fig. 3). The ROC curve analysis of ZnT8A level detected by PLR-ZnT8 revealed an area under the curve (AUC) of ∼0.85, greater than the ∼0.80 for CTD and ∼0.50 for DS-ZnT8 (Fig. 3). Further correlation analysis of ZnT8A level detected by PLR-ZnT8 and CTD showed that the detection of ZnT8A differed between PLR-ZnT8 and CTD (R2 = 0.45) (SI Appendix, Fig. S3), implying PLR-ZnT8 contains more autoreactive sites accessible to ZnT8A, leading to the better sensitivity. Finally, the AUC value of ∼0.50 for ZnT8A detection by ZnT8-free liposomes indicates that liposomes contributed only minimally to ZnT8A detection (SI Appendix, Fig. S4).
For the simplex ZnT8A assay, compared with conventional ZnT8A detection methods, such as ELISA (20), radioimmunoassay (21), electrochemiluminescence (22), and luciferase immunoprecipitation (23), our PLR-ZnT8/pGOLD assay demonstrated improved sensitivity and specificity (∼76% sensitivity and ∼97% specificity for 307 human serum samples), rapid assay processing (∼4 h), a low sample volume requirement (5 µL), and low cost (∼$10 per sample).
Multiplexed Detection of Autoantibodies Toward T1D Diagnosis.
In addition to PLR-ZnT8, we also performed simultaneous detection of IA2A and GADA using robotically printed microarrays of IA2 (fragments encompassing 604–979 aa; Kronus Inc.) and GAD (recombinant protein; Diamyd Medical) antigens, two additional major biomarkers for T1D diagnosis. After incubation with 5 µL of human serum diluted in 100 μL of FBS, washing away excess biomolecules, and labeling with anti-human IgG-IRDye800 conjugates, autoantibodies captured by corresponding antigens were quantified based on the fluorescence intensity.
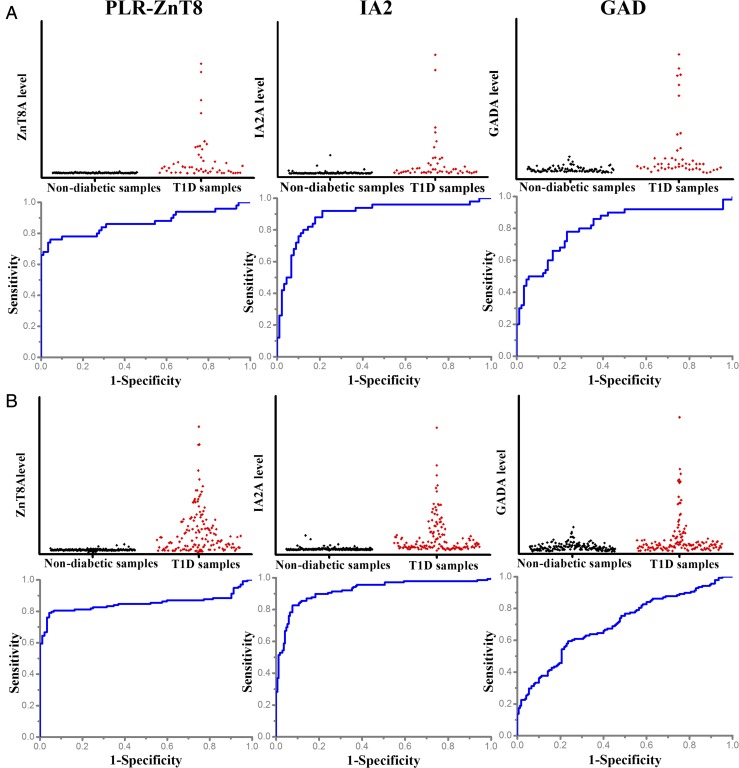
For the samples from the IASP (50 T1D and 90 nondiabetic serum samples), we obtained ∼68% sensitivity and ∼92% specificity for IA2A detection and a relatively poor ∼50% sensitivity and ∼94% specificity for GADA detection (Fig. 4A). For the combined IASP and University of Florida samples (307, including 138 T1D and 169 nondiabetic samples), we still found ∼71% sensitivity and ∼95% specificity for IA2A detection, but only ∼40% sensitivity and ∼86% specificity for GADA detection (Fig. 4B). The poor assay performance for GADA mirrored the observation with DS-ZnT8, suggesting significant loss of the structural integrity of the immobilized GAD on pGOLD for the detection of conformation-specific GADA. Given its high prevalence, ZnT8A obviously overlapped with GADA and IA2A at disease onset (2). Analyzed in terms of level of reactivity, ZnT8A correlated weakly with GADA (R2 = 0.19) and moderately with IA2A (R2 = 0.46) (SI Appendix, Fig. S5), indicating that ZnT8A is likely an independent T1D biomarker, as described previously (2).
Fig. 4.
Multiplexed detection of autoantibodies for T1D diagnosis. (A) Scatterplot and ROC analysis of ZnT8A, IA2A, and GADA levels detected by pGOLD-based microarray in 140 human serum samples from 50 T1D patients and 90 healthy individuals provided by the IASP. The assay performance was ∼76% sensitivity and ∼95% specificity for PLR-ZnT8, ∼68% sensitivity and ∼92% specificity for IA2A, and ∼50% sensitivity and ∼94% specificity for GADA. (B) Scatterplot and ROC analysis of ZnT8A, IA2A, and GADA levels detected by pGOLD-based microarray in 307 human serum samples from 138 T1D patients and 169 healthy individuals provided by the IASP and University of Florida. The assay performance was ∼76% sensitivity and ∼97% specificity for PLR-ZnT8, ∼71% sensitivity and ∼95% specificity for IA2A, and ∼40% sensitivity and ∼86% specificity for GADA.
We further confirmed the reliability of the multiplexed assay by the low intra-assay and interassay variation of antibody detection with coefficients of variation (CV) of <10% and <15%, respectively. CV was calculated based on the formula CV = σ/μ × 100%, where σ is the SD of the sample signals and μ is the mean of the sample signals (SI Appendix, Fig. S6).
ZnT8A is thought to be conformation-specific, recognizing the ZnT8 self-antigen mainly in its native conformation. Purification of multispanning full-length ZnT8 requires detergent solubilization, but the lack of structural stability of DS-ZnT8 poses a major challenge to maintain the proper folding of conformational epitopes. The harsh antigen handling procedures (e.g., drying, rehydration) further exacerbate the loss of bioactivity when full-length ZnT8 is immobilized on pGOLD. The proteoliposome-based approach reported here dramatically improved the structural stability and proper folding of full-length ZnT8 in a protective lipid matrix, making autoreactive sites accessible to ZnT8A with high bioactivity. Even under strict drying and rehydration experimental conditions, a majority of full-length ZnT8 in PLR-ZnT8 remained intact, in contrast to the complete loss of structural integrity of DS-ZnT8 under identical experimental conditions. The scalable production of human ZnT8 from heterologous overexpression in HEK293 cells (18), longer shelf-life of purified ZnT8 antigen in protective proteoliposomes, and miniaturization of ZnT8A detection on pGOLD (10) will facilitate the implementation of point-of-care measurements in the future.
ZnT8A directed to the TMD and CTD may play different roles in stratification of T1D risk. Anti-CTD autoantibody is expected to arise after autoimmune destruction of beta cells, which exposes cytosolic epitopes to extracellular immune surveillance (2). On the other hand, the TMD is displayed on the surface of live beta cells following glucose-stimulated insulin secretion (7). Anti-TMD autoantibody may occur with intact beta cells; indeed, ZnT8A recognizing autoreactive sites outside of the CTD was revealed by the PLR-ZnT8/pGOLD platform, contributing to a 17% improvement in assay sensitivity over CTD/pGOLD detection. This finding opens the possibility of humoral anti-TMD autoimmunity against live beta cells, with implications for T1D initiation and TMD-specific immunotherapy.
Conclusion
In sum, taking advantage of the structure of PLR-ZnT8, we have developed an efficient microarray-based assay for T1D autoantibody detection. Proteoliposomes efficiently maintained the proper folding of full-length ZnT8, allowing ZnT8A to access autoreactive sites. PLR-ZnT8 immobilized on pGOLD achieved ZnT8A detection performance of ∼76% sensitivity with ∼97% specificity for 307 human sera from 138 T1D patients and 169 healthy individuals, superior to the ∼2% sensitivity and ∼94% specificity based on DS-ZnT8 and ∼59% sensitivity and ∼94% specificity based on CTD. pGOLD was also applied for multiplexed detection of other T1D-related autoantibodies, IA2A and GADA, from >300 serum samples, with outcomes of ∼71% sensitivity and ∼95% specificity for the former and ∼40% sensitivity and ∼86% specificity for the latter.
Our present work establishes proteoliposomes on pGOLD as a promising platform for ZnT8A detection with high sensitivity and specificity. A future direction is to improve GADA detection and optimize the overall performance of multiplexed assays for screening for presymptomatic T1D in at-risk populations. Diagnosing the disease at earlier stages will help provide safe and effective therapeutic options for prevention.
Materials and Methods
Details of the materials and methods used in this study, including preparation of recombinant antigens, HPLC analysis of vacuum-dried full-length ZnT8, preparation of T1D-related antigen microarrays on pGOLD, multiplexed detection of T1D-related autoantibodies, and data analysis, are provided in SI Appendix, Materials and Methods. Human serum Institutional Review Board approval for this study was obtained from Stanford University and informed consent was obtained from study participants.
Supplementary Material
Acknowledgments
This study was supported by the National Institutes of Health (Grants R01 HL127113-01A1 and 5R01 GM065137), Shenzhen Peacock Program Grant KQTD20140630160825828, and Cal-BRAIN.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1711169114/-/DCSupplemental.
References
- 1.Jarosz-Chobot P, et al. Rapid increase in the incidence of type 1 diabetes in Polish children from 1989 to 2004, and predictions for 2010 to 2025. Diabetologia. 2011;54:508–515. doi: 10.1007/s00125-010-1993-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wenzlau JM, et al. The cation efflux transporter ZnT8 (Slc30A8) is a major autoantigen in human type 1 diabetes. Proc Natl Acad Sci USA. 2007;104:17040–17045. doi: 10.1073/pnas.0705894104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wenzlau JM. Zinc transporter 8 (ZnT8) autoantibodies. Diapedia. 2016 doi: 10.14496/dia.2105812817.9. [DOI] [Google Scholar]
- 4.Chimienti F, Devergnas S, Favier A, Seve M. Identification and cloning of a beta-cell-specific zinc transporter, ZnT-8, localized into insulin secretory granules. Diabetes. 2004;53:2330–2337. doi: 10.2337/diabetes.53.9.2330. [DOI] [PubMed] [Google Scholar]
- 5.Lu M, Chai J, Fu D. Structural basis for autoregulation of the zinc transporter YiiP. Nat Struct Mol Biol. 2009;16:1063–1067. doi: 10.1038/nsmb.1662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lemaire K, et al. Insulin crystallization depends on zinc transporter ZnT8 expression, but is not required for normal glucose homeostasis in mice. Proc Natl Acad Sci USA. 2009;106:14872–14877. doi: 10.1073/pnas.0906587106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huang Q, Merriman C, Zhang H, Fu D. Coupling of insulin secretion and display of a granule-resident zinc transporter ZnT8 on the surface of pancreatic beta cells. J Biol Chem. 2017;292:4034–4043. doi: 10.1074/jbc.M116.772152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang B, et al. Diagnosis of Zika virus infection on a nanotechnology platform. Nat Med. 2017;23:548–550. doi: 10.1038/nm.4302. [DOI] [PubMed] [Google Scholar]
- 9.Tabakman SM, et al. Plasmonic substrates for multiplexed protein microarrays with femtomolar sensitivity and broad dynamic range. Nat Commun. 2011;2:466. doi: 10.1038/ncomms1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang B, Kumar RB, Dai H, Feldman BJ. A plasmonic chip for biomarker discovery and diagnosis of type 1 diabetes. Nat Med. 2014;20:948–953. doi: 10.1038/nm.3619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li X, et al. Multiplexed anti-toxoplasma IgG, IgM, and IgA assay on plasmonic gold chips: Towards making mass screening possible with dye test precision. J Clin Microbiol. 2016;54:1726–1733. doi: 10.1128/JCM.03371-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu B, et al. High-performance, multiplexed lung cancer biomarker detection on a plasmonic gold chip. Adv Funct Mater. 2016;26:7994–8002. [Google Scholar]
- 13.Koh B, et al. Visible to near-infrared fluorescence enhanced cellular imaging on plasmonic gold chips. Small. 2016;12:457–465. doi: 10.1002/smll.201502182. [DOI] [PubMed] [Google Scholar]
- 14.Rivas L, et al. Triple lines gold nanoparticle-based lateral flow assay for enhanced and simultaneous detection of Leishmania DNA and endogenous control. Nano Res. 2015;8:3704–3714. [Google Scholar]
- 15.Wang J, et al. Synthesis of gold/rare-earth-vanadate core/shell nanorods for integrating plasmon resonance and fluorescence. Nano Res. 2015;8:2548–2561. [Google Scholar]
- 16.Davidson HW, Wenzlau JM, O’Brien RM. Zinc transporter 8 (ZnT8) and β cell function. Trends Endocrinol Metab. 2014;25:415–424. doi: 10.1016/j.tem.2014.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wenzlau JM, et al. A common nonsynonymous single nucleotide polymorphism in the SLC30A8 gene determines ZnT8 autoantibody specificity in type 1 diabetes. Diabetes. 2008;57:2693–2697. doi: 10.2337/db08-0522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Merriman C, Huang Q, Rutter GA, Fu D. Lipid-tuned zinc transport activity of human ZnT8 protein correlates with risk for type-2 diabetes. J Biol Chem. 2016;291:26950–26957. doi: 10.1074/jbc.M116.764605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cheng L, Li T, Zhang D, Chen B. Prokaryotic expression of bioactive zinc transporter 8 antigens and detection of diabetes-specific autoantibodies in a single dot immunogold filtration assay. Clin Lab. 2015;61:1445–1452. doi: 10.7754/clin.lab.2015.150220. [DOI] [PubMed] [Google Scholar]
- 20.Delic-Sarac M, et al. ELISA test for analyzing of incidence of type 1 diabetes autoantibodies (GAD and IA2) in children and adolescents. Acta Inform Med. 2016;24:61–65. doi: 10.5455/aim.2016.24.61-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tiberti C, et al. Detection of four diabetes specific autoantibodies in a single radioimmunoassay: An innovative high-throughput approach for autoimmune diabetes screening. Clin Exp Immunol. 2011;166:317–324. doi: 10.1111/j.1365-2249.2011.04479.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sosenko JM, et al. The use of electrochemiluminescence assays to predict autoantibody and glycemic progression toward type 1 diabetes in individuals with single autoantibodies. Diabetes Technol Ther. 2017;19:183–187. doi: 10.1089/dia.2016.0243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Burbelo PD, Lebovitz EE, Notkins AL. Luciferase immunoprecipitation systems for measuring antibodies in autoimmune and infectious diseases. Transl Res. 2015;165:325–335. doi: 10.1016/j.trsl.2014.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.