Abstract
Components of reading proficiency such as accuracy, fluency, and comprehension require the successful coordination of numerous, yet distinct, cortical regions. Underlying white matter tracts allow for communication among these regions. This study utilized unique residualized tract – based spatial statistics methodology to identify the relations of white matter microstructure integrity to three components of reading proficiency in 49 school - aged children with typically developing phonological decoding skills and 27 readers with poor decoders. Results indicated that measures of white matter integrity were differentially associated with components of reading proficiency. In both typical and poor decoders, reading comprehension correlated with measures of integrity of the right uncinate fasciculus; reading comprehension was also related to the left inferior longitudinal fasciculus in poor decoders. Also in poor decoders, word reading fluency was related to the right uncinate and left inferior fronto - occipital fasciculi. Word reading was unrelated to white matter integrity in either group. These findings expand our knowledge of the association between white matter integrity and different elements of reading proficiency.
Keywords: reading proficiency, white matter, DTI
1. Introduction
As developing readers are exposed to print they acquire a mental framework for representing the relation between text and speech that allows for subsequent mastery of word recognition. This framework is connected via (a) the phonological lexicon, which stores the auditory sound form of words (i.e., phonemes); (b) the semantic lexicon, which stores the meaning of words; and (c) the orthographic lexicon, which is responsible for storing the visual representation of words (Frith, 1985). The development of a semantic and phonological lexicon is a spontaneous process that develops with the exposure to language. On the other hand, the development of an orthographic lexicon is dependent on explicit exposure to print and instruction (Dehaene, 2009)
Although word recognition increases rapidly with adequate phonological awareness, reading comprehension and fluency/automaticity skills are acquired more gradually with increased exposure to print and reading practice. Most children develop accurate and fluent reading skills; however, 6 to 17% of children struggle with reading proficiency even after exposure to standard didactic instruction and appropriate opportunities to learn (Vellutino, Fletcher, Snowling, & Scanlon, 2004).
Learning to read is not a natural process and places a high demand on cognitive resources. It also requires a great deal of reorganization in the brain because it is not “hardwired” for such tasks (Dehaene, 2009). Instead, the brain scaffolds reading onto brain regions allocated for vision and language (Vigneau et al., 2006; Vogel, Petersen, & Schlaggar, 2014). Proficient reading also requires the successful utilization and coordination of a number of cognitive processes that facilitate a coherent representation of text. This implies that reading requires not only the successful activation of multiple, relevant brain regions but also adequate communication among these areas.
1.1 Dual Route Models of Reading
According to dual route models of reading there are two routes involved in the reading process: one corresponding to the semantic and phonological lexicon, the other to the orthographic lexicon (Coltheart, 1985). While several variants of the dual model exist, all incorporate the idea of multiple reading routes (Colheart, Curtis, Atkins, & Haller, 1993; Coltheart, Rastle, Perry, Langdon & Zigler, 2001; Dehaene, 2009). These systems work in parallel to accomplish fluent and proficient reading. Structural and functional magnetic resonance imaging studies have investigated neural structures associated with the dual route model. Research has identified two distinct neural systems, or routes, which operate as a predominantly left – hemispheric network underlying reading ability (Vandermosten, Boets, Wouters, & Ghesquiere, 2012b).The dorsal phonological route, typically associated with sublexical phonological decoding, is comprised of both the left temporoparietal junction as well as frontal regions in and around the inferior frontal gyrus (Broca’s area). The ventral orthographic route is typically associated with the left occiptiotemporal region that includes the Visual Word Form area and is linked to the automatic processing of the orthographic features of written language necessary for automatic word recognition (Cohen et al., 2000; Cohen et al., 2002).
1.2. White Matter Tracts Associated with Reading
Although much is known about the cortical regions involved in reading through lesion studies and through functional neuroimaging studies, much less is known about how these regions are connected. Diffusion tensor imaging (DTI) has allowed for the investigation of white matter tract integrity, including tracts associated with reading–related cortical regions. DTI is a noninvasive, in vivo MRI technique which estimates microstructural properties of white matter tracts by evaluating the diffusion of water molecules in and around nerve fibers (Mori et al., 2006). Quantitative analysis yields many different diffusivity -based measures of white matter integrity with fractional anisotropy (FA) the most widely used (Assaf & Pasternak, 2008). The FA index utilizes eigenvalues that quantify diffusivity parallel and perpendicular to the fibers (also termed principal diffusivities) to measure the fraction of the “magnitude” of anisotropic diffusion (i.e. the normalized standard deviation of the diffusivities) (Assaf & Pasternak, 2008; Pierpaoli, Jezzard, Basser, Barnett, & Di Chiro, 1996). FA values are highest in white matter structures such as the corpus callosum and the ventral internal capsule, where anisotropy is highest, reflecting fast diffusivity parallel to fibers and slower diffusivity perpendicular to them (Assaf & Pasternak, 2008; Pierpaoli et al., 1996). When evaluating white matter tract integrity, it would be expected that FA values would be associated with organization and structure of the tract (Basser & Pierpaoli, 1996).
Relatively little is known about the relation of white matter integrity and reading achievement, particularly when differentiating the role of white matter tracts associated with components of reading proficiency. Recent research has established an association between poor reading proficiency and poor white matter tract integrity associated with the dorsal phonological and ventral orthographic routes of reading (Carter et al., 2009; Feldman, Lee, Yeatman, & Yeom, 2012; Horowitz-Kraus, Wang, Plante, & Holland, 2014; Lebel et al., 2013; Vandermosten et al., 2012). Working together, these white matter pathways underlie coordinated communication among cortical regions essential for proficient reading skills. While research suggests that these white matter pathways are an integral part of the reading network, little is known about the distinct function of each pathway in relation to word reading accuracy, word reading fluency, and/or reading comprehension.
1.2.1 Dorsal Phonological Route
The primary dorsal phonological system is typically associated with phonological decoding or word reading. Functional neuroimaging studies suggest that real word and pseudo word reading skills are associated regions of the phonological route such as the posterior temporoparietal and inferior frontal cortex (Brunswick, McCrory, Price, Firth, & Firth, 1999; Eden & Zeffiro, 1998; Farris et al., 2011; Shaywitz et al, 2002). The superior longitudinal fasciculus (SLF) is the white matter tract most closely associated with the dorsal phonological route of reading. This lateral associative white matter tract connects the temporoparietal area with the inferior frontal gyrus, and has been shown to be involved in language processing (Catani, Jones, & Ffytche, 2005; Catani & Mesulam, 2008) and phonological decoding (Lebel et al., 2013). Longitudinally, measures of white matter integrity (i.e. fractional anisotropy) in the left SLF have been shown to correlate with rate of reading development (J. D. Yeatman, Dougherty, Ben-Shachar, & Wandell, 2012). Fractional anisotropy (FA) values in the SLF are also correlated with measures of word reading accuracy (Frye et al., 2010; Hoeft et al., 2011; Lebel et al., 2013) and reading fluency (Lebel et al., 2013) because of their dependence on phonological processing. Similarly, children and adults with poor word reading ability have lower FA values in the left SLF, which supports a link between SLF integrity and phonological processing (Carter et al., 2009; Vandermosten et al., 2012).
1.2.2. Ventral Orthographic Route
The ventral orthographic route of reading is thought to be involved in more complex components of reading, such as fluency and text comprehension. As such, this route requires the utilization of a greater number of cortical regions Through connections with semantic areas such as those in portions of the inferior temporal and middle temporal gyrus as well as the inferior frontal gyrus, the orthographic route supports automatized word recognition based on orthographic patterns the brain learns to recognize through continued exposure (Vandermosten et al., 2012b). Damage to the orthographic route contributes to a different type of impairment in which phonological decoding remains intact but difficulties arise when attempting to read irregular words, such as “ache” or “enough,” that require more rote memorization because they do not follow standard orthographic to phonemic conversion rules. Because the orthographic route requires the successful coordination of multiple cortical regions it would follow that this reading route would also recruit more white matter to ensure successful communication among those regions (Catani & Mesulam, 2008).
The inferior fronto-occipital fasciculus (IFOF), the inferior longitudinal fasciculus (ILF), and the uncinate fasciculus (UF) are three distinct white matter structures often associated with the ventral orthographic route (Schlaggar & McCandliss, 2007; Vandermosten et al., 2012). The IFOF is an association bundle that connects the ventral occipital lobe and the orbitofrontal cortex (Catani et al., 2005) that correlates with measures of word reading accuracy (Feldman et al., 2012; Odegard, Farris, Ring, McColl, & Black, 2009) and reading fluency (Lebel et al., 2013). The ILF is a ventral associative tract that consists of a bundle of both long and short fibers directly connecting the occipital and anterior temporal lobes (Catani et al., 2005). This tract has been shown to correlate with measures of word reading fluency (Horowitz-Kraus et al., 2014; Lebel et al., 2013) and reading comprehension (Feldman et al., 2012; Horowitz-Kraus et al., 2014).
The UF is a ventral association bundle considered to be part of the limbic system (Catani & Mesulam, 2008). While typically considered part of the ventral route, this tract may also be key in semantic processing by coordinating activity of both dorsal and ventral information streams necessary for language and proficient reading (Feng, Chen, Zhu, He, & Wang, 2015; Mandonnet, Nouet, Gatignol, Capelle, & Duffau, 2007). The UF interconnects the anterior temporal lobe with the orbitofrontal cortex, including the inferior frontal gyrus (Catani, Howard, Pajevic, & Jones, 2002). Portions of this tract connecting the temporal and frontal components of the language and reading networks may account for the significant relation between FA of the UF and measures of single word comprehension in healthy young adults (Cummine et al., 2015; Vigneau et al., 2006).
1.3. Current Study
Neuroimaging research indicates the importance of a left – hemispheric network of inferior frontal, temporal, and occipitotemporal cortical regions in reading, with connecting white matter tracts playing a vital role in communication among these regions. White matter integrity of tracts associated with the dual routes of reading differ in typical and poor decoders, but little is known about how the integrity of these tracts differentially impact reading proficiency in these two reader groups. Investigations into the relations of the IFOF, ILF, SLF, and UF to reading suggest differing relations between each tracts associated with reading and different aspects of reading proficiency. However, simultaneous examination of the role of each of these tracts and their differential relations to word reading accuracy, word reading fluency, and reading comprehension in typical and poor decoders has not been completed. While many studies report mean differences in white matter tract integrity between reader groups, the current study was uniquely interested in the structure – function relations between tract integrity and reading proficiency in poor and typical decoders.
Additionally, previous literature does not account for overlapping regions of white matter from tracts associated with the reading network. This makes it difficult to assess the contribution of individual tracts to behavioral outcomes, such as reading proficiency. A unique methodological feature of this study was the use of tracts that had been residualized to reduce overlap from other tracts thought to contribute to aspects of reading, in order to measure distinct contributions of each tract.
The current study examined white matter tract integrity in school – aged children with poor word reading skills and those with typically developing reading ability. The study examined differences in white matter integrity of four distinct tracts associated with the reading network: the IFOF, ILF, SLF, and UF, as well as their relation to measures of three aspects of reading proficiency. Consistent with previous literature, it was expected that white matter tract integrity (i.e. FA values) would differ in poor and typical readers, impacting structure – function relations. For word reading accuracy, we hypothesized a relation with FA values in the left SLF only in typical readers. This is because proficient word reading ability relies heavily on adequate phonological processing with the SLF playing a key role in the automatization of decoding associated with the dorsal phonological route. For the poor readers, we hypothesized a relation between the left ILF and word reading due to their poor phonological decoding ability, instead requiring use of the orthographic route to support the increased effort required for successful reading in this group.
We hypothesized that word reading fluency would be positively correlated with FA values in the left IFOF and ILF because of the association of these tracts with the ventral orthographic route of reading. A relation between these tracts and fluency in the poor reader group would suggest the effortful processes associated with reading fluency in this group. Similarly, a positive relation of word reading fluency and the UF would support a hypothesized coordinating role of the UF between the dual routes of reading.
Finally, for both groups, we hypothesized a positive relation of reading comprehension with FA values in the left ILF and bilateral UF; however, we expected a stronger relation observed in typical decoders because of the reliance on proficient phonological decoding required for successful reading comprehension. A relation between the UF and reading comprehension was thought to support the role of this tract in the ventral orthographic route.
2. Method
2.1. Participants
Participants were recruited from a longitudinal study of reading intervention (Denton et al., 2011; Vaughn et al., 2010) that began in 2005 and extended through 2010. They included those students who had completed all relevant behavioral measures and received structural MRI scans (including a DTI sequence) as part of participation in the intervention studies. Participants included in the current study had a verbal and/or fluid intelligence score at or above 70 on the Kaufman Brief Intelligence Test – 2 (Kaufman, A. S. & Kaufman, 2004) in order to rule out intellectual disabilities. Children with other neurological conditions or diagnosed attention disorders were excluded. Reader group classification was based on students’ score on the Letter-Word Identification subtest of the Woodcock-Johnson III Tests of Achievement (Woodcock, McGrew, & Mather, 2001). Poor readers were identified as those students who obtained a standard score below 90 (25th percentile), indicating problems with single word reading.
All participants were scanned using the same MRI scanner and using the same DTI sequences, as detailed below. Ninety-three participants from the parent study successfully completed a DTI sequence that qualified for inclusion in the current study. Nine participants were excluded because of incomplete behavioral data. DTI data for remaining participants were assessed for quality with three participants excluded for excessive motion and five for incomplete brain coverage. In all, 76 participants were included in the final analysis; 27 poor and 49 typical readers (see Table 1).
TABLE 1.
Demographic Background for Typical and Poor Decoders
| Variable | Typical (N = 49) | Poor (N = 27) |
|---|---|---|
| Gender (N [% Male]) | 25 (52) | 18 (67) |
| Handedness (N [% Right]) | 43 (90) | 21 (78) |
| Ethnicity (N [%]) | ||
| African American | 31 (65) | 18 (67) |
| Caucasian | 2 (4) | 0 (0) |
| Hispanic | 15 (31) | 9 (33) |
2.2. Reading measures
As part of the larger parent studies, each student was administered a series of standardized reading evaluations in a quiet area of his or her school for one or two sessions over a course of one week. Assessments were administered and scored by a trained member of the research team who was blind to individual group assignment, in accordance with standardized task administration procedures. These included individually administered tests of word reading accuracy, fluency, and comprehension.
2.2.1. Word reading accuracy
Word reading accuracy was assessed using the age – based extended scale score from the Letter - Word Identification (LWID) subtest of the Woodcock - Johnson III Tests of Achievement (Woodcock, et al., 2001). The LWID subtest is an oral test of reading skill which assesses the ability to accurately read a list of real words. The participant reads words aloud from an increasingly difficult list designed for people 4 to 90 years with basal and ceiling rules. Pronunciation is scored by the examiner as correct or incorrect, consistent with standardized task procedures. Coefficient alphas based on a large sample from the parent study ranged from 0.93 to 0.97.
2.2.2. Word reading fluency
The Sight Word Reading Efficiency subtest of the Test of Word Reading Efficiency (TOWRE; Torgesen, Wagner, & Rashotte, 1999) was used to assess reading fluency. The TOWRE measures students’ ability to read words out of context. It consists of a timed measure of real word reading, which measures students’ ability to recognize common words quickly and accurately. The internal consistency for this well standardized test exceeds 0.95. The total number of words read correctly in the 45 second time limit used as a measure of word reading fluency.
2.2.3. Reading comprehension
Reading comprehension was assessed using the age – based extended scaled score from the Passage Comprehension subtest of the Woodcock - Johnson III Tests of Achievement (Woodcock et al., 2001). Passage Comprehension assesses the students’ language comprehension and reading skills using a cloze procedure. This subtest requires the students to read a list of sentences silently and then decide on a specific word needed to complete the sentence, with vocabulary level progressively increasing. Alpha coefficients based on a large sample from the parent study ranged from 0.93 to 0.97.
2.3. Magnetic Resonance Imaging
2.3.1. MRI data acquisition
Whole - brain MRI data was acquired with a 3T Philips Achieva system with a SENSE parallel imaging receiver head coil. The MRI protocol included (a) conventional MRI (3D spoiled gradient-echo, field – of -view= 240 x 240 mm2 (isotropic voxel size= 0.9375 mm), (b) 2D dual spin-echo images TE1/TE2/TR= 10/90/5000 ms, in the axial plane (3 mm slice thickness, square field – of – view= 240 x 240 mm2 at 44 sections), and (c) phase-sensitive MRI in the sagittal and axial planes, in addition to a matching prescription of axial diffusion encoded data.
Diffusion - weighted imaging data was collected using a single - shot spin echo diffusion sensitized echo - planar imaging sequence with the balanced Icosa21 encoding scheme, a diffusion sensitization of b= 1000 s mm−2, repetition and echo times of TR= 6.1 s and TE = 84 ms, respectively (Hasan et al., 2012). Distortion artifacts were reduced using a SENSE acceleration factor or k - space under - sampling of R of two (Hasan, Halphen, Boska, & Narayana, 2008).. Diffusion weighted image volumes were collected with 21 directions as 44 contiguous 3 mm axial slices with no gap, whole – brain coverage, a square field – of – view= 240 x 240 mm2 (non-isotropic voxel size= 0.9 x 0.9 x 3 mm), and a square image matrix of 256 x 256 that matched the 3D-SPGR (spoiled gradient or field echo) and 2D conventional MRI dual spin echo sequences described above. (Hasan et al., 2007). Total DTI acquisition time was approximately 7 minutes.
2.3.2. MRI data processing
DTI data was processed using the FSL package version 5.0.7 (Jenkinson, Beckmann, Behrens, Woolrich, & Smith, 2012). Data from 79 participants was preprocessed including correction for motion and eddy current effects, with data containing excessive motion artifacts excluded from analysis (n= 3). FMRIB’s Diffusion Toolbox was used to fit the tensor model and to compute FA maps.
2.3.3. Tract - based spatial statistics
Voxel – wise analysis was performed using tract – based spatial statistics (TBSS; Smith et al., 2006). TBSS is a fully automated voxel based approach to DTI analysis which has been shown to improve the sensitivity, objectivity, and robustness of multi - subject DTI analyses. TBSS maps individual DTI data onto a white matter ‘skeleton’ created from mean FA in a fashion that resolves the typical issues of alignment and correspondence. Skeletonize DTI scalars produced by TBSS also help to reduce confounds associated with non-isotropic acquisition, such as those of the current study.
All individual FA maps were nonlinearly registered to the FMRIB58_FA standard - space image template and then affine – transformed into standard Montreal Neurological Institute space. A mean skeleton map was generated based on the mean FA image of all subjects. The mean FA skeleton represents the center of white matter tracts that are common to all participants. The mean FA skeleton was thresholded at a value of 0.20 to ensure that analysis was restricted to only points within major white matter tracts, which have been successfully aligned across all participants. Voxels with FA < 0.20 were excluded from analysis to avoid partial volume effect from neighboring gray matter. Finally, each subject’s aligned FA image was then projected onto the mean FA skeleton, resulting in a skeletonized FA map for each individual.
Tract of interest analysis was performed in order to obtain quantitative DTI indices of tracts hypothesized to be involved in reading: the IFOF, ILF, SFL (frontal, temporal, and parietal projections), and UF. A unique methodological feature of this study was the use of FA values of tracts which had been residualized in order to restrict analyses to voxels which were not also included in more than one probability map included in the John Hopkins’s University white matter tract atlas within the FSL software package (Hua et al., 2008, Mori et al., 2006). By utilizing a more restrictive tract delineation, which only consisted of those areas that did not contain voxels also accounted for in maps of other tracts of interest, the current study was able to assess the relation of each restrictive white matter tract to measures of reading proficiency without the confound of overlapping fibers from other tracts of interest.
White matter tracts of interest were masked bilaterally and symmetrically. Bilateral residualized masks were created by subtracting out all voxels that were also accounted for by overlapping tracts. This was done with multiple fslmaths statements (Jenkinson et al., 2012) by cumulatively subtracting out overlapping voxels associated with each of the three additional tracts. This process provided a mask representing the unique segment of each of the four tracts of interest. Resulting masks were overlaid over individual white matter tract skeletons to provide FA values for the tracts of interest for each subject. Mean FA values for skeletonized and residualized IFOF, ILF, SLF, and UF were then correlated with reading proficiency scores.
2.4. Statistical analyses
2.4.1. Group comparisons
Independent sample t-tests were computed to compare typical and poor readers on age-based standardized reading proficiency scores and age. The analysis of covariance was performed to compare groups in terms of white matter microstructure, controlling for age. Independent t-tests and analysis of covariance were estimated using the PROC TTEST and PROC GLM procedures (respectively) in SAS software, Version 9.4 of the SAS System for Windows.
2.4.2. Structure-function relations
The correlation analyses of skeletonized and residualized IFOF, ILF, SLF, and UF tracts with word reading accuracy, word reading fluency, and reading comprehension in typical and poor decoders were estimated using three correlation estimates: the Pearson product-moment correlation, percentage bend correlation, and the skipped correlation using the Donoho-Gasko median (DGM; Wilcox, 2003). Because the Pearson correlation is sensitive to outliers, the two robust correlations were computed to support inferences regarding investigated structure - function relations. Robust correlations are outlier resistant statistical methods that estimate the degree of linear relation between two variables. The percentage bend is robust to univariate outliers while the skipped correlation using DGM is robust to both univariate and multivariate outliers (Wilcox, 2003). The magnitude of the Pearson correlation coefficient is comparable to the magnitude of the robust correlation coefficients only if the two variables have a relatively small number of univariate outliers (Kulesz, Tian, Juranek, Fletcher, & Francis, 2014). Using alternative statistical approaches that vary in their underlying assumptions to estimate structure - function relations not only can aid investigators when confronted with outliers but can also strengthen statistical inferences by discussing patterns of relations across estimates rather than individual estimates (Kulesz et al., 2014), essential for neuroimaging studies where the difficulties of acquiring and analyzing data often result in small sample sizes with elevated risked for Type I and Type II errors. In the context of the current study, the patterns of relations were examined by looking at the magnitude of correlation coefficients across the three estimators. Increased reliability of findings was assumed if the magnitude of correlation coefficients was consistent across the estimators. To be clear, the current study did not focus on looking at significance tests for two reasons: (1) multiple correlation coefficients were estimated, and (2) study was underpowered. Because criteria for interpreting magnitude of correlation coefficients are somewhat arbitrary and vary depending on the context (Cohen, 1988) the following set of criteria was used in the current study: (a) patterns of structure - function relations with the magnitude of correlation coefficients below 0.20 were considered weakly related, (b) patterns of correlation coefficients between 0.20 and 0.40 were considered moderately related, and (c) patterns of correlation coefficients exceeding 0.40 were considered highly related. These criteria were used because structure-function relations are often somewhat difficult to detect. Increased reliability of findings was assumed if the magnitude of correlational estimates was comparable across all estimates. Although a total of 144 correlations were computed, we focused on consistency across estimates as an indicator of the reliability of the findings. Correlation analyses were computed in R version 3.0.2 (R Development Core Team, 2008) using the boot package version 1.3–9, foreign package version 0.8–55, MASS package version 7.3–29, and custom written functions.
3. Results
3.1. Preliminary Analyses
Performance on reading proficiency measures fell roughly within expected ranges for both reader groups (see Table 2). For the typical reader group, word reading fluency and reading comprehension were correlated with word reading accuracy, r = 0.70, p < 0.001 and r = 0.63, p < .001 respectively. Reading fluency and comprehension were also correlated (r = 0.50, p < 0.001) in this reader group. For the poor reader group, word reading fluency and reading comprehension were correlated with word reading accuracy, r = 0.74, p < 0.001 and r = 0.72, p < .001 respectively. Reading fluency and comprehension were also correlated (r = 0.62, p < 0.001) in this reader group.
TABLE 2.
Independent T-tests Demonstrating Significant Differences in Word Reading Accuracy, Word Reading Fluency, Reading Comprehension, and Age at Time of MRI in Typical and Poor Decoders
| Variable | Typical (n = 49) M(SD) |
Poor (n = 27) M(SD) |
t | p |
|---|---|---|---|---|
| Age | 10.47 (2.78) | 11.95 (2.77) | −2.22 | 0.03 |
| Accuracy | 101.90 (8.84) | 75.96 (11.89) | 10.82 | < 0.001 |
| Comprehension | 95.53 (9.83) | 75.96 (10.09) | 8.22 | < 0.001 |
| Fluency | 99.06 (9.80) | 80.48 (12.58) | 7.14 | < 0.001 |
Note: M = mean; SD = standard deviation; Bolded = statistically significant values.
Whole - brain TBSS analysis indicated no significant differences between groups after correction for multiple comparisons. Preliminary comparisons of traditional full tract masks revealed an overlap in voxels associated with multiple tracts of interest (i.e., 65% overlap with left IFOF and UF) (see Figure 1).
FIGURE 1.
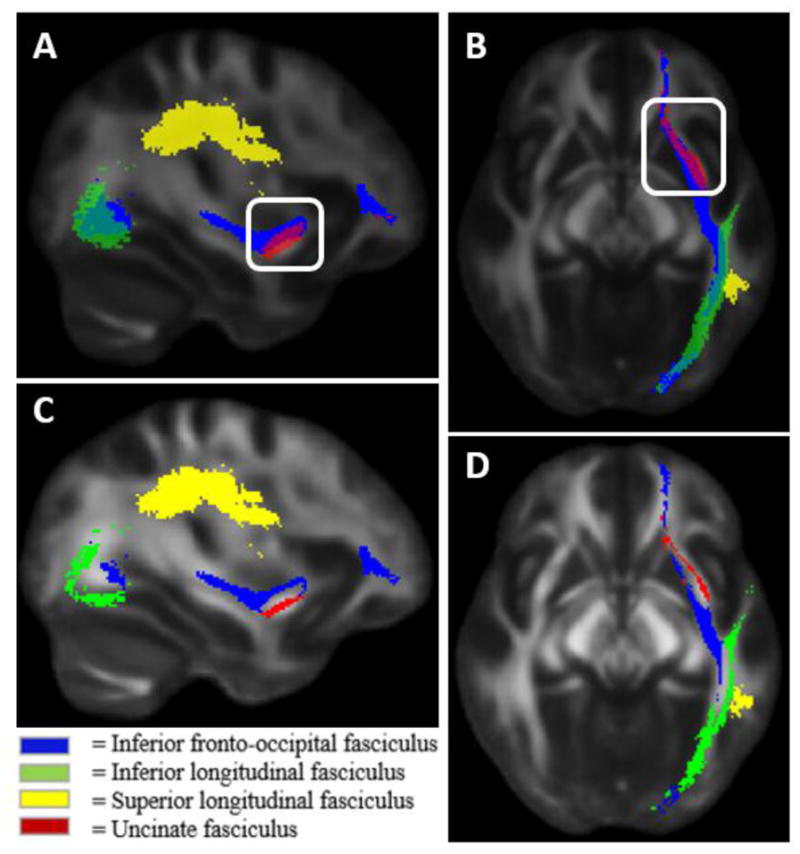
A and B present non-residualized tract – based spatial statistics masks for white matter tracts associated with reading proficiency. Masking inside white box highlight a portion of the 65% overlap between the left inferior fronto - occipital fasciculus and the left uncinate fasciculus. Voxels containing overlap were identified to be subtracted out during the residualization process. C and D present residualized tract masks with overlapping tract voxels subtracted out.
3.2. Group Comparisons of Reading Proficiency and Age at Time of MRI
Table 2 presents means and standard deviations, independent t-tests results, as well as effect sizes for word reading accuracy, word reading fluency, reading comprehension, and age at time of MRI for typical and poor reader groups. As expected, groups significantly differed on all measures of reading ability, with typical readers performing better on all reading proficiency measures (p < .001). Poor readers were, on average, about 15 months older at time of MRI (t = −2.22, p < .05). This likely reflects retention because grade was the selection variable in the intervention studies.
3.3. Group Comparisons of White Matter Microstructure
Table 3 presents means and standard deviations for FA values as a measure of white matter tract integrity along with the results of analysis of covariance in typical and poor readers for tract of interest analysis.
TABLE 3.
Analysis of Covariance of Fractional Anisotropy Values for Tracts of Interest with Age at Time of MRI for Typical Readers and Poor Decoders
| Tract | Typical (n = 49) M(SD) |
Poor (n = 27) M(SD) |
F(3,72) | p |
|---|---|---|---|---|
| Right IFOF | 0.50 (0.02) | 0.50 (0.03) | 1.09 | 0.36 |
| Right ILF | 0.48 (0.02) | 0.47 (0.02) | 1.43 | 0.24 |
| Right SLF | 0.47 (0.02) | 0.47 (0.02) | 0.79 | 0.50 |
| Right UF | 0.52 (0.03) | 0.54 (0.04) | 9.92 | < 0.001 |
| Left IFOF | 0.51 (0.03) | 0.51 (0.03) | 1.19 | 0.32 |
| Left ILF | 0.45 (0.02) | 0.45 (0.02) | 0.87 | 0.46 |
| Left SLF | 0.46 (0.02) | 0.46 (0.02) | 1.44 | 0.24 |
| Left UF | 0.53 (0.04) | 0.55 (0.03) | 8.14 | < 0.001 |
Note: M = mean; SD = standard deviation; Bolded = statistically significant values. IFOF = inferior fronto occipital fasciculus; ILF = inferior longitudinal fasciculus; SLF = superior longitudinal fasciculus; UF = uncinate fasciculus.
The results of analysis of covariance revealed statistically significant differences between typical and poor readers in the mean FA values of right and left UF (F(3,72) = 9.92, p < 0.001; F(3,72) = 8.14, p < 0.001, respectively), controlling for age. Poor readers had higher mean FA values of bilateral UF relative to typical readers.
3.4. Correlational Estimates of Structure-Function Relations for Typical Readers
Table 4 presents the correlational estimates of structure - function relations for the typical reader group. Reading comprehension was negatively correlated with FA values in the right UF (r = −0.21 – −0.28). This finding was observed across all correlational methods, indicating that, contrary to hypotheses, lower reading comprehension performance was related to higher FA values in right UF in typical readers.
TABLE 4.
Correlational Estimates for Structure - Function Relations for Residualized Tracts in Typical and Poor Decoders
| Typical | Poor | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| Pair of Variables | 1 | 2 | 3 | 1 | 2 | 3 |
| Accuracy–Right IFOF | 0.01 | −0.07 | −0.05 | −0.07 | −0.05 | −0.07 |
| Accuracy – Right ILF | −0.02 | −0.15 | −0.04 | −0.08 | −0.10 | −0.08 |
| Accuracy – Right SLF | −0.01 | −0.07 | −0.13 | −0.10 | −0.13 | −0.10 |
| Accuracy – Right UF | −0.13 | −0.13 | −0.09 | 0.10 | 0.26 | 0.14 |
| Accuracy – Left IFOF | −0.04 | −0.10 | −0.09 | 0.01 | 0.04 | 0.01 |
| Accuracy – Left ILF | 0.01 | −0.15 | −0.04 | 0.14 | < −0.01 | 0.14 |
| Accuracy – Left SLF | −0.10 | −0.15 | −0.20 | −0.19 | −0.22 | −0.19 |
| Accuracy – Left UF | −0.21 | −0.14 | −0.11 | 0.04 | 0.19 | 0.04 |
|
| ||||||
| Comprehension – Right IFOF | −0.03 | −0.02 | −0.03 | 0.03 | 0.04 | 0.03 |
| Comprehension – Right ILF | −0.15 | −0.21 | −0.15 | 0.11 | 0.08 | 0.11 |
| Comprehension – Right SLF | −0.11 | −0.12 | −0.11 | −0.03 | −0.05 | −0.03 |
| Comprehension – Right UF | −0.28* | −0.21 | −0.28 | 0.21 | 0.29 | 0.21 |
| Comprehension – Left IFOF | 0.06 | 0.09 | 0.06 | 0.13 | 0.20 | 0.13 |
| Comprehension – Left ILF | −0.02 | −0.08 | −0.02 | 0.30 | 0.21 | 0.30 |
| Comprehension – Left SLF | −0.18 | −0.18 | −0.18 | −0.08 | −0.20 | −0.08 |
| Comprehension – Left UF | −0.29* | −0.19 | −0.29 | 0.10 | 0.16 | 0.10 |
|
| ||||||
| Fluency Right – IFOF | 0.13 | 0.09 | 0.19 | 0.18 | 0.21 | 0.18 |
| Fluency Right – ILF | −0.03 | −0.11 | 0.03 | 0.07 | 0.11 | 0.07 |
| Fluency Right – SLF | 0.03 | 0.02 | 0.04 | 0.12 | 0.17 | 0.12 |
| Fluency – Right UF | 0.04 | 0.01 | 0.06 | 0.32 | 0.39* | 0.32 |
| Fluency –Left IFOF | 0.03 | 0.04 | 0.11 | 0.31 | 0.34* | 0.31 |
| Fluency – Left ILF | 0.05 | 0.01 | 0.05 | 0.17 | 0.22 | 0.18 |
| Fluency –Left SLF | −0.04 | −0.01 | 0.02 | 0.03 | 0.04 | 0.03 |
| Fluency –Left UF | 0.07 | 0.10 | 0.07 | 0.21 | 0.19 | 0.21 |
Note: Typical decoder N = 49; Poor decoder N = 27; Bolded = structure - function relations moderately related across all estimators;
p < 0.05.
IFOF = inferior fronto – occipital fasciculus; ILF = inferior longitudinal fasciculus; SLF= superior longitudinal fasciculus; UF = uncinate fasciculus. 1 = Pearson correlation; 2 = Percentage bend correlation; 3 = Skipped correlation using Donoho - Gasko median.
3.5. Correlational Estimates of Structure-Function Relations for Poor Readers
Table 4 also presents the correlational estimates of structure - function relations for the poor reader group. Consistent with hypotheses, there were positive, moderate relations between comprehension and FA values in the right UF (r = 0.21 – 0.29) and left IFL (r = .021 – 0.30) across all three correlational estimates. Better comprehension performance was associated with the higher FA values in the right UF and left IFL in poor readers. Fluency was also moderately, positively correlated with FA values in the right UF (r = 0.32 – 0.39) and left IFOF (r = 0.31 – 0.34), with better fluency scores related to higher FA values in the right UF and left IFOF in this reader group.
4. Discussion
The current study examined the relations of microstructural integrity of residualized white matter tracts associated with the dual route of reading and three components of reading proficiency. White matter integrity of typical and poor readers differed in only one tract (UF) bilaterally, although results of the current study supported differential relations between the four tracts and different aspects of reading proficiency for each reader group.
In the typical reader group, we hypothesized FA values in the left SLF would be associated with word reading accuracy because of its role in phonological decoding necessary for proficient word reading. A relation between reading comprehension and the left UF was also hypothesized. However, white matter tract integrity was related to reading comprehension, but not accuracy or fluency with a moderate, negative relation between the right UF and reading comprehension. Observed negative correlations suggests that increased FA in this tract may negatively impact comprehension in students with typically developing reading skills.
Previous research has indicated a linear relation between reading comprehension and both the ILF and SLF (Feldman et al., 2012; Horowitz-Kraus et al., 2014). Horowitz-Kraus et al. (2014) found that FA values in the left ILF, as well as bilateral SLF, were significantly correlated reading comprehension. Reading comprehension was hypothesized to be related to the left ILF in the current study, because of its role in the orthographic route of reading. More consistent with previous studies, preliminary traditional full tract analysis in our study did suggest a moderate negative correlation of reading comprehension and right ILF (r = −0.20) and left SLF (r = −0.20) in the typical reader group. However, as in previous studies, the full tract analysis did not account for overlapping fibers from the IFOF and/or UF in these tracts. Different structure – function relations with full and residualized tracts would suggest that how tracts of interest are defined is critical, particularly in tracts with high concentrations of crossing fibers such as those in the current study. Understanding how tracts are defined has important implications for better understanding brain – behavior relations of complex cognitive functions, such as reading comprehension, which utilize multiple cortical regions and connecting white matter tracts.
Taken together, differences in structure – function relations for full and residualized tracts may suggest that the contribution of tracts of interest to reading comprehension may be better explained by the combined utilization of multiple white matter tracts associated with reading comprehension. For example, while no relation between the left UF and comprehension was observed in typical readers in the current study, previous research with typical children and adolescents has shown a significant correlation between the traditionally defined full tract UF and reading comprehension (Feldman et al., 2012). This would indicate that the relation between white matter integrity and reading comprehension may be better explained by the coordinated use of multiple overlapping fibers of tracts associated with the orthographic route (i.e. left IFOF, ILF, and UF) as opposed to any single tract.
The lack of relations to residualized tracts may be due to the coordinated use by typical readers of these tracts and the cortical regions they connect. For students with typically developing decoding skills, accuracy, fluency, and comprehension work together to provide proficient reading (Dehaene, 2009). As such, underlying cortical correlates and their connecting white matter structures must function together during these tasks to facilitate smooth and adequate reading. Therefore, it would follow that no one tract would be associated with different aspects of reading proficiency. Instead, the network as a whole would function together, providing a smooth coordination of these skills.
Conversely, results of the current study would suggest that poor readers may rely more heavily on individual tracts as opposed to more efficient use of the coordinated network. Similar to typical readers, there was a consistent pattern of moderate correlation coefficients in relation to reading comprehension in poor readers. Consistent with hypotheses, the right UF was positively, moderately related to comprehension suggesting that increased FA in the right UF was associated with improved reading comprehension in this reader group. This was a differing pattern of relations from that observed in typical readers. This would suggest that increased FA in this tract would facilitate comprehension in poor readers who rely more heavily on individual tract resources.
Previous research (Horowitz-Kraus et al., 2014; Lebel et al., 2013) also suggested a contribution of bilateral ILF to reading fluency. However, these studies did not take into account the considerable overlap between the ILF with other tracts, such as the IFOF, also associated with reading. Neither the right ILF nor IFOF were correlated with word reading fluency in the current study with residualized tract methodology. The prelimiary full tract analysis indicated a relation between reading fluency and bilateral IFOF (r = 0.25) and right ILF (r = 0.20) for the poor reader group. Instead, the relation between fluency and the left IFOF was highlighted as a result of the residualization process. These findings would suggest that the ILF does not uniquely associate with word reading fluency for readers with poor word reading skills. Instead, as was the case with reading comprehension in typical readers, differences in relations with full and residualized white matter tracts may indicate that tracts associated with the ventral orthographic route of reading function together to account for fluency in reading proficiency.
Brauer, Anwander, and Friederici (2011) argued that during development children distribute language processing across both the dorsal and ventral pathways, as evident by activation of the inferior frontal lobe during functional MRI language tasks. More proficient adults were shown to activate a more precise area of the inferior frontal lobe that corresponds with the termination of the dorsal pathway (SLF). This would suggest that typical readers are more efficient in their recruitment of cortical areas and connecting white matter structures associated with the ventral route; less proficient readers, such as those in our poor reader group, may rely more heavily on the ventral route of reading.
Typically developing readers and those with poor decoding skills, such as those in our poor reader group, may vary in structure - function relations in reading, with poor readers relying more heavily on individual white matter structures in various aspects of reading proficiency. In a case study of an adolescent lacking the SLF, Yeatman and Feldman (2013) found that while language and reading skills were slow to develop, key aspects of reading proficiency (accuracy, phonemic decoding, and comprehension) were roughly within normal ranges. Instead, this individual seemed to utilize structures such as the UF and IFOF to facilitate proficient reading. A redundancy in the dual routes of reading may account for this individual’s ability to utilize the ventral route in the development of adequate reading skills. It may also explain the relations between individual white matter tracts of the ventral route and reading in our poor reader group.
These findings highlight the unique relations of the IFOF, ILF, SLF, and UF to word reading accuracy, fluency, and reading comprehension. These findings also suggest that associations between white matter integrity and reading proficiency may differ in elementary – aged students with typical and poor word reading ability. The reason for these differing associations awaits further investigation. Evidence points to structural variations in brain regions associated with reading, as well as functional abnormalities, in individuals with poor reading skills (Elnakib et al., 2014; Linkersdörfer, Lonnemann, Lindberg, Hasselhorn, & Fiebach, 2012; Maisog, Einbinder, Flowers, Turkeltaub, & Eden, 2008; Schurz et al., 2010). . Longitudinal studies addressing the role of these regions in reading development also indicate that alterations in typical growth of the developing brain may negatively impact reading. Differences in reading ability observed in good versus poor readers could be the result of altered neurodevelopmental changes in brain regions associated with the reading network.
A limitation of the current study was the use of voxel based TBSS methodology. Voxel based approaches limit the interpretation of cross subject differences in FA in areas of partial volume such as where white matter mixes with grey matter or areas where two or more white matter fiber systems overlap (Assaf & Pasternak, 2008; Smith et al., 2006). The mean FA skeleton used in TBSS is thresholded, to account for partial volume where this overlap may occur. However, in smaller tracts, such as the UF, or in areas where tracts from multiple orientations cross, such as in the SLF, it is difficult to determine whether differences in FA is in fact due to within tract FA changes or if it is related to partial voluming associated with overlapping white matter fibers (Smith et al., 2006). The use of residualized tract masks can reduce this likelihood in voxels specifically identified as containing overlapping white matter tracts of interest but this methodology cannot account for individual differences in those areas identified as “unique” to a given tract or in areas where unidentified tracts may also overlap.
Limitations of the current sample include selection based on performance on the measure of word reading accuracy. Therefore, distribution of this measure is truncated and may have limited our ability to assess structure - function relations for this measure. Additionally, the groups differed significantly on age due to selection criteria of the parent study (grade rather than age). While age was accounted for in structural group differences, robust partial correlation estimators that have been extensively used in robust statistics research are not available, and therefore age could not be accounted for in these relations. Pearson partial correlation estimates controlling for age did not alter the patterns reported for the robust correlations except for the relation between the right UF and reading fluency (r = 0.09) in typical readers.
Despite these limitations we believe these findings expand our understanding of white matter microstructure as it relates to different aspects of reading proficiency in typical versus poor readers. Differences in integrity in these tracts associated with the dual route of reading could underlie specific reading deficits.
Highlights.
Typical and poor decoders rely on different white matter tracts for reading proficiency.
Comprehension is related to the right uncinate fasciculus in typical readers and poor decoders.
Left inferior longitudinal fasciculus is related to comprehension in poor decoders.
Reading fluency is related to the right uncinate and left inferior fronto-occipital fasciculi in poor decoders.
Word reading is unrelated to white matter integrity in either decoding group.
Acknowledgments
This research was supported in part by grant P50HD052117 from the Eunice Kennedy Shriver National Institute of Child Health & Human Development. The content is solely the responsibility of the authors and does not necessarily represent the official views of the Eunice Kennedy Shriver National Institute of Child Health & Human Development or the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alexander AL, Lee JE, Lazar M, Field AS. Diffusion tensor imaging of the brain. Neurotherapeutics. 2007;4(3):316–329. doi: 10.1016/j.nurt.2007.05.011. http://doi.org/10.1016/j.nurt.2007.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assaf Y, Pasternak O. Diffusion tensor imaging (DTI)-based white matter mapping in brain research: a review. Journal of Molecular Neuroscience : MN. 2008;34(1):51–61. doi: 10.1007/s12031-007-0029-0. http://doi.org/10.1007/s12031-007-0029-0. [DOI] [PubMed] [Google Scholar]
- Basser PJ, Pierpaoli C. Micostructual and physiological features of tissues eludicated by quantitative-diffusion-tensor MRI. Journal of Magnetic Resonance. 1996;111(Series B):209–219. doi: 10.1006/jmrb.1996.0086. http://doi.org/10.1016/j.jmr.2011.09.022. [DOI] [PubMed] [Google Scholar]
- Brauer J, Anwander A, Friederici AD. Neuroanatomical prerequisites for language functions in the maturing brain. Cerebral Cortex (New York, NY : 1991) 2011;21(2):459–66. doi: 10.1093/cercor/bhq108. http://doi.org/10.1093/cercor/bhq108. [DOI] [PubMed] [Google Scholar]
- Carter JC, Lanham DC, Cutting LE, Clements-Stephens AM, Chen X, Hadzipasic M, … Kaufmann WE. A dual DTI approach to analyzing white matter in children with dyslexia. Psychiatry Research - Neuroimaging. 2009;172(3):215–219. doi: 10.1016/j.pscychresns.2008.09.005. http://doi.org/10.1016/j.pscychresns.2008.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catani M, Howard RJ, Pajevic S, Jones DK. Virtual in Vivo Interactive Dissection of White Matter Fasciculi in the Human Brain. NeuroImage. 2002;17:77–94. doi: 10.1006/nimg.2002.1136. http://doi.org/10.1006/nimg.2002.1136. [DOI] [PubMed] [Google Scholar]
- Catani M, Jones DK, Ffytche DH. Perisylvian language networks of the human brain. Annals of Neurology. 2005;57(1):8–16. doi: 10.1002/ana.20319. http://doi.org/10.1002/ana.20319. [DOI] [PubMed] [Google Scholar]
- Catani M, Mesulam M. The arcuate fasciculus and the disconnection theme in language and aphasia: History and current state. Cortex. 2008;44(8):953–961. doi: 10.1016/j.cortex.2008.04.002. http://doi.org/10.1016/j.cortex.2008.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J. Statistical Power Analysis for the Behavioral Sciences. 2 1988. [Google Scholar]
- Cohen L, Dehaene S, Naccache L, Lehéricy S, Dehaene-Lambertz G, Hénaff MA, Michel F. The visual word form area: Spatial and temporal characterization of an initial stage of reading in normal subjects and posterior split-brain patients. Brain. 2000;123:291–307. doi: 10.1093/brain/123.2.291. http://doi.org/10.1093/brain/123.2.291. [DOI] [PubMed] [Google Scholar]
- Cohen L, Lehéricy S, Chochon F, Lemer C, Rivaud S, Dehaene S. Language-specific tuning of visual cortex? Functional properties of the Visual Word Form Area. Brain. 2002;125(5):1054–1069. doi: 10.1093/brain/awf094. http://doi.org/10.1093/brain/awf094. [DOI] [PubMed] [Google Scholar]
- Coltheart M. In defence of dual-route models of reading. Behavioral and Brain Sciences. 1985;8(04):709. http://doi.org/10.1017/S0140525X0004574X. [Google Scholar]
- Colheart M, Curtis B, Atkins P, Haller M. Models of reading aloud: Dual-route and parallel-distributed-processing approaches. Psychological Review. 1993;100(4):589–608. [Google Scholar]
- Coltheart M, Rastle K, Perry C, Langdon R, Ziegler J. DRC: A dual route cascaded model of visual word recognition and reading aloud. Psychological Review. 2011;108:204–256. doi: 10.1037/0033-295x.108.1.204. [DOI] [PubMed] [Google Scholar]
- Cummine J, Dai W, Borowsky R, Gould L, Rollans C, Boliek C. Investigating the ventral-lexical, dorsal-sublexical model of basic reading processes using diffusion tensor imaging. Brain Structure & Function. 2015;220(1):445–55. doi: 10.1007/s00429-013-0666-8. http://doi.org/10.1007/s00429-013-0666-8. [DOI] [PubMed] [Google Scholar]
- Dehaene S. Reading in the brain. New York: Viking Penguin; 2009. [Google Scholar]
- Denton Ca, Cirino PT, Barth AE, Romain M, Vaughn S, Wexler J, … Fletcher JM. An Experimental Study of Scheduling and Duration of “Tier 2” First-Grade Reading Intervention. Journal of Research on Educational Effectiveness. 2011;4(3):208–230. doi: 10.1080/19345747.2010.530127. http://doi.org/10.1080/19345747.2010.530127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elnakib A, Soliman A, Nitzken M, Casanova MF, Gimel’farb G, El-Baz A. Magnetic resonance imaging findings for dyslexia: A review. Journal of Biomedical Nanotechnology. 2014;10(10):2778–2805. doi: 10.1166/jbn.2014.1895. http://doi.org/10.1166/jbn.2014.1895. [DOI] [PubMed] [Google Scholar]
- Feldman HM, Lee ES, Yeatman JD, Yeom KW. Language and reading skills in school-aged children and adolescents born preterm are associated with white matter properties on diffusion tensor imaging. Neuropsychologia. 2012;50(14):3348–3362. doi: 10.1016/j.neuropsychologia.2012.10.014. http://doi.org/10.1016/j.neuropsychologia.2012.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng G, Chen HC, Zhu Z, He Y, Wang S. Dynamic brain architectures in local brain activity and functional network efficiency associate with efficient reading in bilinguals. NeuroImage. 2015;119:103–118. doi: 10.1016/j.neuroimage.2015.05.100. http://doi.org/10.1016/j.neuroimage.2015.05.100. [DOI] [PubMed] [Google Scholar]
- Frith U. Beneath the surface of developmental dyslexia. In: Patterson M, Marschall KE, Cotheart JC, editors. Surface dyslexia: Cognitive and neuropsychological studies of phonological reading. Hillsdale, NJ: Erlbaum; 1985. [Google Scholar]
- Frye RE, Hasan K, Malmberg B, Desouza L, Swank P, SMITH K, Landry S. Superior longitudinal fasciculus and cognitive dysfunction in adolescents born preterm and at term. Developmental Medicine and Child Neurology. 2010;52(8):760–766. doi: 10.1111/j.1469-8749.2010.03633.x. http://doi.org/10.1111/j.1469-8749.2010.03633.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasan KM, Halphen C, Boska MD, Narayana PA. Diffusion tensor metrics,T2 relaxation, and volumetry of the naturally aging human caudate nuclei in healthy young and middle-aged adults: Possible implications for the neurobiology of human brain aging and disease. Magnetic Resonance in Medicine. 2008;59(1):7–13. doi: 10.1002/mrm.21434. http://doi.org/10.1002/mrm.21434. [DOI] [PubMed] [Google Scholar]
- Hasan KM, Halphen C, Sankar A, Eluvathingal TJ, Kramer L, Stuebing KK, … Fletcher JM. Diffusion tensor imaging-based tissue segmentation: Validation and application to the developing child and adolescent brain. NeuroImage. 2007;34 doi: 10.1016/j.neuroimage.2006.10.029. http://doi.org/10.1016/j.neuroimage.2006.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasan KM, Molfese DL, Walimuni IS, Stuebing KK, Papanicolaou AC, Narayana PA, Fletcher JM. Diffusion tensor quantification and cognitive correlates of the macrostructure and microstructure of the corpus callosum in typically developing and dyslexic children. NMR in Biomedicine. 2012;25(11):1263–1270. doi: 10.1002/nbm.2797. http://doi.org/10.1002/nbm.2797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoeft F, McCandliss BD, Black JM, Gantman A, Zakerani N, Hulme C, … Gabrieli JDE. Neural systems predicting long-term outcome in dyslexia. Proc Natl Acad Sci U S A. 2011;108(1):361–366. doi: 10.1073/pnas.1008950108. http://doi.org/1008950108 [pii]\r10.1073/pnas.1008950108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horowitz-Kraus T, Wang Y, Plante E, Holland SK. Involvement of the right hemisphere in reading comprehension: A DTI study. Brain Research. 2014;1582:34–44. doi: 10.1016/j.brainres.2014.05.034. http://doi.org/10.1016/j.brainres.2014.05.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houston SM, Lebel C, Katzir T, Manis FR, Kan E, Rodriguez GG, Sowell ER. Reading skill and structural brain development. Neuroreport. 2014;25(5):347–52. doi: 10.1097/WNR.0000000000000121. http://doi.org/10.1097/WNR.0000000000000121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hua K, Zhang J, Wakana S, Jian H, Li X, Reich DS, … Mori S. Tract probability maps in stereotaxic spaces: Analyses of white matter anatomy and tract - specific quantification. NeuroImage. 2008;39:336–347. doi: 10.1016/j.neuroimage.2007.07.053. http://doi.org/10.1016/j.neuroimage.2007.07.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkinson M, Beckmann CF, Behrens TEJ, Woolrich MW, Smith SM. FSL. NeuroImage. 2012;62(2):782–790. doi: 10.1016/j.neuroimage.2011.09.015. http://doi.org/10.1016/j.neuroimage.2011.09.015. [DOI] [PubMed] [Google Scholar]
- Kaufman AS, Kaufman NL. Kaufman brief intelligence test. 2. Minneapolis, MN: Pearson Assessment (K-BIT-2); 2004. [Google Scholar]
- Kulesz PA, Tian S, Juranek J, Fletcher JM, Francis DJ. Relations Between Volumetric Measures of Brain Structure and Attentional Function in Spina Bifida: Utilization of Robust Statistical Approaches. Neuropsychology. 2014;(November) doi: 10.1037/neu0000166. http://doi.org/10.1037/neu0000166. [DOI] [PMC free article] [PubMed]
- Lebel C, Shaywitz B, Holahan J, Shaywitz S, Marchione K, Beaulieu C. Diffusion tensor imaging correlates of reading ability in dysfluent and non-impaired readers. Brain and Language. 2013;125(2):215–222. doi: 10.1016/j.bandl.2012.10.009. http://doi.org/10.1016/j.bandl.2012.10.009. [DOI] [PubMed] [Google Scholar]
- Linkersdörfer J, Lonnemann J, Lindberg S, Hasselhorn M, Fiebach CJ. Grey Matter Alterations Co-Localize with Functional Abnormalities in Developmental Dyslexia: An ALE Meta-Analysis. PLoS ONE. 2012;7(8):e43122. doi: 10.1371/journal.pone.0043122. http://doi.org/10.1371/journal.pone.0043122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maisog JM, Einbinder ER, Flowers DL, Turkeltaub PE, Eden GF. A Meta-analysis of Functional Neuroimaging Studies of Dyslexia. Annals of the New York Academy of Sciences. 2008;1145(1):237–259. doi: 10.1196/annals.1416.024. http://doi.org/10.1196/annals.1416.024. [DOI] [PubMed] [Google Scholar]
- Mandonnet E, Nouet A, Gatignol P, Capelle L, Duffau H. Does the left inferior longitudinal fasciculus play a role in language? A brain stimulation study. Brain. 2007;130(3):623–629. doi: 10.1093/brain/awl361. http://doi.org/10.1093/brain/awl361. [DOI] [PubMed] [Google Scholar]
- Mori S, Zhang J, Ahrens ET, Laidlaw DH, Readhead C, Brosnan CF, … Mori S. Principles of diffusion tensor imaging and its applications to basic neuroscience research. Neuron. 2006;51(5):527–39. doi: 10.1016/j.neuron.2006.08.012. http://doi.org/10.1016/j.neuron.2006.08.012. [DOI] [PubMed] [Google Scholar]
- Myers C, Vandermosten M, Farris R, Hancock R, Gimenez P, Black J, … Hoeft F. White Matter Morphometric Changes Uniquely Predict Children’s Reading Acquisition. Psychological …. 2014;(September):9–10. doi: 10.1177/0956797614544511. http://doi.org/10.1177/0956797614544511. [DOI] [PMC free article] [PubMed]
- Odegard TN, Farris EA, Ring J, McColl R, Black J. Brain connectivity in non-reading impaired children and children diagnosed with developmental dyslexia. Neuropsychologia. 2009;47(8–9):1972–1977. doi: 10.1016/j.neuropsychologia.2009.03.009. http://doi.org/10.1016/j.neuropsychologia.2009.03.009. [DOI] [PubMed] [Google Scholar]
- Pierpaoli C, Jezzard P, Basser PJ, Barnett A, Di Chiro G. Diffusion tensor MR imaging of the human brain. Radiology. 1996;201(3):637–48. doi: 10.1148/radiology.201.3.8939209. http://doi.org/10.1148/radiology.201.3.8939209. [DOI] [PubMed] [Google Scholar]
- R Development Core Team. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2008. [Google Scholar]
- Richlan F, Kronbichler M, Wimmer H. Structural abnormalities in the dyslexic brain: a meta-analysis of voxel-based morphometry studies. Human Brain Mapping. 2013;34(11):3055–65. doi: 10.1002/hbm.22127. http://doi.org/10.1002/hbm.22127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rimrodt SL, Peterson DJ, Denckla MB, Kaufmann WE, Cutting LE. White matter microstructural differences linked to left perisylvian language network in children with dyslexia. Cortex. 2010;46(6):739–749. doi: 10.1016/j.cortex.2009.07.008. http://doi.org/10.1016/j.cortex.2009.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAS Institute Inc. SAS/STAT® 9.4 User’s Guide. Cary, NC: SAS Institute Inc; 2014. [Google Scholar]
- Schlaggar BL, McCandliss BD. Development of Neural Systems for Reading. 2007 doi: 10.1146/annurev.neuro.28.061604.135645. http://dx.doi.org/10.1146/annurev.neuro.28.061604.135645. [DOI] [PubMed]
- Schurz M, Sturm D, Richlan F, Kronbichler M, Ladurner G, Wimmer H. A dual-route perspective on brain activation in response to visual words: Evidence for a length by lexicality interaction in the visual word form area (VWFA) NeuroImage. 2010;49(3):2649–2661. doi: 10.1016/j.neuroimage.2009.10.082. http://doi.org/10.1016/j.neuroimage.2009.10.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Johansen-Berg H, Rueckert D, Nichols TE, Mackay CE, … Behrens TEJ. Tract-based spatial statistics: Voxelwise analysis of multi-subject diffusion data. NeuroImage. 2006;31(4):1487–1505. doi: 10.1016/j.neuroimage.2006.02.024. http://doi.org/10.1016/j.neuroimage.2006.02.024. [DOI] [PubMed] [Google Scholar]
- Steinbrink C, Vogt K, Kastrup A, Müller HP, Juengling FD, Kassubek J, Riecker A. The contribution of white and gray matter differences to developmental dyslexia: Insights from DTI and VBM at 3.0T. Neuropsychologia. 2008;46(13):3170–3178. doi: 10.1016/j.neuropsychologia.2008.07.015. http://doi.org/10.1016/j.neuropsychologia.2008.07.015. [DOI] [PubMed] [Google Scholar]
- Torgesen JK, Wagner R, Rashotte C. Test of Word Reading Efficiency. Austin, TX: Pro-Ed; 1999. [Google Scholar]
- Vandermosten M, Boets B, Poelmans H, Sunaert S, Wouters J, Ghesqui??re P. A tractography study in dyslexia: Neuroanatomic correlates of orthographic, phonological and speech processing. Brain. 2012;135(3):935–948. doi: 10.1093/brain/awr363. http://doi.org/10.1093/brain/awr363. [DOI] [PubMed] [Google Scholar]
- Vaughn S, Cirino PT, Wanzek J, Wexler J, Fletcher JM, Denton CD, … Francis DJ. Response to Intervention for Middle School Students With Reading Difficulties: Effects of a Primary and Secondary Intervention. School Psychology Review. 2010;39(1):3–21. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/21479079. [PMC free article] [PubMed] [Google Scholar]
- Vellutino FR, Fletcher JM, Snowling MJ, Scanlon DM. Specific reading disability (dyslexia): what have we learned in the past four decades? Journal of Child Psychology and Psychiatry. 2004;45(1):2–40. doi: 10.1046/j.0021-9630.2003.00305.x. http://doi.org/10.1046/j.0021-9630.2003.00305.x. [DOI] [PubMed] [Google Scholar]
- Vigneau M, Beaucousin V, Hervé PY, Duffau H, Crivello F, Houdé O, … Tzourio-Mazoyer N. Meta-analyzing left hemisphere language areas: phonology, semantics, and sentence processing. NeuroImage. 2006;30(4):1414–32. doi: 10.1016/j.neuroimage.2005.11.002. http://doi.org/10.1016/j.neuroimage.2005.11.002. [DOI] [PubMed] [Google Scholar]
- Vogel AC, Petersen SE, Schlaggar BL. The VWFA: it’s not just for words anymore. Frontiers in Human Neuroscience. 2014;8(March):88. doi: 10.3389/fnhum.2014.00088. http://doi.org/10.3389/fnhum.2014.00088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilcox RR. Inferences based on multiple skipped correlations. Computational Statistics & Data Analysis. 2003;44(1–2):223–236. http://doi.org/10.1016/S0167-9473(03)00043-4. [Google Scholar]
- Woodcock RW, McGrew K, Mather N. Woodcock-Johnson III Tests of Achievement. Itasca, IL: Riverside; 2001. [Google Scholar]
- Yeatman JD, Dougherty RF, Ben-Shachar M, Wandell BA. Development of white matter and reading skills. Proceedings of the National Academy of Sciences. 2012;109(44):E3045, E3053. doi: 10.1073/pnas.1206792109. http://doi.org/10.1073/pnas.1206792109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeatman JD, Feldman HM. Neural plasticity after pre-linguistic injury to the arcuate and superior longitudinal fasciculi. Cortex. 2013;49(1):301–311. doi: 10.1016/j.cortex.2011.08.006. http://doi.org/10.1016/j.cortex.2011.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]


