Abstract
The casper strain of zebrafish is widely used in studies ranging from cancer to neuroscience. casper offers the advantage of relative transparency throughout adulthood, making it particularly useful for in vivo imaging by epifluorescence, confocal, and light sheet microscopy. casper was developed by selective breeding of two previously described recessive pigment mutants: 1) nacre, which harbors an inactivating mutation of the mitfa gene, rendering the fish devoid of pigmented melanocytes; and 2) roy orbison, a mutant with so-far unidentified genetic cause that lacks reflective iridophores. To clarify the molecular nature of the roy orbison mutation, such that it can inform studies using casper, we undertook an effort to positionally clone the roy orbison mutation. We find that roy orbison is caused by an intronic defect in the gene mpv17, encoding an inner mitochondrial membrane protein that has been implicated in human mitochondrial DNA depletion syndrome. The roy orbison mutation is phenotypically and molecularly remarkably similar to another zebrafish iridophore mutant called transparent. Using Cas9-induced crispants and germline mutants with disrupted mpv17 open reading frame, we show in trans-heterozygote embryos that new frameshift alleles of mpv17, roy orbison, and transparent fail to complement each other. Our work provides genetic evidence that both roy orbison and transparent affect the mpv17 locus by a similar if not identical genetic lesion. Identification of mpv17 mutants will allow for further work probing the relationship between mitochondrial function and pigmentation, which has to date received little attention.
INTRODUCTION
The three classes of zebrafish pigment cells - melanophores, xanthophores and iridophores - combine to give the adult fish its characteristic pigmentation pattern (McGowan and Barsh, 2016; Parichy and Spiewak, 2015; Rawls et al., 2001). Mutants with defects in one or more of these cells have yielded important insights into the genes regulating vertebrate pigmentation. For example, the zebrafish nacre (nac) mutant harbors an an inactivating mutation in the mitfa gene and are devoid of nearly all embryonic and adult melanophores (Lister et al., 1999), emphasizing the key role of this transcription factor as a master regulator of this cell type. Mutants with defects in iridophores are somewhat less common, but several have been observed including ltk/shady (shd) (Lopes et al., 2008), ednrba (rose) (Parichy et al., 2000), transparent (tra) (Krauss et al., 2013), and roy orbison (roy) (Ren et al., 2002).
Combinations of these pigmentation mutants have been used to yield adult zebrafish that are more transparent than the typical adult. Combining the mitfa allele nacw2 with the roy mutant yielded the casper strain (White et al., 2008); since casper lacks all light-absorbing melanophores as well as all light-reflecting iridophores, the strain is optimal for imaging of deeper structures throughout the life-cycle of the fish. The casper strain has been broadly applied to numerous imaging applications in the zebrafish field; however, a remaining caveat remains that the affected gene responsible for the roy orbison phenotype has remained unknown. To address this, we aimed to positionally clone and validate the causative mutation. Here, we provide sequencing and genetic evidence that the phenotype of roy is due to a defect in the mitochondrial inner membrane protein mpv17, which has previously been associated with the mitochondrial DNA depletion syndrome in mammals (Löllgen and Weiher, 2015). Our data reveals that the roy allele causes aberrant splicing due to a perturbation in the first intron of mpv17. Importantly, a recent publication linked the highly similar mutant tra to the identical defect in the mpv17 mRNA (Krauss et al., 2013), suggesting these two mutants are caused by similar or even the same lesion. By genetic complementation analysis with Cas9-induced de novo loss-of-function alleles that directly disrupt the open reading frame (ORF) of mpv17, we confirm that both roy and tra are allelic to mpv17 and to each other. Our work underlines the value of newly generated mutant alleles to resolve the causation of genetic lesions. Little information is available connecting mitochondrial function in pigmentation lineages and iridophores specifically, but will be an important future area of investigation for the field of vertebrate pigmentation.
RESULTS
roy orbison contributes to casper by iridophore loss and secondary effects on pigmentation
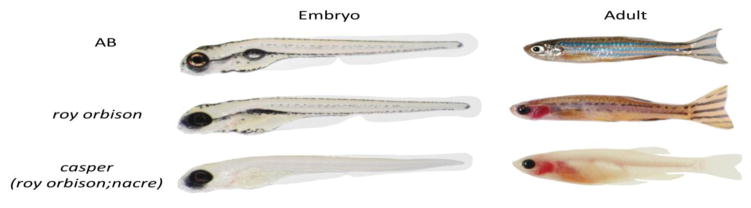
The most obvious phenotype of the recessive roy mutant is a severe, near complete loss of reflective iridophores in both embryos and adults (Figure 1). This gives the eyes its characteristic black appearance (vaguely resembling the popular image of the singer of the same name), in contrast to a wildtype (AB) fish in which the eyes are covered with iridophores. Although embryonic melanocyte development appears largely normal, we noted a severe defect in adult melanocyte formation in roy, which is likely due to the loss of nearby iridophores. We did not observe a strong defect in xanthophores, the other major pigment class found in the zebrafish. The major additional defect in casper is a loss of all pigmented melanocytes due to the mutation in the mitfa gene caused by the nacre allele, as previously described (White et al., 2008), (Lister et al., 1999).
Figure 1.
The roy orbison mutant phenotype, one of the two strains found in the transparent casper strain. During embryogenesis, roy lacks nearly all reflective iridophores, but melanophore development is relatively normal. In contrast, as adults, roy has a severe defect in both iridophores and melanophores. casper is largely similar to roy except that it also has a mutation in mitfa, leading to complete loss of all pigmented melanocytes.
Positional cloning of roy orbison
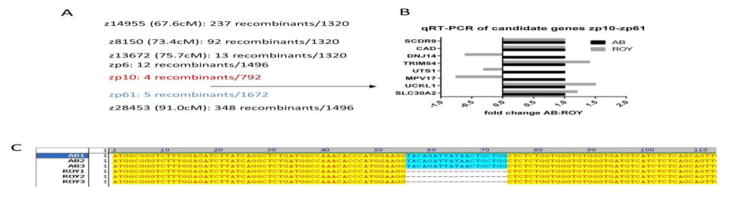
The roy mutant is homozygous viable. We outcrossed adult mutants to the WIK strain of zebrafish to perform low-level genome mapping as previously described (Zhou and Zon, 2011). This revealed the localization of the roy mutation on zebrafish linkage group 20 (Figure 2A). We then used a panel of well-validated SNP markers to narrow down the likely chromosomal location, and found that the roy mutation was located between markers z13672 (75.7cM) and z28453 (91.0cM). We refined this position using further SNP markers zp10 and zp61 that resulted in 4/792 and 5/1496 recombinants, respectively.
Figure 2.
Genetic linkage map of the roy orbison mutant. (A) Chromosome 20 map with SNP markers showing the number of recombinants at each location. The roy mutant was located between markers zp10 and zp61. (B) Quantitative RT-PCR for all 8 genes located in the roy interval, to identify likely candidates showing decreased RNA likely due to nonsense mediated decay. Only three genes, DNJ14, UTS1 and MPV17, showed a decrease in the mRNA. Because MPV17 is expressed in the neural crest, this was the most likely candidate. (C) cDNA sequencing of the MPV17 locus was done in 3 wild-type AB and 3 mutant roy orbison fish. Clones from each animal were sequenced by Sanger sequencing, and in all 3 mutant fish showed a 19bp deletion between exons 2 and 3, leading to an early stop codon.
By examining the candidate region in the UCSC browser, we noted only 8 genes in the region flanked by these markers: scdr9, dnj14, trim54, uts1, mpv17, uckl1, slc20a2. We performed quantitative RT-PCR on whole embryos for each of these 8 genes to narrow down the putative candidates, reasoning that a mutation could lead to nonsense-mediated decay (NMD) of the mRNA (Figure 2B). We detected a clear downregulation of dnj14 and mpv17. Given the identification of mpv17 as affected gene in the transparent mutant (trab6, also referred to as trab18) (Krauss et al., 2013) and given the striking recessive pigmentation phenotype of roy, we reasoned that mpv17 was the likely causative gene for the roy orbison phenotype.
roy orbison features a 19bp deletion in the mpv17 mRNA
We isolated and reverse-transcribed mRNA from AB wildtypes and roy mutants before sequencing multiple cDNA clones to detect potential mutations in mpv17. In all 3 roy clones, we identified a 19 bp deletion that results in aberrant splicing between the exons 2 and 3 of mpv17 (Figure 2C). This leads to a frameshift and early stop codon that likely yields a null for the protein. To identify the cause of the splicing defect, we sequenced genomic DNA corresponding to exons 2 and 3 from AB and mutant fish, but could not detect any exonic mutations that would have caused the splicing defect. We therefore assumed that the underlying genomic defect occurs in the intron between exons 2 and 3, but we repeatedly failed to amplify the genomic DNA from intron 2–3, suggesting there is a deletion in this intron that leads to aberrant splicing and subsequent early stop of the mRNA. Our findings are remarkably similar to the recent reports of cloning the spontaneous trab6 mutant by Krauss et al. (Krauss et al., 2013), who found the identical splice defect and issues with amplifying intron 2–3 in their study.
mpv17 mRNA rescues the roy orbison phenotype
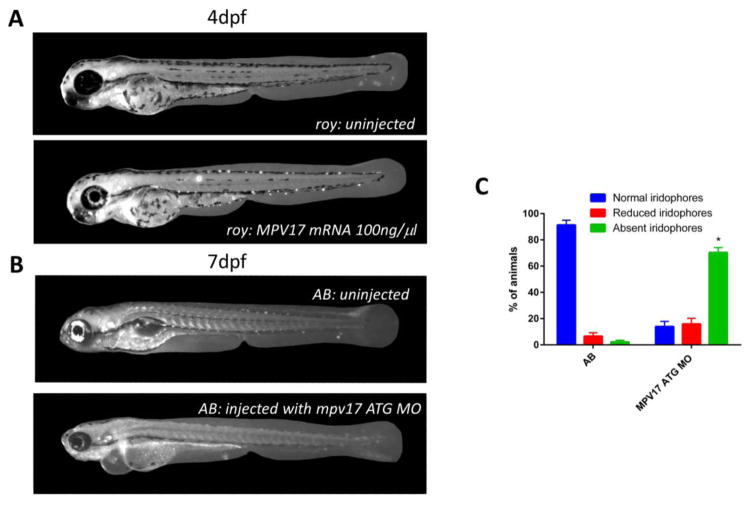
Given that intron 2–3 spans approximately 20kb and we repeatedly failed at isolating any PCR products, we instead decided to functionally test whether mpv17 was the causative gene underlying the roy phenotype. We isolated the full-length mpv17 ORF from the AB strain as above, cloned it into pCS2+, and then used this vector to in vitro-synthesize capped, polyadenylated mpv17 mRNA. We injected the mpv17 mRNA into the yolk of single-cell stage embryos from a homozygous roy incross at a concentration of 100 ng/μl. Of the 20 fish that survived to 4 dpf, 16/20 had fully rescued eye iridophores (Figure 3A). We also injected the mRNA into WT AB strain fish and did not observe any obvious phenotypes. These observations suggest that supplementing homozygous roy embryos with functional mpv17-encoding mRNA rescues the mutant phenotype.
Figure 3.
(A) Injection of full-length mpv17 mRNA leads to rescue of the roy orbison mutant phenotype, as demonstrated by the appearance of iridophores both in the eye and throughout the body of the fish (roy n=0/20 fish with eye iridophores, roy+mRNA injection, n=14/20 fish with eye iridophores). (B) ATG morpholino knockdown of MPV17 in the AB fish leads to a strong phenocopy of the roy phenotype, with loss of eye and body iridophores (AB =71/79 fish with eye iridophores, AB+morpholino injection, n=8/67 fish with eye iridophores). (C) Quantification of eye iridophores after morpholino injection to mpv17 shows a significant increase in # of fish with absent iridophores (*, p<0.05, paired t-test)
Morpholino knockdown of mpv17
We next injected an ATG morpholino against mpv17 into wildtype embryos derived from an incross of the AB strain (Figure 3B,C). We examined the injected embryos at 4–7 dpf and noted a range of phenotypes, with 87% of embryos showing either complete (72%, n=48/67) or moderate (16%, n=11/67) loss of iridophores. Only 12% of the injected fish had normal appearing iridophores, in contrast to 89% of the uninjected AB embryos (p<0.05, uninjected vs. injected embryos, paired t-test). Although the majority of the remaining embryos were phenotypically normal, we did note some animals with defects in heart development, which may represent a nonspecific toxicity of the morpholino since we did not observe a similar phenotype in roy mutants.
Cas9-mediated mutagenesis of the mpv17 ORF reproduces the roy orbison phenotype
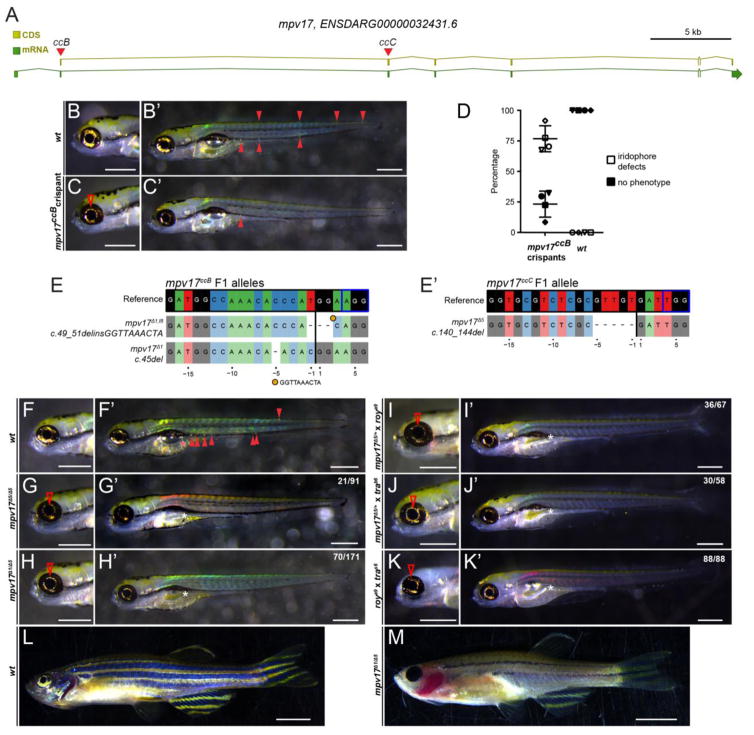
To genetically confirm the mapped mutation and to generate de novo alleles of mpv17 that directly perturb the ORF, we sought to target mpv17 using CRISPR-Cas9 (Figure 4A). We injected wildtype embryos with solubilized, fluorescent Cas9-sgRNA ribonucleoprotein complexes (RNPs) using sgRNAs to target the mpv17 ORF either in exon 2 (sgRNA ccB) or in exon 3 (sgRNA ccC). Both sgRNAs caused comparable effects. Embryos injected with the mpv17ccB sgRNA displayed a specific, strong reduction of iridophores predominantly in the eye and trunk region (Figure 4B,C); an average of 77% (n=172/223) of CRISPR-injected F0 embryos (so-called crispants) consistently featured iridophore loss (Figure 4D). mpv17 crispants showed no apparent other phenotype than iridophore loss and did not have a significantly higher mortality than uninjected siblings at 5 dpf. Sanger sequencing from PCR-isolated clones of the sgRNA target region in injected individuals and allele-level analysis with CrispRVariants (Burger et al., 2016; Lindsay et al., 2016) revealed exceedingly high mutagenesis efficiency, with frameshift deletions as most predominant mutation event that is predicted to result in early termination of translation (Supplemental Figure 1). These results corroborate that direct disruption of the mpv17 ORF results in iridophore defects.
Figure 4.
A) Schematic of the zebrafish mpv17 locus, with coding sequence (CDS) and total mRNA-coding region including introns, red triangles depict sgRNA locations ccB and ccC in coding exons. (B,B′) Brightfield imaging of 5 dpf zebrafish embryos to visualize iridophores, anterior to the left; scale bars represent 500 μm. Wildtype (wt) control showing prominent iridophores surrounding the eye lens and as individual cells along the body (solid arrowheads). (C,C′) Representative Cas9 RNP-injected, somatic-mutant embryo (crispant) where the ORF of mpv17 was targeted with sgRNA ccB in coding exon 1; note diminished iridophores in eye (red arrowhead) and severe reduction of body iridophores. (D) Phenotype distribution in mpv17ccB crispants versus wt controls. (E,E′) Mutant mp 17 alleles isolated in the F1 generation injected with sgRNA ccB (left) and ccC (right) and recovered in complementation analysis; genomic reference sequence depicted on top, black vertical bar indicates Cas9 cleavage site, blue box depicts PAM. (F, F′) Wildtype (wt) forms iridophores in eye and along the body (red arrowhead) and properly inflates the swimbladder (red asterisks). (G–H) Homozygous mp 17Δ5 and trans-heterozygous mp 17Δ1/Δ5 embryos show severe reduction of iridophores. (I,J) The same phenotype occurs in embryos trans-heterozygous for mp 17Δ5 and roya9 (I, I′) or mp 17Δ5 and trab6 (J,J′) as confirmed by genotyping for mp 17Δ5 (n=4). (K,K′) Trans-heterozygous embryos for roya9 and trab6 also show severe iridophore reduction, establishing non-complementation between roya9 and trab6 as mutant alleles of mpv17. (L) 3 month old wildtype with iridophores in the eye and along the body and melanocytes form normally into longitudinal stripes. (M) Trans-heterozygous mp 17Δ1/Δ5 fish completely lack eye iridophores and most iridophores along the body axis. Melanocytes in the stripes are heavily reduced. Scale bars represent 5 mm. White asterisks in whole embryo views mark failed inflation of the swimbladder in all observed mpv17 mutant combinations.
We subsequently raised Cas9 RNP-injected mosaic crispants with iridophore loss to adulthood. While strikingly visible at early stages, we observed a gradual recovery in iridophores over time and crispants were virtually indistinguishable from wildtype controls at 3 months of age (data not shown). We suspect that iridophore recovery is possible due to the mosaic nature of our transient mutagenesis assay. To isolate germline mutants, we incrossed F0 crispant siblings and observed the resulting F1 for phenotypes. Incrosses of mpv17ccB, mpv17ccC as well as trans-heterozygous crosses of adult crispants resulted in F1 embryos with iridophore loss that correlated with mutant mpv17 genotype (Figure 4F–H). Of note, we repeatedly recovered the frameshift allele mpv17c.140_144del (mpv17Δ5) from founders of mpv17ccC, revealing predominant repair preference for this particular 5bp deletions using sgRNA mpv17ccC (Figure 4E,E′). We raised trans-heterozygous mpv17Δ1/Δ5 to adulthood and no longer observed any recovery from the iridophore loss. Compared to wildtype, 3 month old mpv17Δ1/Δ5 were completely devoid of iridophores in the eye and also displayed a nearly complete iridophore loss along the body axis. In addition to the irididophore loss trans-heterozygous mutants also failed to correctly form melanocytes in their longitudinal stripes (Figure 4L, M). Taken together, these experiments confirm that direct perturbation of the mpv17 ORF causes iridophore defects as observed by the roy orbison and transparent lesions.
roya9 and transparentb6 neither complement each other nor mpv17 frameshift alleles
Our sequencing analysis and the data from Krauss et al. (Krauss et al., 2013) indicate that both roy orbison and transparentb6 possibly carry a lesion in intron 2–3 of mpv17. To further corroborate genetic relationship of the different mpv17 alleles, we performed genetic complementation analysis (Figure 4I–K). We crossed mpv17ccB and mpv17ccC F0 crispants to roya9/a9;nacw2/w2 (casper) and to trab6/b6;nacw2/w2. In both crosses, genotype-confirmed offspring harboring frameshift alleles arising from mpv17ccB and mpv17ccC presented a strong iridophore reduction in the eye, trunk, and swimbladder area, revealing non-complementation of roya9 and trab6 by de novo mutants affecting the mpv17 ORF. We also crossed roya9/a9;nacw2/w2 to trab6/b6;nacw2/w2 and observed complete penetrance of the roy orbison and nacre phenotypes, resulting in embryos that are indistinguishable from casper. Taken together, our complementation analysis confirms that the zebrafish mutants roy orbison and transparent form a complementation group and are allelic to mpv17, as further confirmed by our independently derived generated frameshift alleles. In all the allele combinations, the majority of embryos failed to completely inflate their swim bladder by 5 dpf, indicating this phenotype results also as a consequence of mpv17 perturbation. Taken together, mutant zebrafish strains of the genotypes roya9/a9;nacw2/w2 or trab6/b6;nacw2/w2 look virtually identical, and our genetic complementation analysis establishes that both these genotypes result in casper by virtue of the identical defect in roya9 and trab6.
mtDNA content in roy
Given the known role of mpv17 in mtDNA maintenance, we reasoned that the zebrafish mutant might harbor a depletion of mtDNA in the skin. Higdon and Johnson showed by RNA-seq that mpv17 is highly enriched in iridophores compared to other pigment cells such as melanocytes (Higdon et al., 2013). Krauss, et al, also recently noted that zebrafish mpv17 also localizes to the mitochondria, similar the the human and mouse protein. Furthermore, We previously noted that the kidney of the casper strain appears histologically normal, in contrast to the mouse mutant for Mpv17 (Viscomi et al., 2009). To test for loss of mtDNA, we performed array CGH (comparative genomic hybridization) in which we compared the skin and the kidney of both AB fish as well as roy orbison mutant. After isolating DNA from these tissues in 3 AB and 3 roy orbison fish, we hybridized it to Nimblegen zebrafish genome arrays, which contain probes for all autosomes as well as the mitochondrial genome. By comparing the normalized signal of the skin versus kidney in each animal, we could calculate the relative amount of mtDNA in each of these tissues, and across genotypes (Supplemental Figure 2). In the AB strain, we found that the skin generally contained more mtDNA signal compared to the kidney (log2 fold-change of skin:kidney=0.733+/−0.46 SEM). In contrast, in roy orbison, the exact opposite was found, where the mtDNA skin:kidney ratio was −0.742+/−0.19 (p<0.05, AB vs. roy orbison, unpaired t-test). This data are consistent with a relative decrease in mtDNA content in the skin of roy orbison compared to the kidney.
DISCUSSION
The zebrafish strain casper is homozygous for the pigment-affecting mutations of roya9 and nacrew2. Due to its due to its transparency, it is widely used as versatile background for in vivo imaging and transplantation experiments. For casper to maintain its transparency, both the nacw2 and roya9 alleles must be maintained as homozygous. Whereas nac is well-known to be due to an inactivating mutation in mitfa resulting in loss of pigmented melanocytes (Lister et al., 1999), the nature of the roy mutation has remained obscure. Here, we show that the causative lesion in roy is due to a loss of the mitochondrial inner membrane protein mpv17. The same gene was recently shown to be perturbed in the spontaneous zebrafish mutant called transparent, named due to the obvious transparency of its skin initiated by iridophore defects (Krauss et al., 2013). Several lines of evidence presented in our study corroborate that roy orbison and transparent affect the same gene, mpv17, and might be a similar or even the same allele.
Our work establishes that roya9, trab6, and our new Cas9 RNP-induced mpv17 mutants are recessive loss-of-function alleles of the same locus as shown by non-complementation. F0 mosaic Cas9-induced mutants, so-called crispants, readily recapitulate how lesions in the mpv17 ORF cause iridiophore defects. Nonetheless, mpv17 F0 crispants recover and appear wildtype with increasing age; this phenomenon likely reflects how wildtype or hypomorphic clones of cells replace unfit, mutant clones in genetic mosaics, posing a major caveat of somatic mutant analysis. Our readily isolated germline F1 mutants subsequently confirmed the mutant phenotype and non-complementation with roya9 and trab6. These experiments underline the value of using Cas9 to generate de novo alleles of mutant candidate genes to perform complementation analysis and phenotype comparisons.
The most-striking finding of our investigation is the remarkably similar nature of the genetic lesion causing the roya9 and trab6 alleles: without featuring any exon lesions, both mutants feature mis-spliced exons 2 and 3, resulting in the identical frameshift and premature stop codon. As already Krauss et al. discovered for trab6, we find that intron 2–3 of roya9 features a deletion that we also failed to properly chart; hence, roya9 and trab6 must harbor highly similar lesions. It is not clear whether intron 2–3 of mpv17 could have a tendency to accumulate spontaneous mutations. An alternative explanation remains that roya9 and trab6 are indeed descending from the same founder mutation and represent the same strain. Regardless of their origin, our complementation analysis confirms that roy orbison and transparent both affect mpv17. Consequently, compound mutant strains of trab6/b6;nacw2/w2 result in virtually identical animals as roya9/a9;nacw2/w2 compound homozygotes, both establishing the casper phenotype by the same genetic lesions.
The molecular function of mpv17 has remained elusive for nearly two decades. As recently reviewed by Löllgen and Weiher (Löllgen and Weiher, 2015), there is significant inter-species differences in the consequences of mpv17 mutation. In humans, the primary defect seems to occur in the liver, with a severe loss of mtDNA and the clinical syndrome most severe with hepatocerebral disease, often occurring in young children. In contrast, the mouse mutant, which has been extensively analyzed, was originally noted for its severe renal disease resembling focal segmental glomerulosclerosis. Interestingly, the liver in the mouse shows the expected large decrease in mtDNA content, with only 4% of the content compared to WT. In contrast, the kidney is less affected in this regard, with overall 60% of the levels seen in WT, but even within this tissue the effect is heterogeneous, with the glomerular podocytes severely affected while the renal tubules were relatively normal(Spinazzola et al., 2006). In our studies, we find that overall the skin of the roy orbison animals do show a decrease in mtDNA, at least judged by aCGH signal. However, one major limitation of our study is that we could not isolate specific cell types from within the skin such as the iridophores. It is likely that our results could simply reflect the fact that the iridophores are absent from the skin in roy orbison, and if the iridophores are especially rich in mtDNA this would be reflected in the lower mtDNA signal in array CGH. Methods to more specifically isolate and analyze iridophore mtDNA content from the zebrafish will be necessary to elucidate the precise role of mtDNA depletion in the pigment defect we see in the fish.
In contrast to humans or even mice with mutations in mpv17, we see no gross defects in liver, kidney or muscle in our fish, with the majority of defects confined to the skin. It is possible that under certain stressors, defects in these other tissues could become apparent. We did observe an obvious defect or delay in swim bladder inflation, which could potentially hamper proper swimming at early larval stages, with possibly detrimental consequences for survival and behavior. Nonetheless, we find that in general the casper strain maintains excellent health and fecundity throughout its lifespan. Early generations of casper did show poor survival likely caused by inbreeding, as we found that outcrossing casper to the wild EkkWill strain restores full health and potency of casper. Hence, under most laboratory conditions, the mutation in mpv17 functionally impairs only a limited number of cell types within the zebrafish. This makes roy orbison/transparent/mpv17 mutants, and by extension casper itself, an excellent model for investigating tissue-specific defects in mtDNA maintenance and in mitochondrial function.
METHODS
Zebrafish husbandry and maintenance
All zebrafish were maintained in a temperature-controlled (28.5C), light-controlled (14h on/10h off) room as per standard conditions. Embryos were raised in temperature-controlled (28.5°C) incubators in E3 medium. AB and WIK strains were obtained from the ZIRC zebrafish stock center (Eugene, OR); TE was a generous gift from Patrick Müller (MPI Tübingen); roy orbison was originally obtained from the Dowling laboratory (Harvard Medical School); trab6/b6;nacw2/w2 from the Gilmour laboratory (EMBL Heidelberg).
Positional cloning of roy orbison
Positional cloning was performed as previously described (Zhou and Zon, 2011). Briefly, adult roy orbison mutants were bred to homozygous WIK fish to obtain heterozygous progeny. S from this group were then incrossed, and the embryonic offspring screened at 3–4dpf to look for defects in eye iridophores. This revealed Mendelian segregation of the roy orbison phenotype, with ~25% of offspring having severely reduced or absent iridophores. A total mapping panel of 1672 fish were then used for low-resolution linkage analysis, which placed the defect on roy on linkage group 20. We then ped medium and high resolution mapping using previously described SNP markers along chromosome 20, as shown in Figure 2. For the fine-resolution mapping between markers z13672 and z28453, custom designed PCR primers based on the Zv9 zebrafish build were used, which led to few recombinants between markers zp6 and zp10, with only 8 genes in that interval. Based on this small number of genes, we chose a candidate approach and examined expression by ISH and qRT-PCR, revealing the gene mpv17 to be the most likely candidate in this region.
cDNA cloning of mpv17
3 AB adults and and 3 roy orbison adults were tail-clipped, and RNA isolated using Trizol and the RNEasy kits. This was followed by reverse transcription using SuperScript III with both oligo-dT and random hexamers. The entire mpv17 ORF was PCR-amplified, and cloned into the TOPO/TA cloning vector. 4–5 colonies from each TOPO reaction was mini-prepped and subject to Sanger sequencing. The sequence traces were compared to the reference genome sequence.
mRNA rescue
The full-length mpv17 cDNA from the AB strain was subcloned into the pCS2+ vector. Capped mRNA was transcribed using the mMessage machine kit per manufacturer’s protocol. mRNA was resuspended in water and microinjected into 1-cell roy embryos at 100ng/μl. Embryos were scored for rescue of eye iridophores at 4–7 dpf.
Morpholino phenocopy
An ATG translation-blocking morpholino was designed by GeneTools using the AB reference sequence. The morpholino was resuspended in water, and microinjected into 1-cell AB embryos at doses ranging from 0.02mM to 4mM. Embryos were scored for loss of eye iridophores at 4–7 dpf.
Array CGH
Genomic DNA was isolated from the skin and kidney of 3 AB and 3 roy adults. This DNA was hybridized to Nimblegen zebrafish DNA arrays, as previously described (Chen et al., 2013; Freeman et al., 2009). This array has both autosomes in linkage groups 1–25, as well as mitochondrial probes. After normalization for probe intensities, a log2-fold change of skin versus kidney for each individual fish was obtained. The values across each of the three fish for each genotype was averaged.
Statistics
Iridophore counts between groups were compared using unpaired t-tests with a significance level of 0.05. The array CGH data was compared by averaging of replicates and comparison by t-test for mitochondrial chromosome (chrM) DNA signal.
sgRNA synthesis and genotyping
The mpv17 sgRNA was designed using CHOPCHOP (Labun et al., 2016; Montague et al., 2014) (http://chopchop.cbu.uib.no/index.php). sgRNA sequences are (PAM underlined):
sgRNA[mpv17ccB]: 5′-GATGGCCAAACACCCATGGAAGG-3′,
sgRNA[mpv17ccC]: 5′-GGTGCGTCTCGCGTTGTGATTGG-3′
sgRNA synthesis using oligo-based, cloning-free template generation and in vitro transcription (Bassett et al., 2013; Burger et al., 2016). Primers for oligo based sgRNA synthesis were: sgRNA T7fwd: 5′- GAAATTAATACGACTCACTATA-N20-GTTTTAGAGCTAGAAATAGC-3′, where N20 indicates the sgRNA target sequence without PAM and the invariant sgRNArev: 5′-AAAAGCACCGACTCGGTGCCACTTTTTCAAGTTGATAACGGACTAGCCTTATTTTAACTTGCTATTTCTAGCTCTAAAAC-3′ (PAGE-purified, Sigma Aldrich). Primer extension was performed using Phusion polymerase (Thermo Scientific), followed by column purification and in vitro transcription using T7 RNA polymerase (Roche). sgRNAs were assembled with Cas9-EGFP into ribonucleopeotein complexes (RNP) solubilized with 300 mM KCL as described and were injected into 1-cell stage embryos of the TE wildtype strain. F1 animals were genotyped and alleles recovered by amplifying the locus using PCR primers ccBforward: 5′-TAACCGTTTGTCATAATGTGGC-3′, ccBreverse 5′- CTAAACAAACTGCTGCTTAGGGAG-3, ccCforward 5′-AATAGGGAGTGAATGGGG-3′ and ccCreverse 5′-GTGGCCAGCAAAATGTAAA-3′; resulting PCR products were sub-cloned into pJet and sequenced from DNA column-free isolated off colony PCR of individual clones, as established before. The panel plots describing the mpv17 alleles were generated using the CrispRVariantsLite software (Lindsay et al., 2016) (http://imlspenticton.uzh.ch:3838/CrispRVariantsLite/) from clonal Sanger sequencing data of PCR clones from individual embryos.
Crispant and complementation phenotype assessment
Injected clutches were sorted for Cas9-EGFP-positive embryos 3 hours post-injection to quality-control injections and mortality was assessed at 24 hpf. Phenotypes were assessed at 3 dpf by stereomicroscopy. Statistical analysis and graphical representation were performed with GraphPad Prism 7. Embryos for imaging were raised in E3 and treated with 3% MS-222 (pH = 7) during screening and imaging. Image acquisition of mpv17ccB crispants and control embryos was performed with a Leica M205 FA stereomicroscope equipped with a 1x magnification lens at 100x magnification. Iridophores were visualized by illuminating embryos from above with a swan neck light source attached to the base of the stereomicroscope. Images were processed with ImageJ 1.51h and Adobe Photoshop CS6.
Supplementary Material
Supplemental Figure 1: Targeting details for sgRNA ccB and allele spectrum in mpv17ccB crispants. Schematic of mpv17 locus with coding sequence (CDS) and total mRNA-coding region including introns, sgRNA ccB indicated as red triangle. Panel plot shows allele spectrum and mutagenesis efficiency in four individual mpv17ccB crispant embryos. Genomic reference sequence depicted on top, black vertical bar indicates Cas9 cleavage site, blue box depicts PAM. Mutant sequences reveal re-occurring alleles in crispants; note absence of wildtype sequences, suggesting high mutagenesis efficiency for Cas9 RNPs with sgRNA ccB.
Supplemental Figure 2: Mitochondrial DNA (mtDNA) signal from array CGH of skin:kidney from AB shows a severe loss of mtDNA only from the roy orbison skin, consistent with the protein leading to a tissue specific defect in mtDNA maintenance (*=p<0.05, AB vs. roy orbison, unpaired t-test).
Highlights.
-the casper strain of zebrafish is widely used for imaging of adult fish
-casper is a compound mutant composed of both nacre (mitfa) and roy orbison (an unknown gene)
-here the authors identify a mutation in the gene mpv17 as the underlying cause of the roy orbison phenotype
-this leads to a severe defect in iridophore development, and is identical to the recently described transparent mutant
Acknowledgments
We thank the lab of Dr. Nadia Mercader for supplying the Mosimann lab with additional casper adults, and the lab of Dr. Darren Gilmour for transparent mutants. We thank Sibylle Burger for technical assistance, Eliane Escher for sequencing services, Kara Dannenhauer and Dr. Stephan Neuhauss for zebrafish husbandry support.
This work was supported by the Canton of Zürich and the Foundation for Research in Science and the Humanities at the University of Zürich, a Swiss National Science Foundation (SNSF) professorship [PP00P3_139093] and a Marie Curie Career Integration Grant from the European Commission [CIG PCIG14-GA-2013-631984] to C.M.; an UZH URPP “Translational Cancer Research” grant to A.B.; a UZH CanDoc Forschungskredit to G.D. The NIH Director’s New Innovator Award (DP2CA186572), Mentored Clinical Scientist Research Career Development Award (K08AR055368), The Melanoma Research Alliance Young Investigator Award, The Alan and Sandra Gerry Metastasis Research Initiative at the Memorial Sloan Kettering Cancer Center, The Harry J. Lloyd Foundation and Consano (R.M.W.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bassett AR, Tibbit C, Ponting CP, Liu JL. Highly efficient targeted mutagenesis of Drosophila with the CRISPR/Cas9 system. Cell Rep. 2013;4:220–228. doi: 10.1016/j.celrep.2013.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burger A, Lindsay H, Felker A, Hess C, Anders C, Chiavacci E, Zaugg J, Weber LM, Catena R, Jinek M, Robinson MD, Mosimann C. Maximizing mutagenesis with solubilized CRISPR-Cas9 ribonucleoprotein complexes. Development. 2016;143:2025–2037. doi: 10.1242/dev.134809. [DOI] [PubMed] [Google Scholar]
- Chen EY, Dobrinski KP, Brown KH, Clagg R, Edelman E, Ignatius MS, Chen JYH, Brockmann J, Nielsen GP, Ramaswamy S, Keller C, Lee C, Langenau DM. Cross-species array comparative genomic hybridization identifies novel oncogenic events in zebrafish and human embryonal rhabdomyosarcoma. PLoS Genet. 2013;9:e1003727. doi: 10.1371/journal.pgen.1003727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman JL, Ceol C, Feng H, Langenau DM, Belair C, Stern HM, Song A, Paw BH, Look AT, Zhou Y, Zon LI, Lee C. Construction and application of a zebrafish array comparative genomic hybridization platform. Genes Chromosomes Cancer. 2009;48:155–170. doi: 10.1002/gcc.20623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higdon CW, Mitra RD, Johnson SL. Gene expression analysis of zebrafish melanocytes, iridophores, and retinal pigmented epithelium reveals indicators of biological function and developmental origin. PLoS ONE. 2013;8:e67801. doi: 10.1371/journal.pone.0067801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krauss J, Astrinidis P, Frohnhöfer HG, Walderich B, Nüsslein-Volhard C. transparent, a gene affecting stripe formation in Zebrafish, encodes the mitochondrial protein Mpv17 that is required for iridophore survival. Biol Open. 2013;2:703–710. doi: 10.1242/bio.20135132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labun K, Montague TG, Gagnon JA, Thyme SB, Valen E. CHOPCHOP v2: a web tool for the next generation of CRISPR genome engineering. Nucleic Acids Res. 2016;44:W272–6. doi: 10.1093/nar/gkw398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindsay H, Burger A, Biyong B, Felker A, Hess C, Zaugg J, Chiavacci E, Anders C, Jinek M, Mosimann C, Robinson MD. CrispRVariants charts the mutation spectrum of genome engineering experiments. Nat Biotechnol. 2016;34:701–702. doi: 10.1038/nbt.3628. [DOI] [PubMed] [Google Scholar]
- Lister JA, Robertson CP, Lepage T, Johnson SL, Raible DW. nacre encodes a zebrafish microphthalmia-related protein that regulates neural-crest-derived pigment cell fate. Development. 1999;126:3757–3767. doi: 10.1242/dev.126.17.3757. [DOI] [PubMed] [Google Scholar]
- Löllgen S, Weiher H. The role of the Mpv17 protein mutations of which cause mitochondrial DNA depletion syndrome (MDDS): lessons from homologs in different species. Biol Chem. 2015;396:13–25. doi: 10.1515/hsz-2014-0198. [DOI] [PubMed] [Google Scholar]
- Lopes SS, Yang X, Müller J, Carney TJ, McAdow AR, Rauch GJ, Jacoby AS, Hurst LD, Delfino-Machín M, Haffter P, Geisler R, Johnson SL, Ward A, Kelsh RN. Leukocyte tyrosine kinase functions in pigment cell development. PLoS Genet. 2008;4:e1000026. doi: 10.1371/journal.pgen.1000026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGowan KA, Barsh GS. Evolution: How the zebrafish got its stripes. elife. 2016:5. doi: 10.7554/eLife.14239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montague TG, Cruz JM, Gagnon JA, Church GM, Valen E. CHOPCHOP: a CRISPR/Cas9 and TALEN web tool for genome editing. Nucleic Acids Res. 2014;42:W401–7. doi: 10.1093/nar/gku410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parichy DM, Mellgren EM, Rawls JF, Lopes SS, Kelsh RN, Johnson SL. Mutational analysis of endothelin receptor b1 (rose) during neural crest and pigment pattern development in the zebrafish Danio rerio. Dev Biol. 2000;227:294–306. doi: 10.1006/dbio.2000.9899. [DOI] [PubMed] [Google Scholar]
- Parichy DM, Spiewak JE. Origins of adult pigmentation: diversity in pigment stem cell lineages and implications for pattern evolution. Pigment Cell Melanoma Res. 2015;28:31–50. doi: 10.1111/pcmr.12332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawls JF, Mellgren EM, Johnson SL. How the zebrafish gets its stripes. Dev Biol. 2001;240:301–314. doi: 10.1006/dbio.2001.0418. [DOI] [PubMed] [Google Scholar]
- Ren JQ, McCarthy WR, Zhang H, Adolph AR, Li L. Behavioral visual responses of wild-type and hypopigmented zebrafish. Vision Res. 2002;42:293–299. doi: 10.1016/s0042-6989(01)00284-x. [DOI] [PubMed] [Google Scholar]
- Spinazzola A, Viscomi C, Fernandez-Vizarra E, Carrara F, D’Adamo P, Calvo S, Marsano RM, Donnini C, Weiher H, Strisciuglio P, Parini R, Sarzi E, Chan A, DiMauro S, Rötig A, Gasparini P, Ferrero I, Mootha VK, Tiranti V, Zeviani M. MPV17 encodes an inner mitochondrial membrane protein and is mutated in infantile hepatic mitochondrial DNA depletion. Nat Genet. 2006;38:570–575. doi: 10.1038/ng1765. [DOI] [PubMed] [Google Scholar]
- Viscomi C, Spinazzola A, Maggioni M, Fernandez-Vizarra E, Massa V, Pagano C, Vettor R, Mora M, Zeviani M. Early-onset liver mtDNA depletion and late-onset proteinuric nephropathy in Mpv17 knockout mice. Hum Mol Genet. 2009;18:12–26. doi: 10.1093/hmg/ddn309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White RM, Sessa A, Burke C, Bowman T, LeBlanc J, Ceol C, Bourque C, Dovey M, Goessling W, Burns CE, Zon LI. Transparent adult zebrafish as a tool for in vivo transplantation analysis. Cell Stem Cell. 2008;2:183–189. doi: 10.1016/j.stem.2007.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Zon LI. The zon laboratory guide to positional cloning in zebrafish. Methods Cell Biol. 2011;104:287–309. doi: 10.1016/B978-0-12-374814-0.00016-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1: Targeting details for sgRNA ccB and allele spectrum in mpv17ccB crispants. Schematic of mpv17 locus with coding sequence (CDS) and total mRNA-coding region including introns, sgRNA ccB indicated as red triangle. Panel plot shows allele spectrum and mutagenesis efficiency in four individual mpv17ccB crispant embryos. Genomic reference sequence depicted on top, black vertical bar indicates Cas9 cleavage site, blue box depicts PAM. Mutant sequences reveal re-occurring alleles in crispants; note absence of wildtype sequences, suggesting high mutagenesis efficiency for Cas9 RNPs with sgRNA ccB.
Supplemental Figure 2: Mitochondrial DNA (mtDNA) signal from array CGH of skin:kidney from AB shows a severe loss of mtDNA only from the roy orbison skin, consistent with the protein leading to a tissue specific defect in mtDNA maintenance (*=p<0.05, AB vs. roy orbison, unpaired t-test).