Key Points
CCP-224 attenuates neutrophil-platelet aggregation in SCD patient blood.
CCP-224 has the potential to prevent vaso-occlusion in SCD patients.
Introduction
Sickle cell disease (SCD) is a monogenetic disorder1 responsible for at least 100 000 deaths per year worldwide.2 Sickle cell anemia (SS), the most common form of SCD, is caused by a homozygous mutation in the β-globin gene that promotes intraerythrocytic polymerization of deoxygenated hemoglobin, leading to erythrocyte rigidity, dehydration, impaired rheology, and premature hemolysis.1 Vaso-occlusion and intravascular hemolysis are the 2 predominant pathophysiological events in SCD.1,3,4 Vaso-occlusion contributes to the onset of acute painful vaso-occlusive crisis (VOC), which is the primary reason for emergency medical care among SCD patients.1,5 High platelet and leukocyte counts are risk factors for VOC,6 and neutrophil-platelet aggregates are significantly elevated at steady state in the blood circulation of SCD patients.7,8 Neutrophil-platelet aggregation has also been shown to occur in TNF-α–treated cremaster venules of transgenic SCD mice, which was enabled by neutrophil CD11b/CD18 (Mac-1) binding to glycoprotein Ibα (GPIbα) on platelets.9 Recently,10 we used intravital microscopy in transgenic SCD mice to show that large neutrophil-platelet aggregates occlude pulmonary arterioles to promote lung vaso-occlusion in SCD. In the same study,10 we also used quantitative microfluidic fluorescence microscopy (qMFM), an in vitro microfluidic–based approach,11 to reveal that the neutrophil-platelet aggregation under vascular mimetic flow was significantly higher in steady state SCD than race-matched control human blood and partially enabled by Mac-1 on neutrophils binding to GPIbα on platelets. Platelet-neutrophil interactions in SCD human blood were significantly inhibited by function-blocking antibodies (Abs) against CD11b or GPIbα.10 Taken together, these studies7,9,10 suggest that Mac-1–GPIbα interactions also contribute to neutrophil-platelet aggregation in SCD, and GPIbα antagonists can be therapeutically beneficial in preventing VOC. The Mac-1 binding site is situated within the leucine-rich COOH-terminal flanking region of GPIbα (residues 201-268).12 This region includes a regulatory R-loop (residues 227-241), which is also the major binding site for the A1 domain of human von Willebrand factor A1 (VWF-A1).13,14 OS-1,a cyclic peptide (ACTERMALHNLCGG) has been shown to potently inhibit (KD 0.74 nM) human platelet von Willebrand factor (VWF) aggregation by stabilizing the R-loop of GPIbα in an alternative conguration that does not support key interactions with the human VWF-A1.13-15 However, OS-1 is a selective inhibitor of human but not mouse GPIbα, and therefore it cannot be evaluated by intravital studies in transgenic SCD mice. In this study, we used qMFM to show that CCP-224, a PEGylated form of the OS-1 peptide, potently inhibits neutrophil-platelet aggregation in SCD human blood flowing through microfluidic channels in vitro.
Methods
Human subjects
The human blood collection procedure has been described in detail elsewhere.10,11 Blood samples were drawn from steady state (not experiencing a VOC) SCD or control healthy human subjects at the Adult Sickle Cell Clinic of the University of Pittsburgh Medical Center in syringes containing 20 U/mL of heparin as per the protocol approved by the University of Pittsburgh Institutional Review Board. All participants gave written informed consent in accordance with the Declaration of Helsinki. Only nonsmokers who had not undergone exchange transfusion within the last 60 days and who were diagnosed for either SS or sickle/β0 thalassemia were included in the study. Additionally, only those SCD patients who were not experiencing an ongoing VOC were included in the study and referred to as steady state patients. The clinical characteristics of the human subjects are shown in Table 1.
Table 1.
Clinical characterization of human subjects
| Control 1 | Control 2 | Control 3 | SCD 1 | SCD 2 | SCD 3 | |
|---|---|---|---|---|---|---|
| F/M | M | F | F | F | F | M |
| Age, y | 30 | 32 | 35 | 27 | 36 | 47 |
| Hemoglobin, g/dL | 13.8 | 13.2 | 14.4 | 8.8 | 7.1 | 9.1 |
| Hematocrit, % | 38.2 | 39 | 45 | 25.9 | 20.7 | 26.2 |
| Neutrophils, % | NM | 44.16 | 57.6 | 75.3 | 54.4 | 41 |
| Neutrophils, ×109/L | NM | 7.22 | 4.16 | 3.9 | 5.7 | 2.8 |
| Platelets, ×109/L | 227 | 194 | 104 | 310 | 271 | 394 |
| Genotypes | AA | AA | AA | SS | SS | SS |
| Race | African American | African American | White | African American | African American | African American |
| Hydroxyurea | N | N | N | Y | N | Y |
Data represent clinical values based on blood draws.
AA, healthy control; F, female; M, male; N, no; NM, not measured; Y, yes.
CCP-224
CCP-224, a cyclic PEGylated peptide ACTERMALHNLCGGK-polyethylene glycol (PEG 24) polymer with a disulfide bond between Cys2 and Cys12 was provided by Quell Pharma, Inc. (Half Moon Bay, CA). CCP-224 is based on the OS-1 cyclic peptide (ACTERMALHNLCGG), which was derived from a cysteine-constrained phage display library.13,15 The CCP-224 peptide was synthesized, cysteines were oxidized to form a disulfide bond, and the PEG polymer was then conjugated via amine chemistry to the unique lysine. The cyclic peptide somatostatin-14 (AGCKNFFWKTFTSC) with a disulfide bond between Cys3 and Cys14, purchased from AnaSpec, Inc. (Fremont, CA), was used as the control peptide.
qMFM
Fluorescent Abs against CD16 and CD49b were added to the human blood for in situ staining of neutrophils and platelets, respectively. Blood was perfused through a polydimethylsiloxane (silicone)-based microfluidic flow channels with a glass bottom presenting a combination of recombinant human P-selectin, ICAM-1, and interleukin-8 (IL-8) at a physiological wall shear stress of 6 dyn/cm2. Platelet-neutrophil interactions were recorded for 2 minutes by using qMFM (Figure 1A-B). The flow was stopped after 2 minutes of perfusion, and the CCP-224 or control peptide (10 µg/mL) was added to the blood, and the flow was resumed for another 2 minutes to assess the effect of CCP-224 on neutrophil-platelet interactions. The details of the microfluidic device and qMFM approach have been described elsewhere.10,11 See supplemental Methods for details.
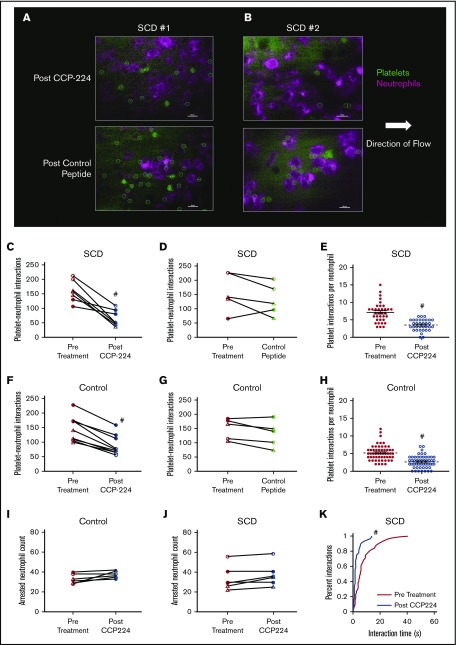
Figure 1.
CCP-224 inhibits platelet-neutrophil aggregation in SCD patient blood. (A-B) Human blood was flown through microfluidic channels presenting P-selectin, ICAM-1, and IL-8, and platelet-neutrophil interactions were assessed by using qMFM. qMFM images showing platelets (green circles) interacting with arrested neutrophils (purple polygons) in the blood of SCD patient 1 (A) and patient 2 (B) following treatment with 10 µg/mL of CCP-224 (top row) or control peptide (bottom row). Neutrophils were stained with Alexa Fluor 647 anti-human CD16 Ab (purple), and platelets were stained with fluorescein isothiocyanate anti-human CD49b Ab (green). qMFM images were recorded by using a Nikon Eclipse Ti inverted microscope equipped with a Zyla-5.5 sCMOS scientific camera and CFI Apochromat TIRF 60× oil objective (numerical aperture: 1.49). All microscope functions and image analyses were conducted by using NIS-Elements software. Borders of platelets are marked with green circles. The arrow indicates the direction of flow. Scale bars, 20 µm. The wall shear stress was 6 dyn/cm2. See supplemental Methods for details. (C-D) Pre- and posttreatment paired analyses showing the effect of CCP-224 (C) or control peptide (D) treatment on total platelet-neutrophil interactions in SCD patient blood. (E) Platelet interactions per arrested neutrophil over a 2-minute observation period pre– and post–CCP-224 treatment in SCD human subject blood. (F-G) Pre- and posttreatment paired analyses showing the effect of CCP-224 (F) or control peptide (G) on control (African American as well as white) subject blood. (H) Platelet interactions per arrested neutrophil over a 2-minute observation period pre– and post–CCP-224 treatment in healthy control (African American as well as white) human subject blood. In panels E and H, 5 neutrophils were randomly selected per experiment, and the number of platelets interacting with each neutrophil were counted. Each data point in panels E and H corresponds to interactions with an individual neutrophil; mean ± SE. (I-J) Pre- and posttreatment paired analyses showing the effect of CCP-224 on the total number of arrested neutrophils in healthy control (I) and SCD (J) human blood. (K) Cumulative probability distribution of the lifetime of platelet-neutrophil interactions pre– and post–CCP-224 treatment in SCD human blood. The lifetime of 10 randomly selected platelet-neutrophil interactions were measured in each experiment. Pre- and posttreatment data points connected by a straight line in panels C-D, F-G, and I-J represent paired data from an individual experiment. Blood samples from 3 SCD and 3 control (2 African American and 1 white) human subjects were used. Two to 3 independent experiments were performed per subject. Closed circles, open circles, and open triangles in panels C-D and J represent independent experiments performed with SCD patient 1, 2, and 3, respectively. Closed circles, open circles, and open triangles in panels F-G and I represent independent experiments performed with control subject 1 (African American), 2 (African American), and 3 (white), respectively. #P < .05 post- vs pretreatment.
Statistics
Total number of neutrophil-platelet interactions (Figure 1C-D, F-G) and the number of arrested neutrophils (Figure 1I-J) pre– vs post–CCP-224 or control peptide treatment were compared by using a paired Student t test. Platelet interactions per arrested neutrophil (Figure 1E,H) were compared using Students t test. The lifetimes of interactions (Figure 1K) were compared by using the nonparametric Kruskal-Wallis H test. P < .05 was used to determine significance. Data in Figure 1E and H represents the mean ± SEM.
Results and discussion
Steady state SCD or control human subject blood with or without the addition of the CCP-224 or the control peptide was allowed to flow through in vitro microfluidic channels, presenting a combination of P-selectin, ICAM-1, and IL-8, and neutrophil-platelet interactions were assessed by using qMFM.11 Identical to our previous findings,10,11 neutrophils were observed to roll, arrest, and capture freely flowing platelets leading to the formation of neutrophil-platelet aggregates. As shown in Figure 1A-B, fewer platelets were observed to nucleate on top of the arrested neutrophils in the blood of SCD patient 1 (Figure 1A) and patient 2 (Figure 1B) following treatment with CCP-224 compared with control peptide treatment. Previously,10,11 we have shown that the neutrophil-platelet aggregation in qMFM studies can be quantified based on 3 parameters; total platelet-neutrophil interactions per field of view, total platelet-neutrophil interactions per arrested neutrophil, and the lifetime distribution of platelet-neutrophil interactions. These parameters were compared by using pre- and posttreatment paired-sample analyses over several independent experiments done with 3 control and 3 SCD subjects (refer to supplemental Methods for details). Paired analysis revealed that CCP-224 (Figure 1C) but not the control peptide (Figure 1D) led to a significant reduction in the total number of platelet-neutrophil interactions in SCD patient blood. Identical to SCD patient blood, CCP-224 also significantly reduced the total number of platelet-neutrophil interactions in African American as well as white healthy control human blood (Figure 1F). As shown in Figure 1G, the control peptide had no effect on platelet-neutrophil interactions in control human subject blood. Treatment with CCP-224 also led to a significant reduction in the number of platelet interactions per arrested neutrophil in both SCD (Figure 1E) and control (Figure 1H) human blood. Platelet-neutrophil aggregation–mediated vaso-occlusion is dependent on the ability of platelets to attach to neutrophils under vascular mimetic flow. Assessment of individual interactions (Figure 1K) revealed that CCP-224 led to a significant reduction in the median lifetime (5 seconds pre–CCP-224 vs 1.7 seconds post–CCP-224) of platelet-neutrophil interactions in SCD human blood. However, the number of arrested neutrophils was unaffected by CCP-224 in both control (Figure 1I) and SCD (Figure 1J) human blood, suggesting that the reduction in platelet-neutrophil interactions was not a consequence of neutrophil detachment from the substrate. The OS-1 peptide, which is the nonPEGylated version of CCP-224 is known to stabilize GPIbα in low affinity configuration.13-15 Thus, the presence or lack of inhibition with the CCP-224 or control peptide, respectively, was not primarily caused by the presence or absence of PEG polymer in the CCP-224 or control peptide, respectively. Taken together, our data suggest that the GPIbα antagonist, CCP-224 is a potent inhibitor of neutrophil-platelet aggregation in SCD patient blood under vascular mimetic flow conditions.
Recent studies have identified a role for P-selectin, E-selectin, and Mac-1 in mediating vaso-occlusion in transgenic SCD mice in vivo.8-10,16 These findings have inspired clinical trials designed to test the efficacy of P-selectin,17 E-selectin,18 and Mac-119 blockers in reducing the frequency of VOC in SCD patients. Our previous10 and current findings suggest that the platelet GPIbα is also a potential target for antiadhesion therapy in SCD. In a recent clinical trial,17 a P-selectin Ab led to a significant reduction in VOC among SCD patients. Thus, a combination therapy that uses both P-selectin and GPIbα inhibitors could possibly be more potent than the individual inhibitors. CCP-224 also inhibits GPIbα binding to human VWF-A1,12,14 and therefore may increase the risk of bleeding complications. However, SCD is associated with elevated plasma levels of hyperadhesive VWF, which is believed to promote microvascular thrombosis.20 Thus, CCP-224 might also prevent hemostatic complications in SCD by downregulating platelet activation by circulating VWF multimers. Our in vitro findings support the need for future clinical studies to test the safety and efficacy of CCP-224 in SCD patients.
Supplementary Material
The full-text version of this article contains a data supplement.
Acknowledgments
This work was supported by National Institutes of Health, National Heart, Lung, and Blood Institute grant 1R01HL128297-01 (P.S.) and Vascular Medicine Institute startup funds (P.S.). M.A.J. was supported by National Institutes of Health, National Heart, Lung, and Blood Institute training grant T32HL076124 and the National Science Foundation Graduate Research Fellowship Program.
Authorship
Contribution: M.A.J. performed and analyzed the qMFM experiments; E.M.N. provided blood samples from human subjects and conducted the clinical characterization of SCD patients; G.D.S. provided CCP-224; P.S. was responsible for the experimental design, manuscript writing, and project supervision; and P.S. and M.A.J. wrote the manuscript with consultation and contribution from all coauthors.
Conflict-of-interest disclosure: G.D.S. is a founder and holds equity in Quell Pharma, Inc. The remaining authors declare no competing financial interests.
Correspondence: Prithu Sundd, University of Pittsburgh School of Medicine, Biomedical Science Tower E1255, 200 Lothrop St, Pittsburgh, PA 15261; e-mail: prs51@pitt.edu.
References
- 1.Rees DC, Williams TN, Gladwin MT. Sickle-cell disease. Lancet. 2010;376(9757):2018-2031. [DOI] [PubMed] [Google Scholar]
- 2.GBD 2013 Mortality and Causes of Death Collaborators. Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2015;385(9963):117-171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Manwani D, Frenette PS. Vaso-occlusion in sickle cell disease: pathophysiology and novel targeted therapies. Blood. 2013;122(24):3892-3898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gladwin MT, Ofori-Acquah SF. Erythroid DAMPs drive inflammation in SCD. Blood. 2014;123(24):3689-3690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang D, Xu C, Manwani D, Frenette PS. Neutrophils, platelets, and inflammatory pathways at the nexus of sickle cell disease pathophysiology. Blood. 2016;127(7):801-809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Curtis SA, Danda N, Etzion Z, Cohen HW, Billett HH. Elevated steady state WBC and platelet counts are associated with frequent emergency room use in adults with sickle cell anemia. PLoS One. 2015;10(8):e0133116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dominical VM, Samsel L, Nichols JS, et al. Prominent role of platelets in the formation of circulating neutrophil-red cell heterocellular aggregates in sickle cell anemia. Haematologica. 2014;99(11):e214-e217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Polanowska-Grabowska R, Wallace K, Field JJ, et al. P-selectin-mediated platelet-neutrophil aggregate formation activates neutrophils in mouse and human sickle cell disease. Arterioscler Thromb Vasc Biol. 2010;30(12):2392-2399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li J, Kim K, Hahm E, et al. Neutrophil AKT2 regulates heterotypic cell-cell interactions during vascular inflammation. J Clin Invest. 2014;124(4):1483-1496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bennewitz MF, Jimenez MA, Vats R, et al. Lung vaso-occlusion in sickle cell disease mediated by arteriolar neutrophil-platelet microemboli. JCI Insight. 2017;2(1):e89761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jimenez MA, Tutuncuoglu E, Barge S, Novelli EM, Sundd P. Quantitative microfluidic fluorescence microscopy to study vaso-occlusion in sickle cell disease. Haematologica. 2015;100(10):e390-e393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Simon DI, Chen Z, Xu H, et al. Platelet glycoprotein ibalpha is a counterreceptor for the leukocyte integrin Mac-1 (CD11b/CD18). J Exp Med. 2000;192(2):193-204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McEwan PA, Andrews RK, Emsley J. Glycoprotein Ibalpha inhibitor complex structure reveals a combined steric and allosteric mechanism of von Willebrand factor antagonism. Blood. 2009;114(23):4883-4885. [DOI] [PubMed] [Google Scholar]
- 14.Chen J, Zhou H, Diacovo A, Zheng XL, Emsley J, Diacovo TG. Exploiting the kinetic interplay between GPIbα-VWF binding interfaces to regulate hemostasis and thrombosis. Blood. 2014;124(25):3799-3807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Benard SA, Smith TM, Cunningham K, et al. Identification of peptide antagonists to glycoprotein Ibalpha that selectively inhibit von Willebrand factor dependent platelet aggregation. Biochemistry. 2008;47(16):4674-4682. [DOI] [PubMed] [Google Scholar]
- 16.Hidalgo A, Chang J, Jang JE, Peired AJ, Chiang EY, Frenette PS. Heterotypic interactions enabled by polarized neutrophil microdomains mediate thromboinflammatory injury. Nat Med. 2009;15(4):384-391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ataga KI, Kutlar A, Kanter J, et al. Crizanlizumab for the Prevention of Pain Crises in Sickle Cell Disease. N Engl J Med. 2017;376(5):429-439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Telen MJ, Wun T, McCavit TL, et al. Randomized phase 2 study of GMI-1070 in SCD: reduction in time to resolution of vaso-occlusive events and decreased opioid use. Blood. 2015;125(17):2656-2664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Manwani D, Chen G, Carullo V, et al. Single-dose intravenous gammaglobulin can stabilize neutrophil Mac-1 activation in sickle cell pain crisis. Am J Hematol. 2015;90(5):381-385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen J, Hobbs WE, Le J, Lenting PJ, de Groot PG, López JA. The rate of hemolysis in sickle cell disease correlates with the quantity of active von Willebrand factor in the plasma. Blood. 2011;117(13):3680-3683. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.