Abstract
Background
Long-term exposure to drugs of abuse causes an up-regulation of the cAMP-signaling pathway in the nucleus accumbens and other forebrain regions, this common neuroadaptation is thought to underlie aspects of drug tolerance and dependence. Phosphodiesterase 4 (PDE4) is an enzyme that the selective hydrolyzes intracellular cAMP. It is expressed in several brain regions that regulate the reinforcing effects of drugs of abuse.
Objective
Here, we review the current knowledge about central nervous system (CNS) distribution of PDE4 isoforms and the effects of systemic and brain-region specific inhibition of PDE4 on behavioral models of drug addiction.
Methods
A systematic literature search was performed using the Pubmed.
Results
Using behavioral sensitization, conditioned place preference and drug self-administration as behavioral models, a large number of studies have shown that local or systemic administration of PDE4 inhibitors reduce drug intake and/or drug seeking for psychostimulants, alcohol, and opioids in rats or mice.
Conclusions
Preclinical studies suggest that PDE4 could be a therapeutic target for several classes of substance use disorder. We conclude by identifying opportunities for the development of subtype-selective PDE4 inhibitors that may reduce addiction liability and minimize the side effects that limit the clinical potential of non-selective PDE4 inhibitors. Several PDE4 inhibitors have been clinically approved for other diseases. There is a promising possibility to repurpose these PDE4 inhibitors for the treatment of drug addiction as they are safe and well-tolerated in patients.
Keywords: PDE4, PDE4 inhibitors, VTA, nucleus accumbens, drug addiction
Drug addiction and alcohol dependence take a high medical, social and economic toll on society. However, options for pharmacological intervention of addictive disorders are very limited, and currently no FDA approved medications are clinically available for the treatment of addiction to psychostimulants. In several animal models, phosphodiesterase 4 (PDE4) inhibitors were found to reduce drug intake and/or drug seeking for psychostimulants, alcohol, and opioids. Here, we review the current knowledge about central nervous system (CNS) distribution of PDE4 isoforms and the effects of systemic and brain-region specific inhibition of PDE4 on behavioral models of drug addiction.
1. The PDE4 family of phosphodiesterases
PDE4 is a cAMP-specific phosphodiesterase expressed in several brain regions that regulates the reinforcing effects of drugs of abuse, including the prefrontal cortex, nucleus accumbens, ventral tegmental area, and amygdala (Cherry and Davis, 1999; Perez-Torres et al., 2000). These brain regions are also targets of drug-associated neuroadaptations in cyclic AMP (cAMP) signaling, and these neuroadaptations are thought to underlie aspects of drug tolerance and dependence (Muschamp and Carlezon, 2013; Nestler, 2015; Wen et al., 2015). In preclinical studies, PDE4 inhibitors reduce drug intake and/or drug seeking for psychostimulants, alcohol, and opioids, suggesting that PDE4 could be a therapeutic target for several classes of substance use disorder. However, the underlying mechanisms for the anti-addictive behavioral effects of PDE4 inhibitors remain poorly understood. cAMP signaling is highly compartmentalized. PDE4 inhibitors can break down such compartmentalization and causes widespread increase in intracellular cAMP levels (Cooper, 2005). PDE4 inhibitors may disrupt or occlude cAMP-related neuroadaptations caused by drugs of abuse. Here, we review the current knowledge about central nervous system (CNS) distribution of PDE4 isoforms and the effects of systemic and brain-region specific inhibition of PDE4 on behavioral models of drug addiction. We conclude by identifying opportunities for the development of subtype-selective PDE4 inhibitors that may reduce addiction liability and minimize the side effects that limit the clinical potential of non-selective PDE4 inhibitors.
1.a. Phosphodiesterases (PDEs) provide the sole means of degrading cAMP and cGMP
Phosphodiesterases (PDEs) are a family of enzymes that hydrolyze intracellular cAMP and cGMP (Conti et al., 2003). There are 11 subtypes of PDEs (PDE1-11) that can be classified into three categories based on their substrate specificity. PDE4, PDE7, and PDE8 are specific for cAMP; PDE5, PDE6, and PDE9 are specific for cGMP; and PDE1, PDE2, PDE3, PDE10, and PDE11 hydrolyze both cAMP and cGMP (Lugnier, 2006; Zhang, 2009). Among these isoforms, PDE4 has emerged as a potential therapeutic target for the treatment of substance abuse and substance use disorders. This review will be focused on PDE4 and PDE4 inhibitors.
1.b. Central nervous system distribution of PDE4 isoforms
PDE4 has four isoforms, PDE4A, PDE4B, PDE4C, and PDE4D. Each of these isoforms are encoded by a unique gene product (MacKenzie and Houslay, 2000; Wang et al., 2015). Further diversity arises from splice variants among each isoform; for example, PDE4D has 11 variants, PDE4D1-11. As a result, PDE4 has over 30 isoforms or splice variants (MacKenzie and Houslay, 2000; Wang et al., 2015). PDE4A, PDE4B, and PDE4D have widespread but segregated expression in different regions of the brain, while PDE4C is largely absent from the rodent brain and found only in a limited number of regions of the human brain (Johansson et al., 2012; Perez-Torres et al., 2000). Below, we summarize findings of PDE4 distribution throughout the central nervous system, with emphasis on brain regions associated with the expression of behaviors by drugs of abuse. For more complete information on central nervous system distribution of the PDE4 isoforms, we refer the reader to (Johansson et al., 2012; Lakics et al., 2010; Perez-Torres et al., 2000).
Striatum/Nucleus accumbens
PDE4B mRNA is high in the human and rodent striatum and nucleus accumbens, while PDE4A and PDE4D mRNA levels are present in lower amounts (Johansson et al., 2012; Lakics et al., 2010; Perez-Torres et al., 2000). Consistent with this, PDE4B-immunopositive neurons and neuropil are observed in the nucleus accumbens (Cherry and Davis, 1999; Perez-Torres et al., 2000). Within the striatum, PDE4B is differentially expressed in two major, functionally distinct subpopulations of medium spiny neurons. Striatal medium spiny neurons are organized into a dopamine D1 receptor-expressing “direct” (striatonigral) output pathway and a dopamine D2 receptor-expressing “indirect” (striatopallidal) output pathway, where the direct pathway is behaviorally stimulating, whereas the indirect pathway is behaviorally suppressant (Graybiel, 1990, 2000). Using transgenic mice with different tags of dopamine D1 and D2 receptors, it was found that the expression of PDE4B was higher in D2 receptor-enriched striatopallidal neurons than that in D1 receptor-enriched striatonigral neurons (Nishi et al., 2008). PDE4B protein is also abundant in the ventral pallidum, the target region of the striatopallidal pathway, and this region has a similar enrichment of PDE4B relative to the other isoforms that the striatum does (Cherry and Davis, 1999). In drug naïve selectively bred alcohol preferring rats, PDE4A mRNA was elevated and PDE4B2 mRNA was reduced in the accumbens relative to the non-preferring rats (Franklin et al., 2015).
Substantia Nigra/Ventral Tegmental Area (VTA)
PDE4A and 4B mRNA are low in the substantia nigra, while PDE4D was not detected in this brain region (Johansson et al., 2012). Human mRNA analyzed by quantitative real-time PCR demonstrated that PDE4B mRNA was approximately 3 fold higher than PDE4A and PDE4D in the substantia nigra (Lakics et al., 2010). Protein expression in rodents largely recapitulated this pattern, where PDE4B immunoreactivity in mouse substantia nigra was moderate and light for PDE4D (Cherry and Davis, 1999). In the rat VTA, presence of mRNA differed somewhat from the substantia nigra, where PDE4A was the most abundant isoform (Perez-Torres et al., 2000). In drug naïve selectively bred high alcohol drinking (HAD1) rats, PDE4B2 mRNA was elevated in the ventral tegmental area relative to the low alcohol drinking (LAD1) rats (Franklin et al., 2015).
Cerebral Cortex
PDE4A, 4B, and 4D are all present in the cortex, although expression is distinct between cortical layers. In layers V/VI of cortex, PDE4A and 4D proteins are expressed in moderate to high amounts, while PDE4B is reported to be present only in layer V and in low amounts (Cherry and Davis, 1999; Kuroiwa et al., 2012). In layers II/III, PDE4B and 4D are both expressed in moderate amounts, although 4A immunoreactivity is absent in these layers (Cherry and Davis, 1999). Quantitative analysis of human frontal cortex including all cell layers indicates that PDE4B is the most abundant isoform, followed by 4A, then 4D (Lakics et al., 2010)
Amygdala
Studies investigating PDE4 in the whole amygdaloid complex of the human and rodent report low to moderate levels of PDE4A and PDE4D, and very low to low levels of PDE4B (Johansson et al., 2012; Perez-Torres et al., 2000). In amygdalar subregions of the rodent, there is considerable diversity in mRNA abundance of each isoform between the subregions. PDE4A is most abundant in the medial amygdala, amygdalopiriform transition area, and posteromedial cortical amygdaloid nuclei. PDE4B and 4D were enriched in the medial amygdala, and in the basolateral amygdala, PDE4D was the only isoform detected (Perez-Torres et al., 2000). In the drug naïve selectively bred alcohol preferring rats, PDE4B1 mRNA was decreased in the central amygdala relative to the non-preferring rats (Franklin et al., 2015).
Brainstem Nausea and Emetic Centers
Although rodents do not have an emetic reflex (Horn et al., 2013), distribution of PDE4 isoforms in brainstem nausea and emetic centers is largely similar between rodents and humans. In the human area postrema, PDE4D mRNA is the highest of the PDE4 isoforms, while PDE4B is present in modest amounts, and PDE4A barely detectable in this region (Mori et al., 2010). A similar pattern was observed in rat brain (Perez-Torres et al., 2000), although while PDE4D protein was detected in mouse, PDE4B protein was not (Cherry and Davis, 1999).
In the nucleus of the solitary tract, patterns of PDE4 mRNA appear to be similar between the to the area postrema. In human brain, PDE4B and 4D are both enriched, while 4A is not detectable (Mori et al., 2010; Perez-Torres et al., 2000). In the same study, relative abundance of rat isoforms was somewhat different: PDE4A and 4D were present in moderate amounts, while 4B was low (Perez-Torres et al., 2000). Despite these differences, protein expression in the rat was similar to that predicted by the human mRNA data: immunoreactivity of PDE4B and 4D were moderate, while PDE4A was undetectable (Cherry and Davis, 1999).
To date, we are only aware of one study measuring PDE4 isoforms in the dorsal vagal motor nucleus. In this study of human brain, PDE4D mRNA was the highest PDE4 isoform in this region, while 4B was lower, and 4A was not detected (Mori et al., 2010).
Box 1. Behavioral Tests Commonly Used in the Study of Drugs of Abuse.
|
Locomotor sensitization (Pierce and Kalivas, 1997; Robinson and Berridge, 1993, 2008) |
Locomotor sensitization is a phenomenon where the locomotor response to a drug (e.g., amphetamine) is increased in animals with a history of repeated drug exposure. Previous studies have observed this increase for up to one year following cessation of drug treatment. The basis for this test is that locomotor sensitization may reflect long-lasting neuroadaptations and behavioral changes following repeated drug exposure, such as increased craving following exposure to drug cues. |
|
Conditioned place preference (Bardo and Bevins, 2000; Liu et al., 2014; Olsen et al., 2010; Tzschentke, 2007) |
The conditioned place preference (CPP) test is a behavioral test that measures an animal’s preference for a place that is associated with previous exposure to a reward. CPP protocols are typically divided into three phases: a pre-test, a conditioning phase, and a post-test. In the pre-test, a drug naïve animal is allowed to explore the entire CPP apparatus (typically composed of two or three distinct, but connected chambers). During the conditioning phase, the animal spends time confined to each of the chambers, however one chamber is paired with a reward (e.g., cocaine), while the other chamber is not (e.g. saline). After several of these pairing sessions, the animal undergoes the post-test in which the animal again is allowed to explore the entire CPP apparatus in a drug free state. The conditioned place preference is typically measured in one of two ways: 1) the difference in time spent in the reward paired chamber between the pre-test and post-test or 2) the difference in the post-test time spent in the reward paired chamber and the non-reward paired chamber. These outcome measures are collected while the animal is in a drug-free state. |
|
Intravenous drug self-administration (Allain et al., 2015; Muelbl et al., 2016; Olsen and Winder, 2006; Thomsen and Caine, 2005) |
Intravenous drug self-administration is a measure of the reinforcing properties of a drug. The basis of this test is that a positive outcome associated with a response will increase the likelihood of the response in the future. Thus, intravenous drug self-administration is a type of operant (instrumental) conditioning that uses intravenous infusion of a drug as the reinforcer. An animal with a chronic venous catheter is placed into an operant conditioning chamber, where it learns that a response on the “active” manipulanda (e.g., a lever) results in a drug infusion, whereas a response on the “inactive” manipulanda has no consequence. Drug self-administration is considered to be acquired when there is selectivity in responses on the active manipulanda relative to the inactive one. The effects of treatments on drug self-administration are typically measured as a change in drug self-administration relative to previously stabilized intake. This outcome measure is collected while the animal is under the influence of the drug of abuse. |
|
Reinstatement of drug seeking (Conrad et al., 2013; Mantsch et al., 2016; Shaham et al., 2003) |
Reinstatement of drug seeking is a measure of drug seeking that is evoked by a stimulus. The basis of this test is that drug seeking (either using the CPP or the operant response in a drug self-administration test) is first extinguished by dissociating the connection between the drug and the drug conditioned response through repeated testing without the drug, then reinstated with a stimulus. Stimuli that are commonly used to reinstate drug seeking include drug-associated cues, stress, or a priming dose of the drug itself. These stimuli are known to also elicit craving in human drug users. The outcome measures are active and inactive manipulandum responding or time spent in the drug and nondrug paired sides (depending on if this is a self-administration or CPP test respectively). These measures are collected while the animal is in a drug-free state (unless drug priming is used to reinstate drug seeking). |
|
Two bottle choice test for alcohol drinking (Crabbe, 2014; Lim et al., 2015; Muelbl et al., 2016; Rodd et al., 2004) |
The two bottle choice test is a measure of voluntary alcohol intake and preference. In this test, alcohol is concurrently available with water and food, so alcohol intake is driven by hedonic processes as opposed to metabolic need. Outcome measures are total alcohol intake and preference (% of total fluid intake that is alcohol), and are collected while the subject is under the influence of the drug. |
|
Intracranial self-stimulation (Britt et al., 2012; Carlezon and Chartoff, 2007; Ikemoto and Bonci, 2014; Negus and Miller, 2014) |
Intracranial self-stimulation measures the reinforcing properties of electrical or optogenetic stimulation of brain reward circuitry. The basis of this test is that direct stimulation of some brain regions (e.g., ventral tegmental area) or specific pathways (e.g., ventral hippocampus to nucleus accumbens) is innately reinforcing. Thus, operant conditioning is performed using stimulation as the primary reinforcer. Data from intracranial self-stimulation experiments are commonly represented as response rates across a series of stimulus intensities (or frequencies), and treatments that enhance brain stimulation reward will produce a left-shift in this intensity-response curve. These outcome measures are collected while the animal is in a drug-free state, unless a treatment is tested for its ability to modulate drug-enhanced brain stimulation reward. |
|
Drug discrimination (Stolerman et al., 2011; Young, 2009) |
Drug discrimination is a test that measures the discriminative stimulus effects of a drug. Psychoactive drugs produce subjective feelings, which can be used to signal that a specific behavioral response is required to earn a reward. For example, an animal can be trained that after a saline infusion, pressing the left lever in an operant conditioning chamber will deliver a food pellet. However, after a cocaine infusion, only presses on the left lever deliver a food pellet. Test sessions occur in the absence of food reward, and the outcome measure is the percentage of cocaine- or saline-appropriate responses after each treatment. There are two main types of drug discrimination tests: stimulus antagonism and stimulus generalization. A stimulus antagonism drug discrimination test can be used to measure the ability of a different drug to reduce the discriminative stimulus properties of the comparator drug. For example, pretreatment with flumazenil (an antagonist of the benzodiazepine binding site of the GABA-A receptor) reduces the percentage of diazepam-appropriate responses in rats trained to discriminate diazepam from saline. A stimulus generalization drug discrimination test assesses the ability of a drug to generalize to another comparative drug. For example, treatment with amphetamine will produce cocaine-appropriate responding in animals trained to discriminate cocaine from saline. Data are collected while the animal is under the influence of the drug. |
2. Effects of PDE4 inhibitors on drug-associated behaviors
2.a. Effects of systemic administration of PDE4 inhibitors
Cocaine
A series of studies by Cherry’s group has shown that PDE4 inhibition reduces cocaine reward, incentive saliency and seeking in rodents. They have examined the effects of the selective PDE4 inhibitor rolipram on cocaine-induced increase in locomotor activity, behavioral sensitization, conditioned place preference (CPP) and self-administration (Janes et al., 2009; Knapp et al., 1999; Thompson et al., 2004). Systemic injection of rolipram 30 min before each of 5 days of cocaine injections greatly reduced cocaine-induced hyperlocomotor activity on days 1 and 5. This treatment also reduced the locomotor response to a challenge dose of cocaine 9 days later, indicating that rolipram treatment during the induction phase could reduce expression of behavioral (locomotor) sensitization to cocaine.
Administration of rolipram 30 minutes after cocaine injection did not affect the cocaine-induced increase in locomotor activity or locomotor sensitization (Janes et al., 2009). Thus, PDE4 inhibition must take place during the exposure to cocaine to prevent the development of locomotor sensitization. More recent studies have shown that intracerebroventricular injection of isobutylmethylxanthine (IBMX), a nonspecific PDE inhibitor that enhances intracellular cAMP levels, does not affect the acute hyperlocomotor response to cocaine, but reduces the development of behavioral sensitization (Schroeder et al., 2012).
The process of locomotor sensitization has been linked with incentive sensitization. The incentive sensitization theory posits that repeated drug exposure leads to increased drug “wanting” and ultimately, compulsive drug taking (Robinson and Berridge, 1993, 2008). Although locomotor sensitization is not a direct measure of incentive sensitization, it is thought to reflect analogous processes in similar neural circuitry. Thus, results suggest that PDE inhibitors may be able to reduce drug “wanting” associated with repeated exposure.
Although PDE4 inhibitors are known to enhance cognitive functions such as learning and memory and have been proposed as potential pharmacotherapy of cognitive decline associate with aging and Alzheimer’s disease (Richter et al., 2013; Wang et al., 2015), they appear to dampen the formation of drug-cue associative memories. Systemic injections of the PDE4 inhibitor rolipram before place conditioning attenuate the expression of cocaine CPP, while injection of rolipram only before the CPP test does not alter a previously established place preference conditioned by cocaine (Thompson et al., 2004). Rolipram itself does not induce CPP or conditioned place aversion, nor does it alter place preference induced by natural reward (food) (Thompson et al., 2004). These results indicate that rolipram attenuates the acquisition, but not the expression, of CPP to cocaine without producing any place aversion or general impairment of associative memory formation.
PDE inhibition has also been demonstrated to facilitate extinction of cocaine CPP. Extinction of drug seeking behavior is not simply passive loss of established memory, but it requires consolidation and formation of new memory (Bardo and Bevins, 2000; Todd et al., 2014). It has been shown that when mice were conditioned by escalating doses of cocaine, the CPP could not be extinguished by free exploration over several sessions (Itzhak and Anderson, 2012). In this escalating dose model, the PDE9 (cGMP specific) inhibitor BAY-73-6691 facilitated extinction and reduced cocaine primed reinstatement of CPP. In the same model, the PDE4 (cAMP specific) inhibitor rolipram did not have a significant effect on the extinction of cocaine CPP (Liddie et al., 2012). These results indicate that different subtypes of PDEs regulate the acquisition vs. extinction of drug-cue associative memories.
In rats trained to self-administer cocaine on a fixed-ratio 5 schedule (5 active responses required per infusion), systemic administration of either rolipram or Ro 20-1724 caused a significant reduction in cocaine intake (Knapp et al., 1999). This reduction was largely attributed to an increase in the latency to initiate operant responding, and food self-administration was reduced in a similar pattern following Ro 20-1724 (Knapp et al., 1999), suggesting that these drugs may have a general effect on inhibiting motivated behavior or reinforcement processes. This finding emphasizes the fact that systemic administration of PDE4 inhibitors can have sedative effects and/or actions in brainstem emetic centers that may non-specifically alter behavioral phenotypes.
Methamphetamine
Beardsley and coworkers have done a series of studies examining the effects of non-selective PDE inhibitor ibudilast and its analogs on methamphetamine abuse. Similar to the aforementioned effects on cocaine-associated hyperlocomotion, they report that ibudilast and its analog AV1013 reduce the methamphetamine-induced increase in locomotor activity and sensitization without significantly affecting locomotor activity by itself (Snider et al., 2012). In addition, ibudilast and AV1013 are effective in attenuating methamphetamine self-administration and drug seeking behavior. Systemic administration of these compounds reduces methamphetamine self-administration on an FR1 schedule in the rat (Snider et al., 2013). Systemic ibudilast and AV1013 significantly reduces footshock- and prime-induced reinstatement of methamphetamine-seeking in the rat (Beardsley et al., 2010). Interestingly, the reduction of drug seeking behavior by ibudilast and its analog may be associated with inflammatory processes. Methamphetamine activates glial cells and increases proinflammatory cytokine production in the brain, and glial cell activation and inflammatory responses have been linked to drug abuse-related behavior (Beardsley and Hauser, 2014). Ibudilast inhibits both PDE and glial proinflammatory activity, while AV1013 shares the anti-inflammatory activity of ibudilast but only negligibly inhibits PDE (Snider et al., 2012). These results suggest that modulation of glial inflammatory activity also contributes to the attenuation of methamphetamine-induced locomotor sensitization and relapse of drug seeking by ibudilast and AV1013, and targeting glial cells may provide a novel approach to pharmacotherapy for treating methamphetamine abuse.
Methamphetamine and cocaine are psychostimulants that share similar mechanisms of increasing extracellular dopamine levels in the brain (Anderson and Pierce, 2005), and many of the behavioral measures discussed above are dopamine-dependent. In vivo microdialysis shows that rolipram increases striatal cAMP levels but does not significantly alter methamphetamine-induced increase in striatal dopamine levels (Iyo et al., 1996), suggesting that rolipram-induced changes in behaviors are not due to direct modulation of striatal dopamine.
Alcohol
Regulation of alcohol drinking by many different subtypes of PDEs and the underlying signaling mechanisms involved have been recently reviewed in great detail (Logrip, 2015). In this review, we will review recent progress of studying behavioral effects of PDE4 inhibitors on alcohol drinking.
Reduction of alcohol drinking by PDE4 inhibitors was first shown in in C57BL/6J mice with unlimited 2-bottle choice access to alcohol. Rolipram and Ro 20-1724 reduce alcohol intake and preference without altering total fluid intake, intake of sucrose or quinine, alcohol-induced sedation, or alcohol metabolism (Hu et al., 2011). Subsequently, this observation was extended to Fawn-Hooded rats and other animal models of alcohol drinking. Acute administration of rolipram dose-dependently reduces operant self-administration of alcohol, and chronic rolipram treatment decreases 2-bottle choice alcohol consumption and preference (Wen et al., 2012).
The non-selective phosphodiesterase inhibitor ibudilast reduces 2-bottle choice alcohol drinking and relapse in alcohol-preferring P rats, high-alcohol drinking HAD1 rats, and in alcohol-dependent C57BL/6J mice at doses which had no effect on alcohol drinking in non-dependent mice (Bell et al., 2015). Taken together, these studies indicate that PDE inhibitors reduce alcohol drinking in multiple rodent models, including models of genetic susceptibility and dependence. Given that ibudilast is a non-selective PDE inhibitor, this study raises the question whether inhibitors to other PDE subtypes reduce alcohol drinking and dependence. Using 24-h two-bottle choice and two-bottle choice with limited (3-h) access to alcohol in C56BL/6J male mice, a recent study has compared the effects of nine PDE inhibitors with different subtype selectivity. Interestingly, only the selective PDE4 inhibitors reduce ethanol intake and preference in the 24-h two-bottle choice test and in the limited access test (Blednov et al., 2014). More recent studies have shown that the PDE10A inhibitor TP-10 reduces alcohol self-administration in conditions predisposing to elevated self-administration. In addition, TP-10 also reduces alcohol self-administration in genetically alcohol-preferring rats, and in alcohol-non-dependent and -dependent rats (Logrip et al., 2014). Taken together, the above studies suggest that PDE4 and PDE10A are potential therapeutic targets for pharmacotherapy of alcohol dependence.
Opioids
PDE4 inhibitors reduce morphine-induced hyperlocomotor activity and CPP. It has been shown that systemic injections of rolipram suppresses morphine-induced hyperlocomotion (Mori et al., 2000). In addition, administration of rolipram 30 min prior to conditioning sessions blocks the acquisition of CPP to morphine, while of administration of rolipram 30 min prior to CPP test does not affect the expression of morphine CPP (Thompson et al., 2004). The PDE inhibitor rolipram also inhibits the discriminative-stimulus effects of morphine in rats (Yan et al., 2006). Taken together, these results suggest that the cAMP signaling cascade may play a key role in the acquisition of conditioned morphine reward.
The PDE4 inhibitor rolipram does not significantly inhibit heroin self-administration under the fixed ratio 1 (FR1) schedule, but dose-dependently decreases the reward values under the progressive ratio schedule. Importantly, rolipram also decreases the cue- and heroin priming-induced reinstatement of heroin seeking (Lai et al., 2014). Rolipram increases the expression of phosphorylated CREB in the nucleus accumbens, which is correlated with its effect on heroin-seeking behavior (Lai et al., 2014). These results suggest that PDE4 inhibitors may be a potential therapeutic target for the treatment of heroin and morphine dependence.
PDE4 inhibitors were shown to attenuate naloxone-precipitated morphine withdrawal symptoms. In rats treated with single morphine, naloxone challenge induces an increase in PDE4 activity on frontal cortex and hippocampus. In contrast, in rats treated repeatedly with morphine, the naloxone challenge produced no significant effects on PDE4 activity although cyclic AMP was significantly increased by naloxone challenge (Kimura et al., 2006). These results suggest that the lack of PDE4 activation leads to an increase in levels of cAMP, which may be involved in naloxone-precipitated morphine withdrawal symptoms (Kimura et al., 2006). Consistent with this premise, subsequent studies have shown that repeated intraperitoneal (i.p.) injections, but not acute injections, of PDE4 inhibitors rolipram dampen naloxone-precipitated behavioral signs of morphine withdrawal, such as teeth-chattering, tremor, piloerection, lacrimation, rhinorrhea, ptosis, spontaneous jumping and wet-dog shakes, and weight loss (Gonzalez-Cuello et al., 2007; Hamdy et al., 2001; Mamiya et al., 2001; Nunez et al., 2009). The attenuation of withdrawal syndrome by the PDE4 inhibitors is likely mediated by the up-regulation of the cAMP signaling (Gonzalez-Cuello et al., 2007; Nunez et al., 2009). Chronic rolipram treatment in combination with morphine in mice blocked the increase in the expression of c-Fos protein induced by naloxone challenge (Hamdy et al., 2001).
PDE4 inhibitors reduce opioid-induced respiratory depression. In rats, intravenous injection of rolipram blocked morphine-induced prolongation and flattening of inspiratory discharge in the phrenic nerve but not did not affect morphine-induced suppression of paw withdrawal responses to nociceptive thermal stimulation. These results suggest that inhibition of PDE4 can block morphine-induced ventilatory depression without loss of analgesia (Kimura et al., 2015).
2.b. Site-specific effects of PDE4 inhibitors
2.b.1. Striatum/Nucleus accumbens
When paired with a distinct environment, cocaine robustly increases levels of the transcription factor c-Fos in the several brain regions in the mesocorticolimbic system. c-Fos is part of the AP-1 transcription complex that results in activation of signaling cascades thought to be important in several functions, including synaptic and behavioral plasticity (Alberini, 2009; Hiroi and Nestler, 1998; Perez-Cadahia et al., 2011). Within the dorsal striatum and nucleus accumbens, rolipram has no effect on cocaine-induced expression of c-Fos, although rolipram itself increases levels in the nucleus accumbens shell (Thompson et al., 2004). These results suggest that either rolipram does not exert effects on induction via altered c-Fos expression, or the site/s of effects on c-Fos lies outside of the striatum.
There is also evidence that PDE activity in the nucleus accumbens can modulate the reinforcing effect of intracranial self-stimulation of the VTA. Infusion of rolipram into the nucleus accumbens decreases the threshold of intracranial self-stimulation and enhances the effects of systemic cocaine on lowering the threshold of intracranial self-stimulation (Knapp et al., 2001). These results suggest that increasing the activity of cyclic AMP (cAMP) pathways within the nucleus accumbens may enhance general brain reward function.
Incubation of craving is a phenomenon where drug or reward seeking increases with longer periods of abstinence (Grimm et al., 2005; Grimm et al., 2001; Lu et al., 2004). Incubation of heroin craving after 14 days of abstinence relative to 1 day was associated with decreased phosphorylated CREB (pCREB) in the nucleus accumbens, and intra-accumbens rolipram reduced drug seeking and increased local pCREB levels (Sun et al., 2015).
2.b.2. Ventral Tegmental area
We have shown that intra-VTA injection of rolipram before cocaine and saline conditioning blocked the acquisition of cocaine CPP, whereas intra-VTA injection of rolipram just before the CPP test did not affect the expression of cocaine CPP (Zhong et al., 2012). Thus, PDE4 inhibition within the VTA is sufficient to reproduce the ability of systemic rolipram to prevent the establishment of CPP.
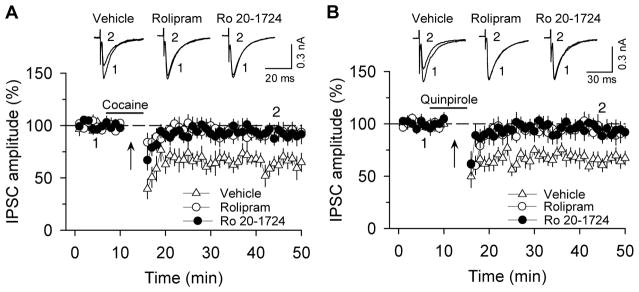
Long-term synaptic plasticity in reward circuitry is thought to underlie reinforcement learning and the development of addictive behavior (Kauer, 2004). Our previous studies have implicated endocannabinoid-mediated long-term depression of inhibitory synaptic transmission (I-LTD) in cocaine-induced inhibitory synaptic plasticity and behavioral effects (Pan et al., 2008a, b; Pan et al., 2011). Cannabinoid receptors (CB1) are Gi/o protein-coupled receptors whose activation leads to the inhibition of adenylyl cyclase (AC), resulting in decreased cAMP/PKA activity (Howlett, 2005), while PDE4 inhibition is known to enhance cAMP levels. We found that PDE4 inhibitors rolipram and Ro 20-1724 blocked endocannabinoid I-LTD and synaptic depression induced by D2 dopamine receptor and cannabinoid CB1 receptor agonists in VTA dopamine neurons [Figure 1, (Zhong et al., 2012)]. These results suggest that blockade of cocaine-induced inhibitory synaptic plasticity within the VTA is a likely cellular mechanism by which PDE4 inhibition impairs CPP to cocaine.
Figure 1.
Selective PDE4 inhibitors rolipram and Ro 20-1724 blocked I-LTD in VTA dopamine neurons. (A) The presence of cocaine (3 μM; indicated by horizontal bar) during the 10 Hz stimulation (indicated by arrow “↑”) induced I-LTD in VTA dopamine neurons (n = 6). This I-LTD was blocked by PDE4 inhibitors rolipram (1 μM; n = 8; p < 0.05 vs. control) and Ro 20-1724 (200 μM; n = 8; p < 0.05 vs. control). The PDE4 inhibitors were present throughout the whole-cell recordings. Sample IPSCs before (indicated by “1”) and after (indicated by “2”) the 10 Hz stimulation are shown on the top. (B) The presence of D2 receptor agonist quinpirole (1 μM) during the 10 Hz stimulation induced I-LTD in VTA dopamine neurons (n = 7). This I-LTD was blocked by rolipram (1 μM; n = 8; p < 0.05 vs. control) or Ro 20-1724 (200 μM; n = 7; p < 0.05 vs. control). Error bars indicate SEM (used with the permission of Neuropsychopharmacology).
3. Therapeutic opportunities of PDE4 inhibitors
3.a. Clinical studies with PDE4 inhibitors
PDE4 inhibitors have been under clinical trials for the treatment of chronic obstructive pulmonary disease (COPD), asthma (Diamant and Spina, 2011; Page and Spina, 2012), and depression (Bertolino et al., 1988; Fleischhacker et al., 1992). Roflumilast is the only approved PDE4 inhibitor for the treatment of COPD, and it reduces COPD exacerbations and modestly improves lung function (Mulhall et al., 2015). More recently, the PDE4 inhibitor MK-0952 has been under a phase II clinical trial for cognition-enhancing effects (Wang et al., 2015). Apremilast is an orally effective PDE4 inhibitor that was approved by US FDA in 2015 for the treatment of moderate-to-severe plaque psoriasis and psoriatic arthritis (Gisondi and Girolomoni, 2016). It is safe and well-tolerated, although it causes nausea and vomiting in some patients (Gisondi and Girolomoni, 2016). As mentioned earlier in this review, preclinical studies with animal models have shown that the non-selective PDE inhibitor ibudilast reduces drug seeking behaviors of methamphetamine (Beardsley et al., 2010; Snider et al., 2013; Snider et al., 2012) and alcohol-dependence (Bell et al., 2015). Ibudilast is currently in a Phase I clinical trial for alcohol use disorders as well as in Phase II clinical trials for methamphetamine dependence and oxycodone abuse (Logrip, 2015). Ibudilast inhibits both PDE and glial proinflammatory activity, which has been linked to its anti-addiction effects (Beardsley and Hauser, 2014). Thus, PDE inhibitors may represent a promising candidate for developing anti-addiction medications. PDE4 inhibitors have adverse side-effects such as nausea and emesis, which limit their clinical utility (O’Donnell and Zhang, 2004). Activation of PDE4D, which is highly expressed in the area postrema, an emetic-trigger zone (Lamontagne et al., 2001), is related to emetic potential of PDE4 inhibitors (Robichaud et al., 2002). Subtype- or isoform-specific PDE4 inhibitors are expected to be developed in the future to minimize their off-target effects (Wang et al., 2015). Paradoxically, non-selective, broader acting drugs such as ibudilast may retain the pharmacological efficacy of PDE inhibition with less side effects. Alternatively, targeting PDE4A or 4B, PDE isoforms with expression in mesocorticolimbic circuitry, may prove to be effective in treatment of substance drug and/or alcohol abuse without inducing the degree of nausea that non-selective PDE4 inhibitors do.
3.b. Opportunities
There are at least 30 isoforms and splice variants of PDE4 (MacKenzie and Houslay, 2000; Wang et al., 2015), which presents challenges and opportunities for pharmacological intervention of drug addiction. As mentioned earlier in this review, a number of PDE4 inhibitors have been approved by the FDA for the treatment of COPD and inflammatory diseases (Gisondi and Girolomoni, 2016; Mulhall et al., 2015). There is a promising possibility to repurpose these PDE4 inhibitors for the treatment of drug addiction as these inhibitors are known to be safe and well-tolerated in patients.
PDE4 inhibitors such as rolipram often cause nausea and emesis, which prevent their clinical utility (Hansen and Zhang, 2015). The emetic effects are likely mediated by PDE4 expressed in the area postrema, the emetic center of the brain (Mori et al., 2010). PDE4A is predominantly expressed in the olfactory system, PDE4B is highly expressed in the mesolimbic dopamine system, and PDE4D is expressed in many parts of the brain, but not in the VTA and nucleus accumbens (Cherry and Davis, 1999). PDE4 inhibition-induced reduction of the effects of drugs of abuse may be mediated primarily by PDE4B. Selective PDE4B inhibitors may represent an effective means to reduce drug seeking behavior while avoiding emetic effects of PDE4 inhibitors that are mediated by activation of the PDE4D. Recently a series of triazine derivatives were found to be PDE4B selective inhibitors, which show >100-fold selectivity over the PDE4D isozyme. These PDE4B inhibitors exhibited potent anti-inflammatory effects in vivo and showed less emesis (Hagen et al., 2014; Naganuma et al., 2009). PDE4B inhibition may also have effects on generalized anxiety, which could influence drug craving during abstinence. Knockout of a large region of the catalytic domain (exons 8–11) resulted in increased anxiety-like behavior in the several assays (Zhang et al., 2008), although there was no difference in the elevated plus maze (Siuciak et al., 2008). In contrast, reduction of cAMP hydrolization via Y358C substitution within the catalytic subregion reduced anxiety-like behavior (McGirr et al., 2016). These conflicting results make it difficult to predict how a particular PDE4B specific inhibitor may influence generalized anxiety during drug abstinence, however inhibitors with selectivity for the PDE4B are predicted to have a strong potential for alleviating drug craving without the emetic effects associated with non-selective PDE4 inhibition.
Acknowledgments
Work in the authors’ laboratories was supported by US NIH Grants DA035217 (QSL), MH101146 (QSL), DA039276 (CMO), and DA041212 (CMO). Research was also supported by the Medical College of Wisconsin Research and Education Initiative Fund, a component of the Advancing a Healthier Wisconsin Endowment at the Medical College of Wisconsin (CMO, QSL).
Footnotes
Compliance with Ethics Guidelines
Christopher M. Olsen and Qing-song Liu declare that they have no conflict of interest. This manuscript is a review article and does not involve a research protocol requiring approval by the relevant institutional review board or ethics committee.
References
- Alberini CM. Transcription factors in long-term memory and synaptic plasticity. Physiol Rev. 2009;89:121–145. doi: 10.1152/physrev.00017.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allain F, Minogianis EA, Roberts DC, Samaha AN. How fast and how often: The pharmacokinetics of drug use are decisive in addiction. Neurosci Biobehav Rev. 2015;56:166–179. doi: 10.1016/j.neubiorev.2015.06.012. [DOI] [PubMed] [Google Scholar]
- Anderson SM, Pierce RC. Cocaine-induced alterations in dopamine receptor signaling: implications for reinforcement and reinstatement. Pharmacology & therapeutics. 2005;106:389–403. doi: 10.1016/j.pharmthera.2004.12.004. [DOI] [PubMed] [Google Scholar]
- Bardo MT, Bevins RA. Conditioned place preference: what does it add to our preclinical understanding of drug reward? Psychopharmacology. 2000;153:31–43. doi: 10.1007/s002130000569. [DOI] [PubMed] [Google Scholar]
- Beardsley PM, Hauser KF. Glial modulators as potential treatments of psychostimulant abuse. Adv Pharmacol. 2014;69:1–69. doi: 10.1016/B978-0-12-420118-7.00001-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beardsley PM, Shelton KL, Hendrick E, Johnson KW. The glial cell modulator and phosphodiesterase inhibitor, AV411 (ibudilast), attenuates prime- and stress-induced methamphetamine relapse. European journal of pharmacology. 2010;637:102–108. doi: 10.1016/j.ejphar.2010.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell RL, Lopez MF, Cui C, Egli M, Johnson KW, Franklin KM, Becker HC. Ibudilast reduces alcohol drinking in multiple animal models of alcohol dependence. Addiction biology. 2015;20:38–42. doi: 10.1111/adb.12106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertolino A, Crippa D, di Dio S, Fichte K, Musmeci G, Porro V, Rapisarda V, Sastre-y-Hernandez M, Schratzer M. Rolipram versus imipramine in inpatients with major, “minor” or atypical depressive disorder: a double-blind double-dummy study aimed at testing a novel therapeutic approach. International clinical psychopharmacology. 1988;3:245–253. doi: 10.1097/00004850-198807000-00006. [DOI] [PubMed] [Google Scholar]
- Blednov YA, Benavidez JM, Black M, Harris RA. Inhibition of phosphodiesterase 4 reduces ethanol intake and preference in C57BL/6J mice. Frontiers in neuroscience. 2014;8:129. doi: 10.3389/fnins.2014.00129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britt JP, Benaliouad F, McDevitt RA, Stuber GD, Wise RA, Bonci A. Synaptic and behavioral profile of multiple glutamatergic inputs to the nucleus accumbens. Neuron. 2012;76:790–803. doi: 10.1016/j.neuron.2012.09.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlezon WA, Jr, Chartoff EH. Intracranial self-stimulation (ICSS) in rodents to study the neurobiology of motivation. Nat Protoc. 2007;2:2987–2995. doi: 10.1038/nprot.2007.441. [DOI] [PubMed] [Google Scholar]
- Cherry JA, Davis RL. Cyclic AMP phosphodiesterases are localized in regions of the mouse brain associated with reinforcement, movement, and affect. The Journal of comparative neurology. 1999;407:287–301. [PubMed] [Google Scholar]
- Conrad KL, Louderback KM, Milano EJ, Winder DG. Assessment of the impact of pattern of cocaine dosing schedule during conditioning and reconditioning on magnitude of cocaine CPP, extinction, and reinstatement. Psychopharmacology (Berl) 2013;227:109–116. doi: 10.1007/s00213-012-2944-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conti M, Richter W, Mehats C, Livera G, Park JY, Jin C. Cyclic AMP-specific PDE4 phosphodiesterases as critical components of cyclic AMP signaling. The Journal of biological chemistry. 2003;278:5493–5496. doi: 10.1074/jbc.R200029200. [DOI] [PubMed] [Google Scholar]
- Cooper DM. Compartmentalization of adenylate cyclase and cAMP signalling. Biochemical Society transactions. 2005;33:1319–1322. doi: 10.1042/BST0331319. [DOI] [PubMed] [Google Scholar]
- Crabbe JC. Rodent models of genetic contributions to motivation to abuse alcohol. Nebr Symp Motiv. 2014;61:5–29. doi: 10.1007/978-1-4939-0653-6_2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamant Z, Spina D. PDE4-inhibitors: a novel, targeted therapy for obstructive airways disease. Pulmonary pharmacology & therapeutics. 2011;24:353–360. doi: 10.1016/j.pupt.2010.12.011. [DOI] [PubMed] [Google Scholar]
- Fleischhacker WW, Hinterhuber H, Bauer H, Pflug B, Berner P, Simhandl C, Wolf R, Gerlach W, Jaklitsch H, Sastre-y-Hernandez M, et al. A multicenter double-blind study of three different doses of the new cAMP-phosphodiesterase inhibitor rolipram in patients with major depressive disorder. Neuropsychobiology. 1992;26:59–64. doi: 10.1159/000118897. [DOI] [PubMed] [Google Scholar]
- Franklin KM, Hauser SR, Lasek AW, McClintick J, Ding ZM, McBride WJ, Bell RL. Reduction of alcohol drinking of alcohol-preferring (P) and high-alcohol drinking (HAD1) rats by targeting phosphodiesterase-4 (PDE4) Psychopharmacology (Berl) 2015;232:2251–2262. doi: 10.1007/s00213-014-3852-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gisondi P, Girolomoni G. Apremilast in the therapy of moderate-to-severe chronic plaque psoriasis. Drug Des Devel Ther. 2016;10:1763–1770. doi: 10.2147/DDDT.S108115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Cuello A, Sanchez L, Hernandez J, Teresa Castells M, Victoria Milanes M, Laorden ML. Phosphodiesterase 4 inhibitors, rolipram and diazepam block the adaptive changes observed during morphine withdrawal in the heart. European journal of pharmacology. 2007;570:1–9. doi: 10.1016/j.ejphar.2007.05.051. [DOI] [PubMed] [Google Scholar]
- Graybiel AM. Neurotransmitters and neuromodulators in the basal ganglia. Trends in neurosciences. 1990;13:244–254. doi: 10.1016/0166-2236(90)90104-i. [DOI] [PubMed] [Google Scholar]
- Graybiel AM. The basal ganglia. Current biology: CB. 2000;10:R509–511. doi: 10.1016/s0960-9822(00)00593-5. [DOI] [PubMed] [Google Scholar]
- Grimm JW, Fyall AM, Osincup DP. Incubation of sucrose craving: effects of reduced training and sucrose pre-loading. Physiol Behav. 2005;84:73–79. doi: 10.1016/j.physbeh.2004.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm JW, Hope BT, Wise RA, Shaham Y. Neuroadaptation. Incubation of cocaine craving after withdrawal. Nature. 2001;412:141–142. doi: 10.1038/35084134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagen TJ, Mo X, Burgin AB, Fox D, 3rd, Zhang Z, Gurney ME. Discovery of triazines as selective PDE4B versus PDE4D inhibitors. Bioorganic & medicinal chemistry letters. 2014;24:4031–4034. doi: 10.1016/j.bmcl.2014.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamdy MM, Mamiya T, Noda Y, Sayed M, Assi AA, Gomaa A, Yamada K, Nabeshima T. A selective phosphodiesterase IV inhibitor, rolipram blocks both withdrawal behavioral manifestations, and c-Fos protein expression in morphine dependent mice. Behavioural brain research. 2001;118:85–93. doi: 10.1016/s0166-4328(00)00315-6. [DOI] [PubMed] [Google Scholar]
- Hansen RT, 3rd, Zhang HT. Phosphodiesterase-4 modulation as a potential therapeutic for cognitive loss in pathological and non-pathological aging: possibilities and pitfalls. Current pharmaceutical design. 2015;21:291–302. doi: 10.2174/1381612820666140826114208. [DOI] [PubMed] [Google Scholar]
- Hiroi N, Nestler EJ. Nuclear memory: gene transcription and behavior. Adv Pharmacol. 1998;42:1037–1041. doi: 10.1016/s1054-3589(08)60924-2. [DOI] [PubMed] [Google Scholar]
- Horn CC, Kimball BA, Wang H, Kaus J, Dienel S, Nagy A, Gathright GR, Yates BJ, Andrews PL. Why can’t rodents vomit? A comparative behavioral, anatomical, and physiological study. PLoS One. 2013;8:e60537. doi: 10.1371/journal.pone.0060537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howlett AC. Cannabinoid receptor signaling. Handbook of experimental pharmacology. 2005:53–79. doi: 10.1007/3-540-26573-2_2. [DOI] [PubMed] [Google Scholar]
- Hu W, Lu T, Chen A, Huang Y, Hansen R, Chandler LJ, Zhang HT. Inhibition of phosphodiesterase-4 decreases ethanol intake in mice. Psychopharmacology. 2011;218:331–339. doi: 10.1007/s00213-011-2290-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikemoto S, Bonci A. Neurocircuitry of drug reward. Neuropharmacology. 2014;76(Pt B):329–341. doi: 10.1016/j.neuropharm.2013.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itzhak Y, Anderson KL. Changes in the magnitude of drug-unconditioned stimulus during conditioning modulate cocaine-induced place preference in mice. Addict Biol. 2012;17:706–716. doi: 10.1111/j.1369-1600.2011.00334.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iyo M, Bi Y, Hashimoto K, Inada T, Fukui S. Prevention of methamphetamine-induced behavioral sensitization in rats by a cyclic AMP phosphodiesterase inhibitor, rolipram. European journal of pharmacology. 1996;312:163–170. doi: 10.1016/0014-2999(96)00479-7. [DOI] [PubMed] [Google Scholar]
- Janes AC, Kantak KM, Cherry JA. The involvement of type IV phosphodiesterases in cocaine-induced sensitization and subsequent pERK expression in the mouse nucleus accumbens. Psychopharmacology. 2009;206:177–185. doi: 10.1007/s00213-009-1594-4. [DOI] [PubMed] [Google Scholar]
- Johansson EM, Reyes-Irisarri E, Mengod G. Comparison of cAMP-specific phosphodiesterase mRNAs distribution in mouse and rat brain. Neurosci Lett. 2012;525:1–6. doi: 10.1016/j.neulet.2012.07.050. [DOI] [PubMed] [Google Scholar]
- Kauer JA. Learning mechanisms in addiction: synaptic plasticity in the ventral tegmental area as a result of exposure to drugs of abuse. Annual review of physiology. 2004;66:447–475. doi: 10.1146/annurev.physiol.66.032102.112534. [DOI] [PubMed] [Google Scholar]
- Kimura M, Tokumura M, Itoh T, Inoue O, Abe K. Lack of cyclic AMP-specific phosphodiesterase 4 activation during naloxone-precipitated morphine withdrawal in rats. Neuroscience letters. 2006;404:107–111. doi: 10.1016/j.neulet.2006.05.014. [DOI] [PubMed] [Google Scholar]
- Kimura S, Ohi Y, Haji A. Blockade of phosphodiesterase 4 reverses morphine-induced ventilatory disturbance without loss of analgesia. Life Sci. 2015;127:32–38. doi: 10.1016/j.lfs.2015.02.006. [DOI] [PubMed] [Google Scholar]
- Knapp CM, Foye MM, Ciraulo DA, Kornetsky C. The type IV phosphodiesterase inhibitors, Ro 20-1724 and rolipram, block the initiation of cocaine self-administration. Pharmacology, biochemistry, and behavior. 1999;62:151–158. doi: 10.1016/s0091-3057(98)00154-3. [DOI] [PubMed] [Google Scholar]
- Knapp CM, Lee K, Foye M, Ciraulo DA, Kornetsky C. Additive effects of intra-accumbens infusion of the cAMP-specific phosphodiesterase inhibitor, rolipram and cocaine on brain stimulation reward. Life Sci. 2001;69:1673–1682. doi: 10.1016/s0024-3205(01)01249-8. [DOI] [PubMed] [Google Scholar]
- Kuroiwa M, Snyder GL, Shuto T, Fukuda A, Yanagawa Y, Benavides DR, Nairn AC, Bibb JA, Greengard P, Nishi A. Phosphodiesterase 4 inhibition enhances the dopamine D1 receptor/PKA/DARPP-32 signaling cascade in frontal cortex. Psychopharmacology (Berl) 2012;219:1065–1079. doi: 10.1007/s00213-011-2436-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai M, Zhu H, Sun A, Zhuang D, Fu D, Chen W, Zhang HT, Zhou W. The phosphodiesterase-4 inhibitor rolipram attenuates heroin-seeking behavior induced by cues or heroin priming in rats. The international journal of neuropsychopharmacology/official scientific journal of the Collegium Internationale Neuropsychopharmacologicum. 2014;17:1397–1407. doi: 10.1017/S1461145714000595. [DOI] [PubMed] [Google Scholar]
- Lakics V, Karran EH, Boess FG. Quantitative comparison of phosphodiesterase mRNA distribution in human brain and peripheral tissues. Neuropharmacology. 2010;59:367–374. doi: 10.1016/j.neuropharm.2010.05.004. [DOI] [PubMed] [Google Scholar]
- Lamontagne S, Meadows E, Luk P, Normandin D, Muise E, Boulet L, Pon DJ, Robichaud A, Robertson GS, Metters KM, Nantel F. Localization of phosphodiesterase-4 isoforms in the medulla and nodose ganglion of the squirrel monkey. Brain research. 2001;920:84–96. doi: 10.1016/s0006-8993(01)03023-2. [DOI] [PubMed] [Google Scholar]
- Liddie S, Anderson KL, Paz A, Itzhak Y. The effect of phosphodiesterase inhibitors on the extinction of cocaine-induced conditioned place preference in mice. Journal of psychopharmacology (Oxford, England) 2012;26:1375–1382. doi: 10.1177/0269881112447991. [DOI] [PubMed] [Google Scholar]
- Lim YW, Meyer NP, Shah AS, Budde MD, Stemper BD, Olsen CM. Voluntary Alcohol Intake following Blast Exposure in a Rat Model of Mild Traumatic Brain Injury. PLoS One. 2015;10:e0125130. doi: 10.1371/journal.pone.0125130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Liu Y, Zhong P, Wilkinson B, Qi J, Olsen CM, Bayer KU, Liu QS. CaMKII activity in the ventral tegmental area gates cocaine-induced synaptic plasticity in the nucleus accumbens. Neuropsychopharmacology. 2014;39:989–999. doi: 10.1038/npp.2013.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logrip ML. Phosphodiesterase regulation of alcohol drinking in rodents. Alcohol. 2015;49:795–802. doi: 10.1016/j.alcohol.2015.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logrip ML, Vendruscolo LF, Schlosburg JE, Koob GF, Zorrilla EP. Phosphodiesterase 10A regulates alcohol and saccharin self-administration in rats. Neuropsychopharmacology. 2014;39:1722–1731. doi: 10.1038/npp.2014.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu L, Grimm JW, Hope BT, Shaham Y. Incubation of cocaine craving after withdrawal: a review of preclinical data. Neuropharmacology. 2004;47(Suppl 1):214–226. doi: 10.1016/j.neuropharm.2004.06.027. [DOI] [PubMed] [Google Scholar]
- Lugnier C. Cyclic nucleotide phosphodiesterase (PDE) superfamily: a new target for the development of specific therapeutic agents. Pharmacology & therapeutics. 2006;109:366–398. doi: 10.1016/j.pharmthera.2005.07.003. [DOI] [PubMed] [Google Scholar]
- MacKenzie SJ, Houslay MD. Action of rolipram on specific PDE4 cAMP phosphodiesterase isoforms and on the phosphorylation of cAMP-response-element-binding protein (CREB) and p38 mitogen-activated protein (MAP) kinase in U937 monocytic cells. The Biochemical journal. 2000;347:571–578. doi: 10.1042/0264-6021:3470571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mamiya T, Noda Y, Ren X, Hamdy M, Furukawa S, Kameyama T, Yamada K, Nabeshima T. Involvement of cyclic AMP systems in morphine physical dependence in mice: prevention of development of morphine dependence by rolipram, a phosphodiesterase 4 inhibitor. British journal of pharmacology. 2001;132:1111–1117. doi: 10.1038/sj.bjp.0703912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantsch JR, Baker DA, Funk D, Le AD, Shaham Y. Stress-Induced Reinstatement of Drug Seeking: 20 Years of Progress. Neuropsychopharmacology. 2016;41:335–356. doi: 10.1038/npp.2015.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGirr A, Lipina TV, Mun HS, Georgiou J, Al-Amri AH, Ng E, Zhai D, Elliott C, Cameron RT, Mullins JG, Liu F, Baillie GS, Clapcote SJ, Roder JC. Specific Inhibition of Phosphodiesterase-4B Results in Anxiolysis and Facilitates Memory Acquisition. Neuropsychopharmacology. 2016;41:1080–1092. doi: 10.1038/npp.2015.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori F, Perez-Torres S, De Caro R, Porzionato A, Macchi V, Beleta J, Gavalda A, Palacios JM, Mengod G. The human area postrema and other nuclei related to the emetic reflex express cAMP phosphodiesterases 4B and 4D. Journal of chemical neuroanatomy. 2010;40:36–42. doi: 10.1016/j.jchemneu.2010.03.004. [DOI] [PubMed] [Google Scholar]
- Mori T, Baba J, Ichimaru Y, Suzuki T. Effects of rolipram, a selective inhibitor of phosphodiesterase 4, on hyperlocomotion induced by several abused drugs in mice. Japanese journal of pharmacology. 2000;83:113–118. doi: 10.1254/jjp.83.113. [DOI] [PubMed] [Google Scholar]
- Muelbl MJ, Nawarawong NN, Clancy PT, Nettesheim CE, Lim YW, Olsen CM. Responses to drugs of abuse and non-drug rewards in leptin deficient ob/ob mice. Psychopharmacology (Berl) 2016 doi: 10.1007/s00213-016-4323-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulhall AM, Droege CA, Ernst NE, Panos RJ, Zafar MA. Phosphodiesterase 4 inhibitors for the treatment of chronic obstructive pulmonary disease: a review of current and developing drugs. Expert Opin Investig Drugs. 2015;24:1597–1611. doi: 10.1517/13543784.2015.1094054. [DOI] [PubMed] [Google Scholar]
- Muschamp JW, Carlezon WA., Jr Roles of nucleus accumbens CREB and dynorphin in dysregulation of motivation. Cold Spring Harb Perspect Med. 2013;3:a012005. doi: 10.1101/cshperspect.a012005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naganuma K, Omura A, Maekawara N, Saitoh M, Ohkawa N, Kubota T, Nagumo H, Kodama T, Takemura M, Ohtsuka Y, Nakamura J, Tsujita R, Kawasaki K, Yokoi H, Kawanishi M. Discovery of selective PDE4B inhibitors. Bioorganic & medicinal chemistry letters. 2009;19:3174–3176. doi: 10.1016/j.bmcl.2009.04.121. [DOI] [PubMed] [Google Scholar]
- Negus SS, Miller LL. Intracranial self-stimulation to evaluate abuse potential of drugs. Pharmacol Rev. 2014;66:869–917. doi: 10.1124/pr.112.007419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestler EJ. Reflections on: “A general role for adaptations in G-Proteins and the cyclic AMP system in mediating the chronic actions of morphine and cocaine on neuronal function”. Brain Res. 2015 doi: 10.1016/0006-8993(91)91111-d. [DOI] [PubMed] [Google Scholar]
- Nishi A, Kuroiwa M, Miller DB, O’Callaghan JP, Bateup HS, Shuto T, Sotogaku N, Fukuda T, Heintz N, Greengard P, Snyder GL. Distinct roles of PDE4 and PDE10A in the regulation of cAMP/PKA signaling in the striatum. J Neurosci. 2008;28:10460–10471. doi: 10.1523/JNEUROSCI.2518-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunez C, Gonzalez-Cuello A, Sanchez L, Vargas ML, Milanes MV, Laorden ML. Effects of rolipram and diazepam on the adaptive changes induced by morphine withdrawal in the hypothalamic paraventricular nucleus. European journal of pharmacology. 2009;620:1–8. doi: 10.1016/j.ejphar.2009.08.002. [DOI] [PubMed] [Google Scholar]
- O’Donnell JM, Zhang HT. Antidepressant effects of inhibitors of cAMP phosphodiesterase (PDE4) Trends in pharmacological sciences. 2004;25:158–163. doi: 10.1016/j.tips.2004.01.003. [DOI] [PubMed] [Google Scholar]
- Olsen CM, Childs DS, Stanwood GD, Winder DG. Operant sensation seeking requires metabotropic glutamate receptor 5 (mGluR5) PloS one. 2010;5:e15085. doi: 10.1371/journal.pone.0015085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen CM, Winder DG. A method for single-session cocaine self-administration in the mouse. Psychopharmacology (Berl) 2006;187:13–21. doi: 10.1007/s00213-006-0388-1. [DOI] [PubMed] [Google Scholar]
- Page C, Spina D. Selective PDE inhibitors as novel treatments for respiratory diseases. Current opinion in pharmacology. 2012 doi: 10.1016/j.coph.2012.02.016. [DOI] [PubMed] [Google Scholar]
- Pan B, Hillard CJ, Liu QS. D2 dopamine receptor activation facilitates endocannabinoid-mediated long-term synaptic depression of GABAergic synaptic transmission in midbrain dopamine neurons via cAMP-protein kinase A signaling. J Neurosci. 2008a;28:14018–14030. doi: 10.1523/JNEUROSCI.4035-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan B, Hillard CJ, Liu QS. Endocannabinoid signaling mediates cocaine-induced inhibitory synaptic plasticity in midbrain dopamine neurons. J Neurosci. 2008b;28:1385–1397. doi: 10.1523/JNEUROSCI.4033-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan B, Zhong P, Sun D, Liu QS. Extracellular signal-regulated kinase signaling in the ventral tegmental area mediates cocaine-induced synaptic plasticity and rewarding effects. J Neurosci. 2011;31:11244–11255. doi: 10.1523/JNEUROSCI.1040-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Cadahia B, Drobic B, Davie JR. Activation and function of immediate-early genes in the nervous system. Biochem Cell Biol. 2011;89:61–73. doi: 10.1139/O10-138. [DOI] [PubMed] [Google Scholar]
- Perez-Torres S, Miro X, Palacios JM, Cortes R, Puigdomenech P, Mengod G. Phosphodiesterase type 4 isozymes expression in human brain examined by in situ hybridization histochemistry and[3H]rolipram binding autoradiography. Comparison with monkey and rat brain. Journal of chemical neuroanatomy. 2000;20:349–374. doi: 10.1016/s0891-0618(00)00097-1. [DOI] [PubMed] [Google Scholar]
- Pierce RC, Kalivas PW. A circuitry model of the expression of behavioral sensitization to amphetamine-like psychostimulants. Brain Res Brain Res Rev. 1997;25:192–216. doi: 10.1016/s0165-0173(97)00021-0. [DOI] [PubMed] [Google Scholar]
- Richter W, Menniti FS, Zhang HT, Conti M. PDE4 as a target for cognition enhancement. Expert opinion on therapeutic targets. 2013;17:1011–1027. doi: 10.1517/14728222.2013.818656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robichaud A, Stamatiou PB, Jin SL, Lachance N, MacDonald D, Laliberte F, Liu S, Huang Z, Conti M, Chan CC. Deletion of phosphodiesterase 4D in mice shortens alpha(2)-adrenoceptor-mediated anesthesia, a behavioral correlate of emesis. The Journal of clinical investigation. 2002;110:1045–1052. doi: 10.1172/JCI15506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Res Brain Res Rev. 1993;18:247–291. doi: 10.1016/0165-0173(93)90013-p. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. Review. The incentive sensitization theory of addiction: some current issues. Philos Trans R Soc Lond B Biol Sci. 2008;363:3137–3146. doi: 10.1098/rstb.2008.0093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodd ZA, Bell RL, Sable HJ, Murphy JM, McBride WJ. Recent advances in animal models of alcohol craving and relapse. Pharmacol Biochem Behav. 2004;79:439–450. doi: 10.1016/j.pbb.2004.08.018. [DOI] [PubMed] [Google Scholar]
- Schroeder JA, Ruta JD, Gordon JS, Rodrigues AS, Foote CC. The phosphodiesterase inhibitor isobutylmethylxanthine attenuates behavioral sensitization to cocaine. Behavioural pharmacology. 2012;23:310–314. doi: 10.1097/FBP.0b013e3283536d04. [DOI] [PubMed] [Google Scholar]
- Shaham Y, Shalev U, Lu L, De Wit H, Stewart J. The reinstatement model of drug relapse: history, methodology and major findings. Psychopharmacology (Berl) 2003;168:3–20. doi: 10.1007/s00213-002-1224-x. [DOI] [PubMed] [Google Scholar]
- Siuciak JA, McCarthy SA, Chapin DS, Martin AN. Behavioral and neurochemical characterization of mice deficient in the phosphodiesterase-4B (PDE4B) enzyme. Psychopharmacology (Berl) 2008;197:115–126. doi: 10.1007/s00213-007-1014-6. [DOI] [PubMed] [Google Scholar]
- Snider SE, Hendrick ES, Beardsley PM. Glial cell modulators attenuate methamphetamine self-administration in the rat. European journal of pharmacology. 2013;701:124–130. doi: 10.1016/j.ejphar.2013.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snider SE, Vunck SA, van den Oord EJ, Adkins DE, McClay JL, Beardsley PM. The glial cell modulators, ibudilast and its amino analog, AV1013, attenuate methamphetamine locomotor activity and its sensitization in mice. European journal of pharmacology. 2012;679:75–80. doi: 10.1016/j.ejphar.2012.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stolerman IP, Childs E, Ford MM, Grant KA. Role of training dose in drug discrimination: a review. Behav Pharmacol. 2011;22:415–429. doi: 10.1097/FBP.0b013e328349ab37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun A, Zhuang D, Zhu H, Lai M, Chen W, Liu H, Zhang F, Zhou W. Decrease of phosphorylated CREB and ERK in nucleus accumbens is associated with the incubation of heroin seeking induced by cues after withdrawal. Neurosci Lett. 2015;591:166–170. doi: 10.1016/j.neulet.2015.02.048. [DOI] [PubMed] [Google Scholar]
- Thompson BE, Sachs BD, Kantak KM, Cherry JA. The Type IV phosphodiesterase inhibitor rolipram interferes with drug-induced conditioned place preference but not immediate early gene induction in mice. The European journal of neuroscience. 2004;19:2561–2568. doi: 10.1111/j.0953-816X.2004.03357.x. [DOI] [PubMed] [Google Scholar]
- Thomsen M, Caine SB. Chronic intravenous drug self-administration in rats and mice. Curr Protoc Neurosci. 2005;Chapter 9(Unit 9):20. doi: 10.1002/0471142301.ns0920s32. [DOI] [PubMed] [Google Scholar]
- Todd TP, Vurbic D, Bouton ME. Behavioral and neurobiological mechanisms of extinction in Pavlovian and instrumental learning. Neurobiol Learn Mem. 2014;108:52–64. doi: 10.1016/j.nlm.2013.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzschentke TM. Measuring reward with the conditioned place preference (CPP) paradigm: update of the last decade. Addict Biol. 2007;12:227–462. doi: 10.1111/j.1369-1600.2007.00070.x. [DOI] [PubMed] [Google Scholar]
- Wang ZZ, Zhang Y, Zhang HT, Li YF. Phosphodiesterase: an interface connecting cognitive deficits to neuropsychiatric and neurodegenerative diseases. Current pharmaceutical design. 2015;21:303–316. doi: 10.2174/1381612820666140826115559. [DOI] [PubMed] [Google Scholar]
- Wen RT, Feng WY, Liang JH, Zhang HT. Role of phosphodiesterase 4-mediated cyclic AMP signaling in pharmacotherapy for substance dependence. Curr Pharm Des. 2015;21:355–364. doi: 10.2174/1381612820666140826114412. [DOI] [PubMed] [Google Scholar]
- Wen RT, Zhang M, Qin WJ, Liu Q, Wang WP, Lawrence AJ, Zhang HT, Liang JH. The phosphodiesterase-4 (PDE4) inhibitor rolipram decreases ethanol seeking and consumption in alcohol-preferring Fawn-Hooded rats. Alcoholism, clinical and experimental research. 2012;36:2157–2167. doi: 10.1111/j.1530-0277.2012.01845.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan Y, Nitta A, Mizuno T, Nakajima A, Yamada K, Nabeshima T. Discriminative-stimulus effects of methamphetamine and morphine in rats are attenuated by cAMP-related compounds. Behavioural brain research. 2006;173:39–46. doi: 10.1016/j.bbr.2006.05.029. [DOI] [PubMed] [Google Scholar]
- Young R. Drug Discrimination. In: Buccafusco JJ, editor. Methods of Behavior Analysis in Neuroscience. 2. Boca Raton (FL): 2009. [Google Scholar]
- Zhang HT. Cyclic AMP-specific phosphodiesterase-4 as a target for the development of antidepressant drugs. Current pharmaceutical design. 2009;15:1688–1698. doi: 10.2174/138161209788168092. [DOI] [PubMed] [Google Scholar]
- Zhang HT, Huang Y, Masood A, Stolinski LR, Li Y, Zhang L, Dlaboga D, Jin SL, Conti M, O’Donnell JM. Anxiogenic-like behavioral phenotype of mice deficient in phosphodiesterase 4B (PDE4B) Neuropsychopharmacology. 2008;33:1611–1623. doi: 10.1038/sj.npp.1301537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong P, Wang W, Yu F, Nazari M, Liu X, Liu QS. Phosphodiesterase 4 inhibition impairs cocaine-induced inhibitory synaptic plasticity and conditioned place preference. Neuropsychopharmacology. 2012;37:2377–2387. doi: 10.1038/npp.2012.93. [DOI] [PMC free article] [PubMed] [Google Scholar]