Abstract
Objective:
To evaluate the effect of ospemifene 60 mg on the lipid and coagulation parameters of postmenopausal women using data from five phase 2 and 3 clinical trials.
Methods:
Data for lipids and coagulation factors for 2,166 postmenopausal women were pooled from five randomized, placebo-controlled studies. Lipid and coagulation parameters included in this analysis were total cholesterol, high-density lipoproteins (HDL), low-density lipoproteins (LDL), triglycerides, activated partial thromboplastin time (aPTT), fibrinogen, antithrombin antigen, protein C Ag, and protein S Ag free.
Results:
Mean percent changes in HDL and LDL were significantly greater with ospemifene versus placebo at month 3 (HDL: 4.4% vs 0.2%; LDL: −5.2% vs 2.4%), month 6 (HDL: 5.1% vs 1.5%; LDL: −6.7% vs 2.4%), and month 12 (HDL: 2.3% vs −1.9%; LDL: −7.0% vs −2.1%; P < 0.05, for all comparisons). Ospemifene significantly reduced total cholesterol at 6 months (−1.8% vs 1.6%; P = 0.0345 versus placebo), and changes in triglycerides with ospemifene were similar to placebo at all three time points. In subgroup analyses based on age, body mass index, and baseline triglyceride level, ospemifene increased HDL and decreased LDL, but had no significant effect on total cholesterol and triglycerides relative to placebo. Ospemifene significantly improved fibrinogen and protein C antigen levels relative to placebo at months 3 (−8.7% vs −0.8% and −2.7% vs 0.5%, respectively), 6 (−6.0% vs 6.7% and −3.6 vs 8.0%), and 12 (−8.7% vs 7.3% and −4.5% vs 6.6%; P < 0.01, for all). The levels of all coagulation factors remained within the normal range throughout the studies.
Conclusion:
Ospemifene 60 mg does not have a detrimental effect on lipid and coagulation parameters of postmenopausal women with up to 12 months of use.
Keywords: Coagulation factors, Dyspareunia, Fibrinogen, Lipids, Ospemifene, Vulvar and vaginal atrophy
Estrogen decline at the onset of menopause can leave the vasculature vulnerable to cardiovascular disease, which is associated with changes in surrogate markers such as lipids and coagulation factors. Postmenopausal women have been shown to have significantly higher levels of total cholesterol, triglycerides, low-density lipoprotein (LDL) cholesterol, and very-LDL (VLDL) cholesterol, and a lower level of high-density lipoprotein (HDL) cholesterol than premenopausal women.1 Hormone therapy (HT) has been shown to offset the detrimental effect of menopause on lipids by decreasing total cholesterol and LDL, and increasing HDL in postmenopausal women.2-8
Clinical studies have shown HT's effect on the risk of thrombotic diseases differs based on the route of administration. Oral HT has been shown to increase fibrinolysis and coagulation in postmenopausal women, with clinical studies showing an increased risk for venous thromboembolism (VTE), and increased fibrinolysis and coagulation with oral compared with transdermal HT.9-12 Activated partial thromboplastin time (aPTT), fibrinogen, antithrombin antigen, protein C Ag, and protein S Ag free are regularly measured as surrogate markers for thrombophilia; however, the clinical impact of abnormal changes in these markers is not fully understood.13-16
Ospemifene is an estrogen receptor agonist/antagonist (ERAA, also referred to as a selective estrogen receptor modulator [SERM]) approved by the US Food and Drug Administration (FDA) for the treatment of moderate-to-severe dyspareunia, a symptom of vulvar vaginal atrophy (VVA, which is also a component of genitourinary syndrome of menopause17) due to menopause.18 Clinical and preclinical studies have shown that ospemifene can elicit tissue-specific estrogenic or antiestrogenic effects in the vagina,19-25 bone,26-30 and breast.27,31,32 However, limited data are available characterizing ospemifene's effect on the surrogate markers for cardiovascular health of postmenopausal women. Two 3-month, phase 2 clinical studies have characterized the effect of ospemifene on lipids and coagulation factors relative to placebo and the SERM raloxifene (60 mg).20,33 Data from both studies suggest that ospemifene may have a beneficial effect on levels of HDL, LDL, and fibrinogen.
A post hoc analysis of pooled data from 5 randomized, placebo-controlled clinical trials16,21-25,33,34 was conducted to assess the effect of systemic ospemifene exposure on lipids and coagulation factors in postmenopausal women.
METHODS
Study design
The effect of once-daily oral ospemifene 60 mg on lipids and coagulation factors in postmenopausal women (40-80 years in age) was evaluated in a post hoc analysis using data from five randomized, double-blind, parallel-group, placebo-controlled, phase 2 and 3 clinical trials (15-50718,25 1506002,16,3315-50615,34 15-50310,21 and 15-5082123,24), including a 40-week extension of a phase 3 study (15-50310x22). Details of the study design and inclusion criteria for all five clinical trials have been previously reported.16,21-25,33,34
Briefly, the studies ranged from 6 weeks to 12 months in length and included healthy, postmenopausal women (150600216,33), and also those with either at least seven moderate to very severe hot flushes per day or 50 per week (15-5061534), or VVA (15-5031021/15-50310x,22 15-50821,23,24 and 15-5071825). Participants were randomized 6:1 to ospemifene 60 mg or placebo in the 52-week safety study (15-5071825) and 1:1 in the four remaining trials (1506002,16,33 15-50615,34 15-5031021/15-50310x,22 and 15-5082123,24). Women who successfully completed a 12-week study (15-5031021) were allowed to continue the randomized therapy for an additional 40 weeks (15-50310x22). The 12-week studies, 15-5031021 and 15-50821,23,24 included women with and without an intact uterus, whereas studies 15-5071825 and the 40-week extension study, 15-50310x,22 were limited to postmenopausal women with an intact uterus.
Study assessments
Lipids evaluated included HDL, LDL, total cholesterol, and triglycerides, whereas coagulation parameters included antithrombin III antigen, aPTT, fibrinogen, protein C antigen, and free protein S antigen. The lipids and coagulation factors evaluated by each study are described in Table 1. The changes from baseline to 3, 6, and 12 months for each lipid and coagulation factor were evaluated in this post hoc analysis. For each parameter, the mean percent change from baseline was calculated and the Welch's t test was used to compare ospemifene 60 mg and placebo.
TABLE 1.
Descriptions of the five phase 2 and 3 clinical trials included in this report
| Study number | Study design | Study duration | Treatment administered | Lipid and coagulation factors measured |
| 15-5061534 | Phase 2, placebo-controlled | 6 wks | Once-daily oral dose of ospemifene 60 mg (n = 100) Placebo (n = 98) | Coagulation factors: Factor V Leidenb and thromboplastin timeEvaluated at screening and wk 6 |
| 150600216,33 | Phase 2, placebo-controlled | 12 wks | Once-daily oral doses of ospemifene 30 mg (n = 40) 60 mg (n = 40) 90 mg (n = 40) Placebo (n = 40) | Lipids: HDL, HDL-2, LDL, LDL-BCD, Lp (a), total cholesterol, and triglyceridesCoagulation factors: Endothelin-1, plasma nitric oxide, prostacyclin, fibrinogen, prothrombin fragments 1 + 2, thrombin-antithrombin III complex, D-dimer, tissue-type plasminogen activator, plasminogen activator inhibitor-1, and homocysteine in plasmaEvaluated at screening and wks 12 and 14 to 16 (after treatment discontinuation) |
| 15-5082123,24 | Phase 3, placebo-controlled | 12 wks | Once-daily oral dose of ospemifene 60 mg (n = 463) Placebo (n = 456) | Lipids: HDL, LDL, total cholesterol, and triglyceridesCoagulation factors: Factor V Leiden,b antithrombin III, fibrinogen, protein C, protein S, and thromboplastin timeEvaluated at screening and wk 12 |
| 15-5031021/15-50310x22 a | 15-50310: Phase 3, placebo-controlled | 12 wks | Once-daily oral dose of ospemifene 30 mg (n = 282) 60 mg (n = 276) Placebo (n = 268) | Lipids: HDL, LDL, total cholesterol, and triglyceridesCoagulation factors: Factor V Leiden,b thromboplastin time, fibrinogen, antithrombin III antigen, protein C antigen, and free protein S antigenEvaluated at screening (coagulation factors) or at randomization (lipids) and at wk 12 |
| 15-50310x: Phase 3, placebo-controlled, safety study | 40-wk extension | Once-daily oral dose of ospemifene 30 mg (n = 62) 60 mg (n = 69) Placebo (n = 49) | Lipids: HDL, LDL, total cholesterol, and triglyceridesCoagulation factors: aPTT, fibrinogen, antithrombin III antigen, protein C antigen, and free protein S antigenEvaluated at wks 26 and 52 | |
| 15-5071825 | Phase 3, placebo-controlled safety study | 52 wks | Once-daily oral dose of ospemifene 60 mg (n = 363) Placebo (n = 63) | Lipids: HDL, LDL, total cholesterol, and triglyceridesCoagulation factors: Factor V Leiden,b antithrombin III, protein C, and protein SEvaluated at screening and wks 12, 26, and 52. |
aPTT, activated partial thromboplastin time; HDL, high-density lipoprotein; LDL, low-density lipoprotein; LDL-BCD, baseline conjugated dienes of low-density lipoprotein; Lp (a), lipoprotein a.
15-50310 and 15-50310x are considered to be one clinical study.
Evaluated only at screening.
Additional subgroup analyses based on age, body mass index (BMI), and triglyceride levels were performed for the lipid parameters using the respective cut-off of 60 years, 32 kg/m2, and 250 mg/dL. Subgroup analysis based on age was performed at 3, 6, and 12 months, whereas the analyses based on BMI and triglyceride levels were limited to 3 months due to small sample size. Statistical analyses were performed using SAS version 9.2 (Cary, NC).
RESULTS
Participant disposition, demographics, and baseline characteristics
Lipid and coagulation factor data were evaluated for 2,166 postmenopausal women participating in five placebo-controlled studies, ranging from 6 weeks to 12 months in length, with 1,242 randomized to ospemifene 60 mg and 924 to placebo (Fig. 1). Study completion rate was similar between the ospemifene (85.4%) and placebo (86.8%) groups. Discontinuation due to adverse events was 7.6% (n = 95) for ospemifene and 3.7% (n = 34) for placebo (Fig. 1).
FIG. 1.
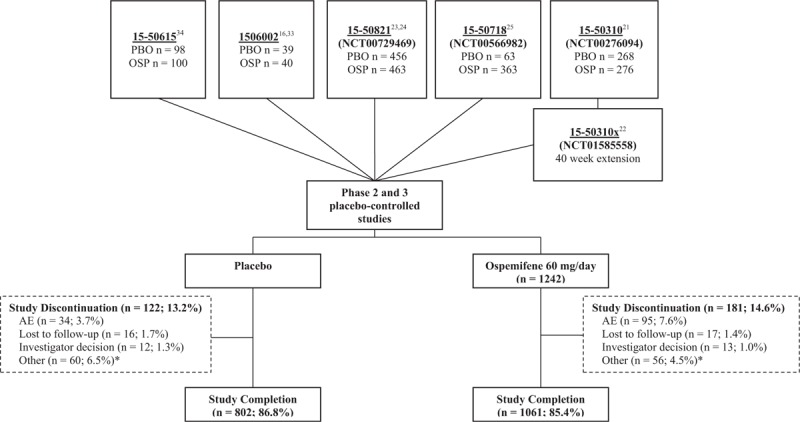
Disposition of participants from the five placebo-controlled phase 2 and 3 clinical studies used to evaluate the effect of ospemifene on the lipid and coagulation factors. ∗Other included withdrew consent, lack of efficacy, and noncompliance. AE, adverse event; OSP, ospemifene; PBO, placebo.
Age, race, and BMI were comparable between the ospemifene and placebo groups (Table 2). The trial participants were predominantly white with a mean age of approximately 59 years and a mean BMI of approximately 26 kg/m2. The percentage of postmenopausal women with an intact uterus was numerically higher for ospemifene (68.5%) versus placebo (58.8%), and a similar percentage of women had a prior history of HT use with ospemifene (21.1%) and placebo (18.8%).
TABLE 2.
Demographics and baseline characteristics of women receiving ospemifene 60 mg/d or placebo in five placebo-controlled trials (15-50718,25 1506002,16,33 15-50615,34 15-5031021/15-50310x,22 and 15-5082123,24)
| Ospemifene 60 mg (n = 1,242) | Placebo (n = 924) | |
| Age, y | ||
| Mean ± SD | 59.4 ± 6.49 | 58.9 ± 6.24 |
| Ethnic origin, n (%) | ||
| White | 1,159 (93.3) | 837 (90.8) |
| Black or African American | 47 (3.8) | 49 (5.1) |
| Asian | 12 (1.0) | 9 (0.9) |
| Other | 24 (1.9) | 29 (2.9) |
| BMI, kg/m2 | ||
| Mean ± SD | 25.7 ± 4.03 | 26.0 ± 4.20 |
| Intact uterus | ||
| n (%) | 851 (68.5) | 543 (58.8) |
| Prior HT use within 6 mos of study entry | ||
| n (%) | 262 (21.1) | 174 (18.8) |
| Lipid parameters, mean ± SD | ||
| HDL, mmol/L | 1.76 ± 0.44 | 1.72 ± 0.45 |
| LDL, mmol/L | 3.32 ± 0.91 | 3.26 ± 0.88 |
| Total cholesterol, mmol/L | 5.68 ± 0.10 | 5.64 ± 1.00 |
| Triglycerides, mmol/L | 1.27 ± 0.65 | 1.32 ± 0.75 |
| Coagulation parameters, mean ± SD | ||
| aPTT, s | 27.5 ± 3.80 | 27.7 ± 4.11 |
| Fibrinogen, μmol/L | 10.2 ± 2.0 | 10.1 ± 2.0 |
| Antithrombin antigen, % | 99.4 ± 12.9 | 100.1 ± 11.8 |
| Protein C Ag, % | 104.7 ± 19.0 | 103.2 ± 22.2 |
| Protein S Ag, free, % | 111.0 ± 21.8 | 111.7 ± 21.1 |
aPTT, activated partial thromboplastin time; BMI, body mass index; HDL, high-density lipoprotein; HT, hormone therapy; LDL, low-density lipoprotein; SD, standard deviation.
Lipids
Mean percent increases in HDL from baseline were significantly greater with ospemifene versus placebo at 3 months (4.4% vs 0.2%; P < 0.0001), 6 months (5.1% vs 1.5%; P = 0.0359), and 12 months (2.3% vs −1.9%; P = 0.0086; Fig. 2A). Similarly, mean percent changes in LDL from baseline were significantly greater with ospemifene versus placebo at 3 months (−5.2% vs 2.4%; P < 0.0001), 6 months (−6.7% vs 2.4%; P = 0.0002), and 12 months (−7.0% vs −2.1%; P = 0.0293; Fig. 2A). Ospemifene significantly reduced total cholesterol at 6 months compared with placebo (−1.8% vs 1.6%; P = 0.0345; Fig. 2B). The increase in triglycerides with ospemifene use was similar to those found with placebo (Fig. 2B).
FIG. 2.
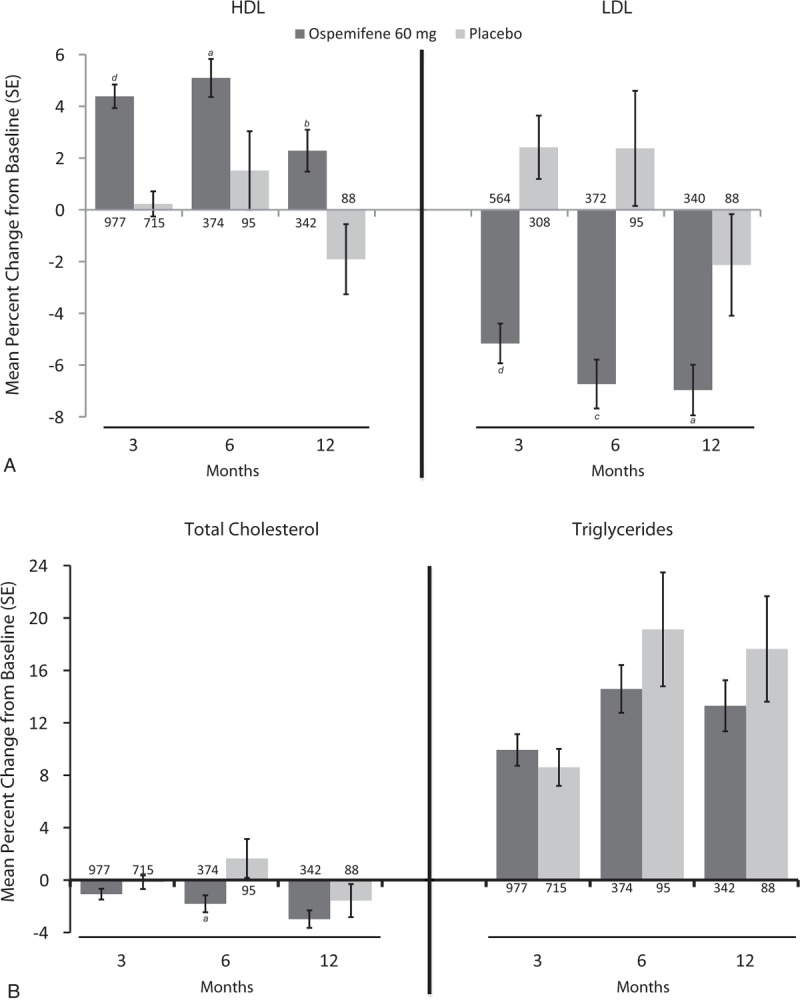
Mean percent change in serum lipid levels (A: HDL- and LDL- Cholesterol; B: Total Cholesterol and Triglycerides) of postmenopausal women treated with ospemifene 60 mg for up to12 months in five placebo-controlled studies (15-50718,25 1506002,16,33 15-50615,34 15-5031021/15-50310x,22 and 15-5082123,24). The values of n for each group appear at the base of each bar. aP < 0.05; bP < 0.01; cP < 0.001; dP < 0.0001. HDL, high-density lipoproteins; LDL, low-density lipoproteins.
The subgroup analyses based on age found that ospemifene significantly increased HDL in postmenopausal women 60 years of age or older at 3, 6, and 12 months, but only at 3 months in women less than 60 years of age (Table 3). LDL levels in contrast were significantly decreased by ospemifene at 3 and 6 months in both age groups. Ospemifene significantly decreased total cholesterol relative to placebo in women less than 60 years of age only at 6 months and had no effect among women aged 60 years or older. Ospemifene had no significant effects, compared with placebo, on the triglyceride levels of postmenopausal women, regardless of their age (Table 3).
TABLE 3.
The effect of age on the mean percent change in serum lipid levels of postmenopausal women treated with ospemifene 60 mg from studies 15-50718,25 1506002,16,33 15-50615,34 15-5031021/15-50310x,22 and 15-5082123,24
| Mean percent change from baseline (n) | |||||||
| Age < 60 y | Age ≥60 y | ||||||
| Time point, mos | Ospemifene 60 mg (n = 664) | Placebo (n = 545) | P | Ospemifene 60 mg (n = 578) | Placebo (n = 379) | P | |
| HDL | 3 | 3.5608 (498) | 0.4212 (418) | 0.0005 | 5.2473 (479) | −0.0429 (297) | <0.0001 |
| 6 | 3.7713 (162) | 4.4413 (43) | 0.7886 | 6.1076 (212) | −0.9000 (52) | 0.0027 | |
| 12 | 2.4799 (150) | −1.0935 (40) | 0.1614 | 2.1321 (192) | −2.5890 (48) | 0.0204 | |
| LDL | 3 | −4.5125 (263) | 2.9522 (163) | <0.0001 | −5.7298 (301) | 1.8122 (145) | 0.0010 |
| 6 | −5.2666 (160) | 3.3872 (43) | 0.0144 | −7.8387 (212) | 1.5365 (52) | 0.0075 | |
| 12 | −6.4397 (148) | −3.3896 (40) | 0.3080 | −7.3690 (192) | −1.0783 (48) | 0.0525 | |
| Triglycerides | 3 | 10.7017 (498) | 8.0056 (418) | 0.2944 | 9.1318 (479) | 9.4436 (297) | 0.9086 |
| 6 | 17.0465 (162) | 19.8375 (43) | 0.6828 | 12.7093 (212) | 18.5546 (52) | 0.3768 | |
| 12 | 12.6197 (150) | 15.7714 (40) | 0.6416 | 13.8306 (192) | 19.1875 (48) | 0.3778 | |
| Total cholesterol | 3 | −0.655 (498) | 0.0740 (418) | 0.3649 | −1.5039 (479) | −0.4904 (297) | 0.3652 |
| 6 | −1.2853 (162) | 3.4364 (43) | 0.0473 | −2.2008 (212) | 0.1701 (52) | 0.2919 | |
| 12 | −2.7399 (150) | −2.0868 (40) | 0.7407 | −3.1654 (192) | −1.1288 (48) | 0.325 | |
HDL, high-density lipoprotein; LDL, low-density lipoprotein.
Ospemifene also significantly increased HDL and decreased LDL levels from baseline to 3 months in women with BMI less than 32 kg/m2 and those with triglyceride levels less than 250 mg/dL (P < 0.0001 vs placebo for all; data not shown). In contrast, ospemifene had no significant effect on HDL or LDL among women with high BMI or triglyceride levels. Total cholesterol and triglyceride levels at 3 months remained unchanged, regardless of the BMI or triglyceride level.
Coagulation factors
Mean percent changes in fibrinogen and protein C antigen levels from baseline were significantly greater with ospemifene versus placebo at 3 months (−8.7% vs −0.8%; P < 0.0001, and −2.7% vs 0.5%; P = 0.0008, respectively), 6 months (−6.0% vs 6.7%; P = 0.0019, and −3.6% vs 8.0%; P < 0.0001), and 12 months (−8.7% vs 7.3%; P = 0.0029, and −4.5% vs 6.6%; P < 0.0001) were significantly greater with ospemifene versus placebo (Fig. 3). Ospemifene numerically lowered aPTT levels from baseline to 3 and 6 months, with the change being significantly greater than that for placebo at 3 months (−1.9% vs 0.7%; P = 0.0009). Antithrombin III antigen levels also decreased from baseline to 3 months with ospemifene, with the change being greater than that with placebo (−2.9% vs −0.8%; P = 0.0004). Ospemifene numerically increased free protein S antigen levels at all measured time points (Fig. 3B), with the change being significantly greater than placebo at 3 months (5.8% vs 1.6%; P < 0.0001). None of these changes were outside the range of normal values for each parameter.
FIG. 3.
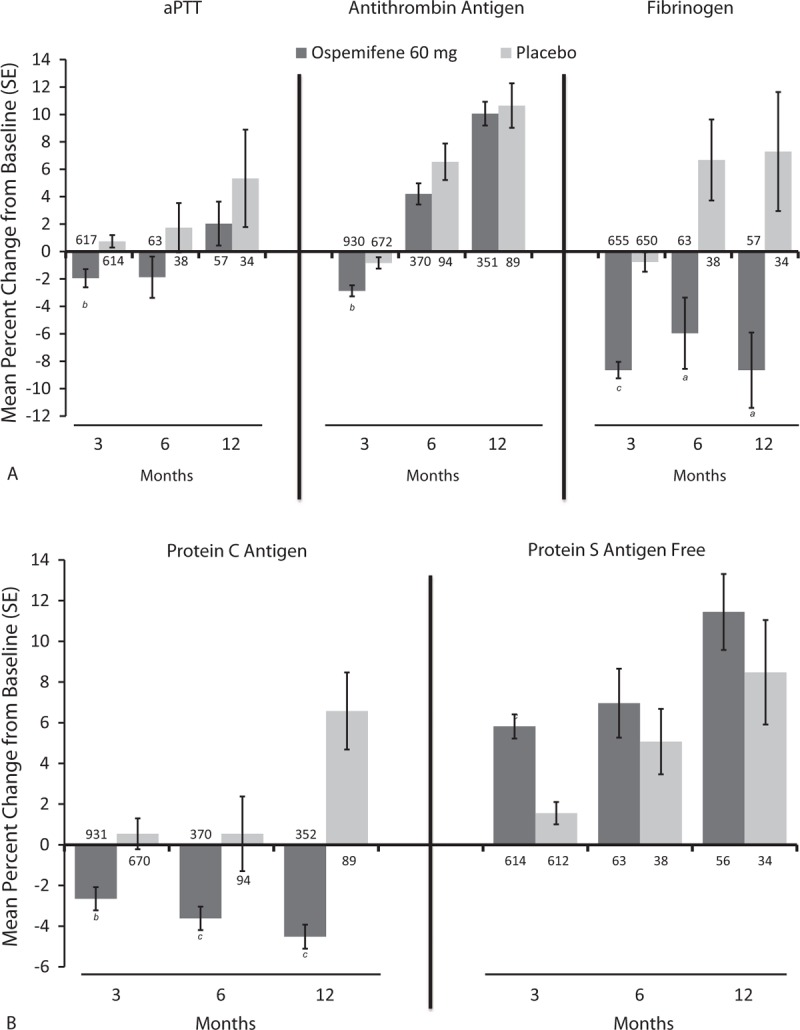
Mean percent change from baseline to 3, 6, and 12 months in the coagulation parameters (A: aPTT, Antithrombin Antigen, and Fibrinogen; B: Protein C Antigen and Protein S Antigen Free) of postmenopausal women treated with ospemifene 60 mg or placebo in five placebo-controlled studies (15-50718,25 1506002,16,33 15-50615,34 15-5031021/15-50310x,22 and 15-5082123,24). The values of n for each group appear at the base of each bar. aP < 0.01; bP < 0.001; cP < 0.0001. aPTT, activated partial thromboplastin time.
DISCUSSION
This post hoc analysis of pooled data from five placebo-controlled clinical studies found ospemifene 60 mg increased HDL and decreased LDL levels in postmenopausal women with no adverse effects on total cholesterol and triglycerides. Ospemifene 60 mg also decreased the levels of fibrinogen (a risk factor for coronary heart disease35) from baseline, with a significant difference from placebo; however, the postbaseline fibrinogen level remained within the normal range for the majority of the participants and would not be expected to have any clinical consequences. Collectively, we found that once-daily ospemifene 60 mg for up to 12 months did not have a negative effect on lipid and coagulation factors in postmenopausal women.
Data characterizing ospemifene's effect on lipid and coagulation factors relative to placebo was previously limited to a 3-month, phase 2 clinical trial (study 1506002, included in this pooled dataset) of 160 healthy, postmenopausal women randomized to either ospemifene at doses of 30, 60, and 90 mg or placebo.33 Ospemifene increased HDL and decreased total cholesterol and LDL from baseline to 3 months, but the changes were not significant versus placebo and vanished within 2 to 4 weeks of treatment cessation.33 Triglycerides increased significantly with ospemifene 90 mg relative to placebo (P = 0.017).33 Ospemifene also decreased plasma fibrinogen levels (P < 0.05), which returned to baseline levels upon treatment cessation.33 More specifically, fibrinogen levels significantly decreased with ospemifene 60 mg (P = 0.0145) and 90 mg (P = 0.0232) relative to placebo at 3 months.33 The results from our post hoc analysis further extend initial data from study 150600233 and demonstrate that ospemifene 60 mg, the clinical US FDA-approved dose, does not negatively influence lipids and coagulation factors in postmenopausal women either in good health or diagnosed with VVA.
The significant improvement in HDL and LDL with ospemifene observed in this post hoc analysis is consistent with the majority of the clinical literature for estrogen therapies and other ERAAs/SERMs, including raloxifene and bazedoxifene.2-5,7,36-38 However, ospemifene has no significant effect on triglycerides, which is in contrast with the increase in triglycerides typically seen with oral estrogens,2,4,6,39 but is consistent with clinical data for bazedoxifene.36,38A 3-month phase 2 clinical study comparing ospemifene (30, 60, and 90 mg) with raloxifene 60 mg showed no significant differences in the triglyceride levels between the four treatment groups (changes from baseline were not reported).20 However, raloxifene's effect on triglycerides varies from no change reported by several studies38,40,41 to a significant increase (P < 0.05) at 4 years in the Multiple Outcomes of Raloxifene Evaluation (MORE) study.42 Additional longer-term, placebo, and active-controlled clinical trials are needed to fully confirm the initial results from this analysis, which suggest ospemifene is likely to improve HDL and LDL levels in postmenopausal women with no detrimental effect on their triglyceride levels.
Ospemifene, like HT4,6,43 and raloxifene,37,41 also does not have a negative effect on fibrinogen levels of postmenopausal women. The significant decrease in fibrinogen levels with ospemifene 60 mg (mean percent change of 8.7%; P = 0.0145) initially observed at 3 months in study 150600233 extends for up to 12 months of treatment (mean percent change of 8.7%) in this post hoc analysis. A 6-month, placebo-controlled study, which compared the effect of raloxifene (60 and 120 mg) with placebo and HT (0.625 mg conjugated equine estrogen and 2.5 mg medroxyprogesterone acetate [CE/MPA]) in 390 healthy, postmenopausal women, reported a 10% to 12% decrease compared with baseline fibrinogen levels with the two raloxifene doses (P < 0.001 vs placebo), whereas CE/MPA did not.41 Assuming a 0.5% reduction in the cardiovascular risk for every 0.01 g/L decrease in fibrinogen level, raloxifene was estimated to reduce the risk of cardiovascular events by 21%.41 Because the decrease in fibrinogen with ospemifene 60 mg was still within the normal range of 1.5 to 4.0 g/L,44 these initial data suggest that ospemifene will not have a negative effect on the fibrinogen levels of postmenopausal women. More rigorous clinical trials are needed to further evaluate ospemifene's effect on coagulation factors in postmenopausal women, and also any potential association between ospemifene use and possible reduction in risk for cardiovascular events.
This post hoc analysis of pooled data is limited by the variations in the duration of treatment and study population across the five trials. Women participating in the trials were predominantly white (90%-93%), with an average BMI of 26 kg/m2, therefore limiting the generalization of the findings. In addition, the individual studies were not designed to evaluate ospemifene's effect on lipids and coagulation factors as primary or secondary endpoints. The lack of statistical significance observed between ospemifene and placebo in the subgroup analyses could also be attributed to the small sample size, particularly at the time point of 12 months and women with high BMI or triglyceride level. The strength of this post hoc analysis lies in the pooling of data from multiple, randomized, placebo-controlled clinical trials. The results of this trial also demonstrate the consistency of ospemifene's effect since they support previously published studies comparing ospemifene's effects on lipids and coagulation factors with placebo or raloxifene.
CONCLUSIONS
Post hoc analysis of pooled data from five randomized, placebo-controlled studies found ospemifene 60 mg to significantly increase HDL and decrease LDL, while having little effect on triglycerides relative to placebo. Significant decreases in fibrinogen were also reported with 12 months of ospemifene treatment; however, this was not considered to be of clinical significance as the values remained within the normal range for both parameters. Taken together, ospemifene 60 mg, when prescribed for postmenopausal symptoms of VVA or GSM, does not have adverse effects on lipid levels and coagulation factors.
Acknowledgments
The authors thank Yasunori Uragari for the statistical analyses, and Disha Patel, PhD, of Precise Publications, LLC, for the medical writing assistance, which was supported by Shionogi, Inc.
Footnotes
Funding/support: Data analyses were conducted by Shionogi, Inc. Also, Shionogi, Inc provided financial support for medical writing assistance supplied by Disha Patel, PhD (Precise Publications, LLC).
Financial disclosure/conflicts of interest: D.F.A. (within the past 3 years) has received research support from Actavis (previously Allergan, Watson Pharmaceuticals, Warner Chilcott), Bayer Healthcare, Endoceutics, Glenmark, Merck (previously Schering Plough, Organon), Radius Health, Shionogi Inc, and TherapeuticsMD; and has served as a consultant to Abbvie (previously Abbott Laboratories), Actavis (previously Allergan, Watson Pharmaceuticals, Warner Chilcott), Agile Therapeutics, Bayer Healthcare, Endoceutics, Exeltis (previously CHEMO), InnovaGyn, Merck (previously Schering Plough, Organon), Pfizer, Radius Health, Sermonix Pharmaceuticals, Shionogi, Inc, Teva Women's Healthcare, and TherapeuticsMD. C.A., W.J., and S.C. are employees of Shionogi, Inc.
REFERENCES
- 1.Reddy KS, Chandala SR. A comparative study of lipid profile and oestradiol in pre- and post-menopausal women. J Clin Diagn Res 2013; 7:1596–1598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Writing Group for the PEPI Trial. Effects of estrogen or estrogen/progestin regimens on heart disease risk factors in postmenopausal women. The Postmenopausal Estrogen/Progestin Interventions (PEPI) trial. JAMA 1995; 273:199–208. [PubMed] [Google Scholar]
- 3.Prestwood KM, Unson C, Kulldorff M, Cushman M. The effect of different doses of micronized 17beta-estradiol on C-reactive protein, interleukin-6, and lipids in older women. J Gerontol A Biol Sci Med Sci 2004; 59:827–832. [DOI] [PubMed] [Google Scholar]
- 4.Lobo RA, Bush T, Carr BR, Pickar JH. Effects of lower doses of conjugated equine estrogens and medroxyprogesterone acetate on plasma lipids and lipoproteins, coagulation factors, and carbohydrate metabolism. Fertil Steril 2001; 76:13–24. [DOI] [PubMed] [Google Scholar]
- 5.Archer DF, Thorneycroft IH, Foegh M, et al. Long-term safety of drospirenone-estradiol for hormone therapy: a randomized, double-blind, multicenter trial. Menopause 2005; 12:716–727. [DOI] [PubMed] [Google Scholar]
- 6.Rossouw JE, Cushman M, Greenland P, et al. Inflammatory, lipid, thrombotic, and genetic markers of coronary heart disease risk in the Women's Health Initiative trials of hormone therapy. Arch Intern Med 2008; 168:2245–2253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Espeland MA, Marcovina SM, Miller V, et al. Effect of postmenopausal hormone therapy on lipoprotein(a) concentration. PEPI Investigators. Postmenopausal Estrogen/Progestin Interventions. Circulation 1998; 97:979–986. [DOI] [PubMed] [Google Scholar]
- 8.Hulley S, Grady D, Bush T, et al. Randomized trial of estrogen plus progestin for secondary prevention of coronary heart disease in postmenopausal women. Heart and Estrogen/progestin Replacement Study (HERS) Research Group. JAMA 1998; 280:605–613. [DOI] [PubMed] [Google Scholar]
- 9.Canonico M, Oger E, Plu-Bureau G, et al. Hormone therapy and venous thromboembolism among postmenopausal women: impact of the route of estrogen administration and progestogens: the ESTHER study. Circulation 2007; 115:840–845. [DOI] [PubMed] [Google Scholar]
- 10.Roach RE, Lijfering WM, Helmerhorst FM, Cannegieter SC, Rosendaal FR, Rosendaal van HV. The risk of venous thrombosis in women over 50 years old using oral contraception or postmenopausal hormone therapy. J Thromb Haemost 2013; 11:124–131. [DOI] [PubMed] [Google Scholar]
- 11.Scarabin PY, Alhenc-Gelas M, Plu-Bureau G, Taisne P, Agher R, Aiach M. Effects of oral and transdermal estrogen/progesterone regimens on blood coagulation and fibrinolysis in postmenopausal women. A randomized controlled trial. Arterioscler Thromb Vasc Biol 1997; 17:3071–3078. [DOI] [PubMed] [Google Scholar]
- 12.Scarabin PY, Oger E, Plu-Bureau G. Differential association of oral and transdermal oestrogen-replacement therapy with venous thromboembolism risk. Lancet 2003; 362:428–432. [DOI] [PubMed] [Google Scholar]
- 13.Marlar RA, Gausman JN. Laboratory testing issues for protein C, protein S, and antithrombin. Int J Lab Hematol 2014; 36:289–295. [DOI] [PubMed] [Google Scholar]
- 14.Grimes DA, Schulz KF. Surrogate end points in clinical research: hazardous to your health. Obstet Gynecol 2005; 105:1114–1118. [DOI] [PubMed] [Google Scholar]
- 15.Franchini M, Martinelli I, Mannucci PM. Uncertain thrombophilia markers. Thromb Haemost 2015; 115:25–30. [DOI] [PubMed] [Google Scholar]
- 16.Rutanen EM, Heikkinen J, Halonen K, Komi J, Lammintausta R, Ylikorkala O. Effects of ospemifene, a novel SERM, on hormones, genital tract, climacteric symptoms, and quality of life in postmenopausal women: a double-blind, randomized trial. Menopause 2003; 10:433–439. [DOI] [PubMed] [Google Scholar]
- 17.Portman DJ, Gass ML. Genitourinary syndrome of menopause: new terminology for vulvovaginal atrophy from the International Society for the Study of Women's Sexual Health and the North American Menopause Society. Menopause 2014; 21:1063–1068. [DOI] [PubMed] [Google Scholar]
- 18.Mirkin S, Komm BS. Tissue-selective estrogen complexes for postmenopausal women. Maturitas 2013; 76:213–220. [DOI] [PubMed] [Google Scholar]
- 19.Voipio SK, Komi J, Kangas L, Halonen K, DeGregorio MW, Erkkola RU. Effects of ospemifene (FC-1271a) on uterine endometrium, vaginal maturation index, and hormonal status in healthy postmenopausal women. Maturitas 2002; 43:207–214. [DOI] [PubMed] [Google Scholar]
- 20.Komi J, Lankinen KS, Harkonen P, et al. Effects of ospemifene and raloxifene on hormonal status, lipids, genital tract, and tolerability in postmenopausal women. Menopause 2005; 12:202–209. [DOI] [PubMed] [Google Scholar]
- 21.Bachmann GA, Komi JO. Ospemifene effectively treats vulvovaginal atrophy in postmenopausal women: results from a pivotal phase 3 study. Menopause 2010; 17:480–486. [DOI] [PubMed] [Google Scholar]
- 22.Simon JA, Lin VH, Radovich C, Bachmann GA. Group tOS. One-year long-term safety extension study of ospemifene for the treatment of vulvar and vaginal atrophy in postmenopausal women with a uterus. Menopause 2013; 20:418–427. [DOI] [PubMed] [Google Scholar]
- 23.Portman D, Palacios S, Nappi RE, Mueck AO. Ospemifene, a non-oestrogen selective oestrogen receptor modulator for the treatment of vaginal dryness associated with postmenopausal vulvar and vaginal atrophy: a randomised, placebo-controlled, phase III trial. Maturitas 2014; 78:91–98. [DOI] [PubMed] [Google Scholar]
- 24.Portman DJ, Bachmann GA, Simon JA. Ospemifene, a novel selective estrogen receptor modulator for treating dyspareunia associated with postmenopausal vulvar and vaginal atrophy. Menopause 2013; 20:623–630. [DOI] [PubMed] [Google Scholar]
- 25.Goldstein SR, Bachmann GA, Koninckx PR, Lin VH, Portman DJ, Ylikorkala O. Ospemifene 12-month safety and efficacy in postmenopausal women with vulvar and vaginal atrophy. Climacteric 2014; 17:173–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Qu Q, Harkonen PL, Vaananen HK. Comparative effects of estrogen and antiestrogens on differentiation of osteoblasts in mouse bone marrow culture. J Cell Biochem 1999; 73:500–507. [PubMed] [Google Scholar]
- 27.Qu Q, Zheng H, Dahllund J, et al. Selective estrogenic effects of a novel triphenylethylene compound, FC1271a, on bone, cholesterol level, and reproductive tissues in intact and ovariectomized rats. Endocrinology 2000; 141:809–820. [DOI] [PubMed] [Google Scholar]
- 28.Michael H, Harkonen PL, Kangas L, Vaananen HK, Hentunen TA. Differential effects of selective oestrogen receptor modulators (SERMs) tamoxifen, ospemifene and raloxifene on human osteoclasts in vitro. Br J Pharmacol 2007; 151:384–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Komi J, Heikkinen J, Rutanen EM, Halonen K, Lammintausta R, Ylikorkala O. Effects of ospemifene, a novel SERM, on biochemical markers of bone turnover in healthy postmenopausal women. Gynecol Endocrinol 2004; 18:152–158. [DOI] [PubMed] [Google Scholar]
- 30.Komi J, Lankinen KS, DeGregorio M, et al. Effects of ospemifene and raloxifene on biochemical markers of bone turnover in postmenopausal women. J Bone Miner Metab 2006; 24:314–318. [DOI] [PubMed] [Google Scholar]
- 31.Taras TL, Wurz GT, DeGregorio MW. In vitro and in vivo biologic effects of ospemifene (FC-1271a) in breast cancer. J Steroid Biochem Mol Biol 2001; 77:271–279. [DOI] [PubMed] [Google Scholar]
- 32.Kangas L, Harkonen P, Vaananen K, Keskitalo J, Eigeliene N. Effects of ospemifene on breast tissue morphology and proliferation: a comparative study versus other selective estrogen receptor modulators in ovariectomized rats. Horm Metab Res 2014; 46:328–332. [DOI] [PubMed] [Google Scholar]
- 33.Ylikorkala O, Cacciatore B, Halonen K, et al. Effects of ospemifene, a novel SERM, on vascular markers and function in healthy, postmenopausal women. Menopause 2003; 10:440–447. [DOI] [PubMed] [Google Scholar]
- 34.Constantine G, Archer DF, Pollycove R, Jiang W, Altomare C, Pinkerton J. Effects of ospemifene on vasomotor symptoms in phase 2 and 3 clinical trials. Menopause 2016; 23:957–964. [DOI] [PubMed] [Google Scholar]
- 35.Danesh J, Lewington S, Thompson SG, et al. Fibrinogen Studies Collaboration. Plasma fibrinogen level and the risk of major cardiovascular diseases and nonvascular mortality: an individual participant meta-analysis. JAMA 2005; 294:1799–1809. [DOI] [PubMed] [Google Scholar]
- 36.Miller PD, Chines AA, Christiansen C, et al. Effects of bazedoxifene on BMD and bone turnover in postmenopausal women: 2-yr results of a randomized, double-blind, placebo-, and active-controlled study. J Bone Miner Res 2008; 23:525–535. [DOI] [PubMed] [Google Scholar]
- 37.Collins P, Mosca L, Geiger MJ, et al. Effects of the selective estrogen receptor modulator raloxifene on coronary outcomes in the raloxifene use for the Heart trial: results of subgroup analyses by age and other factors. Circulation 2009; 119:922–930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Christiansen C, Chesnut Iii CH, Adachi JD, et al. Safety of bazedoxifene in a randomized, double-blind, placebo- and active-controlled phase 3 study of postmenopausal women with osteoporosis. BMC Musculoskelet Disord 2010; 11:130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Anderson GL, Limacher M, Assaf AR, et al. Women's Health Initiative Steering Committee. Effects of conjugated equine estrogen in postmenopausal women with hysterectomy. The Women's Health Initiative randomized controlled trial. JAMA 2004; 291:1701–1712. [DOI] [PubMed] [Google Scholar]
- 40.Delmas PD, Bjarnason NH, Mitlak BH, et al. Effects of raloxifene on bone mineral density, serum cholesterol concentrations, and uterine endometrium in postmenopausal women. N Engl J Med 1997; 337:1641–1647. [DOI] [PubMed] [Google Scholar]
- 41.Walsh BW, Kuller LH, Wild RA, et al. Effects of raloxifene on serum lipids and coagulation factors in healthy postmenopausal women. JAMA 1998; 279:1445–1451. [DOI] [PubMed] [Google Scholar]
- 42.Barrett-Connor E, Grady D, Sashegyi A, et al. Raloxifene and cardiovascular events in osteoporotic postmenopausal women: four-year results from the MORE (Multiple Outcomes of Raloxifene Evaluation) randomized trial. JAMA 2002; 287:847–857. [DOI] [PubMed] [Google Scholar]
- 43.Zegura B, Guzic-Salobir B, Sebestjen M, Keber I. The effect of various menopausal hormone therapies on markers of inflammation, coagulation, fibrinolysis, lipids, and lipoproteins in healthy postmenopausal women. Menopause 2006; 13:643–650. [DOI] [PubMed] [Google Scholar]
- 44.Mackie IJ, Kitchen S, Machin SJ, Lowe GD. Haemostasis and Thrombosis Task Force of the British Committee for Standards in Haematology. Guidelines on fibrinogen assays. Br J Haematol 2003; 121:396–404. [DOI] [PubMed] [Google Scholar]


