Abstract
Background and Purpose
Some of the fucosylation catalyzed by fucosyltransferase-III mediates the epithelial-mesenchymal transition and enhances tumor cell-macrophage signaling, which promotes malignant transforming and immune evasion. The aim of the study was to investigate the association between the expression of fucosyltransferase-III and clinical outcomes of patients with clear-cell renal cell carcinoma after surgery.
Results
High fucosyltransferase-III expression was associated with a greater risk of recurrence (p = 0.002) and shortened overall survival (p < 0.001). We then established a prognostic nomogram including tumor size, pathologic T, N, M stage, coagulative necrosis, lymphovascular invasion and fucosyltransferase-III expression. Furthermore, the predictive accuracy of the Leibovich prognostic score was improved when fucosyltransferase-III expression was added (p = 0.009 for overall survival and p = 0.002 for recurrence-free survival).
Materials and Methods
We conducted a retrospective cohort study of 406 patients who underwent partial or radical nephrectomy between January 2008 and December 2009 in a single institute. Fucosyltransferase-III expression levels were evaluated by immunohistochemical staining in tumor tissues. Kaplan-Meier method was applied to compare survival curves. Cox regression models were fitted to analyze the effect of prognostic factors on recurrence-free and overall survival. Harrell’s concordance index and Akaike’s Information Criteria were calculated to assess predictive accuracy.
Conclusions
Fucosyltransferase-III is a predictive factor for poor overall survival and recurrence free survival in patients with ccRCC. The inhibitor of fucosyltransferase-III might be a potential therapeutic method for the disease.
Keywords: clear cell renal cell carcinoma, fucosyltransferase-III, prognosis, overall survival, recurrence free survival
INTRODUCTION
The incidence of kidney cancer is highest in North America and Western Europe, and increasing quickly in developing country such as China these years [1, 2]. In China, the incidence of kidney cancer was 68.8 per 100,000 and the mortality was 23.4 per 100,000 in 2015. Renal cell carcinoma (RCC) represents about 90 percents of kidney malignancies [2]. RCC is a group of heterogeneous diseases for its diversity of pathologic subtypes which are different in biological behavior and prognosis due to its different pathologic subtype [3]. The RCC patients have a 5-year survival rate for 70% to 90% if their tumor is localized. Nevertheless, the 5-year survival rate was below 10% if the metastasis exists [4, 5]. Clear cell RCC (ccRCC) is the most common pathologic subtype of RCC [3, 6]. It has been elucidated that the deactivation of the von Hippel-Lindau gene, a tumor-suppressor gene, can trigger the formation of ccRCC. However, the theory cannot explain the different biological behavior of ccRCC. Therefore, some biomarkers are needed to predict the prognosis of ccRCC patient more accurately.
Fucosyltransferases (FUTs or Fuc-Ts, FUT-I to FUT-XI) are a family of enzymes catalyzing the reaction of fucosylation. Fucosylation is an important part of post-translational modification found to be associated with tumorigenesis and the malignant potential of the tumor cells [7, 8]. Synthesis of N-linked glycans and O-linked glycans both need the catalysis of the FUTs. Some of the FUTs catalyze the terminal fucosylation, for example, the last step of the synthesis of Sialic-Lewis X(SLex). And some of the FUTs catalyze core fucosylation, such as FUT-VIII is engaged in the addition of α-1,6-fucose to the N-acetylglucosamine (GlcNAc) residue in some molecule in breast cancer and lung cancer [9]. It has been elucidated that the increased core fucosylation of α-fetoprotein can be used to distinguish hepatocellular carcinoma from liver cirrhosis and chronic hepatitis [10]. In breast cancer, the dimerization and phosphorylation of epidermal growth factor receptor (EGFR) is increased if it is over-fucosylated, and this lead to the increase of downstream EGFR-signaling, which promotes the malignant behavior and growth of the tumor [11].
Fucosyltransferase-III (FUT3, FucT-III) is one of the enzymes from FUTs family. FUT3 is coded by FUT3 gene (also called Lewis gene), which located in the 19p13.3. FUT3 is an enzyme with α(1,3)-fucosyltransferase and α(1,4)-fucosyltransferase activities. The most well-known function of FUT3 is the biosynthesis of the Lewis blood-group antigen. In oncologic researches, FUT3 has been found to be up-regulated in cancerous tissue of human colorectal cancer [12]. Down-regulation of FUT3 and FUT5 by shRNA technique can weaken the capability of adhesion to endothelial cell because of the reduced binding to E-selectin and hyaluronic acid [7]. Some cell receptor, such as Transforming growth factor - beta (TGF-β), can transduce a signal for epithelial-mesenchmal transition (EMT) if the TGF-β is fucosylated under the catalysis of FUT3. In addition, they also find the patients with metastatic colorectal cancer (mCRC) have a high expression of FUT3 [13]. Though there are some studies focused on FUTs and fucosylated glycans these years, the function of FUT3 in tumorigenesis and the correlation between FUT3 and malignancies or ccRCC still remains unclear.
In this study, we sought to uncover the relations between FUT3 expression and the prognosis of the ccRCC patients. Our findings demonstrated the high expression of FUT3 could predict a poor prognosis in patients with ccRCC. The expression of FUT3 can stratify the patients into two groups with significant difference in overall survival (OS) and recurrence free survival (RFS). In addition, we built models to predict the OS and RFS of ccRCC patients. Furthermore, we investigated if the predictive accuracy of the existed models, such as TNM stage, was improved after the incorporation of FUT3 expression.
RESULTS
Patient characteristics
To evaluate the level of FUT3 expressed in ccRCC tumor tissues, we conducted the IHC staining to the TMA of 406 patients and analyzed the FUT3 expression of the ccRCC patients. As showed in Table 1 , the mean age of these patients was 55.4 year. The H-score of FUT3 expression ranged from 4 to 220 and representative IHC images were shown in Figure 1. The patients were dichotomized into FUT-3 low group (H-score ranged from 4 to 82; n = 230) and FUT-3 high group (H-score ranged from 85 to 220; n = 176) according to the method of “minimum p value” with the assistance of the X-tile software. The clinical and pathologic features were compared in Table 1 . In general, there was no significant difference of age, gender, tumor size, pathologic N stage, the presence of sarcomatoid change, rhabdoid appearance and LVI between FUT3 high group and FUT3 low group, while pathologic T (p=0.006) and M stage (p=0.015), Fuhrman grade (p=0.005), the presence of coagulative necrosis (p=0.022) and ECOG-PS (p=0.005) showed a significant difference between two groups.
Table 1. Patient characteristics and associations with FUT3 expression.
| Characteristics | Patients (n=406) | FUT3 expression | |||
|---|---|---|---|---|---|
| Number | % | Low (n=230) | High (n=176) | p value | |
| Age at surgery: | 0.447 | ||||
| Mean ± SD (year) | 55.4 ± 12.0 | 55.1 ± 12.0 | 55.9 ± 12.1 | ||
| Gender: | 0.518 | ||||
| Male | 285 | 70.3 | 158 | 127 | |
| Female | 121 | 29.7 | 72 | 49 | |
| Tumor size: | 0.661 | ||||
| Mean ± SD (cm) | 4.4 ± 2.5 | 4.4 ± 2.2 | 4.5 ± 2.8 | ||
| Pathologic T stage: | 0.006 | ||||
| pT1 | 276 | 68.0 | 172 | 104 | |
| pT2 | 27 | 6.7 | 14 | 13 | |
| pT3 | 96 | 23.6 | 42 | 54 | |
| pT4 | 7 | 1.7 | 2 | 5 | |
| Pathologic N stage: | 0.094 | ||||
| pN0 | 399 | 98.3 | 229 | 170 | |
| pN1 | 7 | 1.7 | 1 | 6 | |
| Pathologic M stage: | 0.015 | ||||
| pM0 | 401 | 98.8 | 227 | 174 | |
| pM1 | 5 | 1.2 | 3 | 2 | |
| TNM stage: | 0.003 | ||||
| I | 273 | 67.2 | 172 | 101 | |
| II | 25 | 6.2 | 12 | 13 | |
| III | 96 | 23.6 | 41 | 55 | |
| IV | 12 | 3.0 | 5 | 7 | |
| Fuhrman grade: | 0.005 | ||||
| 1 | 66 | 16.3 | 43 | 23 | |
| 2 | 188 | 46.3 | 116 | 72 | |
| 3 | 99 | 24.4 | 51 | 48 | |
| 4 | 53 | 13.1 | 20 | 33 | |
| Coagulative necrosis: | 0.022 | ||||
| Absent | 316 | 77.8 | 189 | 127 | |
| Present | 90 | 22.2 | 41 | 49 | |
| Sarcomatoid change: | 0.939 | ||||
| Absent | 393 | 96.8 | 223 | 170 | |
| Present | 13 | 3.2 | 7 | 6 | |
| Rhabdoid appearance: | 0.126 | ||||
| Absent | 383 | 94.3 | 221 | 162 | |
| Present | 23 | 5.7 | 9 | 14 | |
| LVI: | 0.198 | ||||
| Absent | 298 | 73.4 | 175 | 123 | |
| Present | 108 | 26.6 | 55 | 53 | |
| ECOG-PS: | 0.005 | ||||
| = 0 | 335 | 82.6 | 201 | 134 | |
| > 1 | 71 | 17.4 | 29 | 42 | |
Abbreviations: FUT3 = Fucosyltransferase 3; LVI = lymphovascular invasion; ECOG-PS: Eastern Cooperative Oncology Group – Performance status.
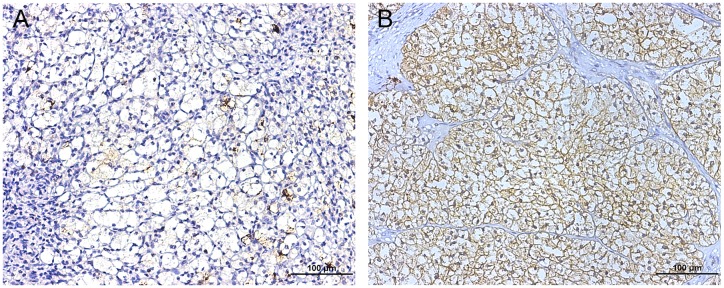
Figure 1. Representative photograph of FUT3 immunostaining in TMA (magnification * 200, scale bar = 100 μm).
(A) showed low densities of FUT3 while (B) showed high densities of FUT3.
High FUT3 expression predicted poor OS and RFS in patients with ccRCC
At the time of last follow-up, 74 (18.2%) patients had recurrence of RCC, including 19 patients with local recurrence only, 46 patients with distant recurrence only and 9 patients with both local and distant recurrence.
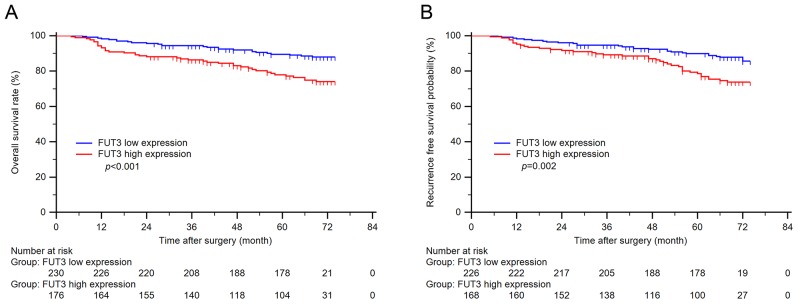
Kaplan-Meier method and log-rank were conducted to evaluate the relationship between FUT3 expression and clinical outcomes in ccRCC patients. As shown in Figure 2 FUT3 expression significantly correlated with OS (p < 0.001) as well as RFS (p = 0.002) in patients with ccRCC.
Figure 2. Kaplan-Meier curves of overall survival and recurrence free survival based on tumor FUT3 expression.
(A) n = 406, p < 0.001; (B) n = 394, p = 0.002.
FUT3 was an independent factor for poor prognosis in ccRCC patients
In order to confirm the prognostic significance of FUT3 expression and other clinicopathologic features in ccRCC, univariate cox analysis was applied. As in Table 2 , in univariate cox regression, tumor size (p < 0.001), pathologic T stage (p < 0.001), N (p < 0.001), M stage (p = 0.003), Fuhrman grade 3 and 4 (p < 0.001), sarcomatoid change (p < 0.001), rhabdoid appearance (p < 0.001), coagulative necrosis (p < 0.001), lymphovascular invasion (p < 0.001), ECOG-PS (p < 0.001) and FUT3 expression (p < 0.001) had significant impacts on OS.
Table 2. Univariate Cox regression analyses of potential prognostic factors for overall survival and recurrence free survival in ccRCC patients.
| Variable | Overall survival | Recurrence free survival | ||
|---|---|---|---|---|
| Hazard ratio (95% CI) | p | Hazard ratio (95% CI) | p | |
| Tumor size: | 1.482 (1.368-1.605) | <0.001 | 1.510 (1.382-1.650) | <0.001 |
| Pathologic T stage: | ||||
| pT2 vs. pT1 | 4.060 (1.660-9.926) | 0.002 | 4.228 (1.597-11.196) | 0.004 |
| pT3 vs. pT1 | 5.219 (3.073-8.864) | <0.001 | 4.938 (2.775-8.786) | <0.001 |
| pT4 vs. pT1 | 11.796 (4.537-30.668) | <0.001 | 13.585 (5.170-35.696) | <0.001 |
| Pathologic N stage: | ||||
| pN1 vs. pN0 | 36.464 (15.261-87.125) | <0.001 | / | / |
| Pathologic M stage: | ||||
| pM1 vs. pM0 | 5.800 (1.815-18.536) | 0.003 | / | / |
| Fuhrman grade: | ||||
| 2 vs. 1 | 1.562 (0.528-4.616) | 0.442 | 1.387 (0.463-4.155) | 0.561 |
| 3 vs. 1 | 4.162 (1.422-12.183) | 0.010 | 3.584 (1.203-10.676) | 0.023 |
| 4 vs. 1 | 17.309 (6.049-49.528) | <0.001 | 15.008 (5.163-43.626) | <0.001 |
| Sarcomatoid change: | ||||
| Present vs. Absent | 19.680 (9.178-42.196) | <0.001 | 26.191 (10.910-62.878) | <0.001 |
| Rhabdoid appearance: | ||||
| Present vs. Absent | 9.308 (5.187-16.704) | <0.001 | 8.602 (4.419-16.745) | <0.001 |
| Coagulative necrosis: | ||||
| Present vs. Absent | 4.418 (2.734-7.140) | <0.001 | 3.967 (2.351-6.693) | <0.001 |
| LVI: | ||||
| Present vs. Absent | 4.501 (2.779-7.289) | <0.001 | 4.466 (2.651-7.525) | <0.001 |
| ECOG-PS: | ||||
| ≥1 vs. 0 | 5.004 (3.037-8.245) | <0.001 | 3.992 (2.270-7.020) | <0.001 |
| FUT3 expression: | ||||
| High vs. Low | 2.342 (1.436-3.821) | <0.001 | 2.076 (1.230-3.505) | 0.006 |
Abbreviations: ccRCC = clear cell renal cell carcinoma; FUT3 = Fucosyltransferase 3; LVI= lymphovascular invasion; ECOG-PS: Eastern Cooperative Oncology Group – Performance status.
On the other hand, as presented in Table 2 , in univariate cox regression, tumor size (p < 0.001), pathologic T stage (p < 0.001), Fuhrman grade 3 and 4 (p < 0.001), sarcomatoid change (p < 0.001), rhabdoid appearance (p < 0.001), coagulative necrosis (p < 0.001), lymphovascular invasion (p < 0.001), ECOG-PS (p < 0.001) and FUT3 expression (p = 0.006) had a significant impact on RFS.
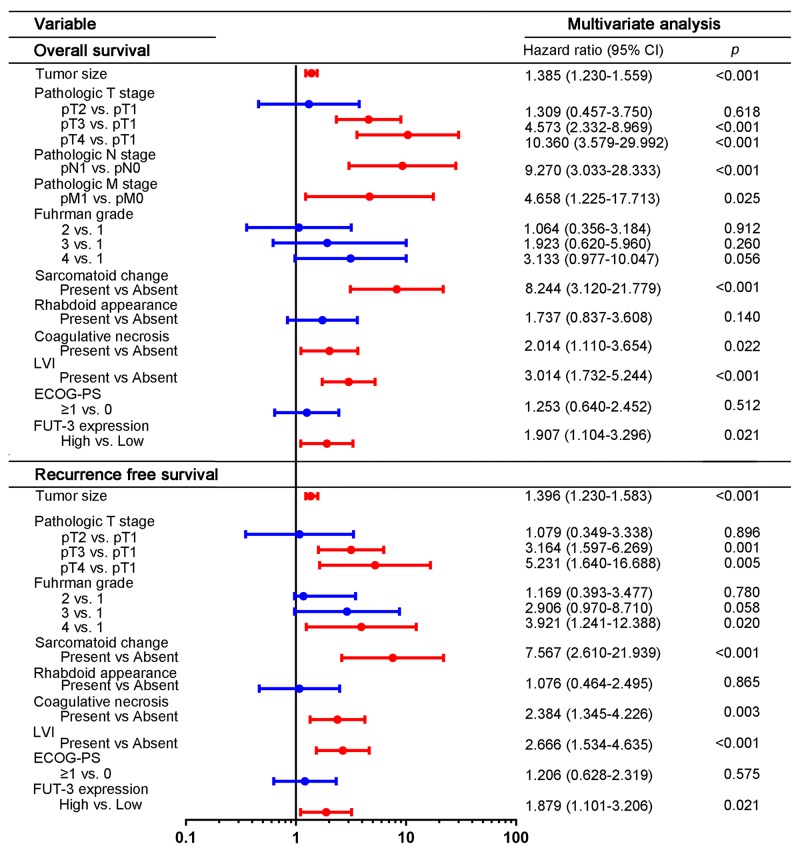
Then the multivariate cox analysis was then conducted to the above significant factors. Result in Figure 3 indicated that FUT3 expression was an independent prognostic factor in ccRCC, both for OS (HR = 1.907; p = 0.021) and RFS (HR = 1.879; p = 0.021). The other factors which considered statistically significant and independent prognostic factors included: pathologic T, N, M stage, presence of sarcomatoid change, coagulative necrosis and lyphovascular invasion for OS and pathologic T stage, Fuhrman grade, presence of sarcomatoid change, coagulative necrosis and lyphovascular invasion for RFS.
Figure 3. Multivariate analyses of conventional prognostic features in OS and RFS.
Figure 3 showed hazard ratio and p-value of each clinical and pathologic feature for OS and RFS. Abbreviation: LVI, lymphovascular invasion; ECOG-PS, Eastern cooperative Oncology Group performance status; FUT3, fucosyltransferase-III.
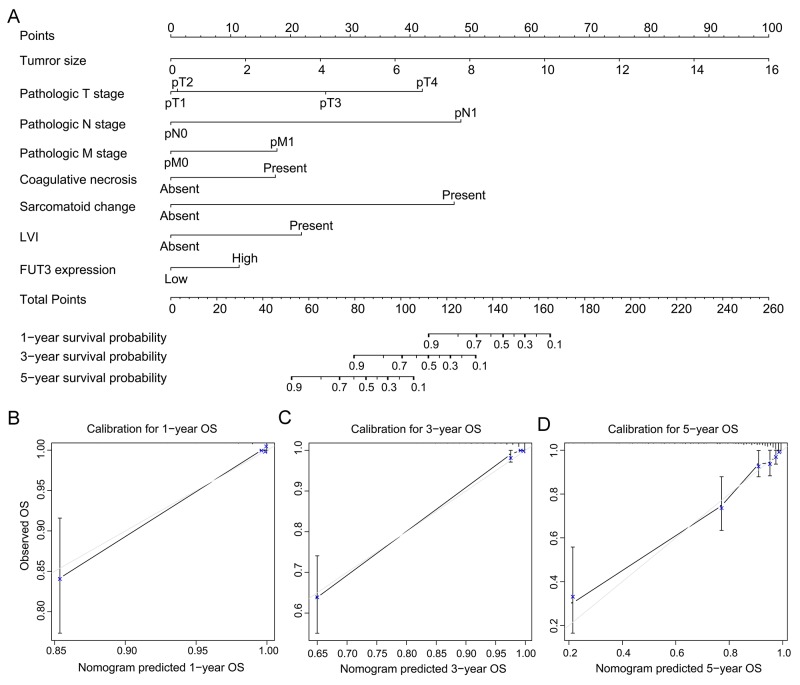
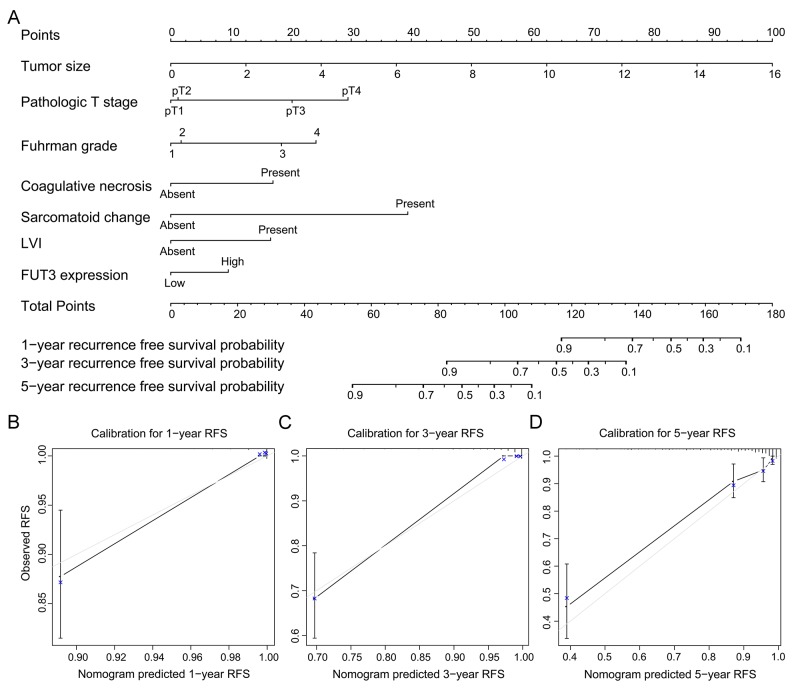
Construction of prognostic nomogram for OS and RFS in patients with ccRCC
All these independent prognosticators were brought into the prognostic nomogram of OS (Figure 4A) and RFS (Figure 5A). The calibration plot for the prognostic accuracy of OS and RFS at 1, 3, 5 years after surgery had a good agreement between the predicted and observed survival (Figure 4B-4D, Figure 5B-5D).
Figure 4. Built-up prognostic nomogram for OS prediction and calibration for it.
(A) showed an eight independent prognostic factors including tumor size, pathologic T, N, M stage, the presence of coagulative necrosis and lymphovascular invasion and FUT3 expression. The total point was generated by adding the scores of the prognostic factors. (B-D) showed the calibration plot for nomogram predicted 1-year, 3-year and 5-year overall survival rate.
Figure 5. Built-up prognostic nomogram for RFS prediction and calibration for it.
(A) showed a seven independent prognostic factors including tumor size, pathologic T stage, Fuhrman grade, the presence of coagulative necrosis and lymphovascular invasion and FUT3 expression. The total point was generated by adding the scores of the prognostic factors. (B-D) showed the calibration plot for nomogram predicted 1-year, 3-year and 5-year recurrence free survival rate.
Ability of FUT3 expression to enhance the established prognostic models
As presented above, high FUT3 expression correlated with reduced survival and high probability in patients with ccRCC. We further investigated whether the addition of FUT3 expression to established prognostic models can improve their predictive accuracies. The C-index, AIC and p value were presented in Table 3 . For OS, the predictive accuracy of Leibovich model improved from 0.829 to 0.854 (p = 0.009). However, the combination of FUT3 expression with TNM grade, SSIGN or UISS did not improve the predictive accuracies of the models. On the other hand, for RFS, the predictive accuracies of TNM grade (p = 0.019), UISS (p = 0.004), SSIGN (p = 0.009) and Leibovich model (p = 0.002) were all improved by the addition of FUT3.
Table 3. The predictive value of FUT3 and the combined hazard models in ccRCC patients.
| Overall survival | Recurrence free survival | |||||
|---|---|---|---|---|---|---|
| Models | C-index | AIC | p value | C-index | AIC | p value |
| FUT3 | 0.606 | 773.041 | 0.608 | 847.870 | ||
| TNM stage | 0.726 | 731.536 | 0.088 | 0.683 | 826.163 | 0.019 |
| TNM stage + FUT3 | 0.751 | 727.496 | 0.725 | 819.997 | ||
| UISS | 0.770 | 701.346 | 0.111 | 0.762 | 777.415 | 0.004 |
| UISS + FUT3 | 0.789 | 699.420 | 0.785 | 774.583 | ||
| SSIGN | 0.778 | 679.953 | 0.042 | 0.761 | 760.206 | 0.009 |
| SSIGN + FUT3 | 0.807 | 675.563 | 0.798 | 754.550 | ||
| Leibovich | 0.829 | 654.813 | 0.009 | 0.818 | 731.214 | 0.002 |
| Leibovich + FUT3 | 0.854 | 652.612 | 0.849 | 727.700 | ||
Abbreviations: ccRCC = clear cell renal cell carcinoma; FUT3 = Fucosyltransferase 3; UISS = University of California Los Angeles Integrated Staging System; SSIGN = Stage Size, Grade and Necrosis; p value = p valuefor AIC.
DISCUSSION
Our study confirms the significance of FUT-3 expression for patients with ccRCC. The high expression of FUT3 can predict poor prognosis of ccRCC patient. Furthermore, we built nomogram models for OS and RFS of ccRCC patients and confirm the models with calibration. In addition, we combine the built-up models and the expression of FUT-3 to improve the predictive accuracy.
FUT-3 is an enzyme from the FUTs family, and is highly expressed in stomach, colon, intestine, lung, and kidney [9]. FUT3 gene is also known as Lewis gene, for its significance in biosynthesis of Lewis antigen (Le) and sialic Lewis antigen (sLe). Many studies from other research groups had similar results to our study, in other words, FUT-3 can promote the malignant behavior of the tumor and lead to a poor prognosis. The high expression of FUT-3 and its product, tetrasaccharide Sialic Lewis x (sLex) has been observed in several kinds of malignant solid tumors, such as oral squamous cell carcinoma [14], breast invasive ductal carcinoma [15], pancreatic cancer [16], ovarian carcinoma and colorectal cancer [17]. sLex is the well-known ligands of the E-selectin [18], one of the cell-adhesion molecules of the endothelium around the blood vessels. E-selectin is a kind of Ca2+-dependent C-type lectins, which mediates cell-cell adhesion in vessels when inflammation takes place [9]. This adhesion is necessary for leukocyte rolling on the endothelium and then escape to inflammatory sites [19]. Nevertheless, this pattern of cell-cell adhesion also allows tumor cell to extravasate to other place during hematogenous metastasis [20], and then promote the metastasis of cancer. A recent study illustrated the proliferation and other malignant properties such as migration and invasion capability of gastric carcinoma cell line can be inhibited by FUT3 gene silencing [21]. In contrast, we cannot confirm that the sLex is all synthesized by FUT3, since FUT4, FUT5, FUT6, FUT-9 and FUT7 also have the α(1,3)-fucosyltransferase activity and their function in Lewis antigen synthesis of different tissue has as well been proved [22–24].
Another well-known fucosylated glycan, sialic Lewis a (sLea, CA19-9), is synthesized specifically by FUT3, because FUT3 has the only α (1,4)-fucosyltransferase activity in the FUTs family. sLea is another ligand for E-selectin and a meaningful circulatory biomarker of pancreatic carcinoma and some colorectal cancer in prognosis prediction and recurrence surveillance [9]. Nevertheless, the significance of sLea in prognosis prediction and recurrence surveillance of ccRCC patients and how it participates in the tumorigenesis and malignant transforming still remains unknown.
Furthermore, CD47 as a glycoprotein on the cellular membrane can give an inhibitory signal to the macrophage by binding to the signal regulatory protein alpha (SIRPα) on the surface of macrophage [25]. Fucosylated glycans, Lewis Y (Ley), can enhance the capacity of CD47-SIRP signaling and then enables the tumor cell to evade the phagocytosis or programmed cell clearance [26, 27].
Some studies demonstrated the opposite results to the studies mentioned above. Low FUT3 mRNA is correlated with the migration and liver metastasis of the colorectal cancer, which means FUT3 is a factor for poor prognosis of patients with colorectal cancer [28]. This paradox may be a result of tumor micro-environment. NK cell can recognize the sLex expressed on the tumor-cell surface and trigger the cytotoxicity mediated by CD94 and NKG2D [29]. On the other hand, the extrinsic apoptosis pathway is mediated by fucosylation in the TNF-related apoptosis-inducing ligand (TRAIL). The pathway is activated when TRAIL is combined to the TRAIL receptors, and then leads to the apoptosis of the target cells [30]. The FUT3 expression upregulates sensitivity of TRAIL pathway in colon cancer patients [31].
A few shortcomings of this study should be noted. The major limitation of this study is the relatively small sample size of the cohort and the retrospective design. A multi-center prospective study in cohorts with larger sample size is needed to confirm these results. TMA technique only displays small representative part of the tumor, and expression of FUT3 is subjectively evaluated.
CONCLUSION
In summary, FUT3 was a predictive factor for poor OS and RFS in patients with ccRCC. FUT3 enhanced the capability of established RCC prognostic models. Inhibitor of FUT3 might be a potential therapeutic method for ccRCC. The FUT3 in blood or tumor tissue would be new biomarkers for ccRCC detection or prognosis prediction soon.
MATERIALS AND METHODS
Patients
The cohort of this retrospective study included 406 ccRCC patients from Department of Urology, Zhongshan Hospital, Fudan University. The inclusion criteria were (1) ccRCC proved by histopathology; (2) underwent a radical nephrectomy or a partial nephrectomy between January, 2008 and December, 2009; (3) no history of another malignant tumor. For each patient, we collected the age at surgery, gender, Eastern cooperative Oncology Group performance status (ECOG-PS), TNM stage [32], Fuhrman grade [33], tumor size, and pathologic features of the tumor, such as coagulative necrosis, rhabdoid appearance, sarcomatoid change, lymphovascular invasion (LVI). Coagulative necrosis, rhabdoid appearance and sarcomatoid change was defined as these pathologic changes observed under the microscope. LVI was defined as the appearance of tumor cells in a space lined with endothelium without underlying muscular walls. SSIGN score, UISS score and Leibovich score were applied to the patients [34–36].
Patients underwent a nephrectomy if their disease was localized while patients with metastatic disease received interferon-α-based immunotherapy after cytoreductive nephrectomy. Patients were followed up with physical examination, laboratory tests, chest X-ray and abdominal CT scan or ultrasound postoperatively every half year for the first two years and annually from then on. The follow-up data was lastly updated March 2015. The study was approved by the clinical research ethic committee of Zhongshan Hospital, Fudan University and informed consent was signed and obtained from each patient.
Immunohistochemistry and assessment
We performed the immunohistochemical (IHC) staining on the tissue microarray (TMA). In the TMA construction, we chose the area away from hemorrhage and necrosis as the representative area. Rabbit polyclonal anti-FUT3 antibody (1:50 dilution, ab110082, Abcam, Cambridge, MA) was used for IHC staining. The IHC score was assessed by two independent pathologists, who were blind to the clinical or pathologic data. A semi-quantitative H-score was generated by multiplying the staining intensities (0 as negative, 1 for weak, 2 for moderate, and 3 for strong staining intensity) by the percentages of positive staining area. So the H-score of each sample ranged from 0 to 300.
Statistical analysis
X-tile plot analysis was applied to select the suitable cutoff value of the H-score according to the “minimum p value” method, in order to set the patients apart into two subgroups of low and high expression of FUT3 [37]. We evaluated the comparison between the expression of FUT3 and clinical and pathological variables by using the student’s t test, χ 2 test and Wilcoxon rank-sum test. OS curves and RFS curves were generated by Kaplan-Meier method and compared by log-rank t test. Independent associations between OS or RFS and clinicopathologic variables were evaluated by using univariable and multivariable Cox proportional-hazard models. Nomogram was built to predict the prognosis, and its prediction accuracy is evaluated by calibration plot. The predictive accuracy and sufficiency of the models were assessed by Harrell’s Concordance index (C-index) and Akaike’s Information Criteria (AIC). The difference between the C-index was compared by Hanley-McNeil test. The difference was considered significant if the two-side p value was less than 0.05.
Data were saved and analyzed by using software as followed: X-tile software version 3.6.1, (Yale University, New Haven, CT, USA), GraphPad Prism version 6.02 (GraphPad Software. Inc.), Stata SE, version 12.1 (Stata, College Station, TX), MedCalc Software 11.4.2.0 (MedCalc, Mariakerke, Belgium) and R software 3.1.2 with the “rms” package (R Foundation for Statistical Computing, Vienna, Austria).
Author contributions
L. Meng, L. Xu and Y. Yang for acquisition of data, analysis and interpretation of data, statistical analysis and drafting of the manuscript; L. Zhou, Y. Chang, T. Shi, C. Tan and H. An for technical and material support; Y. Zhu and J. Xu for study concept and design, analysis and interpretation of data, drafting of the manuscript, obtained funding and study supervision. All authors read and approved the final manuscript.
CONFLICTS OF INTEREST
The authors declare no conflicts of interest.
FUNDING
This study was funded by grants from National Key Projects for Infectious Diseases of China (2012ZX10002012-007, 2016ZX10002018-008), National Natural Science Foundation of China (31270863, 81272936, 81372755, 31470794, 81401988, 81402082, 81402085, 81471621, 81472227, 81472376, 31570803, 81501999, 81671628 and 81672324), Program for New Century Excellent Talents in University (NCET-13-0146) Shanghai Municipal Natural Science Foundation (134119A2700 and 17ZR1417300) and Innovation Project of Shanghai Jiao Tong University School of Medicine (16XJ21001). All these study sponsors have no roles in the study design, in the collection, analysis, and interpretation of data.
REFERENCES
- 1.Ljungberg B, Campbell SC, Choi HY, Jacqmin D, Lee JE, Weikert S, Kiemeney LA. The epidemiology of renal cell carcinoma. Eur Urol. 2011;60:615–21. doi: 10.1016/j.eururo.2011.06.049. Erratum in: Eur Urol. 2011; 60:1317. [DOI] [PubMed] [Google Scholar]
- 2.Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, Jemal A, Yu XQ, He J. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66:115–32. doi: 10.3322/caac.21338. [DOI] [PubMed] [Google Scholar]
- 3.Keegan KA, Schupp CW, Chamie K, Hellenthal NJ, Evans CP, Koppie TM. Histopathology of surgically treated renal cell carcinoma: survival differences by subtype and stage. J Urol. 2012;188:391–97. doi: 10.1016/j.juro.2012.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Motzer RJ, Bander NH, Nanus DM. Renal-cell carcinoma. N Engl J Med. 1996;335:865–75. doi: 10.1056/NEJM199609193351207. [DOI] [PubMed] [Google Scholar]
- 5.Naitoh J, Kaplan A, Dorey F, Figlin R, Belldegrun A. Metastatic renal cell carcinoma with concurrent inferior vena caval invasion: long-term survival after combination therapy with radical nephrectomy, vena caval thrombectomy and postoperative immunotherapy. J Urol. 1999;162:46–50. doi: 10.1097/00005392-199907000-00012. [DOI] [PubMed] [Google Scholar]
- 6.Rini BI, Campbell SC, Escudier B. Renal cell carcinoma. Lancet. 2009;373:1119–32. doi: 10.1016/S0140-6736(09)60229-4. [DOI] [PubMed] [Google Scholar]
- 7.Padró M, Cobler L, Garrido M, de Bolós C. Down-regulation of FUT3 and FUT5 by shRNA alters Lewis antigens expression and reduces the adhesion capacities of gastric cancer cells. Biochim Biophys Acta. 2011;1810:1141–9. doi: 10.1016/j.bbagen.2011.09.011. [DOI] [PubMed] [Google Scholar]
- 8.Kufe DW. Mucins in cancer: function, prognosis and therapy. Nat Rev Cancer. 2009;9:874–85. doi: 10.1038/nrc2761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pinho SS, Reis CA. Glycosylation in cancer: mechanisms and clinical implications. Nat Rev Cancer. 2015;15:540–55. doi: 10.1038/nrc3982. [DOI] [PubMed] [Google Scholar]
- 10.Sato Y, Nakata K, Kato Y, Shima M, Ishii N, Koji T, Taketa K, Endo Y, Nagataki S. Early recognition of hepatocellular carcinoma based on altered profiles of alpha-fetoprotein. N Engl J Med. 1993;328:1802–06. doi: 10.1056/NEJM199306243282502. [DOI] [PubMed] [Google Scholar]
- 11.Takahashi M, Kuroki Y, Ohtsubo K, Taniguchi N. Core fucose and bisecting GlcNAc, the direct modifiers of the N-glycan core: their functions and target proteins. Carbohydr Res. 2009;344:1387–90. doi: 10.1016/j.carres.2009.04.031. [DOI] [PubMed] [Google Scholar]
- 12.Dabrowska A, Baczyńska D, Widerak K, Laskowska A, Ugorski M. Promoter analysis of the human alpha1,3/4-fucosyltransferase gene (FUT III) Biochim Biophys Acta. 2005;1731:66–73. doi: 10.1016/j.bbaexp.2005.08.009. [DOI] [PubMed] [Google Scholar]
- 13.Hirakawa M, Takimoto R, Tamura F, Yoshida M, Ono M, Murase K, Sato Y, Osuga T, Sato T, Iyama S, Miyanishi K, Takada K, Hayashi T, et al. Fucosylated TGF-β receptors transduces a signal for epithelial-mesenchymal transition in colorectal cancer cells. Br J Cancer. 2014;110:156–63. doi: 10.1038/bjc.2013.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Desiderio V, Papagerakis P, Tirino V, Zheng L, Matossian M, Prince ME, Paino F, Mele L, Papaccio F, Montella R, Papaccio G, Papagerakis S. Increased fucosylation has a pivotal role in invasive and metastatic properties of head and neck cancer stem cells. Oncotarget. 2015;6:71–84. doi: 10.18632/oncotarget.2698. https://doi.org/10.18632/oncotarget.2698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Feng X, Zhao L, Gao S, Song X, Dong W, Zhao Y, Zhou H, Cheng L, Miao X, Jia L. Increased fucosylation has a pivotal role in multidrug resistance of breast cancer cells through miR-224-3p targeting FUT4. Gene. 2016;578:232–41. doi: 10.1016/j.gene.2015.12.028. [DOI] [PubMed] [Google Scholar]
- 16.Bassagañas S, Allende H, Cobler L, Ortiz MR, Llop E, de Bolós C, Peracaula R. Inflammatory cytokines regulate the expression of glycosyltransferases involved in the biosynthesis of tumor-associated sialylated glycans in pancreatic cancer cell lines. Cytokine. 2015;75:197–206. doi: 10.1016/j.cyto.2015.04.006. [DOI] [PubMed] [Google Scholar]
- 17.Mare L, Caretti A, Albertini R, Trinchera M. CA19.9 antigen circulating in the serum of colon cancer patients: where is it from? Int J Biochem Cell Biol. 2013;45:792–97. doi: 10.1016/j.biocel.2013.01.004. [DOI] [PubMed] [Google Scholar]
- 18.Lasky LA. Selectin-carbohydrate interactions and the initiation of the inflammatory response. Annu Rev Biochem. 1995;64:113–39. doi: 10.1146/annurev.bi.64.070195.000553. [DOI] [PubMed] [Google Scholar]
- 19.Dube DH, Bertozzi CR. Glycans in cancer and inflammation—potential for therapeutics and diagnostics. Nat Rev Drug Discov. 2005;4:477–88. doi: 10.1038/nrd1751. [DOI] [PubMed] [Google Scholar]
- 20.Burdick MM, Henson KA, Delgadillo LF, Choi YE, Goetz DJ, Tees DF, Benencia F. Expression of E-selectin ligands on circulating tumor cells: cross-regulation with cancer stem cell regulatory pathways? Front Oncol. 2012;2:103. doi: 10.3389/fonc.2012.00103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cai YJ, Zheng XF, Lu CH, Jiang Q, Liu Q, Xin YH. Effect of FUT3 gene silencing with miRNA on proliferation, invasion and migration abilities of human KATO-III gastric cancer cell line. Cell Mol Biol (Noisy-le-grand) 2016;62:15–20. [PubMed] [Google Scholar]
- 22.Nishihara S, Iwasaki H, Nakajima K, Togayachi A, Ikehara Y, Kudo T, Kushi Y, Furuya A, Shitara K, Narimatsu H. Alpha1,3-fucosyltransferase IX (Fut9) determines Lewis X expression in brain. Glycobiology. 2003;13:445–55. doi: 10.1093/glycob/cwg048. [DOI] [PubMed] [Google Scholar]
- 23.Escrevente C, Machado E, Brito C, Reis CA, Stoeck A, Runz S, Marmé A, Altevogt P, Costa J. Different expression levels of alpha3/4 fucosyltransferases and Lewis determinants in ovarian carcinoma tissues and cell lines. Int J Oncol. 2006;29:557–66. [PubMed] [Google Scholar]
- 24.Nordén R, Nyström K, Aurelius J, Brisslert M, Olofsson S. Virus-induced appearance of the selectin ligand sLeX in herpes simplex virus type 1-infected T-cells: involvement of host and viral factors. Glycobiology. 2013;23:310–21. doi: 10.1093/glycob/cws160. [DOI] [PubMed] [Google Scholar]
- 25.Barclay AN, Van den Berg TK. The interaction between signal regulatory protein alpha (SIRPα) and CD47: structure, function, and therapeutic target. Annu Rev Immunol. 2014;32:25–50. doi: 10.1146/annurev-immunol-032713-120142. [DOI] [PubMed] [Google Scholar]
- 26.Jaiswal S, Jamieson CH, Pang WW, Park CY, Chao MP, Majeti R, Traver D, van Rooijen N, Weissman IL. CD47 is upregulated on circulating hematopoietic stem cells and leukemia cells to avoid phagocytosis. Cell. 2009;138:271–85. doi: 10.1016/j.cell.2009.05.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tan M, Zhu L, Zhuang H, Hao Y, Gao S, Liu S, Liu Q, Liu D, Liu J, Lin B. Lewis Y antigen modified CD47 is an independent risk factor for poor prognosis and promotes early ovarian cancer metastasis. Am J Cancer Res. 2015;5:2777–87. [PMC free article] [PubMed] [Google Scholar]
- 28.Petretti T, Kemmner W, Schulze B, Schlag PM. Altered mRNA expression of glycosyltransferases in human colorectal carcinomas and liver metastases. Gut. 2000;46:359–66. doi: 10.1136/gut.46.3.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ohyama C, Kanto S, Kato K, Nakano O, Arai Y, Kato T, Chen S, Fukuda MN, Fukuda M. Natural killer cells attack tumor cells expressing high levels of sialyl Lewis x oligosaccharides. Proc Natl Acad Sci USA. 2002;99:13789–94. doi: 10.1073/pnas.212456599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Johnstone RW, Frew AJ, Smyth MJ. The TRAIL apoptotic pathway in cancer onset, progression and therapy. Nat Rev Cancer. 2008;8:782–98. doi: 10.1038/nrc2465. [DOI] [PubMed] [Google Scholar]
- 31.Wagner KW, Punnoose EA, Januario T, Lawrence DA, Pitti RM, Lancaster K, Lee D, von Goetz M, Yee SF, Totpal K, Huw L, Katta V, Cavet G, et al. Death-receptor O-glycosylation controls tumor-cell sensitivity to the proapoptotic ligand Apo2L/TRAIL. Nat Med. 2007;13:1070–77. doi: 10.1038/nm1627. [DOI] [PubMed] [Google Scholar]
- 32.Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol. 2010;17:1471–4. doi: 10.1245/s10434-010-0985-4. [DOI] [PubMed] [Google Scholar]
- 33.Delahunt B, Cheville JC, Martignoni G, Humphrey PA, Magi-Galluzzi C, McKenney J, Egevad L, Algaba F, Moch H, Grignon DJ, Montironi R, Srigley JR. Members of the ISUP Renal Tumor Panel. The International Society of Urological Pathology (ISUP) grading system for renal cell carcinoma and other prognostic parameters. Am J Surg Pathol. 2013;37:1490–504. doi: 10.1097/PAS.0b013e318299f0fb. [DOI] [PubMed] [Google Scholar]
- 34.Leibovich BC, Blute ML, Cheville JC, Lohse CM, Frank I, Kwon ED, Weaver AL, Parker AS, Zincke H. Prediction of progression after radical nephrectomy for patients with clear cell renal cell carcinoma: a stratification tool for prospective clinical trials. Cancer. 2003;97:1663–71. doi: 10.1002/cncr.11234. [DOI] [PubMed] [Google Scholar]
- 35.Zisman A, Pantuck AJ, Wieder J, Chao DH, Dorey F, Said JW, deKernion JB, Figlin RA, Belldegrun AS. Risk group assessment and clinical outcome algorithm to predict the natural history of patients with surgically resected renal cell carcinoma. J Clin Oncol. 2002;20:4559–66. doi: 10.1200/JCO.2002.05.111. [DOI] [PubMed] [Google Scholar]
- 36.Frank I, Blute ML, Cheville JC, Lohse CM, Weaver AL, Zincke H. An outcome prediction model for patients with clear cell renal cell carcinoma treated with radical nephrectomy based on tumor stage, size, grade and necrosis: the SSIGN score. J Urol. 2002;168:2395–400. doi: 10.1016/S0022-5347(05)64153-5. [DOI] [PubMed] [Google Scholar]
- 37.Camp RL, Dolled-Filhart M, Rimm DL. X-tile: a new bio-informatics tool for biomarker assessment and outcome-based cut-point optimization. Clin Cancer Res. 2004;10:7252–59. doi: 10.1158/1078-0432.CCR-04-0713. [DOI] [PubMed] [Google Scholar]