Abstract
Differentiation of indolent and aggressive prostate carcinoma (PCa) at the time of diagnosis is currently one of the major challenges. This study aimed at identification of prognostic biomarkers to aid in predicting biochemical recurrence (BCR) of the disease. Microarray-based gene expression profiling in tissues of 8 BCR and 8 No-BCR cases revealed expression differences of 455 genes, most of which were down-regulated in BCR cases. Eleven genes were selected for validation by real-time PCR in the first PCa cohort (N = 55), while seven of them were further validated in the second, independent, PCa cohort (N = 53). Down-regulation of MT1E (p < 0.001) and GPR52 (p = 0.002) expression and up-regulated levels of EZH2 (p = 0.025) were specific biomarkers of BCR in at least one of the two PCa cohorts, but only MT1E expression retained the independent prognostic value in a multivariate analysis (p < 0.001). DNA methylation analysis (114 PCa and 24 non-cancerous tissues) showed frequent MT1E methylation in PCa (p < 0.001) and was associated (p < 0.010) with the down-regulated expression in one PCa cohort. The results of our study suggest MT1E down-regulation as a potential feature of aggressive PCa.
Keywords: prostate cancer, biochemical recurrence, gene expression, MT1E
INTRODUCTION
Prostate carcinoma (PCa) is the second most common cancer and the fifth leading cause of death from cancer among men according to GLOBOCAN 2012 [1]. Implementation of prostate-specific antigen (PSA) testing into clinical practice led to marked increase in the numbers of newly diagnosed early stage PCa. However, even localized PCa is a highly heterogeneous disease with variable clinical outcomes ranging from indolent, low-risk disease to tumors with aggressive behavior having a tendency to progress. The risk evaluation based on D’Amico’s classification system (clinical stage, PSA level, and histological features of the tumor on biopsy) is also not informative enough, with nearly one half of cases being upgraded/upstaged at final pathology [2]. Molecular markers may provide more accurate measures of tumor aggressiveness and assist in the discrimination between indolent forms of PCa and clinically aggressive tumors.
During recent years, several molecular changes were identified as PCa-specific biomarkers, while potent prognostic biomarkers for early detection of aggressive forms of PCa are still lacking. In combination with other biomarkers, the fusion of androgen-regulated gene TMPRSS2 with ERG (TMPRSS2-ERG) or other genes of the oncogenic ETS transcription factor family may assist in outcome prediction, but has no independent prognostic value [3, 4–6]. Over-expression of SPINK1 is detectable in a subset of ERG-negative cases and can mark an aggressive course of the disease [7, 8]. However, the prognostic value of ERG or SPINK1 was not confirmed in a study of large multi-institutional cohort of early stage PCa cases [9]. Recent studies have also suggested the prognostic value of several other genetic alterations, including over-expression of the EZH2 gene [10, 11]. However, taking into consideration that multiple genomic alterations in prostate tumors may define patients with different cancer risks, further studies of novel molecular markers in large independent cohorts are needed to improve our understanding of PCa behavior and to provide clinicians with more precise indicators of aggressive course of the disease.
In the present study, genome-wide gene expression profile in tissues of PCa cases that experienced biochemical recurrence (BCR) was compared to non-recurrent cases utilizing microarray technology. A total of 11 gene targets were chosen from microarray analysis or selected from publications [6, 12] and thoroughly validated in two independent cohorts of PCa cases by means of real-time PCR. Possible involvement of DNA methylation in expression changes of the most significantly down-regulated gene MT1E was analyzed in a subset of PCa cases and controls.
RESULTS
Global gene expression profiling
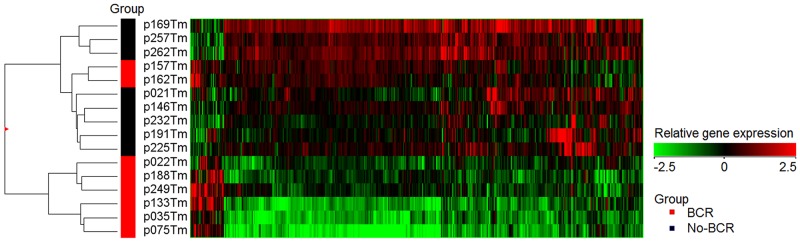
Microarray-based transcriptome comparison of 8 BCR and 8 No-BCR PCa samples identified 455 genes that were significantly deregulated (p < 0.050) with absolute fold change (FC) value of ≥2 (Figure 1). The vast majority of these genes were down-regulated (421 of 455; 92.5%) and only 34 (7.5%) were up-regulated in BCR cases as compared to No-BCR. Among the genes showing the most significant decrease of expression were GPR52 (FC 2.3; p < 0.001), GHRH (FC 2.2; p < 0.001), and FABP7 (FC 3.05; p = 0.009), whereas CHI3L2 (FC 3.6; p = 0.002), SAA2 (FC 6.2; p = 0.014), OLR1 (FC 2.0; p = 0.005) were significantly over-expressed in BCR cases. Comparing PCa cases with early (≤6 mo; N = 4) and late (>6 mo; N = 4) BCR, significant down-regulation of MT1E was detected (FC 3.0; p = 0.026).
Figure 1. Heatmap of gene expression in prostate tissue.
Expression profile of the genes that were differentially expressed (N = 455, p < 0.050) in biochemically recurrent (BCR) and No-BCR cases.
Data validation in PCa1 cohort
In order to explore the prognostic value of the novel biomarkers, seven genes (CHI3L2, FABP7, GHRH, GPR52, MT1E, OLR1, and SAA2) significantly deregulated in the microarray screening were selected for further validation on custom design TaqMan Low Density Array (TLDA) cards using cancerous (N = 55) and non-cancerous (N = 12) prostate tissue (NPT) samples from PCa1 cohort. In addition, several well-known PCa biomarkers (ERG, EZH2, HPN, and TERT) with prognostic value shown in our previous study and in studies of other authors [6, 12] were also included into this analysis.
In PCa1 cohort, comparison of PCa to NPT samples, revealed significant up-regulation of HPN (FC 3.3; p < 0.001), ERG (FC 3.0; p = 0.038), and EZH2 (FC 1.3; p = 0.029) in cancerous tissues, while FABP7 (FC 4.4; p < 0.001) was significantly down-regulated (Supplementary Figure 1A). Other significant changes of gene expression in various clinical sub-groups are presented in Supplementary Figure 1A.
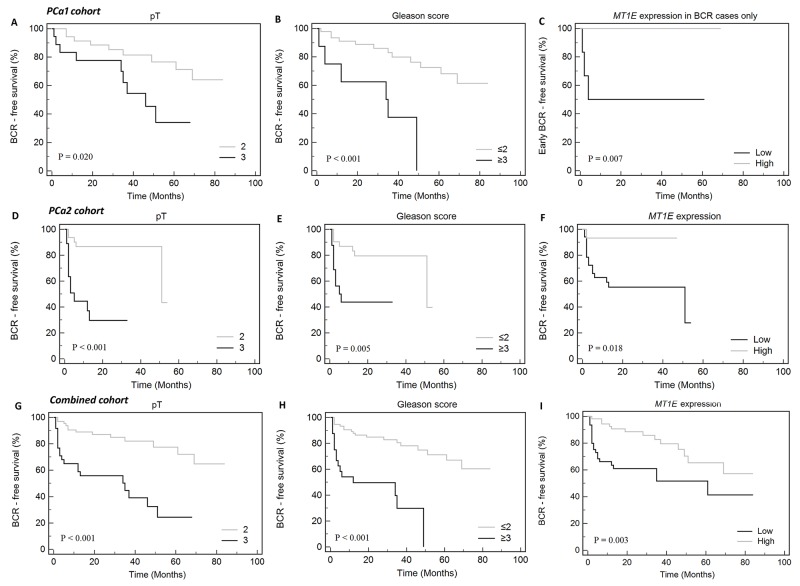
Comparison of BCR to No-BCR cases (N = 18 and N = 35, respectively) revealed significant down-regulation of MT1E (FC 2.0; p < 0.001) and GPR52 (FC 1.7; p = 0.002) in accordance with the disease progression (Supplementary Figure 1A). In Kaplan-Meier analysis, GPR52 (p = 0.033), in addition to tumor stage (pT; p = 0.020) and grade group (G; p < 0.001), was a significant predictor of BCR (Figure 2A, 2B). Moreover, lower MT1E expression level was predictive of the early disease progression (≤6 mo; p = 0.007) (Figure 2C). In univariate Cox regression hazard analysis, clinical variables – G and pT – were significant predictors of BCR, while reduced expression of the MT1E gene in PCa1 cohort showed no significance (p = 0.070; Supplementary Table 1).
Figure 2. Kaplan-Meier curves of biochemical recurrence-free (BCR-free) survival according to clinical-pathological variables and MT1E expression level.
(A and B) – PCa1 cohort, (C) – PCa1 cohort, only cases with BCR, dichotomized defining early BCR (≤6 mo) as a complete event and late BCR (>6 mo) as a censored event, (D-F) – PCa2 cohort, (G-I) – combined cohort (PCa1 & PCa2). Only parameters significantly associated with BCR or early BCR are shown (p < 0.050).
Data validation in PCa2 cohort
The prognostic value of a set of genes showing significant associations with BCR and other clinical variables in PCa1 study (ERG, EZH2, GPR52, HPN, MT1E, OLR1, and TERT) was further analyzed in an independent cohort of 53 PCa cases utilizing TaqMan-based single-assay real-time PCR. Similarly to the PCa1 cohort, comparison of BCR to No-BCR cases (N = 16 and N = 33) revealed significant decrease of MT1E expression (FC 3.8, p < 0.001). In PCa2, BCR was also associated with higher levels of EZH2 (FC 1.4, p = 0.025; Supplementary Figure 1B). Kaplan-Meier curves revealed pT (p < 0.001) and G (p = 0.005) together with MT1E (p = 0.018) expression as significant predictors of BCR (Figure 2D–2F). These findings were supported by univariate Cox regression hazard analysis (Supplementary Table 1). Moreover, EZH2 expression level was also predictive of BCR in univariate Cox model (Supplementary Table 1), although Kaplan-Meier analysis showed no significance (data not shown).
Combined gene expression analysis in both cohorts
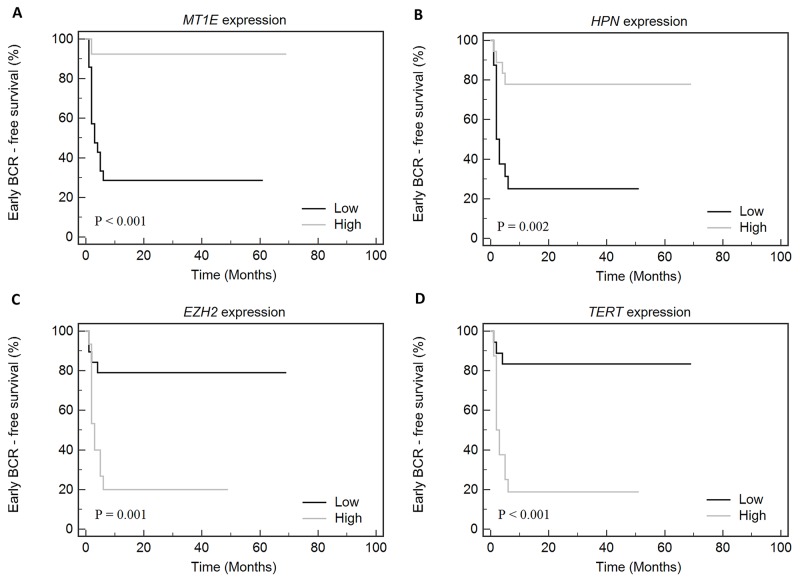
To summarize our study, survival analysis was performed for the combined group of PCa cases (N = 102 in total; 34 BCR and 68 No-BCR cases). Kaplan-Meier analysis revealed that BCR-free survival was significantly impacted by pT (p < 0.001), G (p < 0.001), and MT1E expression (p = 0.003; Figure 2G–2I), while GPR52 expression showed a borderline significance (p = 0.057). In BCR group, early progression was significantly associated with low MT1E and HPN expression levels and high EZH2 and TERT levels (Figure 3A–3D).
Figure 3. Kaplan-Meier curves according to the gene ((A) - MT1E, (B) - HPN, (C) - EZH2, (D) - TERT) expression levels in the combined cohort of cases with early (≤6 mo) and late (>6 mo) biochemical recurrence (BCR).
In the multivariate Cox regression model, backward entering of covariates revealed significant predictive value of pT, G, and MT1E expression (model’s p < 0.001). Out of the gene expression data evaluated, only MT1E expression level maintained an independent predictive value for the BCR in multivariate analysis (p < 0.001; Table 1).
Table 1. Cox regression analysis for clinical-pathological parameters and biomarkers in combined PCa1 and PCa2 cohort.
| Parameter | Univariate | Multivariate | ||||
|---|---|---|---|---|---|---|
| Hazard ratio [95% CI] | P-value | Model’s P-value |
Hazard ratio [95% CI] | P-value | Model’s P-value |
|
| Tumor stage (pT3 vs pT2) | 4.28 [2.11; 8.65] | <0.001 | <0.001 | 2.81 [1.34; 5.88] | 0.006 | <0.001 |
| ISUP Gleason grading group | 5.15 [2.48; 10.70] | <0.001 | <0.001 | 2.67 [1.21; 5.91] | 0.015 | |
| Preoperative PSA | 1.02 [1.00; 1.04] | 0.062 | 0.106 | backward eliminated | ||
| MT1E expression | 0.59 [0.48; 0.72] | <0.001 | <0.001 | 0.68 [0.55; 0.85] | 0.001 | |
| GPR52 expression | 0.78 [0.54; 1.13] | 0.186 | 0.187 | backward eliminated | ||
| EZH2 expression | 1.66 [1.19; 2.31] | 0.003 | 0.003 | backward eliminated | ||
| OLR1 expression | 1.21 [1.04; 1.40] | 0.013 | 0.012 | backward eliminated | ||
| HPN expression | 0.76 [0.59; 0.98] | 0.031 | 0.031 | backward eliminated | ||
Significant P-values are in bold ISUP – International Society of Urological Pathology, PSA – prostate-specific antigen, CI – confidence interval.
Significant associations between clinical variables and between clinical variables and genes’ expression are provided in Supplementary Table 2.
DNA methylation analysis of MT1E
To elucidate the mechanism of MT1E down-regulation in PCa, two MT1E fragments (MT1E-1 and MT1E-2; Supplementary Figure 2) were analyzed by means of methylation-specific PCR (MSP) in most of the cases from PCa1 and PCa2 cohorts (114 PCa and 24 NPT, overall). Aberrant methylation was frequent in PCa and significantly less common in NPT samples (p < 0.001 and p < 0.001 for MT1E-1 and MT1E-2, respectively) (Figure 4A). Both MT1E-1 and MT1E-2 fragments were more frequently methylated in pT3 than pT2 tumors (p = 0.029 and p = 0.009, respectively), but showed no other associations with clinical-pathological parameters. Methylated status of MT1E-1 or MT1E-2 fragments was significantly associated with lower MT1E gene expression level in tumors of PCa2 cohort (p = 0.008 and p = 0.009, respectively; Figure 4B), but no such association was observed in PCa1 tumors.
Figure 4. MT1E methylation analysis.
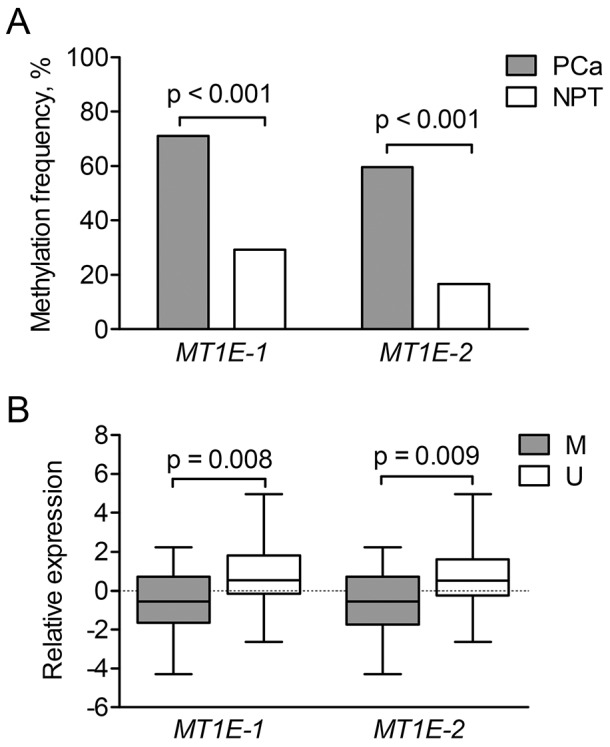
(A) – Aberrant methylation frequencies of the two MT1E gene promoter-associated fragments (MT1E-1 and MT1E-2) in the MSP group. (B) – Relative expression of the MT1E gene according to the methylation status of the two analyzed fragments in tumors of PCa2 cohort. Gene expression values were mean-centered separately for each cohort and represented as median (line) with 25-75th percentiles (box) and range (whiskers). PCa – prostate cancer, NPT – non-cancerous prostate tissue M/ U - methylated/ unmethylated promoter status..
DISCUSSION
During recent years, combinations of molecular biomarkers with the PSA test have suggested some promises for improved detection of PCa, however, their prognostic power remains questionable [10, 13]. Our study aimed at detection of PCa biomarkers prognostic for BCR after the surgery. Genome-wide gene expression profiling in BCR and No-BCR cases with up to 7 years of clinical follow-up data led to the identification of 455 differentially expressed genes. After the validation in two independent PCa cohorts with different duration of follow-up, down-regulation of MT1E was determined as a potential biomarker of early BCR onset in PCa.
Metallothioneins (MTs) are a group of metal binding proteins involved in the intracellular turnover of metal ions and detoxification of heavy metals [14]. The role of MTs has been extensively studied in PCa, but other genes from this superfamily (MT1G, MT1H, MT3) have attracted more attention than MT1E [15–17]. In general, down-regulation of MTs is considered to be an early step of cancer progression, associated with poor prognosis in different cancer patients [14, 18, 19]. In agreement with this, the down-regulation of MT1E expression was strongly associated with BCR in the two PCa cohorts in our study, regardless of the variation in the duration of follow-up. Besides, MT1E down-regulation was predominant in the cases with early BCR and showed associations with clinical markers of poor prognosis, i.e. higher grade group and more advanced tumor stage. In addition, our study revealed DNA methylation of the promoter region of the MT1E gene as a possible mechanism of gene inactivation in PCa. The aberrant MT1E promoter methylation was frequently observed in PCa tissues, but almost never occurred in NPT. MT1E promoter hypermethylation was previously reported only in melanoma and endometrial cancer patients [20, 21], but, to the best of our knowledge, has never been assessed in PCa.
Up-regulation of EZH2 (Enhancer of zeste homolog 2), a subunit of Polycomb repressive complex 2 (PRC2) with a histone methyltransferase activity, is recorded as one of the most common genetic alterations of PCa [22]. Elevated EZH2 gene expression has been associated with metastatic PCa and aggressive course of the disease in clinically localized PCa cases, and independent prognostic value of this biomarker has been demonstrated in several studies [10, 11, 23]. Based on this data, the gene was included in our study and showed significant associations with BCR in one of our cohorts. In addition to the prognostic value of EZH2, a higher expression level of the gene was demonstrated in cancerous than non-cancerous tissues from patients with PCa, showing the potential diagnostic value of this biomarker.
Several biomarkers have been suggested for the stratification of localized PCa into high and low-risk disease and, therefore, could help clinicians in decision making and appropriate treatment selection. Our previous study [6] revealed the prognostic value of a combined TMPRSS2-ERG and TERT expression analysis, while the data of other authors [10, 11, 23] and our present study suggested up-regulation of EZH2 and down-regulation of MT1E as the possible biomarkers of poor prognosis in PCa. Although PCa-specific transcripts are easily detectable in tumors, they are less abundant in blood or urine used for noninvasive monitoring of disease progression after therapy. The data of the present study suggest involvement of promoter DNA methylation as a putative mechanism that might be responsible for the MT1E down-regulation in PCa. The possibility to detect hypermethylated MT1E in body fluids of PCa patients, primarily focusing on evaluating its prognostic potential, remains to be explored in future studies.
In conclusion, our study indicates that reduced expression ofMT1E is an independent predictor of poor prognosis in PCa. Measurement of MT1E level, along with other indicators of the disease progression, may serve for better prognostic stratification of PCa at the time of diagnosis or surgery.
MATERIALS AND METHODS
Tissue samples
Prostate tissue samples were obtained from PSA-screened and biopsy-proven PCa patients treated with radical prostatectomy (RP) at the Urology Centre of Vilnius University Hospital Santaros Clinics during the period 2008-2014. Approval from the Lithuanian Bioethics Committee was obtained for the study and all patients signed informed consent for participation.
The samples, collected during separate periods, composed two independent cohorts: group PCa1 in 2008-2010 and group PCa2 in 2010-2014 (Table 2). Cancerous (≥50% of tumor cells) and non-cancerous (0%) prostatectomy tissues were sampled by expert pathologist as previously reported [6] and prepared for molecular analysis. Gleason score was obtained according to International Society of Urological Pathology (ISUP) 2005 and 2014 recommendations [24]. Grade groups formed according to ISUP 2014 recommendations were merged into two clusters G ≤ 2 and G ≥ 3. In agreement with the EAU guidelines [25], BCR after RP was defined by two consecutive PSA values of ≥0.2 ng/mL and rising.
Table 2. Clinical-pathological parameters of the study groups.
| Variable | Group PCa1a | Group PCa2b | MSP groupc | Combined PCa1 & PCa2 group | ||
|---|---|---|---|---|---|---|
| PCa (N = 55) |
NPT (N = 12) |
PCa (N = 53) |
NPT (N = 24) |
PCa (N = 114) |
PCa (N = 108) |
|
| Mean age, years | 62.1 | 61.5 | 58.4 | 61.5 | 60.5 | 60.3 |
| Tumor stage, N | ||||||
| pT2 | 36 | 34 | 75 | 70 | ||
| pT3 | 19 | 19 | 39 | 38 | ||
| ISUP Gleason grading group, N | ||||||
| G≤2 | 47 | 36 | 92 | 83 | ||
| G≥3 | 8 | 17 | 22 | 25 | ||
| BCR, N | ||||||
| Yes | 18 | 16 | 32 | 34 | ||
| No | 35 | 33 | 74 | 68 | ||
| Unknown | 2 | 4 | 8 | 6 | ||
| Mean follow-up time, months | ||||||
| Mean | 51 | 28 | 32 | 41 | ||
| Median | 54 | 27 | 29.5 | 37 | ||
| Range | [1; 84] | [1; 72] | [1; 84] | [1; 84] | ||
| TMPRSS2-ERG status, N | ||||||
| Positive | 36 | 36 | 76 | 72 | ||
| Negative | 19 | 15 | 36 | 34 | ||
| Unknown | 0 | 2 | 2 | 2 | ||
| Mean preoperative PSA level, ng/ml | 11.46 | 10.18 | 10.89 | 10.82 | ||
BCR – biochemical recurrence; ISUP – International Society of Urological Pathology, MSP – methylation-specific PCR, NPT – non-cancerous prostate tissue, PCa – cancerous prostate tissue, PSA – prostate-specific antigen.
a – Samples were used for gene expression analysis by custom design TaqMan Low Density Arrays (TLDA).
b – Samples were used for gene expression analysis by TaqMan single assays.
c – Samples were used for MT1E gene promoter methylation analysis by MSP.
PCa1 cohort (55 PCa and 12 NPT samples) had a long follow-up period (mean 51 mo after RP) with a follow-up data collected for 96% (53/55) of the cases (Table 2). Based on the BCR status, PCa samples from this cohort were selected for microarray-based gene expression profiling and were also used for the data validation on custom design TLDA cards. PCa2 cohort, comprised of PCa cases with the shorter follow-up period (mean 28 mo), was used for the independent data validation by TaqMan Gene Expression Assays analysis. Follow-up data were collected for 92% (49/53) of PCa2 cohort patients (Table 2). Most of the PCa1 and PCa2 samples and some additional cases and controls (114 PCa and 24 NPT samples in total; Table 2) were assessed for aberrant DNA methylation.
RNA and DNA preparation
Total RNA from snap-frozen sections, ground to powder using liquid nitrogen, was isolated with mirVana Kit (Ambion, Thermo Fisher Scientific, Foster City, CA, USA) according to the manufacturer’s recommendations and used for TLDA and single-gene expression assays. Quantity of the RNA samples was measured spectrophotometrically using the NanoDrop 2000 (Thermo Fisher Scientific, Wilmington, NC, USA). Integrity of the RNA samples (RIN) was assessed with 2100 Bioanalyzer (Agilent Technologies, Santa Clara, CA, USA) prior to analyses, and values ranged up to 9.7 (mean 8.0). Samples having RIN ≥7 were included in the study cohorts.
DNA was extracted from 10-30 mg of homogenized prostate tissues. Samples were digested with proteinase-K (Thermo Fisher Scientific, Vilnius, Lithuania) for up to 16 hours, then purified according to standard phenol-chloroform protocol followed by ethanol precipitation. For methylation analysis, 400 ng of extracted DNA were modified with sodium bisulfite using EZ DNA Methylation Kit (Zymo Research, Irvine, CA, USA) according to manufacturer’s protocol.
Global gene expression profiling
Global gene expression profiling (GEO accession identifier GSE89317) of PCa tissues from 8 BCR and 8 No-BCR cases was performed onto SurePrint G3 Human Gene Expression (v2) 8×60 K microarrays (design ID 028004; Agilent Technologies). Sample processing (100 ng of input RNA) was performed according to One-Color Microarray-Based Gene Expression Analysis Low Input Quick Amp Labeling, version 6.5, protocol using spike RNA (RNA Spike-In kit) and Low Input Quick Amp Labeling Kit, One-Color (Agilent Technologies). Samples were hybridized at 65°C for 17 hours. Then, slides were washed using Gene Expression Wash Buffer kit and scanned with SureScan microarray scanner (Agilent Technologies).
Signal intensities from the images were extracted and evaluated with Feature Extraction software v10.7.3 and further analyzed using GeneSpring GX v12.6.0 (Agilent Technologies). Probes with saturated, non-uniform, and outlier signal values were removed before further preprocessing. Data was normalized according to 75th percentile and the median of all samples was used for the baseline transformation. Probes having raw signal values of ≤10 in more than 50% of the analyzed samples were filtered out. Probe annotations were extracted from eArray platform according to the corresponding microarray design identifier. Fold change (FC) values were estimated and unpaired t-test was used for two group comparison. Differences of gene expression levels were considered significant if absolute FC was ≥2.0 and p-value was <0.050.
Gene expression analysis using TLDA arrays
Gene expression levels of 11 selected target genes and 5 reference genes (Supplementary Table 3) were quantified in triplicates using custom TLDA (Applied Biosystems, Thermo Fisher Scientific, Foster City, CA, USA) in samples from PCa1 cohort. Five hundred ng of total RNA were reverse transcribed (RT) to cDNA using High Capacity cDNA Reverse Transcription Kit with RNase Inhibitor (Applied Biosystems) according to the manufacturer’s instructions. Twenty μL of cDNA (equivalent to 500 ng of total RNA in each port) was immediately used as a template in real-time PCR (RT-qPCR).
Gene expression analysis using TaqMan gene expression assays
Gene expression levels of 7 genes and 2 reference genes were analyzed with TaqMan gene expression assays (Supplementary Table 3) in PCa2 cohort. Only genes expressed in at least 70% of the samples from PCa1 were included in this step.
Genomic DNA from the extracted RNA samples was removed by digesting with 1 unit of DNaseI (Thermo Fisher Scientific, Vilnius, Lithuania). For the RT, 300 ng of total RNA were converted to cDNA using Maxima First Strand cDNA Synthesis Kit for RT-qPCR (ThermoFisher Scientific, Vilnius, Lithuania) in a final volume of 20 μL under the following conditions: 25°C for 10 min, followed by 50°C for 30 min, and 85°C for 5 min. Two μL of cDNA mix were used as a template in a total 20 μL qPCR volume.
RT-qPCR was performed using the TaqMan Universal Master Mix II no UNG following manufacturer’s recommendations (Applied Biosystems). Expression levels of all genes for all samples were analyzed in triplicates and required at least 2 valid wells. Non-template controls were included for each gene in each RT-qPCR run.
DNA methylation analysis
Bisulfite-modified DNA was used as a template for MSP. Two sets of primers covering the promoter region of the MT1E gene (positioned at -112/+105 and -239/-91 from transcription start site, MT1E-1 and MT1E-2, respectively) were designed with Methyl Primer Express software v1.0 (Applied Biosystems) and ordered from Metabion (Martinsried, Germany). The reaction mix (25 μL) contained 1x Maxima Hot Start Taq PCR buffer, 2.5 mM MgCl2, 1.6 mM dNTP mix, 1.25 U Maxima Hot Start Taq DNA polymerase (Thermo Fisher Scientific, Vilnius, Lithuania), 1 μM of each primer, and 1 μL of bisulfite-modified DNA. MSP reaction conditions included 37-38 cycles with primer annealing step at 55-56°C for 45 s. Bisulfite-modified leukocyte DNA from healthy donors served as a negative control for methylated DNA and SssI methyltransferase-treated (Thermo Fisher Scientific, Vilnius, Lithuania) bisulfite-modified leukocyte DNA served as a positive control. Non-template controls were included in each MSP run.
RT-qPCR data processing and statistical analysis
Raw Cq-values were calculated using the Viia7 version 1.1 software (Applied Biosystems), applying automatically selected thresholds. According to NormFinder and GeNorm algorithms, the combination of HPRT1, GUSB, and GAPDH was shown as the most suitable set of reference genes in TLDA analyses, while the HPRT1 and GUSB genes were used for single assays’ data normalization.
Computation of statistical tests was performed using GraphPad Prism version 5.00 for Windows (GraphPad Software, San Diego, CA, USA) and GenEx version 6.0.1 software systems (MultiD Analyses AB, Göteborg, Sweden). Survival analysis was carried out with MedCalc Statistical Software version 14.12.0 (MedCalc Software, Ostend, Belgium). Continuous variables were checked for normal distribution by Shapiro-Wilk statistic and compared by Student’s t-test when normally distributed or by the Mann-Whitney U test for non-normally distributed variables. Spearman’s rank correlation coefficient was calculated to test the associations between two variables with non-normal distribution. Pearson’s Chi-squared test and Fisher’s exact t-test were used for comparison of categorical variables. For survival analysis, Kaplan-Meier curves were compared using Log-rank (Mantel-Cox) test, and Cox proportional-hazards model, applying backward algorithm, was used for multivariate analysis. For regression analysis, gene expression levels were categorized as “high” or “low” if the log-transformed values were above or below the mean value of all samples for a particular gene, respectively. P-value < 0.050 was considered statistically significant.
SUPPLEMENTARY MATERIALS FIGURES AND TABLES
Acknowledgments
Indra Baltrusaityte is kindly acknowledged for her contribution into updating Gleason score values according to ISUP 2014 recommendations.
Abbreviations
- BCR
Biochemical recurrence
- G
Gleason grade group according to ISUP
- ISUP
International Society of Urological Pathology
- MSP
Methylation-specific PCR
- NPT
Non-cancerous prostate tissues
- PCa
Prostate carcinoma
- PSA
Prostate-specific antigen
- pT
Tumor stage
- RP
Radical prostatectomy
- TLDA
TaqMan low density array
Author contributions
RD performed the gene expression experiments, statistical analysis, and drafted the manuscript; KD performed the microarrays experiment, supervised DNA methylation analyses, and drafted the manuscript; AB participated in the sample and clinical data collection; RS analyzed TMPRSS2-ERG status; AS performed DNA methylation analyses; AL and DP performed pathological sample testing; FJ supplied the samples and clinical data, drafted the manuscript; JRL coordinated the sample and data collection, drafted the manuscript; SJ conceived and designed the study, drafted the manuscript.
CONFLICTS OF INTEREST
The authors declare that they have no competing interests.
FUNDING
This research was funded by grant No. LIG-14/2012 from the Research Council of Lithuania.
REFERENCES
- 1.Ferlay J, Soerjomataram I, Ervik M, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:359–86. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 2.Schiffmann J, Wenzel P, Salomon G, Budäus L, Schlomm T, Minner S, Wittmer C, Kraft S, Krech T, Steurer S, Sauter G, Beyer B, Boehm K, et al. Heterogeneity in D’Amico classification-based low-risk prostate cancer: differences in upgrading and upstaging according to active surveillance eligibility. Urol Oncol. 2015;33:13–9. doi: 10.1016/j.urolonc.2015.04.004. [DOI] [PubMed] [Google Scholar]
- 3.Tomlins SA, Rhodes DR, Perner S, Dhanasekaran SM, Mehra R, Sun XW, Varambally S, Cao X, Tchinda J, Kuefer R, Lee C, Montie JE, Shah RB, et al. Recurrent fusion of TMPRSS2 and ETS transcription factor genes in prostate cancer. Science. 2005;310:644–8. doi: 10.1126/science.1117679. [DOI] [PubMed] [Google Scholar]
- 4.Barwick BG, Abramovitz M, Kodani M, Moreno CS, Nam R, Tang W, Bouzyk M, Seth A, Leyland-Jones B. Prostate cancer genes associated with TMPRSS2-ERG gene fusion and prognostic of biochemical recurrence in multiple cohorts. Br J Cancer. 2010;102:570–6. doi: 10.1038/sj.bjc.6605519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Karnes RJ, Cheville JC, Ida CM, Sebo TJ, Nair AA, Tang H, Munz JM, Kosari F, Vasmatzis G. The ability of biomarkers to predict systemic progression in men with high-risk prostate cancer treated surgically is dependent on ERG status. Cancer Res. 2010;70:8994–9002. doi: 10.1158/0008-5472.CAN-10-1358. [DOI] [PubMed] [Google Scholar]
- 6.Sabaliauskaite R, Jarmalaite S, Petroska D, Dasevicius D, Laurinavicius A, Jankevicius F, Lazutka JR. Combined analysis of TMPRSS2-ERG and TERT for improved prognosis of biochemical recurrence in prostate cancer. Genes Chromosomes Cancer. 2012;51:781–91. doi: 10.1002/gcc.21963. [DOI] [PubMed] [Google Scholar]
- 7.Johnson MH, Ross AE, Alshalalfa M, Erho N, Yousefi K, Glavaris S, Fedor H, Han M, Faraj SF, Bezerra SM, Netto G, Partin AW, Trock BJ, et al. SPINK1 defines a molecular subtype of prostate cancer in men with more rapid progression in an at risk, natural history radical prostatectomy cohort. J Urol. 2016;196:1436–44. doi: 10.1016/j.juro.2016.05.092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pan X, Zhang X, Gong J, Tan J, Yin X, Tang Q, Shu K, Shen P, Zeng H, Chen N. The expression profile and prognostic value of SPINK1 in initially diagnosed bone metastatic prostate cancer. Prostate. 2016;76:823–33. doi: 10.1002/pros.23173. [DOI] [PubMed] [Google Scholar]
- 9.Brooks JD, Wei W, Hawley S, Auman H, Newcomb L, Boyer H, Fazli L, Simko J, Hurtado-Coll A, Troyer DA, Carroll PR, Gleave M, Lance R, et al. Evaluation of ERG and SPINK1 by immunohistochemical staining and clinicopathological outcomes in a multi-institutional radical prostatectomy cohort of 1067 patients. PLoS One. 2015;10:e0132343. doi: 10.1371/journal.pone.0132343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huber F, Montani M, Sulser T, Jaggi R, Wild P, Moch H, Gevensleben H, Schmid M, Wyder S, Kristiansen G. Comprehensive validation of published immunohistochemical prognostic biomarkers of prostate cancer - what has gone wrong? A blueprint for the way forward in biomarker studies. Br J Cancer. 2015;112:140–8. doi: 10.1038/bjc.2014.588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Melling N, Thomsen E, Tsourlakis MC, Kluth M, Hube-Magg C, Minner S, Koop C, Graefen M, Heinzer H, Wittmer C, Sauter G, Wilczak W, Huland H, et al. Overexpression of enhancer of zeste homolog 2 (EZH2) characterizes an aggressive subset of prostate cancers and predicts patient prognosis independently from pre- and postoperatively assessed clinicopathological parameters. Carcinogenesis. 2015;36:1333–40. doi: 10.1093/carcin/bgv137. [DOI] [PubMed] [Google Scholar]
- 12.Willard SS, Koochekpour S. Regulators of gene expression as biomarkers for prostate cancer. Am J Cancer Res. 2012;2:620–57. [PMC free article] [PubMed] [Google Scholar]
- 13.Leidinger P, Hart M, Backes C, Rheinheimer S, Keck B, Wullich B, Keller A, Meese E. Differential blood-based diagnosis between benign prostatic hyperplasia and prostate cancer: miRNA as source for biomarkers independent of PSA level, Gleason score, or TNM status. Tumour Biol. 2016;37:10177–85. doi: 10.1007/s13277-016-4883-7. [DOI] [PubMed] [Google Scholar]
- 14.Pedersen MØ, Larsen A, Stoltenberg M, Penkowa M. The role of metallothionein in oncogenesis and cancer prognosis. Prog Histochem Cytochem. 2009;44:29–64. doi: 10.1016/j.proghi.2008.10.001. [DOI] [PubMed] [Google Scholar]
- 15.Henrique R, Jerónimo C, Hoque MO, Nomoto S, Carvalho AL, Costa VL, Oliveira J, Teixeira MR, Lopes C, Sidransky D. MT1G hypermethylation is associated with higher tumor stage in prostate cancer. Cancer Epidemiol Biomarkers Prev. 2005;14:1274–8. doi: 10.1158/1055-9965.EPI-04-0659. [DOI] [PubMed] [Google Scholar]
- 16.Han YC, Zheng ZL, Zuo ZH, Yu YP, Chen R, Tseng GC, Nelson JB, Luo JH. Metallothionein 1 h tumour suppressor activity in prostate cancer is mediated by euchromatin methyltransferase 1. J Pathol. 2013;230:184–93. doi: 10.1002/path.4169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Juang HH, Chung LC, Sung HC, Feng TH, Lee YH, Chang PL, Tsui KH. Metallothionein 3: an androgen-upregulated gene enhances cell invasion and tumorigenesis of prostate carcinoma cells. Prostate. 2013;73:1495–506. doi: 10.1002/pros.22697. [DOI] [PubMed] [Google Scholar]
- 18.Tiwari R, Pandey SK, Goel S, Bhatia V, Shukla S, Jing X, Dhanasekaran SM, Ateeq B. SPINK1 promotes colorectal cancer progression by downregulating metallothioneins expression. Oncogenesis. 2015;4:e162. doi: 10.1038/oncsis.2015.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kuroda K, Aoyama N, Tamura T, Sakashita M, Maekawa S, Inoue T, Wambura C, Shirasaka D, Minami R, Maeda S, Kuroda Y, Kasuga M. Variation in MT expression in early-stage depressed-type and polypoid-type colorectal tumours. Eur J Cancer. 2002;38:1879–87. doi: 10.1016/S0959-8049(02)00233-2. [DOI] [PubMed] [Google Scholar]
- 20.Faller WJ, Rafferty M, Hegarty S, Gremel G, Ryan D, Fraga MF, Esteller M, Dervan PA, Gallagher WM. Metallothionein 1E is methylated in malignant melanoma and increases sensitivity to cisplatin-induced apoptosis. Melanoma Res. 2010;20:392–400. doi: 10.1097/CMR.0b013e32833d32a6. [DOI] [PubMed] [Google Scholar]
- 21.Tse KY, Liu VW, Chan DW, Chiu PM, Tam KF, Chan KK, Liao XY, Cheung AN, Ngan HY. Epigenetic alteration of the metallothionein 1E gene in human endometrial carcinomas. Tumour Biol. 2009;30:93–9. doi: 10.1159/000218032. [DOI] [PubMed] [Google Scholar]
- 22.Yang YA, Yu J. EZH2, an epigenetic driver of prostate cancer. Protein Cell. 2013;4:331–41. doi: 10.1007/s13238-013-2093-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Laitinen S, Martikainen PM, Tolonen T, Isola J, Tammela TL, Visakorpi T. EZH2, Ki-67 and MCM7 are prognostic markers in prostatectomy treated patients. Int J Cancer. 2008;122:595–602. doi: 10.1002/ijc.23145. [DOI] [PubMed] [Google Scholar]
- 24.Epstein JI, Egevad L, Amin MB, Delahunt B, Srigley JR, Humphrey PA, Grading Committee The 2014 International Society of Urological Pathology (ISUP) Consensus Conference on Gleason grading of prostatic carcinoma: definition of grading patterns and proposal for a new grading system. Am J Surg Pathol. 2016;40:244–52. doi: 10.1097/PAS.0000000000000530. [DOI] [PubMed] [Google Scholar]
- 25.Aus G, Abbou CC, Bolla M, Heidenreich A, Schmid HP, van Poppel H, Wolff J, Zattoni F. European Association of Urology. EAU guidelines on prostate cancer. Eur Urol. 2005;48:546–51. doi: 10.1016/j.eururo.2005.06.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.