ABSTRACT
Over the past 10 years there has been an increase in the number of vaccine clinical studies conducted in resource limited countries. These include vaccine trials for diseases such as malaria and dengue fever which are endemic to many low and lower-middle income countries. Concurrent with the increase in the number of trials, has been the increase and improvement in local infrastructure to enable the appropriate conduct and oversight of trials in these settings, including strengthening of local scientific capabilities, ethical and regulatory oversight. While significant advances have been made, there remain gaps to be addressed including strengthening pharmacovigilance in these regions. There are also opportunities to establish novel collaborations to address diseases specific to these populations including strengthening local manufacturers, new ways to engage established large pharmaceutical companies and leveraging established global infrastructure and pathways to develop innovative products beyond vaccines.
KEYWORDS: developing countries, development, regulation, Vaccine trials
Background and introduction
Impact of vaccines on global health
Vaccines are one of the most beneficial and cost-effective disease prevention measures, contributing to long-term health gains in high-income countries and to the decisive eradication of smallpox globally. Vaccination campaigns in developing countries have played an important role in reducing neonatal mortality with immunization preventing about 2.5 million child deaths per year globally.1 There remains a need for new vaccines to address current major public health threats including malaria, dengue, RSV, Ebola, and Zika as well as vaccines for chronic viral infections that may impact cancer such as HPV vaccines including serotypes prevalent in low and lower middle income countries (LMICs).
Globalization of vaccine clinical trials
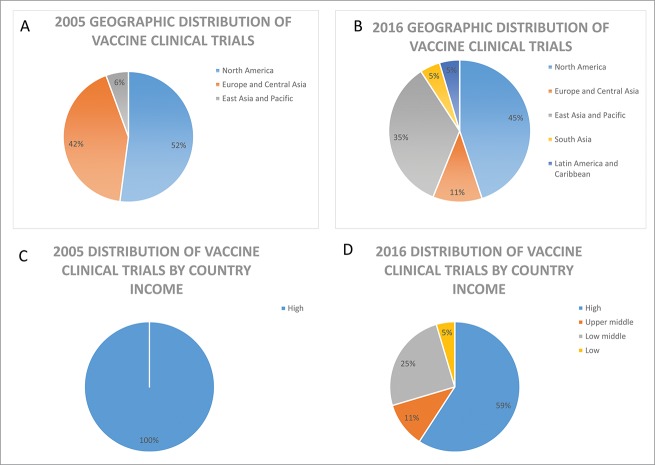
Clinical trials are the cornerstone for the introduction of new medicines; providing the safety and efficacy data to support benefit risk assessments necessary in the evaluation of marketing authorization applications and leading to access to new drugs. For vaccines, clinical trials also provide data to support recommendations for inclusion into national immunization programs. Historically, clinical trials were mostly conducted in North America, Europe and other industrialized regions. However, reports assessing recent trends in global clinical trials show a global expansion in the locations of human clinical trials with the highest growth observed among LMICs.2,3 This trend toward the globalization of clinical trials was also reported for trials supporting marketing applications submitted for review by the European Medicines Agency (EMA) and the United States Food and Drug Administration (US FDA) where an increase in the proportion of clinical data generated in third countries with less clinical trial experience has been observed.4,5 The EMA review of marketing applications from 2005 to 2011 showed a total of 27.8% of patients in pivotal trials from the Rest of World (ROW) region (Africa, Middle East/Asia/Pacific, Australia/New Zealand, Central/South America, Eastern European- non EU) over the review period with several LMICs identified as contributing at least 0.5% of patients. Fig. 1 shows a similar trend toward the globalization of vaccine clinical trials.
Figure 1.
Analysis of top 10 locations for vaccine clinical trials in 2005 and 2016. Geographic locations of vaccine trials are shown in panel A and B and panels C and D show the distribution by country income group classified using the World Bank income group classification (September 2016 update). Numbers on clinical trials are based on data from Citeline Trialtrove for vaccine (infectious disease) trials starting in 2005 or 2016, respectively.
The high burden of disease, increasing costs and complexity of vaccine clinical research and development, as well as the expanding global vaccine market and demand have contributed to the global expansion of vaccine clinical trials into LMICs. Including trial participants from countries with previously under-represented populations, can help to ensure their communities also benefit from advancements in science, contribute to capacity building of local medical infrastructure, and support more timely access to vaccines to manage their health. Clinical trial data should be collected from populations that are representative of those intended to use the vaccine. Therefore the selection of clinical trials sites should also consider potential differences in environmental factors (e.g. nutrition level, prevalence of HIV or other coinfections, local medical practice) across regions where the vaccine is targeted for use in addition to disease prevalence to ensure the resulting safety and efficacy data collected is relevant and applicable to broader populations. Further, to address public health issues endemic to developing countries such as Ebola and malaria, clinical studies must be conducted in these regions where the highest (or only) burden of disease exists.
Historical issues impacting the conduct of vaccine trials in developing countries
All vaccine clinical trials must meet international clinical science, regulatory and ethical standards to ensure the protection of participants and the quality and integrity of the resulting data, regardless of the geographic location of the trial. Historically LMICs have struggled with the need to build adequate local scientific capacity and infrastructure, as well as to provide adequate regulatory and ethical oversight to conduct vaccine trials. Significant progress has been made to strengthen all of these areas in the past decade.
Academic partnerships, product development partnerships, clinical trials networks and investment from national and local governments have contributed to development of scientific capacity and infrastructure to conduct vaccine trials. An example of a product development partnership included non-governmental actors (PATH), a large multi-national pharmaceutical company (GSK), governments and academic institutions with support from the Bill and Melinda Gates Foundation that came together to conduct trials of RTS,S (Mosquirix), GSK's malaria vaccine candidate. This collaboration established a Clinical Trial Partnership Committee that designed and successfully conducted a large randomized, double-blind Phase 3 trial involving 15, 549 infants and young children in 11 clinical trial sites in 7 African countries. The trial was also supported by the Malaria Clinical Trials Alliance, an African-led collaboration that aimed to build capacity for the conduct of clinical trials.8 While the vaccine demonstrated moderate vaccine efficacy, this was a well conducted study that generated data of adequate quality and integrity to support regulatory filings and a positive assessment of the application by the European Medicines Agency (EMA) under Article 58,1 demonstrating that a quality-driven, large multi-center trial could be conducted entirely in low and lower-middle income countries in sub-Saharan Africa.
Partnerships such as that established for RTS,S as well as other regional networks to support specific vaccine clinical trials (e.g., Southeast Asia Influenza Clinical Research Network (SEA ICRN), Meningitis Vaccine Project) and partnerships like the European and Developing Countries Clinical Trials Partnership (EDCTP) established in 2003 have contributed to building sustainable clinical trial infrastructure in developing countries through collaboration and strengthening areas such as Good Clinical Practice (GCP), good laboratory practice (GLP), laboratory infrastructure and training, data management, and epidemiology.9,10 Regulatory oversight, GCP and GLP training, ongoing trial monitoring and the use of data safety monitoring boards help to facilitate data quality produced in a vaccine trial. Ultimately, networks and adherence to the principles of GCP can help sites to address the specific technical and practical issues that can be encountered including recruitment and enrollment, protocol compliance and investigator dependability that will enable them to function on par with sites in more developed settings. Table 1 provides an example of clinical trial partnerships aiming to build sustainable clinical research capacity in developing countries through regional and/or vaccine specific initiatives.
Table 1.
Examples of clinical trial partnerships building research capacity in developing countries.
| Collaboration | Partners | Objectives |
|---|---|---|
| KEMRI Wellcome Trust Research Program (KWTRP), 1989 http://kemri-wellcome.org/about-us/ | •Kenya Medical Research Institute •Wellcome Trust •University of Oxford •National Ministry of Health |
•To conduct research to the highest international scientific and ethical standards on the major causes of morbidity and mortality in the region to provide the evidence base to improve health. •To train an internationally competitive cadre of Kenyan and African research leaders to ensure the long-term development of health research in Africa •Initial work focused on malaria, pneumonia, meningitis, HIV and malnutrition |
| Aeras, 1997 http://www.aeras.org/ Primarily funded by BMGF and European donor governments. Also supported by US government and partnerships with universities and pharmaceutical companies. 2015 Expenses US$44.7M |
Nonprofit biotechnology organization, partners with: individuals, research organizations, academic institutions, funders, policymakers, and others. | •Advance TB vaccine science and development including, •Conduct epidemiological studies •Evaluate TB vaccines from preclinical through post-licensure clinical studies •Improve infrastructure and enhance capacity in high-burden countries for lasting impact •Facilitate community engagement in TB vaccine clinical research |
| European and Developing Countries Clinical Trials Partnership (EDCTP), 2003 http://www.edctp.org/ | Public-public partnership: •countries in Europe •sub-Saharan Africa •European Union |
•Support collaborative research that aims to accelerate the development of new or improved drugs, vaccines, microbicides and diagnostics against HIV/AIDS, tuberculosis and malaria as well as other poverty-related infectious diseases in sub-Saharan Africa •Focus on phase II and III clinical trials. |
| Southeast Asia Influenza Clinical Research Network (SEA ICRN), 2005 | •Hospitals and research institutions in: Thailand, Vietnam, Indonesia | •Conducting protocol-based, multi-institutional studies in accordance with international standards. |
| http://www.seaicrn.org/infobox.aspx?pageID=1 | •National Institute of Allergy and Infectious Diseases (USA) | •Enhancing regional capacities for the conduct of clinical research, particularly local human resources. |
| •Wellcome Trust (UK) | •Promptly disseminating information through presentations at meetings, publications of articles, and advising national authorities. | |
| •Carrying out research that addresses the strategic priorities of partner Governments and others including the Millenium Development Goals. | ||
| Historical Vaccine Specific Collaborations | ||
| Pediatric Dengue Vaccine Initiative, 2001 continued as Dengue Vaccine Initiative, 2011–2016 http://www.denguevaccine.org/ Meningitis Vaccine Project, 2001–2014 http://www.meningvax.org/index.php BMGF awarded PATH US$70 M grant to launch MVP and US$17 M extension grant following development of MenAfriVac® |
Consortium of: •International Vaccine Institute •WHO •International Vaccine Access Center •Sabin Vaccine Institute •PATH •WHO |
•Develop evidence for decision-making regarding the introduction of dengue vaccines •Conduct policy and access-related activities at national, regional and global levels to support vaccine introduction •Enable decision-making for countries interested in early adoption of a dengue vaccine •To develop meningococcal conjugate vaccines that are appropriate for use in Africa. •To monitor the effectiveness and safety of the vaccines in controlled clinical trials. •To create pathways for the licensure of vaccines. •To ensure production in sufficient volume at a price that facilitates wide use in Africa. •To investigate innovative ways to finance the procurement of vaccines through local, country, and other global programs. •To introduce the vaccines through mass and routine immunization in synergy with other public health programs. |
| Malaria Clinical Trials Alliance, 2006 BMGF funded over $6 M from 2006–200833 |
African-led alliance, partnered with •Malaria Vaccine Initiative •Medicines for Malaria Venture |
•To facilitate the development of sufficient near-term capacity in Africa to conduct GCP regulated clinical trials for malaria vaccines and drugs. |
| •To support, strengthen, mentor and network trial sites to facilitate their progression toward self-sustaining clinical research centers. | ||
Perhaps most critical for vaccine clinical trial conduct is strong regulatory and ethical infrastructure, to ensure the safety of research subjects and the scientific integrity of clinical data.11 In many countries the regulatory approval process has been lengthy, ill-defined with significant bureaucracy, and there may be lack of trained staff with the expertise to review Chemistry, Manufacturing and Control (CMC) and preclinical sections of dossiers.11 In the next section of this review the evolving landscape of regulations for vaccine trials is addressed in more detail.
Ethical oversight for a trial should be under a local institutional review board or ethics committee. Historically, there have been weaknesses in review boards in developing countries including lack of procedures for reviewing study protocols and/informed consent forms; lack of trained IRB members; lack of knowledge on the role of the IRB; lack of resources leading to delayed timelines; lack of monitoring systems; lack of independence and lack of archiving systems. In the absence of established ethical oversight infrastructure, national or regional review boards, WHO or commercial review boards could supplement the review and oversight process.
The specific ethical issues encountered that are of particular importance in these settings include obtaining adequate informed consent from trial participants, issues around trial reimbursement as compensation for trial participation as well as trial insurance, issues around standard of care and reasonable availability of future interventions.11-13 Much work has been done to advise on the development of approaches to obtain culturally sensitive informed consent, to ensure reimbursement for trial participation does not serve as an inducement for participation and to ensure patient confidentiality in the process.
Other ethical issues often discussed in the context of vaccine trials in developing countries include standard of care that should be used in research and the reasonable availability of interventions that are proven to be successful in the context of research.13 There should be a firm and mutual understanding of these issues between a sponsor, researchers and host country before undertaking a clinical trial. Emanuel et al have proposed ethical principles and supporting benchmarks for clinical research that can be used as a framework to support the conduct of vaccine trials in developing countries.13
Evolving landscape of vaccine clinical trial regulations in developing countries
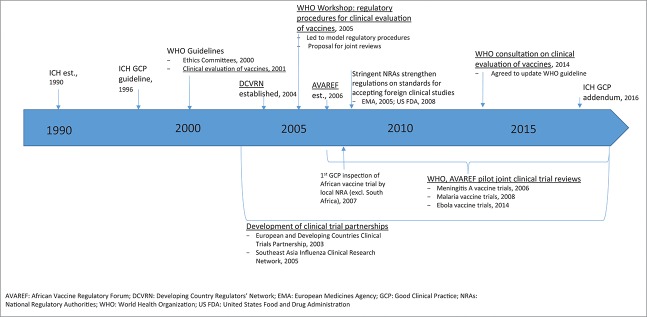
As stated previously, regardless of the geographical region where clinical studies are conducted, clinical trials require regulatory and ethical oversight to ensure the protection of participants as well as to ensure the scientific integrity of the resulting data. Only trials conducted in compliance with international standards such as good clinical practice will be accepted by regulatory authorities in review of future marketing applications. Fig. 2 illustrates key milestones in the development of international guidance to support clinical trial regulation with an emphasis on development of processes to support vaccine clinical trial conduct in developing countries.
Figure 2.
Key milestones in the development of international guidance to support clinical trial regulation with an emphasis on development of processes to support vaccine clinical trial conduct in developing countries.
The first harmonized international guidance on GCP across regions was issued by the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) but was limited in scope to the ICH founding members that included regulatory and industry members in Europe, Japan, and the US. The ICH GCP guideline was adopted in 1996 to provide a unified standard and facilitate the mutual acceptance of data from clinical trials by the regulatory authorities in these jurisdictions (ICH E6, 1996).14 The rationale for convening ICH is similar to that for other global efforts toward harmonization - to address the expansion of drug research and development and marketing across regions as well as concerns on the increasing cost of drug development and the drive to minimize delays in access to new medicines.
Prior to 2005, these clinical trial principles were yet to be fully translated into national laws in developing world countries, and at best, clinical trial applications were limited to ethical review.15 An initial guideline specifically addressing the regulatory expectations for the clinical evaluation of vaccines was issued by WHO and approved by the Expert Committee for Biological Standardization (ECBS) in 2001.16 Further support for strengthening regulatory oversight during the clinical development of vaccines in developing countries came from the establishment of regulator networks in developing countries by WHO. The Developing Countries Vaccine Regulators Network (DCVRN; Table 2) was established in 2004 and was followed by the establishment of the African Vaccine Regulatory Forum (AVAREF) in 2006 to address the growing urgency to conduct vaccine clinical trials to address diseases endemic to Africa. These networks are focused on strengthening the participating National Regulatory Authorities (NRAs), particularly in the area of authorization and evaluation of clinical trials through interactions among the members and Ethics Committees (ECs) as well as information exchange with more experienced regulators.17 Achievements include the joint development of standard operating procedures that facilitated the introduction of standardized GCP inspections by regulators in member countries by the DCVRN,17 development of model procedures to support clinical trial evaluations, and a proposal for joint reviews to aid in capacity building and to optimize resources.18
Table 2.
Vaccine regulatory authorities comprising the Developing Countries Regulators' Network (DCVRN).
| DCVRN Members Representatives from NMRA of: (as of May 2012) |
|---|
| • Brazil |
| • China |
| • Cuba |
| • India |
| • Indonesia |
| • Iran |
| • Republic of Korea |
| • South Africa |
| • Thailand |
Following these efforts, significant progress has been reported in Africa almost 5 y after introduction of WHO initiatives from the 2005 workshop to support NRA oversight of local vaccine clinical trials.15 A few key achievements included training on GCP inspections, with the first GCP inspection of an African vaccine trial to be conducted by a local NRA occurring in 2007, and conduct of joint assessments of vaccine clinical trial applications facilitated by WHO. Further, a recent survey of ethics review systems in the African region also indicates progress in the development of processes to support the increasing conduct of clinical vaccine trials in Africa. While a survey from 2005 revealed that 36% of the respondent countries did not have ECs and 15% indicated that ethical approval of research proposals was not required,19 a subsequent survey reported 90.9% of respondent countries had a national ethics committee (NEC); 79% of which were established by law and noted that more than half of the NECs (54.5%) were formed after the year 2000.20
The continued evolution, growing complexity and globalization of clinical trials has led to recent evaluations of the existing clinical guidelines to ensure these guidelines are still current with the clinical trial landscape. As a result, an addendum to the ICH GCP guideline was issued in November 2016,21 and an update to the 2001 WHO vaccine clinical trial guideline has been adopted to address such issues as the potential role of human challenge studies, adaptive clinical designs, extrapolation of efficacy between geographic/genetically diverse populations, and pharmacovigilance activities.22
Opportunities for the future
Significant progress has been made in vaccine development and the conduct of vaccine clinical trials in the developing world. There are many opportunities for the future as infrastructure continues to develop and unmet medical needs persist. Opportunities exist for novel collaborations and partnerships for research and development; increased transparency and consistency to support efficient regulatory review and licensure and improved pathways for monitoring vaccine safety. The strengthening infrastructure and capacity being developed to support the increasing conduct of vaccine clinical trials in developing countries could also be leveraged to support development of other novel medicinal products such as extended half-life monoclonal antibodies being developed to address relevant medical needs in developing countries. In addition, the progress in building sustainable clinical trial infrastructure and expertise in developing countries to enable the conduct of trials meeting rigorous international standards is likely to lead to a continued trend of an increasing number of patients from developing countries contributing to pivotal trials included in marketing applications for review by developed country regulatory authorities.
Novel collaborations
Novel collaborations and frameworks for vaccine development can be leveraged including partnerships and mechanisms for the public and private sectors to work together to accelerate development and ensure that unmet needs specific to lower and lower-middle income countries are met. One example of a novel approach was that taken for the development of the Rotavac® vaccine in India.23,24 This vaccine was not developed in the traditional manner by a major multinational manufacturer but rather was the work of an international partnership, led by scientists in India. The vaccine was developed in India via a process that began with a key clinical observation, followed by basic virology, immunology and epidemiology research, and progressing through product development to a licensed product. Project financing was buffeted by a public-private partnership between the Indian Government, the local manufacturer Bharat Biotech International, PATH (supported by the Bill & Melinda Gates Foundation), and other academic institutions at different phases of development. Technical support was provided by various members of the team.
This program was undertaken for several reasons including the significant burden of disease in India (about one quarter of the total number of rotavirus deaths worldwide); differences in the disease epidemiology in India from that in high-income countries which required the rotavirus to be delivered on a different vaccine schedule; a key scientific observation that newborn babies in India were becoming infected with an attenuated rotavirus strain in the hospital but not becoming sick; and finally, cost since the available vaccines were highly subsidized by GAVI and many policy makers in low income countries feared that once the subsidies were no longer available, programs would become unsustainable. The Rotavac vaccine was found to be efficacious and well tolerated in infants in India and was subsequently licensed for use.
Other examples of novel partnerships include Aeras Global Tuberculosis Vaccine Foundation, Pediatric Dengue Vaccine Initiative and the RTS,S malaria vaccine partnership that was previously discussed, among others. These partnerships all focus on diseases disproportionately affecting the developing world. Of note, numerous initiatives and efforts can also lead to fragmentation and other nontraditional research –and-development alliances still need to be explored.25 These may include novel ways for pharmaceutical companies to collaborate among themselves, and continued innovation in ways to fund basic research into diseases that may disproportionately impact developing countries. Novel collaborative partnerships could also capitalize on the increasing supply of vaccines from middle-income countries including vaccines manufactured in Brazil, India and China.26
Increased transparency and efficiency
As discussed previously, achieving international standards for clinical trials conduct can be complex and challenging in countries with under resourced regulatory infrastructure and limited formal procedures defining the processes, roles, and responsibilities for clinical trial review and oversight.11,27,28 Recognizing these challenges, clinical research networks and collaborations being facilitated by WHO, are successfully building and strengthening vaccine clinical trial capacity and oversight in developing countries.
Regional collaborative regulatory and ethical review of vaccine clinical trials in developing countries have been piloted in Africa as a method to strengthen regulatory capacity and infrastructure to support robust and efficient reviews. To date, AVAREF with WHO has coordinated several joint reviews of vaccine trial applications to facilitate regulatory capacity building and to enhance the quality of the evaluation for vaccines being developed to address regional public health threats. The process for several of these reviews has been described previously.15 In general, these joint review pilots used model regulatory procedures enabling a single harmonized dossier to be submitted by the sponsor to all participating countries and included a predefined timeline for the procedure. The coordination of the review was noted to increase in complexity and time when the number of countries involved increased. Similar models of collaboration and harmonization of regulatory requirements have been successfully implemented in developed countries to address the globalization of drug development. As one example, the adoption of a common dossier by Europe, Japan and US per the ICH guideline on the electronic common technical document has fostered efficiencies in review and in submission of applications as information is consistently presented and requires minimal modification between national applications.
The continued development of regional and collaborative initiatives in developing countries and defined and transparent harmonized procedures offer advantages to reviewers and applicants by providing clarity regarding regulator expectations and consistency in the presentation of information. Importantly these strategies including collaborative or parallel regulatory and ethics review should also help facilitate efficient and timely quality evaluations to minimize delays to initiating vaccine clinical trials which can also foreseeably translate to accelerating access to vaccines in these regions.
Strengthened pharmacovigilance
Historically, by the time vaccines were introduced in LMICs there was already significant experience understanding their safety profile from countries with more advanced pharmacovigilance systems.29,30 With increased support for new vaccine introduction globally, there is an increased focus on strengthening pharmacovigilance to compensate for the gap on safety intelligence.29,30 There are also new products being developed specifically for use in LMICs such as meningitis A conjugate vaccine, malaria and dengue vaccines and vaccines for specific populations such as pregnant women which will not be able to benefit from safety information garnered elsewhere. To help establish the required systems in all countries WHO developed the Global Vaccine Safety Blueprint, a strategic framework to promote the establishment of effective vaccine pharmacovigilance systems in all countries. Further a Global Vaccine Safety Initiative was launched in early 2012 to implement the Blueprint.29 The Blueprint has 3 main strategic goals: ensuring at least minimum capacity for vaccine safety activities in all countries;31 providing enhanced capacity for specific circumstances; and establishing a global support network to assist national authorities with capacity building and crisis management.29 Another initiative, the Global Alignment of Immunization safety Assessment in pregnancy (GAIA) network has been formed to help establish a global, common understanding of outcomes and approaches to monitoring safety of vaccines used in pregnancy with particular focus on LMICs.32 This initiative is extremely important given the focus on vaccines being developed for use in pregnant women targeting diseases such as RSV and Group B Strep. The anticipated launch of several new vaccines in the coming years will test the impact of these initiatives to improve pharmacovigilance. Further since post-marketing safety initiatives in these settings is underfunded, models for financing will need to include collaboration between governments, donors and industry organizations to effectively remedy the situation.30
Leveraging pathways for novel products
Global pathways and infrastructure developed for traditional vaccines can be used to support the development and widespread accessibility of new products such as extended half-life monoclonal antibodies that can be used to address relevant medical needs in LMICs. For example, WHO can extend its Prequalification Program originally established to ensure the quality of vaccines to ensure the quality of monoclonal antibodies that may be used in passive immunization strategies. The program originally established in 1987, has ensured that vaccines supplied through United Nations procurement agencies are consistently safe and effective under conditions of use in national immunization programs and this standard could be met for other products. The effort has required a major, long-term, commitment on the part of WHO to strengthen national regulatory authorities and, subsequently, undertake site audits of the manufacturing facilities. Over the years, there has also been a dramatic increase in the production of high quality vaccines from emerging market vaccine manufacturers. In 1997, these manufacturers supplied less than 10% of vaccines purchased by UNICEF and by 2012, that proportion rose to approximately 50%.26 Similarly, the Global Alliance for Vaccines and Immunizations reports that, of their 6 suppliers of vaccines in 2001, only one was located in an emerging market country with the remaining ones being from industrialized countries and by 2010, of the 10 manufacturers that supplied vaccines to GAVI, 5 were in emerging market countries.26
Conclusion and recommendation
Millions of people in LMICs are at risk of diseases like malaria, Ebola, HIV/AIDS, tuberculosis and Zika for which vaccines are currently in development to potentially prevent the high morbidity and mortality associated with these pathologies. Although significant efforts and progress have been made to build local scientific capacity and infrastructure and to strengthen regulatory and ethical oversight for vaccine trials in developing countries, innovative approaches such as novel collaborations are needed to incentivize the local and global pharmaceutical industry to invest. Public private partnerships, regional vaccine initiatives, vaccine research networks, support from international health organizations and continued evolution of regulatory infrastructure with an emphasis on pharmacovigilance, is critical for the development of these novel vaccines. The advancements made to support vaccine development including partnerships, financing mechanisms and infrastructure can be leveraged for future novel products that will be widely accessed by populations in LMICs including novel monoclonal antibodies that could potentially be used for passive immunization strategies.
Footnotes
Article 58 of Regulation (EC) No 726/2004 allows the EMA's Committee for Medicinal Products for Human Use (CHMP) to give opinions, in co-operation with the WHO, on medicinal products for human use that are intended exclusively for markets outside of the European Union and address diseases of major public health interest.
Key messages
•From 2005–2016 there has been a change in the geographical distribution of vaccine clinical trials globally with more trials being conducted in low, lower-middle and upper-middle income countries
•There has been a significant improvement in the local scientific capacity, ethical and regulatory oversight to conduct trials in developing countries with several networks and initiatives established to help facilitate these improvements
•Further opportunities exist to strengthen regulatory infrastructure including pharmacovigilance, establish novel collaborations and to establish novel pathways that leverage what has been learned and established for vaccines
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
The authors would like to thank Dr Ibou Thior (PATH), Mary Plank (Astra Zeneca), Therese Takas (MedImmune) and Dr Andreas Seiter (the World Bank) for their review of the paper.
References
- [1].State of the world's vaccines and immunization. Geneva, Switzerland: World Health Organization; 2009 [Google Scholar]
- [2].Drain PK, Robine M, Holmes KK, Bassett IV. Trial watch: Global migration of clinical trials. Nat Rev Drug Discov. 2014; 13:166-7. https://doi.org/ 10.1038/nrd4260. PMID:24577390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Viergever RF, Li K. Trends in global clinical trial registration: An analysis of numbers of registered clinical trials in different parts of the world from 2004 to 2013. BMJ Open. 2015; 5:e008932. https://doi.org/ 10.1136/bmjopen-2015-008932. PMID:26408831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].The Globalization of Clinical Trials: A Growing Challenge in Protecting Human Subjects. Silver Spring, MD: United States Food and Drug Administration; 2001 [PubMed] [Google Scholar]
- [5].Clinical trials submitted in marketing-authorisation applications to the European Medicines Agency. Overview of patient recruitment and the geographical location of investigator sites. Containing data from 2005 to 2011. London, UK: European Medicines Agency; 2013; 39 [Google Scholar]
- [6].Rts SCTP. Efficacy and safety of the RTS,S/AS01 malaria vaccine during 18 months after vaccination: A phase 3 randomized, controlled trial in children and young infants at 11 African sites. PLoS Med. 2014; 11:e1001685. https://doi.org/ 10.1371/journal.pmed.1001685. PMID:25072396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Clemens J, Moorthy V. Implementation of RTS,S/AS01 malaria vaccine–The need for further evidence. N Engl J Med. 2016; 374:2596-7. https://doi.org/ 10.1056/NEJMe1606007. PMID:27355540 [DOI] [PubMed] [Google Scholar]
- [8].Leach A, Vekemans J, Lievens M, Ofori-Anyinam O, Cahill C, Owusu-Agyei S, Abdulla S, Macete E, Njuguna P, Savarese B, et al.. Design of a phase III multicenter trial to evaluate the efficacy of the RTS,S/AS01 malaria vaccine in children across diverse transmission settings in Africa. Malar J 2011; 10:224. https://doi.org/ 10.1186/1475-2875-10-224. PMID:21816029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Higgs ES, Hayden FG, Chotpitayasunondh T, Whitworth J, Farrar J. The Southeast Asian Influenza Clinical Research Network: Development and challenges for a new multilateral research endeavor. Antiviral Res 2008; 78:64-8. https://doi.org/ 10.1016/j.antiviral.2007.10.008. PMID:18295355 [DOI] [PubMed] [Google Scholar]
- [10].Wertheim HF, Puthavathana P, Nghiem NM, van Doorn HR, Nguyen TV, Pham HV, Subekti D, Harun S, Malik S, Robinson J, et al.. Laboratory capacity building in Asia for infectious disease research: Experiences from the South East Asia Infectious Disease Clinical Research Network (SEAICRN). PLoS Med 2010; 7:e1000231. https://doi.org/ 10.1371/journal.pmed.1000231. PMID:20386725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Kochhar S. Challenges and impact of conducting vaccine trials in Asia and Africa: New Technologies in Emerging Markets, October 16th-18th 2012; World Vaccine Congress, Lyon. Hum Vaccin Immunother. 2013; 9:924-7. https://doi.org/ 10.4161/hv.23405. PMID:23321645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Acosta CJ, Galindo CM, Ochiai RL, Danovaro-Holliday MC, Laure-Page A, Thiem VD, Jin Y, Khan MI, Sahito SM, Hamza HB, et al.. Implementation of good clinical practice guidelines in vaccine trials in developing countries. Vaccine. 2007; 25:2852-7. https://doi.org/ 10.1016/j.vaccine.2006.09.079. PMID:17141380 [DOI] [PubMed] [Google Scholar]
- [13].Emanuel EJ, Wendler D, Killen J, Grady C. What makes clinical research in developing countries ethical? The benchmarks of ethical research. J Infect Dis. 2004; 189:930-7. https://doi.org/ 10.1086/381709. PMID:14976611 [DOI] [PubMed] [Google Scholar]
- [14].ICH ICH Harmonised Tripartite Guideline. Guideline for Good Clinical Practice, 1996; 53 https://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Efficacy/E6/E6_R1_Guideline.pdf [PubMed] [Google Scholar]
- [15].Maiga D, Akanmori BD, Chocarro L. Regulatory oversight of clinical trials in Africa: Progress over the past 5 years. Vaccine. 2009; 27:7249-52. https://doi.org/ 10.1016/j.vaccine.2009.08.113. PMID:19748580 [DOI] [PubMed] [Google Scholar]
- [16].WHO WHO Technical Report Series. 2004 http://www.who.int/biologicals/publications/trs/areas/vaccines/clinical_evaluation/035-101.pdf?ua=1) [Google Scholar]
- [17].Nishioka S, Southern J, Dominguez R, Dellepiane N. Helping each other regulate clinical trials: A network of vaccine regulators from developing countries. Clinical Investigation. 2013; 3:113-7. https://doi.org/ 10.4155/cli.12.151 [DOI] [Google Scholar]
- [18].Choccaro L. Workshop on Regulatory Procedures for Clinical Evaluation of Vaccines, Addis Ababa 21–23 September 2005 WHO, 2005:28 http://www.who.int/immunization_standards/vaccine_regulation/meeting_report_addis_sept05.pdf [Google Scholar]
- [19].Kirigia JM, Wambebe C, Baba-Moussa A. Status of national research bioethics committees in the WHO African region. BMC Med Ethics 2005; 6:E10. https://doi.org/ 10.1186/1472-6939-6-10. PMID:16242014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Motari M, Ota MO, Kirigia JM. Readiness of ethics review systems for a changing public health landscape in the WHO African Region. BMC Med Ethics 2015; 16:82. https://doi.org/ 10.1186/s12910-015-0078-9. PMID:26626131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].ICH Integrated Addendum to ICH E6 (R1): Guideline for Good Clinical Practice E6 (R2). 2016:59 http://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Efficacy/E6/E6_R2__Step_4.pdf [Google Scholar]
- [22].World Health Organization WHO guidelines on clinical evaluation of vaccines: Regulatory expectations. WHO technical report series 2017, no. 1004, annex 9 http://www.who.int/biologicals/expert_committee/WHO_TRS_1004_web_Annex_9.pdf?ua=1 [Google Scholar]
- [23].Bhan MK, Glass RI, Ella KM, Bhandari N, Boslego J, Greenberg HB, Mohan K, Curlin G, Rao TS. Team science and the creation of a novel rotavirus vaccine in India: A new framework for vaccine development. Lancet. 2014; 383:2180-3. https://doi.org/ 10.1016/S0140-6736(14)60191-4. PMID:24629993 [DOI] [PubMed] [Google Scholar]
- [24].Bhandari N, Rongsen-Chandola T, Bavdekar A, John J, Antony K, Taneja S, Goyal N, Kawade A, Kang G, Rathore SS, et al.. Efficacy of a monovalent human-bovine (116E) rotavirus vaccine in Indian infants: A randomised, double-blind, placebo-controlled trial. Lancet. 2014; 383:2136-43. https://doi.org/ 10.1016/S0140-6736(13)62630-6. PMID:24629994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Witty A. New strategies for innovation in global health: A pharmaceutical industry perspective. Health Aff (Millwood). 2011; 30:118-26. https://doi.org/ 10.1377/hlthaff.2010.0933. PMID:21209447 [DOI] [PubMed] [Google Scholar]
- [26].Francis DP, Du YP, Precioso AR. Global vaccine supply. The increasing role of manufacturers from middle income countries. Vaccine. 2014; 32:5259-65. https://doi.org/ 10.1016/j.vaccine.2014.07.069. PMID:25110294 [DOI] [PubMed] [Google Scholar]
- [27].Devasenapathy N, Singh K, Prabhakaran D. Conduct of clinical trials in developing countries: A perspective. Curr Opin Cardiol. 2009; 24:295-300. https://doi.org/ 10.1097/HCO.0b013e32832af21b. PMID:19444095 [DOI] [PubMed] [Google Scholar]
- [28].Yusuf S. Clinical research and trials in developing countries. Stat Med. 2002; 21:2859-67. https://doi.org/ 10.1002/sim.1290. PMID:12325102 [DOI] [PubMed] [Google Scholar]
- [29].Amarasinghe A, Black S, Bonhoeffer J, Carvalho SM, Dodoo A, Eskola J, Larson H, Shin S, Olsson S, Balakrishnan MR, et al.. Effective vaccine safety systems in all countries: A challenge for more equitable access to immunization. Vaccine. 2013;31 Suppl 2:B108-14. https://doi.org/ 10.1016/j.vaccine.2012.10.119. PMID:23598471 [DOI] [PubMed] [Google Scholar]
- [30].Olsson S, Pal S, Dodoo A. Pharmacovigilance in resource-limited countries. Expert Rev Clin Pharmacol. 2015; 8:11. https://doi.org/ 10.1586/17512433.2015.1053391. PMID:26041035 [DOI] [PubMed] [Google Scholar]
- [31].World Health Organization Global vaccine safety blueprint vision and strategic goals questions to SAGE. 2011. http://www.who.int/immunization/sage/SAGE_November_2011_Eskola.pdf [Google Scholar]
- [32].WHO Global Advisory Committee on Vaccine Safety, 15–16 June 2016. WHO Weekly Epidemiologocal Record 2016; 91:341-8 [Google Scholar]
- [33].Ogutu BR, Baiden R, Diallo D, Smith PG, Binka FN. Sustainable development of a GCP-compliant clinical trials platform in Africa: The Malaria Clinical Trials Alliance perspective. Malaria Journal 2010; 9:103. [DOI] [PMC free article] [PubMed] [Google Scholar]