Abstract
Objective
To further characterize the association of male infertility with health risks by evaluating semen quality and cancer risk in family members.
Design
Retrospective, cohort study.
Setting
Not applicable.
Patient(s)
A total of 12,889 men undergoing SA and 12,889 fertile control subjects that had first-degree relative (FDR) data (n = 130,689) and 8,032 men with SA and 8,032 fertile control subjects with complete second-degree relative (SDR) data (n = 247,204) were identified through the UPDB. An equal number of fertile population control subjects were matched.
Interventions
None.
Main Outcome Measure(s)
Adult all-site, testicular, thyroid, breast, prostate, melanoma, bladder, ovarian, and kidney cancer diagnoses in FDRs and SDRs.
Result(s)
The FDRs of men with SA had a 52% increased risk of testicular cancer compared with the FDRs of fertile population control subjects. There was no significant difference in testicular cancer risk for the SDRs based on any of the semen parameters. The FDRs and SDRs of azoospermic men had a significantly increased risk of thyroid cancer compared with fertile population control subjects.
Conclusion(s)
These data suggest a link between male infertility and selected cancer risk in relatives. This highlights the possibilities of shared biologic mechanisms between the two diseases, exposure to environmental factors, and an increased level of genetic and/or epigenetic burden in subfertile men and their relatives that may be associated with risk of cancer.
Keywords: Epidemiology, infertility, testicular cancer, semen analysis, andrology
Each year, more than 700,000 men in the United States are estimated to pursue evaluation of malefactor infertility (1). Male subfertility is argued to be a biomarker for overall male somatic health (2, 3). It has been linked to increased risk for testicular, prostate, colon cancer, reduced lifespan, and possibly cardiovascular disease and metabolic syndrome (4–8). Epidemiologic studies have demonstrated the association between infertility and testicular cancer, with the increased risk of developing testicular cancer estimated to be 30%–90% (6, 9). Similarly, infertile men have 2.6 times the risk of high-grade prostate cancer, yet little is known of the cancer risk for an infertile man’s family members (7).
Eisenberg et al. used a medical claims database to compare cancer risk in male infertility patients, men with prior vasectomy, and healthy control subjects and demonstrated an increased cancer risk of genitourinary cancer, particularly testis cancer, in the infertile men (10). However, the study was limited by claims-level analysis and could not examine the association of specific semen quality parameters and cancer risk.
Testis cancer, much like male infertility, may be the result of genetic, epigenetic, and environmental insults. However, unlike male infertility, testis cancer has a well known and documented risk of heritability. If a man has a brother with testicular cancer, his relative risk of testicular cancer is 8–12 times greater than the general population’s, and the relative risk is 2–4 times higher if a man has a father with testicular cancer history (11–13). We hypothesized that the association between infertility and testis cancer might also share a familial component, wherein the genetic risk factors that predispose a man to infertility may also confer additive risk of testicular cancer. Therefore, we sought to determine a familial association between male infertility and cancer risk, with the use of the multigenerational Utah Population Database (UPDB), which is linked to the Utah and Idaho Cancer Registries, as well as the University of Utah UU and Intermountain Health Care (IHC) semen analysis (SA) databases. Our primary objective was to characterize the male infertility phenotype based on cancer risk in the first-degree (FDRs) and second-degree (SDRs) relatives of men who underwent SA. Also, we evaluated if specific semen quality defects were associated with an increased cancer risk in family members.
MATERIALS AND METHODS
Data
This study used the data compiled by the Subfertility Health and Assisted Reproduction (SHARE) study that has been linked to the UPDB. The SHARE database is composed of men who underwent SA at the UU Andrology Clinic from 1996 to 2011 and at IHC from 2002 to 2011. The UPDB is a health data repository that collects and integrates data about residents of Utah, a state in the intermountain west with a population of 2.8 million people. The database includes bio-demographic, health, economic, cancer, and genetic data by linking various sources, including medical records from the two largest health care systems in the state, state driver licenses, and birth, marriage, and death certificate data. It also houses extensive pedigree data from the mid-19th century, which allows researchers to study health outcomes across multiple generations. Many epidemiologic studies have used the complex pedigrees of the UPDB to identify and understand familial diseases (14–17). This integration of data combines biospecimen data with a population resource that contains medical, genealogic, and administrative data to create a unique and comprehensive database for the evaluation of fertility and familial cancer history.
Measures
We evaluated the association of familial cancers and infertility based on the following semen parameters: sperm count (millions [M]), sperm concentration (M/mL), sperm motility (percentage of sperm with forward motility), total motile count (M), sperm head morphology, and vitality. Total motile count, sperm head morphology, and vitality data were available only from the UU database. SAs were performed and processed based on the 2010 World Health Organization guidelines (18). If a man had more than one SA on record, we used the mean value for each semen parameter. A sperm concentration of 0 M/mL was categorized as azoospermia, <15 M/mL as oligozoospermia, 15–178 M/mL as normozoospermia, and >178 M/mL as hyperzoospermia (based on the 90th percentile of data). Total sperm count was categorized as follows: 0 M as azoospermia, <39 M as oligozoospermia, 39–579 M as normozoospermia, and >579 M as hyperzoospermia (based on the 90th percentile). Sperm motility and vitality cut points were made based on quartiles and were as follows: azoospermia, >0–49%, 50%–59%, 60%–69%, and 70%–100%. Total motile count and sperm head morphology were all categorized based on empirically derived quartiles (Q1–Q4).
Cancer diagnoses were obtained from the UPDB with the use of linked data from the Utah and Idaho Cancer Registries and Utah death certificates. The Utah Cancer Registry (UCR) is a National Cancer Institute Surveillance Epidemiology and End Result (SEER) registry and has collected information on all cancer diagnoses from 1966 to 2012 for Utah residents. Before 1966, individuals with cancer were identified with the use of cause of death information listed on the Utah state death certificates, which have been linked to the UPDB from 1905 to the present. This study was approved by the Institutional Review Boards of the UU and IHC by the Utah Resource for Genetic and Epidemiologic Research (www.research.utah.edu/rge/; no. IRB_00069711).
Study Design
We performed a retrospective cohort analysis of cancer risk in FDRs and SDRs of men who underwent SA as part of an infertility work-up at the UU Andrology Clinic from 1996 to 2011, or had SA performed by Intermountain Health Care from 2002 to 2011. Together, these two tertiary medical centers’ andrology labs have captured ~90% of all SAs performed in Utah since 2004.
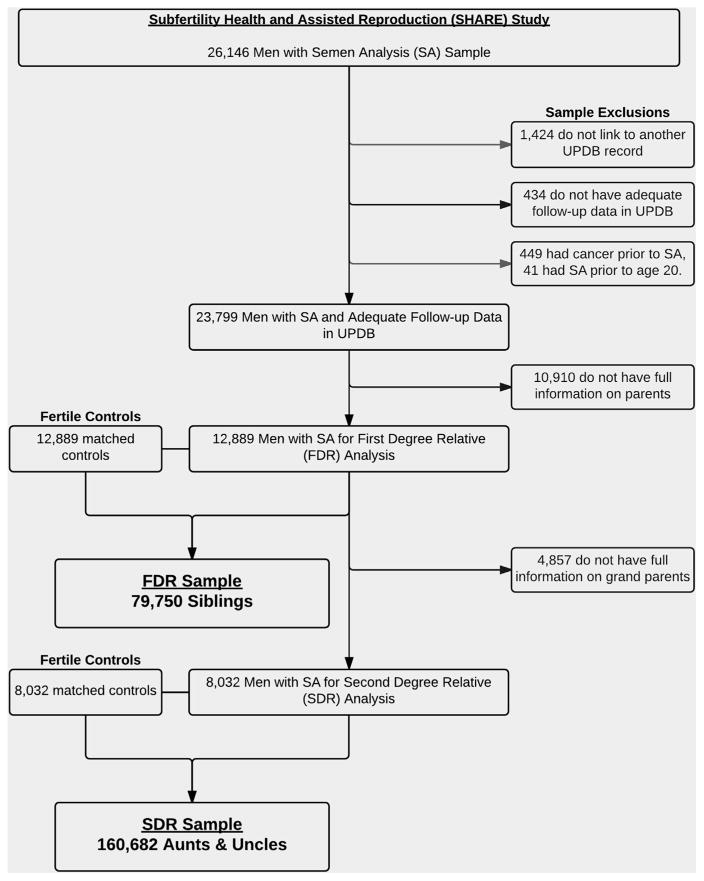
We identified 26,147 men with SAs performed during our study period. This cohort included all men evaluated from these two assisted reproductive technology centers, and therefore both fertile men, infertile men, and men with infertile female partners were included. Men presented for malefactor infertility workup or as part of a couple’s evaluation for infertility. We excluded 1,424 men who did not link to another record in UPDB, 434 with inadequate follow-up, and 449 with cancer before SA. There were 10,910 men without complete pedigree information on their parents, and therefore a total of 12,889 men were available with FDR information. This cohort of men was used to study the cancers diagnosed in FDRs. Another 4,857 men did not have full pedigree information available on their grandparents, which left 8,032 men with complete SDR information available for analysis.
Fertile population control subjects were selected randomly without replacement from the UPDB. Men seen at the IHC or UU clinics were excluded from the pool of potential control subjects. Control subjects were required to be residents of the state of Utah with adequate follow-up data in the UPDB. They were matched by age and birth year at a ratio of 1:1. We used birth certificate data to define fertile as having at least one naturally conceived child. These men did not possess a cancer diagnosis at the time of the matched subfertile man’s SA. A total of 12,889 control subjects were used for the FDR analysis and 8,032 control subjects for the SDR analysis. See Figure 1 for study population inclusion and exclusion criteria as well as final counts of FDRs and SDRs.
FIGURE 1.
Semen analysis cohort inclusion criteria. Sample selection for the men with semen analysis. Matched fertile control subjects were selected for the final samples: 12,889 for the first-degree relative (FDR) analysis and 8,032 for the second-degree relative (SDR) analysis. Total number of FDRs and SDRs for men with semen analysis and their matched control subjects by relationship type. Total numbers of first- and second-degree relatives equal 130,689 and 247,204, respectively.
Relatives were excluded from the analysis if they had incomplete birth and follow-up information in the UPDB, were adopted, did not survive to age 18 years, were born after 1994, or were deceased before 1904 (and therefore did not have death certificate or UCR diagnosis information). FDRs and SDRs of men with SA and their matched control subjects were selected from the UPDB. FDRs included parents, brothers, and sisters, and SDRs were grandparents, aunts, and uncles. With an equal number of control subjects for the FDR and SDR groups, we identified a total of 79,750 siblings, 50,939 parents, 86,522 grandparents, and 160,682 aunts/uncles.
Statistical Methods
Analyses compared cancer risk in relatives of men seen at a fertility clinic and fertile population control subjects, as well as the association between individual semen parameters and cancer in relatives of subfertile men. Cox proportional hazard regression models were used to test the association between semen quality and adult cancer incidence, defined as cancer diagnosis after age 18 years, in FDR and SDR relatives of men with SA and their matched control subjects. The risk in relatives of men who underwent SA compared with relatives of control subjects was determined independently for each relation type (FDR and SDR). To determine the risk of cancer in relatives of men with male-factor infertility and men with normal semen parameters, case-case analyses were also completed in which relatives of men with abnormal semen parameters were compared with relatives of men with normal semen parameters.
All relatives of men who underwent SA and their fertile population control subjects were included in the analyses, even if that relative had been previously counted. For example, for families containing multiple men with semen analyses, each man was included as a separate index case and risk among relatives of each cases was calculated separately, an approach that has been shown to lead to unbiased estimates of risk (19). Huber-White sandwich estimator of variance for clustered data was used to correct for the nonin-dependence of observations within families (20). Relatives of fertile men not presenting in a fertility clinic were used as the reference group.
Analyses were performed for all cancers combined followed by site-specific models for testicular, prostate, melanoma, female breast, ovarian, brain, thyroid, bladder, and kidney cancers. Time to cancer diagnosis was measured as years at risk (age 20 years to age at last follow-up). Death date and last known date residing in Utah were used as the last follow-up date for individuals without a cancer diagnosis. All models controlled for sex, when the cancer was not sex-specific, and birth year.
RESULTS
Our subfertile cohort consisted of 12,889 men with complete FDR data, 8,032 men with complete SDR data, and an equal number of matched control subjects for both. The average age, in years (±SD), at last follow-up was 36.4 ± 10.1 for siblings (n = 79,750), 61.2 ± 11.3 for parents (n = 50,939), 74.4 ± 14.0 for grandparents (n = 86,522), and 57.3 ± 15.2 for aunts/uncles (n = 160,682). The total number of cancers diagnosed in the family members of the subfertile and fertile population control subjects was 20,660 and 23,838, respectively. There were 39,213 siblings in the subfertile group and 40,537 siblings in the control group. The subfertile group of 12,889 men fathered a total of 19,956 children, compared with the control subjects who fathered 33,476 children. There were no differences in general cohort demographics and total counts of the most common and genitourinary cancers diagnosed (Table 1).
TABLE 1.
Number of Cancer Diagnoses by Site and Relation.
| Cancer | Total, n | Men with semen analysis | Fertile | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Parent (n = 25,163) | Sibling (n = 39,213) | Grandparent (n = 37,811) | Aunt/uncle (n = 71,144) | Total (n = 173,331) | Parent (n = 25,776) | Sibling (n = 40,537) | Grandparent (n = 48,708) | Aunt/uncle (n = 89,538) | Total (n = 204,559) | ||||||||||||
| n | % | n | % | n | % | n | % | n | % | n | % | n | % | n | % | n | % | n | % | ||
| Any | 44,498 | 3,426 | 13.6 | 680 | 1.7 | 9,332 | 24.7 | 7,222 | 10.2 | 20,660 | 11.9 | 3,389 | 13.1 | 778 | 1.9 | 11,112 | 22.8 | 8,559 | 9.6 | 23,838 | 11.7 |
| Testicular | 358 | 42 | 0.2 | 39 | 0.1 | 33 | 0.1 | 75 | 0.1 | 189 | 0.1 | 18 | 0.1 | 31 | 0.1 | 29 | 0.1 | 91 | 0.1 | 169 | 0.1 |
| Prostate | 9,547 | 801 | 3.2 | 39 | 0.1 | 2,254 | 6.0 | 1,477 | 2.1 | 4,571 | 2.6 | 747 | 2.9 | 46 | 0.1 | 2,509 | 5.2 | 1,674 | 1.9 | 4,976 | 2.4 |
| Breast | 7,108 | 570 | 2.3 | 121 | 0.3 | 1,427 | 3.8 | 1,258 | 1.8 | 3,376 | 1.9 | 535 | 2.1 | 112 | 0.3 | 1,679 | 3.4 | 1,406 | 1.6 | 3,732 | 1.8 |
| Ovarian | 862 | 37 | 0.1 | 14 | 0.0 | 214 | 0.6 | 140 | 0.2 | 405 | 0.2 | 46 | 0.2 | 12 | 0.0 | 216 | 0.4 | 183 | 0.2 | 457 | 0.2 |
| Melanoma | 3,441 | 378 | 1.5 | 104 | 0.3 | 523 | 1.4 | 655 | 0.9 | 1,660 | 1.0 | 315 | 1.2 | 131 | 0.3 | 566 | 1.2 | 739 | 0.8 | 1,751 | 0.9 |
| Brain | 2,962 | 219 | 0.9 | 38 | 0.1 | 783 | 2.1 | 536 | 0.8 | 1,576 | 0.9 | 231 | 0.9 | 51 | 0.1 | 639 | 1.3 | 465 | 0.5 | 1,386 | 0.7 |
| Kidney | 922 | 82 | 0.3 | 4 | 0.0 | 193 | 0.5 | 157 | 0.2 | 436 | 0.3 | 72 | 0.3 | 14 | 0.0 | 217 | 0.4 | 183 | 0.2 | 486 | 0.2 |
| Bladder | 1,337 | 83 | 0.3 | 5 | 0.0 | 356 | 0.9 | 168 | 0.2 | 612 | 0.4 | 84 | 0.3 | 4 | 0.0 | 430 | 0.9 | 207 | 0.2 | 725 | 0.4 |
| Thyroid | 1,059 | 117 | 0.5 | 68 | 0.2 | 93 | 0.2 | 227 | 0.3 | 505 | 0.3 | 106 | 0.4 | 78 | 0.2 | 122 | 0.3 | 248 | 0.3 | 554 | 0.3 |
Any-site Cancer
There was no difference in any-site cancer risk in the FDRs (hazard ratio [HR] 0.98, 95% confidence interval [CI] 0.93–1.02) or the SDRs (HR 0.98, 95% CI 0.96–1.01) of men who underwent SA compared with the relatives of fertile population control subjects. Similar results were seen when the men who underwent SA were further categorized by semen parameters. See Supplemental Tables 1–3 (available online at www.fert-stert.org) for complete FDR and SDR cancer risk for each semen parameter, reported for all cancer subtypes examined.
Testicular Cancer
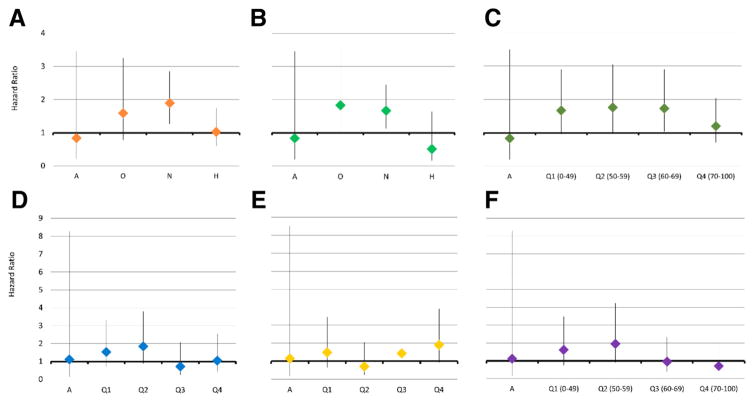
The FDRs of men who underwent SA had a 52% increased risk of testicular cancer compared with FDRs of the fertile population control subjects (HR 1.52, 95% CI 1.05–2.22). For individual semen parameters, we found that both normozoospermic count (HR 1.67, 95% CI 1.13–2.45) and normozoospermic concentration (HR 1.90, 95% CI 1.26–2.85) were associated with increased risk of testicular cancer. There was no significant difference in testicular cancer risk for the SDRs for any of the semen parameters. Azoospermia was not associated with an increased risk of testicular cancer in the FDRs or SDRs compared with the relatives of fertile population control subjects (Fig. 2). FDRs of both the hyperzoospermic men and the fertile population control subjects had a decreased risk of testicular cancer (HR 0.54, 95% CI 0.32–0.91; HR 0.53, 95% CI 0.35–0.79; respectively). See Supplemental Table 1 for each semen parameter in our risk modeling.
FIGURE 2.
Risk of testicular cancer for first-degree relatives of men with semen analysis compared with first-degree relatives (FDRs) of fertile control subjects. (A) Concentration (model 2; a = azoospermia; o = oligozoospermia; n = normozoospermia; h = hyperzoospermia). (B) Sperm count (model 3). (C) Motility (model 4; a = azoospermia; q1 = 1st quartile, 0%–49%; q2 = 2nd quartile, 50%–59%; q3 = 3rd quartile, 60%–69%; q4 = 4th quartile, 70%–100%). (D) Total motile count (model 8). (E) Morphology (model 6). (F) Vitality (model 5; a = azoospermia; q1 =1st quartile, 0%–49%; q2 = 2nd quartile, 50%–59%; q3 = 3rd quartile, 60%–69%; q4 = 4th quartile, 70%–100%).
Prostate Cancer
FDRs and SDRs of men who underwent SA did not have an increased risk of prostate cancer compared with fertile population control subjects (HR 1.00, 95% CI 0.91–1.11; HR 1.03, 95% CI 0.98–1.09; respectively). Only one of the semen parameters met statistical significance for increased risk of prostate cancer; the SDRs of the highest quartile of total motile count displayed a 13% increase risk of prostate cancer compared with control subjects (Supplemental Table 2).
Breast Cancer
We did not find an overall increased risk of breast cancer in the female FDRs or SDRs of men who underwent SA compared with fertile population control subjects (HR 1.08, 95% CI 0.97–1.20; HR 1.04, 95% CI 0.98–1.10; respectively). Relative to the female FDRs of fertile control subjects, female FDRs of hyperzoospermic men had an increased risk of breast cancer (HR 1.26, 95% CI 1.00–1.57). The same was true for female FDRs of men in the highest quartile of total motile count (HR 1.32, 95% CI 1.07–1.63). Again, this was not consistent with any of the other semen parameters, nor was there any linear trend of significance, and therefore is likely not clinically significant (Supplemental Table 2).
Thyroid Cancer
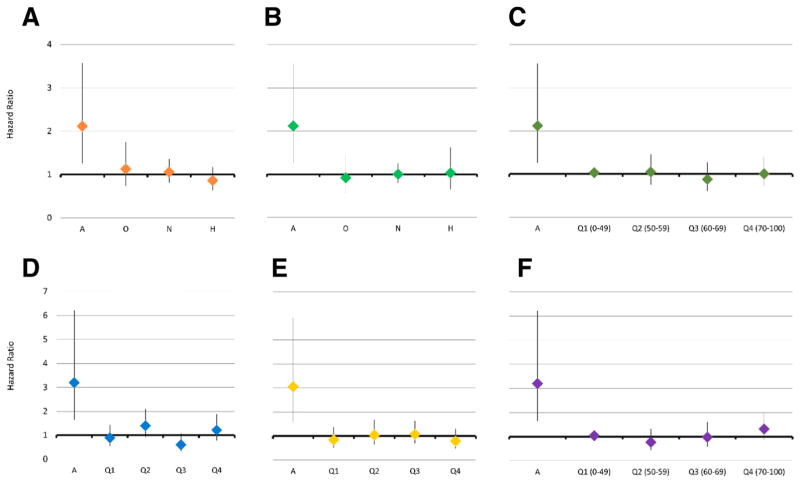
Overall, there was no significant difference in the risk of thyroid cancer for FDRs or SDRs of men who underwent SA compared with relatives of fertile population control subjects (HR 1.04, 95% CI 0.84–1.29; HR 1.01, 95% CI 0.85–1.18; respectively). When we examined each semen parameter individually we found a consistent increased risk for the relatives of azoospermic men. The FDRs of azoospermic men had a twofold increased risk of being diagnosed with thyroid cancer compared with FDRs of fertile population control subjects (HR 2.12, 95% CI 1.26–3.57; Fig. 3). A similar increased risk was seen for SDRs of azoospermic men (HR 1.57, 95% CI 1.03–2.39) compared with SDRs of fertile population control subjects. In the case-case analysis, we found that compared with FDRs of normozoospermic men, FDRs and SDRs of azoospermic men had a similar increased risk of thyroid cancer (HR 2.01, 95% CI 1.18–3.43; HR 1.62, 95% CI 1.06–2.45; respectively; Supplemental Table 1).
FIGURE 3.
Risk of thyroid cancer in first-degree relatives (FDRs) of men with semen analysis compared with FDRs of fertile control subjects. (A) Concentration (model 2; a = azoospermia; o = oligozoospermia; n = normozoospermia; h = hyperzoospermia). (B) Sperm count (model 3). (C) Motility (model 4; a = azoospermia; q1 = 1st quartile, 0%–49%; q2 = 2nd quartile, 50%–59%; q3 = 3rd quartile, 60%–69%; q4 = 4th quartile, 70%–100%). (D) Total motile count (model 8). (E) Morphology (model 6). (F) Vitality (model 5; a = azoospermia; q1 = 1st quartile, 0%–49%; q2 = 2nd quartile, 50%–59%; q3 = 3rd quartile, 60%–69%; q4 = 4th quartile, 70%–100%).
Melanoma
There was no overall difference in the risk of melanoma for FDRs or SDRs of men who underwent SA compared with relatives of fertile control subjects (HR 1.07, 95% CI 0.93–1.22; HR 1.09, 95% CI 1.00–1.19; respectively). We found an increased risk of melanoma in SDR of men with normozoospermic count and normozoospermic concentration (HR 1.13, 95% CI 1.02–1.25; HR 1.12, 95% CI 1.02–1.22; Supplemental Table 2).
Bladder, Renal, and Ovarian Cancers
There was no significant difference in the risk of bladder, renal, and ovarian cancers seen in the relatives of men who underwent SA compared with fertile population control subjects (Supplemental Table 3).
DISCUSSION
In this retrospective cohort study, we examined the familial cancer risk for men who underwent SA compared with age-matched fertile population control subjects with the use of links between two large SA databases and the UPDB. We found a significantly increased risk of thyroid cancer in relatives of azoospermic men, an increased risk of testicular cancer in relatives of normozoospermic men, and no increase of prostate cancer for relatives of men undergoing a SA compared with fertile control subjects.
Male infertility is a heterogeneous set of diseases that ultimately leads to the inability to naturally conceive children, and the etiology of more than one-half of male infertility cases that complete a formal infertility work-up will remain unknown (21). Known monogenetic causes of infertility contribute to <30% of the diagnoses of male infertility, and >1,500 genes are thought to contribute to spermatogenesis alone (22, 23). As we unravel more of the genetic mechanisms of cancer and infertility, common pathways are emerging. Recombination abnormalities that lead to aneuploidy, microsatellite instability, genetic polymorphisms in mismatch repair genes, and germline mutations are associated with both infertility and cancer (24–27). Evidence from rodent models demonstrates that deletions of meiotic regulators in the DNA-mismatch repair proteins leads to infertility and tumorigenesis, and these same deletions have been identified in some infertile men (28). Additionally, epigenetic factors may contribute to genitourinary cancers as well as to normal sperm function (29). We sought to determine if the genetic and epigenetic factors that predispose men to infertility and cancer would also predispose their relatives to cancer.
We found the FDRs of normozoospermic men had an increased risk of testicular cancer. In addition, we found that azoospermia was not associated with an increased risk of testicular cancer in either FDRs or SDRs compared with the relatives of fertile population control subjects. This may indicate that the molecular pathways involved in spermatogenic arrest are distinct from those involved in testicular cancers. However, subfertility (as indicated by undergoing SA despite having a normozoospermic SA) does appear to be associated with increased familial risk of testis cancer. We hypothesize that this may be due to subtle genetic and epigenetic disturbances that affect sperm and somatic cell DNA fidelity and lead to familial risk of infertility and cancer. It is unclear why FDRs of azoospermic men do not demonstrate this elevated cancer risk, but it may be that the germline DNA in these families have severe mutations that lead to spermatogenic arrest and do not elevate cancer risk, because they result in severe mutations that stop cell division.
Thyroid cancer risk for the FDRs of azoospermic men was two times greater than for fertile population control subjects. This risk is also elevated when we used the normozoospermic men as the comparison group, suggesting that it is not likely due to our sample selection. This was the strongest association we found for any of the cancers investigated, and it was consistent for both FDRs and SDRs. Interestingly, there was no increased risk of cancer for any of the other semen parameter groups; only relatives of azoospermic men demonstrated the association with increased thyroid cancer risk. These data are similar to those of Eisenberg et al., who reported a 30%–90% increased risk for thyroid cancer in their cohort of infertile men from U.S. claims data (10), with the use of vasectomy procedure codes as a surrogate for assumed fertility in their control subjects. This highlights another strength of the UPDB, which is the ability to identify our control subjects such that we know that they have each naturally conceived at least one child.
We examined the risk of genitourinary and gynecologic cancers because previous studies demonstrated associations of these cancers in infertile men and women. We did not see any difference in the familial risk of ovarian, bladder, or kidney cancers for men who underwent SA compared with their fertile counterparts.
Prostate cancer risk for the relatives of men who underwent SA compared with fertile population control subjects was not significantly different. This finding was also contrary to what we expected given that earlier studies show an increased risk of prostate cancer in infertile men (6, 8). Unfortunately, our cohort of brothers and fathers of the young men undergoing SA has not reached the average age of prostate cancer diagnosis (the average age at last follow-up was 36.4 years for brothers and 61.2 years for fathers). Likewise, we did not identify a linear relationship between the semen parameters and risk of prostate cancer. Additionally, we did not find an association between prostate cancer risk in the grandfathers of men who underwent SA compared with fertile population control subjects. SDRs, but not FDRs, of men with the highest quartile of total motile count demonstrated a 13% increased risk of prostate cancer. It is unclear why this was only seen in SDRs, but perhaps this was due to the young age of the FDRs compared with the SDRs. Thus, a signal could develop as the cohort ages.
We hypothesize that a common genetic insult is shared in the pathways that lead to both thyroid cancer and complete arrest of spermatogenesis. For example, mutations in thyroid receptors are associated with increased risk of papillary thyroid cancer, and aberrant alpha subunits have displayed an infertile phenotype (30, 31). We do not have the ability to investigate if the thyroid cancers in our database were papillary subtype, and it is unclear if the infertile phenotype identified with some alpha-receptor subtype mutations resulted in azoospermia. Previous epidemiologic studies have demonstrated a slightly increased cancer incidence and mortality for men with Klinefelter syndrome, including breast and mediastinal tumors (32, 33). Biologic mechanisms such as these can be the focus of future research, given our large biobank of specimens from the men who underwent SA.
Our study assembled a unique fertile population control group of men and their associated relatives to attempt to minimize the inherent shortcomings of this, and any other, retrospective cohort study design. Another limitation of this paper is that we did not have medical comorbidity indexes or smoking status for either the men who underwent SA in our original cohort or the relatives in our comparison groups. However, our cohort is predominantly represented by members of the Latter Day Saints, who do not usually smoke or drink. This study involved only Utah residents, and this state has less ethnic and racial diversity compared with other regions. Another limitation is that we do not know the proportion of female partners with diagnosed infertility. We chose not to subcategorize each infertile man by infertile diagnosis, because our goal was to understand how semen parameters influenced familial cancer incidence. Therefore, we did not report the number of azoospermic men with Klinefelter syndrome, and this is a possible limitation. There may be a selection bias based on socioeconomic status because this cohort of men also had the means to be evaluated as an individual or couple seeking infertility evaluation. Another limitation is that we did not have SA data on our fertile control subjects.
The novel aspects of the study supersede these limitations for three distinct reasons. First, the UPDB’s extensive familial linkages allowed reporting of cancer risk not only for men with SA data, but also for multiple generations of family members. Second, we are the first to report specific HRs for the cancer risk of relatives based on six common semen parameters. Finally, we used known fertile age-matched population control subjects from the same state. These findings have important implications for population health and would be difficult to replicate in almost any other database in North America owing to the lack of pedigree data.
CONCLUSION
The FDRs of men who underwent SA had an increased risk of testicular cancer compared with the relatives of fertile age-matched control subjects. Azoospermia was associated with an increased risk of thyroid cancer in relatives of men who underwent went SA, but there was no other association we identified with increased risk of cancer for relatives of these azoospermic men. Second-degree relatives of men in the highest quartile of total motile count demonstrated an increased risk of prostate cancer.
Supplementary Material
Acknowledgments
Supported by the National Institutes of Health–National Institute of Aging (grant 2R01 AG022095). Database support provided to the Pedigree and Population Resource of the Huntsman Cancer Institute (HCI), University of Utah by the Huntsman Cancer Foundation. Partial support for all datasets within the UPDB was provided by the HCI Cancer Center Support Grant P30 CA42014 from the National Cancer Institute.
The authors thank Alison Fraser, Justin Berger, Diana Phuong Thai, and Diana Lane Reed for valuable assistance in managing data.
Footnotes
Discuss: You can discuss this article with its authors and with other ASRM members at http://fertstertforum.com/andersonr-semen-quality-familial-cancer-risk/
R.E.A. has nothing to disclose. H.A.H. has nothing to disclose. D.P.P. has nothing to disclose. E.J. has nothing to disclose. K.I.A. has nothing to disclose. D.T.C. has nothing to disclose. W.T.L. has nothing to disclose. K.R.S. has nothing to disclose. J.M.H. has nothing to disclose.
References
- 1.Anderson JE, Farr SL, Jamieson DJ, Warner L, Macaluso M. Infertility services reported by men in the United States: national survey data. Fertil Steril. 2009;91:2466–70. doi: 10.1016/j.fertnstert.2008.03.022. [DOI] [PubMed] [Google Scholar]
- 2.Eisenberg ML. Parenthood, host resistance to the common cold, and impaired fertility. Psychosom Med. 2012;74:988. doi: 10.1097/PSY.0b013e318273880f. [DOI] [PubMed] [Google Scholar]
- 3.Eisenberg ML, Park Y, Hollenbeck AR, Lipshultz LI, Schatzkin A, Pletcher MJ. Fatherhood and the risk of cardiovascular mortality in the NIH-AARP Diet and Health Study. Hum Reprod. 2011;26:3479–85. doi: 10.1093/humrep/der305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Walsh TJ, Pera RR, Turek PJ. The genetics of male infertility. Semin Reprod Med. 2009;27:124–36. doi: 10.1055/s-0029-1202301. [DOI] [PubMed] [Google Scholar]
- 5.Salonia A, Matloob R, Gallina A, Abdollah F, Saccá A, Briganti A, et al. Are infertile men less healthy than fertile men? Results of a prospective case-control survey. Eur Urol. 2009;56:1025–32. doi: 10.1016/j.eururo.2009.03.001. [DOI] [PubMed] [Google Scholar]
- 6.Walsh TJ, Croughan MS, Schembri M, Chan JM, Turek PJ. Increased risk of testicular germ cell cancer among infertile men. Arch Intern Med. 2009;169:351–6. doi: 10.1001/archinternmed.2008.562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Walsh TJ, Schembri M, Turek PJ, Chan JM, Carroll PR, Smith JF, et al. Increased risk of high-grade prostate cancer among infertile men. Cancer. 2010;116:2140–7. doi: 10.1002/cncr.25075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eisenberg ML, Betts P, Herder D, Lamb DJ, Lipshultz LI. Increased risk of cancer among azoospermic men. Fertil Steril. 2013;100:681–5. doi: 10.1016/j.fertnstert.2013.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jacobsen R, Bostofte E, Engholm G, Hansen J, Olsen JH, Skakkebaek NE, et al. Risk of testicular cancer in men with abnormal semen characteristics: cohort study. BMJ. 2000;321:789–92. doi: 10.1136/bmj.321.7264.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eisenberg ML, Li S, Brooks JD, Cullen MR, Baker LC. Increased risk of cancer in infertile men: analysis of US claims data. J Urol. 2015;193:1596–601. doi: 10.1016/j.juro.2014.11.080. [DOI] [PubMed] [Google Scholar]
- 11.Westergaard T, Olsen JH, Frisch M, Kroman N, Nielsen JW, Melbye M. Cancer risk in fathers and brothers of testicular cancer patients in Denmark. A population-based study. Int J Cancer. 1996;66:627–31. doi: 10.1002/(SICI)1097-0215(19960529)66:5<627::AID-IJC8>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 12.Sonneveld DJ, Sleijfer DT, Schraffordt Koops H, Sijmons RH, van der Graaf WT, Sluiter WJ, et al. Familial testicular cancer in a single-centre population. Eur J Cancer. 1999;35:1368–73. doi: 10.1016/s0959-8049(99)00140-9. [DOI] [PubMed] [Google Scholar]
- 13.Hemminki K, Chen B. Familial risks for cervical tumors in full and half siblings: etiologic apportioning. Cancer Epidemiol Biomarkers Prevent. 2006;15:1413–4. doi: 10.1158/1055-9965.EPI-05-0933. [DOI] [PubMed] [Google Scholar]
- 14.Kerber RA, O’Brien E. A cohort study of cancer risk in relation to family histories of cancer in the Utah population database. Cancer. 2005;103:1906–15. doi: 10.1002/cncr.20989. [DOI] [PubMed] [Google Scholar]
- 15.Oakley GM, Curtin K, Layfield L, Jarboe E, Buchmann LO, Hunt JP. Increased melanoma risk in individuals with papillary thyroid carcinoma. JAMA Otolaryngol Head Neck Surg. 2014;140:423–7. doi: 10.1001/jamaoto.2014.78. [DOI] [PubMed] [Google Scholar]
- 16.Gibson SB, Figueroa KP, Bromberg MB, Pulst SM, Cannon-Albright L. Familial clustering of ALS in a population-based resource. Neurology. 2014;82:17–22. doi: 10.1212/01.wnl.0000438219.39061.da. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tuohy TM, Rowe KG, Mineau GP, Pimentel R, Burt RW, Samadder NJ. Risk of colorectal cancer and adenomas in the families of patients with adenomas: a population-based study in Utah. Cancer. 2014;120:35–42. doi: 10.1002/cncr.28227. [DOI] [PubMed] [Google Scholar]
- 18.World Health Organization. WHO laboratory manual for the examination and processing of human semen. 5. Geneva: World Health Organization; 2010. [Google Scholar]
- 19.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 20.Jorde LB. Inbreeding in the Utah Mormons: an evaluation of estimates based on pedigrees, isonymy, and migration matrices. Ann Hum Genet. 1989;53:339–55. doi: 10.1111/j.1469-1809.1989.tb01803.x. [DOI] [PubMed] [Google Scholar]
- 21.Visser L, Repping S. Unravelling the genetics of spermatogenic failure. Reproduction. 2010;139:303–7. doi: 10.1530/REP-09-0229. [DOI] [PubMed] [Google Scholar]
- 22.Schultz N, Hamra FK, Garbers DL. A multitude of genes expressed solely in meiotic or postmeiotic spermatogenic cells offers a myriad of contraceptive targets. Proc Natl Acad Sci U S A. 2003;100:12201–6. doi: 10.1073/pnas.1635054100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Aston KI, Conrad DF. A review of genome-wide approaches to study the genetic basis for spermatogenic defects. Spermatogenesis. 2012;927:397–410. doi: 10.1007/978-1-62703-038-0_34. [DOI] [PubMed] [Google Scholar]
- 24.Gonsalves J, Sun F, Schlegel PN, Turek PJ, Hopps CV, Greene C, et al. Defective recombination in infertile men. Hum Mol Genetics. 2004;13:2875–83. doi: 10.1093/hmg/ddh302. [DOI] [PubMed] [Google Scholar]
- 25.Maduro MR, Casella R, Kim E, Levy N, Niederberger C, Lipshultz LI, et al. Microsatellite instability and defects in mismatch repair proteins: a new aetiology for Sertoli cell–only syndrome. Mol Hum Reprod. 2003;9:61–8. doi: 10.1093/molehr/gag013. [DOI] [PubMed] [Google Scholar]
- 26.Bonadona V, Bonaïti B, Olschwang S, Grandjouan S, Huiart L, Longy M, et al. Cancer risks associated with germline mutations in MLH1, MSH2, and MSH6 genes in Lynch syndrome. JAMA. 2011;305:2304–10. doi: 10.1001/jama.2011.743. [DOI] [PubMed] [Google Scholar]
- 27.Ji G, Long Y, Zhou Y, Huang C, Gu A, Wang X. Common variants in mismatch repair genes associated with increased risk of sperm DNA damage and male infertility. BMC Med. 2012;10:49. doi: 10.1186/1741-7015-10-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mukherjee S, Ridgeway AD, Lamb DJ. DNA mismatch repair and infertility. Curr Opin Urol. 2010;20:525–32. doi: 10.1097/MOU.0b013e32833f1c21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Qiao D, Zeeman AM, Deng W, Looijenga LH, Lin H. Molecular characterization of hiwi, a human member of the piwi gene family whose overexpression is correlated to seminomas. Oncogene. 2002;21:3988–99. doi: 10.1038/sj.onc.1205505. [DOI] [PubMed] [Google Scholar]
- 30.Puzianowska-Kuznicka M, Krystyniak A, Madej A, Cheng SY, Nauman J. Functionally impaired TR mutants are present in thyroid papillary cancer. J Clin Endocrinol Metab. 2002;87:1120–8. doi: 10.1210/jcem.87.3.8296. [DOI] [PubMed] [Google Scholar]
- 31.Fozzatti L, Kim DW, Park JW, Willingham MC, Hollenberg AN, Cheng SY. Nuclear receptor corepressor (NCOR1) regulates in vivo actions of a mutated thyroid hormone receptor α. Proc Natl Acad Sci U S A. 2013;110:7850–5. doi: 10.1073/pnas.1222334110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Swerdlow AJ, Higgins CD, Schoemaker MJ, Wright AF, Jacobs PA. Mortality in patients with Klinefelter syndrome in Britain: a cohort study. J Clin Endocrinol Metab. 2005;90:6516–22. doi: 10.1210/jc.2005-1077. [DOI] [PubMed] [Google Scholar]
- 33.Hasle H, Mellemgaard A, Nielsen J, Hansen J. Cancer incidence in men with Klinefelter syndrome. Br J Cancer. 1995;71:416–20. doi: 10.1038/bjc.1995.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.