Abstract
The aim of the study was to draw a comparison between the characteristics of infective endocarditis (IE) in patients with cancer and those of IE in noncancer patients.
Patients with IE, according to the modified Duke criteria, were prospectively included in the GAMES registry between January 2008 and February 2014 in 30 hospitals. Patients with active cancer were compared with noncancer patients.
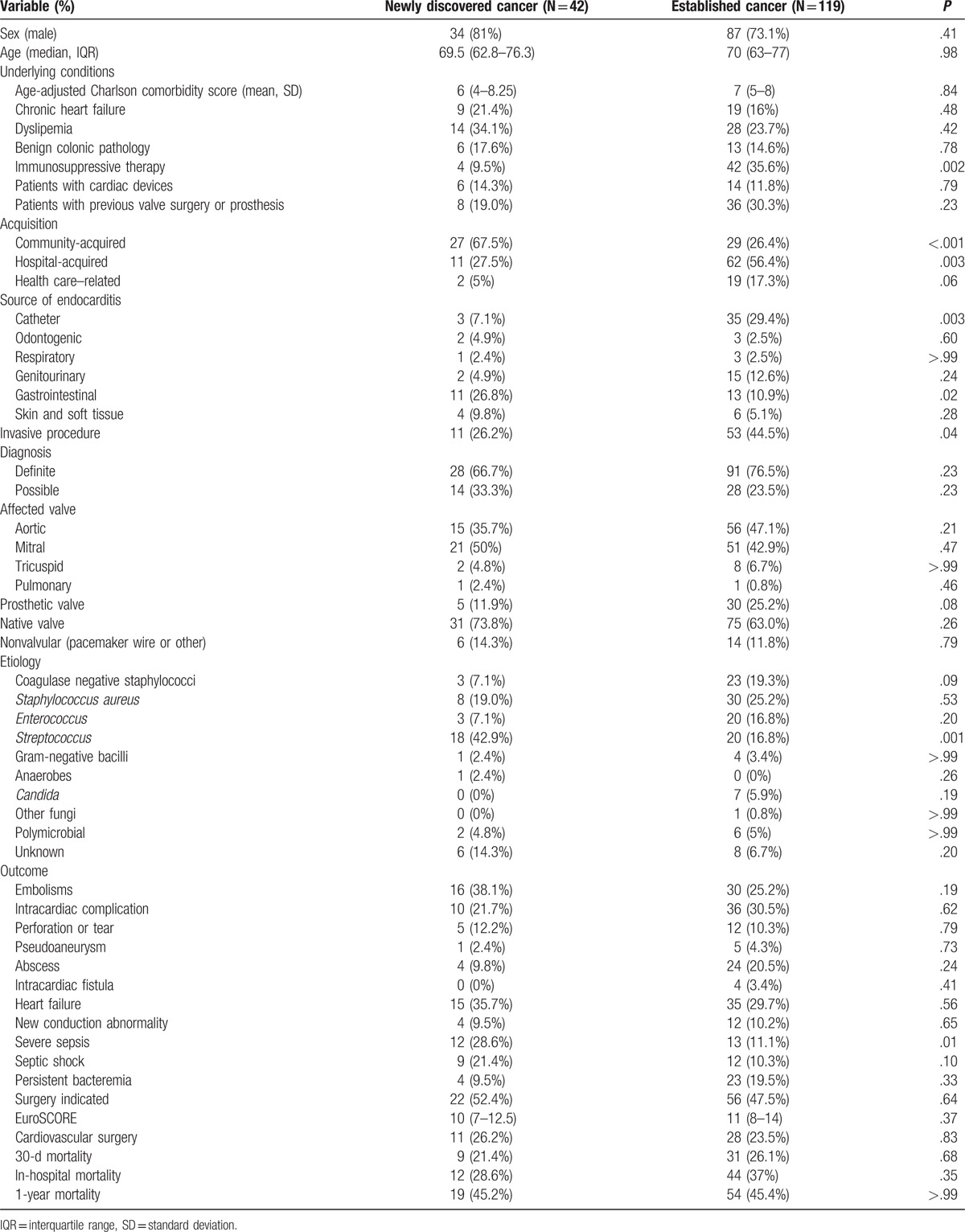
During the study period, 161 episodes of IE fulfilled the inclusion criteria. We studied 2 populations: patients whose cancer was diagnosed before IE (73.9%) and those whose cancer and IE were diagnosed simultaneously (26.1%). The latter more frequently had community-acquired IE (67.5% vs 26.4%, P < .01), severe sepsis (28.6% vs 11.1%, P = .013), and IE caused by gastrointestinal streptococci (42.9% vs 16.8%, P < .01). However, catheter source (7.1% vs 29.4%, P = .003), invasive procedures (26.2% vs 44.5%, P = .044), and immunosuppressants (9.5% vs 35.6%, P = .002) were less frequent.
When compared with noncancer patients, patients with cancer were more often male (75.2% vs 67.7%, P = .049), with a higher comorbidity index (7 vs 4). In addition, IE was more often nosocomial (48.7% vs 29%) and originated in catheters (23.6% vs 6.2%) (all P < .01). Prosthetic endocarditis (21.7% vs 30.3%, P = .022) and surgery when indicated (24.2% vs 46.5%, P < .01) were less common. In-hospital mortality (34.8% vs 25.8%, P = .012) and 1-year mortality (47.8% vs 30.9%, P < .01) were higher in cancer patients, although 30-day mortality was not (24.8% vs 19.3%, P = .087).
A significant proportion of cases of IE (5.6%) were recorded in cancer patients, mainly as a consequence of medical interventions. IE may be a harbinger of occult cancer, particularly that of gastrointestinal or urinary origin.
Keywords: cancer, infective endocarditis, neoplasm
1. Introduction
The importance of active cancer as an underlying disease in patients with infective endocarditis (IE) has not been specifically addressed, although neoplasms are common in patients with IE.[1]
The reciprocal and negative influence of cancer and IE on the management of both conditions is easy to envision but not frequently addressed. Potential higher mortality has been suggested in patients with cancer.[2] Some studies report an association between intra-abdominal cancer and endocarditis,[3] whereas others report a high incidence of nosocomial IE caused by staphylocci.[2] However, specific characteristics and prognostic factors have not been analyzed. Studies describing IE in patients with cancer or vice versa are subject to limitations: they analyze single-center cohorts,[2] do not draw comparisons with a population without cancer,[4] focus only on nonbacterial thrombotic endocarditis[5] or endocarditis associated with specific kinds of cancer,[3] and report the association in specific population subsets.[6]
Our objective was to describe the clinical characteristics and prognosis of IE in cancer patients in a large, multicenter cohort of patients with IE by comparing them with those of IE in noncancer patients. We assessed 2 populations: patients in whom cancer had been diagnosed before IE and patients in whom IE was diagnosed before cancer, thus pointing to cancer as a potential portal of entry for IE.
2. Materials and methods
2.1. Setting
In 2008, in association with the International Collaboration on Endocarditis (ICE),[1] a national cooperative endocarditis study group, Grupo de Apoyo al Manejo de la Endocarditis Infecciosa en España (GAMES [the Spanish Collaboration on Endocarditis]), was created in Spain with the objective of improving the care of IE patients and conducting research. GAMES is a prospective registry managed by a multicenter multidisciplinary group dedicated to improving the management of IE.[7]
2.2. Patients
Consecutive patients with IE were prospectively included in the GAMES registry between January 2008 and February 2014 in 30 Spanish hospitals. Multidisciplinary teams completed a standardized case report form. Patients were followed for 1 year. Patients with and without active cancer were compared.
2.3. Definitions
We defined active cancer as hematological neoplasm or solid tumors diagnosed <5 years before IE or any cancer managed with active cancer therapy at admission for IE. In the case of nonadvanced cancer, only those diagnosed <6 months before IE were considered active.[8] Cancer was stratified according to the stage of the disease at diagnosis of IE. Stage was considered advanced when the tumor was locally advanced or metastatic (solid tumors), or when the patient had received reinduction therapy, or a recurrence had been diagnosed (hematological cancer). We analyzed 2 subsets: patients with a diagnosis of cancer before the diagnosis of IE (established cancer) and patients in whom cancer was diagnosed simultaneously with IE (same admission) or subsequently (newly discovered cancer).
IE was defined according to the modified Duke criteria.[9]
Site of acquisition of IE was defined following ICE recommendations.[10] In brief, community-acquired IE was defined as IE diagnosed within the first 48 hours of admission in a patient who did not fulfill the criteria for nosocomial or health care-associated infection. Nosocomial IE was defined as IE in a patient who had been hospitalized for >48 hours before the onset of signs or symptoms consistent with IE. Health care-associated IE was diagnosed within 48 hours of admission of an outpatient with any of the following criteria [11]: intravenous therapy, wound care, or specialized nursing care at home within the 30 days before the onset of IE; attendance at a hospital or hemodialysis clinic or receipt of intravenous chemotherapy within the 30 days before the onset of IE; hospitalization in an acute care hospital for ≥2 days during the 90 days before the onset of IE; or residence in a nursing home or long-term care facility.
The source of endocarditis was considered to be the alleged source when the same microorganism was isolated in blood cultures and the potential source (e.g., catheter), when there was a clinical source compatible with the microorganism (e.g., Enterococcus and evidence of urinary infection in a patient with a permanent urinary catheter) and/or when an invasive intervention was performed before the diagnosis of IE that could be temporally and microbiologically related to the etiology of the endocarditis.
An implantable cardiac device was defined as a permanent pacemaker and/or cardioverter-defibrillator.
Prosthetic valve IE was defined as an endovascular infection affecting a prosthetic valve or reconstructed native heart valve, irrespective of whether the prosthesis was a mechanical prosthesis and/or bioprosthetic xenograft (stented or unstented) and/or repaired native valve with implantation of an annular ring.
The EuroSCORE was used to assess operative risk in heart surgery.[12,13] We used the age-adjusted Charlson comorbidity index to categorize comorbidities.[14]
All patients were evaluated by cardiac surgeons following international indications to determine the need for surgery.[15] The final decision on surgery was made in agreement with the multidisciplinary endocarditis team at each center, including the oncologist.
Both in-hospital mortality (overall mortality rate during the hospital stay) and 30-day mortality (considered likely related to the infection[16]) were analyzed. Long-term mortality was defined as mortality at 1 year.
2.4. Data analysis
Patients with active cancer at admission for IE were analyzed and compared with the rest of the patients in the database.
Among patients with cancer, those with a previous diagnosis of cancer were compared with those who had a simultaneous diagnosis of IE and cancer (same month).
Quantitative variables were expressed as mean and standard deviation or as median and interquartile range (IQR), as appropriate; qualitative variables were expressed as frequency and percentage. Continuous variables were compared using the t test, and categorical variables were compared using the χ2 test or Fisher exact test when the χ2 test was not appropriate. Adjusted odds ratios (ORs) were computed using logistic regression analysis. Stepwise logistic regression analysis was performed including variables with a P < .1 in the univariate analysis. All statistical analyses were performed using PASW Statistics for Windows, version 18.0 (SPSS Inc, Chicago, IL).
2.5. Ethics
The study and the common case report form were approved by the local and national institutional review boards and ethics committees (E.C. 18/07).
3. Results
3.1. Incidence and etiology of IE in cancer patients
During the 6-year study period, 161 cases in 160 patients from 30 Spanish hospitals fulfilled the inclusion criteria (5.6% of all cases of IE diagnosed during the same period-2888 episodes of endocarditis). The characteristics of patients with and without cancer are summarized in Table 1.
Table 1.
Characteristics of infective endocarditis in patients with and without cancer.
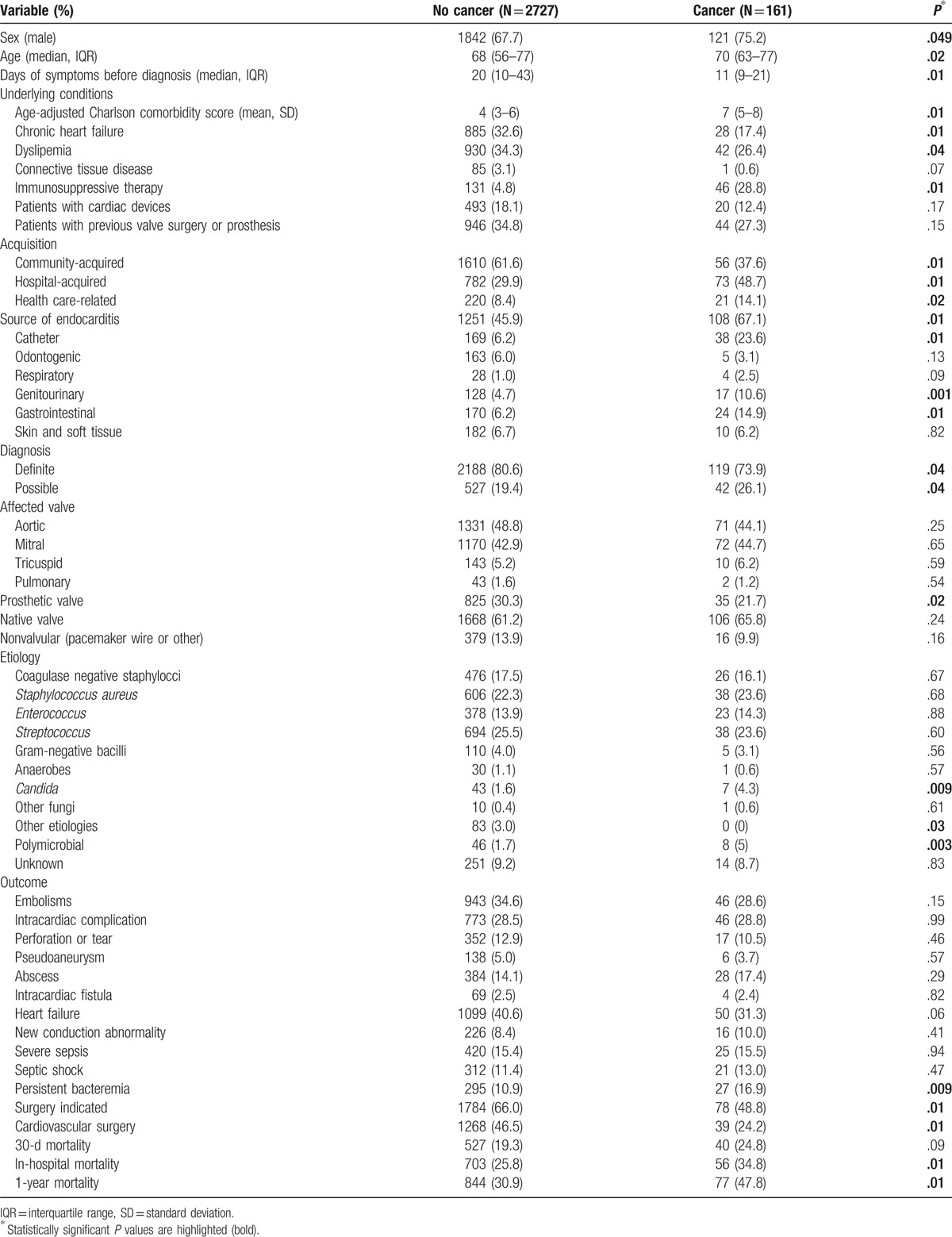
The etiology of IE in cancer patients is shown in Figure 1. A significant association was found between colon cancer and streptococcal etiology (32.7% vs 18.3%, P = .048). We failed to find any further association between other etiologies and other kinds of cancer.
Figure 1.
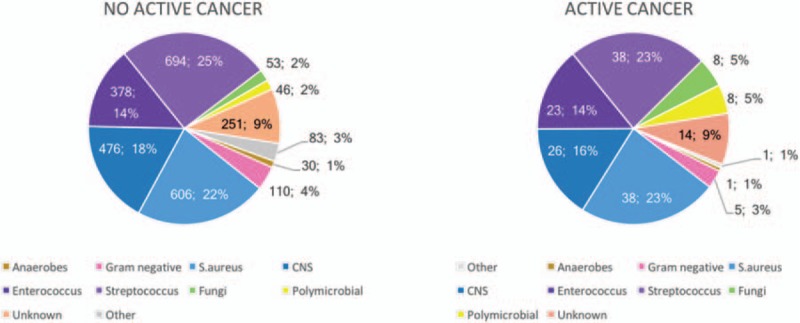
Etiology of infective endocarditis in patients with and without cancer. CNS = coagulase-negative staphylococci.
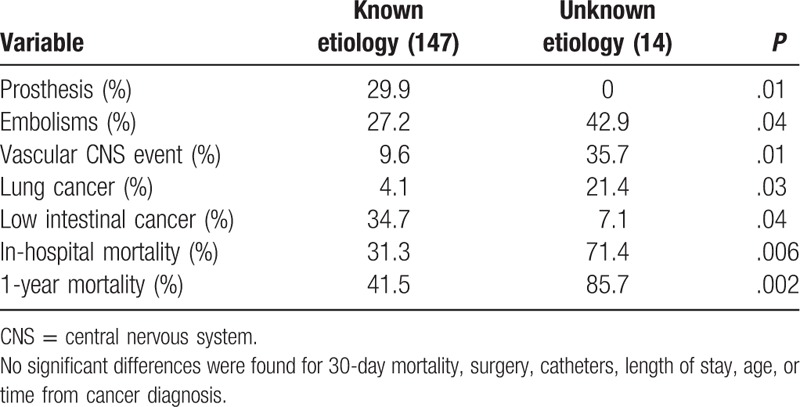
Of note, an etiological diagnosis for IE was not attained in 14 cases (8.7%); therefore, it was not possible to exclude nonbacterial thrombotic endocarditis. Significant differences between cases with and without a microbial etiology are summarized in Table 2. Cases without an etiologic diagnosis had significantly more embolisms and higher in-hospital mortality and were found more often in patients without a prosthetic valve.
Table 2.
Differences between cases of infective endocarditis with a known and unknown etiology.
3.2. Characteristics of IE according to the time of cancer diagnosis
The underlying neoplasm was hematological in 19.4% and a solid tumor in 80.6% (Fig. 2). The most common malignant neoplasm was colon cancer (33.5%), followed by prostate cancer (9.7%), lymphoma (8.4%), and urothelial tumors (8.4%).
Figure 2.
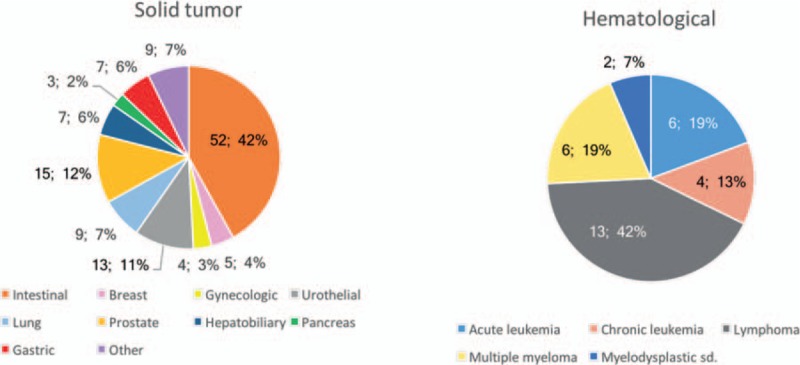
Underlying cancer.
We identified 2 different populations: patients with a cancer diagnosis before IE (established cancer) (119, 73.9%) and patients in whom IE was diagnosed simultaneously with cancer (same admission) or subsequently (newly discovered cancer) (42, 26.1%).
In patients with established cancer, the median time from cancer to IE was 257 days (IQR 67–809). Cancer was at an advanced stage in 56.1%. At diagnosis of IE, only 1 patient was neutropenic, although 42 (25.6%) had recently received chemotherapy.
Patients with established and newly discovered cancer were compared (Table 3). Cases where endocarditis was a harbinger of cancer were more likely to be community-acquired, were less frequently treated with immunosuppressors, presented with severe sepsis, and were caused by gastrointestinal streptococci. On the contrary, catheter source and invasive procedures before the IE episode were less frequent. There were no differences in surgical management or outcome.
Table 3.
Characteristics of infective endocarditis according to when cancer was diagnosed.
3.3. Comparison between patients with and without cancer
When we compared patients with and without cancer (Table 1), cancer patients were more often male (75.2% vs 67.7%, P = .049) and significantly older (median age 70 [IQR 63–77] vs 68 [IQR 56–77], P = .02) and had a higher age-adjusted Charlson comorbidity index (mean 7 vs 4, P < .01).
IE was more often nosocomial in cancer patients (48.7% vs 29.9%, P = .01). In 23.6% of cases, a central venous catheter was purportedly the source of IE compared with only 6.2% in noncancer patients (P = .01). Genitourinary or intestinal sources of endocarditis were also significantly more frequent in cancer patients.
Clinical presentation was unspecific, although the diagnostic criteria were less often definite than in noncancer patients (73.9% vs 80.6%, P = .04). Prosthetic IE was less frequent in patients with cancer (21.7% vs 30.3%, P = .02). Interestingly, 4 cases had mural IE (among 16 cases of nonvalvular IE).
Candida and polymicrobial IE were significantly more frequent among cancer patients (4.3% vs 1.6%, P = .009; and 5% vs 1.7%, P = .003, respectively) (Fig. 2). Persistent bacteremia was more common in cancer patients (16.9% vs 10.9%, P = .009).
Surgery, although indicated, was performed less often in cancer patients (24.2% vs 46.5%, P = .01). The reasons for not performing surgery were death before surgery (18.4%), poor prognosis after surgery (7.9%), poor prognosis of underlying disease (55.3%), hemodynamic instability (5.3%), and cirrhosis, stroke, or patient/surgeon refusal (2.6% each). The reasons for not operating were unknown in 2 cases.
There were no differences in 30-day mortality (24.8% vs 19.3%, P = .09), although in-hospital mortality was higher in cancer patients (34.8% vs 25.8%, P = .01). One-year mortality was significantly higher in cancer patients (47.8% vs 30.9%, P = .01) (Fig. 3).
Figure 3.
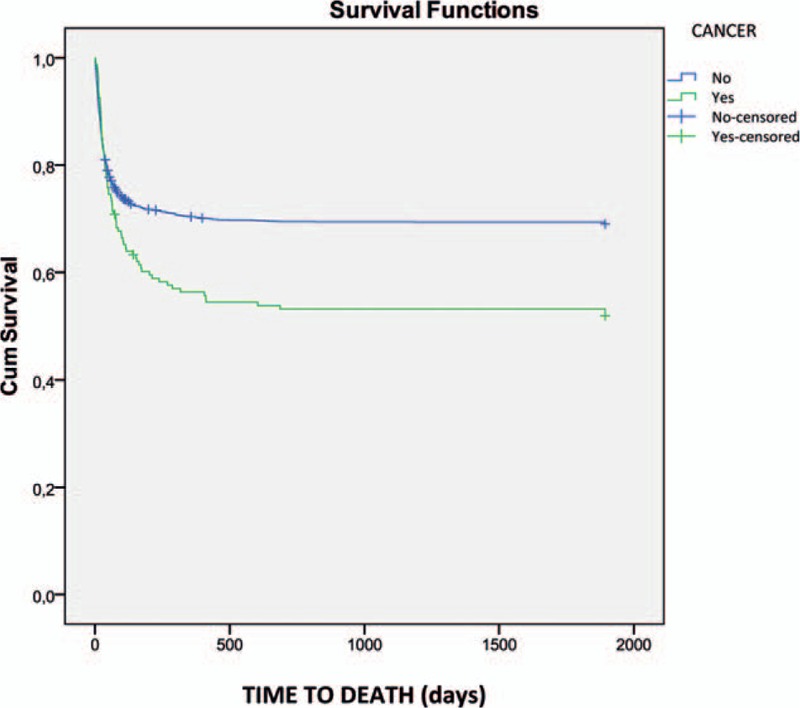
One-year survival in patients with and without cancer.
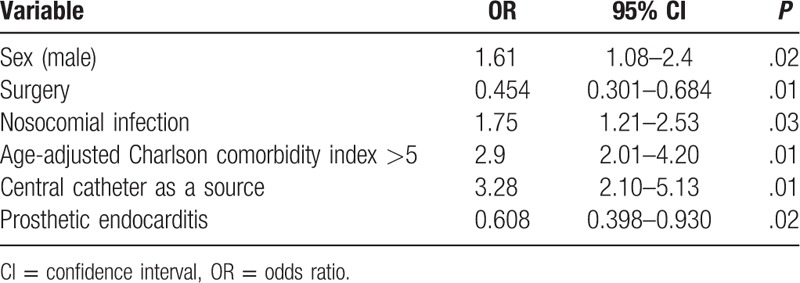
The multivariate analysis (Table 4) confirmed that IE in cancer patients more often affected men, with a higher age-adjusted comorbidity index, and was more often hospital-acquired and originated in catheters. Prosthetic valve endocarditis was less frequent, and cancer patients underwent surgery significantly less frequently when surgery was indicated.
Table 4.
Independent differential factors for infective endocarditis (cancer vs noncancer).
3.4. Risk factors for mortality in cancer patients
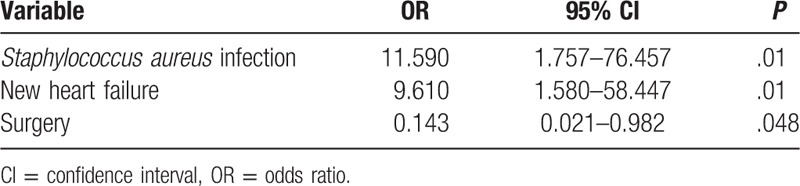
Among patients with active cancer, Staphylococcus aureus IE, new heart failure, and nonsurgical therapy were independent prognostic factors for 30-day mortality. No cancer-related factors were associated with 30-day mortality (Table 5).
Table 5.
Risk factors for 30-day mortality in cancer patients with infective endocarditis.
In-hospital mortality in cancer patients was independently associated with a higher age-adjusted Charlson comorbidity index and development of heart failure, whereas a streptococcal etiology was a protective factor (Table 6). Among patients who died during admission, death was from endocarditis in 41% of cases, cancer in 10.7%, other causes in 17.8%, and unknown origin in 30.4%.
Table 6.
Risk factors for in-hospital mortality in cancer patients with infective endocarditis.
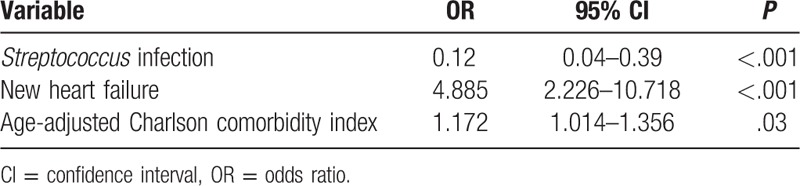
We were not able to identify independent risk factors for 1-year mortality.
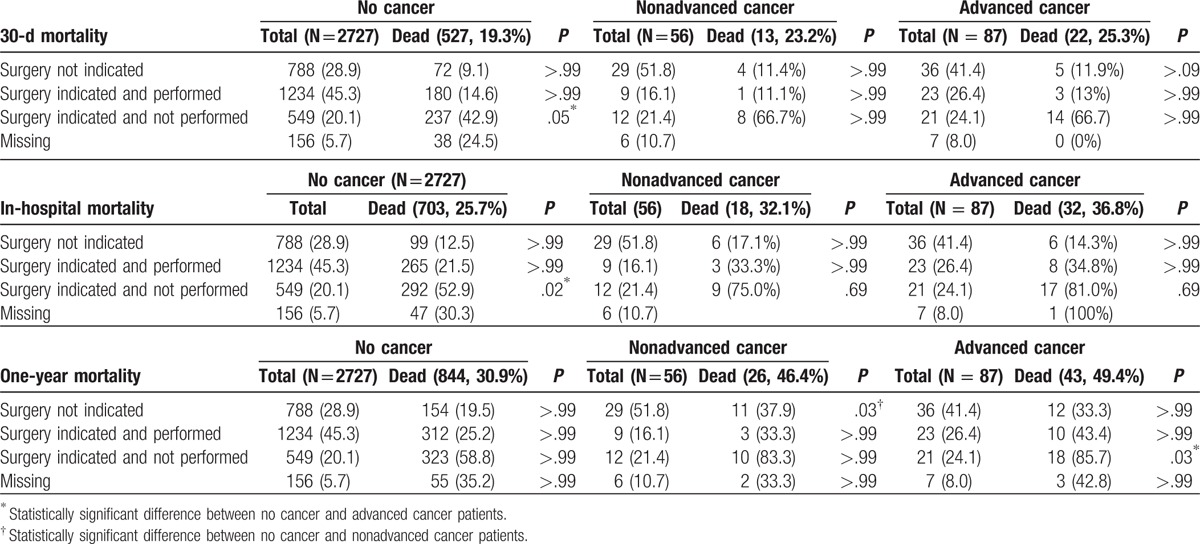
In patients who underwent surgery, mortality at discharge, 30 days, and 1 year was similar for cancer and noncancer patients, and lower than in nonoperated patients for whom surgery was indicated, independently of cancer stage (Table 7).
Table 7.
Mortality associated with surgery for infective endocarditis in patients with and without cancer according to stage.
4. Discussion
Our study shows that a significant proportion of IE patients (5.6%) have underlying active cancer and that IE may be a harbinger or a consequence of cancer. Endocarditis behaves differently in cancer patients, although risk factors for 30-day mortality (which we consider attributable mortality) are similar to those for the general population of patients with endocarditis.[7] Long-term prognosis is related to the underlying disease.
Data on the prevalence of cancer among patients with IE are scarce in the literature, and it is generally not determined whether cancer is active or not when IE is diagnosed.[1] In our series, we only included patients with active cancer, thus possibly accounting for the somewhat lower prevalence in the present study (5.6% vs 8% elsewhere[1]).
We identified 2 different populations in cancer patients with IE: one with a previous diagnosis of cancer, in which IE is a consequence of cancer management, and another, in which IE is diagnosed after the diagnosis of cancer. There are opportunities both for early diagnosis of cancer and for prevention of endocarditis.
The association between colon cancer and IE is well known[3,6,17,18]; however, in our series, a high risk of IE was also present for lymphoma, prostate cancer, and genitourinary cancer. In many cases, IE occurs concurrently with, and even leads to, a diagnosis of cancer (42; 26% cases).[19] The only association we were able to find between the etiology of IE and the underlying tumor was streptococcal endocarditis in patients with colon cancer. An association between Streptococcus bovis (S. gallolyticus subsp gallolyticus) and colon cancer has been thoroughly described,[18] and several mechanisms have been proposed.[17] Although an association between other types of streptococcal endocarditis and colon cancer has been reported (Kestler et al, in press), this is not as strong as in the case of S. gallolyticus. Some authors suggest that the risk of being diagnosed with cancer is higher in patients with IE than in those without IE, in particular during the first 3 months of follow-up, although this period can be as long as 4 years.[19,20] This is an important issue for early diagnosis of cancer, particularly when made based on colonoscopy in patients with streptococcal endocarditis. The current European guidelines[15] recommend ruling out cancer in cases of IE caused by S. bovis (gallolyticus). The increasing use of PET/CT in the extension study can help diagnose occult cancer in patients with IE.[7]
In other cases, endocarditis is diagnosed after cancer and application of its diagnostic and therapeutic measures. Previous cancer facilitates IE by means of associated thrombotic phenomena in cardiac valves that favor bacterial colonization or as a consequence of medical management (e.g., catheter-associated disease).
Nonbacterial thrombotic endocarditis is also found in cancer patients. Yusuf et al[4] reported a 42% frequency of culture-negative endocarditis in a retrospective series of consecutive cancer patients. Classically, embolisms are more frequent in nonbacterial thrombotic endocarditis, which occurs more frequently in patients with lung, pancreas, and gastric cancer and healthy valves.[21,22] In our series, it was not possible to reach a microbiological diagnosis in 8.7% of cases, and we cannot rule out the possibility that these cases involved nonbacterial thrombotic endocarditis. Consistent with this finding, patients with no known etiology had significantly more native valve endocarditis, more embolisms, and higher long-term mortality. It is important to bear in mind the possibility of noninfectious endocarditis, which needs different management strategies.[21]
Hospital and health care-related acquisition were significantly more frequent among patients with cancer, probably as a result of the use of invasive techniques and devices, such as catheters.[23] Long-term catheters are necessary in cancer patients, and although the risk of infection in totally implantable catheters is lower than in other catheters, these stay in place for longer periods of time and are eventually responsible for a considerable number of infections.[24] In a retrospective series of endocarditis in patients with cancer, 60% of those with culture-positive endocarditis had a central venous catheter.[4] In our series, the catheter was purportedly the source of endocarditis in 23.6%. Preventive measures have successfully prevented catheter-related bloodstream infections in other settings.[25] As suggested by Chu,[26] appropriate management of catheter-related bacteremia is essential for source control in endocarditis. Both prevention and management of catheter-related bacteremia are necessary to prevent the development of endocarditis in cancer patients. Programs specifically addressing long-term catheters are essential.
Interestingly, we found 4 cases of mural endocarditis (all right-sided). It is possible that chemotherapy administered through central venous catheters irritates the atrial wall and favors the development of atrial endocarditis. Other authors have described an association between thrombotic complications and catheter-related bloodstream infections in long-term catheters.[27] Nonvalvular endocarditis is characteristically associated with central catheters.[27–30]
Risk factors for 30-day mortality were similar to those of the general population and were all noncancer-related factors, whereas in-hospital mortality was also related to underlying disease and comorbidity. Cancer was a risk factor for 1-year mortality after IE, although not for short-term mortality, as previously reported by our group.[7]
Surgery is rarely offered because of concern over major postoperative complications and deterioration of an already compromised health status. Whether endocarditis affects cancer mortality[2,6] or does not affect it[3] remains a controversial issue. In our series, there were no differences in postoperative mortality between patients with and without cancer who had undergone surgery. When evaluating a particular patient's prognosis, we need to bear in mind that short-term prognosis is similar to that of any other patient with endocarditis, and in cases with a favorable cancer prognosis and no other comorbidity, intensive management and surgical therapy should be considered.
Our series may not represent the situation of IE in countries where levels of health care differ from those of Spain, which has a universal public health system.
In conclusion, a significant proportion of cases of IE (5.6%) occur in cancer patients, mainly as a consequence of medical interventions in established cancers. IE should prompt a search for occult cancer, particularly in the gastrointestinal or urinary tract. Our results will facilitate informed clinical decisions in patients with IE and active cancer, thus enabling adequate preventive measures to be established.
Acknowledgments
We thank Thomas O’Boyle for his help in the preparation of the manuscript. We thank Iván Adán (GAMES) for his coordination work in this study.
We thank the members of the GAMES study group for their contribution to the work:
Hospital Costa del Sol (Marbella): Fernando Fernández Sánchez, Mariam Noureddine, Gabriel Rosas, Javier de la Torre Lima; Hospital Universitario de Cruces (Bilbao): José Aramendi, Elena Bereciartua, María Victoria Boado, Itxasne Cabezón Estébanez, Marta Campaña Lázaro, Josune Goikoetxea, Juan José Goiti, José Ramón Iruretagoyena, Josu Irurzun Zuazabal, Leire López-Soria, Miguel Montejo, Pedro María Pérez, Regino Rodríguez, Roberto Voces; Hospital Universitario Virgen de la Victoria (Málaga): Mª Victoria García López, Radka Ivanova Georgieva, Manuel Márquez Solero, Isabel Rodríguez Bailón, Josefa Ruiz Morales; Hospital Universitario Donostia-Policlínica Gipuzkoa (San Sebastián): Ana María Cuende, Tomás Echeverría, Ana Fuerte, Eduardo Gaminde, Miguel Ángel Goenaga, Pedro Idígoras, José Antonio Iribarren, Alberto Izaguirre Yarza, Xabier Kortajarena Urkola, Carlos Reviejo; Hospital General Universitario de Alicante (Alicante): Rafael Carrasco, Vicente Climent, Patricio Llamas, Esperanza Merino, Joaquín Plazas, Sergio Reus; Complejo Hospitalario Universitario A Coruña (A Coruña): Nemesio Álvarez, José María Bravo-Ferrer, Laura Castelo, José Cuenca, Pedro Llinares, Enrique Miguez Rey, María Rodríguez Mayo, Efrén Sánchez, Dolores Sousa Regueiro; Complejo Hospitalario Universitario de Huelva (Huelva): Francisco Javier Martínez; Hospital Universitario de Canarias (Canarias): Mª del Mar Alonso, Beatriz Castro, Dácil García Rosado, Mª del Carmen Durán, Mª Antonia Miguel Gómez, Juan Lacalzada, Ibrahim Nassar; Hospital Regional Universitario de Málaga (Málaga): Antonio Plata Ciezar, José Mª Reguera Iglesias; Hospital Universitario Central Asturias (Oviedo): Víctor Asensi Álvarez, Carlos Costas, Jesús de la Hera, Jonathan Fernández Suárez, Lisardo Iglesias Fraile, Víctor León Arguero, José López Menéndez, Pilar Mencía Bajo, Carlos Morales, Alfonso Moreno Torrico, Carmen Palomo, Begoña Paya Martínez, Ángeles Rodríguez Esteban, Raquel Rodríguez García, Mauricio Telenti Asensio; Hospital Clínic-IDIBAPS, Universidad de Barcelona (Barcelona): Manuel Almela, Juan Ambrosioni, Manuel Azqueta, Mercè Brunet, Marta Bodro, Ramón Cartañá, Carlos Falces, Guillermina Fita, David Fuster, Cristina García de la Mària, Marta Hernández-Meneses, Jaume Llopis Pérez, Francesc Marco, José M. Miró, Asunción Moreno, David Nicolás, Salvador Ninot, Eduardo Quintana, Carlos Paré, Daniel Pereda, Juan M. Pericás, José L. Pomar, José Ramírez, Irene Rovira, Elena Sandoval, Marta Sitges, Dolors Soy, Adrián Téllez, José M. Tolosana, Bárbara Vidal, Jordi Vila; Hospital General Universitario Gregorio Marañón (Madrid): Iván Adán, Javier Bermejo, Emilio Bouza, Gregorio Cuerpo Caballero, Ana Fernández Cruz, Mª Eugenia García Leoni, Víctor González Ramallo, Martha Kestler Hernández, Mercedes Marín, Manuel Martínez-Sellés, Mª Cruz Menárguez, Patricia Muñoz, Cristina Rincón, Hugo Rodríguez-Abella, Marta Rodríguez-Créixems, Blanca Pinilla, Ángel Pinto, Maricela Valerio, Pilar Vázquez, Eduardo Verde Moreno; Hospital Universitario La Paz (Madrid): Isabel Antorrena, Belén Loeches, Alejandro Martín Quirós, Mar Moreno, Ulises Ramírez, Verónica Rial Bastón, María Romero, Araceli Saldaña; Hospital Universitario Marqués de Valdecilla (Santander): Jesús Aguero Balbín, Carlos Armiñanzas Castillo, Ana Arnaiz, Francisco Arnaiz de las Revillas, José Ramón de Berrazueta, Manuel Cobo Belaustegui, María Carmen Fariñas, Concepción Fariñas-Álvarez, Rubén Gómez Izquierdo, Claudia González Rico, Manuel Gutiérrez-Cuadra, José Gutiérrez Díez, Rafael Martín Durán, Marcos Pajarón, José Antonio Parra, Ramón Teira, Jesús Zarauza; Hospital Universitario Puerta de Hierro (Madrid): Fernando Domínguez, Pablo García Pavía, Jesús González, Beatriz Orden, Antonio Ramos; Hospital Universitario Ramón y Cajal (Madrid): Tomasa Centella, José Manuel Hermida, José Luis Moya, Pilar Martín-Dávila, Enrique Navas, Enrique Oliva, Alejandro del Río, Soledad Ruiz; Hospital Universitario Virgen de las Nieves (Granada): Carmen Hidalgo Tenorio; Hospital Universitario Virgen Macarena (Sevilla): Manuel Almendro Delia, Omar Araji, José Miguel Barquero, Román Calvo Jambrina, Marina de Cueto, Juan Gálvez Acebal, Irene Méndez, Isabel Morales, Luis Eduardo López-Cortés; Hospital Universitario Virgen del Rocío (Sevilla): Arístides de Alarcón, Emilio García, Juan Luis Haro, José Antonio Lepe, Francisco López, Rafael Luque; Hospital San Pedro (Logroño): Luis Javier Alonso, Pedro Azcárate, José Manuel Azcona Gutiérrez, José Ramón Blanco, Lara García-Álvarez, José Antonio Oteo, Mercedes Sanz; Hospital de la Santa Creu i Sant Pau (Barcelona): Natividad de Benito, Mercé Gurguí, Cristina Pacho, Roser Pericas, Guillem Pons; Complejo Hospitalario Universitario de Santiago de Compostela (A Coruña): M. Álvarez, A. L. Fernández, Amparo Martínez, A. Prieto, Benito Regueiro, E. Tijeira, Marino Vega; Hospital Santiago Apóstol (Vitoria): Andrés Canut Blasco, José Cordo Mollar, Juan Carlos Gainzarain Arana, Oscar García Uriarte, Alejandro Martín López, Zuriñe Ortiz de Zárate, José Antonio Urturi Matos; Hospital SAS Línea de la Concepció (Cádiz): Gloria García Domínguez, Antonio Sánchez-Porto; Hospital Clínico Universitario Virgen de la Arrixaca (Murcia): José Mª Arribas Leal, Elisa García Vázquez, Alicia Hernández Torres, Ana Blázquez, Gonzalo de la Morena Valenzuela; Hospital de Txagorritxu (Vitoria): Ángel Alonso, Javier Aramburu, Felicitas Elena Calvo, Anai Moreno Rodríguez, Paola Tarabini-Castellani; Hospital Virgen de la Salud (Toledo): Eva Heredero Gálvez, Carolina Maicas Bellido, José Largo Pau, Mª Antonia Sepúlveda, Pilar Toledano Sierra, Sadaf Zafar Iqbal-Mirza; Hospital Rafael Méndez (Lorca-Murcia):, Eva Cascales Alcolea, Pilar Egea Serrano, José Joaquín Hernández Roca; Hospital Universitario San Cecilio (Granada): Eduardo Moreno Escobar, Alejandro Peña Monje, Valme Sánchez Cabrera, David Vinuesa García; Hospital Son Llàtzer (Palma de Mallorca): María Arrizabalaga Asenjo, Carmen Cifuentes Luna, Juana Núñez Morcillo, Mª Cruz Pérez Seco, Aroa Villoslada Gelabert; Hospital Universitario Miguel Servet (Zaragoza): Carmen Aured Guallar, Nuria Fernández Abad, Pilar García Mangas, Marta Matamala Adell, Mª Pilar Palacián Ruiz, Juan Carlos Porres.
Footnotes
Abbreviations: GAMES = Grupos de Ayuda al Manejo de la Endocarditis (Spanish Collaboration on Endocarditis), ICE = International Collaboration on Endocarditis, IE = infective endocarditis, IQR = interquartile range, OR = odds ratio.
This study was previously presented in part as a Poster (#15) at the “IV Congreso SEICAV”, Santander, October 29–31, 2015, and as a Poster (#P1148) at the 26th ECCMID conference in Amsterdam, The Netherlands, April 9–12, 2016.
JMM received a personal intensification research grant [#INT15/00168] in 2016 from Instituto de Salud Carlos III, Madrid, Spain.
The authors have no conflicts of interest to disclose.
References
- [1].Murdoch DR, Corey GR, Hoen B, et al. Clinical presentation, etiology, and outcome of infective endocarditis in the 21st century: the International Collaboration on Endocarditis-Prospective Cohort Study. Arch Intern Med 2009;169:463–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Mesa Del Castillo-Paya C, Rodriguez-Esteban M, Quijada-Fumero A, et al. [Infective endocarditis in patients with oncological diseases]. Enferm Infecc Microbiol Clin 2016;Dec 13. pii: S0213-005X(16)30344-5. doi: 10.1016/j.eimc.2016.10.011. [Epub ahead of print] Spanish. [DOI] [PubMed] [Google Scholar]
- [3].Ouaissi M, Studer AS, Mege D, et al. Characteristics and natural history of patients with colorectal cancer complicated by infectious endocarditis. Case control study of 25 patients. Anticancer Res 2014;34:349–53. [PubMed] [Google Scholar]
- [4].Yusuf SW, Ali SS, Swafford J, et al. Culture-positive and culture-negative endocarditis in patients with cancer: a retrospective observational study, 1994–2004. Medicine (Baltimore) 2006;85:86–94. [DOI] [PubMed] [Google Scholar]
- [5].Edoute Y, Haim N, Rinkevich D, et al. Cardiac valvular vegetations in cancer patients: a prospective echocardiographic study of 200 patients. Am J Med 1997;102:252–8. [DOI] [PubMed] [Google Scholar]
- [6].Garcia-Albeniz X, Hsu J, Lipsitch M, et al. Infective endocarditis and cancer in the elderly. Eur J Epidemiol 2016;31:41–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Munoz P, Kestler M, De Alarcon A, et al. Current epidemiology and outcome of infective endocarditis: a multicenter, prospective, cohort study. Medicine (Baltimore) 2015;94:e1816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Rabello LS, Silva JR, Azevedo LC, et al. Clinical outcomes and microbiological characteristics of severe pneumonia in cancer patients: a prospective cohort study. PLoS One 2015;10:e0120544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Li JS, Sexton DJ, Mick N, et al. Proposed modifications to the Duke criteria for the diagnosis of infective endocarditis. Clin Infect Dis 2000;30:633–8. [DOI] [PubMed] [Google Scholar]
- [10].Baddour LM, Wilson WR, Bayer AS, et al. Infective endocarditis: diagnosis, antimicrobial therapy, and management of complications: a statement for healthcare professionals from the Committee on Rheumatic Fever, Endocarditis, and Kawasaki Disease, Council on Cardiovascular Disease in the Young, and the Councils on Clinical Cardiology, Stroke, and Cardiovascular Surgery and Anesthesia, American Heart Association: endorsed by the Infectious Diseases Society of America. Circulation 2005;111:e394–434. [DOI] [PubMed] [Google Scholar]
- [11].Cockerill FR, 3rd, Wilson JW, Vetter EA, et al. Optimal testing parameters for blood cultures. Clin Infect Dis 2004;38:1724–30. [DOI] [PubMed] [Google Scholar]
- [12].Roques F, Nashef SA, Michel P, et al. Risk factors and outcome in European cardiac surgery: analysis of the EuroSCORE multinational database of 19030 patients. Eur J Cardiothorac Surg 1999;15:816–22. [DOI] [PubMed] [Google Scholar]
- [13].Nashef SA, Roques F, Hammill BG, et al. Validation of European System for Cardiac Operative Risk Evaluation (EuroSCORE) in North American cardiac surgery. Eur J Cardiothorac Surg 2002;22:101–5. [DOI] [PubMed] [Google Scholar]
- [14].Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987;40:373–83. [DOI] [PubMed] [Google Scholar]
- [15].Habib G, Lancellotti P, Antunes MJ, et al. 2015 ESC Guidelines for the management of infective endocarditis: the Task Force for the Management of Infective Endocarditis of the European Society of Cardiology (ESC). Endorsed by: European Association for Cardio-Thoracic Surgery (EACTS), the European Association of Nuclear Medicine (EANM). Eur Heart J 2015;36:3075–128. [DOI] [PubMed] [Google Scholar]
- [16].Ternhag A, Cederstrom A, Torner A, et al. A nationwide cohort study of mortality risk and long-term prognosis in infective endocarditis in Sweden. PLoS One 2013;8:e67519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Boleij A, Tjalsma H. The itinerary of Streptococcus gallolyticus infection in patients with colonic malignant disease. Lancet Infect Dis 2013;13:719–24. [DOI] [PubMed] [Google Scholar]
- [18].Boleij A, van Gelder MM, Swinkels DW, et al. Clinical importance of Streptococcus gallolyticus infection among colorectal cancer patients: systematic review and meta-analysis. Clin Infect Dis 2011;53:870–8. [DOI] [PubMed] [Google Scholar]
- [19].Thomsen RW, Farkas DK, Friis S, et al. Endocarditis and risk of cancer: a Danish nationwide cohort study. Am J Med 2013;126:58–67. [DOI] [PubMed] [Google Scholar]
- [20].Sun LM, Wu JN, Lin CL, et al. Infective endocarditis and cancer risk: a population-based cohort study. Medicine (Baltimore) 2016;95:e3198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].el-Shami K, Griffiths E, Streiff M. Nonbacterial thrombotic endocarditis in cancer patients: pathogenesis, diagnosis, and treatment. Oncologist 2007;12:518–23. [DOI] [PubMed] [Google Scholar]
- [22].Asopa S, Patel A, Khan OA, et al. Non-bacterial thrombotic endocarditis. Eur J Cardiothorac Surg 2007;32:696–701. [DOI] [PubMed] [Google Scholar]
- [23].Llinares Mondejar P, Nunez Fernandez M, Cordero Lorenzana L, et al. [Nosocomial infective endocarditis in patients without hear prosthesis]. Rev Clin Esp 1997;197:814–8. [PubMed] [Google Scholar]
- [24].Lebeaux D, Larroque B, Gellen-Dautremer J, et al. Clinical outcome after a totally implantable venous access port-related infection in cancer patients: a prospective study and review of the literature. Medicine (Baltimore) 2012;91:309–18. [DOI] [PubMed] [Google Scholar]
- [25].Palomar M, Alvarez-Lerma F, Riera A, et al. Impact of a national multimodal intervention to prevent catheter-related bloodstream infection in the ICU: the Spanish experience. Crit Care Med 2013;41:2364–72. [DOI] [PubMed] [Google Scholar]
- [26].Chu VH. When the cat's out of the bag: searching for portals of entry in infective endocarditis. J Am Coll Cardiol 2016;67:159–61. [DOI] [PubMed] [Google Scholar]
- [27].Raad II, Luna M, Khalil SA, et al. The relationship between the thrombotic and infectious complications of central venous catheters. JAMA 1994;271:1014–6. [PubMed] [Google Scholar]
- [28].Keino D, Tsuzuki Y, Mori T, et al. Infective endocarditis associated with acute leukemia: report of two cases. Pediatr Int 2015;57:1017–20. [DOI] [PubMed] [Google Scholar]
- [29].Chrissoheris MP, Libertin C, Ali RG, et al. Endocarditis complicating central venous catheter bloodstream infections: a unique form of health care associated endocarditis. Clin Cardiol 2009;32:E48–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Thakar S, Janga KC, Tolchinsky T, et al. Superior vena cava and right atrium wall infective endocarditis in patients receiving hemodialysis. Heart Lung 2012;41:301–7. [DOI] [PubMed] [Google Scholar]


