Supplemental Digital Content is available in the text
Keywords: gestational diabetes mellitus, glyburide, insulin, metformin, network meta-analysis, treatment
Abstract
Background:
The safety and efficacy of different drugs in treatment of gestational diabetes mellitus (GDM) patients who could not maintain normal glucose level only through diet and exercise remains to be debated. We performed this network meta-analysis (NAM) to compare and rank different antidiabetic drugs in glucose level control and pregnancy outcomes in GDM patients.
Methods:
We searched PubMed, Cochrane Library, Web of Science, and Embase up to December 31, 2016. Randomized controlled trials (RCTs) related to different drugs in the treatment of GDM patients were enrolled. We extracted the relevant information and assessed the risk of bias with the Cochrane risk of bias tool. We did pair-wise meta-analyses using the fixed-effects model or random-effects model and then adopted random-effects NAM combining both direct and indirect evidence within a Bayesian framework, to calculate the odds ratio (OR) or standardized mean difference (SMD) and to draw a surface under the cumulative ranking curve of the neonatal and maternal outcomes of different treatments in GDM patients.
Results:
Thirty-two randomized controlled trials (RCTs) were included in this NAM, including 6 kinds of treatments (metformin, metformin plus insulin, insulin, glyburide, acarbose, and placebo). The results of the NAM showed that regarding the incidence of macrosomia and LGA, metformin had lower incidence than glyburide (OR, 0.5411 and 0.4177). In terms of the incidence of admission to the NICU, insulin had higher incidence compared with glyburide (OR, 1.844). As for the incidence of neonatal hypoglycemia, metformin had lower incidence than insulin and glyburide (OR, 0.6331 and 0.3898), and insulin was lower than glyburide (OR, 0.6236). For mean birth weight, metformin plus insulin was lower than insulin (SMD, -0.5806), glyburide (SMD, -0.7388), and placebo (SMD, -0.6649). Besides, metformin was observed to have lower birth weight than glyburide (SMD, 0.2591). As for weight gain, metformin and metformin plus insulin were lower than insulin (SMD, -0.9166, -1.53). Ranking results showed that glyburide might be the optimum treatment regarding average glucose control, and metformin is the fastest in glucose control for GDM patients; glyburide have the highest incidence of macrosomia, preeclampsia, hyperbilirubinemia, neonatal hypoglycemia, shortest gestational age at delivery, and lowest mean birth weight; metformin (plus insulin when required) have the lowest incidence of macrosomia, PIH, LGA, RDS, low gestational age at delivery, and low birth weight. Besides, insulin had the highest incidence of NICU admission, acarbose had the lowest risk of neonatal hypoglycemia.
Conclusion:
Our study concluded that metformin is fastest in glucose control, with a more favorable pregnancy outcomes—would be a better option, but its rate of glucose control is the lowest.However, glyburide is the optimumtreatment regarding the rate of glucose control, but withmore adverse outcomes. This NAMbased on 32 RCTs will strongly help to guide further development of management for GDM patients, clinicians should carefully balance the risk–benefit profile of different treatments according to various situations.
1. Introduction
Gestational diabetes mellitus (GDM) is a major global public health issue, with prevalence increasing in recent years due to the epidemic of obesity and type 2 diabetes.[1,2]
GDM is defined as a condition in which a woman without diabetes develops the glucose intolerance resulting in hyperglycemia of variable degree during pregnancy.[3] Risk factors of developing GDM include being overweight, polycystic ovary syndrome, maternal age, and a family history with type 2 diabetes. GDM generally exhibit no symptoms, but it increases the risk of preeclampsia, depression, and the incidence of cesarean section. Moreover, children born to mothers with badly treated GDM are at higher risk of LGA, hypoglycemia, jaundice or at increased risk of being overweight and developing type 2 diabetes.[4] So the management of GDM is primarily aimed at glycemic control to reduce the incidence of adverse pregnancy outcomes.[5]
Most women are able to control their blood sugar with proper diet or plus exercise, if not, insulin treatment is considered as the gold standard for GDM.[6,7] However, several disadvantages of insulin treatment are recognized such as frequent injections, increased risk of hypoglycemia, and higher cost,[8] which could reduce patient's compliance. Furthermore, the dose of insulin needs to be individualized according to the women's body mass index (BMI), glucose control levels, and lifestyle.[9] By contrast, oral agents (metformin and glyburide) present the advantages of easier management and lower cost, so that they become an attractive alternative to insulin with better acceptance,[10] which enhance adherence to the treatment.[11] Metformin is a biguanide that achieves euglycemia primarily by suppressing hepatic gluconeogenesis and enhancing peripheral glucose uptake.[12] Glyburide acts by binding to and inhibiting the ATP-sensitive potassium channels (KATP) in pancreatic beta cells, and leads to an increase in intracellular calcium in the beta cell and subsequent stimulation of insulin secretion.[13]
Several previous studies have compared efficacy and safety of oral antidiabetic drugs (OADs) and insulin in treating GDM, with somewhat inconsistent results. A recent meta-analysis[14] including 11 RCTs found metformin was comparable with insulin in glycemic control, and could significantly reduce several adverse pregnancy outcomes. Another study[15] suggested that glyburide is as effective as insulin, but the risk of macrosomia, neonatal hypoglycemia, and fetal birth weight were higher. However, there is 1 RCT[16] concluded that metformin was equivalent to glyburide both for women and newborns. Moreover, another review[17] mentioned that glyburide is more effective in lowering blood sugar in women with GDM, and with a lower treatment failure rate than metformin. Therefore, there is still debate about which would be the most favorable hypoglycemic drugs in GDM patients.
In recent years, several previous traditional meta-analyses (TMAs) have been performed to compare the efficacy and safety of OADs with insulin. Nevertheless, the results were inconsistent due to the lack of evidence from head-to-head RCTs. However, network meta-analysis (NMA), also known as mixed treatment comparisons (MTC), allows to compare more than 2 treatments (e.g., treatments A, B, C), by including both direct and indirect comparisons, and thereby making it possible to rank all the treatments, and to pool all the available evidence.[18,19] In 2014, one NMA[20] including 18 RCTs revealed that both metformin and glyburide are suitable for use in the management of GDM, but glyburide was associated with more adverse pregnancy outcomes. However, there are increasing number of new clinical trials conducted to evaluate the relative efficacy and safety of OADs in GDM, we therefore performed an updated NMA to provide a more comprehensive assessment for available treatments by incorporating additional trials published since the last review. The NMA presented here aimed to provide more powerful evidence about the efficacy and safety of different treatments in GDM.
2. Materials and methods
2.1. Ethnic statement
The meta-analysis was based on previous published studies, thus no ethical approval and patient consent are required.
2.2. Search strategy and selection criteria
We searched the databases including Medline, PubMed, Embase, Cochrane Library (last search was updated on December 31, 2016). The terms used to search were “Gestational Diabetes” or “GDM” and “oral hypoglycemic agents,” “oral antidiabetic drugs,” “glibenclamide,” “metformin,” “glyburide,” or “acarbose,” in combination with RCT. Finally, we searched for additional eligible trials in reference lists of retrieved publications and relevant meta-analyses.
Studies were included if they met the following criteria: subjects were women with gestational diabetes requiring drug treatment; randomized control trials (RCTs) of comparing efficacy and safety parameters of different OADs or OADs versus insulin for GDM; studies offering information at least 1 maternal or fetal outcome; maternal outcomes were glycohemoglobin (HbA1c), fasting blood glucose (FBG), 2-hour postprandial glucose (2HPG), pregnancy-induced hypertension (PIH), weight gain and preeclampsia; neonatal outcomes were hypoglycemia, mean birth weight, macrosomia, large for gestational age (LGA), preterm birth, neonatal intensive care unit (NICU), hyperbilirubinemia, respiratory distress syndrome (RDS) and gestational age at delivery. The exclusion criteria were as follows: Reviews, letters, and comments were excluded; studies published with insufficient information; duplicate studies were excluded, in the case that significant overlap with multiple publications by the same group; studies involving pregnant women with preexisting diabetes were excluded. No language restrictions were set.
2.3. Data collection and quality assessment
Two investigators independently reviewed trials for eligibility and extracted relevant information from included trials with a standard protocol, and assessed the risk of bias with the Cochrane risk of bias tool.[21] We extracted study characteristics (author name, publication year, country, BMI of study subjects, sample size), intervention, outcomes (maternal and neonatal outcomes), and risk of bias. Any disagreements between reviewers were resolved by discussion.
2.4. Outcomes of interest
Outcomes of interest were divided into 2 categories: neonatal outcomes and maternal outcomes. Neonatal outcomes included macrosomia, LGA births, hypoglycemia, mean birth weight, neonatal intensive care unit (NICU), hyperbilirubinemia, RDS, gestational age at delivery. Maternal outcomes included glycohemoglobin (HbA1c), FBG, 2HBG, PIH, weight gain, and preeclampsia. The endpoint definitions as applied in each trial were incorporated.
2.5. Statistical analysis
2.5.1. Pairwise meta-analysis
We conducted pairwise meta-analyses with a fixed effects model or random effects model. The standardized mean difference (SMD) was calculated as the effect size for continuous variables and the odds ratio (OR) was calculated for dichotomous variables, both with 95% CI. The I2 statistic and P-value was used to quantify heterogeneity in each pairwise comparison. I2 > 50% or P < .01 indicated the existence of heterogeneity across the studies.[21] The Egger test was used to detect publication bias. All statistical analysis was conducted using STATA version 12.0 (Stata Corp, College Station, TX).
2.5.2. Network meta-analysis (NMA)
The Bayesian NMA is a generalization of pair-wise meta-analysis, which was performed within Bayesian inference with the use of Gibbs sampling methods that allow combined direct and indirect comparisons. An advantage of this approach is that it is straightforward to extend to shared parameter models where different RCTs outcomes in different formats but from a common underlying model.[18] Then, a random-effects model was selected to allow for heterogeneity among trials on the assumption that different treatment effects originated from a normal distribution. Bayesian inference with WinBUGS software (version 1.4.3, MRC Biostatistics Unit, Cambridge, UK)[21] uses Markov Chain Monte Carlo (MCMC) simulation to calculate the posterior distributions within the framework of the chosen model and likelihood function and on the basis of some prior assumptions.[22,23]
Further analysis performed using R version 3.3.1 (The R Foundation for Statistical Computing) and STATA 12.0 software (Stata Corp).[24] The results of NMA with effect sizes (SMD or OR) and their credible intervals (CI) were obtained by the MCMC method. See Appendix for details about the WinBUGS codes used. Three Markov chains ran simultaneously with different initial values chosen arbitrarily, with 40,000 iterations, and the first 10,000 simulations were discarded due to the burn-in period.[25,26] A network plot was drawn with the nodes representing interventions, the node size representing sample sizes, and the line thicknesses indicating the available direct comparisons between pairs of interventions.
We did the inconsistency analysis with RoR (the ratio of 2 ORs) values in every closed loop and drawn inconsistency plot to assess inconsistency between direct and indirect sources of evidence. RoR values close to 1 mean that the 2 sources are in agreement.[27]
The surface under the cumulative ranking curve (SUCRA) is used to provide a hierarchy of the treatments. The SUCRA value was presented as the percentage of the area under the curve, the larger the SUCRA value, the better the treatment or the lower the incidence of adverse effects. The presence of small-study effects in a meta-analysis is assessed by comparison-adjusted funnel plot.[28]
3. Results
3.1. Characteristics of the included studies
Figure 1 shows the study selection process of included trials. A total of 583 studies were initially identified by literature research, among which 464 studies were excluded for not RCTs or duplicated studies. Then after screening titles, abstracts, and full text, 86 studies were discarded because of irrelevant interventions, review or letter, duplicated study, not relevant outcomes, intervention or included population that did not meet inclusion criteria, case control study. Eventually, we enrolled 31 studies[16,29–58] (corresponding to 32 RCTs) in the NMA. The characteristics of the included studies are presented in Table 1. Of all these 32 RCTs, 30 were 2-arm trials and 2 were 3-arm trials, with a total of 4723 GDM patients were enrolled. Among them, 10 and 13 RCTs reported patients with or without obesity (defined as BMI ≥ 30), respectively, the rest of 8 RCTs did not mention clearly. Subjects involved in this meta-analysis were treated with metformin (A), metformin plus insulin (B), insulin (C), glyburide (D), placebo (E), and acarbose (F). Figure 2A–E and Appendix Fig. 1 show the network plot of eligible comparisons for different treatments, and contribution plot are shown in Appendix Fig. 2.
Figure 1.
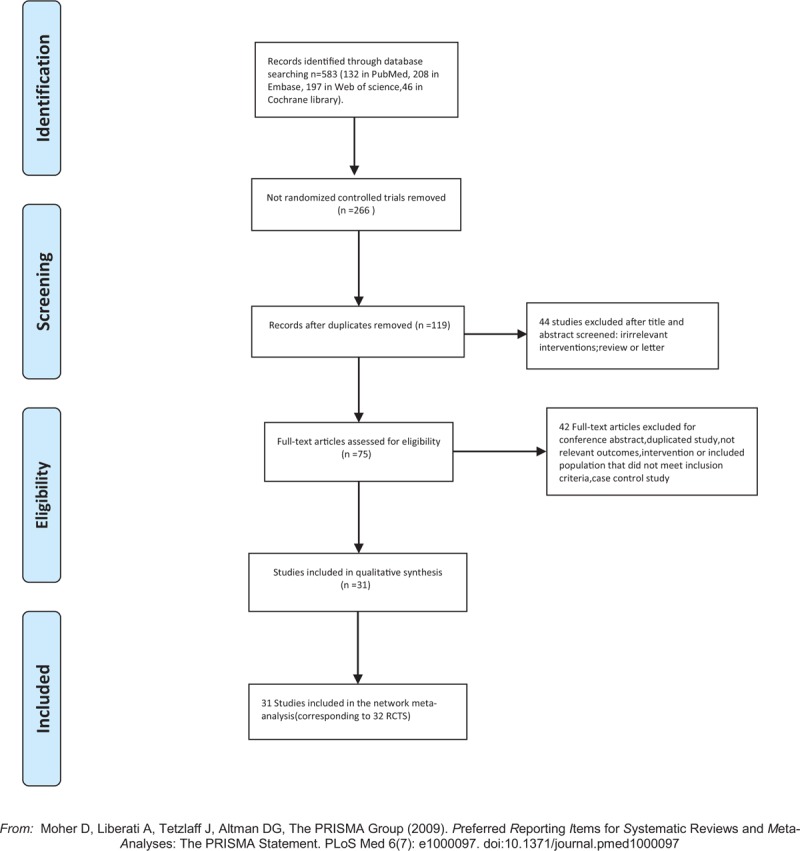
Flow diagram of study selection.
Table 1.
Main characteristics of the randomized trials included in the network meta-analysis.
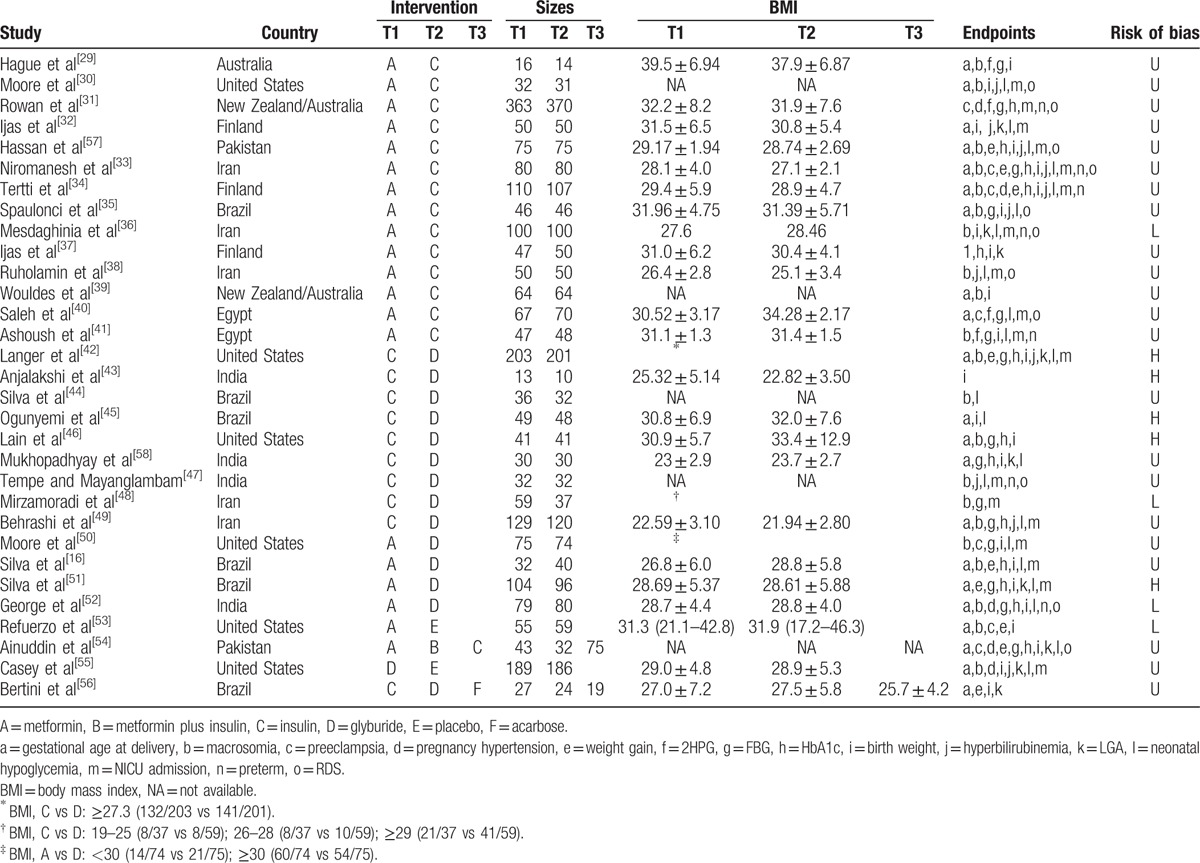
Figure 2.
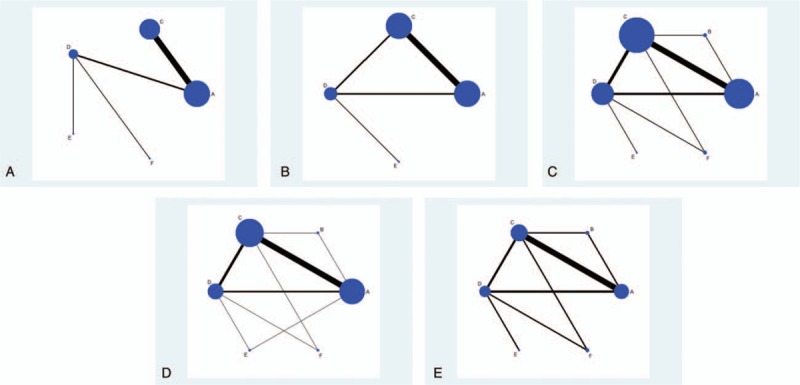
(A–E) The network plot of eligible comparisons for different treatments.
3.2. Results from pairwise meta-analysis and network meta-analysis
The results of the Pairwise meta-analysis and NMA are presented as a league table in Table 2 and Appendix Tables 1 and 2.
Table 2.
ORs or SMD and 95% CI of 6 treatments according to the network meta-analysis.
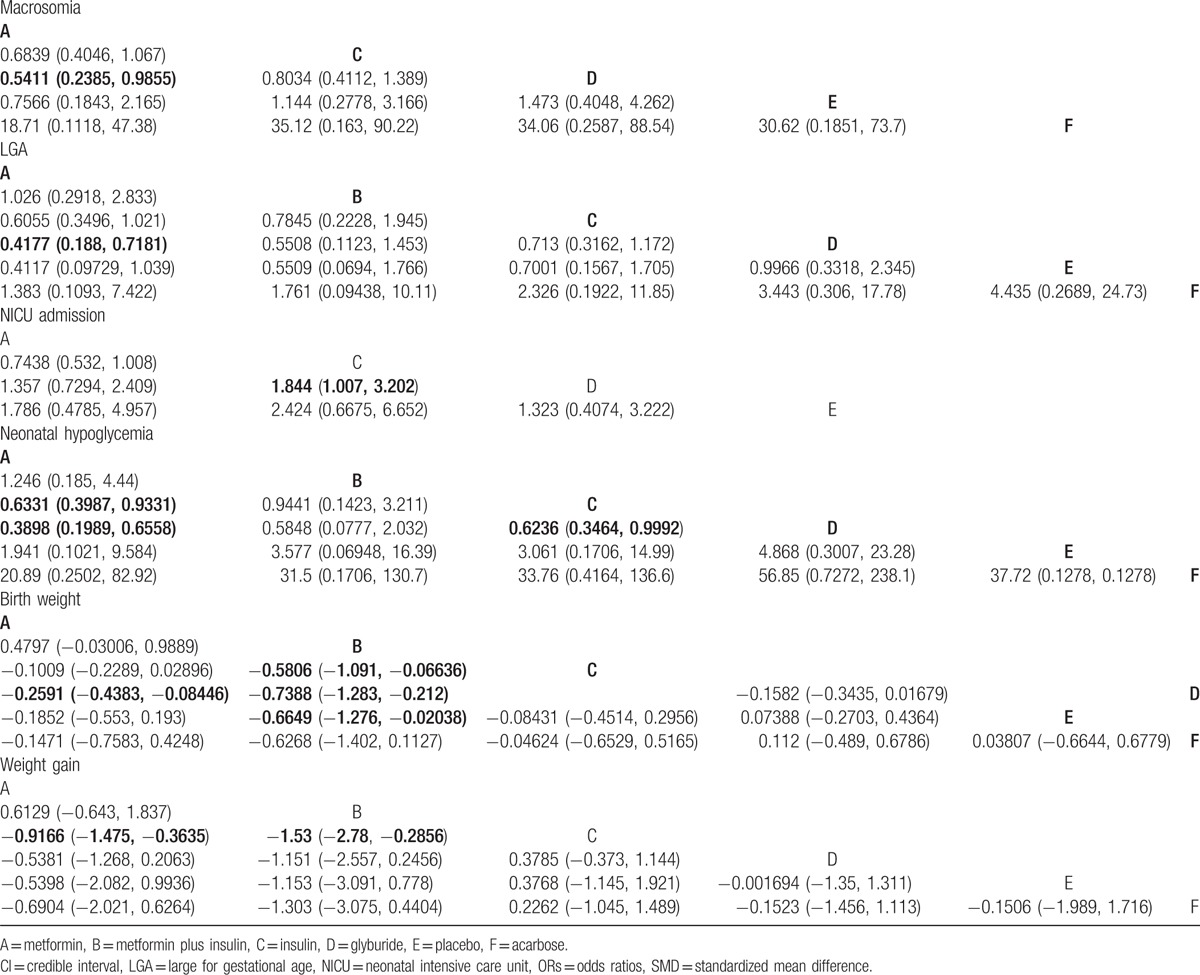
3.3. Macrosomia
Twenty-five studies involving 3412 GDM patients reported the macrosomia. In the pairwise meta-analysis, insulin showed no statistical significance compared with glyburide (OR, 0.788; 95% CI, 0.510–1.219); metformin was significantly lower compared with insulin (OR, 0.729; 95% CI, 0.545–0.974), but had no significant difference compared with glyburide (OR, 0.587; 95% CI, 0.239–1.442).
The NMA revealed that metformin was significantly lower compared with glyburide (OR, 0.5411; 95% CI, 0.2385–0.9855), but there were no significance between metformin and insulin or insulin and glyburide.
3.4. LGA
Fifteen studies involving 1813 GDM patients reported the incidence of LGA. In the pairwise meta-analysis, metformin was significantly lower than insulin (OR, 0.647; 95% CI, 0.438–0.956), and glyburide (OR, 0.431; 95% CI, 0.229–0.814), but had no significant difference between insulin and glyburide (OR, 0.838; 95% CI, 0.542–1.295).
In the NMA, metformin was observed to have lower incidence of LGA than glyburide (OR, 0.4177; 95% CI, 0.188–0.7181). No other significant results were observed about the incidence of LGA.
3.5. Preterm
Nine studies involving 1879 GDM patients were involved in the analysis of incidence of preterm. In the pairwise meta-analysis, metformin showed a significant increase compared with glyburide (OR, 2.887; 95% CI, 1.087–7.666), but showed no significance compared with insulin (OR, 1.332; 95% CI, 0.939–1.890).
In the NMA, we did not find any significant results about the incidence of preterm.
3.6. Admission to the NICU
Eighteen studies involving 3635 GDM patients focused on the incidence of admission to the NICU. In the pairwise meta-analysis, we only observed that metformin has a lower incidence of admission to the NICU than insulin (OR, 0.772; 95% CI, 0.644–0.927).
In the NMA, glyburide had significant lower incidence of admission to the NICU compared with insulin (OR, 0.542; 95% CI, 0.312–0.993), no other significant results were found.
3.7. Neonatal hypoglycemia
Twenty-six studies involving 3360 GDM patients reported the incidence of neonatal hypoglycemia. In the pairwise meta-analysis, metformin had lower incidence of neonatal hypoglycemia than insulin (OR, 0.636; 95% CI, 0.486–0.832), and insulin was lower than glyburide (OR, 0.647; 95% CI, 0.423–0.991).
In the NMA, metformin was significantly lower compared with insulin (OR, 0.6331; 95% CI, 0.3987–0.9331), and glyburide (OR, 0.3898; 95% CI, 0.1989–0.6558). Besides, insulin was significantly lower than glyburide (OR, 0.6236; 95% CI, 0.3464–0.9992). No other significant results were found.
3.8. Birth weight
Thirty studies involving 4060 GDM patients reported the mean birth weight. In the pairwise meta-analysis, metformin was significantly lower than insulin (SMD, −0.111; 95% CI, −0.194 to −0.028), and glyburide (SMD, −0.235; 95% CI, −0.399 to −0.071); insulin was significantly lower compared with glyburide (SMD, −0.180; 95% CI, −0.327 to −0.033).
In the NMA, we observed that metformin plus insulin has lower birth weight than insulin, glyburide and placebo (SMD, −0.5806; 95% CI, −1.091 to −0.06636; SMD, −0.7388; 95% CI, −1.283 to −0.212; and SMD, −0.6649; 95% CI, −1.276 to −0.02038, respectively). Besides, metformin was observed to have significantly lower birth weight than glyburide (SMD, −0.2591; 95% CI, −0.4383 to −0.08446).
3.9. 2-hour postprandial glucose (2HPG)
Six studies involving 1345 GDM patients focused on the 2HPG. In the pairwise meta-analysis, metformin showed lower 2HPG than insulin (SMD, −0.285; 95% CI, −0.417 to −0.154), and insulin was lower than glyburide (SMD, −0.302; 95% CI, −0.493 to −0.111). In the NMA, there were no significant results.
3.10. Fasting blood glucose (FBG)
Seventeen studies involving 2769 GDM patients reported the FBG. In the pairwise meta-analysis, only metformin showed higher FBG than glyburide (SMD, 0.192; 95% CI, 0.018–0.366). No other significant results were observed. In the NMA, however, we did not get significant results between groups.
3.11. Glycohemoglobin (HbA1c)
Seventeen studies involving 2887 GDM patients reported the HbA1c. However, we did not obtain significant results from pairwise meta-analysis or NMA.
3.12. Gestational age at delivery
Twenty-seven studies involving 4146 GDM patients focused on the gestational age at delivery. In the pairwise meta-analysis, metformin and metformin plus insulin were significantly lower than insulin (SMD, −0.126; 95% CI, −0.212 to −0.040; SMD, −0.284; 95% CI, −0.521 to −0.048, respectively), and insulin was significantly lower than glyburide (SMD, −0.180; 95% CI, −0.303 to −0.057). In the NMA, no significant difference was identified between groups.
3.13. Weight gain
Fourteen studies involving 1893 GDM patients were enrolled in the analysis of weight gain. In the pairwise meta-analysis, metformin was significantly lower compared with insulin (SMD, −0.774; 95% CI, −0.928 to −0.620), and glyburide (SMD, −0.321; 95% CI, −0.560 to −0.081).
In the NMA, metformin and metformin plus insulin were observed to have significantly lower weight gain than insulin (SMD, −0.9166; 95% CI, −1.475 to −0.3635; SMD, −1.53; 95% CI, −2.78 to −0.2856, respectively). No other significant differences were observed about the weight gain.
3.14. Other outcomes
Thirteen studies involving 2008 GDM patients were enrolled in the analysis of the incidence of RDS, 10 studies involving 1906 patients focused on the incidence of hyperbilirubinemia, and 17 studies involving 2887 patients reported HbA1c. Besides, 7 studies involving 1634 patients were included in the analysis of PIH, 11 studies involving 1754 patients regarding the incidence of preeclampsia. However, both pairwise meta-analysis and NMA results show no significant differences between groups among these outcomes.
3.15. Relative ranking of 6 kinds of treatments in GDM patients
We compared the relative rank probabilities of different treatments based on SUCRA values (Table 3) and cumulative probability plots (Appendix Fig. 3A–O). The larger the SUCRA value, the better the rank of the treatment or the lower the incidence of adverse effects. According to the result, metformin ranked the best with the lowest incidence of macrosomia, 2HPG, LGA, and RDS; metformin plus insulin ranked the best regarding the risk of PIH, gestational age at delivery, weight gain, mean birth weight, and FBG; glyburide ranked the worst regarding the risk of macrosomia, preeclampsia, hyperbilirubinemia, neonatal hypoglycemia, and gestational age at delivery and mean birth weight, but ranked the best regarding the risk of NICU admission and HbA1C. Besides, insulin ranked the worst regarding the incidence of NICU admission. Acarbose ranked the best regarding the risk of neonatal hypoglycemia.
Table 3.
SUCRA values of 6 treatments under different pregnancy outcomes.

3.16. The efficacy of OADs between GDM patients with and without obesity
Ten studies involving 1577 obese GDM patients were enrolled in the analysis of the efficacy of OADs. As for 2HPG, metformin ranked the best, followed by insulin and glyburide. For FBG, metformin also ranked the best, followed by glyburide and insulin. However, regarding HbA1c, glyburide ranked the best, followed by metformin and insulin.
Thirteen studies involving 2035 nonobese GDM patients were enrolled focusing on the efficacy of OADs. As for FBG, insulin ranked the best, followed by glyburide, metformin ranked the worst. For HbA1c, glyburide ranked the best, followed by insulin, metformin ranked the worst. Detailed results are shown in Appendix Tables 3 and 4.
3.17. Publication bias
As suggested by the Egger test (Appendix Table 1) and comparison-adjusted funnel plot for each outcome from NMA (Fig. 3A–E and Appendix Fig. 4), there was no significant publication bias among various studies.
Figure 3.
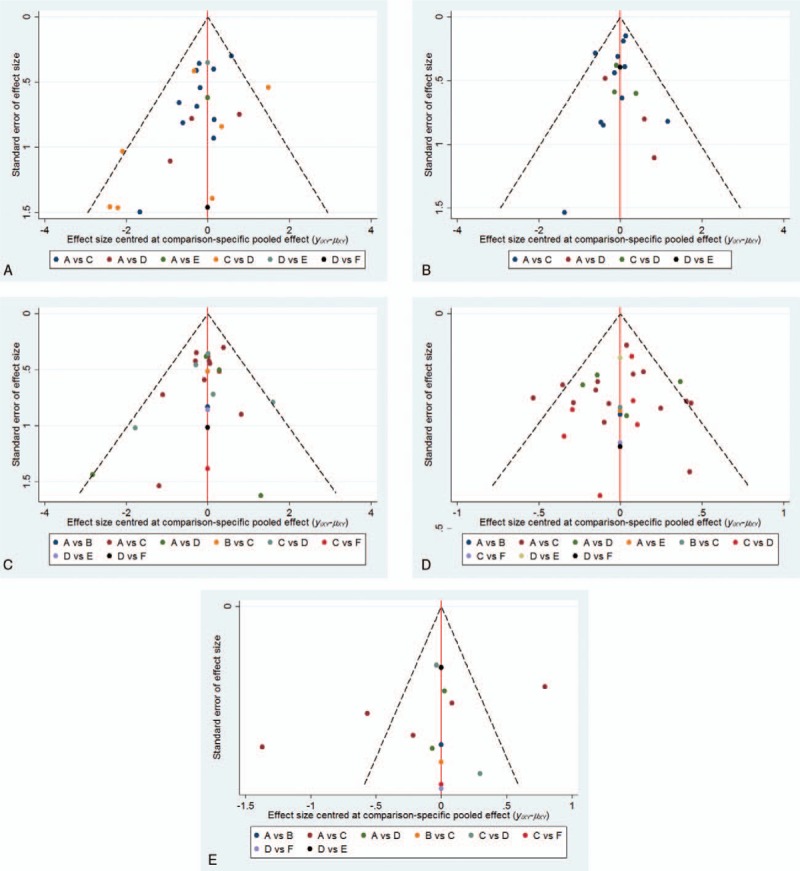
(A–E) The comparison-adjusted funnel plot from network meta-analysis.
3.18. Comparisons between pairwise meta-analysis and network meta-analysis
The results of pairwise meta-analysis and NMA are shown in Table 2 and Appendix Tables 1 and 2. Although the pooled estimates for the outcome showed minor differences, the confidence intervals from pairwise meta-analysis and NMA are generally consistent in majority comparisons. Tests of inconsistency showed that there was no significant inconsistency between direct and indirect comparisons (Appendix Fig. 5A–K).
4. Discussion
The purpose of this NMA was to evaluate the efficacy and safety of all commonly used pharmaceutical treatment for GDM, compare with each other, and rank them. In most of our included studies, we observed that the baseline BMI were slightly higher in metformin group, however, there was no statistical significance in all but one of the baseline BMI. The mean BMI of the GDM patients in Moore et al show that a significant number of them are obese in metformin group. Our findings show that glyburide might be the optimum treatment regarding average glucose control, and metformin is the fastest in glucose control for GDM patients. Then, we further explored the efficacy of OADs between GDM patients with and without obesity, and found that in obese GDM patients, metformin is generally superior to glyburide and insulin, but for nonobese GDM patients, glyburide is better than insulin and metformin, which is supported by a previous systematic review.[59] Moreover, glyburide ranked the worst with the highest incidence of macrosomia, preeclampsia, hyperbilirubinemia, neonatal hypoglycemia, preterm birth, and low birth weight; metformin (plus insulin when required) has the lowest risk of macrosomia, pregnancy hypertension, LGA, RDS, preterm birth, and low birth weight. Besides, insulin had the highest incidence of NICU admission, acarbose had the lowest risk of neonatal hypoglycemia. These findings are an important addition to our knowledge about which drugs are most optimal in treatment of GDM patients.
Our findings confirm and extend previous focused studies, but go beyond them, because the network technique makes us can synthesize the data from trials with more than 2 interventions, incorporate both direct and indirect evidence, increases the accuracy in the estimates, and produces a relative rank for all kinds of treatments.[28,60] All previous meta-analyses[15,20,61,62] drawn the conclusion that OADs and insulin are comparable in glucose control simply from pairwise meta-analysis. In our paper, we firstly adopted network technique to combine and rank all kinds of treatments for GDM patients from different variables. For 2HPG and FBG, metformin (plus insulin when required) ranked the best indicating it reached glucose targets sooner; but for HbA1c, glyburide ranked the best, followed by insulin, metformin ranked the worst; However, FBG and 2-hour postprandial blood glucose are susceptible to eating, glucose metabolism and other related factors, merely reflecting the level of blood sugar in a specific time. HbA1c can be stable and reliable to reflect the average blood glucose level within 120 days, which has become the gold standard for diabetes monitoring. Thus, our finding suggests glyburide might be the optimum treatment regarding average glucose control, and metformin is the fastest in glucose control for GDM patients. However, every treatment may have some extent failure rate in glucose control,[30] and the failure of treatment was related to the severity of GDM. Thus, clinicians should also inform patients the risk of failure when choose to utilize OADs.
In terms of glyburide, it ranked the worst with highest risk of macrosomia, preeclampsia, hyperbilirubinemia, neonatal hypoglycemia, and higher gestational age at delivery and mean birth weight. Previous reviews[20,63] also found glyburide had increased incidence of macrosomia than metformin.
Moreover, metformin (plus insulin when required) ranked the best with the lowest incidence of macrosomia, PIH, LGA, RDS. But in terms of preterm, metformin ranked the worst with the highest risk of preterm birth. Furthermore, as for NICU admission, previous meta-analyses[14,64,65] showed that metformin presented significantly lower incidence of NICU compared with insulin, which is in line with our pairwise meta-analysis (RR, 0.772; 95% CI, 0.644–0.927), did not provide more detailed results about glyburide. However, in our study, we found insulin has the highest risk of NICU admission, followed by metformin, glyburide ranked the best in reducing the risk of NICU admission. Besides, acarbose ranked the best in reducing the risk of neonatal hypoglycemia, followed by metformin (plus insulin when required), insulin, and glyburide.
Jiang et al[20] reported a NMA result about GDM pharmaceutical treatment that also integrate direct and indirect evidence, which were not completely coincident with our results. Their analysis included fewer interventions than did in our analysis; have not included the intervention of metformin plus insulin and the outcomes of RDS and hyperbilirubinemia; and have not presented the contribution plot and cumulative probability plot. The most important is that we added new RCTs and ranked all the treatments in various outcomes, our results were more detailed and maybe more reliable.
To our knowledge, this is the largest and most comprehensive synthesis of data to date for available pharmacological treatments for GDM patients. The NMA synthesizes direct and indirect evidence that allowed comparison of all available treatments for GDM and ranking them in a single analysis, rather than separate and disconnected meta-analyses for individual pairs of treatments, which increases the precision in the estimates.[66] Thus, results from NMAs are more likely to be helpful to clinicians when making choices among multiple alternatives.
Several limitations are worth noting. First, despite the sample size of the present study is largest up to date, we can only analyze 12 outcomes reported in the original RCTs and do not consider every possible relevant outcome because of too few studies were included and a small number of events. Second, indirect evidence is susceptible to confounding,[67] and thus should be regarded with caution since it does not always consistent with the corresponding direct estimates.[68,69] However, our analysis yielded low heterogeneity and little evidence for inconsistency. Third, we cannot explore treatment outcomes in different ethnicity without access to individual patient data. Fourth, to reduce heterogeneity, we enrolled only trials comparing among OADs or insulin, excluding trials comparing other treatment strategy.
In conclusion, metformin have more favorable pregnancy outcomes and the fastest rate of glucose control, especially in obese GDM patients, but with lowest rate of average glucose control; glyburide have the highest rate of average glucose control, particularly in nonobese GDM patients, but with more adverse outcomes. Clinicians should carefully balance the risk and benefit of different treatments according to various situations in selecting different GDM treatment strategy.
Acknowledgment
The authors thank all the anonymous reviewers and editors for their suggestions, which will be helpful for us to improve our paper.
Supplementary Material
Footnotes
Abbreviations: 2HPG = 2-hour postprandial glucose, FBG = fasting blood glucose, GDM = gestational diabetes mellitus, LGA = large for gestational age, NAM = network meta-analysis, NICU = neonatal intensive care unit, OADs = oral antidiabetic drugs, OR = odds ratio, PIH = pregnancy-induced hypertension, RCTs = randomized control trials, RDS = respiratory distress syndrome, SMD = standardized mean difference, SUCRA = surface under the cumulative ranking curve, TMA = traditional meta-analysis.
Funding: This paper received no specific grant from any funding agency.
Authors’ contributions: H-lL contributed to the study inception, literature search and selection, data extraction, analysis and writing the manuscript. S-jM contributed to the quality assessment and manuscript revising, Y-nX contributed to the quality assessment. H-zT contributed to the manuscript revising and study supervision. All authors gave comments on the revised manuscript and approved the final version of the manuscript.
The authors have no conflicts of interest to disclose.
Supplemental Digital Content is available for this article.
References
- [1].Ferrara A. Increasing prevalence of gestational diabetes mellitus: a public health perspective. Diabetes Care 2007;30(suppl 2):S141–6. [DOI] [PubMed] [Google Scholar]
- [2].Coustan DR. Gestational diabetes mellitus. Clin Chem 2013;59:1310–21. [DOI] [PubMed] [Google Scholar]
- [3].American Diabetes A. Diagnosis and classification of diabetes mellitus. Diabetes Care 2014;37(suppl 1):S81–90. [DOI] [PubMed] [Google Scholar]
- [4].Wikipedia contributors. Gestational diabetes. Wikipedia, The Free Encyclopedia. August 2017. [Google Scholar]
- [5].Coetzee EJ. Pregnancy and diabetes scenario around the world: Africa. Int J Gynaecol 2009;104(suppl 1):S39–41. [DOI] [PubMed] [Google Scholar]
- [6].Glueck CJ, Goldenberg N, Streicher P, et al. The contentious nature of gestational diabetes: diet, insulin, glyburide and metformin. Expert Opin Pharmacother 2002;3:1557–68. [DOI] [PubMed] [Google Scholar]
- [7].Nicholson W, Baptisteroberts K. Oral hypoglycaemic agents during pregnancy: the evidence for effectiveness and safety. Best Pract Res Clin Obstet Gynaecol 2011;25:51–63. [DOI] [PubMed] [Google Scholar]
- [8].Norman RJ, Wang JX, Hague W. Should we continue or stop insulin sensitizing drugs during pregnancy? Curr Opin Obstet Gynecol 2004;16:245–50. [DOI] [PubMed] [Google Scholar]
- [9].Petry CJ. Gestational diabetes: risk factors and recent advances in its genetics and treatment. Br J Nutr 2010;104:775–87. [DOI] [PubMed] [Google Scholar]
- [10].Maymone AC, Baillargeon JP, Menard J, et al. Oral hypoglycemic agents for gestational diabetes mellitus? Expert Opin Drug Saf 2011;10:227–38. [DOI] [PubMed] [Google Scholar]
- [11].Conway DL, Gonzales O, Skiver D. Use of glyburide for the treatment of gestational diabetes: the San Antonio experience. J Matern Fetal Neonatal Med 2004;15:51–5. [DOI] [PubMed] [Google Scholar]
- [12].Collier CA, Bruce CR, Smith AC, et al. Metformin counters the insulin-induced suppression of fatty acid oxidation and stimulation of triacylglycerol storage in rodent skeletal muscle. Am J Physiol 2006;291:182–9. [DOI] [PubMed] [Google Scholar]
- [13].Serrano-Martin X, Payares G, Mendoza-Leon A. Glibenclamide, a blocker of K+ATP channels, shows antileishmanial activity in experimental murine cutaneous leishmaniasis. Antimicrob Agents Chemother 2006;50:4214–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Li G, Zhao S, Cui S, et al. Effect comparison of metformin with insulin treatment for gestational diabetes: a meta-analysis based on RCTs. Arch Gynecol Obstet 2015;292:111–20. [DOI] [PubMed] [Google Scholar]
- [15].Zeng YC, Li MJ, Chen Y, et al. The use of glyburide in the management of gestational diabetes mellitus: a meta-analysis. Adv Med Sci 2014;59:95–101. [DOI] [PubMed] [Google Scholar]
- [16].Silva JC, Pacheco C, Bizato J, et al. Metformin compared with glyburide for the management of gestational diabetes. Int J Gynaecol Obstet 2010;111:37–40. [DOI] [PubMed] [Google Scholar]
- [17].Kalra B, Gupta Y, Singla R, et al. Use of oral anti-diabetic agents in pregnancy: a pragmatic approach. N Am J Med Sci 2015;7:6–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Dias S, Sutton AJ, Ades AE, et al. Evidence synthesis for decision making 2: a generalized linear modeling framework for pairwise and network meta-analysis of randomized controlled trials. Med Decis Making 2013;33:607–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Caldwell DM, Ades AE, Higgins JP. Simultaneous comparison of multiple treatments: combining direct and indirect evidence. BMJ 2005;331:897–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Jiang YF, Chen XY, Ding T, et al. Comparative efficacy and safety of OADs in management of GDM: network meta-analysis of randomized controlled trials. J Clinical Endocrinol Metab 2015;100:2071–80. [DOI] [PubMed] [Google Scholar]
- [21].Lumley T. Network meta-analysis for indirect treatment comparisons. Stat Med 2002;21:2313–24. [DOI] [PubMed] [Google Scholar]
- [22].Katsanos K, Spiliopoulos S, Karunanithy N, et al. Bayesian network meta-analysis of nitinol stents, covered stents, drug-eluting stents, and drug-coated balloons in the femoropopliteal artery. J Vasc Surg 2014;59:1123.e8–33.e8. [DOI] [PubMed] [Google Scholar]
- [23].Song G-M, Tian X, Zhang L, et al. Immunonutrition support for patients undergoing surgery for gastrointestinal malignancy: preoperative, postoperative, or perioperative? A Bayesian Network Meta-Analysis of Randomized Controlled Trials. Medicine 2015;94:e1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Salanti G, Higgins JP, Ades AE, et al. Evaluation of networks of randomized trials. Stat Methods Med Res 2008;17:279–301. [DOI] [PubMed] [Google Scholar]
- [25].Spiegelhalter D, Thomas A, Best N, et al. WinBugs Version 1.4 User Manual. Cambridge, UK: MRC Biostatistics Unit; 2003. [Google Scholar]
- [26].Ades AE, Welton NJ, Caldwell D, et al. Multiparameter evidence synthesis in epidemiology and medical decision-making. J Health Serv Res Policy 2008;13(suppl 3):12–22. [DOI] [PubMed] [Google Scholar]
- [27].Chaimani A, Higgins JP, Mavridis D, et al. Graphical tools for network meta-analysis in STATA. PLoS ONE 2013;8:e76654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Salanti G, Ades AE, Ioannidis JP. Graphical methods and numerical summaries for presenting results from multiple-treatment meta-analysis: an overview and tutorial. J Clin Epidemiol 2011;64:163–71. [DOI] [PubMed] [Google Scholar]
- [29].Hague WM, Davoren PM, Oliver J, et al. Contraindications to use of metformin. Metformin may be useful in gestational diabetes. BMJ 2003;326:762.author reply 762. [PubMed] [Google Scholar]
- [30].Moore LE, Briery CM, Clokey D, et al. Metformin and insulin in the management of gestational diabetes mellitus: preliminary results of a comparison. J Reprod Med 2007;52:1011–5. [PubMed] [Google Scholar]
- [31].Rowan JA, Hague WM, Gao W, et al. Metformin versus insulin for the treatment of gestational diabetes. N Engl J Med 2008;358:2003–15. [DOI] [PubMed] [Google Scholar]
- [32].Ijas H, Vaarasmaki M, Morin-Papunen L, et al. Metformin should be considered in the treatment of gestational diabetes: a prospective randomised study. BJOG 2011;118:880–5. [DOI] [PubMed] [Google Scholar]
- [33].Niromanesh S, Alavi A, Sharbaf FR, et al. Metformin compared with insulin in the management of gestational diabetes mellitus: a randomized clinical trial. Diabetes Res Clin Pract 2012;98:422–9. [DOI] [PubMed] [Google Scholar]
- [34].Tertti K, Ekblad U, Koskinen P, et al. Metformin vs. insulin in gestational diabetes. A randomized study characterizing metformin patients needing additional insulin. Diabetes Obes Metab 2013;15:246–51. [DOI] [PubMed] [Google Scholar]
- [35].Spaulonci CP, Bernardes LS, Trindade TC, et al. Randomized trial of metformin vs insulin in the management of gestational diabetes. Am J Obstet Gynecol 2013;209:34.e1–7. [DOI] [PubMed] [Google Scholar]
- [36].Mesdaghinia E, Samimi M, Homaei Z, et al. Comparison of newborn outcomes in women with gestational diabetes mellitus treated with metformin or insulin: a randomised blinded trial. Int J Prev Med 2013;4:327–33. [PMC free article] [PubMed] [Google Scholar]
- [37].Ijas H, Vaarasmaki M, Saarela T, et al. A follow-up of a randomised study of metformin and insulin in gestational diabetes mellitus: growth and development of the children at the age of 18 months. BJOG 2015;122:994–1000. [DOI] [PubMed] [Google Scholar]
- [38].Ruholamin S, Eshaghian S, Allame Z. Neonatal outcomes in women with gestational diabetes mellitus treated with metformin in compare with insulin: a randomized clinical trial. J Res Med Sci 2014;19:970–5. [PMC free article] [PubMed] [Google Scholar]
- [39].Wouldes TA, Battin M, Coat S, et al. Neurodevelopmental outcome at 2 years in offspring of women randomised to metformin or insulin treatment for gestational diabetes. Arch Dis Child Fetal Neonatal Ed 2016;72: 2015-309602. [DOI] [PubMed] [Google Scholar]
- [40].Saleh HS, Abdelsalam WA, Mowafy HE, et al. Could metformin manage gestational diabetes mellitus instead of insulin? Int J Reprod Med 2016;2016:3480629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Ashoush S, El-Said M, Fathi H, et al. Identification of metformin poor responders, requiring supplemental insulin, during randomization of metformin versus insulin for the control of gestational diabetes mellitus. J Obstet Gynaecol Res 2016;42:640–7. [DOI] [PubMed] [Google Scholar]
- [42].Langer O, Conway DL, Berkus MD, et al. A comparison of glyburide and insulin in women with gestational diabetes mellitus. N Engl J Med 2000;343:1134–8. [DOI] [PubMed] [Google Scholar]
- [43].Anjalakshi C, Balaji V, Balaji MS, et al. A prospective study comparing insulin and glibenclamide in gestational diabetes mellitus in Asian Indian women. Diabetes Res Clin Pract 2007;76:474–5. [DOI] [PubMed] [Google Scholar]
- [44].Silva JC, Bertini AM, Taborda W, et al. [Glibenclamide in the treatment for gestational diabetes mellitus in a compared study to insulin]. Arq Bras Endocrinol Metabol 2007;51:541–6. [DOI] [PubMed] [Google Scholar]
- [45].Ogunyemi D, Jesse M, Davidson M. Comparison of glyburide versus insulin in management of gestational diabetes mellitus. Endocr Pract 2007;13:427–8. [DOI] [PubMed] [Google Scholar]
- [46].Lain KY, Garabedian MJ, Daftary A, et al. Neonatal adiposity following maternal treatment of gestational diabetes with glyburide compared with insulin. Am J Obstet Gynecol 2009;200:501.e1–6. [DOI] [PubMed] [Google Scholar]
- [47].Tempe A, Mayanglambam RD. Glyburide as treatment option for gestational diabetes mellitus. J Obstet Gynaecol Res 2013;39:1147–52. [DOI] [PubMed] [Google Scholar]
- [48].Mirzamoradi M, Heidar Z, Faalpoor Z, et al. Comparison of glyburide and insulin in women with gestational diabetes mellitus and associated perinatal outcome: a randomized clinical trial. Acta Med Iran 2015;53:97–103. [PubMed] [Google Scholar]
- [49].Behrashi M, Samimi M, Ghasemi T, et al. Comparison of glibenclamide and insulin on neonatal outcomes in pregnant women with gestational diabetes. Int J Prev Med 2016;7:88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Moore LE, Clokey D, Rappaport VJ, et al. Metformin compared with glyburide in gestational diabetes: a randomized controlled trial. Obstet Gynecol 2010;115:55–9. [DOI] [PubMed] [Google Scholar]
- [51].Silva JC, Fachin DR, Coral ML, et al. Perinatal impact of the use of metformin and glyburide for the treatment of gestational diabetes mellitus. J Perinat Med 2012;40:225–8. [DOI] [PubMed] [Google Scholar]
- [52].George A, Mathews JE, Sam D, et al. Comparison of neonatal outcomes in women with gestational diabetes with moderate hyperglycaemia on metformin or glibenclamide—a randomised controlled trial. Aust N Z J Obstet Gynaecol 2015;55:47–52. [DOI] [PubMed] [Google Scholar]
- [53].Refuerzo JS, Viteri OA, Hutchinson M, et al. The effects of metformin on weight loss in women with gestational diabetes: a pilot randomized, placebo-controlled trial. Am J Obstet Gynecol 2015;212:389.e1–9. [DOI] [PubMed] [Google Scholar]
- [54].Ainuddin J, Karim N, Hasan AA, et al. Metformin versus insulin treatment in gestational diabetes in pregnancy in a developing country: a randomized control trial. Diabetes Res Clin Pract 2015;107:290–9. [DOI] [PubMed] [Google Scholar]
- [55].Casey BM, Duryea EL, Abbassi-Ghanavati M, et al. Glyburide in women with mild gestational diabetes: a randomized controlled trial. Obstet Gynecol 2015;126:303–9. [DOI] [PubMed] [Google Scholar]
- [56].Bertini AM, Silva JC, Taborda W, et al. Perinatal outcomes and the use of oral hypoglycemic agents. J Perinat Med 33, 2005, 519–523. [DOI] [PubMed] [Google Scholar]
- [57].Hassan JA, Karim N, Sheikh Z. Metformin prevents macrosomia and neonatal morbidity in gestational diabetes. Pak J Med Sci 2012;28:384–9. [Google Scholar]
- [58].Mukhopadhyay P, Bag TS, Kyal A, et al. Oral hypoglycemic glibenclamide: can it be a substitute to insulin in the management of gestational diabetes mellitus? A comparative study. J SAFOG 2012;4:28–31. [Google Scholar]
- [59].Lautatzis ME, Goulis DG, Vrontakis M. Efficacy and safety of metformin during pregnancy in women with gestational diabetes mellitus or polycystic ovary syndrome: a systematic review. Metabolism 2013;62:1522–34. [DOI] [PubMed] [Google Scholar]
- [60].Bucher HC, Guyatt GH, Griffith LE, et al. The results of direct and indirect treatment comparisons in meta-analysis of randomized controlled trials. J Clin Epidemiol 1997;50:683–91. [DOI] [PubMed] [Google Scholar]
- [61].Gui J, Liu Q, Feng L. Metformin vs insulin in the management of gestational diabetes: a meta-analysis. PLoS ONE 2013;8:e64585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Su DF, Wang XY. Metformin vs insulin in the management of gestational diabetes: a systematic review and meta-analysis. Diabetes Res Clin Pract 2014;104:353–7. [DOI] [PubMed] [Google Scholar]
- [63].Balsells M, Garcia-Patterson A, Sola I, et al. Glibenclamide, metformin, and insulin for the treatment of gestational diabetes: a systematic review and meta-analysis. BMJ 2015;350:h102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Kitwitee P, Limwattananon S, Limwattananon C, et al. Metformin for the treatment of gestational diabetes: an updated meta-analysis. Diabetes Res Clin Pract 2015;109:521–32. [DOI] [PubMed] [Google Scholar]
- [65].Zhu B, Zhang L, Fan YY, et al. Metformin versus insulin in gestational diabetes mellitus: a meta-analysis of randomized clinical trials. Ir J Med Sci 2016;185:371–81. [DOI] [PubMed] [Google Scholar]
- [66].Loymans RJ, Gemperli A, Cohen J, et al. Comparative effectiveness of long term drug treatment strategies to prevent asthma exacerbations: network meta-analysis. BMJ 2014;348:g3009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Baker SG, Kramer BS. The transitive fallacy for randomized trials: if A bests B and B bests C in separate trials, is A better than C? BMC Med Res Methodol 2002;2:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Song F, Altman DG, Glenny AM, et al. Validity of indirect comparison for estimating efficacy of competing interventions: empirical evidence from published meta-analyses. BMJ 2003;326:472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Song F, Harvey I, Lilford R. Adjusted indirect comparison may be less biased than direct comparison for evaluating new pharmaceutical interventions. J Clin Epidemiol 2008;61:455–63. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


