Abstract
The aim of this study was to assess the role of 18F-FDG PET/CT in preoperative staging of vulvar cancer patients.
29 pts (69 years, range 51–88) with vulvar cancer (clinical apparent stage I-II), underwent preoperative FDG-PET/CT scan followed by radical vulvectomy and bilateral (or monolateral in case of tumor >2 cm from midline) inguinal lymphadenectomy ± sentinel node biopsy. PET/CT images were analyzed in consensus and correlated to histological findings according to a pt-based and a groin-based analyses. SUVmax of the nodal uptake of each inguinal area (if present) was calculated and correlated to histological findings. The presence of distant metastases was also considered and confirmed.
PET/CT analysis in consensus resulted negative at the inguinal LN level in 17 pts (10 true negative, 7 false negative) and positive in 12 pts (7 true positive, 5 false positive). Incidence of LN metastases resulted 48%. On pt-based analysis, sensitivity, specificity, accuracy, and negative and positive predictive value of PET/CT in detecting LN metastases were 50%, 67%, 59%, 59%, and 58%, respectively. On a groin-based analysis, considering overall 50 LN-sites, sensitivity, specificity, accuracy, and negative and positive predictive value of PET/CT were 53%, 85%, 73%, 67%, and 76%, respectively. The mean value of SUVmax was 6.1 (range 0.7–16.2) for metastatic nodes, whereas 1.6 (range 0.7 – 5.4) for negative lymph-nodes (P = .007). PET/CT detected pelvic (n = 1) and both pelvic/paraortic (n = 1) nodal metastases.
In clinical early stage vulvar cancer FDG PET/CT showed low sensitivity and moderate specificity for N-staging; therefore, it is not an accurate tool for the nodal status assessment. PET/CT may not be cost-effective in detecting the rare event of distant metastases, but further studies are needed.
Keywords: PET/CT, preoperative staging, vulvar cancer
1. Introduction
Vulvar cancer, of which 90% is squamous in origin, accounts for 4% of gynecological malignancies.[1] The standard treatment for squamous cell carcinoma of the vulva is radical surgery, which in all but stage Ia disease includes inguinofemoral lymphadenectomy (unilateral or bilateral) or sentinel lymph-node (SLN) biopsy in selected cases.[2–4] In early stage vulvar cancer (clinical stage I-II), the frequency of metastases in regional lymph nodes is approximately 25% to 35%.[5]
Because the lymph node status has been identified as the most important prognostic factor, careful evaluation and determination of nodal status is crucial. Groin node dissection carries associated significant short- and long-term morbidity: up to 50% of patients undergoing inguinofemoral lymphadenectomy will suffer postoperative complications (wound infection, wound breakdown, and debilitating lymphedema).[6] Increasing evidences suggest that the use of SLN biopsy of the inguinofemoral LN is an alternative standard-of-care approach in selected women with squamous cell carcinoma of the vulva, including patients with negative clinical groin examination and imaging. On one hand, the assessment by clinical palpation of the groins is inadequate; of patients with clinically normal lymph nodes, 16% to 24% have metastases, whereas 24% to 41% of those with clinically involved nodes are negative at histological examination.[7,8] On the other hand, an accurate imaging approach to the identification of groin node metastasis has not been yet defined.
18F-FDG positron emission tomography/computed tomography (PET/CT) is a diagnostic tool widely used in oncology for staging and restaging cancer patients.[9,10] In particular for vulvar cancer, according to NCCN Guidelines, whole body PET/CT scan is considered for T2 or larger tumors or if metastases is suspected (indications may include abnormal physical exam, such as palpable new mass or adenopathy or pelvic/abdominal/symptoms).[2] However, the definitive role of the technique in this scenario has not been yet defined, as few published data are available.[11–15]
The aim of this study was to evaluate the utility of preoperative PET/CT in detecting groin and distant metastases in vulvar cancer patients.
2. Material and methods
2.1. Patient population
From 2007 to 2015, patients with histologically confirmed vulvar cancer T1-T2 < 4 cm with a stromal invasion greater than 1 mm, who underwent 18F-FDG PET/CT followed by surgery at the Gynecologic Oncology Department of San Gerardo Hospital, Monza, Italy, were considered for the analysis. Exclusion criteria were: histopatologic findings different from primary vulvar cancer; relapsed vulvar cancer; patients who transferred their care to a different institution, resulting in insufficient clinical data for evaluation of subsequent management. All patients signed a written informed consent. All procedures were in accordance with the ethical standards; considering the monocentric and retrospective nature of the study, a formal local ethical approval was not necessary.
2.2. PET/CT imaging
All studies were performed with a PET/CT scanner (Discovery 600 and Discovery DST, GE Healthcare, Milwaukee, WI), consisting of a PET scanner and a multidetector CT scanner, which allows the acquisition of co-registered CT and PET images from the same patient in 1 session. Patients fasted for at least 6 hours before the intravenous administration of 3.7 MBq/kg of 18F-FDG; patients’ blood glucose levels had to be above 170 mg/dL. All patients were orally hydrated (500 mL of water) during the FDG uptake period and were asked to empty their bladder before positioning for the scan. The combined examination was started about approximately 60 minutes after the FDG injection. CT was acquired first, during shallow breathing, with 140 kV, 60 mA, and 3.75 mm of slice thickness. No oral contrast or intravenous contrast was used for the CT component of the examination. PET was acquired in 3D mode with 3 minutes in each bed position; PET images were reconstructed with an iterative algorithm (ordered subset expectation maximization–OSEM), attenuation, random, and scatter correction. Attenuation correction was performed on the basis of CT scan data.
2.3. Image analysis
Images were evaluated by 3 nuclear medicine physicians in consensus informed about the clinical data of patients at the moment of the scan. Image analysis was performed as follows: attenuation-corrected PET images, CT images, and co-registered PET/CT images were displayed together on the workstation (AW4.6 GE Healthcare, Milwaukee, WI). FDG uptake in the anatomical structure was indicated suspicious for malignancy when tracer accumulation was moderately to markedly increased relative to comparable surrounding background activity, excluding the areas of physiologically increased uptake. The metabolic findings were correlated to their anatomical sites on the basis of the CT images. In particular, the lymph nodes inguinal sites were identified on 18F-FDG PET/CT images and carefully analyzed. The diagnosis of metastatic lymph nodes on 18F-FDG PET/CT images was based on the presence of focal increased tracer uptake, regardless of their size on CT. Conversely, lymph nodes with no significant tracer uptake were reported as negative, independent of their size on CT images. This method of image analysis of PET/CT images was derived from previous reports, in which this combined technique was used for tumor staging.[16–18] Semiquantitative analysis required identifying regions of interest (ROI) in areas of abnormality and the highest standard uptake value (SUVmax) of each inguinal site was calculated, based on the following formula:
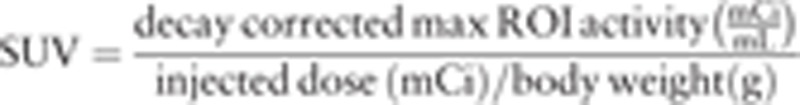 |
2.4. Surgical procedure
All patients underwent to radical vulvectomy and reconstruction. In the case of lateral lesion ≥ 2 cm from vulvar midline, ipsilateral groin node evaluation was performed: SLNs or ipsilateral groin lymph-node dissection. In the case of vulvar midline lesion (anterior or posterior), a bilateral inguino-femoral groin node surgery (SLNs or bilateral groin LN dissection) was performed. A selective monolateral or bilateral groin lympahedenectomy was performed in case SLNs were not detected.[2]
2.5. SLN procedure
Few hours before surgery, in each patient, approximately 18 MBq of Tc-99m nanocolloid (Nanocoll, GE Healthcare, Milwaukee, WI) in 0.2 mL was injected intradermally at 4 locations around the primary tumor. Images acquisition was performed using a single-head gamma camera with a low-energy high-resolution collimator.[19] The first persistent focal uptake to appear was considered to be an SLN and the sites of hot spots in the peritumoral areas were marked with a pencil on the overlying skin. After lymphoscintigraphy patients were transported to the operating room. SLN biopsy was performed under general anesthesia, by using an intraoperative gamma probe (C-Trak Galaxy CW4000 System, Southern Scientific, Ltd, UK) to localize SLNs. The marked lymph nodes were removed with the aid of probe-guided surgery while finding the hottest area of radioactivity in vivo and checked for radioactive counts ex vivo.
2.6. Standard of reference and data analysis
Histopathological findings were analyzed by an experienced pathologist, who was blinded to the 18F-FDG PET/CT results and served as the standard of reference. In particular, lymph nodes were reported by the pathologist as normal, reactive (follicular or sinusoidal hyperplasia), or metastatic.
Descriptive analysis utilized absolute values and percentages for categorical data. Sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), and overall accuracy of 18F-FDG PET/CT imaging in the diagnosis of inguino-femoral nodal metastases were calculated on the basis of per patient and per-groin analysis. A true-positive (TP) finding was a lesion detected on 18F-FDG PET/CT as a metastatic site and then confirmed at the subsequent histologic analysis; a false-positive (FP) lesion was a lesion seen on 18F-FDG PET/CT images but found to be negative for tumor tissue at histologic analysis. A true-negative (TN) lesion was indicated when no pathologic uptake was seen on 18F-FDG PET/CT images and the result of the histologic sample was negative or tumor tissue. A false-negative (FN) lesion was a lesion that was missed at image analysis but was found to be positive for neoplastic tissue at histologic analysis. SUVmax of positive groin-site at histopatholigical analysis was compared to SUVmax of negative sites, by using the Kruskal-Wallis test. Distant metastases were confirmed by imaging and clinical follow-up.
3. Results
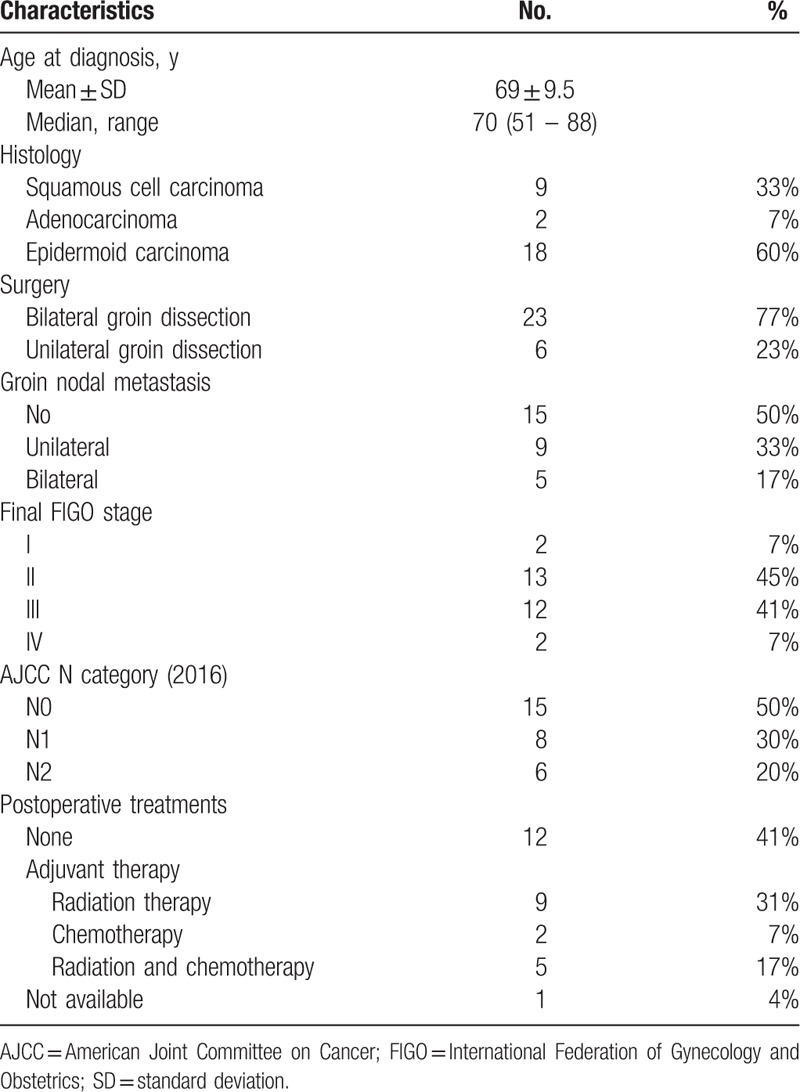
Twenty-nine vulvar cancer patients met the inclusion criteria. Patients characteristics were resumed in Table 1. The median age was 69 years (range 51–88).
Table 1.
Patient population characteristics.
3.1. Histopathologic findings
Twenty-three women underwent bilateral and 6 unilateral (3 right and 3 left) groin surgery. A total of 329 nodes were removed and analyzed. The median number of lymph nodes sampled was 11 (range 1–26); in particular, the median number was 13 in case of bilateral lymphadenectomy and 9 in unilateral. SLN biopsy was performed in 17 of 29 patients; however, in 15/17 patients, lymphadenectomy was performed, as these cases were performed during the learning curve.
Fourteen patients (48%) had histologically proven nodal metastases, of which 9 monolateral and 5 bilateral. Among women with nodal metastases, the median number of positive nodes was 1 (range 1–3).
3.2. PET/CT findings
Table 2 shows the patient-based and groin-based analysis for the detection of nodal metastases by 18F-FDG PET/CT. PET/CT correctly identified histologically proven metastatic nodes in 7 of 14 affected women, and the absence of metastatic nodes in 10 of the 15 negative patients, resulting in a per-patient sensitivity of 50%, specificity of 67%, PPV of 58%, and NPV of 59%.
Table 2.
Diagnostic accuracy of PET/CT scan in a patient-based and site-based analysis.
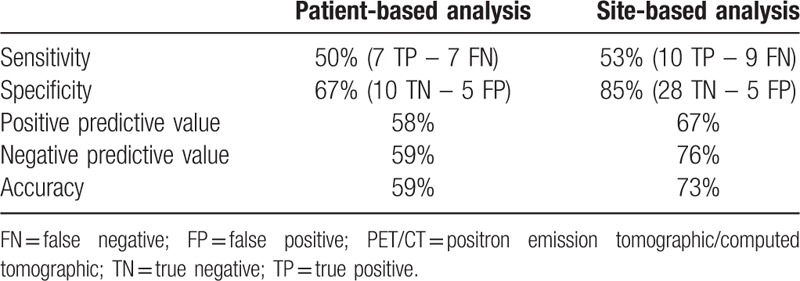
As groin-based performances of 18F-FDG PET/CT sensitivity resulted 53%, specificity 85%, PPV 67%, and NPV 76%. The mean value of SUVmax of groin site was 6.1 (range 0.7–16.2) for metastatic sites, whereas 1.6 (range 0.7 – 5.44) for negative sites (P = .005).
PET/CT detected pelvic (n = 1) and both pelvic/paraortic (n = 1) nodal metastases; in 1 patient, PET/CT showed an inflammatory uptake at right lung hilum.
4. Discussion
The primary status of inguinal LN and the presence of extravulvar lesions play an important role in stage, treatment, and prognosis of patients with vulvar cancer.[20]
LN metastasis is considered the most important prognostic factor. Additional factors that have been showed to be predictive of recurrence and/or survival include depth of invasion, tumor thickness, margin distance, and presence of lympho-vascular space invasion (LVSI).[2] The presence of infiltrative growth patterns, compared with a pushing pattern, is associated with a higher local recurrence rate.[20] The presence of prominent fibromyxoid stroma at the invasive edge is associated with poorer outcome[21] and LVSI is also associated with an increased local recurrence rate.[20]
Advances in molecular oncology have provided better understanding of the tumorigenesis of vulvar cancer, investigating on biological pathways and mutations that drive both HPV-associated and HPV-independent vulvar cancer,[22] as opportunity to improve the targeted therapies.
Although these available cellular markers, it is still lacking an appropriate image modality to accurately detect the groin LNs and extravulvar metastasis.[23]
A noninvasive approach to the identification of groin node metastasis is attractive because of its acceptability to the patient and in the reduction of potential adverse events.[24] Magnetic resonance imaging (MRI) provides important information about local invasion, but it is not accurate enough for routine assessment of groin node status.[24]
Several studies, in small groups of patients, have suggested that FDG-PET/CT could be useful for detecting nodal and distant metastases in vulvar cancer and Table 3 resumes the main characteristics and findings of these published studies.[11–15]
Table 3.
Diagnostic accuracy of 18F-FDG-PET and PET/CT scan in staging nodal metastases in other published studies.
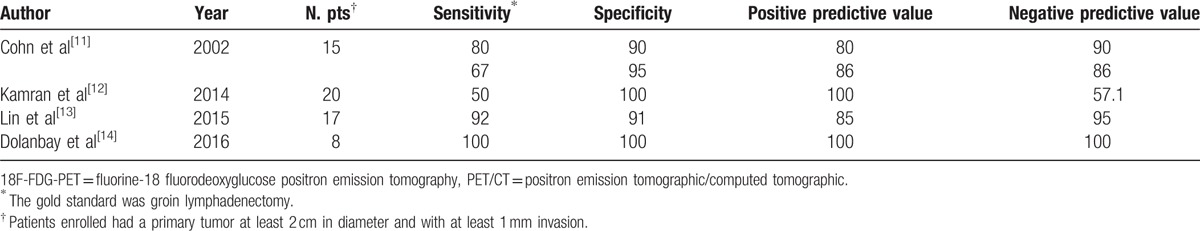
Our data are similar to those obtained by Cohn et al[11] by using a PET-only scanner; although the development of hybrid technique, we similarly confirmed a low sensitivity also for PET/CT in detection of nodal metastases. Thus, these findings can be explained by biological and metabolic characteristics of tumor such as the presence of necrotic tumors that are metabolically inactive, leading to false-negative images. However, Cohn et al[11] found a necrotic nodal tissue in only 33% of false-negative studies, and similarly in our experience, we found it in 28% of FN findings (Fig. 1). Thus, necrosis does not entirely explain the relatively low sensitivity and negative predictive value of PET imaging. The low sensitivity of 18F-FDG-PET/CT was probably due to the presence of small metastatic deposits in LN, as less than 5 mm metastases could not be detectable, due to the spatial resolution limit of 18F-FDG-PET/CT imaging.[16] In particular amongst 7 FN patients: 2 patients had a massive nodal metastases with necrosis, 1 had micrometastases (<2 mm) at SLN biopsy, and 4 had partial nodal metastases.
Figure 1.
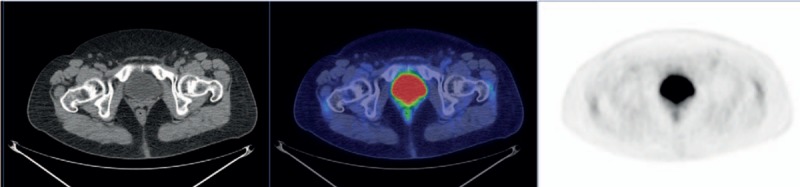
From the left, axial CT, fused PET/CT, and PET images of a false negative case. PET showed no FDG groin uptake bilaterally, whereas a partial metastasis was found at histology in a left inguino-femoral LN. CT = computed tomography, FDG = fluoro-deoxy-glucose, PET/CT = positron emission tomographic/computed tomographic.
Conversely, other authors demonstrated a great sensitivity and specificity of PET/CT in nodal staging of vulvar cancer.[13,14] The high sensitivity obtained by Lin et al[13] could be related to images interpretation, as also the probable lesions were scored as positive, whereas the higher specificity could be explained by the lack of factors such as inflammation in the considered sample size.
Considering high specificity reported in previous experiences,[12,14] PET/CT was proposed as an attractive imaging modality either to plan groin exploration or radiation, or in combination with SLN biopsy to assist the surgeon in identifying the positive node.[12] In our series, although nodal FDG uptake was higher in nodal metastases, 5 of 12 positive PET resulted falsely positive (PPV = 58%); a clinical case is shown in Fig. 2. Similarly Lin et al[13] found FP lymph nodes or distant metastatic PET findings in 7/17 (41%) of cases, concluding that PET can have a positive impact; however, it should be interpreted with caution. These findings are related to inflammatory phenomena frequently present at inguinal sites; in particular, vulvar malignancies often present as ulcerative lesions with concomitant inflammatory enlarged nodes, which may contribute to false-positive results.[13]
Figure 2.
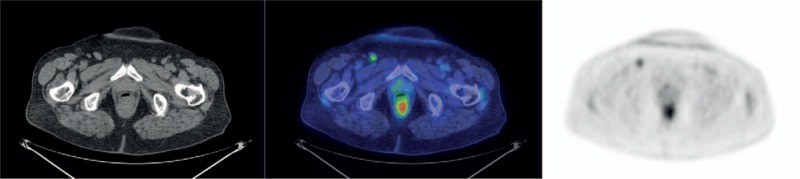
From the left, axial CT, fused PET/CT, and PET images of a false positive case. PET showed a pathological FDG uptake at the right inguinal site (SUVmax 3.53), whereas no metastases were found at histology. CT = computed tomography, FDG = fluoro-deoxy-glucose, PET/CT = positron emission tomographic/computed tomographic.
There is a growing body of evidence demonstrating that SLN biopsy is an alternative standard-of- care approach to lymphadenectomy in selected women.[2,4,23,25] Multicenter studies[26,27] demonstrated the safety and practicality of this intervention with significant improvements in postoperative morbidity without any significant compromise in accuracy or outcomes in terms of relapse.[3,28] According to NCCN guidelines,[2] candidates for SLN biopsy should have clinically/radiologically negative groin nodes, unifocal primary tumor less than 4 cm, and no history of previous vulvar surgery. PET/CT scan could be supposed to select patients to refer to surgery, for example, to perform lymphadenectomy in case of positive PET/CT and SLN biospsy in case of negative PET/CT. However, in our experience on the basis of a positive PET/CT at inguinal site, considering the low PPV, the selection of patients for SLN does not seem adequate.
Considering the important established role of SLN for N staging, PET/CT scan has been proposed as a screening tool for distant metastasis only.[29] In our population, PET/CT detected pelvic nodes in 1 patient and both pelvic and paraortic nodal metastases in 1 case; the small number of distant metastases could be related to the clinical early stage of our population. However, the occurrence of distant metastasis from vulvar cancer is a rare event (about 5%) with very limited prognosis.[30] As regards the impact of PET/CT, Robertson et al[31] found that following FDG-PET/CT, the physician's prognostic impression changed in 29/54 (54%) and a change in patient management was documented in 30/83 (36%) of studies from patients with primary or recurrent vulvar and vaginal cancer.
The main limitation of our study resides in the small sample size; however, published studies (Table 3) have similar population. In addition, there is a big interest in evaluation of FDG metabolic features of primary lesion as prognostic factors; however, these characteristics were not evaluated because vulvar region is frequently site of contamination from urinary uptake. Thus, SUV values may not be a reliable index of the metabolic activity of the primary lesion.
In conclusion, FDG PET/CT has low sensitivity and moderate specificity in nodal staging; thus, it is not an accurate tool for the nodal status assessment. PET/CT may not be cost-effective in detecting the rare event of distant metastases in early stages; however, further studies, such as cost-effectiveness analysis in larger population, are needed to clarify the role of PET/CT in this setting.
Footnotes
Abbreviations: CT = computed tomography, FDG = fluoro-deoxy-glucose, LN = lymph-node, PET = positron emission tomography, SLN = sentinel lymph-node, SUV = standardized uptake value.
The authors have no funding and conflicts of interest to disclose.
References
- [1].Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin 2016;66:7–30. [DOI] [PubMed] [Google Scholar]
- [2].Koh WJ, Greer BE, Abu-Rustum NR, et al. Vulvar cancer, version 1.2017, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw 2017;15:92–120. [DOI] [PubMed] [Google Scholar]
- [3].Guidelines for the Diagnosis and Management of Vulval Carcinoma. 2014. Available at: https://www.rcog.org.uk/globalassets/documents/guidelines/vulvalcancerguideline.pdf. [Google Scholar]
- [4].Hacker NF, Eifel PJ, van der Velden J. Cancer of the vulva. Int J Gynaecol Obstet 2015;131(suppl 2):S76–83. [DOI] [PubMed] [Google Scholar]
- [5].Zweizig S, Korets S, Cain JM. Key concepts in management of vulvar cancer. Best Pract Res Clin Obstet Gynaecol 2014;28:959–66. [DOI] [PubMed] [Google Scholar]
- [6].Gaarenstroom KN, Kenter GG, Trimbos JB, et al. Postoperative complications after vulvectomy and inguinofemoral lymphadenectomy using separate groin incisions. Int J Gynecol Cancer 2003;13:522–7. [DOI] [PubMed] [Google Scholar]
- [7].Sedlis A, Homesley H, Bundy BN, et al. Positive groin lymph nodes in superficial squamous cell vulvar cancer. A Gynecologic Oncology Group Study. Am J Obstet Gynecol 1987;156:1159–64. [DOI] [PubMed] [Google Scholar]
- [8].Homesley HD, Bundy BN, Sedlis A, et al. Prognostic factors for groin node metastasis in squamous cell carcinoma of the vulva (a Gynecologic Oncology Group study). Gynecol Oncol 1993;49:279–83. [DOI] [PubMed] [Google Scholar]
- [9].Boellaard R, Delgado-Bolton R, Oyen WJ, et al. FDG PET/CT: EANM procedure guidelines for tumour imaging: version 2.0. Eur J Nucl Med Mol Imaging 2015;42:328–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Picchio M, Mansueto M, Crivellaro C, et al. PET/CT and contrast enhanced CT in single vs. two separate sessions: a cost analysis study. Q J Nucl Med Mol Imaging 2012;56:309–16. [PubMed] [Google Scholar]
- [11].Cohn DE, Dehdashti F, Gibb RK, et al. Prospective evaluation of positron emission tomography for the detection of groin node metastases from vulvar cancer. Gynecol Oncol 2002;85:179–84. [DOI] [PubMed] [Google Scholar]
- [12].Kamran MW, O’Toole F, Meghen K, et al. Whole-body [18F]fluoro-2-deoxyglucose positron emission tomography scan as combined PET-CT staging prior to planned radical vulvectomy and inguinofemoral lymphadenectomy for squamous vulvar cancer: a correlation with groin node metastasis. Eur J Gynaecol Oncol 2014;35:230–5. [PubMed] [Google Scholar]
- [13].Lin G, Chen CY, Liu FY, et al. Computed tomography, magnetic resonance imaging and FDG positron emission tomography in the management of vulvar malignancies. Eur Radiol 2015;25:1267–78. [DOI] [PubMed] [Google Scholar]
- [14].Dolanbay M, Ozcelik B, Abdulrezzak U, et al. F-18 fluoro-D-glucose (FDG)-positron emission tomography (PET)/computed tomography (CT) in planning of surgery and sentinel lymph node screening in vulvar cancers. Arch Gynecol Obstet 2016;293:1319–24. [DOI] [PubMed] [Google Scholar]
- [15].Peiro V, Chiva L, Gonzalez A, et al. Utility of the PET/CT in vulvar cancer management. Rev Esp Med Nucl Imagen Mol 2014;33:87–92. [DOI] [PubMed] [Google Scholar]
- [16].Signorelli M, Guerra L, Montanelli L, et al. Preoperative staging of cervical cancer: is 18-FDG-PET/CT really effective in patients with early stage disease? Gynecol Oncol 2011;123:236–40. [DOI] [PubMed] [Google Scholar]
- [17].Signorelli M, Guerra L, Pirovano C, et al. Detection of nodal metastases by 18F-FDG PET/CT in apparent early stage ovarian cancer: a prospective study. Gynecol Oncol 2013;131:395–9. [DOI] [PubMed] [Google Scholar]
- [18].Signorelli M, Crivellaro C, Buda A, et al. Staging of high-risk endometrial cancer with PET/CT and sentinel lymph node mapping. Clin Nucl Med 2015;40:780–5. [DOI] [PubMed] [Google Scholar]
- [19].Elisei F, Crivellaro C, Giuliani D, et al. Sentinel-node mapping in endometrial cancer patients: comparing SPECT/CT, gamma-probe and dye. Ann Nucl Med 2016;31:93–9. [DOI] [PubMed] [Google Scholar]
- [20].Heaps JM, Fu YS, Montz FJ, et al. Surgical-pathologic variables predictive of local recurrence in squamous cell carcinoma of the vulva. Gynecol Oncol 1990;38:309–14. [DOI] [PubMed] [Google Scholar]
- [21].Ambros RA, Malfetano JH, Mihm MC., Jr Clinicopathologic features of vulvar squamous cell carcinomas exhibiting prominent fibromyxoid stromal response. Int J Gynecol Pathol 1996;15:137–45. [DOI] [PubMed] [Google Scholar]
- [22].Clancy AA, Spaans JN, Weberpals JI. The forgotten woman's cancer: vulvar squamous cell carcinoma (VSCC) and a targeted approach to therapy. Ann Oncol 2016;27:1696–705. [DOI] [PubMed] [Google Scholar]
- [23].Yen TC, Lai CH. Positron emission tomography in gynecologic cancer. Semin Nucl Med 2006;36:93–104. [DOI] [PubMed] [Google Scholar]
- [24].Selman TJ, Luesley DM, Acheson N, et al. A systematic review of the accuracy of diagnostic tests for inguinal lymph node status in vulvar cancer. Gynecol Oncol 2005;99:206–14. [DOI] [PubMed] [Google Scholar]
- [25].Ramirez PT, Levenback C. Long-term outcomes of sentinel node mapping in vulvar cancer: a time to cheer with enthusiasm or pause and question current practice? Gynecol Oncol 2016;140:1–2. [DOI] [PubMed] [Google Scholar]
- [26].Oonk MH, van Hemel BM, Hollema H, et al. Size of sentinel-node metastasis and chances of non-sentinel-node involvement and survival in early stage vulvar cancer: results from GROINSS-V, a multicentre observational study. Lancet Oncol 2010;11:646–52. [DOI] [PubMed] [Google Scholar]
- [27].Te Grootenhuis NC, van der Zee AG, van Doorn HC, et al. Sentinel nodes in vulvar cancer: long-term follow-up of the GROningen INternational Study on Sentinel nodes in Vulvar cancer (GROINSS-V) I. Gynecol Oncol 2016;140:8–14. [DOI] [PubMed] [Google Scholar]
- [28].Van der Zee AG, Oonk MH, De Hullu JA, et al. Sentinel node dissection is safe in the treatment of early-stage vulvar cancer. J Clin Oncol 2008;26:884–9. [DOI] [PubMed] [Google Scholar]
- [29].Perry LJ, Guralp O, Al-Niaimi A, et al. False positive PET-CT scan and clinical examination in a patient with locally advanced vulvar cancer. Gynecol Oncol Case Rep 2013;4:29–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Prieske K, Haeringer N, Grimm D, et al. Patterns of distant metastases in vulvar cancer. Gynecol Oncol 2016;142:427–34. [DOI] [PubMed] [Google Scholar]
- [31].Robertson NL, Hricak H, Sonoda Y, et al. The impact of FDG-PET/CT in the management of patients with vulvar and vaginal cancer. Gynecol Oncol 2016;140:420–4. [DOI] [PMC free article] [PubMed] [Google Scholar]


