Abstract
The aim of this study was to evaluate the surgical outcome of slide tracheoplasty.
Eighty-one patients who underwent slide tracheoplasty were retrospectively reviewed. Before and after operation, all patients were examined by computed tomography (CT) and bronchoscopy regularly.
There were 8 deaths and the mortality was 9.9%. They all died of respiratory failure associated with the formation of granulation tissue in the airway postoperatively. The mortality was 15.8% from 2009 to 2012 and decreased to 8.1% from 2013 to 2016. The mortality of patients aged 10 to 24 months was 5.7%, which was significantly lower than those younger than 10 months and those older than 24 months. After surgery, 11 patients had granulation tissue growing at anastomosis edges and 8 of them died eventually. Twenty patients had mucosa varus at the site of anastomosis which mainly happened in the early time. Between different time periods and different age groups, there was significant difference in the incidence of granulation tissue and mucosa varus (P < .01). Clinical symptoms of tracheal stenosis disappeared and the results of CT were satisfactory after operation.
Slide tracheoplasty is an effective surgical method for congenital tracheal stenosis associated with congenital heart disease. With the continuous improvement of surgical technique, the mortality has been reduced and the incidence of granulation tissue and mucosa varus also has been reduced. The period of 10 to 24 months of age is the optimal cure time.
Keywords: congenital heart disease, congenital tracheal stenosis, granulation tissue, slide tracheoplasty
1. Introduction
Congenital tracheal stenosis is a rare and life-threatening malformation in infants and children.[1,2] The spectrum of stenosis encompasses a variety of tracheobronchial anomalies and the most common are long-segment tracheal stenosis and bridging bronchus. The typical characteristic is the presence of complete tracheal rings which are always younger than normal C-shaped rings.[3,4] Tracheal stenosis is usually associated with congenital cardiovascular anomalies, which should be addressed concomitantly by surgery.[5–7] During the past 30 years, various surgical techniques have emerged to correct tracheal stenosis.[8–12] Among them, slide tracheoplasty has shown superior clinical results and been advocated as the technique of choice for most cases of tracheal stenosis.[13–17] Over the past 7 years, we performed slide tracheoplasty of congenital tracheal stenosis complicated with congenital heart disease in 81 patients and obtained satisfactory surgical outcomes. The aim of this study was to evaluate our surgical outcomes of slide tracheoplasty.
2. Materials and methods
2.1. Patients
This retrospective study was approved by Shanghai Children's Medical Center and the affiliated Medical College of Jiao Tong University of Medicine Human Investigation Committee. Data of patients’ characteristics, operation notes, and postoperative results were obtained by reviewing medical records of our center. Patients who underwent slide tracheoplasty for tracheal stenosis from 2009 to 2016 were identified.
From December 2009 to June 2016, slide tracheoplasty of congenital tracheal stenosis and corresponding correction of congenital heart disease were performed in 81 patients (41 males and 40 females) in our center. According to the time of surgery, patients were divided into 2 groups: Group 1, from December 2009 to December 2012 and Group 2, from January 2013 to June 2016. At the time of surgery, the mean age was (18.6 ± 11.9) months (range: 2–71 months) and the mean weight was (9.9 ± 5.5) kg (rang: 3.8–52.0) kg. According to the age, patients were divided into 3 groups: Group A, younger than 10 months old (n = 11, 27.3%), Group B, between 10 and 24 months old (n = 53, 65.4%), and Group C, older than 24 months old (n = 17, 21.0%). To diagnose the anomalies and the degree of tracheal stenosis, enhanced computed tomography (CT) with 3-dimensional reconstruction was performed before surgery and bronchoscopy during surgery in all patients. Among them, long-segment tracheal stenosis was present in 49 patients (60.5%) and bridging bronchus in 32 patients (39.5%). Complete tracheal or bronchial rings were identified in all cases. Seventy-nine patients (97.5%) had associated cardiovascular anomalies, including 53 patients (65.4%) with pulmonary artery sling, 13 patients (16.1%) with ventricular septal defect (VSD), 8 patients (9.9%) with vascular ring, 3 patients (3.7%) with tetralogy of Fallot, 1 patient (1.2%) with patent ductus arteriosus (PDA) and 1 patient (1.2%) with double outlet right ventricle (DORV). Patients’ general characteristics and statistic results are summarized in Table 1.
Table 1.
Patients’ general characteristics and statistic results.
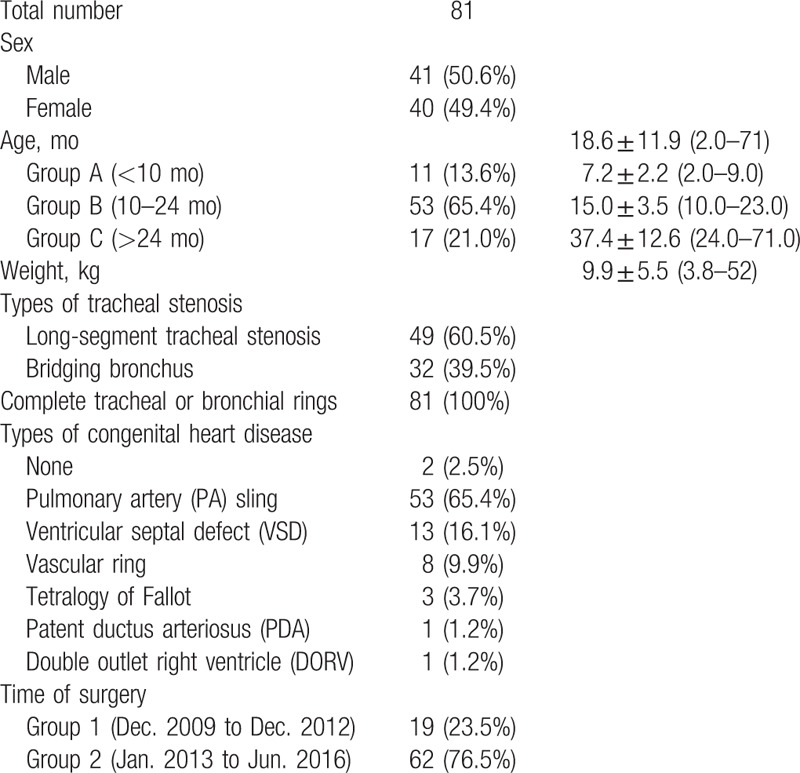
2.2. Surgical technique
The surgery of congenital tracheal stenosis and associated congenital heart disease was performed through a median sternotomy with cardiopulmonary bypass (CPB) at the same time. The cardiovascular anomalies were corrected before the tracheoplasty. The procedure of slide tracheoplasty was stated as follows. Firstly, flexible bronchoscopy was used to identify the limits of the stenosis. Then the trachea was exposed completely to beyond the limits of complete rings or until normal trachea were seen. After mobilization, the trachea was divided at the midpoint of the stenosis. Longitudinal incisions were made on opposite side of proximal and distal segments and the ends are spatulated. The proximal segment was incised anteriorly and the distal segment posteriorly to point just beyond the entire stenosis. Lastly, the 2 segments were slid together and a sliding oblique anastomosis was performed with continuous running 5-0 polydioxanone (PDS, Ethicon, NJ) suture. To avoid restenosis, we created an everting suture line along the entire length, which was achieved by suturing from the outside of the lumen to the lining edge. After anastomosis, the suture line could hardly be seen in the lumen. Immediate postoperative bronchoscopy and a leak test with gradual insufflation of the lungs could be used to assess the effectiveness of repair. Postoperatively, patients were placed in intensive care unit (ICU) and bronchoscopy was repeated to assess the outcome of slide tracheoplasty and recovery of trachea.
2.3. Postoperation
At 1, 3, 6, 12 months after surgery and then every 6 months, patients were asked for CT reexamination to measure the tracheal diameter and to evaluate the outcome of slide tracheoplasty.
2.4. Data analysis
Data are analyzed with SPSS software version (IBM SPSS Statistics, NY) and presented as mean ± standard deviation, medians and ranges. Nonparametric tests are performed to analyze differences between groups.
3. Results
3.1. Mortality
Totally, the operative mortality was 9.9% (n = 8, 8 out of 91). They all died of respiratory failure associated with the formation of granulation tissue in the airway postoperatively. From 2009 to 2012, the mortality was 15.8% (n = 3; 3 out of 19), while from 2013 to 2016, it decreased to 8.1% (n = 5, 5 out of 62). Although statistical analysis revealed no significant difference of mortality between different time periods, we still can consider that, with the continuous improvement of surgical techniques, the surgical outcome of slide tracheoplasty was much more satisfactory. In addition, the mortality of patients who were 10 to 24 months was 5.7% (n = 3, 3 out of 53), which was significantly lower than those who were younger than 10 months (n = 3, 27.3%, 3 out of 11) and those who were older than 24 months (n = 2, 11.8%, 2 out of 17). According to these, we think the period of 10 to 24 months could be the best time for surgery and would increase survival rate. The information of mortality is summarized in Table 2.
Table 2.
Statistical analysis of surgical outcome.
3.2. Bronchoscopy results
After surgery, all patients were examined by bronchoscopy to evaluate recovery condition of anastomosis. Postoperative tracheal or bronchial malacia was present in all 81 patients, but was not shown influence on surgical outcome and postoperative result. Eleven patients (13.6%) had granulation tissue growing around the anastomosis edges and 8 of them died eventually. Twenty patients (24.7%) had mucosa varus at the site of anastomosis which mainly happened in the early time. Between different time periods, there was significant difference in the incidence of granulation tissue and mucosa varus (P < .01). It is worth noting that the formation of granulation tissue had significant relation to mortality (P < .01). We believed that the use of everting suture technique in slide tracheoplasty would improve surgical outcomes. Similarly, significant difference was found among different age groups (P < .01), suggesting that the period of 10 to 24 months could be the optimal surgery time. The statistical results are summarized in Table 2.
3.3. Postoperative results
Forty-seven patients came back to clinics for reexamination 1 month to 7 years after discharges. Clinical symptoms of tracheal stenosis disappeared and the results of CT were satisfied. Before surgery, the mean diameter of stenotic trachea was (2.4 ± 0.6) mm (rang: 1.2–4.7) mm. One month after surgery, the mean diameter of trachea was (4.0 ± 1.1) mm (rang: 2.0–6.2) mm. One year after surgery, the mean diameter was (5.1 ± 1.1) mm (rang: 3.8–7.5) mm and 2 years after surgery, the mean diameter was (6.8 ± 1.2) mm (rang: 5.0–8.0) mm. Significant difference in the diameter of trachea was found between before and after surgery (P < .01). The statistical results are summarized in Table 3.
Table 3.
Comparison of tracheal diameter before and after surgery.

4. Discussion
Since the first tracheoplasty for congenital tracheal stenosis was reported in 1982,[18,19] various surgical techniques have been proposed to correct tracheal stenosis. But there is no uniform surgical technique because of the lack of surgical experience due to low incidence and high mortality. In 1989, slide tracheoplasty was first described by Tsang et al,[20] which was initially designed for long-segment tracheal stenosis and had shown superior results to other techniques. Since then, side tracheoplasty has been adopted increasingly as the preferred surgical technique at a number of centers.
According to previous studies, the mortality of slide tracheoplasty ranged from 5% to 30%.[2,16,21] As shown in many studies, the mortality was 11%,[2] 7%,[7] 14%,[15] 5%,[16] and 11.8%.[17] Comparably, the mortality of our study was 9.9%. Notably, the mortality between 2013 and 2016 was 8.1%, which was lower than the mortality of 15.8% between 2009 and 2012. Similarly, results of bronchoscopy after surgery showed significant difference in the incidence of granulation tissue between 2009–2012 and 2013–2016 from 31.6% to 8.1%, respectively (P < .01). The results of mucosa varus were the same. The incidence was 52.6% and 16.1% in 2009–2012 and 2013–2016, respectively. The reduction of mortality can be considered to be the result of continuous improvement of surgical technique.
In the early years, we incised the proximal segment posteriorly and the distal segment anteriorly (Fig. 1), as described by Grillo.[22] But it seems a little difficult to suture because there are vessels blocking in the front of proximal trachea. When suturing, the proximal segment need to be turned over, so the operative field is greatly limited. Subsequently, we reversed the vertical incisions. We incised the proximal segment anteriorly and the distal segment posteriorly (Fig. 2), so as to make the operative field clear and easy to suture.
Figure 1.
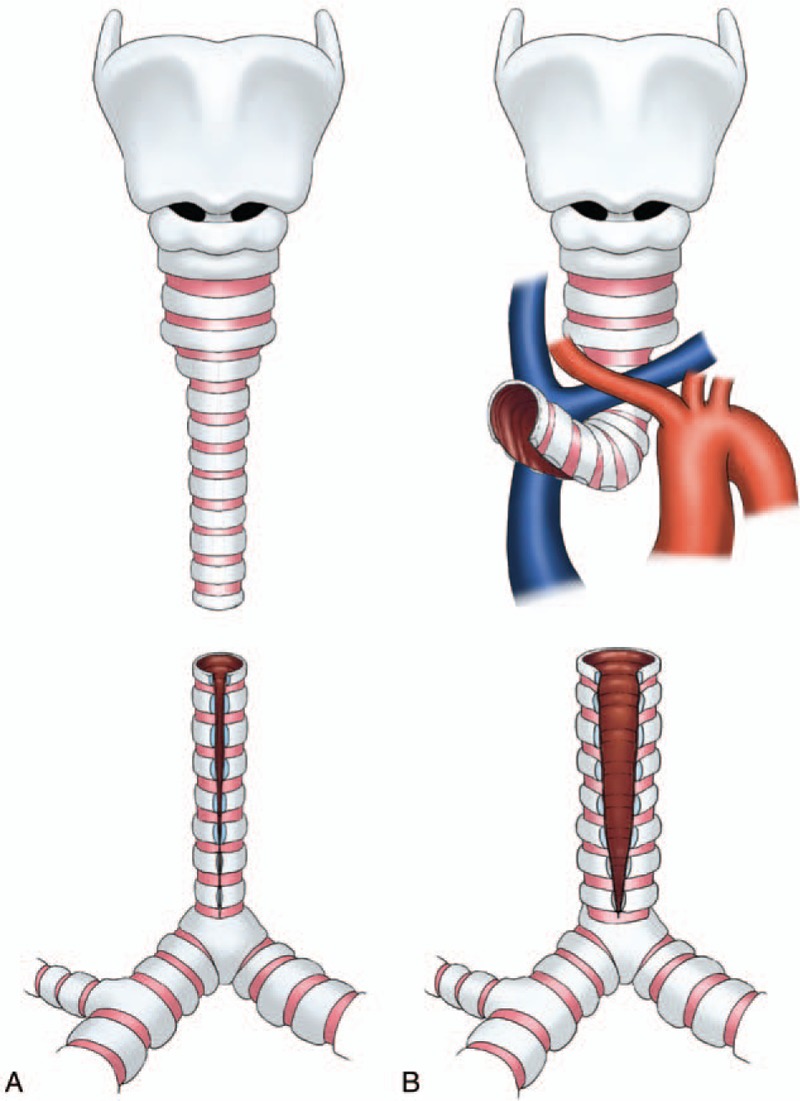
Technique of slide trachoplasty in the early years. (A) The stenotic segment was divided at the midpoint, the proximal segment was incised posteriorly and the distal segment anteriorly. (B) There were vessels blocking in the front of proximal trachea, which makes suture a little difficult.
Figure 2.
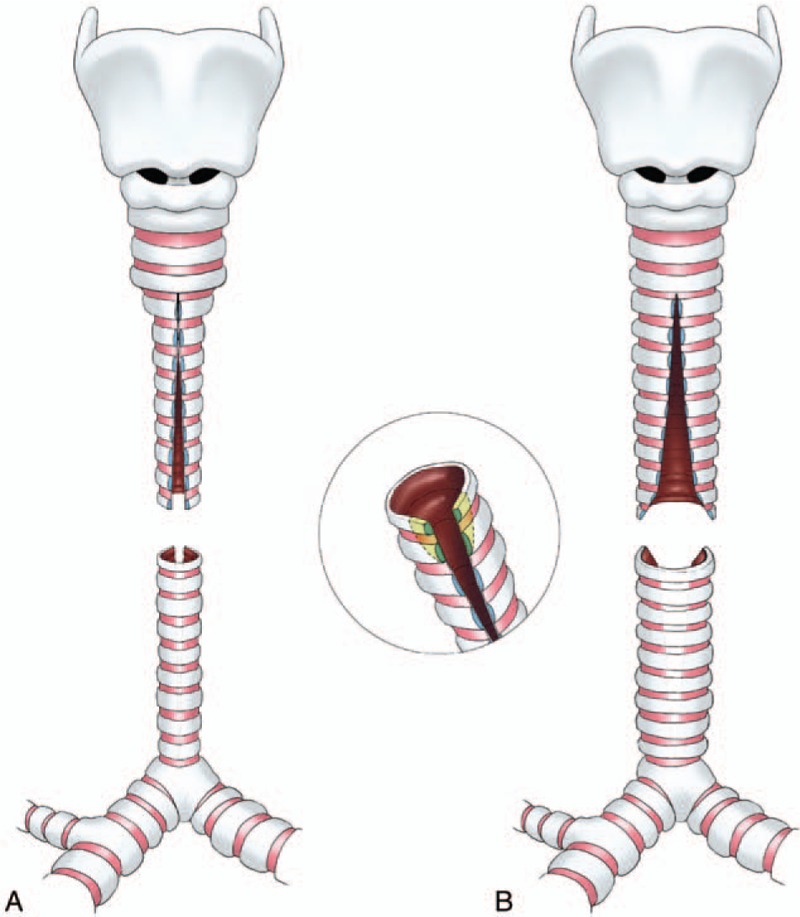
Modified technique of slide trachoplasty. (A) The vertical incisions were reversed, the proximal segment was incised anteriorly and the distal segment posteriorly. (B) The edges of both upper and lower segments were trimmed away.
Originally, we just incised the tracheal wall vertically and did not cut off the cartilage tissues around incision (Fig. 1). We found it was difficult to suture because the shape of 2 segments are not match. Then, we cut off a little cartilage tissues and made the incision like a triangle (Fig. 2). At the beginning, the width of the triangular incision was shorter than the diameter of tracheal ring, which had been intended to retain more cartilage tissues. But gradually we found that it was easier to form a type of 8-shaped trachea, leading to mucosa varus and growing of granulation tissue. At present, we have extended the width of resection to the diameter of tracheal ring. The edges of both proximal and distal segments are trimmed away, which look like tongue. The cartilage corners from the top of each segment are trimmed also in order to avoid being inserted into the lumen (Fig. 3).
Figure 3.
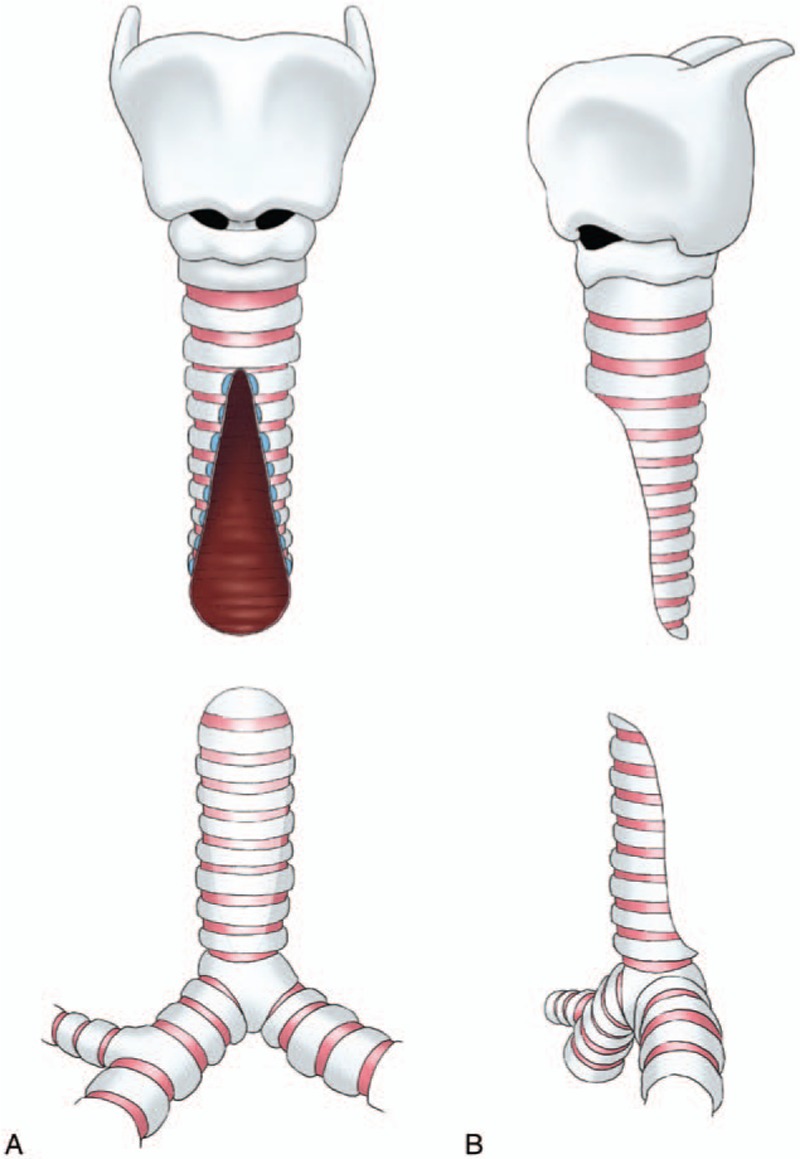
Technique of slide trachoplasty used at present. (A and B) The ends of both proximal and distal segments were trimmed away, which look like tongue. The cartilage corners from the top of each segment were trimmed also.
For suture techniques, we adopted an everting suture using 5-0 polydioxanone (PDS, Ethicon) suture. Along the entire length, we created an everting suture line, which was achieved by suturing from the outside of the lumen to the lining edge. The posterior segment of the anastomosis was performed first, as it was more difficult to expose. After anastomosis, the suture line could hardly be seen in the lumen (Fig. 4). With these improvements, the incidences of mucosa varus and granulation tissue were decreased significantly, resulting in the reduction of mortality eventually.
Figure 4.
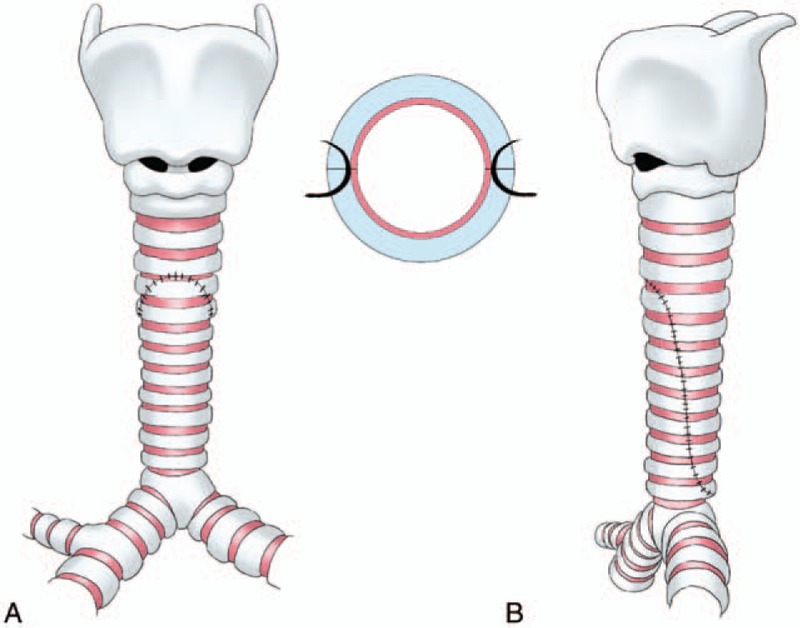
The 2 segments were slid together and a sliding oblique anastomosis was sutured. (A) The front view of sutural trachea. (B) The lateral view of sutural trachea.
In 2006, Chiu and Kim[23] published the first report on the prognostic factors determining surgical outcomes for congenital tracheal stenosis. They reported the highest mortality rate was observed in patients younger than 1 month. In 2011, Manning et al[24] also reported age was associated with mortality. In our study, we divided patients into 3 groups: younger than 10 months, aged 10 to 24 months, and older than 24 months. We found that the mortality was lowest in patients aged 10 to 24 months, and increased significantly in patients younger than 10 months and older than 24 months. Further analysis showed that the incidences of mucosa varus and granulation tissue were the same. The possible reasons could be that tracheal stenosis in children younger than 10 months was usually more severe, so the surgery and the postoperative management were usually more difficult. For children older than 24 months, tracheal stenosis is usually longer, so that the anastomosis is usually long, anastomotic tension is usually high, and the duration of operation is prolonged, all of which may increase the rate of complications and mortality. Therefore, we believe that the optimal age for surgery is between 10 and 24 months.
After operations, the trachea keeps on growing and the tracheal diameter significantly increased with time. These results were the same as previous studies,[25–27] suggested that slide tracheoplasty does not inhibit tracheal growth.
In conclusion, slide tracheoplasty is an effective surgical method for congenital tracheal stenosis. We think the optimal age for surgery is between 10 and 24 months. With the improvement of surgical techniques, the surgical outcome of slide tracheoplasty was excellent and satisfactory.
Footnotes
Abbreviations: CPB = cardiopulmonary bypass, DORV = double outlet right ventricle, ICU = intensive care unit, PDA = patent ductus arteriosus, VSD = ventricular septal defect.
Funding: This work was supported by National Nature Science Fund of China (81370117).
HZ and SW contributed equally to this work.
The authors have no conflicts of interest to disclose.
References
- [1].Benjamin B, Pitkin J, Cohen D. Congenital tracheal stenosis. Ann Otol Rhinol Laryngol 1981;90(4 Pt 1):364–71. [DOI] [PubMed] [Google Scholar]
- [2].Elliott M, Hartley BE, Wallis C, et al. Slide tracheoplasty. Curr Opin Otolaryngol Head Neck Surg 2008;16:75–82. [DOI] [PubMed] [Google Scholar]
- [3].Andrews TM, Cotton RT, Bailey WW, et al. Tracheoplasty for congenital complete tracheal rings. Arch Otolaryngol Head Neck Surg 1994;120:1363–9. [DOI] [PubMed] [Google Scholar]
- [4].Rutter MJ, Cotton RT, Azizkhan RG, et al. Slide tracheoplasty for the management of complete tracheal rings. J Pediatr Surg 2003;38:928–34. [DOI] [PubMed] [Google Scholar]
- [5].Herrera P, Caldarone C, Forte V, et al. The current state of congenital tracheal stenosis. Pediatr Surg Int 2007;23:1033–44. [DOI] [PubMed] [Google Scholar]
- [6].Loukanov T, Sebening C, Springer W, et al. Simultaneous management of congenital tracheal stenosis and cardiac anomalies in infants. J Thorac Cardiovasc Surg 2005;130:1537–41. [DOI] [PubMed] [Google Scholar]
- [7].Mainwaring RD, Shillingford M, Davies R, et al. Surgical reconstruction of tracheal stenosis in conjunction with congenital heart defects. Ann Thorac Surg 2012;93:1266–72. discussion 1263–1272. [DOI] [PubMed] [Google Scholar]
- [8].Anton-Pacheco JL, Cano I, Comas J, et al. Management of congenital tracheal stenosis in infancy. Eur J Cardiothorac Surg 2006;29:991–6. [DOI] [PubMed] [Google Scholar]
- [9].Backer CL, Mavroudis C, Gerber ME, et al. Tracheal surgery in children: an 18-year review of four techniques. Eur J Cardiothorac Surg 2001;19:777–84. [DOI] [PubMed] [Google Scholar]
- [10].Elliott M, Roebuck D, Noctor C, et al. The management of congenital tracheal stenosis. Int J Pediatr Otorhinolaryngol 2003;67(suppl 1):S183–92. [DOI] [PubMed] [Google Scholar]
- [11].Hamelink MC, Beare PG. Surgical management of congenital tracheal stenosis. Heart Lung 1989;18:178–83. [PubMed] [Google Scholar]
- [12].Valencia D, Overman D, Tibesar R, et al. Surgical management of distal tracheal stenosis in children. Laryngoscope 2011;121:2665–71. [DOI] [PubMed] [Google Scholar]
- [13].Butler CR, Speggiorin S, Rijnberg FM, et al. Outcomes of slide tracheoplasty in 101 children: a 17-year single-center experience. J Thorac Cardiovasc Surg 2014;147:1783–9. [DOI] [PubMed] [Google Scholar]
- [14].Kocyildirim E, Kanani M, Roebuck D, et al. Long-segment tracheal stenosis: slide tracheoplasty and a multidisciplinary approach improve outcomes and reduce costs. J Thorac Cardiovasc Surg 2004;128:876–82. [DOI] [PubMed] [Google Scholar]
- [15].Li X, Cheng LC, Cheung YF, et al. Management of symptomatic congenital tracheal stenosis in neonates and infants by slide tracheoplasty: a 7-year single institution experience. Eur J Cardiothorac Surg 2010;38:609–14. [DOI] [PubMed] [Google Scholar]
- [16].Manning PB, Rutter MJ, Lisec A, et al. One slide fits all: the versatility of slide tracheoplasty with cardiopulmonary bypass support for airway reconstruction in children. J Thorac Cardiovasc Surg 2011;141:155–61. [DOI] [PubMed] [Google Scholar]
- [17].Tsugawa C, Nishijima E, Muraji T, et al. Tracheoplasty for long segment congenital tracheal stenosis: analysis of 29 patients over two decades. J Pediatr Surg 2003;38:1703–6. [DOI] [PubMed] [Google Scholar]
- [18].Ein SH, Friedberg J, Williams WG, et al. Tracheoplasty—a new operation for complete congenital tracheal stenosis. J Pediatr Surg 1982;17:872–8. [DOI] [PubMed] [Google Scholar]
- [19].Kimura K, Mukohara N, Tsugawa C, et al. Tracheoplasty for congenital stenosis of the entire trachea. J Pediatr Surg 1982;17:869–71. [DOI] [PubMed] [Google Scholar]
- [20].Tsang V, Murday A, Gillbe C, et al. Slide tracheoplasty for congenital funnel-shaped tracheal stenosis. Ann Thorac Surg 1989;48:632–5. [DOI] [PubMed] [Google Scholar]
- [21].Yong MS, d’Udekem Y, Robertson CF, et al. Tracheal repair in children: reduction of mortality with advent of slide tracheoplasty. ANZ J Surg 2014;84:748–54. [DOI] [PubMed] [Google Scholar]
- [22].Grillo HC. Slide tracheoplasty for long-segment congenital tracheal stenosis. Ann Thorac Surg 1994;58:613–9. discussion 619–621. [DOI] [PubMed] [Google Scholar]
- [23].Chiu PP, Kim PC. Prognostic factors in the surgical treatment of congenital tracheal stenosis: a multicenter analysis of the literature. J Pediatr Surg 2006;41:221–5. discussion 221–225. [DOI] [PubMed] [Google Scholar]
- [24].Manning PB, Rutter MJ, Border WL. Slide tracheoplasty in infants and children: risk factors for prolonged postoperative ventilatory support. Ann Thorac Surg 2008;85:1187–91. discussion 1182–1191. [DOI] [PubMed] [Google Scholar]
- [25].Grillo HC, Wright CD, Vlahakes GJ, et al. Management of congenital tracheal stenosis by means of slide tracheoplasty or resection and reconstruction, with long-term follow-up of growth after slide tracheoplasty. J Thorac Cardiovasc Surg 2002;123:145–52. [PubMed] [Google Scholar]
- [26].Macchiarini P, Dulmet E, de Montpreville V, et al. Tracheal growth after slide tracheoplasty. J Thorac Cardiovasc Surg 1997;113:558–66. [DOI] [PubMed] [Google Scholar]
- [27].Speggiorin S, Gilbert TW, Broadhead M, et al. Do tracheas grow after slide tracheoplasty? Ann Thorac Surg 2012;93:1083–6. [DOI] [PubMed] [Google Scholar]


