Abstract
To assess the safety and efficacy of enhanced recovery after surgery (ERAS) as compared with the traditional care in patients undergoing liver surgery and optimization of enhanced recovery programs.
Literature, until August 2016, was searched to identify the comparative studies evaluating preoperative hospital stay time, complications, and C-reactive protein (CRP). Pooled odds ratios (OR) or weighted mean differences (WMDs) were calculated with either the fixed or random effect model.
These studies included a total of 524 patients: 254 treated with ERAS and 270 with traditional care. The postoperative recovery time and length of hospital stay were significantly better than the control group (WMD −2.72; 95% confidence interval [CI] −3.86 to −1.57; WMD −2.67; 95% CI −3.68 to −1.65, respectively). The overall complications, grade I, and Grand II–V complications were significantly favorable to the ERAS group (OR, 0.45 [95% CI, 0.30–0.67]; OR, 0.55 [95% CI, 0.31–0.98]; OR, 0.49 [95% CI, 0.32–0.76], respectively). The concentration of CRP in the control group was significantly higher than that in the ERAS group on postoperative day 5 (WMD −21.68; 95% CI −29.30 to −14.05). Time to first flatus (WMD −0.93; 95% CI −1.41 to −0.46) was significantly shortened in the ERAS group.
The evidence indicates that ERAS following liver surgery is safe, effective, and feasible. Therefore, further are essential for optimizing the ERAS protocols.
Keywords: enhanced recovery after surgery, hepatectomy, meta-analysis
1. Introduction
Liver cancer is an increasing global concern owing to the spread of hepatitis B and C infections, as well as, the rise in the rates of alcohol abuse and non-alcoholic steatohepatitis.[1–3] The optimal treatment for hepatocellular carcinoma (HCC) is liver transplantation; however, hepatectomy is yet the key approach for HCC. The surgical complications led to a 10% rate of mortality and a considerable morbidity during early liver resection in HCC.[3] The rates of mortality <5% can be achieved due to improvements in patient selection, early diagnosis, preoperative and postoperative management, surgical technique, and development of new technologies.[4] However, the morbidity rates remain high at 15% to 50%.[5]
More than 20 years after the pioneering studies[6] and a few years after the publication of several randomized studies, cohort studies, and meta-analyses proved the efficacy of enhanced recovery programs (ERP) with respect to reduction of morbidity and short hospital stay.[7,8] ERP aimed to minimize the pain and stress during and after surgery in order to decrease the organ dysfunction and morbidity, enhance recovery, enable early hospital discharge, and improve cost effectiveness. ERP was initially evaluated within the framework of colorectal surgery. However, indications have rapidly extended to other specialties in gastrointestinal (bariatric, pancreatic, gastric, esophageal), orthopedic, thoracic, urologic, gynecological, and cardiovascular surgery.[9–15]
Formal guidelines for enhanced recovery after surgery (ERAS) in liver surgery have not yet been published. Therefore, we conducted a meta-analysis on the available literature in order to assess the safety and efficacy of ERAS in comparison with the traditional care in patients undergoing liver surgery. In addition, ERP was also analyzed for further optimization.
2. Methods
This work does not contain any studies involving human participants or animals performed by any of the authors. Therefore, the ethical approval was not necessary.
2.1. Publication search
We searched MEDLINE, PubMed, EMBASE, and the Cochrane Central Register of Controlled Trials (CENTRAL) for literature until August 2016. The search terminology included perioperative care, preoperative care, postoperative care, convalescence, ERAS, fast track, enhanced recovery, and enhanced rehabilitation combined with liver, hepatic, hepato-, resection, segmentectomy, and hepatectomy. To ensure a complete meta-analysis, we used a maximally sensitive search for randomized controlled trials (RCTs). Language restrictions were not applied.
2.2. Data extraction and quality assessment
Data were extracted by 2 independent observers using standardized forms. The recorded data included the number of patients, ERP, preoperative length of hospital stay, complications, readmissions, and C-reactive protein. The quality of all the selected articles was ranked according to the score of the randomized controlled clinical trial quality evaluation standard.
2.3. Inclusion and exclusion criteria
Studies selected for the meta-analysis fulfilled the following inclusion criteria: the study evaluated ERAS in comparison with the conventional care in adult patients undergoing elective open or laparoscopic liver surgery; the outcome measures included complications and duration of hospital stay; the study was an RCT; studies compared the ERAS programs with conventional care and described an ERAS program with a minimum of 7 items in the ERAS groups. The following exclusion criteria were applied: the study was not an RCT; the study did not compare ERAS with conventional care; the study reported on emergency, non-elective, or transplantation surgery; the study consisted of unpublished data with only the abstract presented at a national or international meeting.
2.4. Statistical analysis
Meta-analyses were conducted using odd ratios (ORs) for dichotomous outcomes, and weighted mean differences (WMDs) were used for continuous outcomes. Pooled estimates were presented with 95% confidence intervals (CIs). If the included studies provided medians and interquartile ranges, the mean ± Standard Deviation (SD) was calculated according to the method described by Hozo et al.[16] When heterogeneity was found to be statistically significant (P < .05 or I2 > 50%), a random effects model was applied.[17] Conversely, a fixed effects model was adopted to calculate the pooled ORs or WMDs. Funnel plots were generated to determine the presence of publication bias. In the event that a study presented significant heterogeneity, sensitivity analyses assessed the effect of SDs from medians and interquartile ranges in poor quality studies on the overall results. We further identified the sources of heterogeneity and assessed the robustness and consistency of the statistical techniques used in the present study. For all other comparisons, statistical significance was defined by P < .05, and all tests were 2-tailed. All statistical analyses were performed using the Review Manager (RevMan; http://tech.cochrane.org/) software, version 5.3 from the Cochrane Collaboration.
2.5. Publication bias
A funnel plot was used to evaluate the bias. The asymmetry in the funnel plot of trial size against treatment effect was used to assess the risk of bias.
3. Results
3.1. Study characteristics
In total, 862 records were retrieved from the initial literature search. After the removal of duplicates (179 records), we identified 683 records by screening the titles and abstracts. Six hundred seventy one articles were excluded; thus, 12 articles remained for further evaluation. Subsequently, 7 articles were excluded after full-text reading, including 3 that had <7 ERAS items and 4 were non-randomized controlled trials. Lu et al[18] demonstrated that the ERP reduced the time of anesthesia and controlled the duration of operation, which could artificially affect the postoperative results. In addition, the present study was only single-blind, and hence, we excluded the article by Lu et al from the analysis. Eventually, 4 RCTs were included in the meta-analysis (Fig. 1).[19–22]
Figure 1.
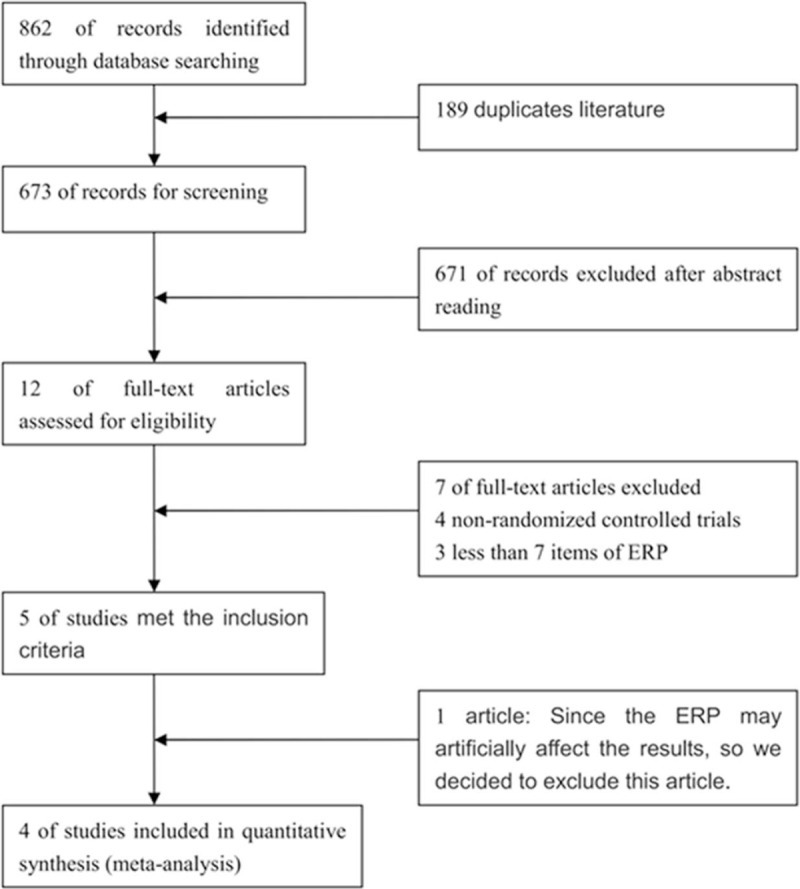
Identification of studies for inclusion.
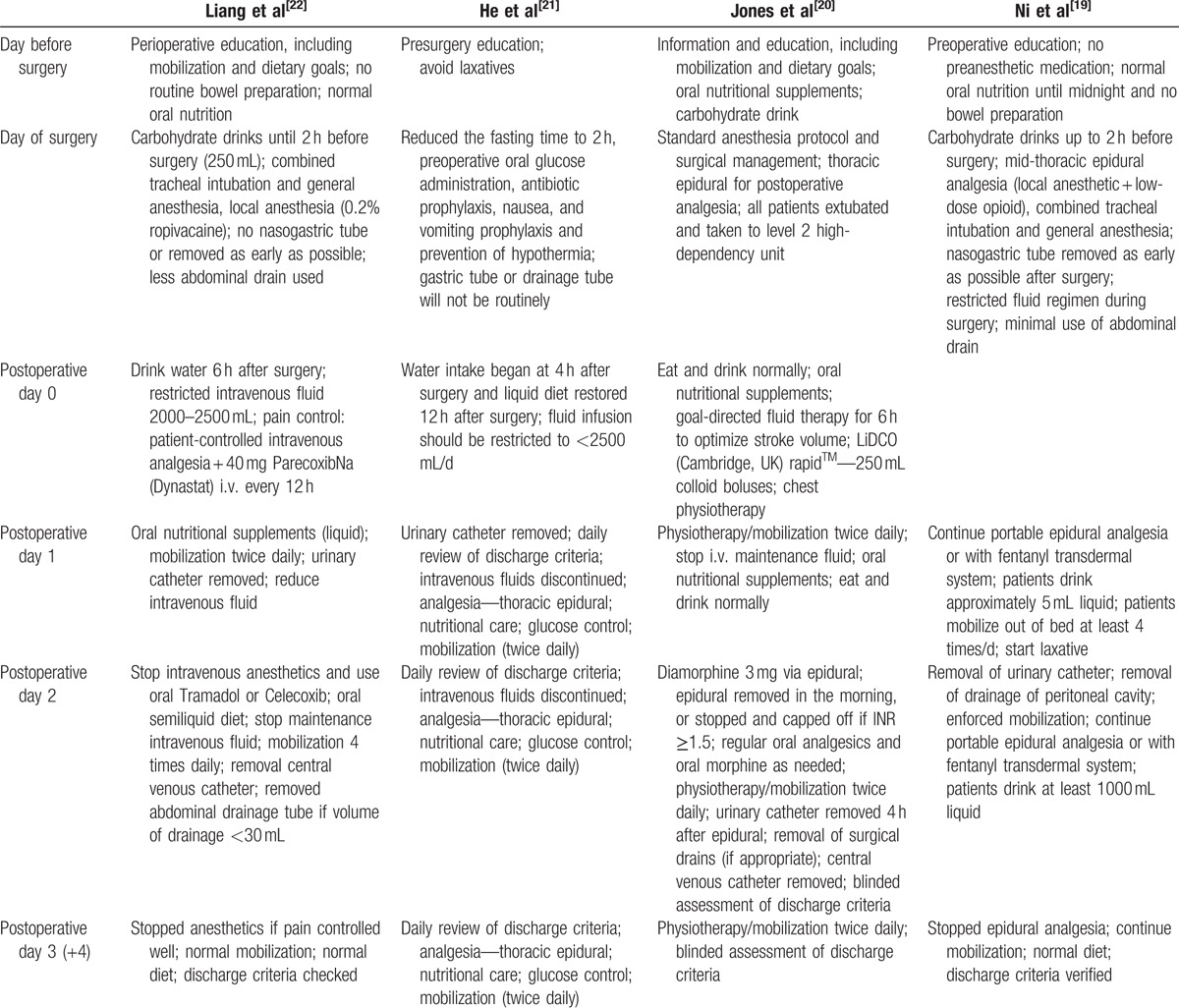
The trials were spanned over a period from 2013 to 2016. A total of 254 patients underwent liver resection according to an ERAS protocol, and 270 were managed on a conventional care pathway after liver resection. The majority of the operations were conducted for benign diseases, colorectal liver metastases, or HCC. The participants’ characteristics are summarized in Table 1. All the studies explicitly described an ERAS protocol. The individual components utilized and rates of adherence to the protocol are displayed in Table 2.
Table 1.
Characteristics of included trials.
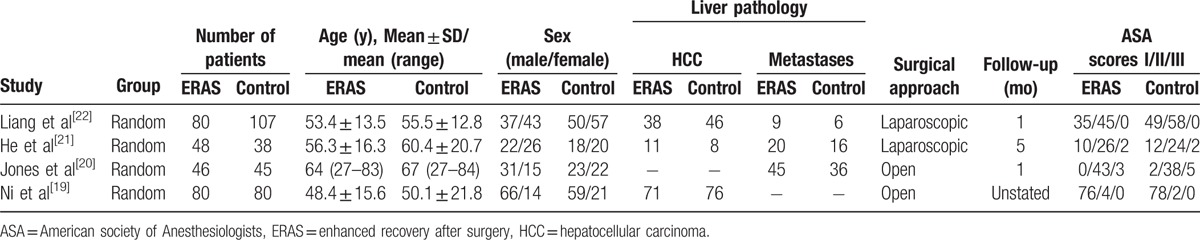
Table 2.
Enhanced recovery programs of included trial.
3.2. Postoperative hospital stay
3.2.1. The length of postoperative hospital stay
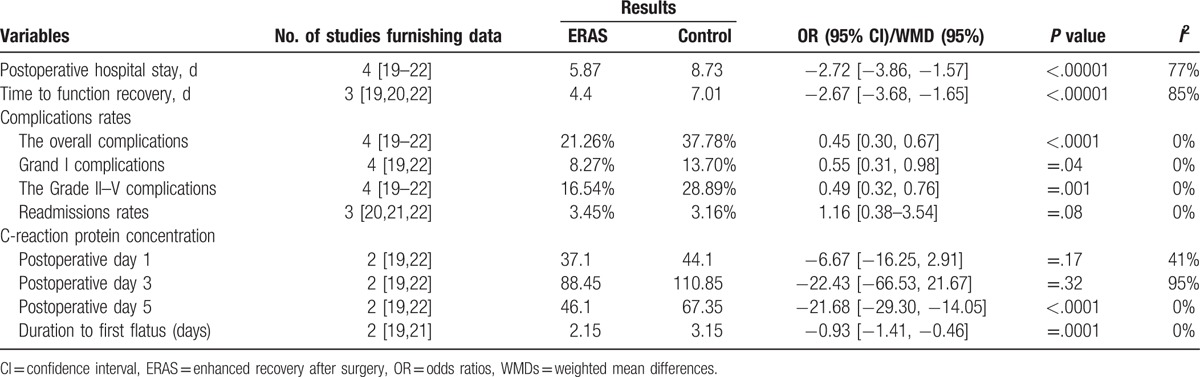
The meta-analysis (all trials reported these data) showed a significant difference favorable to the ERAS group (WMD −2.72; 95% CI −3.86 to −1.57; P < .00001), with certain heterogeneity (Table 3).
Table 3.
Summary of the comparison between ERAS versus conditional care in hepatectomy.
3.2.2. Time to function recovery
The meta-analysis (3 trials reported these data) showed a significant difference favorable to the ERAS group (WMD −2.67; 95% CI −3.68 to −1.65; P < .00001), with certain heterogeneity.
3.3. Complications
3.3.1. The overall complications
The meta-analysis (all trials reported these data) showed a significant difference and was favorable to the ERAS group (OR, 0.45 [95% CI, 0.30–0.67]; P < .0001), with no evidence of significant heterogeneity (Fig. 2).
Figure 2.
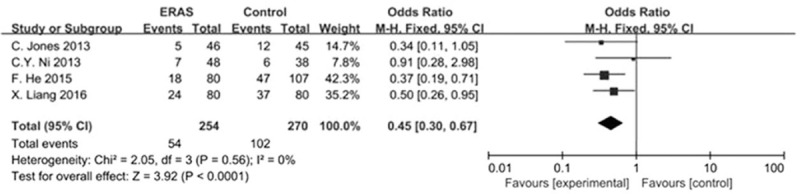
Forest plot: the overall complications by ERAS versus conventional care after hepatectomy. ERAS = enhanced recovery after surgery.
3.3.2. Grade I complications
The meta-analysis (all trials reported these data) revealed a significant difference and was favorable to the ERAS group (OR, 0.55 [95% CI, 0.31–0.98]; P = .04), with no evidence of significant heterogeneity.
3.3.3. Grade II–V complications
The meta-analysis (all trials reported these data) showed a significant difference that was favorable to the ERAS group (OR, 0.49 [95% CI, 0.32–0.76]; P = .001), with no evidence of significant heterogeneity.
3.4. Readmissions
The meta-analysis (3 trials reported these data) showed no statistical differences (OR, 1.16 [95% CI, 0.38–3.54]; P = .08).
3.5. C-reaction protein (CRP) concentration
3.5.1. Postoperative day 1
The meta-analysis (2 trials reported these data) showed that there was no statistics (WMD −6.67; 95% CI −16.25–2.91; P = .17).
3.5.2. Postoperative day 3
The meta-analysis (2 trials reported these data) demonstrated no statistical differences (WMD −22.43; 95% CI −66.53–21.67; P = .32).
3.5.3. Postoperative day 5
The meta-analysis (2 trials reported these data) showed that the experimental group of ERAS was significantly lesser than the control group (WMD −21.68; 95% CI −29.30–14.05; P < .0001), with no evidence of significant heterogeneity.
3.6. Duration to first flatus
The meta-analysis (2 trials reported these data) showed that the experimental group of ERAS was significantly shorter than the control group (WMD −0.93; 95% CI −1.41 to −0.46; P = .0001), with no evidence of significant heterogeneity.
3.7. Sensitivity analysis and publication bias
The complication, postoperative hospital stay, readmissions, CRP concentration, and duration to first flatus following ERAS or control in hepatectomy were calculated by both fixed-effect and random-effect models. The results obtained by both the methods were similar, and the combined results were highly reliable. Moreover, the results of subgroup analysis were also in agreement (laparoscopic and open liver surgery).
The funnel plots of the study results were shown in Fig. 3. The funnel plots on overall complications following ERAS versus control after hepatectomy showed symmetry, which did not indicate a significant publication bias.
Figure 3.
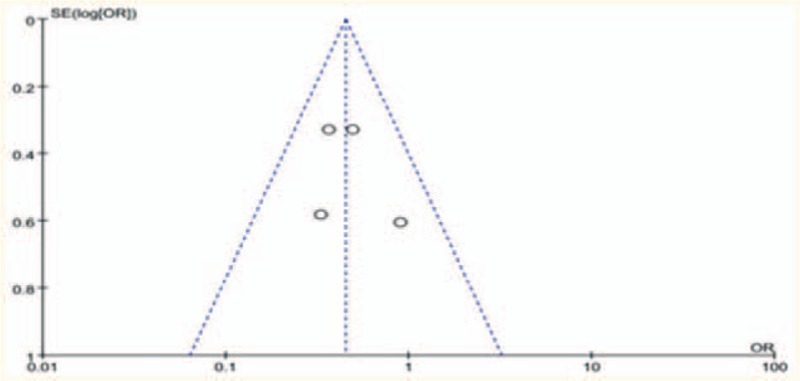
Funnel plot: the overall complications by ERAS versus conventional care after hepatectomy. ERAS = enhanced recovery after surgery.
4. Discussion
The present meta-analysis evaluated the effects of ERAS programs in comparison with the conventional care in patient recovery after liver surgery. The primary outcome parameters reflect the safety and efficacy of the intervention, which is always the greatest concern in clinical practice.
The length of stay is not an ideal index to judge the success of an ERAS program. Because, several factors make the patients able to or keen to stay in the hospital.[23] Functional recovery, which offered the modest reporting of recovery milestones, was assessed in the present studies frequently. This study showed that the durations of postoperative recovery time and hospital stay were significantly better than the control group, indicating the effectiveness of ERAS.
The ERAS patients presented a significant reduction in overall morbidity. Simultaneously, the Grade I and Grade II–V complications were significantly favorable to the ERAS group. However, the rates of readmission were not statistically significant; thus, the ERAS program can be designated as safe.
The levels of serum CRP were used to evaluate the surgical stress response.[24] The meta-analysis showed that although the comparison is not statistically significant on postoperative days 1 and 3, the CRP concentration in the control group was significantly higher than that in the ERAS group on postoperative day 5, thereby indicating that ERAS regulates the postoperative inflammation.
We also compared the duration to first flatus, which is commonly assessed by recovery bowel function. The experimental group of ERAS was found to be superior to the control group, which could be attributed to no bowel preparation and less fasting time preoperative, early mobilization and feeding postoperative.
In 2005 and 2009, the consensus guidelines of ERAS programs were developed and modified by a group of colorectal surgeons.[25,26] However, there is no similar guideline in liver surgery. The current analysis revealed that the ERAS programs were heterogeneous in all the included articles. Therefore, optimization of the ERAS programs is imperative. We found that the following areas may optimize the ERAS programs to facilitate recovery.
4.1. Family support
Family support was a very big element while considering early discharge. Early discharge was thinked with trepidation and apprehension regarding patients with limited family support. Patients with sick family members, other family commitments, and those living alone were often concerned about the actual care arrangements at home, especially during early discharge.[27]
4.2. Preoperative nutritional assessment and adjustment
Malnutrition was found in 17% to 46% of patients in general surgery.[28] Several studies have shown that protein-energy malnutrition (PEM), an imbalance between the needs and expenditure of an organism, significantly impairs the postoperative course and increases morbidity,[29–31] especially infectious complications, and postoperative mortality.[32] Moreover, the surgical stress increases metabolic needs and releases cytokines, which worsen anorexia and muscle wasting. Thus, malnutrition leads to increased postoperative complications, and surgical stress worsens malnutrition.
4.3. Type of incision
Two systematic reviews[33,34] demonstrated that the “long transverse incision” group harbored fewer postoperative pulmonary complications, short- and long-term wound complications and postoperative pain than the “long longitudinal incisions” group in the gastrointestinal and abdominal aortic surgeries. Similarly, concerning a mini-incision approach for locally advanced colonic cancer, a transverse incision seems to be advantageous with respect to minimal invasiveness and early recovery as compared with the longitudinal incision. The mechanism underlying less pain after a transverse incision is a 2-sided approach. First, it could potentially avoid cutaneous somatic nerve injuries. In addition, the approach did not divide the rectus abdominis muscle and retracted it laterally, also avoiding relevant nerve injuries. Second, compared with the longitudinal incision, a transverse incision might decrease the tension on the wound itself as demonstrated in human and animal studies, leading to less pain.[34,35]
4.4. Incision suture
Evidence-based medicine concluded that continuous sutures of midline incision were beneficial as compared with interrupted sutures owing to lower incidence of incisional hernia and time saving, and therefore strongly recommended.[36,37] The continuous suture minimized the number of knots and an evenly distributed tension across the suture line,[38] which was associate with an equivalent or lower rate of an incisional hernia and decreased operation time.[39] In liver resection with transverse or oblique incisions, no difference was confirmed in the short-term and long-term wound complications between continuous and interrupted sutures. However, the continuous sutures significantly saved time and healed than interrupted layered sutures.[40]
4.5. Infrahepatic inferior vena cava clamping (IIVCC)
The decrease in intraoperative blood loss and the need for blood transfusion were considered as principal contributors towards the decrease in morbidity and mortality.[41] The observed low blood loss and transfusion rates potentially originated from low central venous pressure (CVP) during liver transection,[42] although with the risk of various side-effects. Besides impairment of kidney function and tissue oxygenation, hypovolemic patients are at risk of circulatory instability, especially in the case of unexpected intraoperative hemorrhage. IIVCC were significantly less than the low CVP in total blood loss and transection-related blood loss, albeit with fewer side-effects. The key reasons would be: IIVCC decreases CVP and controls venous backflow bleeding, and IIVCC causes fewer hemodynamic disturbances in cirrhosis patients than low CVP.[43–45]
4.6. Omega-3 polyunsaturated fatty acids (ω-3 PUFAs)
Previous studies have proved that ω-3 PUFAs effectively reduced severe hepatic steatosis and protected the liver from ischemia-reperfusion injury. The regenerative capacity and oncological outcomes await confirmatory studies in humans.[46,47]
5. Limitations of the study
As with all systematic reviews, our review is limited by the quality of the studies available for inclusion. First, the small number of RCTs prevents any meaningful meta-analysis. Second, the randomization procedure was unclear or inadequate in the trials, which could lead to selection bias. Finally, the nature of the surgical research often precludes blinding of personnel and participants in the RCT, which leads to an increased risk for both performance and measurement bias. Therefore, our pooled RR might be an overestimate of the true effect.
Furthermore, in the included articles, they had different patient eligible standards, different evaluation time, different treatment programs, different pathological types, and different discharge criteria, which can result in heterogeneity.
6. Optimized ERAS groups
6.1. Preoperative
Preoperative education, no routine bowel preparation, no routine use of nasogastric tube, preoperative nutritional assessment and adjustment, carbohydrate drinks until 2 hours before surgery (500 mL).
6.2. Intraoperative
Multimodal analgesia; less abdominal drain used; antibiotic prophylaxis, nausea, and vomiting prophylaxis and prevention of hypothermia; target guiding fluid, transverse incision; continuous sutures; reasonable blood flow control technology.
6.3. Postoperative
Multimodal analgesia; water intake began at 4 hours after surgery and liquid diet restored 12 hours after surgery; restricted intravenous fluid; the urinary catheter was removed 1 day after surgery; early activity; remove the abdominal drainage tube as soon as possible, etc.
7. Conclusion
In summary, the evidence suggested that ERAS following liver surgery is safe, effective, and feasible. Nevertheless, additional studies are essential for optimizing ERAS protocols.
Footnotes
Abbreviations: ω-3 PUFAs = Omega-3 polyunsaturated fatty acid, CI = confidence interval, CRP = C-reactive protein, CVP = central venous pressure, ERAS = enhanced recovery after surgery, ERP = enhanced recovery programs, HCC = hepatocellular carcinoma, IIVCC = infrahepatic inferior vena cava clamping, OR = odds ratios, PEM = protein-energy malnutrition, RCTs = randomized controlled trials, WMDs = weighted mean differences.
Contributors: LL carried out the studies, participated in collecting data, and drafted the manuscript. JMC and ZHL performed the statistical analysis and participated in its design. QL and YS helped to draft the manuscript. All authors read and approved the final manuscript.
Conflicts of Interest: All authors declare that they have no conflict of interests.
References
- [1].Kudo M. Surveillance, diagnosis, treatment, and outcome of liver cancer in Japan. Liver Cancer 2015;4:39–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Zhang Y, Ren JS, Shi JF, et al. International trends in primary liver cancer incidence from 1973 to 2007. BMC Cancer 2015;15:94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Lau WY. Management of hepatocellular carcinoma. J Royal Coll Surg Edinburgh 2002;47:389–99. [PubMed] [Google Scholar]
- [4].Palavecino M, Kishi Y, Chun YS, et al. Two-surgeon technique of parenchymal transection contributes to reduced transfusion rate in patients undergoing major hepatectomy: analysis of 1,557 consecutive liver resections. Surgery 2010;147:40–8. [DOI] [PubMed] [Google Scholar]
- [5].Virani S, Michaelson JS, Hutter MM, et al. Morbidity and mortality after liver resection: results of the patient safety in surgery study. J Am Coll Surg 2007;204:1284–92. [DOI] [PubMed] [Google Scholar]
- [6].Bardram L, Funch-Jensen P, Jensen P, et al. Recovery after laparoscopic colonic surgery with epidural analgesia, and early oral nutrition and mobilisation. Lancet 1995;345:763–4. [DOI] [PubMed] [Google Scholar]
- [7].Simpson JC, Moonesinghe SR, Grocott MP, et al. Enhanced recovery from surgery in the UK: an audit of the enhanced recovery partnership programme 2009–2012. Br J Anaesth 2015;115:560–8. [DOI] [PubMed] [Google Scholar]
- [8].ERAS Compliance Group The impact of enhanced recovery protocol compliance on elective colorectal cancer resection: results from an international registry. Ann Surg 2015;261:1153–9. [DOI] [PubMed] [Google Scholar]
- [9].Varadhan KK, Neal KR, Dejong CH, et al. The enhanced recovery after surgery (ERAS) pathway for patients undergoing major elective open colorectal surgery: a meta-analysis of randomized controlled trials. Clin Nutr 2010;29:434–40. [DOI] [PubMed] [Google Scholar]
- [10].Kim JW, Kim WS, Cheong JH, et al. Safety and efficacy of fast-track surgery in laparoscopic distal gastrectomy for gastric cancer: a randomized clinical trial. World J Surg 2012;36:2879–87. [DOI] [PubMed] [Google Scholar]
- [11].Muehling B, Schelzig H, Steffen P, et al. A prospective randomized trial comparing traditional and fast-track patient care in elective open infrarenal aneurysm repair. World J Surg 2009;33:577–85. [DOI] [PubMed] [Google Scholar]
- [12].Kirsh EJ, Worwag EM, Sinner M, et al. Using outcome data and patient satisfaction surveys to develop policies regarding minimum length of hospitalization after radical prostatectomy. Urology 2000;56:101–6. discussion 06-7. [DOI] [PubMed] [Google Scholar]
- [13].Santillan A, Govan L, Zahurak ML, et al. Feasibility and economic impact of a clinical pathway for pap test utilization in Gynecologic Oncology practice. Gynecol Oncol 2008;109:388–93. [DOI] [PubMed] [Google Scholar]
- [14].Jones EL, Wainwright TW, Foster JD, et al. A systematic review of patient reported outcomes and patient experience in enhanced recovery after orthopaedic surgery. Ann R Coll Surg Engl 2014;96:89–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Xiong J, Szatmary P, Huang W, et al. Enhanced recovery after surgery program in patients undergoing pancreaticoduodenectomy: A PRISMA-Compliant Systematic Review and Meta-Analysis. Medicine 2016;95:e3497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol 2005;5:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ 2003;327:557–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Lu H, Fan Y, Zhang F, et al. Fast-track surgery improves postoperative outcomes after hepatectomy. Hepatogastroenterology 2014;61:168–72. [PubMed] [Google Scholar]
- [19].Ni CY, Yang Y, Chang YQ, et al. Fast-track surgery improves postoperative recovery in patients undergoing partial hepatectomy for primary liver cancer: a prospective randomized controlled trial. Eur J Surg Oncol 2013;39:542–7. [DOI] [PubMed] [Google Scholar]
- [20].Jones C, Kelliher L, Dickinson M, et al. Randomized clinical trial on enhanced recovery versus standard care following open liver resection. Br J Surg 2013;100:1015–24. [DOI] [PubMed] [Google Scholar]
- [21].He F, Lin X, Xie F, et al. The effect of enhanced recovery program for patients undergoing partial laparoscopic hepatectomy of liver cancer. Clin Transl Oncol 2015;17:694–701. [DOI] [PubMed] [Google Scholar]
- [22].Liang X, Ying H, Wang H, et al. Enhanced recovery program versus traditional care in laparoscopic hepatectomy. Medicine 2016;95:e2835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Maessen JM, Dejong CH, Kessels AG, et al. Length of stay: an inappropriate readout of the success of enhanced recovery programs. World J Surg 2008;32:971–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Miller RJ, Sutherland AG, Hutchison JD, et al. C-reactive protein and interleukin 6 receptor in post-traumatic stress disorder: a pilot study. Cytokine 2001;13:253–5. [DOI] [PubMed] [Google Scholar]
- [25].Lassen K, Soop M, Nygren J, et al. Consensus review of optimal perioperative care in colorectal surgery: Enhanced Recovery After Surgery (ERAS) Group recommendations. Arch Surg 2009;144:961–9. [DOI] [PubMed] [Google Scholar]
- [26].Fearon KC, Ljungqvist O, Von Meyenfeldt M, et al. Enhanced recovery after surgery: a consensus review of clinical care for patients undergoing colonic resection. Clin Nutr 2005;24:466–77. [DOI] [PubMed] [Google Scholar]
- [27].Vandrevala T, Senior V, Spring L, et al. ‘Am I really ready to go home?’: a qualitative study of patients’ experience of early discharge following an enhanced recovery programme for liver resection surgery. Supportive Care Cancer 2016;24:3447–54. [DOI] [PubMed] [Google Scholar]
- [28].Larsson J, Akerlind I, Permerth J, et al. The relation between nutritional state and quality of life in surgical patients. Eur J Surg 1994;160:329–34. [PubMed] [Google Scholar]
- [29].Alves A, Panis Y, Mathieu P, et al. Postoperative mortality and morbidity in French patients undergoing colorectal surgery: results of a prospective multicenter study. Arch Surg 2005;140:278–83. discussion 84. [DOI] [PubMed] [Google Scholar]
- [30].Peng PD, van Vledder MG, Tsai S, et al. Sarcopenia negatively impacts short-term outcomes in patients undergoing hepatic resection for colorectal liver metastasis. HPB (Oxford) 2011;13:439–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Merli M, Nicolini G, Angeloni S, et al. Malnutrition is a risk factor in cirrhotic patients undergoing surgery. Nutrition 2002;18:978–86. [DOI] [PubMed] [Google Scholar]
- [32].Slim K, Panis Y, Alves A, et al. Predicting postoperative mortality in patients undergoing colorectal surgery. World J Surg 2006;30:100–6. [DOI] [PubMed] [Google Scholar]
- [33].Grantcharov TP, Rosenberg J. Vertical compared with transverse incisions in abdominal surgery. Eur J Surg 2001;167:260–7. [DOI] [PubMed] [Google Scholar]
- [34].Brown SR, Goodfellow PB. Transverse verses midline incisions for abdominal surgery. Cochrane Database Syst Rev 2005;19:Cd005199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Ishida H, Sobajima J, Yokoyama M, et al. Comparison between transverse mini-incision and longitudinal mini-incision for the resection of locally advanced colonic cancer. Int Surg 2014;99:216–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Diener MK, Voss S, Jensen K, et al. Elective midline laparotomy closure: the INLINE systematic review and meta-analysis. Ann Surg 2010;251:843–56. [DOI] [PubMed] [Google Scholar]
- [37].Muysoms FE, Antoniou SA, Bury K, et al. European Hernia Society guidelines on the closure of abdominal wall incisions. Hernia 2015;19:1–24. [DOI] [PubMed] [Google Scholar]
- [38].Martyak SN, Curtis LE. Abdominal incision and closure. A systems approach. Am J Surg 1976;131:476–80. [DOI] [PubMed] [Google Scholar]
- [39].Rucinski J, Margolis M, Panagopoulos G, et al. Closure of the abdominal midline fascia: meta-analysis delineates the optimal technique. Am Surg 2001;67:421–6. [PubMed] [Google Scholar]
- [40].Zhang J, Zhang HK, Zhu HY, et al. Mass continuous suture versus layered interrupted suture in transverse abdominal incision closure after liver resection. World J Surg 2016;40:2237–44. [DOI] [PubMed] [Google Scholar]
- [41].Wei AC, Tung-Ping Poon R, Fan ST, et al. Risk factors for perioperative morbidity and mortality after extended hepatectomy for hepatocellular carcinoma. Br J Surg 2003;90:33–41. [DOI] [PubMed] [Google Scholar]
- [42].Wang WD, Liang LJ, Huang XQ, et al. Low central venous pressure reduces blood loss in hepatectomy. World J Gastroenterol 2006;12:935–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Rahbari NN, Zimmermann JB, Koch M, et al. IVC CLAMP: infrahepatic inferior vena cava clamping during hepatectomy—a randomised controlled trial in an interdisciplinary setting. Trials 2009;10:94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Zhu P, Lau WY, Chen YF, et al. Randomized clinical trial comparing infrahepatic inferior vena cava clamping with low central venous pressure in complex liver resections involving the pringle manoeuvre. Br J Surg 2012;99:781–8. [DOI] [PubMed] [Google Scholar]
- [45].Rahbari NN, Koch M, Zimmermann JB, et al. Infrahepatic inferior vena cava clamping for reduction of central venous pressure and blood loss during hepatic resection: a randomized controlled trial. Ann Surg 2011;253:1102–10. [DOI] [PubMed] [Google Scholar]
- [46].Marsman HA, de Graaf W, Heger M, et al. Hepatic regeneration and functional recovery following partial liver resection in an experimental model of hepatic steatosis treated with omega-3 fatty acids. Br J Surg 2013;100:674–83. [DOI] [PubMed] [Google Scholar]
- [47].Gong Y, Liu Z, Liao Y, et al. Effectiveness of omega-3 polyunsaturated fatty acids based lipid emulsions for treatment of patients after hepatectomy: a prospective clinical trial. Nutrients 2016;8:357. [DOI] [PMC free article] [PubMed] [Google Scholar]


