Abstract
Sepsis and AKI are frequently combined in critical care patients. They are both independently associated with increased mortality and morbidity. AKI may precede, coincide with, or follow sepsis diagnosis. Risk factors for sepsis followed by AKI differ from those associated with AKI preceding or coinciding with sepsis and the pathophysiologic mechanisms may be different. In this article, we review the available clinical, laboratory and imaging tools available for the recognition of septic AKI. Early identification of high-risk patients and targeted preventive and therapeutic measures are key in reducing mortality and morbidity of the complex syndrome of septic AKI.
Introduction
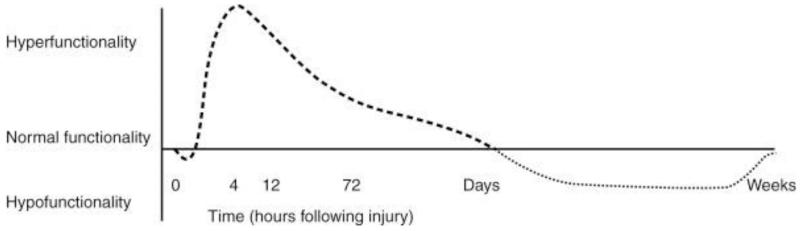
Acute kidney injury (AKI) is frequently associated with sepsis. Its incidence varies from 11 to 42% [1, 2] and may be as high as 67% in a septic surgical population.[3] Sepsis is the most common cause of AKI in critical care patients, accounting for 50% of cases in the ICU.[4] AKI incidence rate and severity correlate with the severity of the underlying sepsis.[5] Septic AKI is a hallmark of severe sepsis and septic shock and is associated with worse outcomes including prolonged hospital length of stay, fewer ventilator-free days and increased mortality when compared to patients with non-septic AKI.[2, 3] It appears that septic AKI is different than non-septic AKI with respect to the underlying contributing factors, and severity of injury and outcomes. Septic patients develop more severe AKI than in non-septic patients and even patients with non-severe infections (e.g. pneumonia) have a significantly higher incidence of AKI.[2, 6]. Recent studies have identified several pathophysiological mechanisms that are discussed in detail elsewhere in this journal. Several factors have been implicated in the pathogenesis of septic AKI. Hemodynamic changes in the macro circulation (i.e.: vasodilatation and increased cardiac output), and systemic and renal microcirculation contribute to renal hyperemia coupled with inefficient cellular oxygen extraction. The renal medulla is particularly sensitive to these hemodynamic perturbations and resultant hypoxemia, since it is already functioning at a lower PaO2 level, especially in the nephrons of the cortico-medullary junction. Sepsis is also associated with systemic inflammation and endothelial dysfunction, which also have been shown to contribute to renal injury and enhance microcirculation perturbations.[7, 8] The stress response is altered in sepsis; the earliest phase characterized by a short-lived hypo-responsiveness, which is followed by a dramatic phase of hyper-responsiveness. In the hyper-reponsive phase, both pro- and anti-inflammatory cytokines are released in the systemic circulation, and endothelial exposure of local adhesion receptors leads to platelet aggregation with microthrombi formation and enhanced leucocyte recruitment. This excessive immune response with deregulation between the pro- and anti-inflammatory mediators contributes to further downstream or distant organ damage such as AKI. The later phase of sepsis is characterized by hypofunctionality of the immune system, which may last from several days to weeks, and increases susceptibility to new or recurrent infections. The complex interplay of various factors during the course of sepsis makes it difficult to identify the exact mechanism and pathways in septic AKI.
Although there is a significant body of literature supporting an important role of inflammation in the pathogenesis of septic AKI, the use (to date)of interventions that reduce the inflammatory state seen in sepsis have not been successful in reducing AKI risk. In a prospective cohort study by Murugan et al, the use of statins (which have a pleiotropic anti-inflammatory effect) in patients presenting with pneumonia was associated with a reduction in the risk of AKI that did not remain statistically significant after adjusting for confounders (OR 0.72, p=0.09).[9]
Sepsis and AKI: Timing and Risk Factors
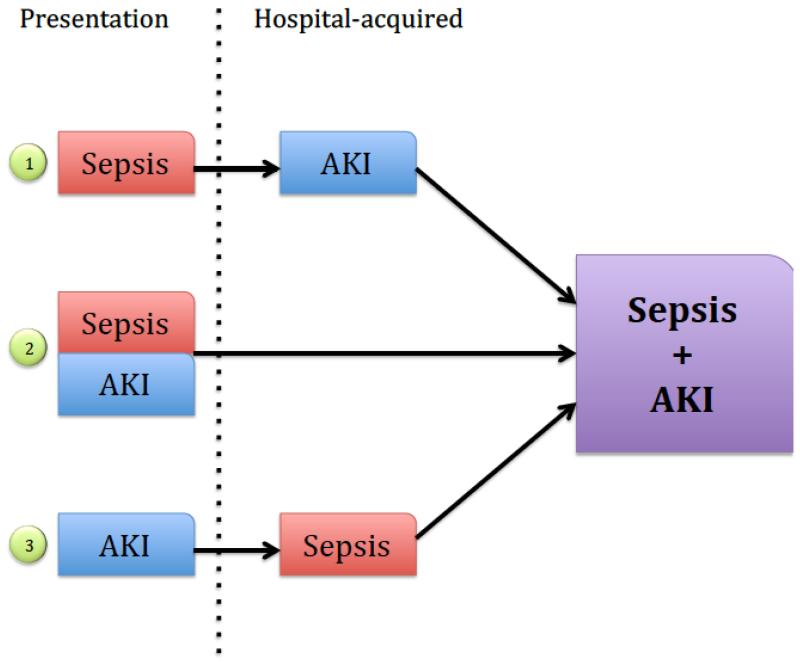
AKI in the setting of sepsis can be considered in three different domains: Sepsis preceding AKI, concurrent presentation of sepsis and AKI and sepsis following AKI.
It is generally well accepted that sepsis greatly increases the risk of AKI, but there is growing evidence that AKI itself increases the risk of sepsis. In a post-hoc analysis of the prospective multicenter PICARD study (AKI patients), 40% of the patients developed sepsis after they developed AKI (median of 5 days), compared to 28% in which sepsis preceded AKI. Mortality was similar between groups, but when they were compared to a group of AKI patients without sepsis, both groups had higher mortality, risk of requiring dialysis and longer hospital length of stay. Significant predictors of sepsis in AKI patients identified in this study were fluid accumulation, oliguria, severity of illness score, non-surgical procedures and dialysis.[10] Different mechanisms may explain increased risk of sepsis in AKI patients. Uremia appears to affect distant organ function. For example, it is associated with immune system dysfunction, impaired leukocyte trafficking, cytokine regulation and vascular permeability.[11] Immunoparalysis has been described in chronic kidney disease and especially in the end-stage renal disease population with increased risks of pneumonia and sepsis.[12] It is now increasingly believed that similar changes occur with AKI. Critical care patients with AKI have impaired monocyte cytokine production associated with high levels of plasma cytokines.[13] Impaired local protective barriers mechanisms associated with the fluid overload often seen with AKI may also contribute to increasing sepsis risk (e.g. edema, third spacing, skin or gastro-intestinal barrier breakdown with bacterial translocation, and edema with poor wound healing leading ultimately to infection). Patients with AKI requiring dialysis are also at increased risk of bacteremia and endocarditis through central venous catheter insertion or peritoneal dialysis catheter placement. AKI increases length of stay in hospital, which itself is a well-known risk factor for nosocomial infections. AKI treatment may also increase the risk of infection or sepsis, by mechanisms beyond simple under-dosing of antimicrobial drugs (by either inadequate supplemental dosing to correct for drug removal by RRT, or failure to augment dose during AKI recovery). A rapid reduction through dialysis of neutrophil gelatinase-associated lipocalin (NGAL), a known antibacterial factor of natural immunity, may perhaps increase risk of subsequent infection or sepsis. [14] Erythropoietin, also reduced with AKI, may similarly have an immunomodulatory effect.
The risk of developing AKI after sepsis is higher in patients with older age, male sex, increased severity of illness, lower urinary output, higher central venous filling pressures, vasopressor requirements and pre-existing treatment with ACEI/ARB.[1, 15-17] Serum creatinine at presentation and pH of <7.3 have also been identified as predictive of AKI in septic patients.[1] A recent retrospective study analyzing over 4,000 septic patients found that the presence of septic AKI varied significantly based on source of infection, with non-pulmonary infections having higher risks for AKI development. After multivariable analysis, no specific type of pathogen was associated with increased septic AKI risk compared to others.[18] On the other hand, another study did identify a higher number of positive blood cultures, especially gram-negative bacilli and fungi, in septic AKI patients compared to septic patients who did not develop AKI.[17] Additionally, several clinical characteristics differ between patients with septic AKI and those with non-septic AKI. Septic AKI patients tend to be older, have more co-morbid disease, are more likely to be admitted to the medical intensive care unit (ICU), have higher severity of underlying illness scores, greater abnormalities in vitals signs, markers of inflammation and blood chemistry.[2]
Considering all of these arguments, it must be emphasized that not only is sepsis a risk factor for AKI, but AKI itself appears to be a risk factor for sepsis. In some situations the sepsis clearly precedes the kidney injury, but other cases might not be so clear leading one to wonder: “Is the kidney a victim or the cause of the sepsis?” AKI may therefore be a cause and a consequence of sepsis. The fact that mortality rates associated with early versus late development of sepsis in AKI patients do not differ indicates that while the latter has been under-recognized in the past, it carries significant consequences.
Recognition
Clinical manifestations of sepsis with AKI depend on many factors. The sequence of insults (AKI preceding sepsis, versus sepsis preceding AKI, or simultaneous presentation) may influence the patient’s initial clinical features. One must therefore keep sepsis features in mind when evaluating a patient with AKI and conversely evaluate for AKI when a patient presents with sepsis.
Signs and symptoms of sepsis vary not only with organ involvement, but also from one individual to another due to patient- and disease-specific characteristics and susceptibilities. Signs of sepsis reflect the phase of the disease and range from features limited to the primary organ (e.g. pneumonia) to severe multi-organ dysfunction syndrome (MODS) and septic shock. Caregivers must therefore be alert for any signs of infection, sepsis or septic shock when evaluating patients for renal failure, and conversely it is important to, frequently monitor renal function (along with other organ involvement) in patients with documented or suspected sepsis.
Septic AKI is defined by AKI in the presence of sepsis without another significant contributing factor explaining AKI. Recent diagnostic and staging criteria for AKI included an absolute increase of serum creatinine of 0.3mg/dl over 48 hours, a relative change in serum creatinine 1.5-1.9 times baseline over 7 days, or a urine output of less than 0.5 ml/kg/h for six hours.[94] Severity of septic AKI may be classified using the well documented consensus KDIGO criteria for AKI staging, and outcomes appear to be correlated with the presence and severity of AKI as defined by this classification system. [2, 19] Several pitfalls are associated with the use of serum creatinine and urine output for the diagnosis of septic AKI. Serum creatinine is a late, insensitive marker of renal injury, for a number of reasons. Because of the half-life of circulating creatinine, increments in serum creatinine lag decrements in glomerular filtration rate (GFR) by hours. Furthermore, the time to achieve a new steady state concentration that fully reflects the degree of GFR loss is delayed by (3-5) multiples of a prolonged serum creatinine half-life, reflected in changes over days rather than hours. Additionally, in critically ill septic patients hemodilution in hypotensive patients receiving aggressive fluid resuscitation with positive fluid balance masks serum creatinine increments, and has been shown to delay AKI diagnosis by a further day. Sepsis has also been show to reduce muscular production of creatinine, even without weight loss, further reducing the utility of serum creatinine as a marker of septic AKI.[20] Finally, patients receiving diuretics may not meet AKI diagnosis criteria based on reduced urine output due diuretic action. Other urinary biochemistry indices (see later text) may similarly unreliable with diuretic use.
Early identification of AKI in septic patients is crucial, because supportive and therapeutic maneuvers in septic patients are often nephrotoxic (e.g. use of vancomycin and aminoglycosides; or the use of vasopressor therapy with inadequate fluid resuscitation) and can aggravate the renal injury. In most sepsis trials, septic AKI is associated with poor survival, which is influenced by the magnitude of renal recovery. A recent retrospective trial by Sood et al. showed that septic patients who experienced reversible AKI or improved AKI (within 24 hours of diagnosis) had better survival rates than patients who didn’t recover from AKI and even those who didn’t develop AKI at all. Factors independently associated with AKI reversibility in this study were early administration of anti-microbial therapy, lower Acute Physiology and Chronic Health Evaluation II score, lower age, and a smaller number of failed organs (excluding renal) on the day of shock, as well as community-acquired infection. [21]
Additional tools may be useful to confirm or complement AKI diagnosis, by informing differential diagnostic and prognostic assessments. Urinalysis (by dipstick) and measurement of urine biochemistry indices such as the urinary sodium (UNa), fractional excretion of sodium (FeNa) and fractional secretion of urea (FeU) are commonly used to help differentiate pre-renal AKI from acute tubular necrosis (ATN), but they remain insensitive and non-specific tools offering often little reliable information to AKI diagnosis. Their use in sepsis appears to be even more limited, since septic AKI is a complex pathology that affects more than simply tubular reabsorption. A prospective cohort study of 83 patients failed to show any clinical significant differences amongst theses indices to help differentiate septic versus non-septic AKI, nor were they predictive of AKI worsening, renal replacement therapy requirements, or death. 50% of patients in this population showing significant microscopic evidence of tubular damage were found to have a FeNa <1%. Urine indices were not correlated with “damage” tubular marker such as NGAL.[22] In another studyl, a low FeNa and FeU were highly prevalent in the first hours of sepsis and a combination of both was predictive of transient AKI, whereas oliguria was predictive of impending AKI.[23] These contradictions further question the use of urine biochemistry in critical care patients, especially in septic patients since it may be unreliable due to the heterogeneity of the kidney disease and confounding factors (i.e.: timing, pre-existing CKD, vasopressors, fluid resuscitation, or diuretics). Unfortunately, there is currently no urine biochemistry test available to accurately differentiate septic AKI from non-septic AKI. A urinary scoring system based on the presence of granular casts and renal epithelial cells has been used to differentiate pre-renal AKI and ATN with the presence of different types of urinary casts being associated with a higher likelihood of dialysis.[24] These urine microscopy scores have also been demonstrated to be significantly higher in septic AKI patients than in non-septic AKI patients. Urine microscopy scores also correlate well with urinary NGAL levels, more modestly with plasma NGAL, and are predictive of worsening AKI. [25] Of course, the use of urine microscopy for the diagnostic assessment of AKI of AKI is further supported by the potential to disclose significant, treatable causes of AKI apart from prerenal azotemia or ATN, such as rapidly progressive glomerulonephritis (erythrocyte casts, “active” sediment with proteinuria, hematuria, leukocyturia), allergic interstitial nephritis (leukocyturia, leukocyte casts in the absence of UTI, and perhaps with eosinophiluria). It must, however, be emphasized that studies of urinalysis, biochemistry and microscopy are often confounded by numerous factors such as the unknown timing of renal insult, varying degrees of sepsis severity and fluctuating clinical course of different patients. All of these factors make inter-patient and inter-study comparison limited.
A promising investigational tool allowing earlier AKI diagnosis is Doppler-based renal resistance index (RI). Higher RI may predictive of AKI in patients with sepsis.[26] Doppler-based studies additionally may be useful in measuring renal perfusion during vasopressor therapy and may help differentiate between transient and persistent AKI.[26-28] A major downfall in the clinical use of renal RI is that it is influenced by numerous factors including patient age, arterial stiffness, pulse intra-abdominal pressure and other systemic hemodynamic factors such as pressure index, mean arterial pressure and heart rate. Additionally, these RI measurement results may differ between operators and centers and comparison of results must take these factors in consideration. Data regarding the effect of systemic hemodynamics factors on RI are still contradictory, with influences seen in some studies and not in others.[26, 28, 29]
Most of these diagnostic tools remain imperfect and AKI diagnosis by serum creatinine elevation or oliguria if often made after the window of opportunity for therapeutic or preventative intervention has already passed. For these reasons, newer biomarkers are increasingly being studied for rapid AKI diagnosis. These biomarkers can be classified as “functional” biomarkers (i.e.: serum creatinine and cystatin C) and “damage” biomarkers (i.e.: urinary albumin, NGAL, interleukin-18, KIM-1, L-FABP, TIMP-2, IGFBP7 and more). In addition to AKI, elevation of “functional” biomarkers with normal “damage” biomarkers may represent pre-renal state or in some cases CKD. On the other hand, elevation of “damage” biomarkers without elevation of “functional” biomarkers may represent a sub-clinical form of AKI that can subsequently progress into AKI as defined by serum creatinine elevation or resolve back to normal state. The ultimate goal would be to have a marker for septic AKI that would help identify the high-risk or sub-clinical AKI patients in whom prevention and support would play a critical role in outcome. These would also be the ideal patients to involve in interventional trials as they might benefit from the early treatment more than the patient with advanced, acutely irreversible form of septic AKI. The concern with some of these biomarkers is that they are non-specific to kidney injury and may be elevated in sepsis without AKI. NGAL, for example, is released by activated neutrophils in response to infection. IL-18 is also increased by inflammation and infection.[30] Several trials have evaluated these biomarkers’ role in septic AKI diagnosis. A pediatric study demonstrated elevated and discriminatory levels of urinary NGAL, serum and urinary cystatin C in children with septic AKI compared to septic children without AKI. Serum NGAL levels were not different between these groups in this study and caution should be used when interpreting levels this biomarker in septic patients.[31] Results from an adult population trial confirmed the same findings, where urinary NGAL, serum and urinary cystatin C showed significant discrimination for AKI in septic patients.[32] Urinary liver fatty acid-binding protein is significantly higher in ICU patients with AKI compare to those without AKI, and has been shown to be predictive of mortality in septic patients.[33, 34] Netrin, a laminin-like protein, may be an early marker of AKI. It appears to be excreted in the urine 1 hour after insult reaching a peak 30-fold increase by 6 hours. This could represent an important opportunity for eventual early intervention in these high-risk patients. These results, although not consistent, appear to support the conduct of further studies o their general use, and will perhaps result in the development, validation, and implementation of diagnostic tools for early septic AKI diagnosis.[30]
The exact attribution of the etiology of renal injury in the septic patient may not always be as straightforward as one would wish. These patients often have a significant number of co-morbidities (i.e.: immunosuppression, diabetes, hypertension, CKD, heart disease) and pre-existing medications (i.e.: NSAIDS, ACEI/ARB, calcineurin inhibitors or other) that may contribute to the renal insult. Once hospitalized, they are often exposed to addition risks and procedures, such as radiologic imaging or procedures requiring intravenous radiocontrast administration, or require specific treatment with nephrotoxic antimicrobial agents as aminoglycosides or amphotericin B. Although we tend to limit nephrotoxic agent use, most if not all patients will receive antimicrobial therapy, which may also rarely cause allergic interstitial nephritis. All of these interventions may cause hospital-acquired or iatrogenic AKI, which may further contribute or confuse septic AKI diagnosis. When there is doubt on AKI diagnosis, renal biopsy should be considered. Histology studies in septic patients have shown alternative diagnosis to acute tubular necrosis or “normal renal histology” (prerenal azotemia), including glomerular disease, acute interstitial nephritis, pyelonephritis or signs of vascular disease (eg. atheroembolism).[35]
Response
Management of sepsis involves three aspects: prevention, treatment and rehabilitation. Prevention remains the ultimate goal to reduce downstream patient and economic consequences and involves rapid treatment of infections, development of preventative measures against hospital acquired infections, glycemic control and sometimes use of prophylactic antibiotics. Sepsis specific treatment has seen little progress since anti-microbial therapy discovery in the mid-1900s. For the moment, most of the available interventions on AKI are based on prevention of further renal insult or organ support. Timely recognition of sepsis and response is therefore the first key element in its treatment. Supportive treatment is key to preventing further organ damage and improving survival in sepsis. Early administration of appropriate anti-microbial therapy (within 6 hours and ideally within one hour) is the cornerstone treatment in sepsis and improves survival.[36, 37] In a multicenter retrospective cohort study with 3373 hypotensive patients with septic shock, longer delays before administration of appropriate anti-microbial therapy were associated with early AKI development.[38]
Restoration of tissue perfusion and optimization of hemodynamic status are important goals of supportive therapy in sepsis and septic shock. Fluid therapy and vasopressor infusion are the main treatments available for hemodynamic support. Renal auto-regulation of renal blood flow (RBF) and glomerular filtration rate is usually maintained if mean arterial blood pressure (MAP) is in the range of 80 to 180 mm Hg. Within these values, fluctuations in blood pressure have only marginal effects on renal blood flow and glomerular filtration rate. Renal hypoperfusion however does not appear to be the main contributor to septic AKI.
Rather, human and animal studies have shown that RBF in septic patients was either preserved or increased [39-41], which strongly challenged the hypothesis that hypoperfusion resulting in either prerenal azotemia or ischemic renal injury with ATN are the predominant mechanisms of septic AKI. Of course, this also raises questions concerning the importance of a target MAP in septic patients, at least on renal standpoint. There is currently no accepted bedside method to evaluate renal perfusion.
Early goal directed therapy (EGDT) is an integrated approach designed to guide the physician with treatment goals and algorithms to treat septic patients. In 2001, Rivers et al. published a randomized-controlled single center emergency department trial of 263 patients in which they demonstrated improved survival of patients with severe sepsis or septic shock treated with EGDT (targeting normalization of central venous oxygen saturation) within 6 hours of emergency room (ER) arrival, compared to patients treated with protocolized standard therapy (30.5% vs 46.5%).[42] Soon after publication of the Rivers’ protocol it was rapidly integrated into Surviving Sepsis Campaign guidelines.[43] Additionally to standard of care, the EGDT added serial measurements of central venous oxygen saturation (ScvO2,), which was used to guide treatment through fluid therapy, vasopressors, inotropes and red blood cell transfusions to achieve an ScvO2 higher than or equal to 70%. Unfortunately renal outcomes were not evaluated in seminal the Rivers EGDT trial. Renal outcomes have been evaluated in a small group of ICU patients treated with EGDT protocol compared to historical controls, in a study that found a trend (that did not reach statistical significan) towards a lowerAKI incidence in the EGDT group compared to standard therapy.[44] Since the publication of the initial EGDT study more than a decade ago, much progress has been made in the early recognition of sepsis, timely administration of anti-microbial therapy and goal-directed or protocolized hemodynamic support, which has lead physicians to question if the results from this relatively small, single-centre study are still applicable in modern practice. The ProCESS trial, a 31 centers randomized-controlled ER trial was designed to evaluate the generalizability and necessity of the 2001 EGDT protocol. Patients were randomized to one of three groups: EGDT, usual care or a protocol-based standard therapy that did not involve central venous catheter placement, transfusion or inotropes administration. There were no differences in 60, 90 days and one-year mortality amongst the three groups. New renal failure, defined here by the need for dialysis within the first week, appeared to be lower in the usual care group and EGDT groups compared to the protocol-based standard therapy (2.8% vs 3.1% vs 6.0%; p=0.04). It must be mentioned that patients in the protocol-based standard therapy had received the greatest volume of fluid followed by the EGDT group and usual care group. The duration of RRT did not differ amongst groups.[45]
Advances in the care of ICU patient over the last decade such as implementation of lung-protective mechanical ventilation strategies, lower threshold for blood transfusion, and improved glycemic control may account for at least a part of the similarity in mortality rates in this study, rendering the contribution of EGDT less perceptible.
The Surviving Sepsis Campaign’s most recent revision still recommends achieving a MAP of 65 mmHg, central venous pressure of 8-12 mmHg when available, aiming a urine output of at least 0.5 ml/kg/h and ScvO2 higher than or equal to 70%.[37] It must be noted that these recommendations were published before the results of the ProCESS trial were available and evidence for use of ScvO2 is now weaker and might not be included in the next guidelines. The group also recommends aiming for normalization of serum lactate. The Surviving Sepsis Campaign care bundle suggest administration of 30 ml/kg crystalloid bolus for patient with hypotension within the first three hours and the use of vasopressors within the first six hours for patients’ whose MAP remains below 65 mmHg despite fluid therapy. Formal evaluation of optimal fluid parameters or hemodynamic targets for AKI prevention or management has been limited. It has been suggested in one study that targeting a MAP of 70-80 mmHg would be necessary to prevent AKI in septic shock, but this needs further validation..[46] A recent multicenter study randomized 776 septic patients to resuscitation plus high-target MAP (80-85 mmHg) versus low-target group (65-70 mmHg). The 28 and 90 day mortality rate did not differ between groups. Interestingly, patients with pre-existing chronic hypertension in the high-MAP group required less RRT than the ones of the low-target group.[47] The focus should be to restore organ perfusion and treatment should be individualized according to patient co-morbidities and clinical condition. Whether this should be achieved by fluid therapy or vasopressors depends on clinical evaluation of the patient’s volume status. The optimal method for evaluating volume status is not well established.[48] Clinicians should rely on an ensemble of clinical findings as opposed to results from a single test and interpret them according to patient’s co-morbidities and dynamic assessments of the patient’s condition.
Fluid administration should be the part of initial therapy as recommended above, but should be re-evaluated serially during the day, because septic alterations of hemodynamic status and vascular permeability is an evolving process. Septic AKI patients tend to have lower urinary outputs, receive more fluid therapy and/or diuretics and are more likely to develop fluid retention than non-septic AKI patients.[38, 49, 50] Fluid overload has been associated worse patient outcome in numerous studies.[50, 51] A recent retrospective trial by Legrand et al. identified an association between new or persistent AKI and elevated central venous pressure (CVP).[52] These findings go against the previous belief that when it came to fluid administration in septic patients, “there was no such thing as less is more”. A high CVP is no longer a desirable target and fluid resuscitation-induced venous congestion is increasingly believed to be contributing to renal injury.[53] In light of this information, it would be advisable that boluses would be administered at patient presentation and then fluid responsiveness evaluated within a few hours. Crystalloids are the fluid of choice for resuscitation and hydroxyethyl starches (HES) or hyperchloremic solutions are not recommended.[37] Numerous trials have evaluated the use of synthetic colloids in the past decade. A 2013 meta-analysis of these has shown increased association with RRT requirements with no demonstrated benefit on survival. Septic patients treated with HES also appear to develop more AKI and have increased requirements of RRT. [54] Fluid administration should be tempered after the initial bolus phase and eventually ceased when the patient reaches equilibrium phase after which the aim should be fluid mobilization by withholding fluids and allowing diuresis (spontaneous or with diuretics as needed). It should be noted that if at anytime the patient appears unresponsive to fluid therapy, at which point vasopressors should be prioritized over fluid therapy to avoid unnecessary fluid accumulation. Vasopressor support is often needed in septic shock since fluid therapy alone does not correct sepsis induced systemic vasodilatation and endothelial dysfunction. Norepinephrine is the drug of choice for septic patients requiring vasopressors.[55] Vasopressin has been compared to norepinephrine and did not appear to offer any benefit on mortality.[56] This study however noted that patients with mild forms of AKI were less likely to progress to more severe AKI but this was observed in a post-hoc analysis.[57]
Another key element in the management of septic patient in regards to AKI prevention is the avoidance of potentially nephrotoxic medication and contrast agents when possible, especially in high-risk patients (i.e.: diabetes, older age or CKD). [58, 59] Fenoldopam, a vasodilator with immunological properties has been tried as a preventive treatment in numerous AKI etiologies including sepsis which has showed conflicting results and is not currently recommended in practice.[60, 61]
The enzyme alkaline phosphatase (AP) has shown promising results in treatment of sepsis, predominantly through a renal protective effect in two phase 2a trials. [62, 63] In these trials, the administration of AP prevented septic AKI development and reduced the severity of AKI when it was present with few patients requiring renal replacement therapy (RRT), shorter duration of RRT and better creatinine clearance. It is believed that AP reduces inflammation through dephosphorylation and therefore detoxification of endotoxins and conversion of adenosine triphosphate into a form of adenosine with anti-inflammatory and tissue protective effects. These two mechanisms are believed to work against the hypoxic and inflammatory injuries encountered in septic AKI. Pharmacologic properties and safety of AP has been tested. [64] In light of this, AP can be considered as a potential future treatment for sepsis-induced AKI, if pivotal confirmatory clinical trials are similarly successfu.[65]
Nutritional support is an important but often overlooked aspect of global patient care. It is especially important in septic AKI, a hypercatabolic state that requires adapted protein and caloric intake. Numerous aspects need to be considered when calculating a septic AKI patient’s nutritional requirements such as his baseline characteristics, underlying condition, volume status and possible protein loss through RRT. For these reasons, each patient requires an individual approach for nutritional support.[66-68]
Extracorporeal Blood Purification
The utility of extracorporeal blood purification therapies for septic patients can be evaluated (and debated) for two different purposes: renal support and immunomodulation therapy.
The first and more commonly used application is renal replacement therapy in patients whose renal function fails to provide sufficient function to maintain body homeostasis. Traditional RRT indications for organ support purposes such as uremia, metabolic disturbances, and fluid overload apply in septic AKI just as in non-septic AKI. Timing of RRT initiation remains heterogeneous in clinical practice and is not yet firmly supported by uniform scientific evidence although excessive delays have been linked to higher mortality and worse renal function in retrospective analysis including septic patients.[69] The only published randomized controlled trial available to date did not find significant differences in renal outcomes or patient survival between early and late initiation of hemofiltration.[70] Two trials are now ongoing and will hopefully answer the optimal timing for RRT question: STARRT-AKI trial [71] and the IDEAL-ICU study.[72] The latter is addressing this question specifically in septic AKI patients.
RRT modality choice may have important implications for survivors of septic AKI, because CRRT appears to be associated with better renal recovery than intermittent modalities.[73, 74] Along the same lines, a recently published retrospective study of septic AKI patients from China found that initial therapy with continuous venovenous hemodiafiltration (CVVHDF) was associated with greater renal recovery at 60 days (defined as dialysis independence) compared to patients treated with extended daily hemofiltratrion (EDHF).[75] This different was observed despite the fact that CVVHDF patient had lower blood pressure, were more acidotic and oliguric than the other group treated with EDHF. Even though they did not observe a significant difference in mortality, the difference in dialysis dependence observed remains a relevant clinical outcome.
Even though CVVDHF is more costly than intermittent dialysis modalities in the ICU, the development of ESRD requiring chronic dialysis, an important cost to society, was lower in the long run. The differences in renal recovery may be due to the fact that better fluid control was achieved with fewer episodes of hypotension with CRRT.
Optimal RRT dosing has been evaluated in two major critical care trials (without specifically focusing on AKI) and the current recommended adequate effluent rate (delivered RRT dose) for CRRT is 25-30 ml/kg/h. [76-78] In one of these trials, a post hoc analysis of septic patients showed a tendency towards reduced mortality in the group or patients treated with the higher intensity approach (40 ml/kg/h vs 25 ml/kg/h).[70] Clinicians must take into consideration the fact that prescribed and delivered CRRT doses may differ, because treatment is interrupted for numerous reasons during a patient’s stay and should therefore over-prescribe with a 25% safety margin (30-35 ml/kg/h) to insure adequate delivered dose.[78, 79] High volume hemofiltration (HVHF) is defined as effluent rate above 35 ml/kg/h although some advocate that the criteria for this definition should be higher. HVHF has been hypothesized to clear sepsis-associated inflammatory mediators and therefore perhaps helps reduce inflammation-induced organ damage and improve septic shock survival. Since CRRT at standard “renal-dose” does not appear to improve outcomes in septic shock without renal failure[80, 81], studies using higher effluent rates (70-85 ml/kg/h) have been conducted to evaluate this approach. These trials and a recent meta-analysis have all failed to demonstrate any impact on patient survival, hemodynamic status or organ improvement. [82-84] Two possible factors explaining the absence of significant difference in outcomes in HVHF trials are the low cut-off points of the hemofilters used (which don’t remove larger mediators), and technical difficulties in delivering and maintaining the prescribed CRRT dose.
Rehabilitation and follow up
Early detection and reversal of AKI was associated with better outcomes.[18] Additionally, a prospective observational international study of 1753 patients, showed that patients with septic AKI showed a trend towards higher chances of recovery and dialysis independence compared to non-septic AKI patients even though they had higher risk of death and longer hospital length of stay.[85]
Although survival data is available, the renal prognosis of septic AKI has not been well described in the literature. Renal recovery is highly unlikely when sepsis is not controlled since the mechanisms of insult persist. Once sepsis is resolved, the likelihood of renal recovery depends on a number of factors such as the patients underlying characteristics (age, underlying CKD, diabetes and other co-morbidities), the severity of underlying insult (prolonged hypotension, sepsis severity and multiple organ involvement) and iatrogenic insults associated with process of care (fluid overload, hypotension associated with RRT, nephrotoxic antibiotics or contrast exposure). In clinical practice, the kidney is often one of the last organs to recover in patients with multiple organ failure due to sepsis and they may require weeks to months of dialysis.
Patients should be monitored for renal recovery during hospital stay, before hospital discharge and if no recovery has occurred by that time, it should also be assessed at regular intervals post discharge. We suggest that a useful way of monitoring renal recovery in these patients would be timed urinary creatinine and urea clearances repeated at periodic intervals.
As for all AKI cases, septic AKI patients should be scheduled for follow up within three months as suggested by the KDIGO AKI clinical practice guidelines to monitor kidney function and address recovery or optimize CKD treatment.[94]
Research
The incidence of sepsis has been increasing by 8.7% yearly and its mortality has not changed.[86] Despite all the progress achieved in general medicine in the past decades, the mortality of septic AKI remains unacceptably high. Contributing factors to this dilemma are perhaps the fact that the underlying pathophysiology of septic AKI remains to be fully understood with complete histological information, and that the tools currently used to assess it (serum creatinine and urine output) are of relatively low reliability in the septic patient. Despite numerous trials attempting to find a pharmacologic treatment for septic AKI, very few have been successful. This may be due to several factors such as late recognition of AKI where significant renal damage has already occurred. Another important factor to be considered is the heterogeneity of the septic population being studied, with variable organ involvement and multiple possible pathogenetic factors contributing to renal injury.[87] As mentioned earlier in this text, early identification of high-risk patients may be the key in preventing septic AKI and ultimately obtaining significant results in AKI treatment trials.
Future research should focus on two major aspects of prevention and treatment of septic AKI: identification of high-risk patients at earlier stages of renal injury, and targeted treatment of AKI once it has developed. Novel biomarker and imaging studies should be designed to select patient with early injury, to facilitate the design of specific therapeutic trials and complement clinical ascertainment of modifiable patient risk factors. Another key in septic AKI prevention is the establishment of appropriate criteria for surveillance of septic AKI in hospitalized patients, whether it be through pharmacy identification of high risk medication in a patient’s profile, judicious use of contrast in patients at high risk of contrast-induced AKI, or careful use of fluid, diuretics and nephrotoxic medication. Further understanding underlying pathophysiologic models of septic AKI will be crucial to achieve successful prevention and therapeutic trials designs and results.
Conclusion
AKI associated with sepsis may present in different forms and is independently associated with increased mortality and morbidity. AKI may precede or follow sepsis. Differentiating septic AKI from other forms of AKI is important, as underlying pathophysiologic mechanisms and outcomes differ between these two groups. Identification of high-risk patients and those with early AKI is crucial in influencing patient outcome. Serum creatinine and urine output are imperfect markers of early AKI in septic patients, and other novel tools need to be implemented to identify these patients.
While there are no specific treatments for septic AKI, early antibiotic administration, avoidance of hypotension (through fluid administration or vasopressors), nephrotoxic agents and fluid overload (through judicious use of fluid therapy, diuretics and RRT) can minimize AKI risk. CRRT has been associated with improved renal recovery, and should perhaps be started earlier in AKI evolution, but this need to be validated in future studies. Future trials should be designed to identify high-risk patients with early injury and focus on targeted therapy.
Figure 1.
Immune hypo and hyper responsiveness phases in sepsis here
Figure 2.
Three models of sepsis and AKI classified by sequence of injury. 1) Patient presents with sepsis and later develops AKI (late septic AKI). 2) Patient presents with simultaneous AKI and sepsis (early septic AKI). 3) Patient presents with AKI and later develops sepsis.
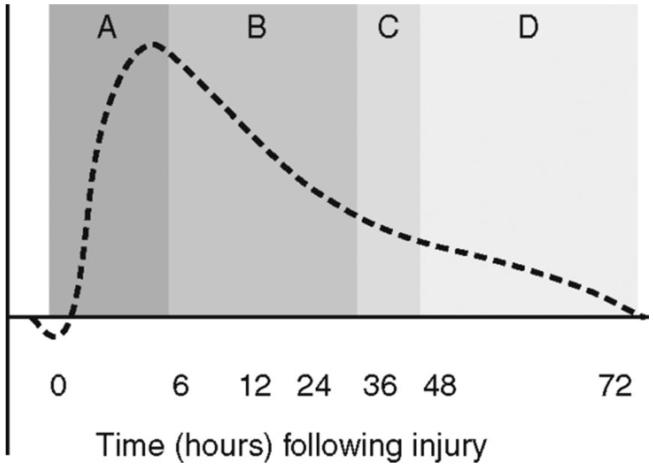
Figure 3.
Fluid resuscitation strategy during the stress response. Phase A: 0 to 6 hours = aggressive volume resuscitation. Phase B: 6 to 36 hours = decelerating fluid resuscitation; fluid boluses administered to compensate for extravascular sequestration. Phase C: 36 to 48 hours = equilibrium phase; stop administering intravenous fluids. Phase D: 48 to 72 hours = mobilization fluids; withhold fluids and allow spontaneous diuresis (or diurese if necessary)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Bibliography
- 1.Hoste EA, et al. Acute renal failure in patients with sepsis in a surgical ICU: predictive factors, incidence, comorbidity, and outcome. J Am Soc Nephrol. 2003;14(4):1022–30. [Google Scholar]
- 2.Bagshaw SM, et al. Early acute kidney injury and sepsis: a multicentre evaluation. Crit Care. 2008;12(2):R47. doi: 10.1186/cc6863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.White LE, et al. Acute kidney injury is surprisingly common and a powerful predictor of mortality in surgical sepsis. J Trauma Acute Care Surg. 2013;75(3):432–8. doi: 10.1097/TA.0b013e31829de6cd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Uchino S, et al. Acute renal failure in critically ill patients: a multinational, multicenter study. JAMA. 2005;294(7):813–8. doi: 10.1001/jama.294.7.813. [DOI] [PubMed] [Google Scholar]
- 5.Rangel-Frausto MS, et al. The natural history of the systemic inflammatory response syndrome (SIRS). A prospective study. JAMA. 1995;273(2):117–23. [PubMed] [Google Scholar]
- 6.Murugan R, et al. Acute kidney injury in non-severe pneumonia is associated with an increased immune response and lower survival. Kidney Int. 2010;77(6):527–35. doi: 10.1038/ki.2009.502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ergin B, et al. The renal microcirculation in sepsis. Nephrol Dial Transplant. 2014 doi: 10.1093/ndt/gfu105. [DOI] [PubMed] [Google Scholar]
- 8.Wan L, et al. Pathophysiology of septic acute kidney injury: what do we really know? Crit Care Med. 2008;36(4 Suppl):S198–203. doi: 10.1097/CCM.0b013e318168ccd5. [DOI] [PubMed] [Google Scholar]
- 9.Murugan R, et al. Association of statin use with risk and outcome of acute kidney injury in community-acquired pneumonia. Clin J Am Soc Nephrol. 2012;7(6):895–905. doi: 10.2215/CJN.07100711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mehta RL, et al. Sepsis as a cause and consequence of acute kidney injury: Program to Improve Care in Acute Renal Disease. Intensive Care Med. 2011;37(2):241–8. doi: 10.1007/s00134-010-2089-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Scheel PJ, Liu M, Rabb H. Uremic lung: new insights into a forgotten condition. Kidney Int. 2008;74(7):849–51. doi: 10.1038/ki.2008.390. [DOI] [PubMed] [Google Scholar]
- 12.Coresh J. CKD prognosis: beyond the traditional outcomes. Am J Kidney Dis. 2009;54(1):1–3. doi: 10.1053/j.ajkd.2009.05.005. [DOI] [PubMed] [Google Scholar]
- 13.Himmelfarb J, et al. Impaired monocyte cytokine production in critically ill patients with acute renal failure. Kidney Int. 2004;66(6):2354–60. doi: 10.1111/j.1523-1755.2004.66023.x. [DOI] [PubMed] [Google Scholar]
- 14.Bolignano D, et al. Neutrophil gelatinase-associated lipocalin (NGAL): a new piece of the anemia puzzle? Med Sci Monit. 2010;16(6):RA131–5. [PubMed] [Google Scholar]
- 15.Yegenaga I, et al. Clinical characteristics of patients developing ARF due to sepsis/systemic inflammatory response syndrome: results of a prospective study. Am J Kidney Dis. 2004;43(5):817–24. doi: 10.1053/j.ajkd.2003.12.045. [DOI] [PubMed] [Google Scholar]
- 16.Leedahl DD, et al. Derivation of Urine Output Thresholds That Identify a Very High Risk of AKI in Patients with Septic Shock. Clin J Am Soc Nephrol. 2014;9(7):1168–74. doi: 10.2215/CJN.09360913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Suh SH, et al. Acute kidney injury in patients with sepsis and septic shock: risk factors and clinical outcomes. Yonsei Med J. 2013;54(4):965–72. doi: 10.3349/ymj.2013.54.4.965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sood M, et al. Non-pulmonary infections but not specific pathogens are associated with increased risk of AKI in septic shock. Intensive Care Med. 2014 doi: 10.1007/s00134-014-3361-1. [DOI] [PubMed] [Google Scholar]
- 19.Bellomo R, et al. Acute renal failure - definition, outcome measures, animal models, fluid therapy and information technology needs: the Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care. 2004;8(4):R204–12. doi: 10.1186/cc2872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Doi K, et al. Reduced production of creatinine limits its use as marker of kidney injury in sepsis. J Am Soc Nephrol. 2009;20(6):1217–21. doi: 10.1681/ASN.2008060617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sood MM, et al. Early reversible acute kidney injury is associated with improved survival in septic shock. 2014 doi: 10.1016/j.jcrc.2014.04.003. [DOI] [PubMed] [Google Scholar]
- 22.Bagshaw SM, et al. Urine biochemistry in septic and non-septic acute kidney injury: a prospective observational study. J Crit Care. 2013;28(4):371–8. doi: 10.1016/j.jcrc.2012.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vanmassenhove J, et al. Urinary output and fractional excretion of sodium and urea as indicators of transient versus intrinsic acute kidney injury during early sepsis. Crit Care. 2013;17(5):R234. doi: 10.1186/cc13057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kidney Disease: Improving Global Outcomes (KDIGO) Acute Kidney Injury Work Group KDIGO clinical practice guideline for acute kidney injury. Kidney Int Suppl. 2012;2:1–138. [Google Scholar]
- 25.Bagshaw SM, et al. A prospective evaluation of urine microscopy in septic and non-septic acute kidney injury. Nephrol Dial Transplant. 2012;27(2):582–8. doi: 10.1093/ndt/gfr331. [DOI] [PubMed] [Google Scholar]
- 26.Lerolle N, et al. Renal failure in septic shock: predictive value of Doppler-based renal arterial resistive index. Intensive Care Med. 2006;32(10):1553–9. doi: 10.1007/s00134-006-0360-x. [DOI] [PubMed] [Google Scholar]
- 27.Deruddre S, et al. Renal arterial resistance in septic shock: effects of increasing mean arterial pressure with norepinephrine on the renal resistive index assessed with Doppler ultrasonography. Intensive Care Med. 2007;33(9):1557–62. doi: 10.1007/s00134-007-0665-4. [DOI] [PubMed] [Google Scholar]
- 28.Darmon M, et al. Diagnostic accuracy of Doppler renal resistive index for reversibility of acute kidney injury in critically ill patients. Intensive Care Med. 2011;37(1):68–76. doi: 10.1007/s00134-010-2050-y. [DOI] [PubMed] [Google Scholar]
- 29.Schnell D, et al. Renal perfusion assessment by renal Doppler during fluid challenge in sepsis. Crit Care Med. 2013;41(5):1214–20. doi: 10.1097/CCM.0b013e31827c0a36. [DOI] [PubMed] [Google Scholar]
- 30.Bagshaw SM, et al. Urinary biomarkers in septic acute kidney injury. Intensive Care Med. 2007;33(7):1285–96. doi: 10.1007/s00134-007-0656-5. [DOI] [PubMed] [Google Scholar]
- 31.Di Nardo M, et al. Impact of severe sepsis on serum and urinary biomarkers of acute kidney injury in critically ill children: an observational study. Blood Purif. 2013;35(1-3):172–6. doi: 10.1159/000346629. [DOI] [PubMed] [Google Scholar]
- 32.Aydogdu M, et al. The use of plasma and urine neutrophil gelatinase associated lipocalin (NGAL) and Cystatin C in early diagnosis of septic acute kidney injury in critically ill patients. Dis Markers. 2013;34(4):237–46. doi: 10.3233/DMA-130966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Doi K, Noiri E, Sugaya T. Urinary L-type fatty acid-binding protein as a new renal biomarker in critical care. Curr Opin Crit Care. 2010;16(6):545–9. doi: 10.1097/MCC.0b013e32833e2fa4. [DOI] [PubMed] [Google Scholar]
- 34.Nakamura T, Sugaya T, Koide H. Urinary liver-type fatty acid-binding protein in septic shock: effect of polymyxin B-immobilized fiber hemoperfusion. Shock. 2009;31(5):454–9. doi: 10.1097/SHK.0b013e3181891131. [DOI] [PubMed] [Google Scholar]
- 35.Bagshaw SM, Bellomo R. Urine abnormalities in acute kidney injury and sepsis. Contrib Nephrol. 2010;165:274–83. doi: 10.1159/000313767. [DOI] [PubMed] [Google Scholar]
- 36.Kumar A, et al. Duration of hypotension before initiation of effective antimicrobial therapy is the critical determinant of survival in human septic shock. Crit Care Med. 2006;34(6):1589–96. doi: 10.1097/01.CCM.0000217961.75225.E9. [DOI] [PubMed] [Google Scholar]
- 37.Dellinger RP, et al. Surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock: 2012. Crit Care Med. 2013;41(2):580–637. doi: 10.1097/CCM.0b013e31827e83af. [DOI] [PubMed] [Google Scholar]
- 38.Bagshaw SM, et al. Acute kidney injury in septic shock: clinical outcomes and impact of duration of hypotension prior to initiation of antimicrobial therapy. Intensive Care Med. 2009;35(5):871–81. doi: 10.1007/s00134-008-1367-2. [DOI] [PubMed] [Google Scholar]
- 39.Bradley VE, et al. Renal hemodynamic response to furosemide in septic and injured patients. Surgery. 1976;79(5):549–54. [PubMed] [Google Scholar]
- 40.Brenner M, et al. Detection of renal blood flow abnormalities in septic and critically ill patients using a newly designed indwelling thermodilution renal vein catheter. Chest. 1990;98(1):170–9. doi: 10.1378/chest.98.1.170. [DOI] [PubMed] [Google Scholar]
- 41.Langenberg C, et al. Renal blood flow in experimental septic acute renal failure. Kidney Int. 2006;69(11):1996–2002. doi: 10.1038/sj.ki.5000440. [DOI] [PubMed] [Google Scholar]
- 42.Rivers E, et al. Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med. 2001;345(19):1368–77. doi: 10.1056/NEJMoa010307. [DOI] [PubMed] [Google Scholar]
- 43.Dellinger RP, et al. Surviving Sepsis Campaign guidelines for management of severe sepsis and septic shock. Intensive Care Med. 2004;30(4):536–55. doi: 10.1007/s00134-004-2210-z. [DOI] [PubMed] [Google Scholar]
- 44.Ahmed W, et al. Outcome of patients with acute kidney injury in severe sepsis and septic shock treated with early goal-directed therapy in an intensive care unit. Saudi J Kidney Dis Transpl. 2014;25(3):544–51. doi: 10.4103/1319-2442.132171. [DOI] [PubMed] [Google Scholar]
- 45.Pro CI, et al. A randomized trial of protocol-based care for early septic shock. N Engl J Med. 2014;370(18):1683–93. doi: 10.1056/NEJMoa1401602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Badin J, et al. Relation between mean arterial pressure and renal function in the early phase of shock: a prospective, explorative cohort study. Crit Care. 2011;15(3):R135. doi: 10.1186/cc10253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Asfar P, Teboul JL, Radermacher P. High versus low blood-pressure target in septic shock. N Engl J Med. 2014;371(3):283–4. doi: 10.1056/NEJMc1406276. [DOI] [PubMed] [Google Scholar]
- 48.Godin M, Bouchard J, Mehta RL. Fluid balance in patients with acute kidney injury: emerging concepts. Nephron Clin Pract. 2013;123(3-4):238–45. doi: 10.1159/000354713. [DOI] [PubMed] [Google Scholar]
- 49.Van Biesen W, et al. Relationship between fluid status and its management on acute renal failure (ARF) in intensive care unit (ICU) patients with sepsis: a prospective analysis. J Nephrol. 2005;18(1):54–60. [PubMed] [Google Scholar]
- 50.Payen D, et al. A positive fluid balance is associated with a worse outcome in patients with acute renal failure. Crit Care. 2008;12(3):R74. doi: 10.1186/cc6916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bouchard J, et al. Fluid accumulation, survival and recovery of kidney function in critically ill patients with acute kidney injury. Kidney Int. 2009;76(4):422–7. doi: 10.1038/ki.2009.159. [DOI] [PubMed] [Google Scholar]
- 52.Legrand M, et al. Association between systemic hemodynamics and septic acute kidney injury in critically ill patients: a retrospective observational study. Crit Care. 2013;17(6):R278. doi: 10.1186/cc13133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rajendram R, Prowle JR. Venous congestion: are we adding insult to kidney injury in sepsis? Crit Care. 2014;18(2):104. doi: 10.1186/cc13709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zarychanski R, Turgeon AF, Abou-Setta AM. Meta-analyses of hydroxyethyl starch for volume resuscitation--reply. JAMA. 2013;309(21):2209. doi: 10.1001/jama.2013.5820. [DOI] [PubMed] [Google Scholar]
- 55.Bellomo R, Wan L, May C. Vasoactive drugs and acute kidney injury. Crit Care Med. 2008;36(4 Suppl):S179–86. doi: 10.1097/CCM.0b013e318169167f. [DOI] [PubMed] [Google Scholar]
- 56.Russell JA, et al. Vasopressin versus norepinephrine infusion in patients with septic shock. N Engl J Med. 2008;358(9):877–87. doi: 10.1056/NEJMoa067373. [DOI] [PubMed] [Google Scholar]
- 57.Gordon AC, et al. The effects of vasopressin on acute kidney injury in septic shock. Intensive Care Med. 2010;36(1):83–91. doi: 10.1007/s00134-009-1687-x. [DOI] [PubMed] [Google Scholar]
- 58.Zahar JR, et al. Inappropriate prescribing of aminoglycosides: risk factors and impact of an antibiotic control team. J Antimicrob Chemother. 2006;58(3):651–6. doi: 10.1093/jac/dkl288. [DOI] [PubMed] [Google Scholar]
- 59.Barrett BJ, Parfrey PS. Clinical practice. Preventing nephropathy induced by contrast medium. N Engl J Med. 2006;354(4):379–86. doi: 10.1056/NEJMcp050801. [DOI] [PubMed] [Google Scholar]
- 60.Morelli A, et al. Prophylactic fenoldopam for renal protection in sepsis: a randomized, double-blind, placebo-controlled pilot trial. Crit Care Med. 2005;33(11):2451–6. doi: 10.1097/01.ccm.0000186413.04875.ef. [DOI] [PubMed] [Google Scholar]
- 61.Patel NN, Angelini GD. Pharmacological Strategies For The Prevention Of Acute Kidney Injury Following Cardiac Surgery: An Overview Of Systematic Reviews. Curr Pharm Des. 2014 doi: 10.2174/1381612820666140325113422. [DOI] [PubMed] [Google Scholar]
- 62.Heemskerk S, et al. Alkaline phosphatase treatment improves renal function in severe sepsis or septic shock patients. Crit Care Med. 2009;37(2):417–23. e1. doi: 10.1097/CCM.0b013e31819598af. [DOI] [PubMed] [Google Scholar]
- 63.Pickkers P, et al. Alkaline phosphatase for treatment of sepsis-induced acute kidney injury: a prospective randomized double-blind placebo-controlled trial. Crit Care. 2012;16(1):R14. doi: 10.1186/cc11159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pickkers P, et al. Clinical pharmacology of exogenously administered alkaline phosphatase. Eur J Clin Pharmacol. 2009;65(4):393–402. doi: 10.1007/s00228-008-0591-6. [DOI] [PubMed] [Google Scholar]
- 65.Peters E, et al. Alkaline phosphatase: a possible treatment for sepsis-associated acute kidney injury in critically ill patients. Am J Kidney Dis. 2014;63(6):1038–48. doi: 10.1053/j.ajkd.2013.11.027. [DOI] [PubMed] [Google Scholar]
- 66.Bellomo R. How to feed patients with renal dysfunction. Blood Purif. 2002;20(3):296–303. doi: 10.1159/000047024. [DOI] [PubMed] [Google Scholar]
- 67.Btaiche IF, et al. Amino Acid requirements in critically ill patients with acute kidney injury treated with continuous renal replacement therapy. Pharmacotherapy. 2008;28(5):600–13. doi: 10.1592/phco.28.5.600. [DOI] [PubMed] [Google Scholar]
- 68.McCarthy MS, Phipps SC. Special nutrition challenges: current approach to acute kidney injury. Nutr Clin Pract. 2014;29(1):56–62. doi: 10.1177/0884533613515726. [DOI] [PubMed] [Google Scholar]
- 69.Liu KD, et al. Timing of initiation of dialysis in critically ill patients with acute kidney injury. Clin J Am Soc Nephrol. 2006;1(5):915–9. doi: 10.2215/CJN.01430406. [DOI] [PubMed] [Google Scholar]
- 70.Bouman CS, et al. Effects of early high-volume continuous venovenous hemofiltration on survival and recovery of renal function in intensive care patients with acute renal failure: a prospective, randomized trial. Crit Care Med. 2002;30(10):2205–11. doi: 10.1097/00003246-200210000-00005. [DOI] [PubMed] [Google Scholar]
- 71.Smith OM, et al. Standard versus accelerated initiation of renal replacement therapy in acute kidney injury (STARRT-AKI): study protocol for a randomized controlled trial. Trials. 2013;14:320. doi: 10.1186/1745-6215-14-320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Barbar SD, et al. Impact on mortality of the timing of renal replacement therapy in patients with severe acute kidney injury in septic shock: the IDEAL- ICU study (initiation of dialysis early versus delayed in the intensive care unit): study protocol for a randomized controlled trial. Trials. 2014;15(1):270. doi: 10.1186/1745-6215-15-270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wald R, et al. The association between renal replacement therapy modality and long-term outcomes among critically ill adults with acute kidney injury: a retrospective cohort study*. Crit Care Med. 2014;42(4):868–77. doi: 10.1097/CCM.0000000000000042. [DOI] [PubMed] [Google Scholar]
- 74.Schneider AG, et al. Choice of renal replacement therapy modality and dialysis dependence after acute kidney injury: a systematic review and meta-analysis. Intensive Care Med. 2013;39(6):987–97. doi: 10.1007/s00134-013-2864-5. [DOI] [PubMed] [Google Scholar]
- 75.Sun Z, et al. Continuous venovenous hemofiltration versus extended daily hemofiltration in patients with septic acute kidney injury: a retrospective cohort study. Crit Care. 2014;18(2):R70. doi: 10.1186/cc13827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Investigators, R.R.T.S. Intensity of continuous renal-replacement therapy in critically ill patients. N Engl J Med. 2009;361(17):1627–38. doi: 10.1056/NEJMoa0902413. [DOI] [PubMed] [Google Scholar]
- 77.Network, V.N.A.R.F.T. et al. Intensity of renal support in critically ill patients with acute kidney injury. N Engl J Med. 2008;359(1):7–20. doi: 10.1056/NEJMoa0802639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kellum JA, Ronco C. Dialysis: Results of RENAL--what is the optimal CRRT target dose? Nat Rev Nephrol. 2010;6(4):191–2. doi: 10.1038/nrneph.2010.15. [DOI] [PubMed] [Google Scholar]
- 79.Vesconi S, et al. Delivered dose of renal replacement therapy and mortality in critically ill patients with acute kidney injury. Crit Care. 2009;13(2):R57. doi: 10.1186/cc7784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Cole L, et al. A phase II randomized, controlled trial of continuous hemofiltration in sepsis. Crit Care Med. 2002;30(1):100–6. doi: 10.1097/00003246-200201000-00016. [DOI] [PubMed] [Google Scholar]
- 81.Payen D, et al. Impact of continuous venovenous hemofiltration on organ failure during the early phase of severe sepsis: a randomized controlled trial. Crit Care Med. 2009;37(3):803–10. doi: 10.1097/CCM.0b013e3181962316. [DOI] [PubMed] [Google Scholar]
- 82.Zhang P, et al. Effect of the intensity of continuous renal replacement therapy in patients with sepsis and acute kidney injury: a single-center randomized clinical trial. Nephrol Dial Transplant. 2012;27(3):967–73. doi: 10.1093/ndt/gfr486. [DOI] [PubMed] [Google Scholar]
- 83.Joannes-Boyau O, et al. High-volume versus standard-volume haemofiltration for septic shock patients with acute kidney injury (IVOIRE study): a multicentre randomized controlled trial. Intensive Care Med. 2013;39(9):1535–46. doi: 10.1007/s00134-013-2967-z. [DOI] [PubMed] [Google Scholar]
- 84.Clark E, et al. High-volume hemofiltration for septic acute kidney injury: a systematic review and meta-analysis. Crit Care. 2014;18(2):R7. doi: 10.1186/cc13184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Bagshaw SM, et al. Septic acute kidney injury in critically ill patients: clinical characteristics and outcomes. Clin J Am Soc Nephrol. 2007;2(3):431–9. doi: 10.2215/CJN.03681106. [DOI] [PubMed] [Google Scholar]
- 86.Martin GS, et al. The epidemiology of sepsis in the United States from 1979 through 2000. N Engl J Med. 2003;348(16):1546–54. doi: 10.1056/NEJMoa022139. [DOI] [PubMed] [Google Scholar]
- 87.Jo SK, Rosner MH, Okusa MD. Pharmacologic treatment of acute kidney injury: why drugs haven’t worked and what is on the horizon. Clin J Am Soc Nephrol. 2007;2(2):356–65. doi: 10.2215/CJN.03280906. [DOI] [PubMed] [Google Scholar]